Abstract
Since the time of the Greeks, philosophers and scientists have wondered about the origins of structure and function. Plato proposed that the origins of structure and function lie in the organism's nature whereas Aristotle proposed that they lie in its nurture. This nature/nurture dichotomy and the emphasis on the origins question has had a powerful effect on our thinking about development right into modern times. Despite this, empirical findings from various branches of developmental science have made a compelling case that the nature/nurture dichotomy is biologically implausible and, thus, that a search for developmental origins must be replaced by research into developmental processes. This change in focus recognizes that development is an immensely complex, dynamic, embedded, interdependent, and probabilistic process and, therefore, renders simplistic questions such as whether a particular behavioral capacity is innate or acquired scientifically uninteresting.
“The use of “explanatory” categories such as “innate” and “genetically fixed” obscures the necessity of investigating developmental processes in order to gain insight into the actual mechanisms of behavior and their interrelations” (Lehrman, 1953) p. 345.
“When developmentalists assign causality to autonomous change (maturation), to mental structures that are there from the beginning (innate knowledge), or to factors inherited from parents (genetics), they often stop looking for process, that is, mechanisms of change” (Thelen & Adolph, 1992) p. 378.
When Plato and his pupil Aristotle asked about the origins of human knowledge, they came up with radically different answers. Plato argued that our sense data do not provide sufficient information to specify the abstract ideas and knowledge that humans possess and concluded that we are endowed with such ideas at birth. Plato's view spawned the rationalist school of thought whose basic tenet that all knowledge is innate was later championed by Descartes, Spinoza, Leibniz, and Kant. In contrast to Plato, Aristotle argued that our sense data are sufficient to specify abstract concepts and ideas and, therefore, that human knowledge is acquired through everyday experience. Aristotle's views spawned the empiricist school of thought whose basic belief in the power of experience was subsequently championed by Locke, Berkeley, Hume, and Mill. The divergent views expressed by the rationalists and empiricists gave rise to the nature-nurture dichotomy. This dichotomy has exerted a powerful influence on Western thought in general and on developmental thinking in particular.
In a larger sense, Plato's and Aristotle's arguments about the origins of knowledge are concerned with questions about the origins of structure and function. One way in which Aristotle attempted to shed some light on these questions was to conduct systematic observations of growing chick embryos. Through these observations he discovered that as embryos grow, their various structures and functions undergo transformation. Aristotle named this process epigenesis. Later, with the advent of embryology, this term came to denote the emergence of new structures and functions. Today, in the field of developmental molecular biology, epigenesis has various meanings that generally refer to context-dependent processes that underlie developmental plasticity and canalization, with much of the current focus being on how these processes affect inheritance patterns that are not due to changes in DNA structure (Jablonka & Raz, 2009). Aristotle's focus on epigenesis and the underlying transformations that characterized it provided a key insight into the process underlying development. Unfortunately, because he lacked the basic investigative tools that we take for granted today, Aristotle assumed that the transformations that led to the emergence of biological form were guided by some unknown but predetermined vital force, an entelechy.
THE NEW VITALISM
Aristotle's vitalism has persisted over the centuries. It was particularly in fashion during the 19th century in developmental biology when Hans Driesch, one of the early embryologists, proposed that the development of the embryo is guided by the Aristotelian entelechy. Subsequently, the discovery of the material (i.e., physical) causes of developmental transformation led to the rejection of vitalism by most biologists. Despite this, however, as noted by Noble (2008) and Oyama (2010), vitalism continues to provide a conceptual framework in some biological quarters. This modern version of vitalism accepts biological materialism and merely shifts the burden of control of the developmental process from some previously unknown life-force to some undefined function of the gene.
Psychology often takes its cues from biology and developmental psychologists who subscribe to the rationalist philosophy of innate determinants have adopted the modern form of biological gene-centric vitalism. In essence, they rely on this modern form of vitalism for thinking about their principal question of interest: the developmental origins of knowledge. In this quest, nativists believe that evolution has endowed the human species with something like primitives, core cognitive capacities, or principles that are directly related to specific domains of knowledge including language, object, number, geometry, space, social relations, morality, and religious belief (Baillargeon, 2008; Bloom, 2007; Landau, 2009; Marcus, 2004; Pinker & Bloom, 1992; Spelke & Newport, 1998; Spelke & Kinzler, 2007). These primitives, core capacities, or principles are assumed to have been acquired through natural selection and presumably direct the subsequent developmental acquisition of the knowledge needed for a particular domain. Critically, they are believed to derive from genetic mechanisms whose processes are currently undefined but thought to be definable eventually by developmental molecular neurobiology. Thus, the nativists who subscribe to this view must accept the central biological dogma that genes determine the development of organic structure and resulting function. By extension, they also must subscribe to the view that genes are the ultimate source of knowledge because genes must be in place before conception to guide the development of the individual.
The gene-centric “vitalism” that drives nativist thinking is rather curious given the overwhelming evidence that the central biological dogma that DNA is the repository of all developmental information is a serious distortion of, both, the facts of biology and the complexity of the developmental process (Gottlieb, 1998; Gottlieb, Wahlsten, & Lickliter, 2006; Gottlieb, 2007; Johnston & Edwards, 2002; Karmiloff-Smith, 2009; Keller, 2000, 2005, 2010; Lehrman, 1953, 1970; Mameli & Bateson, 2006; Michel & Moore, 1995; Noble, 2006, 2008, 2011; Oyama, 2000, 2010; Robert, 2001; Schneirla, 1957). Despite this evidence, nativists defer to their gene-centric colleagues in biology and readily accept the infocentric dogma that genes drive development. By doing so, they have replaced Aristotle's vitalism with the material process of gene action and combined it with Plato's rationalist philosophy to yield a gene-centric framework that is explicitly designed to answer the origins question.
Here, I argue that the nature-nurture dichotomy should be abandoned and that gene-centrism and the single-minded focus on the origins question both miss key aspects of the developmental process. They miss the fact that development is an immensely complex, dynamic, and emergent process that does not always yield the perfectly adapted organism. Rather, because of its probabilistic nature (Gottlieb, 1991b, 2007), development is a variable process and, as a consequence, even in the absence of genetic mutations it sometimes yields organisms that are not well adapted. Conversely, even major mutations (either “natural” or produced in the lab via knock-out procedures) sometimes have minimal or unnoticed consequences on development. This variability of developmental outcomes is key for it shows that development is an emergent, rather than predetermined, process and indicates that only a focus on this emergent process can reveal underlying mechanisms (Karmiloff-Smith, 2009; Thelen & Smith, 1994). To begin our examination of the developmental process, we will first consider what might seem to be the heart of the issue - at least for those who subscribe to dichotomous thinking – namely, gene action. Once we do, it will become clear that genes are not where the action is. Rather, we will see that the action is in the system and the complex interactions among its various components.
GENES AND THEIR FUNCTION
Genes have been and continue to be viewed as providing some sort of blueprint. It has repeatedly been noted, however, that this metaphor is highly problematic (Gottlieb, 1998; Keller, 2000, 2005; Lehrman, 1970; Michel & Moore, 1995; Noble, 2006, 2008, 2011; Oyama, 2000). For example, Lehrman (1970) noted that a blueprint specifies an isomorphism between the blueprint and the structure for which it stands for two reasons. The ratios of the lengths and widths in the blueprint are the same as in the structure and the topographic relationships of the parts of the blueprint correspond to the topographic relationship of the parts of the structure. The fact is, however, that the relationship between the genome and the phenotype is nothing like that implied by the blueprint metaphor. In addition, genes code for ribonucleic acid (RNA) which then gets translated into proteins. If so, how do we get from RNA and protein to behavior?
The answer is complicated by the fact that gene expression itself - the transcription of the DNA sequence specifying a particular protein – is a highly regulated and complex process that is intimately dependent on the DNA's cellular environment. That is, gene expression occurs in a context and, thus, one must explicate whether and how the context plays a role in this process. For example, as noted by Noble (2008), one of the most overlooked but critical parts of this context is the maternal egg cell and all of the biochemical substances and machinery therein. The interaction between genes and all of the chemicals in the maternal egg cell (and the paternal sperm cell too) determines what genes are expressed and what proteins are produced and, as a result, it is not just the genome that is inherited but the maternal and paternal germ cells with their constituents as well. In addition, the developmental context created by the mother and the developing organism's species tends to remain constant and is passed down through generations (Bjorklund, 2006; Gottlieb, 2002). Given this overall picture, we might ask how the complex and “low-level” molecular interactions that occur in the fertilized and developing egg cell - and that are so far removed from the behaviors that developmental psychologists study – might be related to behavioral development? The answer is that such complex interactions occur at all levels of organization including the cellular, tissue, organ, system, and environment (Gottlieb, 1991b; Johnston & Edwards, 2002; Michel & Moore, 1995; Oyama, 2000).
The discovery of the complexity and interdependence of gene transcription and the cellular environment has led to a major revision of the very definition of what constitutes a gene (Michel, 2010). A gene is no longer seen as a single well-defined unit of DNA that contains specific information that is read out during protein production. Even developmental biology, which studies how genes control the processes of cell growth, differentiation, and morphogenesis and which, as a result, has the tendency to rely on gene-centric thinking, has long ago recognized the basic fact that genes are not the only ones doing developmental work and that they only code for RNA and proteins. Also, developmental biologists recognized a long time ago that the path from gene to mature structure and function is complex and full of interactions and that these include gene-gene interactions as well as the interactions of genes with their proximal and distal environments (Wright, 1968). Similarly, evolutionary developmental biology - which investigates how a set of “master genes” can account for the enormous diversity of life-forms and their different bodily structures - recognizes that developmental outcomes are due to the interactions among many different genes and the interactions of those genes with their environments (Carroll, 2005). Unfortunately, evolutionary developmental biology has not yet recognized the fact that master genes are themselves regulated by other genes (Robert, 2001) and that genes themselves participate in cascades of developmental events (Noble, 2011).
The revision of the modern gene concept is due to the discovery that genes constitute only 2–3% of the total DNA normally found in the cell nucleus and that the rest of the DNA, previously thought to be “junk”, contains regions that code for regulatory RNA. This RNA is intimately involved in the control of gene transcription by turning specific genes on or off depending on the specific conditions in the cell/organism (Carroll, 2005; Meaney, 2010). In other words, gene expression does not involve the simple read-out of the linearly arranged set of nucleotide base pairs. Instead, it is controlled by a cascade of factors all of which interact with one another in a sequential fashion. Specifically, transcription requires the action of two regions of DNA that are adjacent to the gene. These regions, the promoter and the enhancer, become activated by transcription proteins floating in the cell. Without the action of these proteins and the promoter and enhancer regions, no transcription occurs. These proteins, in turn, are regulated by signals from outside the cell that can be as far removed as the behaviors of others (Champagne, 2008; Meaney & Szyf, 2005; Meaney, 2010).
One example that illustrates the complex sets of reciprocal interactions and the interdependence of the developmental system is the glucocorticoid receptor. It plays a central role in the body's response to stress, is found in nearly all the cells in the body, and is a protein that is present inside the cells and their nucleus. Its function is to bind to glucocorticoids (e.g., cortisol, the steroid hormone involved in the human body's stress response) and once it does, it enters the nucleus where it regulates gene transcription involved in the body's response to the stress. Often, the activated glucocorticoid receptor combines with another protein (a cofactor) and depending on which cofactor that might be, the ultimate effect on subsequent gene expression can be very different because different sets of genes are activated (Meaney, 2010).
Given that genes and their milieu are completely interdependent and that gene expression is the result of a complex and embedded process, it makes little sense to imbue them with privileged status. Developmental control resides in the process itself and no single factor in this process takes precedence. Of course, nativists may not be troubled by this scenario because the concept of interaction (at least between the innate and the learned) is very much a part of their contemporary theoretical framework. Unfortunately, their framework has two problems. First, given the complex processes involved at the cellular level - never mind at the other higher levels and the interactions between them - the concept of the innate and its sister concept of a blueprint do not square with biological reality. Second, the learning part of the nativist dichotomy only refers to the traditional concept of learning that includes classical or operant conditioning, training, practice, and imitation through observation. It misses all the other forms of external and internal stimulation and its developmental trace effects that don't qualify as traditional learning effects but that can have profound effects on organisms and their development. All of these effects, together with traditional learning effects, are part of the broader concept of experience (Gottlieb, 1991b; Lehrman, 1970; Michel, 2010; Schneirla, 1957).
HISTORY REPEATS ITSELF: LESSONS FROM THE PAST
The recent advances in our understanding of the molecular mechanisms involved in gene expression show that a gene-centric, predeterministic developmental framework fails to recognize biological reality. Interestingly, this failure is not so surprising when put into its historical context. Even before the molecular mechanisms described above were discovered, many writers criticized and rejected dichotomous and deterministic thinking as being non-explanatory (Gottlieb, 1997; Lehrman, 1953, 1970; Schneirla, 1957). Perhaps because genetic determinism appears to offer such a seemingly elegant and powerful explanation for a very complex phenomenon, the lessons and the arguments of the past either have been lost on many contemporary researchers or simply have not penetrated contemporary thinking. These lessons and arguments date back to ethology's heyday when concepts such as instinct and fixed action pattern were used to account for and explain the developmental origins and adaptive value of species-specific behaviors (Hinde, 1966; Lorenz, 1965; Tinbergen, 1951). The concept of instinct became associated with Lorenz's work on imprinting in ducks and with Tinbergen's work on the mating behavior of the three-spined stickleback fish. According to Lorenz, an instinctive behavior was a stereotyped action pattern that was innate because it was genetically inherited and immune to the effects of experience. These central ethological concepts had such broad appeal and carried such seeming explanatory power that the assumption that the process of imprinting was an evolutionarily inherited adaptation was incorporated into explanations of the formation of social attachment in humans (Bowlby, 1969). Needless to say, the process underlying the formation of social bonds in birds and humans is vastly different. In birds it depends on the young learning the call and visual characteristics of the mother over a relatively short period of time, whereas in humans it depends on a myriad of sensory, perceptual, cognitive, affective and social factors that all interact over a long period of time.
In his well-known critiques of the central ethological concepts and claims, Lehrman (1953, 1970) pointed out that substantiating such claims requires careful and detailed studies of the developmental processes that contribute to the ontogeny of the behaviors in question. As Lehrman as well as others (Schneirla, 1957) pointed out, such studies were absent in Lorenz's and Tinbergen's work. As a result, their assumption of innateness was unsupported and, indeed, later Lorenz noted that his interest was not development but adaptiveness (Lorenz, 1965). In addition, the use of unitary concepts to explain what are seemingly similar but in reality very different behaviors across different species is risky at best (Schneirla, 1949). For example, given the radically different conditions under which attachment develops in ducks and humans, there is little doubt that the processes underlying its emergence in each species are very different.
Lehrman's critiques of ethological concepts stemmed from his careful analysis of the myriad problems associated with dichotomous conceptualizations in developmental work. His powerful critiques led to serious questioning of ethological concepts and to subsequent experimental studies substantiating the basic principle that the emergence of any behavior is the result of highly dynamic and complex processes. Unfortunately, the lessons learned from the debates surrounding ethological concepts either have been forgotten or ignored in some contemporary developmental quarters.
EXAMPLES OF A MODERN NATIVIST APPROACH & ITS LIMITATIONS
Two recent studies illustrate the point that the lessons from the past have been forgotten and that dichotomous thinking with a nativist focus continues unabated today. The first is a study by Hamlin, Wynn, and Bloom (2007) in which the investigators asked whether 6- and 10-month-old infants can evaluate others' social intentions. Infants were first habituated to an event in which an inanimate “climber” object (a round disk with “googly” eyes on it) could be seen climbing a hill and another object with eyes on it (a triangle or a square) could be seen either helping it climb by pushing it up or hindering it from climbing by pushing it down. Following habituation, infants' preference for the helper or hinderer was measured in two ways. First, the climber was shown alternately sitting close to the helper or the hinderer and visual preferences were measured. Then, infants were allowed to reach either for the helper or the hinderer.
Results indicated that the 10-month-old infants looked longer at the hinderer but that the 6-month-old exhibited no visual preference. The authors interpreted the 10-month-olds' preference for the hinderer as a reflection of their being surprised that the climber would spend time sitting next to the hinderer. In contrast to the visual preference results, the results from the reaching trial indicated both age groups reached more for the helper. To determine whether the eyes were critical in signaling social interactions, the experiment was repeated with a climber who did not have eyes. Only a reaching test was administered and this time infants no longer preferred the helper. In follow-up work, Hamlin and colleagues (Hamlin, Wynn, & Bloom, 2010; Hamlin & Wynn, 2011) have extended this work to 3-month-old infants and to new objects (puppets) and have reported that 3-month-olds also prefer the helper. This time, however, the preference was based on visual fixation.
In the original report, Hamlin, Wynn, and Bloom (2007) concluded that preverbal infants can assess individuals on the basis of their behavior towards others. Furthermore, the authors concluded that “the capacity to evaluate individuals on the basis of their social interaction is universal and unlearned” (p. 559) and that this capacity reflects the operation of a “biological adaptation” (p. 558). Though not explicitly defined by the authors, the concept of biological adaptation usually implies that the trait in question is encoded in the organism's evolutionary (i.e., genetic) history (Chomsky, 1965). What is surprising is that the authors draw such farreaching conclusions despite the lack of any direct evidence to support their twin claims of universality and biological adaptiveness and despite the myriad problems associated with genecentric thinking that render the very idea of encoded traits questionable. In addition, it is surprising that the authors are willing to rule out the likely contribution of social experience given findings from humans and non-humans showing that social experience plays a critical role in development in a myriad of ways (Gottlieb, 1991a, 1991b, 1997; Lewkowicz & Ghazanfar, 2009; Maurer, Mondloch, & Lewis, 2007; Michel & Tyler, 2005; Nelson, 2001; Werker & Tees, 2005).
In addition to the general conceptual issues, the Hamlin et al. studies raise a number of specific process-related issues. First, why should infants generalize their social knowledge - especially if it is universal and, therefore, a reflection of how real humans behave - to highly unrealistic objects and why should they do this as early as three months of age? Put differently, might infants evaluate social relations performed by humans in the same way that they evaluate them with non-human objects? Second, no data are provided on infant hand preference in any of the studies and on whether this might be related to the performance of the 5–6 month-old and 9–10 month-old infants. Infant hand use changes during this age period (Michel, 2002) and, thus, it is legitimate to ask whether the types of reaches differed as a function of age in their form and latency, and, if so what that means about infant choice. Third, what would happen if the stimuli were not withdrawn too quickly? Would the infants' contact with the initially chosen object persist or might infants switch to the other object and, if so, what might that tell us about their choice? Fourth, the experimenter interacted with and spoke to the infants while presenting the objects to them. What role might the experimenter's behavior have played in the infants' reaching behavior? Might the experimenter's behavior have either aroused the infants and/or introduced some sort of social “contagion” during the choice test?
Perhaps the biggest problem with the Hamlin et al. results is that the visual preference data are inconsistent across the various ages and experiments and that the visual preference procedures used across the ages were not the same. Specifically, the 3-month-olds exhibited a visual preference for the helper (Hamlin, et al., 2010; Hamlin & Wynn, 2011), the 10-month-olds exhibited a preference for the hinderer (Hamlin, et al., 2007), and the 6-month-olds exhibited no visual preference for either object (Hamlin, et al., 2007). In a follow-up to the original study (Hamlin, et al., 2010), 6-month-olds were found to look more at the helper but the problem here is that the findings from this study come from a reaching task and not the same visual preference test that was given in the original study nor in the 3-month studies. These inconsistent visual preference results beg the question of why the 10-month-olds were surprised and why they looked more at the hinderer and why the 3- and 6-month-old infants were not surprised. This is especially worrisome because the authors explicitly made predictions in the original study based on the violation-of-expectancy procedure (VEP) where infants are supposed to exhibit surprise when confronted with “unusual” events. The VEP procedure has been used in scores of studies to demonstrate infant understanding of objects and surprise has been reported in infants as young as three months of age (Baillargeon & DeVos, 1991). Indeed, studies using the VEP have been used to overturn the traditional Piagetian dogma that an understanding of objects and their properties emerges slowly during early development. The principal rationale underlying the studies using the VEP has been that reaching is not sufficiently sensitive to reveal infants' true knowledge but that looking is. Interestingly, however, for Hamlin et al. reaching and looking measures are interchangeable despite the inconsistent picture that they provide.
I have extensively examined the Hamlin et al. studies only because they illustrate the principal logic that drives so much “nativist-minded” research in human infancy. Researchers with this perspective posit a priori that some behavioral capacity is innate and/or part of the species' core knowledge system and this assumption is, in turn, based either on an underlying and sometimes explicit assumption that this capacity is encoded in the organism's biological make-up (i.e., genetic endowment). They then conduct tests with infants who are unlikely to have had a great deal of experience and when they find that this particular behavioral capacity is present, they then conclude that this capacity must be predetermined. It is interesting to note that this is exactly the approach that was initially used by ethologists in their developmental research and that it changed after it was so effectively critiqued by Lehrman.
Some researchers who subscribe to the dichotomous nature-nurture framework practice a slightly weaker form of nativism. They tend to be more agnostic about the ultimate origins of structure and function but, like those who practice the stronger version of nativism, they still seek to distinguish between innate and learned. For these researchers, as long as those abilities appear at birth, they are considered to be inborn (and, thus, innate) because no postnatal learning could have contributed to their acquisition. This weaker form of nativism usually does not explicitly posit genetic origins, although it comes close to it. For example, Slater and Kirby (1998) reviewed evidence on newborn infant response to faces and to multisensory relations and concluded that infants have an innate representation of faces and that they are prepared to perceive multisensory relations. Moreover, they suggested that these abilities are evolutionary in origin, implying that some sort of hereditary mechanism is likely to be involved. Slater (2004) repeated this conclusion in a review of the development of face perception by asserting that even though in-utero propioceptive feedback may contribute to newborns' representation of faces, the in-utero experience interacts with “innate evolutionary biases” (p. 21). Although Slater does not specify what he means by innate evolutionary biases, others who practice the strong version of nativism are clear about this. For example, the subtitle of Marcus' (2004) book is: “How a Tiny Number of Genes Creates the Complexities of Human Thought”.
A second study that illustrates the nativist approach - though a somewhat weaker version of it - is a recent one by Walker et al. (2010). These investigators asked whether infants are synaesthetes. In synaesthesia, sensations in one modality evoke sensations in a different modality suggesting that the senses work in an integrated fashion. Although synaesthesia is a rare case of unusual multisensory perception in adults (Cytowic, 2003; Marks, 1978), it is special because synaesthetic experiences appear to be automatic and, thus, obligatory. The expectation that infants might be synaesthetes is based on the theory that the exuberant neural connections that are typically found in early neural development create opportunities for cross-modal cross-talk (Spector & Maurer, 2009). If infants are synaesthetes then this would suggest that infants perceive their multisensory world as an integrated and coherent place rather than as a collection of unrelated sights, sounds, touches, smells, and tastes. If they don't perceive their multisensory world as a coherent place this would mean that they experience William James' blooming, buzzing, confusion.
Walker et al.'s (2010) explicit aim was to test the “perceptual innateness of synaesthetic cross-modality correspondences (p. 22)” in the absence of learning based on language comprehension. To test it, these investigators conducted two experiments with 3–4 month-old infants by examining the amount of time they looked at events that either were consistent or inconsistent with synaesthetic multisensory relations. One type of event consisted of a sound varying in pitch and a visual object that varied in height and the other of a sound varying in pitch and an object that varied in its pointedness. Thus, in one experiment, infants saw a bouncing ball rising and falling together with a sound whose pitch rose and fell (congruent condition) or together with a sound whose pitch fell and rose (incongruent condition). In a second experiment, infants watched a geometric shape morphing constantly between two extreme forms of pointedness together with a sound whose pitch either rose and fell as the shape became more and less pointed (congruent condition) or together with a sound that fell and rose (incongruent condition). Walker et al. found that infants looked more at the congruent events in both experiments and concluded that this is evidence of synaesthesia, that the perception of synaesthetic correspondences is unlearned, and that this ability is phylogenetic in origin.
These findings and the conclusions drawn from them raise several questions. With specific regard to the study design and the results reported, it is not clear on a priori grounds why infants should look longer at the congruent than the incongruent event (no predictions are offered) and what the preference actually means. As Haith (1998) has noted, it is not always clear what infant looking preferences mean unless we can make strong a priori predictions. The most parsimonious conclusion that can be drawn from the Walker et al. data is that infants can discriminate between the two events and that they prefer to look at one of them, but why they do so is not clear. In other words, even though the Walker et al. study addresses a fundamental question, it does not provide direct evidence of synaesthetic perception in infants. Such evidence could, however, be obtained with the standard intersensory matching procedure where two different visual events are presented side-by-side together with a sound that corresponds to one of them. That is, two objects bouncing out of phase with respect to one another can be presented together with a sound that bears a synaesthetic relationship with respect to one of them and a non-synaesthetic one with respect to the other. Previous studies using this procedure and similar stimuli, in which 4-month-old infants' ability to make auditory-visual (A-V) matches based on non-synaesthetic relations were investigated, have yielded evidence of matching (Lewkowicz, 1992). As a result, by substituting a sound that increases and decreases in pitch, it would be possible to determine whether young infants can perceive synaesthetic relations.
Assuming for a moment that the outcome of the proposed experiment were consistent with synaesthesia, such findings would still not justify the conclusion that this is an innate ability. Walker et al. justify their conclusion by indicating that it is difficult to identify natural co-occurrences that might support the kinds of auditory-visual correspondences presented in their study. Often, however, experience can have non-obvious and surprisingly indirect effects (Goldstein & Schwade, 2008; Gottlieb, 1991a). One such non-obvious influence might be the exposure that young infants have to infant-directed speech (IDS) and their robust preference for it (Cooper & Aslin, 1990; Fernald, 1985). IDS is characterized by high overall pitch, expanded pitch excursions, slow tempo, and overall rhythmicity and it is used from birth through the first months of the infant's life (Fernald & Simon, 1984; Fernald & Mazzie, 1991; Papoušek, Papoušek, & Haekel, 1987). At birth, 77% of the speech that mothers direct to their newborns consists of expanded pitch contours - these are rarely seen in adult speech - and these expanded contours consist of 37% rising contours and 24% falling contours (Fernald & Simon, 1984). The story is similar at three months of age where around 65% of all mother as well as father utterances directed to their infants are unidirectional in frequency contour and consist of rising and falling contours, with about 10% more of them being rising (Papoušek, et al., 1987). These findings indicate that parents produce lots of rising and falling contours when they produce infant-directed speech. In addition, this kind of speech is usually accompanied by exaggerated mouth, head, and body movements that often visually punctuate the tops or bottoms of the contours. If that is the case, and if infants can perceive synaesthetic cross-modal relations, then audiovisual attributes of IDS may contribute to this ability but in a non-obvious way.
A second non-obvious influence on the development of early synaesthetic perception might be the ways in which caregivers move infants whenever they play with them. Infants love to be bounced to a song or rocked to a lullaby. Each time they are bounced or rocked, they receive concurrent vestibular stimulation that is usually associated with visual movement. These kinds of early cross-modal experiences are more than likely to influence infants' developing preferences for specific types of cross-modal relations. For example, 7-month-old infants take advantage of vestibular-auditory relations in their learning of specific metrical patterns in music. When they listen to a non-rhythmical sequence of sounds but experience concurrent rhythmical body movement, they end up preferring a sound sequence with the same rhythmical pattern (Phillips-Silver & Trainor, 2005). This suggests that infant-directed speech or play - where infants have access to concurrent auditory, visual, and vestibular information – offers infants lots of opportunities to develop and tune their preferences for specific types of cross-modal relations that may include synaesthetic ones. This scenario provides an alternative process-oriented approach to the very interesting question investigated by Walker et al. without any a priori assumptions of predetermined causes.
THE NATURE-NURTURE DICHOTOMY & ITS LIMITATIONS
As seen above, the nature-nurture dichotomy continues to motivate some developmental studies. Although the dichotomy may have been a reasonable starting point for the philosophical and scientific consideration of developmental questions, it is now patently clear that the dichotomy has serious conceptual limitations on both sides. As already discussed, dichotomous thinking is biologically implausible. Even if this were not the case, however, the rationalists' assumption that structure and function are predetermined by genes is a non sequitur because no organism can possibly develop in a vacuum; its environment must in some measure contribute to its development. Certainly, everyone would agree that no organism can develop in the absence of oxygen, proper nutrition, and the correct temperature, never mind the usual stimulation that organisms receive from their caregivers. Of course, some might argue that these types of environmental factors should be considered as supportive whereas the “biological” factors (i.e., genes) should be considered as critical because they are what gets passed down through heredity. As Noble (2008) points out, however, “…by itself, DNA does nothing at all” p. 3003. Similarly, Meaney (2010) notes that “… at the level of biology, there are no genes for intelligence, depression, athletic abilities, fashion sense, or any such complex trait.” p. 45. Consequently, the more reasonable view – adopted by those who accept the validity of dichotomous thinking (but see below where such thinking is rejected outright) - might be that both nature and nurture matter in development. Of course, this means that the rationalist argument is flawed from the start. On the empiricist side, the logical problems are no less serious. The empiricists' assumption that structure and function are fully determined by environmental influences is equally problematic in that an organism's biological endowment (however loosely it might be defined) obviously contributes in a major way to its development.
Reflecting these basic and obvious conceptual problems, most contemporary developmental scientists now acknowledge the complexity of the developmental process and recognize the basic fact that the emergence of particular structure and function is the result of the reciprocal interaction of biology and environment. Even ardent nativists (Marcus, 2004; Spelke & Newport, 1998; Spelke & Kinzler, 2007) agree with this characterization of development. Unfortunately, despite their seeming interactionist position, the dichotomy between different and separable parts – the innate and the acquired – is fundamental to the nativists' view that some forms of knowledge are part of our inherited system of core knowledge. As seen earlier, nativists motivate their experiments in terms of the nature-nurture dichotomy and ask origins-oriented rather than process-oriented questions. The problem is that the dichotomy ignores the fact that developing organisms are fused systems wherein organismic and environmental factors are in such continuous interaction that it makes no heuristic sense to treat them as separable influences (Bateson, 2005; Gottlieb, 1997; Griffiths & Gray, 2004; Lehrman, 1953, 1970; Overton, 2006; Oyama, 2000; Schneirla, 1957). Complicating matters even more is the fact that the typical organism-environment interaction is at one scale of many. Although we usually think of every stimulative influence outside of the organism as constituting the environment, there are also “internal” environments that provide developmental contexts (Michel, 2010). For example, DNA resides in and interacts with the microscopic environment of its host cell (Noble, 2008) as well as the external environment of the organism (e.g., epigenetic effects of maternal behavior).
The shift in focus from gene to organism is critical for understanding the role of genes in development because genes are embedded in the organism's many other levels of organization including the cellular, tissue, organ, and system levels and there are interactions across all of these levels (Gottlieb, 1992; Johnston & Edwards, 2002; Michel & Moore, 1995). This means that genes are completely embedded within the organism and, therefore, are not autonomous agents driving development. For example, beginning at the genetic level, gene expression depends intimately on the contents of the maternal and paternal germ cells (Meaney, 2010; Noble, 2008). The same is true at the other end of the hierarchy where the effects of externally generated as well as self-generated stimulation can influence perceptual and neural function. For instance, at the behavioral level, the specific coordination dynamics of the motor system at a particular point in development play a key role in the infants' ability to locomote under specific environmental challenges and in their ability to perceive and understand their world (Adolph, 2000; Corbetta & Bojczyk, 2002; Thelen & Smith, 1994). At the neurobehavioral level, the development of the neural mechanisms underlying the emergence of cognitive abilities is determined by the bidirectional co-actions of genes, brain, and the child's environment rather than the unidirectional action of genes (Karmiloff-Smith, 2009). Most remarkable and as discussed below, the organism's environment and its stimulative effects can have effects that penetrate all the way down to the genetic level.
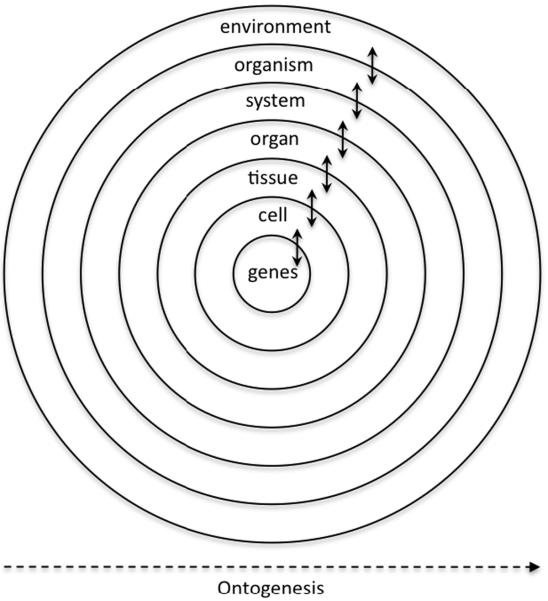
Figure 1 illustrates one way to conceptualize the dynamic and complex nature of the developmental process. Shown here is the complexity of the interactions that occur at each level of organization and the embeddedness of the developmental process. In particular, the figure illustrates the fact that each respective level of organization is embedded in all the other levels of organization and that each level of organization interacts bidirectionally with all the other levels. In this system, there are bottom-up and top-down influences and, critically, one depends on the other – the system is fully bidirectional. How the bidirectional interactions at one level are related to the interactions at the other levels is currently still poorly understood and the answer to this question requires a systems perspective and a process approach to the design of research. What is clear, however, is that this kind of a system has the power to self-organize and to produce emergent properties. It is also clear that such a highly dynamic and embedded system has no single driver nor blueprint; novel behaviors arise as a function of the many possible interactions. Thus, knowledge is created through the dynamics of the developmental process rather than through some predetermined process that endows organisms with a static representation of the world at the beginning of life (Edelman, 1992; Lewis, 2000; Michel & Moore, 1995; Quartz & Sejnowski, 1997; Smith & Thelen, 2003; Thelen, 2000).
Figure 1.
The embedded nature of the developmental system. Each lower level of organization is embedded within the next higher level and each interacts directly with its neighbors and, ultimately, indirectly with all the other levels.
WHY DOES THE DICHOTOMY PERSIST?
Despite the fact that the developmental process is dynamic, embedded, and reciprocally interactive and that neither the nature/nurture dichotomy nor its more contemporary interactionist cousin provide a satisfactory framework for thinking about development (Johnston, 1987; Lehrman, 1970; Spencer, Samuelson, et al., 2009; Spencer, Blumberg, et al., 2009), dichotomous thinking persists (Scholl, 2005). If anything, in some quarters, dichotomous thinking has now evolved to a more nuanced form that on its surface appears to be consistent with a systems perspective but in reality is still dichotomous and predeterministic. This is illustrated by the theory of the growth of the mind by Marcus (2004). In his theory, Marcus proposes that development is driven by gene-environment interactions and not by some genetic blueprint but then, in a strange twist of argument, he assigns privileged and autonomous status to genes. This is clear from his assertion that the genome is a “complex, dynamic set of self-regulating recipes that actively modulate every step of life” (p. 169). In addition, Marcus does not view experience as an equal partner in development but rather as something that merely refines what has first been created by genes. This is evident in his contention that “embryos are endowed both with systems for creating structure independently of experience and with mechanisms for recalibrating those structures on the basis of experience” (p. 166).
The nativists' dogged adherence to the dichotomous and gene-centric framework begs the question of why it persists. Michel and Moore (1995) suggest that one reason is that this kind of thinking is consistent with the concept of reaction range (even though this concept and some of the empirical evidence used to argue in its favor have been the subject of serious criticism (Gottlieb, 2007; Platt & Sanislow, 1988)). According to the concept of reaction range, the genotype sets a priori limits on psychological potential and, thus, the genotype predetermines the range of possible psychological characteristics (phenotypes) that can emerge during development. This general view has been held by those interested in behavioral development long before cognitive developmental psychologists began to link specific early capacities with innate causation. For example, as Thelen and Adolph (1992) show in their review of Arnold Gesell's contributions to developmental psychology, Gesell firmly held to the maturationist view that the ultimate cause of development is the unfolding of the genetic program. In addition, although Gesell recognized developmental plasticity and individual differences, he felt that plasticity does not reflect environmental influences but rather that it itself is genetically determined. This belief in the genetic control of plasticity is similar to the idea behind the reaction range concept that distinct genotypes code for a distinct range of phenotypic outcomes.
GENE X ENVIRONMENT INTERACTIONS
Earlier it was noted that organisms and environments are in constant interaction and that those interactions can take place at many different scales that can range from genes and their immediate cellular environments to genes and the organism's external environment (see Fig. 1). Thus, it is legitimate to ask how genes and the environments in which they are embedded interact with one another. Two often-cited studies provide good illustrations of such interactions. One of these studies by Newman and colleagues (Newman, et al., 2005) investigated whether the relationship between a gene involved in the production of circulating serotonin levels – the monoamine oxidase A (MAOA) gene - and aggression might be mediated by early rearing experience. MAOA is an enzyme that oxidizes and, thereby, reduces circulating serotonin levels in the brain; low levels of MAOA (i.e., high levels of serotonin) are associated with aggressive behavior. Newman and colleagues raised low- and high-activity MAOA genotype groups of rhesus monkeys either with their mothers or with their peers and then assessed their aggressive behavior in a food-competition and a social interaction condition. Findings indicated that genotype affected aggressive behavior in mother-reared but not peer-reared monkeys in both aggression conditions. In the competitive condition, low-activity MAOA/mother-reared monkeys had higher competitive aggressive behaviors than, both, high-activity MAOA/mother-reared monkeys and low-activity MAOA/peer-reared monkeys. Similarly, in the social interaction condition, low-activity MAOA/mother-reared monkeys spent more time engaged in aggressive behavior than, both, high-activity MAOA/mother-reared monkeys and peer-reared subjects with either allele. In other words, a specific genotype does not determine a specific phenotype; rather, it interacts with specific experience to determine the phenotype.
Another study by Caspi and colleagues (Caspi, et al., 2003) investigated the relationship between a genetic polymorphism in the promoter region of the serotonin transporter gene and depression. This genetic polymorphism can result in individuals who either have the short or long allele of the serotonin transporter gene. Those with the short allele produce less transporter protein while those with the long allele produce more of it. This protein is involved in the re-uptake of circulating brain serotonin levels and, thus, short-allele individuals who have less of the protein have more circulating serotonin and, as a result, are more anxious, fearful, and depressed. The situation is opposite in individuals with the long allele of the transporter gene.
Caspi and colleagues assessed the effects of stress experienced between 21 and 26 years of age on three groups of individuals who either had two copies of the short allele, one copy of the short allele, or two copies of the long allele. Findings indicated that the relation between the participants' report of depressive symptoms at age 26 as a function of stress was significantly stronger for those individuals with the short allele than for those with two copies of the long allele. Particularly interesting from a developmental standpoint was the additional finding that there was a relationship between the degree of maltreatment between 3 and 11 years of age and adult depression and that this relationship was related to specific genotype. Individuals who had two one or two copies of the short allele reported increasing depression as a function of degree of maltreatment whereas those who had two copies of the long allele exhibited no such relationship.
The Caspi et al. (2003) findings initially led to a great deal of excitement in the psychiatric community because they seemed to provide a marker for depression that in interaction with knowledge of stressful life-events could predict depression. Unfortunately, some subsequent studies have failed to replicate the Caspi et al. study. In a meta-analysis (Risch, et al., 2009) of 14 studies that attempted to replicate the gene x environment interaction reported by Caspi et al., Risch and colleagues (Risch, et al., 2009) found a clear relationship between stressful events and depression but they did not find any evidence that depression due to exposure to stressful events was linked to the allele for the serotonin transporter gene. In a reply to Risch et al., Caspi and his colleagues (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010) have argued that the meta-analysis overlooks key differences among the various attempts at replication and that these are likely to account for the different findings. In particular, Caspi et al. (2010) note that many of the studies that have failed to replicate their findings are plagued by poor measurement of early life-stress. These studies rely on brief self-report measures of stress, whereas studies that have replicated the Caspi et al. (2003) findings used objective indicators or face-to-face interviews to assess stress exposure. In sum, Caspi et al. (2010) point out that it is necessary to focus on a specific, homogeneous, developmentally relevant, and clearly operationalized depression-inducing events in order to decrease between-subject heterogeneity in exposure to such events and, thus, maximize the internal validity of the study design.
The Caspi et al. (2003) findings and subsequent attempts to replicate them raise interesting questions regarding gene x environment interactions. Caspi et al.'s (2010) response to the failures to replicate is certainly sensible in that careful measures of early developmental stress events are essential to the validity of these types of studies. In addition, it is possible that the gene x stress interaction that presumably disposes individuals with a particular genetic profile for depression may be mediated by the interaction of life stress and the multiple genes that are involved in the synthesis and regulation of brain serotonin levels. Of course, even if that is the case, a multiple-gene x environment interaction does not tell us very much about the developmental conditions that lead up to a particular outcome. Many different phenotypic outcomes are possible given a particular genotype and a priori predictions are difficult without a full understanding of the developmental conditions that mediate the expression of the phenotype. Although Caspi et al. (2003) did examine the relationship between early maltreatment and subsequent depression, the measures of maltreatment only scratch the surface of the day-to-day developmental conditions that each individual enrolled in the study experienced during his/her early years (Gottlieb, 2007; Michel, 2010). Consequently, the Caspi et al. (2003) study leaves unanswered the question of how specific and ongoing early experience contributes to the observed phenotypic outcome.
As indicated earlier, behavioral development is a dynamic, embedded, and reciprocally interactive system. If experience - broadly defined as any stimulative activity that includes trace effects from earlier times in ontogeny (Schneirla, 1966) - plays a key role then this requires careful studies of the interaction between the many experiential factors during an individual's ontogeny and the organism (including its genotype). Such careful studies might reveal that one of the reasons for the failures to replicate the initial Caspi et al. study may be that the ontogenetic histories of the participants in those different studies were quite different and not, as Risch et al. (2009) have concluded, that the genotype plays no role in the overall developmental process.
EXTRAORDINARY PRODUCTS OF DEVELOPMENT
One of the reasons that the reaction range concept has been so compelling to psychologists is because development usually produces organisms that exhibit a relatively restricted range of species-specific structural and functional characteristics. That is, all humans resemble one another, possess a certain set of well-defined and common motor and cognitive abilities, and differ from other primates in that the latter share some structural and functional characteristics with humans but differ in some critical ways (e.g., non-human primates do not possess a fully opposable thumb nor language). Sometimes, however, development yields individuals who fall outside the normal range (Blumberg, 2008). At the “high” end, such individuals can be people like Albert Einstein, with his extraordinary mathematical powers, Amadeus Mozart, with his musical genius, and Michael Jordan, with his amazing athletic prowess. At the “low” end, such individuals are illustrated by two-headed monsters and one-eyed cyclops. How might we explain these extraordinary products of development and what might they tell us about the developmental process? From an origins perspective, one might posit that a single gene or a complex of genes determine whether a developing individual ends up at one or the other extreme end of the developmental spectrum. Indeed, traditional biological approaches usually engage in a search for a single gene or multiple genes and this search is based on the gene-centric assumption that genes provide the blueprint for developmental outcomes.
Development Gone Awry
If gene action is a complex and embedded process, and if the broader concept of experience allows for non-learning types of influences on gene expression, then how might such effects manifest themselves? To answer this question, let's consider the seemingly simple question of how we get from gene expression to anomalous organic structure by considering the development of one-eyed monsters. In sheep, this anomaly, known as holoprosencephaly, arises when pregnant sheep graze on the lily Veratrum californicum. The offspring of these sheep are born with a single central eye, missing nasal and jaw structures, and incompletely developed brain hemispheres. As might be expected, these developmental anomalies are lethal and these offspring do not survive. Studies have shown that the malformations associated with holoprosencephaly are caused by exposure of the developing embryo to the chemical cyclopamine that is found in the lily. Critically, the embryo must be exposed to the teratogen at day 14 of gestation and holoprosencephaly only results when the teratogen interacts with a protein known as sonic hedgehog that is expressed by the sonic hedgehog gene (Shh)). Introduction of cyclopamine at different times into the developing system leads to different sets of deformities (Cordero, et al., 2004). Thus, the timing of exposure to the teratogen during early development matters and this demonstrates that it is neither the Shh gene alone nor cyclopamine alone that produce the malformation; rather, it is their co-action and its specific timing that leads to the anomaly. This illustrates the fundamental fact about development that the specific timing of the interaction between specific factors (usually defined as the sensitive period) is crucial in structural as well as functional development (Bateson, 1979; Blumberg, 2008; Lewkowicz & Ghazanfar, 2009; Michel & Tyler, 2005; Oppenheim, 1981; Turkewitz & Kenny, 1982; Werker & Tees, 2005). More broadly, the development of holoprosencephaly is consistent with the norm of reaction concept and its primary assumption that the products of genotype x environment interaction cannot be determined a priori because they truly depend on specific interactions taking place at particular points in the developmental process.
Despite the myriad problems with the concept of genetic blueprint, the biological/genetic approach to the study of development is very alluring because it offers the possibility of gaining insights into what are undoubtedly fundamental processes underlying developmental outcome. Earlier it was noted that one reason why developmental psychologists of the nativist persuasion still frame their explanations in dichotomous terms and why they like to resort to gene-centric interpretation is because of the wide acceptance of the reaction range concept. A second and more recent reason for the nativists' gene-centric approach is the emergence of evolutionary psychology. Its main goal is to link behavioral traits with our evolutionary past (while ignoring developmental processes). According to evolutionary psychology, the behavioral traits must have a biological (i.e., genetic) cause because of the neo-Darwinian dogma that natural selection operates on genes and their population frequency during evolution. As a result, linking biology with behavioral abilities provides a gateway to not only understanding the evolution of behavior but to the relationship between evolution and development (Bjorklund, 2006). While this may be a fair extension of evolutionary theory, the evolution of any trait involves the passing on of the species genome as well as the species' developmental/experiential histories acquired during each generation (Bateson, 2005; Gottlieb, 1992, 2002; Griffiths & Gray, 1994). These generational histories, known as developmental manifolds (Gottlieb, 2002), are passed down from generation to generation and natural selection operates on them rather than on genes alone. If so, the mechanism that provides cross-generational developmental stability to a species is not genetic transmission alone but transmission of genes embedded in the developmental process.
GENERATIONAL TRANSMISSION OF MATERNAL BEHAVIORS
The emphasis on the whole organism and its developmental context requires us to focus on the resources that are available in earlier generations and ask whether their availability in subsequent generations makes a difference. If it does then this would suggest that species-typical traits are reconstructed in the next generation through the interaction of all the developmental resources that include the genome and the organism's developmental resources. In other words, individual organisms and their ontogenies are constrained by species-specific developmental constancies that persist across generations and, together with the species' genetic endowment, create structural and functional continuity across generations. The results of studies of the cross-generational transmission of individual differences in the maternal behavior of primates and rodents (Francis, Diorio, Liu, & Meaney, 1999; Maestripieri, 2005) illustrate this process in an especially compelling way.
In rodents, mothers lick and groom their pups after giving birth. Some mothers exhibit low levels of licking and grooming (low-LG mothers) while others exhibit high levels of licking and grooming (high-LG mothers). The daughters and grand-daughters of low-LG mothers become low-LG mothers while the daughters and grand-daughters of high-LG mothers become high-LG mothers. The offspring of high-LG versus low-LG mothers differ in a number of ways. That is, the offspring of low-LG mothers are less able to regulate the release of stress hormones when placed in a stressful situation and exhibit less exploratory behavior, greater behavioral inhibition, and poor maternal behavior.
Using the cross-fostering technique, both primate and rodent studies have shown that it is maternal behavioral style that determines offspring maternal behavior (Francis, et al., 1999; Maestripieri, 2005). For example, when pups from low-LG mothers are cross-fostered to high-LG mothers, they become high-LG mothers (Francis, et al., 1999) indicating that instead of being an inherited phenotypic characteristic, maternal style is a function of the female's early experience. Especially interesting, the rodent studies also have revealed how different types of maternal experience can initiate different patterns of gene expression (Champagne, 2008). In essence, different types of maternal behaviors can induce different patterns of methylation of DNA sequences and this can lead to the silencing of particular parts of the genome. To understand these epigenetic effects it is first necessary to understand the structure of the genome. Normally, DNA is wrapped around a complex of histone proteins and together they form clusters that are known as chromatin. In order for DNA to be expressed, it must first come into contact both with the enzyme RNA polymerase and transcription factors. Before that happens, DNA must be unwrapped from the histone proteins. When it is unwrapped, the nucleic acid sequences are exposed and it is at this point that environmental factors can exert their epigenetic effects. These epigenetic effects result in changes in gene expression - and therefore phenotype – and, crucially, these effects are not due to changes in the sequence of base pairs that make up DNA (as is the case in genetic mutation). During the methylation process that is involved in maternal behavior, methyl groups attach to cytosine (one of the DNA bases) in the promoter region of the DNA that is upstream from the site where transcription (i.e., protein production) takes place. When the promoter is methylated, transcription factors lose access to the gene and the gene is silenced. In the case of maternal behaviors, in the low-LG females, the methylation occurs in the estrogen receptor alpha (ERα) - this is a ligand-activated transcription factor that is essential for gene transcription in response to the circulating hormone estrogen following parturition. Because estrogen mediates maternal behavior, and because low-LG offspring have low levels of ERα, they cannot bind circulating estrogen and, as a result, don't engage in maternal behavior.
The methylation that occurs in low-LG female offspring demonstrates that inheritance of maternal behavior in subsequent generations is due to the dynamic response of the genome to the environmental exigencies imposed on the developing organism by specific early experience. That is, transmission of individual differences in maternal behavior is neither under genetic control nor under environmental control; it is under the control of the dynamics of the developmental process. This is a remarkable finding because it shows that gene expression is deeply embedded in the developmental process and reveals how it works hand-in-hand with early experience. Equally remarkable is the finding that the DNA methylation patterns that are induced by differential levels of LG are very stable and, although reversible, are passed down to the offspring including the granddaughters. This is a particularly clear example of the mechanism by which a developmental manifold can be transmitted across generations.
WE MUST REJECT DICHOTOMOUS/ORIGINS THINKING
As I have argued and as Fig. 1 shows, genes are embedded within organisms which, in turn, are embedded in external environments. As a result, even though genes are a critical part of developmental systems, they are only one part of such systems where interactions occur at all levels of organization during both ontogeny and phylogeny. Despite this and despite all the problems associated with gene-centric thinking, nativists continue to rely on the nature/nurture dichotomy and continue to search for predetermined causes. By dichotomizing behavioral capacities into those that are innate and those that are learned, and by assuming that the former are coded in the organism's genome - without providing direct evidence for this claim - these investigators ignore the basic facts of biology and the various problems posed by gene-centric thinking. By doing so, the nativists' interpretations of empirical findings are doomed to be nothing more than philosophical conjectures that hark back to vitalism.
I echo the many prior calls to abandon dichotomous developmental thinking and its focus on the origins question. It is time to shift our focus to the processes question. This shift requires that we adopt a developmental systems approach pioneered by Schneirla and Kuo and later championed by Lehrman, Gottlieb and others (Lickliter, 2007; Turkewitz, 1987). This general approach has spawned a number of variants such as probabilistic epigenesis (Gottlieb, 1998; Gottlieb, et al., 2006; Kuo, 1967; Schneirla, 1957), transactionalism (Sameroff & Chandler, 1975), dynamical systems (Thelen & Smith, 1994), and developmental contextualism (Lerner & Kaufman, 1985). All developmental systems views acknowledge the complexity of the developmental process, eschew any simplistic notions of causality and the kinds of reductionsm that nativists practice, and advocate for a focus on process (Blumberg, 2008; Gottlieb, 1976, 1991b, 1992, 1997, 1998; Griffiths & Gray, 1994; Johnston & Edwards, 2002; Keller, 2000; Kuo, 1967; Lehrman, 1953, 1970; Lickliter, 2007; Mameli & Bateson, 2006; Michel & Moore, 1995; Moore, 2003; Oyama, 2000; Schneirla, 1957; Spencer, Blumberg, et al., 2009).
In general, the developmental systems approach considers the behavior of an organism at any specific time during its development as an emergent property of a complex, co-actional, dynamic system. In such a system, there are horizontal co-actions (e.g., the effects of a particular factor at one time during development on behavior at a subsequent time) and vertical co-actions (e.g., the effects of bottom-up factors such as genes on subsequently emerging organs and/or the effects of top-down factors such as maternal stimulation on gene expression). It is these co-actions that are at the heart of a process that leads to the emergence of novel functions. Because the developmental systems approach makes the fundamental assumption that a developing organism is a fused system, it makes little heuristic sense to separate intrinsic and extrinsic factors and assign separate proportions of influence to each. This does not mean that one cannot study the influence of each factor on the other. Rather, it means that our interpretation of the resulting data should never lose sight of the fact that the influence of one factor always occurs in the context of other factors.
One of the key principles of the developmental systems approach is Schneirla's (1949, 1957, 1966) levels principle. Schneirla proposed this principle as an alternative to reductionism in the study of behavior and its evolution. According to this principle, all organic structures and functions are hierarchically organized such that (a) each level of organization is fully integrated, (b) each successive level of organization is more complex than the lower one, and (c) the rules of integration are different at each successive level of organization. For example, at the genetic level, the genome interacts with the myriad chemical substances and structures (e.g., ribosomes) located in the cytoplasm in an integrated fashion to produce proteins. Understanding these interactions and the rules governing them is not sufficient to understand the interactions among each of these cells when they make up tissues. Indeed, this problem becomes increasingly more difficult as we move up the hierarchy of levels of organization (see Fig. 1) and as we attempt to relate the rules of organization and integration across several of them. For example, relating the rules of organization and integration at the genetic level and the behavioral level - crossing from the genetic to the behavioral level as is typically done by nativists - is fraught with difficulty because one must first explain what role the intervening levels and their organizational properties play in the gene-behavior relationship. For example, as shown earlier, rodent maternal behavior can have effects on gene expression in the offspring and this effect becomes permanent and persists into future generations. Crucially, this seemingly simple behavior-to-gene relationship is the result of an immensely complex cascade of bottom-up and top-down co-acting influences that include behavioral activity, sensory responsiveness, hormone secretion, and gene expression. Such a complex, hierarchically organized, and interdependent system makes causal statements such as “gene X causes behavior X” or statements like “behavior X is innate” inappropriate.
Despite the many calls to reject dichotomous thinking, some nativists claim that it is essential for making progress in developmental science (Landau, 2009). Why might that be? There are at least two reasons for the persistence of the dichotomy. First, this kind of thinking is firmly rooted in folk wisdom (Mameli & Bateson, 2006) and, thus, is so ingrained in people's thinking that it has now taken on the status of an unquestioned principle. Certainly, this is true in our everyday culture where we constantly see headlines trumpeting this or that being determined by genes. In addition, witness the current popular use of the expression: “it's in his/her DNA” as a convenient short-cut for “explaining” why someone might engage in a particular kind of behavior without really explaining what specific underlying mechanisms are involved. Finally, dichotomous thinking, and especially the rationalist argument that initial structure and function is innate, makes it easy to seemingly account for phenomena that we actually do not understand. For example, some nativists claim that primitives provide the essential initial conditions for development and that without them development has no starting point (Landau, 2009). Of course, even if one assumes that primitives exist, their development must be explained too. Instead of doing so, nativists ask us to take their existence on faith. Needless to say, this approach fails to deal with the hard questions in developmental science.
ABANDONING THE ORIGINS QUESTION
Abandoning the origins question in favor of the process question requires a major shift in the way we conceptualize our research enterprise, the way we design our experiments, and the way we interpret our findings. The major advantage of doing this is that we can begin to move our theoretical analyses to the next and necessary analytic level and, thus, begin to investigate the hard questions. In other words, instead of positing that some early appearing behavioral skill is hard-wired, predetermined, and/or innate - when we simply don't know enough about it and can't explain the underlying developmental mechanisms that account for its emergence - we must focus on the underlying process.
As I have indicated throughout this paper, the call for a shift in theoretical and empirical focus is not new. In fact, it is not even new in the field of human infancy studies represented by this journal. Infancy has previously featured articles by two society presidents who have similarly argued against approaches that do not focus on the hard questions. Esther Thelen (2000), who devoted her career to studying the complex processes underlying the development of sensorimotor behavior and cognition, pointed out in her Presidential Address that behavior is the result of an “emergent pattern of multiple cooperating components, all of which count and none of which are privileged” (p.7). Using this embodied cognition approach, Thelen offered a framework that enables us to ask how the moment-moment fluctuations in an organism's sensori-motor activity are linked to emerging perceptions, actions, and cognitive structures. She offered this as an alternative to static views of the mind that make the basic assumption that the mind consists of dedicated and specialized modules or that it contains evolutionarily acquired modules of core knowledge. Similarly, Arnold Sameroff (2005) discussed his work on the effects of social context on long-range developmental outcomes and noted that he and his colleagues ultimately came to the conclusion that developmental outcome was a probabilistic rather than predetermined affair where the best predictor of developmental outcome was a constellation of organismic and environmental variables. For Sameroff, outcome depends on the transaction between the organism and its environment where individuals are constantly being changed by and changing their environments.
EXAMPLE OF A PROCESS-ORIENTED APPROACH
To demonstrate how the broad concept of experience can be used to frame developmental process questions I end by discussing our recent work on perceptual narrowing. This phenomenon is characterized by the finding that younger infants can perceive and discriminate a broader set of stimuli than can older infants. Perceptual narrowing has been found in audition in the speech and music perception domains and in vision in the face perception domain (for reviews of this work see Lewkowicz and Ghazanfar (2009) and Scott, Pascalis, and Nelson (2007)). For example, in the speech perception domain, it has been found that young infants can perceive native and nonnative phonetic contrasts but that older infants and adults no longer do (Best, McRoberts, & Sithole, 1988; Rivera-Gaxiola, Silva-Pereyra, & Kuhl, 2005; Werker & Tees, 1984) and that younger infants respond to native and non-native musical meter but that older infants respond only to native meter (Hannon & Trehub, 2005a). Similarly, in the face perception domain, it has been found that young infants can perceive and discriminate human and monkey faces as well as the faces of their own race and the faces of other races but that older infants can only discriminate human and same-race faces (Kelly, et al., 2007; Pascalis, Haan, & Nelson, 2002). Perceptual narrowing also has been found in birds. For example, mallard duck embryos who are prevented from hearing both their self-generated vocalizations and the vocalizations from their siblings prior to hatching respond equally to mallard and chicken maternal calls after hatching. In contrast, embryos who are not prevented from hearing such calls respond only to mallard maternal calls after hatching.
The mallard experiments indicate that normal early perceptual experience narrows the initially broad auditory tuning sot that by the time the birds hatch they only respond to their own species' maternal calls (Gottlieb, 1991a). Studies have shown that experience plays a similar role in human infants. That is, the usual decline that has been found in older infants' responsiveness to non-native auditory and visual inputs can be prevented to some extent by giving infants extra experience with such inputs. This is the case for non-native phonetic contrasts (Kuhl, Tsao, & Liu, 2003; Yeung & Werker, 2009), musical meter (Hannon & Trehub, 2005b), faces (Pascalis, et al., 2005; Scott & Monesson, 2009), and other-race faces (Sangrigoli, Pallier, Argenti, Ventureyra, & de Schonen, 2005).
Overall, the findings on perceptual narrowing indicate that this is a domain-general phenomenon. What was not known until recently, however, is whether this is also a pan-sensory phenomenon. We decided to test the latter possibility by using the intersensory matching procedure and allowing 4-, 6-, 8-, and 10-month-old infants to choose to look at one of two identical rhesus monkey faces while they listened to a monkey vocalization whose onset and offset was synchronized with the onset and offset of the corresponding vocalizing face (Lewkowicz & Ghazanfar, 2006). As predicted, we found that the younger infants (4- and 6-month-olds) matched the faces and vocalizations but that the older infants did not. This finding provided the first evidence of multisensory perceptual narrowing (MPN).
In subsequent studies we have found that the younger infants perform the cross-species cross-modal matches on the basis of audio-visual synchrony in that when the faces and vocalizations are desynchronized, neither the younger nor older infants match (Lewkowicz, Sowinski, & Place, 2008). In another study, we have found that the broad perceptual tuning is present at birth in that newborns also are able to match corresponding monkey faces and vocalizations and, in addition, can do so even when a tone of the same duration as the audible vocalization is presented instead (Lewkowicz, Leo, & Simion, 2010). The latter finding indicates that at birth the ability to make matches is based on nothing more than the detection of corresponding multisensory energy onsets and offsets rather than on the extraction of higher-level audio-visual dynamic correlations and/or correlated multisensory speech features. Finally, in our most recent studies we have found that MPN also occurs in the audiovisual speech domain and that the mechanism underlying MPN changes dramatically over the first months of life. In these studies, we tested 6- and 11-month-old English- and Spanish-learning infants' ability to match an audible /ba/ or /va/ with one of two human faces seen producing each of these vocalizations (Pons, Lewkowicz, Soto-Faraco, & Sebastián-Gallés, 2009). We expected to find narrowing in the Spanish-learning infants because the /ba/-/va/ distinction is not phonemically relevant in Spanish. To test for cross-modal matching, here we used a familiarization/test technique in order to eliminate any synchrony cues. Thus, infants first heard one of the syllables and then watched the two faces utter the syllables in silence. Consistent with MPN, we found that both 6-month-old English- and Spanish-learning infants made successful matches but that only the 11-month-old English-learning infants made the matches. Because here matching was only possible on the basis of the extraction and cross-modal transfer of speech-related perceptual attributes, these results demonstrate that MPN in the speech perception domain does not depend on A-V synchrony as does MPN in cross-species face-voice matching.
Overall, our findings suggest that the mechanisms underlying MPN change during early life. Initially, because infants are sensitive to A-V synchrony cues from birth on (Lewkowicz, 2010; Lewkowicz, et al., 2010), they can begin to construct a coherent multisensory world, albeit a very crude and imprecise one. Nonetheless, they begin life with a powerful mechanism for bootstrapping their initial attempt at constructing a coherent multisensory world. With experience, that includes perceptual learning and differentiation of increasingly finer perceptual structure, the breadth of acceptable multisensory coherence begins to decline. Crucially, each time infants discover new and increasingly more complex perceptual features, they tend to integrate multisensory inputs broadly at first on the basis of these newly discovered perceptual features, but as they continue to acquire additional experience, this broad tuning declines too.
The theoretical picture outlined above eschews any questions of developmental origins. The aim is not to search for the origin of MPN nor is it motivated by any a priori assumptions regarding its origins. Instead, the focus is on the developmental process in the context of a developmental system beginning with very young infants who already have a developmental history and who bring this history to the experimental task. This is best illustrated by our newborn study. Here, the aim was not to ask whether the broad perceptual tuning that we initially found in 4-month-old infants is an innate aspect of perceptual organization. Rather, the aim was to determine whether the newborn's state of neural organization and the vast prenatal experience that the newborn brings with it may be sufficient to set up broad multisensory tuning. The newborn findings suggest that it is and, thus, one next step might be to look for non-obvious prenatal factors that might facilitate the discovery of multisensory coherence prior to birth. What makes the newborn findings fascinating is that they suggest that the sort of broad multisensory tuning that we found may reflect an ontogenetic adaptation (Oppenheim, 1981) that is adaptive in that it provides newborns with a way to organize the onslaught of multisensory information. This, in turn, raises interesting questions about the various ways in which experience contributes to the narrowing that then occurs as infants become expert perceivers.
The most important point is that inquiry does not stop once a particular behavioral skill is identified at some early point in development. Rather, its identification serves as a starting point for process-oriented questions that, when pursued, help unpack the underlying process. The aim is not to determine whether a behavioral skill is learned or innate based on whether the skill is present earlier or later in life; rather, it is to determine how the dynamics of the processes at different levels of organization become integrated to produce the skill in question.
CONCLUSION
Modern psychobiological conceptualizations of the process of development and the vast array of empirical evidence from developmental studies amassed in the last several decades demonstrate convincingly that the dilemma of having to choose between conceptualizations that pose origins versus process-oriented questions disappears once we reject the false dichotomy imposed by the nature-nurture dichotomy. A focus on the origins question is what has forced developmentalists to look for the initiating cause and genes have become the cause célèbre because of their vaunted status as the traditional vehicle by which evolution has been thought to ensure the continuity of species across time. If, however, we accept the notion that it is ontogenies, and not adult organisms, that evolve and that evolution ensures the continuity of species through the preservation of developmental manifolds, then we must shift our focus to the developmental process. This, in turn, requires us to come to terms with the exquisitely dynamic, embedded, interdependent, and probabilistic nature of development. Once we do, we will finally be in a position to obtain insights into the complex causes of developmental change that have eluded thinkers for millennia.
Acknowledgements
I am grateful for constructive and helpful comments on a previous version of this article by David Bjorklund, Robert Lickliter, George Michel, and Susan Oyama. The writing of this article was supported, in part, by NSF grant BCS-0751888 and NIH grant HD057116.
References
- Adolph KE. Specificity of learning: Why infants fall over a veritable cliff. Psychological Science. 2000;11(4):290–295. doi: 10.1111/1467-9280.00258. [DOI] [PubMed] [Google Scholar]
- Baillargeon R, DeVos J. Object permanence in young infants: Further evidence. Child development. 1991;62(6):1227–1246. [PubMed] [Google Scholar]
- Baillargeon R. Innate ideas revisited: For a principle of persistence in infants' physical reasoning. Perspectives on Psychological Science. 2008;3(1):2. doi: 10.1111/j.1745-6916.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P. How do sensitive periods arise and what are they for? Animal Behaviour. 1979;27:470–486. [Google Scholar]
- Bateson P. The return of the whole organism. Journal of Biosciences. 2005;30(1):31–39. doi: 10.1007/BF02705148. [DOI] [PubMed] [Google Scholar]
- Best CT, McRoberts GW, Sithole NM. Examination of perceptual reorganization for nonnative speech contrasts: Zulu click discrimination by English-speaking adults and infants. Journal of Experimental Psychology: Human Perception and Performance. 1988;14(3):345–360. doi: 10.1037//0096-1523.14.3.345. [DOI] [PubMed] [Google Scholar]
- Bjorklund D. Mother knows best: Epigenetic inheritance, maternal effects, and the evolution of human intelligence. Developmental Review. 2006;26(2):213–242. [Google Scholar]
- Bloom P. Religion is natural. Developmental Science. 2007;10(1):147–151. doi: 10.1111/j.1467-7687.2007.00577.x. [DOI] [PubMed] [Google Scholar]
- Blumberg M. Freaks of nature: What anomalies tell us about development and evolution. Oxford University Press; USA: 2008. [Google Scholar]
- Bowlby J. Attachment and loss. Vol. 1. Basic Books; New York: 1969. [Google Scholar]
- Carroll S. Endless forms most beautiful: The new science of evo devo and the making of the animal kingdom. WW Norton & Company; 2005. [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri A, Holmes A, Uher R, Moffitt T. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167(5):509. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F. Epigenetic mechanisms and the transgenerational effects of maternal care. Frontiers in neuroendocrinology. 2008;29(3):386–397. doi: 10.1016/j.yfrne.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomsky N. Aspects of the Theory of Syntax. The MIT press; Cambridge, MA: 1965. [Google Scholar]
- Cooper RP, Aslin RN. Preference for infant-directed speech in the first month after birth. Child Development. 1990;61:1584–1595. [PubMed] [Google Scholar]
- Corbetta D, Bojczyk KE. Infants return to two-handed reaching when they are learning to walk. Journal of Motor Behavior. 2002;34(1):83–95. doi: 10.1080/00222890209601933. [DOI] [PubMed] [Google Scholar]
- Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms J. Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. Journal of Clinical Investigation. 2004;114(4):485–494. doi: 10.1172/JCI19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cytowic R. The man who tasted shapes. MIT Press; Cambridge, MA: 2003. [Google Scholar]
- Edelman GM. Bright air, brilliant fire. Basic Books; New York: 1992. [Google Scholar]
- Fernald A, Simon T. Expanded intonation contours in mothers' speech to newborns. Developmental Psychology. 1984;20:104–113. [Google Scholar]
- Fernald A. Five-month-old infants prefer to listen to motherese. Infant Behavior and Development. 1985;8:181–195. [Google Scholar]
- Fernald A, Mazzie C. Prosody and focus in speech to infants and adults. Developmental Psychology. 1991;27(2):209–221. [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286(5442):1155. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Goldstein MH, Schwade JA. Social feedback to infants' babbling facilitates rapid phonological learning. Psychological Science. 2008;19(5):515. doi: 10.1111/j.1467-9280.2008.02117.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Conceptions of prenatal development: Behavioral embryology. Psychological Review. 1976;83(3):215–234. [PubMed] [Google Scholar]
- Gottlieb G. Experiential canalization of behavioral development: Results. Developmental Psychology. 1991a;27(1):35–39. [Google Scholar]
- Gottlieb G. Experiential canalization of behavioral development: Theory. Developmental Psychology. 1991b;27(1):4–13. [Google Scholar]
- Gottlieb G. Individual development & evolution: the genesis of novel behavior. Oxford University Press; New York: 1992. [Google Scholar]
- Gottlieb G. Synthesizing nature-nurture: Prenatal roots of instinctive behavior. Erlbaum; Mahwah, NJ: 1997. [Google Scholar]
- Gottlieb G. Normally occurring environmental and behavioral influences on gene activity: From central dogma to probabilistic epigenesis. Psychological Review. 1998;105:792–802. doi: 10.1037/0033-295x.105.4.792-802. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Emergence of the developmental manifold concept from an epigenetic analysis of instinctive behavior. In: Lewkowicz DJ, Lickliter R, editors. Conceptions of development: Lessons from the laboratory. Taylor & Francis; New York: 2002. pp. 31–56. [Google Scholar]
- Gottlieb G, Wahlsten D, Lickliter R. The significance of biology for human development: A developmental psychobiological systems view. In: Lerner R, editor. Handbook of child psychology. Vol. 1. Wiley; New York: 2006. pp. 210–257. [Google Scholar]
- Gottlieb G. Probabilistic epigenesis. Developmental Science. 2007;10(1):1–11. doi: 10.1111/j.1467-7687.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Griffiths PE, Gray RD. Developmental systems and evolutionary explanation. The Journal of Philosophy. 1994;91(6):277–304. [Google Scholar]
- Griffiths PE, Gray RD. Studying the ecology and evolution of complex phenotypes. Oxford University Press; New York: 2004. The Developmental Systems Perspective: Organism-environment systems as units of development and evolution Phenotypic integration; pp. 409–431. [Google Scholar]
- Haith MM. Who put the cog in infant cognition? Is rich interpretation too costly? Infant Behavior & Development. 1998;21(2):167–179. [Google Scholar]
- Hamlin JK, Wynn K, Bloom P. Social evaluation by preverbal infants. Nature. 2007;450(7169):557–559. doi: 10.1038/nature06288. [DOI] [PubMed] [Google Scholar]
- Hamlin JK, Wynn K, Bloom P. Three-month-olds show a negativity bias in their social evaluations. Developmental Science. 2010;13(6):923–929. doi: 10.1111/j.1467-7687.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin JK, Wynn K. Young infants prefer prosocial to antisocial others. Cognitive Development. 2011;26:30–39. doi: 10.1016/j.cogdev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon EE, Trehub SE. Metrical categories in infancy and adulthood. Psychological Science. 2005a;16(1):48–55. doi: 10.1111/j.0956-7976.2005.00779.x. [DOI] [PubMed] [Google Scholar]
- Hannon EE, Trehub SE. Tuning in to musical rhythms: infants learn more readily than adults. Proceedings of the National Academy of Sciences USA. 2005b;102(35):12639–12643. doi: 10.1073/pnas.0504254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde R. Animal Behaviour: a synthesis of ethology and comparative psychology. McGraw-Hill; New York: 1966. [Google Scholar]
- Jablonka E, Raz G. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Quarterly Review of Biology. 2009;84(2):131–176. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- Johnston TD. The persistence of dichotomies in the study of behavioral development. Developmental Review. 1987;7:149–182. [Google Scholar]
- Johnston TD, Edwards L. Genes, interactions, and the development of behavior. Psychological Review. 2002;109(1):26–34. doi: 10.1037/0033-295x.109.1.26. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Nativism versus neuroconstructivism: rethinking the study of developmental disorders. Developmental Psychology. 2009;45(1):56–63. doi: 10.1037/a0014506. [DOI] [PubMed] [Google Scholar]
- Keller EF. The century of the gene. Harvard University Press; Cambridge, MA: 2000. [Google Scholar]
- Keller EF. The century beyond the gene. Journal of Biosciences. 2005;30(1):3–10. doi: 10.1007/BF02705144. [DOI] [PubMed] [Google Scholar]
- Keller EF. The Mirage of a Space between Nature and Nurture. Duke University Press Books; 2010. [Google Scholar]
- Kelly DJ, Quinn PC, Slater A, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science. 2007;18(12):1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Tsao FM, Liu HM. Foreign-language experience in infancy: effects of short-term exposure and social interaction on phonetic learning. Proceedings of the National Academy of Sciences USA. 2003;100(15):9096–9101. doi: 10.1073/pnas.1532872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo ZY. The dynamics of behavior development: An epigenetic view. Plenum; New York: 1967. [Google Scholar]
- Landau B. The importance of the nativist-empiricist debate: Thinking about primitives without primitive thinking. Child development perspectives. 2009;3(2):88–90. [Google Scholar]
- Lehrman DS. A critique of Konrad Lorenz's theory of instinctive behavior. Quarterly Review of Biology. 1953;28(4):337–363. doi: 10.1086/399858. [DOI] [PubMed] [Google Scholar]
- Lehrman DS. Semantic and conceptual issues in the nature-nurture problem. In: Aronson LR, Lehrman DS, Tobach E, Rosenblatt JS, editors. Development and evolution of behavior. W. H. Freeman; San Francisco: 1970. pp. 17–52. [Google Scholar]
- Lerner RM, Kaufman MB. The concept of development in contextualism. Developmental Review. 1985;5:309–333. [Google Scholar]
- Lewis MD. The promise of dynamic systems approaches for an integrated account of human development. Child Development. 2000;71(1):36–43. doi: 10.1111/1467-8624.00116. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ. Infants' response to temporally based intersensory equivalence: The effect of synchronous sounds on visual preferences for moving stimuli. Infant Behavior & Development. 1992;15(3):297–324. [Google Scholar]
- Lewkowicz DJ, Ghazanfar AA. The decline of cross-species intersensory perception in human infants. Proceedings of the National Academy Sciences USA. 2006;103(17):6771–6774. doi: 10.1073/pnas.0602027103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowicz DJ, Sowinski R, Place S. The decline of cross-species intersensory perception in human infants: Underlying mechanisms and its developmental persistence. Brain Research. 2008;1242:291–302. doi: 10.1016/j.brainres.2008.03.084. Epub (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowicz DJ, Ghazanfar AA. The emergence of multisensory systems through perceptual narrowing. Trends in Cognitive Sciences. 2009;13(11):470–478. doi: 10.1016/j.tics.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ. Infant perception of audio-visual speech synchrony. Developmental Psychology. 2010;46(1):66–77. doi: 10.1037/a0015579. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ, Leo I, Simion F. Intersensory perception at birth: Newborns match non-human primate faces & voices. Infancy. 2010;15(1):46–60. doi: 10.1111/j.1532-7078.2009.00005.x. [DOI] [PubMed] [Google Scholar]
- Lickliter R. The dynamics of development and evolution: insights from behavioral embryology. Dev Psychobiol. 2007;49(8):749–757. doi: 10.1002/dev.20270. [DOI] [PubMed] [Google Scholar]
- Lorenz K. Evolution and modification of behavior. University of Chicago Press; Chicago: 1965. [Google Scholar]
- Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9726. doi: 10.1073/pnas.0504122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Bateson P. Innateness and the sciences. Biology and Philosophy. 2006;21(2):155–188. [Google Scholar]
- Marcus G. The birth of the mind. Vol. 165. Basic Books; New York: 2004. [Google Scholar]
- Marks L. The unity of the senses. Academic Press; New York: 1978. [Google Scholar]
- Maurer D, Mondloch C, Lewis T. Effects of early visual deprivation on perceptual and cognitive development. Progress in Brain Research. 2007;164:87–104. doi: 10.1016/S0079-6123(07)64005-9. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends in Neurosciences. 2005;28(9):456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Michel GF, Moore C. Developmental psychobiology: An interdisciplinary science. The MIT Press; 1995. [Google Scholar]
- Michel GF. Development of infants handedness. In: Lewkowicz DJ, Lickliter R, editors. Conceptions of development: Lessons from the laboratory. Psychology Press; New York: 2002. [Google Scholar]
- Michel GF, Tyler AN. Critical period: A history of the transition from questions of when, to what, to how. Developmental Psychobiology. Special Issue: Critical Periods Re-examined: Evidence from Human Sensory Development. 2005;46(3):156–162. doi: 10.1002/dev.20058. [DOI] [PubMed] [Google Scholar]
- Michel GF. The roles of environment, experience, and learning in behavioral development. In: Hood K, Halpern C, Greenberg G, Lerner R, editors. Handbook of Developmental Science, Behavior and Genetics. Wiley; Malden, MA: 2010. pp. 123–165. [Google Scholar]
- Moore D. The dependent gene: The fallacy of nature vs. nurture. Holt Paperbacks; 2003. [Google Scholar]
- Nelson CA. The development and neural bases of face recognition. Infant & Child Development. 2001;10(1–2):3–18. [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch K-P. Monoamine Oxidase A Gene Promoter Variation and Rearing Experience Influences Aggressive Behavior in Rhesus Monkeys. Biological Psychiatry. 2005;57(2):167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Noble D. The music of life. Oxford University Press; Oxford: 2006. [Google Scholar]
- Noble D. Genes and causation. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2008;366(1878):3001. doi: 10.1098/rsta.2008.0086. [DOI] [PubMed] [Google Scholar]
- Noble D. Differential and integral views of genetics in computational systems biology. Interface Focus. 2011;1(1):7. doi: 10.1098/rsfs.2010.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW. Ontogenetic adaptations and retrogressive processes in the development of the nervous system and behavior: A neuroembryological perspective. In: Connolly KJ, Prechtl HFR, editors. Maturation and Development: Biological and Psychological Perspectives. Lippincott; Philadelphia: 1981. pp. 73–109. [Google Scholar]
- Overton WF. Developmental Psychology: Philosophy, Concepts, Methodology. In: Lerner RM, editor. Handbook of child psychology (6th ed.): Theoretical models of human development. Vol. 1. John Wiley & Sons Inc.; Hoboken, NJ: 2006. pp. 18–88. [Google Scholar]
- Oyama S. The ontogeny of information. Duke University Press; Durham, NC: 2000. [Google Scholar]
- Oyama S. Biologists behaving badly: vitalism and the language of language. History and philosophy of the life sciences. 2010;32(2–3):401. [PubMed] [Google Scholar]
- Papoušek M, Papoušek H, Haekel M. Didactic adjustments in fathers' and mothers' speech to their 3-month-old infants. Journal of Psycholinguistic Research. 1987;16(5):491–516. [Google Scholar]
- Pascalis O, Haan M. d., Nelson CA. Is face processing species-specific during the first year of life? Science. 2002;296(5571):1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Scott LS, Kelly DJ, Shannon RW, Nicholson E, Coleman M, Nelson CA. Plasticity of face processing in infancy. Proceedings of the National Academy of Sciences USA. 2005;102(14):5297–5300. doi: 10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Silver J, Trainor LJ. Feeling the Beat: Movement Influences Infant Rhythm Perception. Science. 2005;308(5727):1430–1430. doi: 10.1126/science.1110922. [DOI] [PubMed] [Google Scholar]
- Pinker S, Bloom P. Natural language and natural selection. The adapted mind: Evolutionary psychology and the generation of culture. 1992:451–493. [Google Scholar]
- Platt S, Sanislow C. Norm-of-reaction: Definition and misinterpretation of animal research. Journal of Comparative Psychology. 1988;102(3):254–261. doi: 10.1037/0735-7036.102.3.254. [DOI] [PubMed] [Google Scholar]
- Pons F, Lewkowicz DJ, Soto-Faraco S, Sebastián-Gallés N. Narrowing of intersensory speech perception in infancy. Proceedings of the National Academy of Sciences USA. 2009;106(26):10598–10602. doi: 10.1073/pnas.0904134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartz S, Sejnowski T. The neural basis of cognitive development: A constructivist manifesto. Behavioral and brain sciences. 1997;20(04):537–556. doi: 10.1017/s0140525x97001581. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas K. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Jama. 2009;301(23):2462. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Gaxiola M, Silva-Pereyra J, Kuhl P. Brain potentials to native and non-native speech contrasts in 7- and 11-month-old American infants. Developmental Science. 2005;8(2):162–172. doi: 10.1111/j.1467-7687.2005.00403.x. [DOI] [PubMed] [Google Scholar]
- Robert J. Interpreting the homeobox: metaphors of gene action and activation in development and evolution. Evolution & Development. 2001;3(4):287–295. doi: 10.1046/j.1525-142x.2001.003004287.x. [DOI] [PubMed] [Google Scholar]
- Sameroff A, Chandler M. 4 Reproductive Risk and the Continuum of Caretaking Casualty. Review of child development research. 1975:187. [Google Scholar]
- Sameroff A. The science of infancy: Academic, social, and political agendas. Infancy. 2005;7(3):219–242. doi: 10.1207/s15327078in0703_1. [DOI] [PubMed] [Google Scholar]
- Sangrigoli S, Pallier C, Argenti AM, Ventureyra VAG, de Schonen S. Reversibility of the other-race effect in face recognition during childhood. Psychological Science. 2005;16(6):440–444. doi: 10.1111/j.0956-7976.2005.01554.x. [DOI] [PubMed] [Google Scholar]
- Schneirla TC. Levels in the psychological capacities of animals. In: Sellars RW, McGill VJ, Farber M, editors. Philosophy for the future. Macmillan; New York: 1949. pp. 243–286. [Google Scholar]
- Schneirla TC. The concept of development in comparative psychology. In: Harris DB, editor. The concept of development. University of Minnesota Press; Minneapolis: 1957. pp. 78–108. [Google Scholar]
- Schneirla TC. Behavioral development and comparative psychology. The Quarterly Review of Biology. 1966;41(3):283–302. doi: 10.1086/405056. [DOI] [PubMed] [Google Scholar]
- Scholl B. Innateness and (Bayesian) visual perception. In: Carruthers P, Laurence S, Stich S, editors. The innate mind: structure and contents. Oxford University Press; USA: 2005. p. 34. [Google Scholar]
- Scott LS, Pascalis O, Nelson CA. A domain general theory of the development of perceptual discrimination. Current Directions in Psychological Science. 2007;16(4):197–201. doi: 10.1111/j.1467-8721.2007.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LS, Monesson A. The origin of biases in face perception. Psychological Science. 2009;20(6):676–680. doi: 10.1111/j.1467-9280.2009.02348.x. [DOI] [PubMed] [Google Scholar]
- Slater A, Kirby R. Innate and learned perceptual abilities in the newborn infant. Experimental Brain Research. 1998;123:90–94. doi: 10.1007/s002210050548. [DOI] [PubMed] [Google Scholar]
- Slater A. Visual perception. In: Bremner JG, Fogel A, editors. Blackwell handbook of infant development. Wiley-Blackwell; Malden, MA: 2004. pp. 5–34. [Google Scholar]
- Smith LB, Thelen E. Development as a dynamic system. Trends in Cognitive Sciences. 2003;7(8):343–348. doi: 10.1016/s1364-6613(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Spector F, Maurer D. Synesthesia: a new approach to understanding the development of perception. Dev Psychol. 2009;45(1):175–189. doi: 10.1037/a0014171. [DOI] [PubMed] [Google Scholar]
- Spelke E, Newport E. Nativism, empiricism, and the development of knowledge. In: Damon W, Lerner R, editors. Handbook of child psychology: Vo. 1. Theoretical models ofhuman development. 5th ed. Wiley; New York: 1998. pp. 275–340. [Google Scholar]
- Spelke ES, Kinzler KD. Core knowledge. Dev Sci. 2007;10(1):89–96. doi: 10.1111/j.1467-7687.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Samuelson LK, Blumberg MS, McMurray B, Robinson SR, Bruce Tomblin J. Seeing the World Through a Third Eye: Developmental Systems Theory Looks Beyond the Nativist-Empiricist Debate. Child development perspectives. 2009;3(2):103–105. doi: 10.1111/j.1750-8606.2009.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP, Blumberg MS, McMurray B, Robinson SR, Samuelson LK, Tomblin JB. Short arms and talking eggs: Why we should no longer abide the nativist-empiricist debate. Child Development Perspectives. 2009;3(2):79–87. doi: 10.1111/j.1750-8606.2009.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E, Adolph KE. Arnold L. Gesell: The paradox of nature and nurture. Developmental Psychology. 1992;28(3):368–380. [Google Scholar]
- Thelen E, Smith LB. A dynamic systems approach to the development of cognition and action. MIT Press; Cambridge, MA: 1994. [Google Scholar]
- Thelen E. Grounded in the world: Developmental origins of the embodied mind. Infancy. 2000;1(1):3–28. doi: 10.1207/S15327078IN0101_02. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. The study of instinct. Clarendon Press; Oxford: 1951. [Google Scholar]
- Turkewitz G, Kenny PA. Limitations on input as a basis for neural organization and perceptual development: A preliminary theoretical statement. Developmental Psychobiology. 1982;15:357–368. doi: 10.1002/dev.420150408. [DOI] [PubMed] [Google Scholar]
- Turkewitz G. Psychobiology and developmental psychology: The influence of TC Schneirla on human developmental psychology. Developmental Psychobiology. 1987;20(4):369–375. doi: 10.1002/dev.420200402. [DOI] [PubMed] [Google Scholar]
- Walker P, Bremner JG, Mason U, Spring J, Mattock K, Slater A, Johnson SP. Preverbal infants' sensitivity to synaesthetic cross-modality correspondences. Psychol Sci. 2010;21(1):21–25. doi: 10.1177/0956797609354734. [DOI] [PubMed] [Google Scholar]
- Werker JF, Tees RC. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavior & Development. 1984;7(1):49–63. [Google Scholar]
- Werker JF, Tees RC. Speech perception as a window for understanding plasticity and commitment in language systems of the brain. Developmental Psychobiology. Special Issue: Critical Periods Re-examined: Evidence from Human Sensory Development. 2005;46(3):233–234. doi: 10.1002/dev.20060. [DOI] [PubMed] [Google Scholar]
- Wright S. Evolution and the Genetics of Populations, Volume 1: Genetic and Biometric Foundations. University of Chicago Press; 1968. [Google Scholar]
- Yeung H, Werker J. Learning words' sounds before learning how words sound: 9-Month-olds use distinct objects as cues to categorize speech information. Cognition. 2009;113(2):234–243. doi: 10.1016/j.cognition.2009.08.010. [DOI] [PubMed] [Google Scholar]