Abstract
In the past decade we have witnessed important advances in the treatment of gynecological cancers and have recognized their potential immunogenicity. This has opened the door to explore immune therapy not only in HPV-induced cancers but also in ovarian and endometrial cancers. Here we will review the off target immune effects of select chemotherapy drugs commonly used to treat gynecologic cancers and novel tools that can stimulate both the adaptive and innate immune mechanisms such as novel pleiotropic cytokines, Toll-like receptors, and powerful antibodies that can target inhibitory checkpoints thereby activating effector cellular immune mechanisms and neutralizing suppressor cells. We will also review how existing drugs can be used for combinatorial tumor therapy.
INTRODUCTION
Significant advances have been made in the therapy of gynecologic cancers in the past two decades. Chemotherapy regimens have been optimized with respect to dose, schedule and combinations, and novel targeted therapies have emerged that can selectively neutralize signals that drive or maintain the oncogenic process. Although the cancer cell remains the main target of oncologic therapy, it is becoming progressively clear that the tumor microenvironment provides critical support to tumor growth and therefore opportunities for therapy. Inhibition of tumor angiogenesis is an obvious example of effective biological therapy that has produced clinical results. Importantly, complex mechanisms regulating immune response and inflammation interface with angiogenesis at the tumor microenvironment, and their balance can greatly affect the fate of tumors. The overall balance of tumor inflammatory mechanisms is polarized to promote angiogenesis, tumor cell survival and immune escape, all contributing to tumor growth. However, it is becoming clear that many patients with gynecologic malignancies mount a spontaneous antitumor immune response. Although ineffective to reject tumor, this can be potentially harnessed therapeutically. Here we will review how existing drugs can capitalize on and manipulate natural antitumor immunity and thus be used for combinatorial tumor therapy.
The use of immunomodulatory therapy is predicated on the notion that gynecologic cancers are potentially immunogenic tumors, i.e they can be recognized and attacked by cell based immune mechanisms. Cervical and lower genital tract cancers induced by human papillomavirus (HPV) are the prototype of potentially immunogenic tumors that can elicit a spontaneous immune response. HPV xenoantigens expressed by tumor cells are readily recognized by the immune system. Cell-mediated immune responses are important in controlling HPV infections as well as HPV-associated neoplasms (for review, see [1]). The prevalence of HPV-related diseases is increased in patients with impaired cell-mediated immunity, including transplant recipients [2] and HIV-infected patients [3, 4]. Infiltrating CD4+ (T helper cells) and CD8+ (cytotoxic) T cells have been observed in spontaneously regressing warts [5] and, warts often disappear in patients who are on immunosuppressive therapy when treatment is discontinued [6]. In addition, animals immunized with viral proteins are protected from HPV infection or the development of neoplasia, and experience regression of existing lesions [7, 8]. Nevertheless, patients with invasive cervical cancer exhibit exhausted and tolerized T cells that recognize antigen in vitro but are unable to reject tumors in vivo [9, 10]. The emergence of immunomodulatory therapies revives opportunities to activate and invigorate such T cell immunity and warrants clinical testing.
Although tumor-associated antigens have not undergone rigorous scrutiny in other gynecologic malignancies (reviewed in [11]), similar mechanisms of spontaneous antitumor immune response have been convincingly demonstrated. Tumor-reactive T cells and antibodies have been detected in peripheral blood of patients with advanced stage ovarian cancer at diagnosis [12, 13], while oligoclonal tumor reactive T cells have been isolated from tumors or ascites [14–22]. Importantly, the detection of intratumoral or intraepithelial tumor infiltrating lymphocytes (TIL), i.e. T cells infiltrating tumor islets predicts significantly improved progression survival and overall survival in ovarian cancer. We first reported in an Italian cohort that patients whose tumors had intraepithelial T cells experienced 3.8-fold longer median progression-free survival and 2.8-fold longer overall survival as compared to patients whose tumors lacked intraepithelial T cells. Remarkably, survival rate at five years was 38% in patients whose tumors had intraepithelial T cells (n=102) and 4.5% in patients lacking them (n=72). The impact of intraepithelial T cells was confirmed by multiple independent studies on ethnically diverse populations [23–29]. Similar observations were made in endometrial cancer [30–32] and other solid tumors [33]. Retrospective studies showing that the incidence of many non-virally induced solid tumor types is in fact 4–30 fold increased in immunosuppressed transplant recipients [34–38], provide evidence that immune recognition is probably a universal mechanism in tumors.
CHEMOTHERAPY AS AN IMMUNE MODULATOR
Although it has been traditionally thought that chemotherapy antagonizes immune mechanisms altogether, recent evidence has challenged this view. Indeed, agents such as cyclophosphamide, doxorubicin and paclitaxel increase the number and function of antigen-specific T cells and thus may enhance cancer immunity [39]. It is becoming progressively clear that conventional chemotherapy has important “off-target” immunologic effects, and in fact, may depend on activation of immune mechanisms to achieve its full efficacy. In mouse models of solid tumors, increased tumor inflammation following administration of chemotherapy predicts better prognosis [40], while tumors grown in immunodeficient mice fail to respond to chemotherapy [41], clearly highlighting a role for the immune system in cancer clearance in the context of cytotoxic therapy. Similar events may occur in humans; tumor-infiltrating lymphocytes predicted complete pathologic response in breast cancer patients after neoadjuvant chemotherapy [42]. Furthermore, neoadjuvant taxol therapy was found to increase TIL [43]. Interestingly, breast cancer patients bearing a loss-of-function Asp299Gly polymorphism of the Toll-like receptor (TLR) 4 receptor (see below) exhibit a higher risk ofrelapse after treatment with chemotherapyand radiation therapy [44].
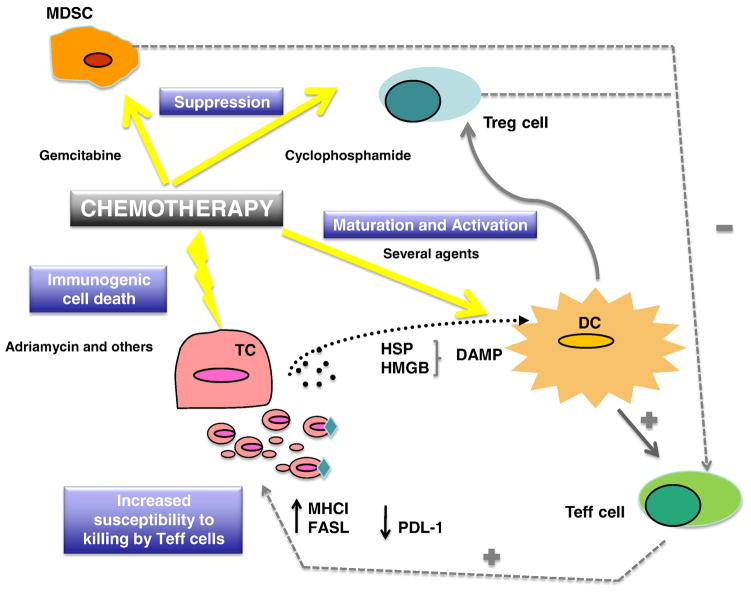
The immunomodulatory effects of chemotherapy can be broadly grouped in three mechanisms: a) induction of immunogenic cancer cell death, which facilitates tumor antigen presentation (in situ vaccination); b) direct activation of antigen presenting or effector mechanisms; and c) suppression of immune inhibitory cells, thereby releasing regulatory breaks on antitumor immune response (Figure 1). These mechanisms are quite complex and our understanding is still in its infancy, but effects appear to dependent on drug type, dose and schedule as well as the immune cell type. The available evidence is reviewed below.
Figure 1.
a) In situ Vaccination from Immunogenic Tumor Cell Death
Several recent studies have demonstrated that a number of chemotherapeutic agents are able to induce “immunogenic cell death”. In general, necrotic cell death is considered inflammatory, while apoptotic cell death induces less inflammation. Recently, it was found that doxorubicin, idarubicin, mitoxanthrone, oxaliplatin and other drugs induce apoptotic cell death, which however leads to greatly increased antigen uptake by dendritic cells (DCs), the professional antigen presenting cells, and to DC maturation, leading to in situ immunization against tumor antigens [44, 45]. The underlying molecular mechanism is related to the preapoptotic exposure of calreticulin on the cell surface, which mediates DC uptake and maturation [46].
b) Activation of APCs and Effector Mechanisms
Dendritic cells are the most potent antigen-presenting cells in the immune arsenal. High doses of immunogenic chemotherapy may be transiently pro-inflammatory, however they do not promote DC longevity. Cyclophosphamide has interesting immunomodulatory properties, and in combination with cisplatin demonstrated synergy with subcutaneous interferon-gamma in a phase III study in patients with advanced ovarian cancer [47]. Since the 1980s, it has been recognized that cyclophosphamide administered at standard dose prior to cancer vaccines significantly enhanced immune therapy, however the mechanism of this phenomenon remained unclear [48]. A recent study in the mouse reported that cyclophosphamide at myelosuppressive dose induces rebound myelopoiesis and leads to the emergence of tumor-infiltrating DCs that secrete more IL-12 and less IL-10, which are fully capable of priming T cell responses [49]. In addition, metronomic, or low dose non-myelotoxic administration of paclitaxel, doxorubicin, vincristine and other drugs can cause activation and maturation of DCs, comprising increased IL-12 secretion, a critical factor required for T cell priming. Signaling via STAT4 and Rho GTPases may account for these effects [50].
An additional mechanism through which chemotherapy enhances immune mechanisms is by rendering tumor cells more susceptible to killing by immune effector cells. Fas is a membrane-bound protein belonging to the tumor necrosis factor (TNF) family of surface receptors, which, upon ligation with Fas-ligand (FasL), induces apoptosis. FasL is expressed by activated effector immune cells, namely CD8+ T and NK cells. A recent in vitro study showed that treatment of cancer cells with topotecan causesupregulation of Fas expression, and this can lead to significant T cell activation within the tumor microenvironment [51]. Cisplatin has also been shown to increase the susceptibility of tumor cells to tumor-infiltrating lymphocytes or NK cells [52, 53].
Although cytotoxic chemotherapy drugs activate antigen presentation mechanisms and tumor cell susceptibility to immune effector cells, their direct effects on immune effector mechanisms remain poorly understood. High dose cytotoxic chemotherapy could deplete activated tumor-reactive T cells. However, Nowak et al showed that relatively high doses of gemcitabine suppressed IgG antibody production, but did not block T lymphocyte recall responses and were not detrimental to specific anti-tumor immunity [54]. Furthermore, topotecan at MTD did not impair the frequency or response of virus-reactive lymphocytes in ovarian cancer patients [55]. Finally, cisplatin and paclitaxel did not interfere with T cell function in vitro. [56]. However, in the mouse cyclophosphamide, doxorubicin and paclitaxel at maximally tolerated dose (MTD) dampen the efficacy of vaccine if given after the vaccine, although they increase tumor-reactive T cells when given prior to vaccine [39]. Low dose metronomic chemotherapy may be a better approach for combinations with immunotherapy. In a mouse model, metronomic cyclophosphamide provided significantly greater synergism with vaccine than MTD cyclophosphamide. Both metronomic and MTD cyclophosphamide caused deletion of proliferating tumor-specific CTL in blood, but the metronomic dosing schedule caused deletion with slower kinetics and did not delete CD43low memory cells, which maintained potent capacity to respond to repeat tumor challenge [57].
c) Suppression of Inhibitory Cells
T regulatory (Treg) cells are characterized as CD25+CD4+Foxp3+ T cells which function to suppress immune responses and maintain peripheral tolerance. We have shown significantly increased proportions of TGF-β secreting Treg in patients with ovarian cancer [58], capable of suppressing tumor-specific T cell immunity and contributing to growth of human tumors in vivo [59]. Increased frequency of Treg in ovarian tumors predicts reduced survival [23, 59].
Intravenous cyclophosphamide has been used in humans to augment cancer vaccines [60, 61]. In addition, intravenous cyclophosphamide, doxorubicin and paclitaxel at maximally tolerated dose (MTD) were shown to help break tolerance to “self” cancer antigens and enhanced cancer vaccine efficacy [39]. The exact molecular function of these drugs on particular immune cells subsets has recently begun to be clarified. In mouse models, cyclophosphamide enhanced tumor immunotherapy by elimination of tumor-induced suppressor T cells [62] independently of cytotoxic reduction in tumor burden [63], and enhanced the magnitude of secondary but not primary cytotoxic lymphocyte responses induced by cancer vaccines [64, 65]. In the human, oral administration of metronomic cyclophosphamide was shown to induce a profound and selective reduction of circulating CD4+CD25+ regulatory T cells and restored T and NK effector functions in end-stage cancer patients [66]. Cyclophosphamide may have additional effects contributing to restoration of the immune response. For example, it can enhance IFN-γ production by splenocytes in a mouse model [67]. Conventional paclitaxel therapy also caused a significant decline in both numbers and activity of Treg, enhancing CD4+ and CD8+ activity systemically in patients with non-small cell lung cancer [68].
Myeloid-derived suppressor cells (MDSCs) is a recently described cell population characterized phenotypically as CD11b+Gr-1+ in the mouse. In the human, CD11b+Gr+ MDSC have not been clearly identified, but HLA-DRneg monocytes have functional features of suppressor cells and may be MDSC. A high frequency of HLA-DRneg monocytes secreting IL-10 have been described in ovarian cancer ascites in the human [69]. The main mechanism by which MDSC suppress T effector function is through the production of L-arginase, which reduces the availability of L-arginine, an essential amino acid fundamental for the function of T lymphocytes [70]. Importantly, gemcitabine used at conventional doses was found to significantly reduce the number of MDSCs in the spleens of treated mice, whereas other leukocytes were not affected [71].
In summary, chemotherapy drugs commonly used in gynecologic malignancies have specific immunomodulatory properties and could be used rationally to harness preexisting or induce a de novo antitumor immune response. It is thus expected that rational combination of these drugs with other immunomodulatory drugs may produce important clinical results, especially in patients with gynecologic malignancies who show pre-existing antitumor immune response. Below we will review some of the bona fide immunomodulatory tools already in the clinic that are available for clinical testing in immuno-chemotherapy combinations.
NON-SPECIFIC IMMUNE ACTIVATION
Multifaceted, pleiotropic immune activation can be achieved with cytokines and Toll-like receptor agonist therapy and is suitable for combination with immunomodulatory chemotherapy. Below we summarize experience accumulated to date in gynecologic cancers with older, more traditional cytokines, and will review newer cytokines and TLR agonists.
a) Interferons
Interferons were first described as antiviral cytokines, but have since been shown to be secreted in response to a vast number of stimulatory factors other than viruses. They are divided into two broad categories; type I and type II interferons. Type I interferons are subdivided into two classes, known as alpha and beta. Interestingly, 12 forms of IFN-α have been identified, while only one form of IFN-β has been isolated. Signaling through their corresponding receptors on target cells is mediated by a series of Jak/STAT proteins and results in several antiviral activities. Additionally, they have potent effects on cell proliferation. Mouse models have demonstrated that gene therapy with IFN-β can greatly enhance tumor cell death in the context of several different malignancies [72]. Many clinical trials have demonstrated the efficacy of type I interferon therapy in the treatment of hematologic malignancies [73–75], melanoma [76–80] and renal cell carcinoma [81–83]. Phase I/II clinical studies have examined the therapeutic value of type I IFNs in ovarian cancer. Intraperitoneal recombinant IFN-α alone or combined with cisplatin as salvage therapy for persistent ovarian cancer after primary chemotherapy has shown clinical efficacy in small volume disease [84, 85], but there was no significant effect in a cohort of patients with recurrent, platinum-resistant disease [86]. Although encouraging, these results did not support additional clinical development of type I interferon in ovarian cancer.
One of the limitations of interferon therapy relates to the high intratumoral cytokine levels required to induce antitumor responses, which cannot be achieved without eliciting systemic toxicity and cannot be sustained owing to the short half-life of recombinant proteins. Cytokine gene therapy using recombinant viral vectors can achieve much higher and sustained cytokine levels at the tumor site than those resulting from systemic or regional administration of recombinant cytokine proteins without engendering systemic toxicity [87]. A trial of intrapleural adenovirus delivering human IFN-β was recently completed at the University of Pennsylvania. Toxicity was minimal. One patient with recurrent, platinum-resistant low-grade ovarian carcinoma achieved complete objective and cytologic response of both pleural and intraperitoneal disease following a single intrapleural injection of adenovirus vector in this trial [88]. Disease stability or objective responses were also observed in patients with malignant pleural mesothelioma enrolled in the study [89]. These data present promising evidence that IFN-β can serve as a potent anticancer agent, and its use in combination with other forms of chemo and immunotherapy certainly warrants further consideration.
Structurally unrelated to type I interferons, IFN-γ is secreted by activated effector T cells and NK cells in response to target recognition. IFN-γ has been shown to have direct anti-proliferative activity on ovarian cancer cells in vitro, which proved to be synergistic with cisplatin and doxorubicin [90–92]. In vitro and in vivo, IFN-γ upregulates HLA class I and class II molecules and antigen presentation in ovarian tumor cells [93], a requisite for recognition by T cells. In fact, HLA class I expression by the tumor correlates with the intensity of T cell infiltration [94], a predictor of longer survival. Furthermore, IFN-γ has antiangiogenic effects [95].
Encouraging results have been reported with recombinant human (rh)IFN-γ either as intraperitoneal monotherapy or in combinations in early phase trials [96–99]. Theoretically, the effects are likely to be greatest in women who are also receiving chemotherapy because of IFN-γ’s non-specific immunomodulatory effects [100]. Confirming expectations, a three-fold prolongation of progression-free survival was observed in a phase-III multi-center study from Europe with subcutaneous administration of rhIFN-γ combined with MTD cisplatin and cyclophosphamide chemotherapy, with minimal added toxicity [47]. However, in a subsequent randomized phase-III trial conducted in the U.S., addition of subcutaneous rhIFN-γ to carboplatin and paclitaxel did not improve survival [101]. Although one cannot exclude that racial and other demographic differences may account for opposite results, this data may indicate that the choice of chemotherapy drugs is in fact critical in combinatorial approaches with immune therapy. Indeed, whereas cyclophosphamide has potent immunomodulatory effects on many cell subsets including suppressing T regulatory (Treg) cells (see below), high dose steroids, which are necessarily given with paclitaxel to prevent acute hypersensitivity reactions, are immunosuppressive and induce Treg in the setting of antigen presentation.
b) Interleukins
Interleukin-2 (IL-2) promotes expansion and enhances the cytotoxicity of effector immune cells [102]. In addition, IL-2 can restore T cell function following suppression by negative regulatory receptors such as PD-1 (see below). IL-2 represents the most widely investigated cytokine for use in cancer therapy, having shown clinical efficacy in malignant melanoma and renal cell carcinoma [103, 104], for which it is now FDA approved. Additionally, it has been used to enhance the efficacy of immunotherapy including vaccines and adoptive T-cell therapy [105]. However, its use has several limitations. In monotherapy and in the context of adoptive immunotherapy, IL-2 is used at MTD, which induces a systemic inflammatory response, with significant morbidity including multiple organ toxicities, most significantly the heart, lungs, kidneys, and central nervous system. Other manifestation of IL-2 toxicity is capillary leak syndrome, resulting in a hypovolemic state and fluid accumulation in the extravascular space [106].
Because ovarian cancer patients exhibit spontaneous antitumor immune response, IL-2 therapy may be a rational approach to activate preexisting immunity or enhance immunomodulatory therapy. Intraperitoneal IL-2 was used in a phase I/II study in 41 patients with laparotomy-confirmed persistent or recurrent ovarian cancer. Weekly IL-2 infusion was relatively well tolerated and demonstrated evidence of long-term efficacy in a modest number of patients. Twenty-percent of patients had a negative third look, i.e. exhibited pathologic evidence of complete response and no residual disease at repeat abdominal exploration [107]. Reflecting pre-therapy T cell activity, low expression of the CD3-zeta chain in peripheral blood T cells prior to therapy, a biomarker of T cell functional suppression by tumor-derived factors, predicted poor of response to IL-2 therapy [108]. Importantly, IL-2 is essential for the peripheral homeostasis of CD4+CD25+Foxp3+ Treg cells, and it is now known that IL-2 is also an important activator of Treg suppressive activity in vivo [109]. After IL-2 cessation, the number of Treg cells more efficiently dropped in patients who experienced a clinical response than in non-responders [110]. Together, this data indicate that patients with pre-existing tumor-reactive, functional T cells and low prevalence of Treg are those likely to benefit from IL-2 monotherapy. In another phase II study, 44 patients with EOC responding to primary chemotherapy were treated with subcutaneous low dose IL-2 and oral retinoic acid for one year and with intermittent schedules for up to five years. Patients experienced significantly improved progression-free and overall survival relative to 82 well-matched controls treated with standard therapy [111].
Alternate cytokines that selectively support activation of effector cells without promoting Treg cells may prove even more effective. IL-7, IL-15, IL-18 and IL-21 provide possible alternatives to IL-2, however their function and clinical use are still under investigation. The function of IL-7 has not been completely appreciated until recently. It serves an essential role not only in lymphopoeisis, but also T cell activity and maintenance and can promote antitumor immunity [112, 113]. A recent study using a mouse model of lung cancer examined the effects of IL-7 administration and found significant reduction in tumor burden, with a correlating increase in CD4+ and CD8+ T cells [114, 115]. IL-15 has similar functions to IL-2 in its effects of T cells, but also potentiates NK cell maturation and activity [116]. IL-21 is a promising cytokine as it enhances the cytolytic activity of CD8+ T cells and NK cells, but also modulates the activity of CD4 T cells, B cells and suppresses Treg cells [117]. A recent phase II trial demonstrated that administration of IL-21 was associated with antitumor activity in patients with unresectable metastatic melanoma [118].
IL-18 is a novel cytokine that has been shown to have very potent immunostimulatory effects, including induction if IFN-γ, TNF-α, IL-1α, and GM-CSF, augmentation of NK cell cytotoxicity, activation of effector T cells, and promotion of Th1 responses, which are critical for tumor rejection. In a recent study, rhIL-18 was found to expand human effector T cells and reduce human Treg in a mouse model transplanted with human peripheral blood lymphocytes [119]. Clearly this biology points to a strong potential for the use of IL-18 in cancer immunotherapy. The immunostimulatory activity of IL-18 in vivo has been demonstrated in non-human primates [120] and humans [121]. In phase I clinical evaluation, recombinant human (rh)IL-18 was safely administered as monotherapy to 28 patients with solid tumors, with minimal dose-limiting toxicities and two partial tumor responses [121]. Toxicity has generally been mild to moderate even with repeat administration and a maximum tolerated dose has not been reached to date [122]. IL-18 enhanced activation of peripheral blood CD8+ T cells, NK cells and monocytes and induced a transient increase in the frequency and expression level of Fas ligand (FasL) in peripheral blood CD8+ T cells and NK cells [122]. The relatively minor toxicity of rhIL-18, compared with other immunostimulatory cytokines that have undergone clinical development, is remarkable and renders IL-18 a well suited drug for combinatorial approaches with chemotherapy. In mice with established ovarian carcinoma, administration of IL-18 alone was shown to have modest effects on tumor immunity, but when combined with pegylated liposomal doxorubicin chemotherapy its effects were greatly enhanced (Coukos et al, unpublished data). Clearly the use of IL-18 therapy with other immunogenic chemotherapy warrants further investigation. A phase I study is currently under way to test this hypothesis.
c) Toll-like Receptor Agonists
One of the most basic mechanisms for activation of the immune system is through the Toll-like receptors (TLRs). TLRs belong to the type I transmembrane receptor family. Their expression is ubiquitous, from epithelial to immune cells. The TLR-family members are pattern-recognition receptors that collectively recognize lipid, carbohydrate, peptide and nucleic-acid structures that are broadly expressed by different groups of microorganisms. Some TLRs are expressed at the cell surface, whereas others are expressed on the membrane of endocytic vesicles or other intracellular organelles. There are at least 10 known TLRs in humans grouped in six major families, based on their phylogenetic background [123]. Each family is attributed to a general class of PAMPs. TLRs 3, 7, 8 and 9 are located mainly in endosomes; double-stranded RNA are ligands for TLR3 [124], while TLRs 7 and 8 recognize single-stranded viral RNA [125]. The other TLRs are located on the cell surface [126]; TLRs 1, 2, 5, 6 and 10 respond to bacterial, fungal and viral PAMPs [127–129]. Lipopolysaccharides are TLR4 ligands [130].
TLR engagement alerts the immune system and leads to the activation of innate immune cells. Two major signaling pathways are generally activated in response to a TLR ligand [131]. One pathway involves the MyD88-independent production of type I interferons. The second uses MyD88 to activate nuclear factor-kappa B (NF-κB), JUN kinase (JNK) and p38, finally resulting in the production of proinflammatory cytokines such as TNF-α, IL-12 and IL-1 and induction of innate effector mechanisms [132, 133]. Additionally, TLR triggering induces DC maturation, which leads to the upregulation of costimulatory molecules such as CD40, CD80 and CD86, and secretion of immune modulatory cytokines and chemokines. In addition, TLRs can directly stimulate the proliferation of CD4+ and CD8+ T cells as well as reverse the suppressive function of Treg cells [126][134][135]. Adding TLR 3, 4, 7 or 9 ligands was shown to activate CD8+ cytotoxic T cells with increased IFN-γ production and promote a stimulatory cytokine milieu at the tumor microenvironment [136, 137]. Optimal antitumor immunity requires robust enhancement of the effector T cell response induced by tumor antigenic peptides and control or elimination of Treg suppressive function. Thus, the combination of peptide-based vaccines with TLR agonists, in particular a TLR8 agonist, may greatly improve the therapeutic potential of cancer vaccines.
Several clinical trials have demonstrated that administration of agonists for TLRs 3,4,7 and 9 can enhance activity of cancer vaccines in the context of non-small cell lung cancer [138], non-Hodgkins lymphoma [139, 140], glioblastoma [141], and superficial basal cell carcinoma [142]. Multiple TLR agonists have also been explored in melanoma. TLR 7 or 9 agonists were used in combination with melanoma antigen vaccine in advanced melanoma [31, 143, 144]. In addition, the TLR ligand Ribomunyl has been used in conjunction with a dendrtic cell vaccine in a phase I/II trial, which reported a median survival of 10.5 months in patients with advanced melanoma [145].
The use of TLR agonists in the clinic requires careful preclinical evaluation. For example, in the absence of specific cell-mediate antitumor immunity, non-specific activation of inflammation could in fact promote tumor growth rather than reducing it, because of the potent tumor-promoting effects of inflammation [146]. Thus, combinations with active immunization or adoptive immunotherapy seem ideal, as these approaches greatly benefit from concomitant activation of innate immune response. If combination with chemotherapy is designed, it seems rational to combine TLR agonists with chemotherapy drugs that can activate cellular immune mechanisms. Finally, the choice of TLR agonists may matter. Whereas TLR 3 and 9 agonists induce apoptosis of TLR-expressing tumor cells [147]. TLR4 agonists were shown to promote tumor cell survival, tumor growth and paclitaxel resistance in a proportion of ovarian cancer cells [148, 149].
ACTIVATION OF CELLULAR IMMUNITY
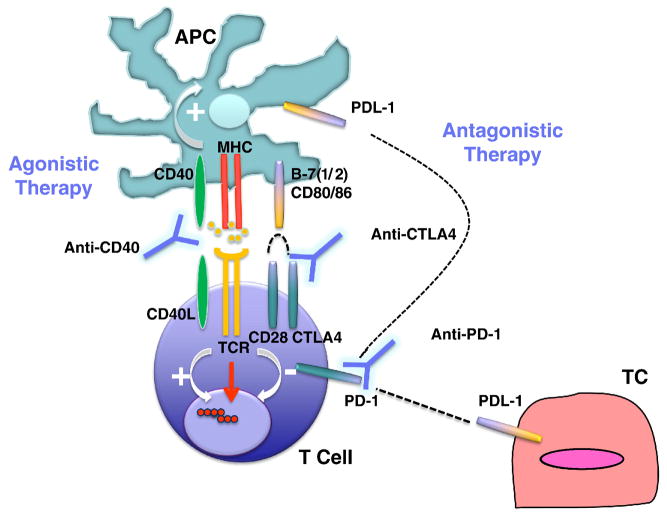
Generation of a successful antitumor adaptive immune response requires first and foremost the primary signal provided by the binding of T cell receptor to cognate tumor antigen. However, multiple secondary signals can activate or suppress this response. Characterization of these pathways in tumors and the development of specific agonistic or antagonistic antibodies or ligands has created new opportunities for powerful stimulation of antitumor immune response (Figure 2).
Figure 2.
a) DC Activation via CD40
The CD40 receptor is a member of the TNF receptor family expressed by antigen presenting cells and B cells. Its ligand, CD40L, is transiently upregulated on activated T cells, activated B cells and platelets; and under inflammatory conditions is also induced on monocytes and other innate immune cells. CD40 is a potent stimulator of antigen presenting cells and cellular immunity, and CD40/CD40L interaction is critical in the development of protective anti-tumor immunity. Mice deficient in CD40 fail to mount a protective anti-tumor immune response following vaccination. In addition, neutralizing anti-CD40L monoclonal antibody can abrogate the therapeutic value of potent tumor vaccines [150]. Vice versa, a CD40 agonistic antibody was shown to be able to overcome peripheral tolerance and generate antitumor immunity able to reject tumors [151]. The main mechanism of immune stimulation by CD40 ligands is activation of DCs resulting in increased survival, upregulation of costimulatory molecules, and secretion of critical cytokines for T cell priming such as IL-12. This promotes antigen presentation, priming and cross-priming of CD4+ and CD8+ effector T cells [152]. However, agonistic anti-CD40 antibody alone can have adverse effects on antitumor immunity as in the mouse it can ultimately impair the development of tumor-specific T cells [153] or accelerate the deletion of tumor-specific cytotoxic lymphocytes in the absence of antigen vaccination [154]. CD40 ligation could thus be best used in combinatorial approaches including vaccines and TLR agonists [152, 155]. Based on the imunomodulatory effects of select chemotherapy agents, the combination of CD40 ligands with chemotherapy is also a rational approach that warrants thorough investigation. For example, in mice with established solid tumors, the administration of gemcitabine with CD40L triggered potent antitumor immune response that eliminated tumor burden, and these mice became also resistant to repeated tumor challenge [156].
Interestingly, the CD40 receptor is expressed on a variety of tumors including melanoma, lung, bladder and prostate cancers [152], but also cervix [157] and the majority of ovarian cancers [158–162]. Because tumor cells also express the CD40L, it is likely that low-level constitutive engagement of CD40 facilitates malignant cell growth. However, transient potent activation of CD40 on carcinomas with ligand results in direct anti-proliferative effects and apoptosis. CD40 agonists promoted apoptosis and resulted in growth inhibition of ovarian carcinoma lines expressing CD40. CD40 ligation also induced NF-κB activation, and TNF-α, IL-6 and IL-8 production in most EOC cell lines [158, 163]. In vivo, administration of rhuCD40L inhibited the growth of several ovarian adenocarcinoma xenografts in severe combined immunodeficient mice through a direct effect causing apoptosis, fibrosis and tumor destruction. The antitumor effect of rhuCD40L was further increased by cisplatin [164]. Interestingly, rIFN-γ enhanced expression of CD40 on tumor cells and the efficacy of on EOC cell lines [159]. Thus, CD40 agonists can have a direct cytotoxic effects on tumors, even in the absence of any additional immune responses and cells.
Early clinical experience with monoclonal IgG agonistic antibodies is encouraging. In a recent phase I study, patients with advanced solid tumors received single doses of CD40 agonistic antibody CP-870,893 intravenously. CP-870,893 was well tolerated; the most common adverse event being cytokine release syndrome including chills, rigors, and fever; 14% of all patients and 27% of melanoma patients had objective partial responses [165].
b) Activation of T Effector Cells via Blockade of Inhibitory Checkpoints
T-cell activation is triggered through the T-cell receptor by recognition of the cognate antigen complexed with MHC. T cell activation is regulateD by complex signals downstream of the diverse family of CD28 family immune receptors, which includes costimulatory (CD28 and ICOS) and inhibitory receptors (CTLA-4, PD-1 and BTLA). CD28 and CTLA-4 share the same ligands, B7-1 (CD80) and B7-2 (CD86), whereas PD-1 interacts with PD ligand 1 (PD-L1), also named B7-H1, and PD-L2, also named B7-DC. Simultaneous recognition of the cognate MHC-peptide complex by the TCR (signal 1) and CD80 or CD86 by CD28 (signal 2) results in T cell activation, proliferation, and differentiation, as well as effector cytokine production. PD-1 and CTLA-4 are induced on T cells following a TCR signal, and result in cell cycle arrest and termination of T cell activation. The importance of the PD-1 and CTLA-4 pathways in the physiologic regulation of T cell activation is demonstrated by the autoimmune diseases occurring in CTLA-4 and PD-1 knockout mice [166] and further illustrated by the inflammatory side effects that can result from a therapeutic blockade of CTLA-4 in vivo, both in animal models and in humans [167–170]. The use of blocking CTLA-4or PD-1mAbs can sustain the activation and proliferation of tumor-specificT cells, preventing anergy or exhaustion and thereby allowing the development of an effectivetumor-specific immune response.
b.1) CTLA-4 Blockade
CTLA-4 is the best characterized among the inhibitory B7 receptors; like CD28, CTLA-4 also binds CD80 and 86 expressed by APCs, but provides inhibitory signals to theT cell, serving as a negative feedback loop. In animal models, CTLA-4 proved critical in the normal functioning immune system; CTLA-4 knockout mice developed uncontrolled lymphoproliferation with early lethalities. Furtermore, administration of CTLA-4 blockade significantly enhanced antitumor immunity in a variety of mouse models and with various therapeutic combinations including vaccine, TLR agonist, chemotherapy and radiation therapy. The mechanism by which CTLA-4 blockade induces tumor regression is not fully understood. Although Treg constitutively express CTLA-4, CTLA-4 blockade had no effect on the number or function of Treg in cancer patients [171]. It seems that CTLA-4 blockade activates directly CD4 and CD8+ T effector cells by removing an inhibotory checkpoint on proliferation and function [172], and combination of direct enhancement of Teff cell function and inhibition of T reg activity is essential for mediating the full therapeutic effects of anti-CTLA-4 antibodies during cancer immunotherapy [173].
Based on these data, several clinical studies have been undertaken to assess the efficacy of a CTLA-4 antagonist antibodies in the setting of human cancer. The majority of clinical data to date have emerged from studies inpatients with melanoma (reviewed in [174]). Phase II studies with ipilimumab monotherapy showed objective responses at a dose of 3 to 9 mg/kg every3 weeks, with 10 mg/kg showing the best risk-benefit profile. Ipilimumab (3 mg/kg every 4 weeks × 4) administeredin combination with chemotherapy (dacarbazine) resulted in enhanced objective response rates without added toxicity. Ipilumimab has also been combined successfully with IL-2 (720,000 U/kg every8 hours × 15 (ORrate of 22% - three CRs) or melanoma vaccine (OR rateof 13% - two complete responses). In addition, tremelimumab has also produced OR and CRs in melanoma patients. To date, CTLA-4 antibody has not shown superiority over standard chemotherapy trial in treatment-naive melanoma patients in a phase III randomized clinical, which was discontinued after interim analysis. In melanoma patients, the most common grade 3 to 4 adverse events were colitis/enterocolitis (increased frequency of diarrhea/stools), dermatitis, andhypophysitis.
Eleven patients with ovarian carcinoma, previously vaccinated with GM-CSF modified, irradiated autologous tumor cells (GVAX), received ipilumimab (1 month to 3 years following GVAX). Significant antitumor effects were observed in a minority of the patients. One patient achieved a dramatic fall in CA-125 levels several months after an initial dose of ipilumimab; although the response was not maintained, a second infusion resulted in a more rapid decline in CA-125 values. Nine additional infusions of the anti-CTLA-4 antibody spaced at 3- to 5-month intervals over nearly 4 years have maintained profound disease control, with grade 1 rash as the only adverse event. An objective radiographic response was noted in parallel with the reduction in CA125 Moreover, this patient’s course was initially characterized by a remarkable periodicity to the rise and fall of CA-125 levels, but this gradually dampened as a function of time and/or prolonged therapy, demonstrating a lack of acquired resistance to ipilimumab commonly observed with cytotoxic chemotherapy [169]. In contrast to the melanoma cohort, a single dose of 3 mg/kg Ipilumimab triggered two cases of grade 3 gastrointestinal inflammation among the 9 ovarian carcinoma patients, consistent of significant diarrhea. Endoscopic biopsies revealed mucosal damage associated with abundant granulocytes, macrophages, CD4+ and CD8+ T cells, and FoxP3+ Treg. The remaining seven patients showed only minor inflammatory toxicities including papular rash, or urticarial-like reactions at sites of prior vaccination [169]. Tumor regression in these patients correlated with the CD8+/Treg ratio, suggesting that other forms of therapy that target Treg depletion may provide a highly effective form of treatment when combined with the tumor vaccine and CTLA-4 antibody arsenal [169].
b.2) PD-1 Blockade
Programmed death receptor-1 is a negative regulator of cell activation, primarily expressed on both B and T lymphocytes, which binds PD-L1 and PD-L2 ligands. PD-L2 is restricted to professional antigen presenting cells, while PD-L1 is expressed on many tissues, and plays a pivotal role in maintaining peripheral tolerance. Importantly, cancer cells have adopted expression of PD-L1 into their immunosuppressive arsenal [175]; a variety of epithelial cancers express PD-L1 [176–178]. It has also recently been shown that ovarian carcinoma cells as well as tumor-infiltrating tolerogenic DCs and myeloid derived suppressor cells express PD-L1 [179, 180], and indeed expression levels correlate with disease course.1 Although PD-1 signaling can occur at very low levels of receptor expression, elevated levels of PD-1 have recently been attributed functional significance; during conditions of chronic antigen persistence (such as in chronic viral infections), antigen specific effector T cells expressing high levels of PD-1 were demonstrated to be functionally exhausted, i.e. unable to proliferate, secrete IL-2 or kill target cells [181]. It has been recently shown that tumor infiltrating lymphocytes in metastatic melanoma and other tumors expresses higher levels of PD-1 than CD4+ and CD8+ cells in the peripheral blood of healthy patients and exhibit phenotypic and functional characteristics of exhausted T cells with impaired effector function. These findings suggest that the tumor microenvironment can lead to up-regulation of PD-1 on tumor-reactive T cells and contribute to impaired antitumor immune responses [182]. Indeed, constitutive or inducible expression of PD-L1by tumors conferred resistance to immunotherapy in mice related to failure of antigen-specific CD8+ CTLs to destroy tumor cells but without impairment of CTL function [183].
Following evidence that PD-1 blockade through gene therapy approaches in the tumor microenvironment activates antitumor immunity [184], antibodies blocking PD-L1 or PD-1 were found to profoundly enhance the efficacy of immune therapy [183, 185]. Anti-PD-1 antibody has been combined with cell-based vaccines and demonstrated increased infiltration, potency and duration of activity of CD8 T cells in the tumor microenvironment in the setting of B16 melanoma and CT26 colon carcinoma in mice.
Despite their similarities, the regulatory roles of CTLA-4 and PD-1 may differ substantially in cancer patients. First, Even though PD-1 and CTLA-4 ligation efficiently block T-cell responses, the homeostatic function of the two inhibitory receptors differs. For example, CTLA-4 ligation does not inhibit PI3-kinase signaling, while PD-1 engagement blocks the induction of PI3K activity. PD-1-mediated signaling blocks T-cell activation more effectively than CTLA-4-mediated signaling. In addition, PD-1 signaling may be related to T cell apoptosis. Second, whereas the ligands for CTLA-4, CD80 and CD86, are primarily expressed by mature antigen presenting cells, PD-L1 (on tumor cells or myeloid cells) and PD-1 (on effector T cells) are significantly upregulated in the tumor microenvironment. Thus, the effects of PD-1 blockade may be more pronounced in the tumor than in periphery. Indeed, a phase I study using PD-1 blocking antibody showed the antibody to be safe and well tolerated in patients with hematologic malignancies. No single maximum tolerated dose was defined in this study. Clinical benefit was observed in 33% of the patients with one complete remission [186].
c) Depletion of T Regulatory Cells
As mentioned previously, CD4+CD25+Foxp3+ T regulatory cells are responsible for maintaining peripheral tolerance by inhibiting T cell activity therefore forming an obstacle for cancer immunotherapy. Depletion of Treg in vivo enhances tumor immunity in numerous animal models [187–189]. In clinical trials, a number of Treg-depleting strategies have been investigated [39, 105, 190–192]. An example is the use of cyclophosphamide in combination with vaccines [61, 193]. Other strategies for Treg depletion is through targeting the IL-2 receptor alpha chain, also known as CD25. In mouse models, the use of anti-CD25 monoclonal antibody before vaccination led to complete tumor rejection and establishment of long-lasting tumor immunity with essentially no autoimmune complications [194, 195]. Preclinical studies have shown that administration of anti-CD25 antibody linked to a potent pro-inflammatory toxin can selectively reduce Treg concentrations in vitro while maintaining other T cell populations [196]. This same molecule was used in patients with metastatic melanoma and showed consistent reduction in CD4+CD25+ Treg cells [197]. However, the effect was transient, with a half-life of the immunotoxin of only 2 hours. Another clinical appproach of targeting CD25 is through Denileukin diftitox, a fusion protein of IL-2 and diphtheria toxin that targets CD25-expressing cells used in patients with melanoma, ovarian cancer and renal cell carcinoma [198–201]. Although effective in short-term infusions, these conjugates are quite immunogenic and induce neutralizing antibodies, which hamper their long-term application.
Another agent is Daclizumab (Roche), which is an FDA-approved humanized IgG1-kappa mAb that binds specifically to CD25 [202]. Daclizumab inhibits T cell proliferation to recall antigens, but does not inhibit IL-15 effects. It has been used in autoimmune disorders [203, 204], acute graft-versus-host disease [205], and in cancer patients with CD25+ T cell malignancies [206]. The advantage of Daclizumab is that it extremely well tolerated, and has a half-life of 20 days [207]. Furthermore, It is significantly less immunogenic than immunotoxins and can be given for longer periods of time. In a recent study, Daclizumab was used in a single dose of 1 mg/m2 prior to hTERT peptide vaccine for metastatic breast cancer. Total CD4+CD25+ and CD4+CD25+FoxP3+ cells remained suppressed for several weeks during vaccination. There have been no significant toxicities. Importantly, administration of CD25 antibody was compatible with effective vaccination (Robert Vonderheide, unpublished observations).
CONCLUSIONS
In the past decade we have witnessed important advances. First, gynecologic cancers were proposed as potentially immunogenic tumors, a characterization formerly reserved only for melanoma and renal cell cancer. This has opened the door to explore immune therapy not only in HPV-induced cancers but also in ovarian and endometrial cancers. Second, the a priori notion that chemotherapy drugs antagonize immune mechanisms altogether was challenged by evidence that select chemotherapy drugs commonly used to treat gynecologic cancers have important immunomodulatory effects. This has opened the door to explore interactions of these drugs with natural antitumor immunity. Third, over the past decade several mechanisms of tumor immune escape, accounting for failure of immunotherapy, have been deciphered, and the importance of combinatorial immunotherapy targeting both adaptive and innate, effector and suppressor mechanisms has been proven. Fourth, this decade has produced novel and potent bona fide stimulants of innate and adaptive immunity, such as novel pleiotropic cytokines and Toll-like receptors, as well as powerful antibodies activating cellular immune mechanisms. Finally, the development of several syngeneic mouse models of ovarian and other gynecologic cancers in immunocompetent animals is an important step forward in the preclinical development of these agents. The next decade will be the time to test and optimize these combinations to maximize efficacy and decrease toxicity. Rational combinations of agents will require understanding of their precise mechanism of action in order to select combinations yielding positive interactions. Careful preclinical evaluation in well-characterized animal models will be necessary to screen combinations before undertaking clinical studies.
Acknowledgments
This work was supported by NIH R01-CA098951; NIH P50-CA083638 Ovarian Cancer SPORE and NIH R01-CA112162 and The Immune Therapy Initiative for Ovarian Cancer
Footnotes
CONFLICT OF INTEREST
All authors declare that there are no conflicts of interest except Dr. Jonathan Berek who is a research consultant for Genentech, GlaxoSmithKline and Menarini.
References
- 1.Wu TC. Immunology of the human papilloma virus in relation to cancer. Curr Opin Immunol. 1994;6:746–754. doi: 10.1016/0952-7915(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 2.Halpert R, Fruchter RG, Sedlis A, Butt K, Boyce JG, Sillman FH. Human papillomavirus and lower genital neoplasia in renal transplant patients. Obstet Gynecol. 1986;68:251–8. [PubMed] [Google Scholar]
- 3.Laga M, Icenogle JP, Marsella R, Manoka AT, Nzila N, Ryder RW, Vermund SH, Heyward WL, Nelson A, Reeves WC. Genital papillomavirus infection and cervical dysplasia--opportunistic complications of HIV infection. Int J Cancer. 1992;50:45–8. doi: 10.1002/ijc.2910500110. [DOI] [PubMed] [Google Scholar]
- 4.Schafer A, Friedmann W, Mielke M, Schwartlander B, Koch MA. The increased frequency of cervical dysplasia-neoplasia in women infected with the human immunodeficiency virus is related to the degree of immunosuppression. Am J Obstet Gynecol. 1991;164:593–9. doi: 10.1016/s0002-9378(11)80029-3. [DOI] [PubMed] [Google Scholar]
- 5.Tagami H. Regression phenomenon of numerous flat warts--an experiment on the nature of tumor immunity in man. Int J Dermatol. 1983;22:570–1. doi: 10.1111/j.1365-4362.1983.tb02126.x. [DOI] [PubMed] [Google Scholar]
- 6.Benton C, Shahidullah H, Hunter JAA. Papillomavirus Report. 1992. Human papillomavirus in the immunosuppressed; pp. 23–26. [Google Scholar]
- 7.Brandsma JL. Papillomavirus Report. 1994. Animal models for HPV vaccine development; pp. 105–111. [Google Scholar]
- 8.Selvakumar R, Borenstein LA, Lin YL, Ahmed R, Wettstein FO. Immunization with nonstructural proteins E1 and E2 of cottontail rabbit papillomavirus stimulates regression of virus-induced papillomas. J Virol. 1995;69:602–5. doi: 10.1128/jvi.69.1.602-605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilders CG, Ras L, van Eendenburg JD, Nooyen Y, Fleuren GJ. Isolation and characterization of tumor-infiltrating lymphocytes from cervical carcinoma. Int J Cancer. 1994;57:805–13. doi: 10.1002/ijc.2910570608. [DOI] [PubMed] [Google Scholar]
- 10.Sheu BC, Chang WC, Lin HH, Chow SN, Huang SC. Immune concept of human papillomaviruses and related antigens in local cancer milieu of human cervical neoplasia. J Obstet Gynaecol Res. 2007;33:103–13. doi: 10.1111/j.1447-0756.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- 11.Chu CS, Kim SH, June CH, Coukos G. Immunotherapy opportunities in ovarian cancer. Expert Rev Anticancer Ther. 2008;8:243–57. doi: 10.1586/14737140.8.2.243. [DOI] [PubMed] [Google Scholar]
- 12.Schlienger K, Chu CS, Woo EY, Rivers PM, Toll AJ, Hudson B, Maus MV, Riley JL, Choi Y, Coukos G, Kaiser LR, Rubin SC, Levine BL, Carroll RG, June CH. TRANCE- and CD40 ligand-matured dendritic cells reveal MHC class I-restricted T cells specific for autologous tumor in late-stage ovarian cancer patients. Clin Cancer Res. 2003;9:1517–27. [PubMed] [Google Scholar]
- 13.Goodell V, Salazar LG, Urban N, Drescher CW, Gray H, Swensen RE, McIntosh MW, Disis ML. Antibody immunity to the p53 oncogenic protein is a prognostic indicator in ovarian cancer. J Clin Oncol. 2006;24:762–8. doi: 10.1200/JCO.2005.03.2813. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi K, Yonamine K, Masuko-Hongo K, Iida T, Yamamoto K, Nishioka K, Kato T. Clonal expansion of T cells that are specific for autologous ovarian tumor among tumor-infiltrating T cells in humans. Gynecol Oncol. 1999;74:86–92. doi: 10.1006/gyno.1999.5430. [DOI] [PubMed] [Google Scholar]
- 15.Halapi E, Yamamoto Y, Juhlin C, Jeddi-Tehrani M, Grunewald J, Andersson R, Hising C, Masucci G, Mellstedt H, Kiessling R. Restricted T cell receptor V-beta and J-beta usage in T cells from interleukin-2-cultured lymphocytes of ovarian and renal carcinomas. Cancer Immunol Immunother. 1993;36:191–7. doi: 10.1007/BF01741091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisk B, Blevins TL, Wharton JT, Ioannides CG. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med. 1995;181:2109–17. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kooi S, Freedman RS, Rodriguez-Villanueva J, Platsoucas CD. Cytokine production by T-cell lines derived from tumor-infiltrating lymphocytes from patients with ovarian carcinoma: tumor-specific immune responses and inhibition of antigen-independent cytokine production by ovarian tumor cells. Lymphokine Cytokine Res. 1993;12:429–37. [PubMed] [Google Scholar]
- 18.Peoples GE, Goedegebuure PS, Smith R, Linehan DC, Yoshino I, Eberlein TJ. Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc Natl Acad Sci U S A. 1995;92:432–6. doi: 10.1073/pnas.92.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peoples GE, Anderson BW, Fisk B, Kudelka AP, Wharton JT, Ioannides CG. Ovarian cancer-associated lymphocyte recognition of folate binding protein peptides. Ann Surg Oncol. 1998;5:743–50. doi: 10.1007/BF02303486. [DOI] [PubMed] [Google Scholar]
- 20.Dadmarz RD, Ordoubadi A, Mixon A, Thompson CO, Barracchini KC, Hijazi YM, Steller MA, Rosenberg SA, Schwartzentruber DJ. Tumor-Infiltrating Lymphocytes from Human Ovarian Cancer Patients Recognize Autologous Tumor in an MHC Class II-Restricted Fashion. Cancer J Sci Am. 1996;2:263. [PubMed] [Google Scholar]
- 21.Santin AD, Bellone S, Ravaggi A, Pecorelli S, Cannon MJ, Parham GP. Induction of ovarian tumor-specific CD8+ cytotoxic T lymphocytes by acid-eluted peptide-pulsed autologous dendritic cells. Obstet Gynecol. 2000;96:422–30. doi: 10.1016/s0029-7844(00)00916-9. [DOI] [PubMed] [Google Scholar]
- 22.Peoples GE, Schoof DD, Andrews JV, Goedegebuure PS, Eberlein TJ. T-cell recognition of ovarian cancer. Surgery. 1993;114:227–34. [PubMed] [Google Scholar]
- 23.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams SF, Levine DA, Cadungog MG, Hammond R, Facciabene A, Gimotty PA, Coukos G. Intraepithelial T Cells and Tumor Proliferation: Impact on the Benefit from Surgical Cytoreduction in Advanced Serous Ovarian Cancer. Cancer. 2009 doi: 10.1002/cncr.24317. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke B, Tinker AV, Lee C, Subramanian S, van de Rijn M, Turbin D, Kalloger S, Cadungog MG, Huntsman D, Coukos G, Gilks CB. Intraepithelial T cells and Prognosis in Ovarian Carcinoma: Novel Associations with Stage, Tumor Type and BRCA1 Loss. Modern Pathology. 2008 doi: 10.1038/modpathol.2008.191. In Press. [DOI] [PubMed] [Google Scholar]
- 26.Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, Lin YG, Merritt WM, Spannuth WA, Deavers MT, De Geest K, Gershenson DM, Lutgendorf SK, Ferrone S, Sood AK. HLA class I antigen processing machinery component expression and intratumoral T-Cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res. 2008;14:3372–9. doi: 10.1158/1078-0432.CCR-07-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah CA, Allison KH, Garcia RL, Gray HJ, Goff BA, Swisher EM. Intratumoral T cells, tumor-associated macrophages, and regulatory T cells: Association with p53 mutations, circulating tumor DNA and survival in women with ovarian cancer. Gynecol Oncol. 2008 doi: 10.1016/j.ygyno.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomsova M, Melichar B, Sedlakova I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol. 2008;108:415–20. doi: 10.1016/j.ygyno.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 30.de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, Nijman HW. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114:105–10. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Adams S, O’Neill DW, Nonaka D, Hardin E, Chiriboga L, Siu K, Cruz CM, Angiulli A, Angiulli F, Ritter E, Holman RM, Shapiro RL, Berman RS, Berner N, Shao Y, Manches O, Pan L, Venhaus RR, Hoffman EW, Jungbluth A, Gnjatic S, Old L, Pavlick AC, Bhardwaj N. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181:776–84. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santin AD. HER2/neu overexpression: has the Achilles’ heel of uterine serous papillary carcinoma been exposed? Gynecol Oncol. 2003;88:263–5. doi: 10.1016/s0090-8258(02)00094-x. [DOI] [PubMed] [Google Scholar]
- 33.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 34.Birkeland SA, Storm HH, Lamm LU, Barlow L, Blohme I, Forsberg B, Eklund B, Fjeldborg O, Friedberg M, Frodin L, et al. Cancer risk after renal transplantation in the Nordic countries, 1964–1986. Int J Cancer. 1995;60:183–9. doi: 10.1002/ijc.2910600209. [DOI] [PubMed] [Google Scholar]
- 35.Adami J, Gabel H, Lindelof B, Ekstrom K, Rydh B, Glimelius B, Ekbom A, Adami HO, Granath F. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221–7. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pham SM, Kormos RL, Landreneau RJ, Kawai A, Gonzalez-Cancel I, Hardesty RL, Hattler BG, Griffith BP. Solid tumors after heart transplantation: lethality of lung cancer. Ann Thorac Surg. 1995;60:1623–6. doi: 10.1016/0003-4975(95)00120-4. [DOI] [PubMed] [Google Scholar]
- 37.Bordea C, Wojnarowska F, Millard PR, Doll H, Welsh K, Morris PJ. Skin cancers in renal-transplant recipients occur more frequently than previously recognized in a temperate climate. Transplantation. 2004;77:574–9. doi: 10.1097/01.tp.0000108491.62935.df. [DOI] [PubMed] [Google Scholar]
- 38.Chapman JR, Sheil AG, Disney AP. Recurrence of cancer after renal transplantation. Transplant Proc. 2001;33:1830–1. doi: 10.1016/s0041-1345(00)02698-1. [DOI] [PubMed] [Google Scholar]
- 39.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–97. [PubMed] [Google Scholar]
- 40.Kepp O, Tesniere A, Schlemmer F, Michaud M, Senovilla L, Zitvogel L, Kroemer G. Immunogenic cell death modalities and their impact on cancer treatment. Apoptosis. 2009;14:364–75. doi: 10.1007/s10495-008-0303-9. [DOI] [PubMed] [Google Scholar]
- 41.Obeid M, Panaretakis T, Tesniere A, Joza N, Tufi R, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Leveraging the immune system during chemotherapy: moving calreticulin to the cell surface converts apoptotic death from “silent” to immunogenic. Cancer Res. 2007;67:7941–4. doi: 10.1158/0008-5472.CAN-07-1622. [DOI] [PubMed] [Google Scholar]
- 42.Hornychova H, Melichar B, Tomsova M, Mergancova J, Urminska H, Ryska A. Tumor-infiltrating lymphocytes predict response to neoadjuvant chemotherapy in patients with breast carcinoma. Cancer Invest. 2008;26:1024–31. doi: 10.1080/07357900802098165. [DOI] [PubMed] [Google Scholar]
- 43.Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, Muggia F, Symmans WF. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–30. [PubMed] [Google Scholar]
- 44.Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 2008;68:4026–30. doi: 10.1158/0008-5472.CAN-08-0427. [DOI] [PubMed] [Google Scholar]
- 45.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Metivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, Kroemer G. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 47.Windbichler GH, Hausmaninger H, Stummvoll W, Graf AH, Kainz C, Lahodny J, Denison U, Muller-Holzner E, Marth C. Interferon-gamma in the first-line therapy of ovarian cancer: a randomized phase III trial. Br J Cancer. 2000;82:1138–44. doi: 10.1054/bjoc.1999.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berd D, Mastrangelo MJ. Active immunotherapy of human melanoma exploiting the immunopotentiating effects of cyclophosphamide. Cancer Invest. 1988;6:337–49. doi: 10.3109/07357908809080657. [DOI] [PubMed] [Google Scholar]
- 49.Radojcic V, Bezak KB, Skarica M, Pletneva MA, Yoshimura K, Schulick RD, Luznik L. Cyclophosphamide resets dendritic cell homeostasis and enhances antitumor immunity through effects that extend beyond regulatory T cell elimination. Cancer Immunol Immunother. 2009 doi: 10.1007/s00262-009-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183:137–44. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei J, DeAngulo G, Sun W, Hussain SF, Vasquez H, Jordan J, Weinberg J, Wolff J, Koshkina N, Heimberger AB. Topotecan enhances immune clearance of gliomas. Cancer Immunol Immunother. 2009;58:259–70. doi: 10.1007/s00262-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins JL, Kao MS. The anticancer drug, cisplatin, increases the naturally occurring cell-mediated lysis of tumor cells. Cancer Immunol Immunother. 1989;29:17–22. doi: 10.1007/BF00199911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaue H, Tanimura H, Noguchi K, Iwahashi M, Tsunoda T, Tani M, Tamai M, Hotta T, Mizobata S, Arii K. Cisplatin treatment renders tumor cells more susceptible to attack by lymphokine-activated killer cells. J Clin Lab Immunol. 1991;35:165–70. [PubMed] [Google Scholar]
- 54.Nowak AK, Robinson BW, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 2002;62:2353–8. [PubMed] [Google Scholar]
- 55.Ferrari S, Rovati B, Cucca L, Scarabelli C, Presti M, Beccaria C, Collova E, Porta C, Danova M. Impact of topotecan-based chemotherapy on the immune system of advanced ovarian cancer patients: an immunophenotypic study. Oncol Rep. 2002;9:1107–13. [PubMed] [Google Scholar]
- 56.Markasz L, Skribek H, Uhlin M, Otvos R, Flaberg E, Eksborg S, Olah E, Stuber G, Szekely L. Effect of frequently used chemotherapeutic drugs on cytotoxic activity of human cytotoxic T-lymphocytes. J Immunother. 2008;31:283–93. doi: 10.1097/CJI.0b013e3181628b76. [DOI] [PubMed] [Google Scholar]
- 57.Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 2003;63:8408–13. [PubMed] [Google Scholar]
- 58.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- 59.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 60.Curti BD, Ochoa AC, Powers GC, Kopp WC, Alvord WG, Janik JE, Gause BL, Dunn B, Kopreski MS, Fenton R, Zea A, Dansky-Ullmann C, Strobl S, Harvey L, Nelson E, Sznol M, Longo DL. Phase I trial of anti-CD3-stimulated CD4+ T cells, infusional interleukin-2, and cyclophosphamide in patients with advanced cancer. J Clin Oncol. 1998;16:2752–60. doi: 10.1200/JCO.1998.16.8.2752. [DOI] [PubMed] [Google Scholar]
- 61.Berd D, Maguire HC, Jr, Mastrangelo MJ. Induction of cell-mediated immunity to autologous melanoma cells and regression of metastases after treatment with a melanoma cell vaccine preceded by cyclophosphamide. Cancer Res. 1986;46:2572–7. [PubMed] [Google Scholar]
- 62.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–74. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Awwad M, North RJ. Cyclophosphamide-induced immunologically mediated regression of a cyclophosphamide-resistant murine tumor: a consequence of eliminating precursor L3T4+ suppressor T-cells. Cancer Res. 1989;49:1649–54. [PubMed] [Google Scholar]
- 64.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 65.Taieb J, Chaput N, Schartz N, Roux S, Novault S, Menard C, Ghiringhelli F, Terme M, Carpentier AF, Darrasse-Jeze G, Lemonnier F, Zitvogel L. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol. 2006;176:2722–9. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 66.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, Proietti E. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–30. [PubMed] [Google Scholar]
- 68.Zhang L, Dermawan K, Jin M, Liu R, Zheng H, Xu L, Zhang Y, Cai Y, Chu Y, Xiong S. Differential impairment of regulatory T cells rather than effector T cells by paclitaxel-based chemotherapy. Clin Immunol. 2008;129:219–29. doi: 10.1016/j.clim.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 69.Melichar B, Savary C, Kudelka AP, Verschraegen C, Kavanagh JJ, Edwards CL, Platsoucas CD, Freedman RS. Lineage-negative human leukocyte antigen-DR+ cells with the phenotype of undifferentiated dendritic cells in patients with carcinoma of the abdomen and pelvis. Clin Cancer Res. 1998;4:799–809. [PubMed] [Google Scholar]
- 70.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–91. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 72.Lu W, Fidler IJ, Dong Z. Eradication of primary murine fibrosarcomas and induction of systemic immunity by adenovirus-mediated interferon beta gene therapy. Cancer Res. 1999;59:5202–8. [PubMed] [Google Scholar]
- 73.Allan NC, Richards SM, Shepherd PC. UK Medical Research Council randomised, multicentre trial of interferon-alpha n1 for chronic myeloid leukaemia: improved survival irrespective of cytogenetic response. The UK Medical Research Council’s Working Parties for Therapeutic Trials in Adult Leukaemia. Lancet. 1995;345:1392–7. doi: 10.1016/s0140-6736(95)92596-1. [DOI] [PubMed] [Google Scholar]
- 74.Anger B, Porzsolt F, Leichtle R, Heinze B, Bartram C, Heimpel H. A phase I/II study of recombinant interferon alpha 2a and hydroxyurea for chronic myelocytic leukemia. Blut. 1989;58:275–8. doi: 10.1007/BF00320165. [DOI] [PubMed] [Google Scholar]
- 75.Foon KA, Roth MS, Bunn PA., Jr Alpha interferon treatment of low-grade B-cell non-Hodgkin’s lymphomas, cutaneous T-cell lymphomas, and chronic lymphocytic leukemia. Semin Oncol. 1986;13:35–42. [PubMed] [Google Scholar]
- 76.Hersey P, Hasic E, MacDonald M, Edwards A, Spurling A, Coates AS, Milton GW, McCarthy WH. Effects of recombinant leukocyte interferon (rIFN-alpha A) on tumour growth and immune responses in patients with metastatic melanoma. Br J Cancer. 1985;51:815–26. doi: 10.1038/bjc.1985.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Creagan ET, Ahmann DL, Green SJ, Long HJ, Frytak S, O’Fallon JR, Itri LM. Phase II study of low-dose recombinant leukocyte A interferon in disseminated malignant melanoma. J Clin Oncol. 1984;2:1002–5. doi: 10.1200/JCO.1984.2.9.1002. [DOI] [PubMed] [Google Scholar]
- 78.Creagan ET, Ahmann DL, Green SJ, Long HJ, Rubin J, Schutt AJ, Dziewanowski ZE. Phase II study of recombinant leukocyte A interferon (rIFN-alpha A) in disseminated malignant melanoma. Cancer. 1984;54:2844–9. doi: 10.1002/1097-0142(19841215)54:12<2844::aid-cncr2820541205>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 79.Kirkwood JM, Ernstoff M. Melanoma: therapeutic options with recombinant interferons. Semin Oncol. 1985;12:7–12. [PubMed] [Google Scholar]
- 80.Kirkwood JM, Ernstoff MS, Davis CA, Reiss M, Ferraresi R, Rudnick SA. Comparison of intramuscular and intravenous recombinant alpha-2 interferon in melanoma and other cancers. Ann Intern Med. 1985;103:32–6. doi: 10.7326/0003-4819-103-1-32. [DOI] [PubMed] [Google Scholar]
- 81.Quesada JR, Swanson DA, Gutterman JU. Phase II study of interferon alpha in metastatic renal-cell carcinoma: a progress report. J Clin Oncol. 1985;3:1086–92. doi: 10.1200/JCO.1985.3.8.1086. [DOI] [PubMed] [Google Scholar]
- 82.Levens W, Rubben H, Ingenhag W. Long-term interferon treatment in metastatic renal cell carcinoma. Eur Urol. 1989;16:378–81. doi: 10.1159/000471621. [DOI] [PubMed] [Google Scholar]
- 83.Rinehart JJ, Young D, Laforge J, Colborn D, Neidhart JA. Phase I/II trial of interferon-beta-serine in patients with renal cell carcinoma: immunological and biological effects. Cancer Res. 1987;47:2481–5. [PubMed] [Google Scholar]
- 84.Berek JS, Markman M, Stonebraker B, Lentz SS, Adelson MD, DeGeest K, Moore D. Intraperitoneal interferon-alpha in residual ovarian carcinoma: a phase II gynecologic oncology group study. Gynecol Oncol. 1999;75:10–4. doi: 10.1006/gyno.1999.5532. [DOI] [PubMed] [Google Scholar]
- 85.Berek JS, Welander C, Schink JC, Grossberg H, Montz FJ, Zigelboim J. A phase I-II trial of intraperitoneal cisplatin and alpha-interferon in patients with persistent epithelial ovarian cancer. Gynecol Oncol. 1991;40:237–43. doi: 10.1016/0090-8258(90)90284-r. [DOI] [PubMed] [Google Scholar]
- 86.Markman M, Belinson J, Webster K, Zanotti K, Morrison B, Jacobs B, Borden E, Lindner D. Phase 2 trial of interferon-beta as second-line treatment of ovarian cancer, fallopian tube cancer, or primary carcinoma of the peritoneum. Oncology. 2004;66:343–6. doi: 10.1159/000079480. [DOI] [PubMed] [Google Scholar]
- 87.Liu M, Acres B, Balloul JM, Bizouarne N, Paul S, Slos P, Squiban P. Gene-based vaccines and immunotherapeutics. Proc Natl Acad Sci U S A. 2004;101 (Suppl 2):14567–71. doi: 10.1073/pnas.0404845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sterman DH, Gillespie CT, Carroll RG, Coughlin CM, Lord EM, Sun J, Haas A, Recio A, Kaiser LR, Coukos G, June CH, Albelda SM, Vonderheide RH. Interferon beta adenoviral gene therapy in a patient with ovarian cancer. Nat Clin Pract Oncol. 2006;3:633–9. doi: 10.1038/ncponc0658. [DOI] [PubMed] [Google Scholar]
- 89.Sterman DH, Recio A, Carroll RG, Gillespie CT, Haas A, Vachani A, Kapoor V, Sun J, Hodinka R, Brown JL, Corbley MJ, Parr M, Ho M, Pastan I, Machuzak M, Benedict W, Zhang XQ, Lord EM, Litzky LA, Heitjan DF, June CH, Kaiser LR, Vonderheide RH, Albelda SM, Kanther M. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin Cancer Res. 2007;13:4456–66. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]
- 90.Nehme A, Julia AM, Jozan S, Chevreau C, Bugat R, Canal P. Modulation of cisplatin cytotoxicity by human recombinant interferon-gamma in human ovarian cancer cell lines. Eur J Cancer. 1994;30A:520–5. doi: 10.1016/0959-8049(94)90430-8. [DOI] [PubMed] [Google Scholar]
- 91.Melichar B, Hu W, Patenia R, Melicharova K, Gallardo ST, Freedman R. rIFN-gamma-mediated growth suppression of platinum-sensitive and -resistant ovarian tumor cell lines not dependent upon arginase inhibition. J Transl Med. 2003;1:5. doi: 10.1186/1479-5876-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wall L, Burke F, Smyth JF, Balkwill F. The anti-proliferative activity of interferon-gamma on ovarian cancer: in vitro and in vivo. Gynecol Oncol. 2003;88:S149–51. doi: 10.1006/gyno.2002.6707. [DOI] [PubMed] [Google Scholar]
- 93.Freedman RS, Kudelka AP, Kavanagh JJ, Verschraegen C, Edwards CL, Nash M, Levy L, Atkinson EN, Zhang HZ, Melichar B, Patenia R, Templin S, Scott W, Platsoucas CD. Clinical and biological effects of intraperitoneal injections of recombinant interferon-gamma and recombinant interleukin 2 with or without tumor-infiltrating lymphocytes in patients with ovarian or peritoneal carcinoma. Clin Cancer Res. 2000;6:2268–78. [PubMed] [Google Scholar]
- 94.Kooi S, Zhang HZ, Patenia R, Edwards CL, Platsoucas CD, Freedman RS. HLA class I expression on human ovarian carcinoma cells correlates with T-cell infiltration in vivo and T-cell expansion in vitro in low concentrations of recombinant interleukin-2. Cell Immunol. 1996;174:116–28. doi: 10.1006/cimm.1996.0301. [DOI] [PubMed] [Google Scholar]
- 95.Duda DG, Sunamura M, Lozonschi L, Kodama T, Egawa S, Matsumoto G, Shimamura H, Shibuya K, Takeda K, Matsuno S. Direct in vitro evidence and in vivo analysis of the antiangiogenesis effects of interleukin 12. Cancer Res. 2000;60:1111–6. [PubMed] [Google Scholar]
- 96.Pujade-Lauraine E, Guastalla JP, Colombo N, Devillier P, Francois E, Fumoleau P, Monnier A, Nooy M, Mignot L, Bugat R, Marques C, Mousseau M, Netter G, Maloisel F, Larbaoui S, Brandely M. Intraperitoneal recombinant interferon gamma in ovarian cancer patients with residual disease at second-look laparotomy. J Clin Oncol. 1996;14:343–50. doi: 10.1200/JCO.1996.14.2.343. [DOI] [PubMed] [Google Scholar]
- 97.Chen JT, Hasumi K, Masubuchi K. Interferon-alpha, interferon-gamma and sizofiran in the adjuvant therapy in ovarian cancer--a preliminary trial. Biotherapy. 1992;5:275–80. doi: 10.1007/BF02179044. [DOI] [PubMed] [Google Scholar]
- 98.Colombo N, Peccatori F, Paganin C, Bini S, Brandely M, Mangioni C, Mantovani A, Allavena P. Anti-tumor and immunomodulatory activity of intraperitoneal IFN-gamma in ovarian carcinoma patients with minimal residual tumor after chemotherapy. Int J Cancer. 1992;51:42–6. doi: 10.1002/ijc.2910510109. [DOI] [PubMed] [Google Scholar]
- 99.Schmeler KM, Vadhan-Raj S, Ramirez PT, Apte SM, Cohen L, Bassett RL, Iyer RB, Wolf JK, Levenback CL, Gershenson DM, Freedman RS. A phase II study of GM-CSF and rIFN-gamma1b plus carboplatin for the treatment of recurrent, platinum-sensitive ovarian, fallopian tube and primary peritoneal cancer. Gynecol Oncol. 2009;113:210–5. doi: 10.1016/j.ygyno.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Berek JS. Interferon plus chemotherapy for primary treatment of ovarian cancer. Lancet. 2000;356:6–7. doi: 10.1016/S0140-6736(00)02422-3. [DOI] [PubMed] [Google Scholar]
- 101.Alberts DS, Marth C, Alvarez RD, Johnson G, Bidzinski M, Kardatzke DR, Bradford WZ, Loutit J, Kirn DH, Clouser MC, Markman M. Randomized phase 3 trial of interferon gamma-1b plus standard carboplatin/paclitaxel versus carboplatin/paclitaxel alone for first-line treatment of advanced ovarian and primary peritoneal carcinomas: results from a prospectively designed analysis of progression-free survival. Gynecol Oncol. 2008;109:174–81. doi: 10.1016/j.ygyno.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 102.Ohta M, Mitomi T, Kimura M, Habu S, Katsuki M. Anomalies in transgenic mice carrying the human interleukin-2 gene. Tokai J Exp Clin Med. 1990;15:307–15. [PubMed] [Google Scholar]
- 103.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–19. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McDermott DF. The application of high-dose interleukin-2 for metastatic renal cell carcinoma. Med Oncol. 2009;26 (Suppl 1):13–7. doi: 10.1007/s12032-008-9152-1. [DOI] [PubMed] [Google Scholar]
- 105.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schwartz RN, Stover L, Dutcher J. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park) 2002;16:11–20. [PubMed] [Google Scholar]
- 107.Edwards RP, Gooding W, Lembersky BC, Colonello K, Hammond R, Paradise C, Kowal CD, Kunschner AJ, Baldisseri M, Kirkwood JM, Herberman RB. Comparison of toxicity and survival following intraperitoneal recombinant interleukin-2 for persistent ovarian cancer after platinum: twenty-four-hour versus 7-day infusion. J Clin Oncol. 1997;15:3399–407. doi: 10.1200/JCO.1997.15.11.3399. [DOI] [PubMed] [Google Scholar]
- 108.Kuss I, Rabinowich H, Gooding W, Edwards R, Whiteside TL. Expression of zeta in T cells prior to interleukin-2 therapy as a predictor of response and survival in patients with ovarian carcinoma. Cancer Biother Radiopharm. 2002;17:631–40. doi: 10.1089/108497802320970235. [DOI] [PubMed] [Google Scholar]
- 109.Brandenburg S, Takahashi T, de la Rosa M, Janke M, Karsten G, Muzzulini T, Orinska Z, Bulfone-Paus S, Scheffold A. IL-2 induces in vivo suppression by CD4(+)CD25(+)Foxp3(+) regulatory T cells. Eur J Immunol. 2008;38:1643–53. doi: 10.1002/eji.200737791. [DOI] [PubMed] [Google Scholar]
- 110.Wei S, Kryczek I, Edwards RP, Zou L, Szeliga W, Banerjee M, Cost M, Cheng P, Chang A, Redman B, Herberman RB, Zou W. Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Res. 2007;67:7487–94. doi: 10.1158/0008-5472.CAN-07-0565. [DOI] [PubMed] [Google Scholar]
- 111.Recchia F, Saggio G, Cesta A, Candeloro G, Nuzzo A, Lombardo M, Carta G, Rea S. Interleukin-2 and 13-cis retinoic acid as maintenance therapy in advanced ovarian cancer. Int J Oncol. 2005;27:1039–46. [PubMed] [Google Scholar]
- 112.Capitini CM, Chisti AA, Mackall CL. Modulating T-cell homeostasis with IL-7: preclinical and clinical studies. J Intern Med. 2009;266:141–53. doi: 10.1111/j.1365-2796.2009.02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ribas A. Update on immunotherapy for melanoma. J Natl Compr Canc Netw. 2006;4:687–94. doi: 10.6004/jnccn.2006.0058. [DOI] [PubMed] [Google Scholar]
- 114.Andersson A, Yang SC, Huang M, Zhu L, Kar UK, Batra RK, Elashoff D, Strieter RM, Dubinett SM, Sharma S. IL-7 promotes CXCR3 ligand-dependent T cell antitumor reactivity in lung cancer. J Immunol. 2009;182:6951–8. doi: 10.4049/jimmunol.0803340. [DOI] [PubMed] [Google Scholar]
- 115.Sharma S, Wang J, Huang M, Paul RW, Lee P, McBride WH, Economou JS, Roth MD, Kiertscher SM, Dubinett SM. Interleukin-7 gene transfer in non-small-cell lung cancer decreases tumor proliferation, modifies cell surface molecule expression, and enhances antitumor reactivity. Cancer Gene Ther. 1996;3:302–13. [PubMed] [Google Scholar]
- 116.Shanmugham LN, Petrarca C, Frydas S, Donelan J, Castellani ML, Boucher W, Madhappan B, Tete S, Falasca K, Conti P, Vecchiet J. IL-15 an immunoregulatory and anti-cancer cytokine. Recent advances. J Exp Clin Cancer Res. 2006;25:529–36. [PubMed] [Google Scholar]
- 117.Brandt K, Singh PB, Bulfone-Paus S, Ruckert R. Interleukin-21: a new modulator of immunity, infection, and cancer. Cytokine Growth Factor Rev. 2007;18:223–32. doi: 10.1016/j.cytogfr.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 118.Thompson JA, Curti BD, Redman BG, Bhatia S, Weber JS, Agarwala SS, Sievers EL, Hughes SD, DeVries TA, Hausman DF. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008;26:2034–9. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 119.Carroll RG, Carpenito C, Shan X, Danet-Desnoyers G, Liu R, Jiang S, Albelda SM, Golovina T, Coukos G, Riley JL, Jonak ZL, June CH. Distinct effects of IL-18 on the engraftment and function of human effector CD8 T cells and regulatory T cells. PLoS ONE. 2008;3:e3289. doi: 10.1371/journal.pone.0003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Herzyk DJ, Soos JM, Maier CC, Gore ER, Narayanan PK, Nadwodny KL, Liu S, Jonak ZL, Bugelski PJ. Immunopharmacology of recombinant human interleukin-18 in non-human primates. Cytokine. 2002;20:38–48. doi: 10.1006/cyto.2002.1978. [DOI] [PubMed] [Google Scholar]
- 121.Robertson MJ, Mier JW, Logan T, Atkins M, Koon H, Koch KM, Kathman S, Pandite LN, Oei C, Kirby LC, Jewell RC, Bell WN, Thurmond LM, Weisenbach J, Roberts S, Dar MM. Clinical and biological effects of recombinant human interleukin-18 administered by intravenous infusion to patients with advanced cancer. Clin Cancer Res. 2006;12:4265–73. doi: 10.1158/1078-0432.CCR-06-0121. [DOI] [PubMed] [Google Scholar]
- 122.Robertson MJ, Kirkwood JM, Logan TF, Koch KM, Kathman S, Kirby LC, Bell WN, Thurmond LM, Weisenbach J, Dar MM. A dose-escalation study of recombinant human interleukin-18 using two different schedules of administration in patients with cancer. Clin Cancer Res. 2008;14:3462–9. doi: 10.1158/1078-0432.CCR-07-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD, Hood LE, Aderem A. The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci U S A. 2005;102:9577–82. doi: 10.1073/pnas.0502272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 125.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 126.Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309:1380–4. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 127.Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–40. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 128.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–4. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 129.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 130.Yang H, Young DW, Gusovsky F, Chow JC. Cellular events mediated by lipopolysaccharide-stimulated toll-like receptor 4. MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J Biol Chem. 2000;275:20861–6. doi: 10.1074/jbc.M002896200. [DOI] [PubMed] [Google Scholar]
- 131.Spaner DE, Masellis A. Toll-like receptor agonists in the treatment of chronic lymphocytic leukemia. Leukemia. 2007;21:53–60. doi: 10.1038/sj.leu.2404456. [DOI] [PubMed] [Google Scholar]
- 132.Ausubel FM. Are innate immune signaling pathways in plants and animals conserved? Nat Immunol. 2005;6:973–9. doi: 10.1038/ni1253. [DOI] [PubMed] [Google Scholar]
- 133.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 134.Crellin NK, Garcia RV, Hadisfar O, Allan SE, Steiner TS, Levings MK. Human CD4+ T cells express TLR5 and its ligand flagellin enhances the suppressive capacity and expression of FOXP3 in CD4+CD25+ T regulatory cells. J Immunol. 2005;175:8051–9. doi: 10.4049/jimmunol.175.12.8051. [DOI] [PubMed] [Google Scholar]
- 135.Tabiasco J, Devevre E, Rufer N, Salaun B, Cerottini JC, Speiser D, Romero P. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J Immunol. 2006;177:8708–13. doi: 10.4049/jimmunol.177.12.8708. [DOI] [PubMed] [Google Scholar]
- 136.Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine. 2008;26:5046–57. doi: 10.1016/j.vaccine.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 137.Ramakrishna V, Vasilakos JP, Tario JD, Jr, Berger MA, Wallace PK, Keler T. Toll-like receptor activation enhances cell-mediated immunity induced by an antibody vaccine targeting human dendritic cells. J Transl Med. 2007;5:5. doi: 10.1186/1479-5876-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Manegold C, Gravenor D, Woytowitz D, Mezger J, Hirsh V, Albert G, Al-Adhami M, Readett D, Krieg AM, Leichman CG. Randomized phase II trial of a toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3979–86. doi: 10.1200/JCO.2007.12.5807. [DOI] [PubMed] [Google Scholar]
- 139.Link BK, Ballas ZK, Weisdorf D, Wooldridge JE, Bossler AD, Shannon M, Rasmussen WL, Krieg AM, Weiner GJ. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006;29:558–68. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- 140.Leonard JP, Link BK, Emmanouilides C, Gregory SA, Weisdorf D, Andrey J, Hainsworth J, Sparano JA, Tsai DE, Horning S, Krieg AM, Weiner GJ. Phase I trial of toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non Hodgkin’s lymphoma. Clin Cancer Res. 2007;13:6168–74. doi: 10.1158/1078-0432.CCR-07-0815. [DOI] [PubMed] [Google Scholar]
- 141.Carpentier A, Laigle-Donadey F, Zohar S, Capelle L, Behin A, Tibi A, Martin-Duverneuil N, Sanson M, Lacomblez L, Taillibert S, Puybasset L, Van Effenterre R, Delattre JY, Carpentier AF. Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro Oncol. 2006;8:60–6. doi: 10.1215/S1522851705000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stockfleth E, Trefzer U, Garcia-Bartels C, Wegner T, Schmook T, Sterry W. The use of Toll-like receptor-7 agonist in the treatment of basal cell carcinoma: an overview. Br J Dermatol. 2003;149 (Suppl 66):53–6. doi: 10.1046/j.0366-077x.2003.05626.x. [DOI] [PubMed] [Google Scholar]
- 143.Koido S, Hara E, Homma S, Torii A, Toyama Y, Kawahara H, Watanabe M, Yanaga K, Fujise K, Tajiri H, Gong J, Toda G. Dendritic cells fused with allogeneic colorectal cancer cell line present multiple colorectal cancer-specific antigens and induce antitumor immunity against autologous tumor cells. Clin Cancer Res. 2005;11:7891–900. doi: 10.1158/1078-0432.CCR-05-1330. [DOI] [PubMed] [Google Scholar]
- 144.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Toonen LW, Figdor CG, Ruers TJ, Adema GJ. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006;66:7285–92. doi: 10.1158/0008-5472.CAN-06-0206. [DOI] [PubMed] [Google Scholar]
- 145.Lesimple T, Neidhard EM, Vignard V, Lefeuvre C, Adamski H, Labarriere N, Carsin A, Monnier D, Collet B, Clapisson G, Birebent B, Philip I, Toujas L, Chokri M, Quillien V. Immunologic and clinical effects of injecting mature peptide-loaded dendritic cells by intralymphatic and intranodal routes in metastatic melanoma patients. Clin Cancer Res. 2006;12:7380–8. doi: 10.1158/1078-0432.CCR-06-1879. [DOI] [PubMed] [Google Scholar]
- 146.Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4:641–8. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 147.Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176:4894–901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 148.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–68. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 149.Kim KH, Xie Y, Tytler EM, Woessner R, Mor G, Alvero AB. KSP inhibitor ARRY-520 as a substitute for Paclitaxel in Type I ovarian cancer cells. J Transl Med. 2009;7:63. doi: 10.1186/1479-5876-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mackey MF, Gunn JR, Ting PP, Kikutani H, Dranoff G, Noelle RJ, Barth RJ., Jr Protective immunity induced by tumor vaccines requires interaction between CD40 and its ligand, CD154. Cancer Res. 1997;57:2569–74. [PubMed] [Google Scholar]
- 151.Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, Offringa R, Toes RE. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774–9. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 152.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–72. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Berner V, Liu H, Zhou Q, Alderson KL, Sun K, Weiss JM, Back TC, Longo DL, Blazar BR, Wiltrout RH, Welniak LA, Redelman D, Murphy WJ. IFN-gamma mediates CD4+ T-cell loss and impairs secondary antitumor responses after successful initial immunotherapy. Nat Med. 2007;13:354–60. doi: 10.1038/nm1554. [DOI] [PubMed] [Google Scholar]
- 154.Kedl RM, Jordan M, Potter T, Kappler J, Marrack P, Dow S. CD40 stimulation accelerates deletion of tumor-specific CD8(+) T cells in the absence of tumor-antigen vaccination. Proc Natl Acad Sci U S A. 2001;98:10811–6. doi: 10.1073/pnas.191371898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Scarlett UK, Cubillos-Ruiz JR, Nesbeth YC, Martinez DG, Engle X, Gewirtz AT, Ahonen CL, Conejo-Garcia JR. In situ Stimulation of CD40 and Toll-like Receptor 3 Transforms Ovarian Cancer-Infiltrating Dendritic Cells from Immunosuppressive to Immunostimulatory Cells. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–6. [PubMed] [Google Scholar]
- 157.Altenburg A, Baldus SE, Smola H, Pfister H, Hess S. CD40 ligand-CD40 interaction induces chemokines in cervical carcinoma cells in synergism with IFN-gamma. J Immunol. 1999;162:4140–7. [PubMed] [Google Scholar]
- 158.Gallagher NJ, Eliopoulos AG, Agathangelo A, Oates J, Crocker J, Young LS. CD40 activation in epithelial ovarian carcinoma cells modulates growth, apoptosis, and cytokine secretion. Mol Pathol. 2002;55:110–20. doi: 10.1136/mp.55.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Melichar B, Patenia R, Gallardo S, Melicharova K, Hu W, Freedman RS. Expression of CD40 and growth-inhibitory activity of CD40 ligand in ovarian cancer cell lines. Gynecol Oncol. 2007;104:707–13. doi: 10.1016/j.ygyno.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 160.Hakkarainen T, Hemminki A, Pereboev AV, Barker SD, Asiedu CK, Strong TV, Kanerva A, Wahlfors J, Curiel DT. CD40 is expressed on ovarian cancer cells and can be utilized for targeting adenoviruses. Clin Cancer Res. 2003;9:619–24. [PubMed] [Google Scholar]
- 161.Jiang E, He X, Chen X, Sun G, Wu H, Wei Y, Zhao X. Expression of CD40 in ovarian cancer and adenovirus-mediated CD40 ligand therapy on ovarian cancer in vitro. Tumori. 2008;94:356–61. doi: 10.1177/030089160809400312. [DOI] [PubMed] [Google Scholar]
- 162.Ciaravino G, Bhat M, Manbeian CA, Teng NN. Differential expression of CD40 and CD95 in ovarian carcinoma. Eur J Gynaecol Oncol. 2004;25:27–32. [PubMed] [Google Scholar]
- 163.Toutirais O, Gervais A, Cabillic F, Le Gallo M, Coudrais A, Leveque J, Catros-Quemener V, Genetet N. Effects of CD40 binding on ovarian carcinoma cell growth and cytokine production in vitro. Clin Exp Immunol. 2007;149:372–7. doi: 10.1111/j.1365-2249.2007.03426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ghamande S, Hylander BL, Oflazoglu E, Lele S, Fanslow W, Repasky EA. Recombinant CD40 ligand therapy has significant antitumor effects on CD40-positive ovarian tumor xenografts grown in SCID mice and demonstrates an augmented effect with cisplatin. Cancer Res. 2001;61:7556–62. [PubMed] [Google Scholar]
- 165.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, Green SJ, O’Dwyer PJ, Running KL, Huhn RD, Antonia SJ. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–83. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 166.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–24. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2009 doi: 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 168.Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823–30. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I, Korman A, Lautz D, Russell S, Jaklitsch MT, Ramaiya N, Chen TC, Neuberg D, Allison JP, Mihm MC, Dranoff G. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M, Nichol G, White DE, Steinberg SM, Rosenberg SA. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–8. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–54. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–83. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 175.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–7. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173–82. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–61. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 178.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–9. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 179.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–7. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 180.Liu SM, Meng Q, Zhang QX, Wang SD, Liu ZJ, Zhang XF. Expression and significance of B7-H1 and its receptor PD-1 in human gastric carcinoma. Zhonghua Zhong Liu Za Zhi. 2008;30:192–5. [PubMed] [Google Scholar]
- 181.Kaufmann DE, Walker BD. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J Immunol. 2009;182:5891–7. doi: 10.4049/jimmunol.0803771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–45. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–96. [PubMed] [Google Scholar]
- 184.He YF, Zhang GM, Wang XH, Zhang H, Yuan Y, Li D, Feng ZH. Blocking programmed death-1 ligand-PD-1 interactions by local gene therapy results in enhancement of antitumor effect of secondary lymphoid tissue chemokine. J Immunol. 2004;173:4919–28. doi: 10.4049/jimmunol.173.8.4919. [DOI] [PubMed] [Google Scholar]
- 185.Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R, Mackensen A. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119:317–27. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 186.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–51. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 187.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 188.Gallimore A, Sakaguchi S. Regulation of tumour immunity by CD25+ T cells. Immunology. 2002;107:5–9. doi: 10.1046/j.1365-2567.2002.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Steitz J, Bruck J, Lenz J, Knop J, Tuting T. Depletion of CD25(+) CD4(+) T cells and treatment with tyrosinase-related protein 2-transduced dendritic cells enhance the interferon alpha-induced, CD8(+) T-cell-dependent immune defense of B16 melanoma. Cancer Res. 2001;61:8643–6. [PubMed] [Google Scholar]
- 190.Berd D, Sato T, Maguire HC, Jr, Kairys J, Mastrangelo MJ. Immunopharmacologic analysis of an autologous, hapten-modified human melanoma vaccine. J Clin Oncol. 2004;22:403–15. doi: 10.1200/JCO.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 191.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–7. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.MacLean GD, Miles DW, Rubens RD, Reddish MA, Longenecker BM. Enhancing the effect of THERATOPE STn-KLH cancer vaccine in patients with metastatic breast cancer by pretreatment with low-dose intravenous cyclophosphamide. J Immunother Emphasis Tumor Immunol. 1996;19:309–16. doi: 10.1097/00002371-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 194.Benencia F, Coukos G. T Regulatory Cell Depletion Can Boost DC-Based Vaccines. Cancer Biol Ther. 2005:4. [Google Scholar]
- 195.Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J Immunol. 2005;174:90–8. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 196.Attia P, Powell DJ, Jr, Maker AV, Kreitman RJ, Pastan I, Rosenberg SA. Selective elimination of human regulatory T lymphocytes in vitro with the recombinant immunotoxin LMB-2. J Immunother (1997) 2006;29:208–14. doi: 10.1097/01.cji.0000187959.45803.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Powell DJ, Jr, Attia P, Ghetie V, Schindler J, Vitetta ES, Rosenberg SA. Partial reduction of human FOXP3+ CD4 T cells in vivo after CD25-directed recombinant immunotoxin administration. J Immunother. 2008;31:189–98. doi: 10.1097/CJI.0b013e31815dc0e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Barnett B, Kryczek I, Cheng P, Zou W, Curiel TJ. Regulatory T cells in ovarian cancer: biology and therapeutic potential. Am J Reprod Immunol. 2005;54:369–77. doi: 10.1111/j.1600-0897.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 200.Mahnke K, Schonfeld K, Fondel S, Ring S, Karakhanova S, Wiedemeyer K, Bedke T, Johnson TS, Storn V, Schallenberg S, Enk AH. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007;120:2723–33. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- 201.Rasku MA, Clem AL, Telang S, Taft B, Gettings K, Gragg H, Cramer D, Lear SC, McMasters KM, Miller DM, Chesney J. Transient T cell depletion causes regression of melanoma metastases. J Transl Med. 2008;6:12. doi: 10.1186/1479-5876-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Waldmann TA. Daclizumab (anti-Tac, Zenapax) in the treatment of leukemia/lymphoma. Oncogene. 2007;26:3699–703. doi: 10.1038/sj.onc.1210368. [DOI] [PubMed] [Google Scholar]
- 203.Kreijveld E, Koenen HJ, Klasen IS, Hilbrands LB, Joosten I. Following anti-CD25 treatment, a functional CD4+CD25+ regulatory T-cell pool is present in renal transplant recipients. Am J Transplant. 2007;7:249–55. doi: 10.1111/j.1600-6143.2006.01604.x. [DOI] [PubMed] [Google Scholar]
- 204.Nussenblatt RB, Fortin E, Schiffman R, Rizzo L, Smith J, Van Veldhuisen P, Sran P, Yaffe A, Goldman CK, Waldmann TA, Whitcup SM. Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc Natl Acad Sci U S A. 1999;96:7462–6. doi: 10.1073/pnas.96.13.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Przepiorka D, Kernan NA, Ippoliti C, Papadopoulos EB, Giralt S, Khouri I, Lu JG, Gajewski J, Durett A, Cleary K, Champlin R, Andersson BS, Light S. Daclizumab, a humanized anti-interleukin-2 receptor alpha chain antibody, for treatment of acute graft-versus-host disease. Blood. 2000;95:83–9. [PubMed] [Google Scholar]
- 206.Lehky TJ, Levin MC, Kubota R, Bamford RN, Flerlage AN, Soldan SS, Leist TP, Xavier A, White JD, Brown M, Fleisher TA, Top LE, Light S, McFarland HF, Waldmann TA, Jacobson S. Reduction in HTLV-I proviral load and spontaneous lymphoproliferation in HTLV-I-associated myelopathy/tropical spastic paraparesis patients treated with humanized anti-Tac. Ann Neurol. 1998;44:942–7. doi: 10.1002/ana.410440613. [DOI] [PubMed] [Google Scholar]
- 207.Vincenti F, Nashan B, Light S. Daclizumab: outcome of phase III trials and mechanism of action. Double Therapy and the Triple Therapy Study Groups. Transplant Proc. 1998;30:2155–8. doi: 10.1016/s0041-1345(98)00571-5. [DOI] [PubMed] [Google Scholar]