Abstract
Although the presence of an olfactory impairment in Parkinson's disease (PD) has been recognized for 25 years, its cause remains unclear. Here we suggest a contributing factor to this impairment, namely, that PD impairs active sniffing of odorants. We tested 10 men and 10 women with clinically typical PD, and 20 age- and gender-matched healthy controls, in four olfactory tasks: (i) the University of Pennsylvania smell identification test; (ii and iii) detection threshold tests for the odorants vanillin and propionic acid; and (iv) a two-alternative forced-choice detection paradigm during which sniff parameters (airflow peak rate, mean rate, volume, and duration) were recorded with a pneomatotachograph-coupled spirometer. An additional experiment tested the effect of intentionally increasing sniff vigor on olfactory performance in 20 additional patients. PD patients were significantly impaired in olfactory identification (P < 0.0001) and detection (P < 0.007). As predicted, PD patients were also significantly impaired at sniffing, demonstrating significantly reduced sniff airflow rate (P < 0.01) and volume (P < 0.002). Furthermore, a patient's ability to sniff predicted his or her performance on olfactory tasks, i.e., the more poorly patients sniffed, the worse their performance on olfaction tests (P < 0.009). Finally, increasing sniff vigor improved olfactory performance in those patients whose baseline performance had been poorest (P < 0.05). These findings implicate a sniffing impairment as a component of the olfactory impairment in PD and further depict sniffing as an important component of human olfaction.
Parkinson's disease (PD) is a neurodegenerative disorder characterized pathologically by progressive neuronal loss, particularly of aminergic brainstem neurons. PD primarily affects motor control, but it also affects cognitive and sensory processing (1). Of the sensory impairments associated with PD, olfactory impairment has received most attention. PD patients exhibit impairments in odor detection, identification, and discrimination (2–6) and a reduced olfactory evoked response (7, 8). This occurs so consistently that the olfactory impairment may have diagnostic value for PD (9–12). Although the presence of an olfactory impairment in PD has been acknowledged for 25 years (2), its cause remains unknown.
Olfaction requires complex sensory–motor integration (13). Sniffing, the motor component of olfaction, involves transportation of airborne stimuli to the olfactory receptors. Smelling, the sensory component of olfaction, involves transduction of odorants into a neural signal and further processing of that neural signal. The olfactory impairment in PD has always been attributed to pathology in the neural substrates of smelling (2–4), consisting of depletion of neurotransmitters, and the postmortem finding of Lewy bodies, the pathologic hallmark of PD, throughout the olfactory cortex (9, 15). Here we suggest that what had long been considered a smelling impairment in PD is actually, in part, a sniffing impairment. To address this suggestion, we compared the dissociable abilities of sniffing and smelling in PD patients with those of healthy matched controls.
Methods
Subjects.
We tested 10 men and 10 women with clinically typical idiopathic PD and 20 age- and gender-matched healthy controls. Subjects diagnosed as potentially atypical PD were excluded. The patients were mostly stage II on the Hoehn and Yahr staging of PD (mean = 2.3, SD = 0.65), with a mean disease duration of 8.1 years (SD = 4.3). All patients were taking dopaminergic replacement medication and were tested in the “on” condition (i.e., typically in the middle of the time window between drug administrations). There were no differences between patients and controls in age [PD mean = 67.1, SD = 9.9; control mean = 68, SD = 7.6, t(38)= 0.32, P = 0.75], in cognitive state as assessed by the Mini Mental State Examination [PD mean = 28.1, SD = 1.9; control mean = 28.75, SD = 2, t(38) = 0.99, P = 0.33], in years of formal education [PD mean = 15.8, SD = 3; control mean = 16.9, SD = 2.7, t(38) = 1.1, P = 0.27], in handedness (PD = 18R, 2L; control = 17R, 3L), or in smoking behavior (none of the patients or controls was a current smoker; nine of the patients and 10 of the controls had smoked in the past). Patients' scores on the unified Parkinson's disease rating scale (UPDRS) were as follows: part I (mentation behavior and mood), mean = 2.4, SD = 2.1; part II (activities of daily living), mean = 11.9, SD = 5.2; part III (motor examination), mean = 18.7, SD = 12.4 (UPDRS scores were not obtained for one of the 20 patients).
Smell Identification.
All subjects performed the University of Pennsylvania smell identification test (UPSIT). This test consists of 40 scratch-and-sniff presentations of odorants in a four-alternative forced-choice identification paradigm. The UPSIT is widely used in clinical and experimental settings and enables comparison to published standardized scores based on large normative data sets (16). To reduce potential sources of variance, rather than permitting self-administration, the UPSIT was administered to the subjects (both PD and control) by the experimenters. The odorant was scratched by the experimenter and then held to the subject's nose for smelling. Because PD patients may display specific anosmias (17), the order of UPSIT stimulus presentation was randomized and balanced across subjects.
Smell Detection Threshold.
Detection thresholds for the odorants vanillin and propionic acid were tested in all subjects. These odorants were chosen so as to target both the olfactory (vanillin) and trigeminal (propionic) systems (18, 19). Dilution series of the odorants were prepared in double-distilled di-ionized water. Pairs consisting of an odorant and a diluent were then presented to blindfolded subjects in a randomized two-alternative forced-choice ascending staircase procedure (20), with five consecutive hits as criteria for threshold.
Airflow.
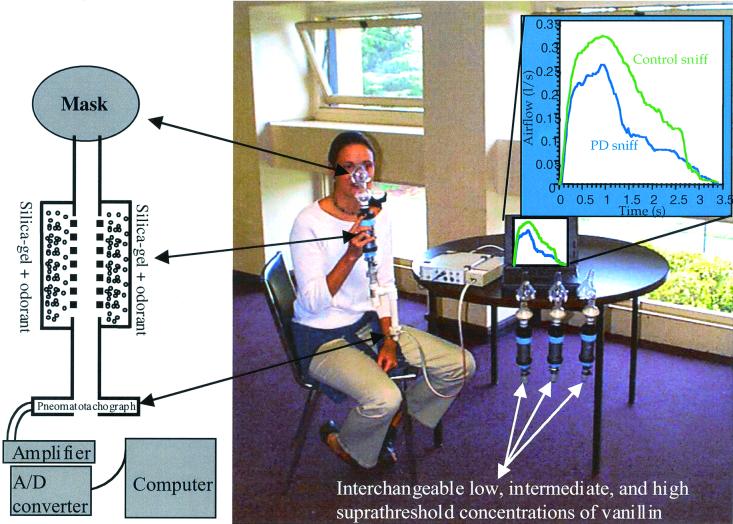
Sniff parameters were recorded during a two-alternative forced-choice detection paradigm with methods described in refs. 22 and 27 (Fig. 1). Subjects were presented with a nose mask (Respronics, Murrysville, PA) coupled to an odorant source and a pneomatotachograph that relayed changes in pressure to an amplifier (spirometer; A. D. Instruments, Milford, MA). The transduced signal was displayed and recorded on a computer. Measures of airflow rate, duration, and volume were extracted with commercially available software designed specifically for these measures (chart 3.6.3 with spirometry extension; A. D. Instruments). The test consisted of 24 odorant presentations: 12 no-odorant trials randomly interspersed with 12 suprathreshold vanillin trials (four low concentration, four intermediate concentration, and four high concentration). In a later described control experiment, we also measured sniffing during the UPSIT before and after a manipulation that consisted of verbally instructing patients to increase their sniff vigor. In this control, the odorant source was cut out of the UPSIT and placed within the nose mask. UPSIT stimuli were randomized and balanced between the pre- and postmanipulation batch, across subjects. In all experiments, subjects were unaware that airflow was being measured.
Figure 1.
Methods for recording airflow. A schematic drawing and image of the recording apparatus as used in refs. 22 and 27 (note the three additional interchangeable odorant sources at the table). Subjects were told that the tubes connected to the mask were the odorant supply (they were in fact the pressure transduction tubes). Subsequent questioning revealed that no subject was aware of the ongoing airflow recording. The data in the upper right quadrant is the actual mean first sniff of 20 patients and 20 controls (the person demonstrating the apparatus is not a patient).
Finger Tapping.
As an additional measure of motor performance, each subject used the index finger to tap on a counter as many times as possible within 10 s. The task was repeated four times, twice with each hand. The score was the mean of the four performances.
Results
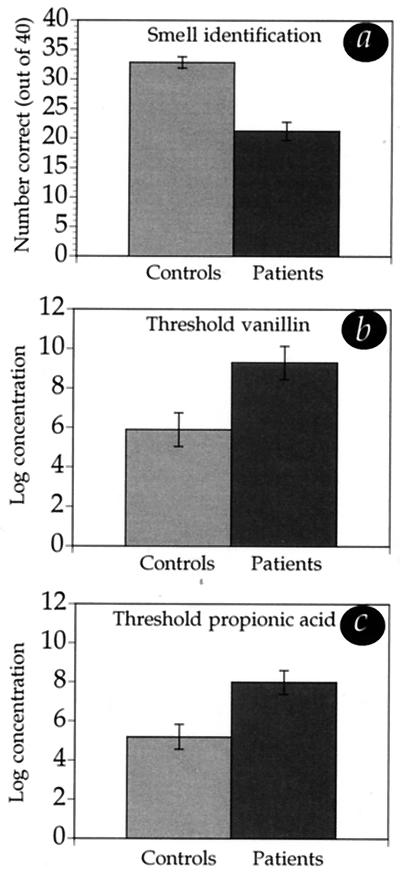
PD patients were impaired on all measures of olfaction (Fig. 2). Two-tailed unpaired t tests revealed significantly reduced UPSIT performance for patients (mean = 21.2, SD = 6.7) compared with controls (mean = 32.8, SD = 4.3) [t(38) = 6.52, P < 0.0001], significantly reduced vanillin detection for patients (mean = 9.3, SD = 3.7) compared with controls (mean = 5.9, SD = 3.8) [t(38) = 2.86, P = 0.007], and significantly reduced propionic acid detection for patients (mean = 8, SD = 2.7) compared with controls (mean = 5.2, SD = 2.8) [t(38) = 3.2, P = 0.003]. The PD deteriorations in vanillin detection and propionic acid detection were similar [F(1,38) = 0.25, P = 0.62].
Figure 2.
Olfaction in patients and controls. Patients performed worse than controls in all olfactory tasks. (a) Relative to the controls, who performed within the range of published norms (16), patients' performance on the UPSIT was near anosmia (complete olfactory loss). The detection threshold scores achieved represent the following concentrations. (b) For vanillin, controls = 1.09 × 10−4 M in H2O, patients = 1.13 × 10−3 M in H2O. (c) For propionic acid, controls = 5.27 × 10−4 M in H2O, patients = 3.8 × 10−3 M in H2O. As previously shown (16), olfactory performance was not significantly correlated in patients with finger tapping, age, disease duration, cognitive state (Mini Mental State Examination), or stage of disease progression (Hoehn and Yahr scale).
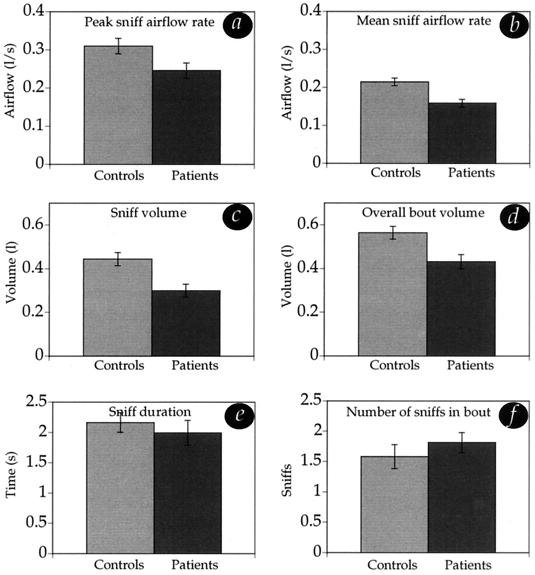
PD patients were impaired at sniffing (Fig. 3). Two-tailed unpaired t tests revealed significantly reduced values in patients compared with controls in sniff peak airflow rate [PD mean = 0.25 liter/s, SD = 0.08; control mean = 0.31 liter/s, SD = 0.08, t(38) = 2.6, P = 0.01], sniff mean airflow rate [PD mean = 0.16 liter/s, SD = 0.05; control mean = 0.21 liter/s, SD = 0.05, t(38) = 3.6, P = 0.0009], sniff volume [PD mean = 0.3 liter, SD = 0.13; control mean = 0.44 liter, SD = 0.14, t(38) = 3.38, P = 0.0017], and bout volume [PD mean = 0.43 liter, SD = 0.14; control mean = 0.56 liter, SD = 0.13, t(38) = 3.042, P = 0.0042]. There were no significant differences in sniff duration [PD mean = 1.9 s, SD = 0.9; control mean = 2.16 s, SD = 0.73, t(38) = 0.66, P = 0.51] or number of sniffs in bout [PD mean = 1.8, SD = 0.73; control mean = 1.6, SD = 0.48, t(38) = 1.59, P = 0.12]. The foregoing analysis of sniffing considered only the 12 sniffs from the randomly interspersed no-odorant trials, and not the 12 sniffs from the odorant trials, because the presence of an odorant will alter a sniff in a predicted manner (the higher the odorant concentration, the shorter the sniff) (21, 22). In the no-odorant trials, sniffs constitute an olfactory search that is unaffected by odorant presence, i.e., the sniffing component of olfaction is unaffected by the smelling component. When analysis was made of all sniffs, however, PD patients exhibited an identical pattern of impairment.
Figure 3.
Sniffing in patients and controls. Patients displayed significantly reduced sniff airflow (a) peak rate, (b) mean rate, (c) volume, and (d) overall bout volume. There was no difference between patients and controls in (e) sniff duration and (f) number of sniffs attempted in a bout before making a decision. Sniffing performance was not significantly correlated in patients with finger tapping, age, disease duration, cognitive state (Mini Mental State Examination), or stage of disease progression (Hoehn and Yahr scale).
Thus, PD patients displayed reduced sniffing capabilities in comparison with healthy controls in the context of an olfactory task. The absence of significant differences between controls and patients in sniff duration and number of sniffs per bout indicates that the patients were trying to perform the task. The PD deficit in sniff parameters, therefore, is not a reflection of reduced patient effort at participation.
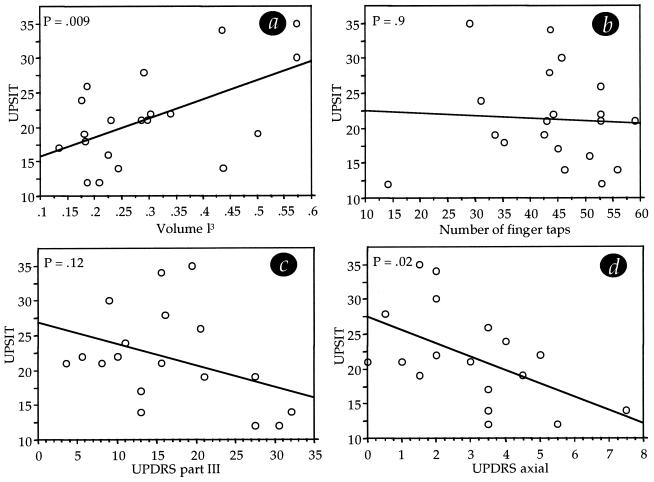
Among sniff parameters, sniff volume has been determined as most important for maximizing olfactory performance (21). To determine whether sniffing capability was related to olfactory performance within the PD patients, we examined the relation between sniff volume and olfactory performance as measured with the UPSIT. There was a significant correlation between these measures (r = 0.56, P = 0.009), such that greater sniff volume predicted better performance on the separately conducted UPSIT in PD patients. In other words, patients with greater sniff volume performed better at olfaction; patients with lesser sniff volume performed worse at olfaction (Fig. 4a).
Figure 4.
Predictors of olfactory performance in PD. (a) Better sniffing predicted better olfactory performance in PD. (b) Motor performance as assessed by finger tapping did not significantly predict olfactory performance in PD. (c) Motor performance as assessed by a standard test (UPDRS part III) did not significantly predict olfactory performance in PD. (d) A subset of the UPDRS part III, representing only axial function, significantly predicted olfactory performance in PD (note that one subject had a significantly outlying UPDRS score and is therefore not depicted in the scattergram. Graph c remained insignificant, and d remained significant, with or without this subject).
Because a sniff is in part a motor action, this finding may be taken to suggest a motor component in the olfactory impairment in PD. Previous work, however, had suggested that the olfactory impairment in PD is unrelated to the extent of motor deterioration as assessed by finger tapping (16). This apparent contradiction between our finding and previous work by others may be resolved if the PD-related deterioration in finger tapping were dissociated from the PD-related deterioration in sniffing. To address this possibility we compared finger tapping with sniffing in all patients. There was no correlation between any of the sniff components and finger tapping (correlations between finger tapping and sniff parameters: peak airflow rate, r = 0.23, P = 0.32; mean airflow rate, r = 0.27, P = 0.25; volume, r = 0.02, P = 0.9; duration, r = 0.1, P = 0.66; bout volume, r = 0.08, P = 0.72; number sniffs in bout, r = 0.01, P = 0.9). Finger tapping was not correlated with olfactory performance (r = 0.06, P = 0.9) (Fig. 4b). Furthermore, olfactory performance was not correlated with the UPDRS motor score (r = 0.39, P = 0.12) (Fig. 4c). Only the subset of questions in UPDRS part III that probe axial rather than proximal function (questions 1, 2, 3 face + lips + chin, 5 neck) were significantly correlated with olfactory performance (r = 0.50, P = 0.02) (Fig. 4d) and tended toward a correlation with sniff volume (r = 0.42, P = 0.07).
Effect of Manipulating Sniffing in PD Patients.
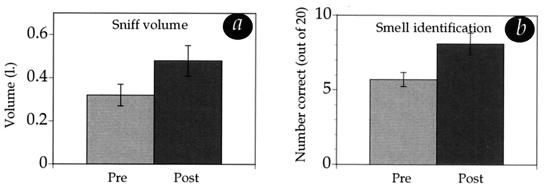
The above findings suggest that weaker sniffing is predictive of poorer olfactory performance in PD. Next, we sought to determine whether an improvement in sniffing could improve olfactory performance in PD patients. An additional 20 PD patients (16 men, four women) were tested in a control experiment where sniff airflow parameters were measured while the UPSIT was performed. For each patient, we divided the UPSIT into two 20-trial sessions. After the first 20 trials were completed, we then verbally asked the patient to increase his or her sniff volume during the second 20-trial session. Because visual feedback can assist PD patients in the coordination of movement (23), we allowed each patient to take a few sniffs while he or she saw visual feedback representing their sniff on the computer monitor. UPSIT odorants were randomized and balanced across subjects and sessions. Our aim was to test (i) whether patients could increase sniff volume and (ii) whether PD patients could improve olfactory performance by increasing sniff volume. Of the 20 patients, 18 increased sniff volume. Mean volume increased from 0.29 liter (SD = 0.16) to 0.445 liter (SD = 0.2) [t(19), = 3.8, P = 0.001], a value virtually identical to that in healthy controls (0.444 liter). Overall, increasing sniff volume led to only a small and insignificant improvement in UPSIT scores, from a mean of 9 of 20 before the sniff increase manipulation to a mean of 10 of 20 after the manipulation. There appeared, however, to be a bimodal distribution of patients. Patients who had performed worse at baseline improved, but those who performed best at baseline did not improve. To address the possibility of bimodal distribution, we conducted a median split. The median score at baseline was 7.5 of 20. The 10 patients who scored below the median at baseline had a significant improvement in olfactory performance, from a mean of 5.7 of 20 at baseline to a mean of 8.1 after sniff volume increase [t(9) = 2.27, P = 0.049] (Fig. 5). Two concerns may be raised regarding this analysis. First, improvement from the first to the second half of the UPSIT could have occurred as a result of practice, regardless of the sniff improvement manipulation. To address this concern, we compared the first and second halves of the UPSIT scores previously obtained from 20 patients. In these UPSIT tests, patients in fact performed slightly worse in the second compared with the first half of the UPSIT, presumably because of fatigue [mean first half = 11, SD = 3.9; mean second half = 10.2, SD = 2.9, t(19) = 2, P = 0.056]. This negates the concern regarding a practice effect. Second, the improvement seen in the worst performers (at baseline) could have represented regression toward the mean. To address this concern, we conducted a median split of the initial 20 patients. In contrast to the patients in the group with increased sniff volume, the worst of those performers at baseline did not improve from the first to the second half of the UPSIT [mean first half = 8, SD = 1.7; mean second half = 8.2, SD = 1.6, t(9) = 0.48, P = 0.64]. Thus these analyses indicate that the improvement was related to the sniff volume increase manipulation and did not occur otherwise. The improvement, however, still left patients at a level of performance far below that of healthy controls.
Figure 5.
Effects of increasing sniff volume on olfactory performance. (a) Increases in sniff volume from spontaneous sniffing (Pre) to sniffing after instructions (Post), in the 10 worst olfactory performing patients. (b) Improvement in olfactory performance between baseline (Pre) and after sniff volume increases (Post) in the same 10 worst olfactory performing patients. Increasing sniff volume led to a significant improvement in performance.
Discussion
Here we have shown that sniffing is impaired in PD. The importance of sniffing as a component of human olfaction has been suggested in psychophysical, physiological, and imaging studies. These studies have shown that sniffs not only (i) transport the stimulus, but also (ii) affect patterns of neural activity at all levels of the olfactory system (24–26), (iii) are modulated to account for different odorant concentrations (21, 22, 27), (iv), affect the type of odorant that is perceived (28), and (v) are a fundamental component of the olfactory percept (21, 22, 26–32). None of these studies, however, had addressed the consequence of a neurally based impairment in sniffing for olfactory processing. Here we have addressed this issue in a neurodegenerative disease and have shown that (i) PD patients are impaired at sniffing, (ii) sniffing capability predicts olfactory capability within patients, and (iii) in the worst performers among the patients, increasing sniff volume improved olfactory performance. These findings point to an essential role for sniffing in human olfaction and combine to offer an alternative explanation for the olfactory deficit in PD.
A key finding was the correlation between sniffing capability and olfactory performance in PD (Fig. 4a). One may raise the concern, however, that this correlation did not imply causation. This concern was largely negated by the continuation study where improving sniffing improved olfactory performance, suggesting a causal link between poor sniffing and poor olfaction in PD. Furthermore, PD patients had detection thresholds for the pure olfactant vanillin, and the strong trigeminal propionic acid equally deteriorated. If the source of the deficit was in the olfactory system alone, one might expect a greater impairment in vanillin than in propionic acid detection. That they were equally impaired suggests a deficit in a common processing phase. Sniffing is one such common mechanism subserving perception of both of these odorants. In turn, in contrast to the robust sniff impairment finding of this study, the sniff improvement effect was not as extremely robust. Only a slight but significant improvement in performance was evident in the worst of performers. Thus a noncausal link between the sniffing impairment we have shown and the olfactory impairment in PD remains plausible.
Our findings do not suggest that the sniff impairment is the sole cause of the olfactory impairment in PD. Increasing sniff volume only helped the worst of the performers and did not bring them to normal performance. The remaining nonsniffing component of the olfactory impairment is most likely a smelling impairment related to the disease-induced damage found in the neural substrates of the olfactory system (9, 15). This neural damage may be the result of pathological Parkinsonian processes unrelated to sniffing. Alternatively, this damage in itself may be the result of altered sniffing. The olfactory system is highly sensitive to sensory deprivation. For example, in rats, merely reducing airflow in a nostril results in extensive damage and deterioration throughout the olfactory system (33, 34). Thus, the sniffing impairment in PD may constitute a form of olfactory deprivation that in turn leads to degradation throughout the olfactory system. In this manner, the sniffing impairment may not only be a component of the eventual olfactory impairment, but also potentially the cause of the nonsniffing components in this impairment. The latter is merely our speculation regarding the significance of the sniff impairment documented here. This speculation, however, is significant in that it suggests a reversal in the current concept of cause and effect in the formation of the typical Parkinsonian lesions throughout olfactory cortex.
The sniffing impairment did not appear to be a simple extension of the generalized motor impairment in PD. Neither sniffing nor olfactory performance was significantly correlated with either the UPDRS motor scale or finger tapping ability. Furthermore, 18 of the 20 patients were able to increase their sniff volume upon request. In other words, most PD patients were able to sniff more than they did spontaneously. In fact, patients increased sniff volume by 72%, bringing their sniff volume to a value almost identical to that of controls (0.444 liter in controls and 0.445 liter in improved patients). Thus, whereas in healthy individuals a sniff was an automatic implicit response, in PD patients a sniff was a cue-dependent explicit response. This pattern is in agreement with the deficiency in internal triggering and cueing of movement sequences (35) and hampered timing of sensory acquisition motor sequences (36) associated with PD. Therefore, rather than “motor impairment” per se, we propose that the sniff impairment represents an impairment in the coordination of sensory, attentional, and motor mechanisms that combine to produce a sniff. That said, olfactory performance was significantly correlated with an axial subset of the UPDRS motor scale (i.e., motor measures related to the torso, head, and neck, and not arms and legs), leaving the possibility that the sniffing impairment in PD is but another purely motor symptom of PD.
Finally, although the improvement in olfactory performance witnessed in this study was small, this effect was nevertheless striking, as it is unusual that a simple behavioral suggestion partially ameliorates a sensory impairment induced by a long-term neurodegenerative disease. This amelioration is important, considering the implications of reductions in olfactory sensitivity (termed anosmia) on quality of life. Anosmia can alter food choices and intake, resulting in impaired nutritional status, challenged immunity, and weight loss (37, 38). Anosmia may also deprive patients of a valuable warning mechanism for life hazards, such as smoke, gas, spoiled food, and chemical contaminations (39). Last but not least, anosmia deprives patients of the hedonic pleasures related to good food and wine, as well as the pleasures related to the smell of a new car, a flower, or a loved one. Our findings, combined with previous anecdotal olfactory improvements reported in two PD patients (14), suggest that this loss may be at least partially reversed.
Acknowledgments
N.S. thanks the Parkinson's Institute staff and 40 anonymous PD patients for their helpfulness; Gordon Bower for his editorial help; Ilana Hairston, Eve DeRosa, and Adam Anderson for their advice on the manuscript; and Elite Ha Arak for continued support. This work was supported by National Institutes of Health Grants AA10723 (to E.V.S) and NS40467 and ES10803 (to C.M.T.).
Abbreviations
- PD
Parkinson's disease
- UPSIT
University of Pennsylvania smell identification test
- UPDRS
unified Parkinson's disease rating scale
References
- 1.Quinn N P. Baillieres Clin Neurol. 1997;6:1–13. [PubMed] [Google Scholar]
- 2.Ansari K A, Johnson A. J Chronic Dis. 1975;28:493–497. doi: 10.1016/0021-9681(75)90058-2. [DOI] [PubMed] [Google Scholar]
- 3.Doty R L, Riklan M, Deems D A, Reynolds C, Stellar S. Ann Neurol. 1989;25:166–171. doi: 10.1002/ana.410250210. [DOI] [PubMed] [Google Scholar]
- 4.Hawkes C H, Shephard B C, Daniel S E. J Neurol Neurosurg Psychiatry. 1997;62:436–446. doi: 10.1136/jnnp.62.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward C D, Hess W A, Calne D B. Neurology. 1983;33:943–946. doi: 10.1212/wnl.33.7.943. [DOI] [PubMed] [Google Scholar]
- 6.Quinn N P, Rossor M N, Marsden C D. J Neurol Neurosurg, Psychiatry. 1987;50:88–89. doi: 10.1136/jnnp.50.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobal G, Hummel T. Toxicol Ind Health. 1994;10:587–596. [PubMed] [Google Scholar]
- 8.Barz S, Hummel T, Pauli E, Majer M, Lang C J, Kobal G. Neurology. 1997;49:1424–1431. doi: 10.1212/wnl.49.5.1424. [DOI] [PubMed] [Google Scholar]
- 9.Daniel S E, Hawkes C H. Lancet. 1992;340:186. doi: 10.1016/0140-6736(92)93275-r. [DOI] [PubMed] [Google Scholar]
- 10.Doty R L, Bromley S M, Stern M B. Neurodegeneration. 1995;4:93–97. doi: 10.1006/neur.1995.0011. [DOI] [PubMed] [Google Scholar]
- 11.Hawkes C H, Shephard B C, Daniel S E. Q J Med. 1999;92:473–480. doi: 10.1093/qjmed/92.8.473. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery E B, Jr, Lyons K, Koller W C. Movement Disorders. 2000;15:47–48. doi: 10.1002/1531-8257(200005)15:3<474::AID-MDS1008>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 13.Tucker D. Gen Physiol. 1963;46:453–489. doi: 10.1085/jgp.46.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandyk R. Int J Neurosci. 1999;97:225–233. doi: 10.3109/00207459909000662. [DOI] [PubMed] [Google Scholar]
- 15.Pearce R K, Hawkes C H, Daniel S E. Movement Disorders. 1995;3:283–287. doi: 10.1002/mds.870100309. [DOI] [PubMed] [Google Scholar]
- 16.Doty R L, Deems D A, Stellar S. Neurology. 1988;38:1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- 17.Hawkes C H, Shephard B C. Lancet. 1993;13:435–436. [PubMed] [Google Scholar]
- 18.Doty R L, Brugger W E, Jurs P C, Orndorff M A, Snyder P J, Lowry L D. Physiol Behav. 1978;20:175–185. doi: 10.1016/0031-9384(78)90070-7. [DOI] [PubMed] [Google Scholar]
- 19.Cometto-Muniz J E, Cain W S. Int Arch Occup Environ Health. 1998;71:105–110. doi: 10.1007/s004200050256. [DOI] [PubMed] [Google Scholar]
- 20.Cain W S, Gent J F, Goodspeed R B, Leonard G. Laryngoscope. 1988;98:83–88. doi: 10.1288/00005537-198801000-00017. [DOI] [PubMed] [Google Scholar]
- 21.Laing D G. Perception. 1983;100:99–117. doi: 10.1068/p120099. [DOI] [PubMed] [Google Scholar]
- 22.Sobel N, Khan R, Hartely C, Sullivan E, Gabrieli J D E. Chem Senses. 2000;25:1–8. doi: 10.1093/chemse/25.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Jobst E E, Melnick M E, Byl N N, Dowling G A, Aminoff M. Arch Neurol. 1997;54:450–454. doi: 10.1001/archneur.1997.00550160080020. [DOI] [PubMed] [Google Scholar]
- 24.Adrian E D. J Physiol (London) 1942;100:459–473. doi: 10.1113/jphysiol.1942.sp003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes J R, Hendrix D E, Wetzel N, Johnston J W. In: Olfaction and Taste. Pfaffmann C, editor. Vol. 3. New York: Academic; 1969. pp. 172–191. [Google Scholar]
- 26.Sobel N, Prabhakaran V, Desmond J, Glover G, Goode R L, Sullivan E, Gabrieli J D E. Nature (London) 1998;392:282–286. doi: 10.1038/32654. [DOI] [PubMed] [Google Scholar]
- 27.Youngentob S L, Stern N M, Mozell M M, Leopold D A, Hornung D E. Am J Otolaryngol. 1986;7:187–193. doi: 10.1016/s0196-0709(86)80005-9. [DOI] [PubMed] [Google Scholar]
- 28.Sobel N, Khan R M, Saltman A, Sullivan E V, Gabrieli J D. Nature (London) 1999;402:35. doi: 10.1038/46944. [DOI] [PubMed] [Google Scholar]
- 29.Teghtsoonian R, Teghtsoonian M, Berglund B, Berglund U. J Exp Psychol. 1978;4:144–152. doi: 10.1037//0096-1523.4.1.144. [DOI] [PubMed] [Google Scholar]
- 30.Mozell M M, Sheehe P R, Swieck S W, Kurtz D B, Hornung D E. J Gen Physiol. 1984;83:233–267. doi: 10.1085/jgp.83.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornung D E, Chin C, Kurtz D B, Kent P F, Mozell M M. Chem Senses. 1997;22:177–180. doi: 10.1093/chemse/22.2.177. [DOI] [PubMed] [Google Scholar]
- 32.Kurtz D B, Mozell M M. J Gen Physiol. 1985;86:329–352. doi: 10.1085/jgp.86.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson D A, Wood J G. Neuroscience. 1992;49:183–192. doi: 10.1016/0306-4522(92)90086-h. [DOI] [PubMed] [Google Scholar]
- 34.Cummings D M, Brunjes P C. Exp Neurol. 1997;148:360–366. doi: 10.1006/exnr.1997.6660. [DOI] [PubMed] [Google Scholar]
- 35.Georgiou N, Iansek R, Bradshaw J L, Phillips J G, Mattingley J B, Bradshaw J A. Brain. 1993;116:1575–1587. doi: 10.1093/brain/116.6.1575. [DOI] [PubMed] [Google Scholar]
- 36.Ivry R B. Curr Opin Neurobiol. 1996;6:851–857. doi: 10.1016/s0959-4388(96)80037-7. [DOI] [PubMed] [Google Scholar]
- 37.Murphy C. Crit Rev Food Sci Nutr. 1993;33:3–15. doi: 10.1080/10408399309527607. [DOI] [PubMed] [Google Scholar]
- 38.Schiffman S S. J Am Med Assoc. 1997;278:1357–1362. [PubMed] [Google Scholar]
- 39.Schiffman S S. N Engl J Med. 1983;22:1337–1343. doi: 10.1056/NEJM198306023082207. [DOI] [PubMed] [Google Scholar]