Abstract
The Ras family GTPases (Ras, Rap1, and Rap2) and their downstream mitogen-activated protein kinases (ERK, JNK, and p38MAPK) and PI3K signaling cascades control various physiological processes. In neuronal cells, recent studies have shown that these parallel cascades signal distinct forms of AMPA-sensitive glutamate receptor trafficking during experience-dependent synaptic plasticity and adaptive behavior. Interestingly, both hypo- and hyper-activation of Ras/Rap signaling impair the capacity of synaptic plasticity, underscoring the importance of a “happy-medium” dynamic regulation of the signaling. Moreover, accumulating reports have linked various genetic defects that either up- or down-regulate Ras/Rap signaling with a number of mental disorders associated with learning disability (e.g., Alzheimer’s disease, Angelman syndrome, autism, cardio-facio-cutaneous syndrome, Coffin-Lowry syndrome, Costello syndrome, Cowden and Bannayan-Riley-Ruvalcaba syndromes, fragile X syndrome, neurofibromatosis type 1, Noonan syndrome, schizophrenia, tuberous sclerosis, and X-linked mental retardation), highlighting the necessity of happy-medium dynamic regulation of Ras/Rap signaling in learning behavior. Thus, the recent advances in understanding of neuronal Ras/Rap signaling provide a useful guide for developing novel treatments for mental diseases.
Keywords: AMPA receptors, MAPK, neuromodulator, NMDA receptors, plasticity, Ras, Rap1, Rap2, sensory experience, subcellular compartment, synaptic transmission, trafficking
The sustained interest in synaptic plasticity stems from the belief that this process underlies key aspects of adaptive cognitive function such as learning and memory (Bliss and Collingridge 1993; Malenka and Nicoll 1999). Long-term synaptic plasticity, the sustained synaptic modification after periods of repetitive synaptic activity, was first discovered in the hippocampus (Bliss and Lomo 1973). The phenomenon has since been described in many other brain regions and used extensively as an experimental model for exploring the physiology and pathology of human learning and memory. Significant progress has been made in understanding different forms of synaptic plasticity, with the cellular, molecular, and signaling mechanisms best illustrated for N-methyl-D-Aspartate (NMDA)-sensitive glutamate (Glu) receptor (-R)-dependent forms of synaptic plasticity (Derkach and others 2007; Gu and Stornetta 2007; Isaac and others 2007; Kauer and Malenka 2007; Shepherd and Huganir 2007; Kerchner and Nicoll 2008; Kessels and Malinow 2009). Early physiology studies have identified the opening of NMDA-Rs and influx of calcium ions to be the two essential cellular events that trigger synaptic plasticity. Subsequent investigations have established trafficking of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)-sensitive receptors at postsynaptic sites as a common mechanism for altering synaptic strength during plasticity. More recent works have implicated that the Ras family GTPases (i.e., Ras, Rap1 and Rap2) and their downstream mitogen-activated protein kinases (MAPK) and phosphoinositide 3-kinase (PI3K) signaling pathways serve as the key biochemical cascades linking activation of NMDA-Rs and calcium influx with phosphorylation and trafficking of AMPA-Rs during synaptic plasticity. In the meantime, accumulating reports have associated genetic mutations of various molecules involved in Ras and Rap signaling with a number of mental disorders causing learning disability, further underscoring the essential role of Ras and Rap signaling in both the physiology and pathology of human learning and memory. Here we review the current models for NMDA-R-dependent AMPA-R trafficking in synaptic plasticity, learning and memory, and mental diseases with learning disability, focusing on the regulation and dysregulation of Ras and Rap signaling in these physiological and pathological processes.
AMPA-R Trafficking and Synaptic Plasticity
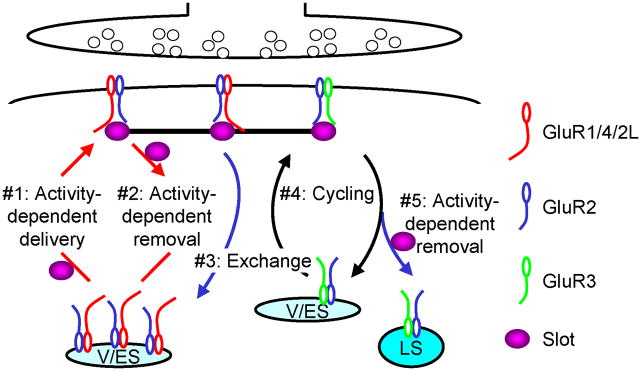
Tetrameric AMPA-Rs can be classified into two major groups based on the composition of subunits encoded by four genes, GluR1-4 (Hollmann and Heinemann 1994; Schoepfer and others 1994). One group consists of AMPA-Rs containing subunits with long cytoplasmic termini. These are GluR1, GluR2L (a long splice form of GluR2) and GluR4. The other group includes AMPA-Rs containing subunits with short cytoplasmic termini, i.e., GluR2, GluR3 and GluR4c (a short splice form of GluR4). These distinct AMPA-Rs are involved in different synaptic trafficking events (Fig. 1) (Malinow and Malenka 2002; Collingridge and others 2004; Gu and Stornetta 2007). Whereas GluR1, GluR4 and GluR2L take part in synaptic AMPA-R delivery during synaptic potentiation and synaptic AMPA-R removal during depotentiation, GluR2, GluR3 and GluR4c participate in synaptic AMPA-R removal during synaptic depression and one-to-one replacement during synaptic AMPA-R cycling. Additionally, these two groups of AMPA-Rs are involved in parallel, opposite trafficking during synaptic AMPA-R exchange. Among these trafficking events, the regulation of synaptic trafficking of AMPA-Rs with long cytoplasmic termini, particularly, GluR1-containing AMPA-Rs, has been most extensively examined (Kessels and Malinow 2009).
Fig. 1. Model for synaptic AMPA-R trafficking.
LS: lysosome; Slot: hypothesized protein(s) that are delivered and removed with AMPA-Rs during activity-dependent synaptic potentiation and depression, respectively; V/ES: vesicles/endosomes.
Synaptic AMPA-R delivery during LTP
AMPA-Rs with long cytoplasmic termini (i.e., GluR1-, GluR2L- or GluR4-containing AMPA-Rs) are normally restricted from synapses (Fig. 1) (Malinow and Malenka 2002). They can be driven into synapses during synaptic enhancement (e.g., long-term potentiation or LTP) within 15–20 min with a rate time constant of ~4 min (Fig. 2). Interestingly, dependent on input origin, cell type, and developmental stage, different central synapses may selectively express one or multiple types of AMPA-Rs with long cytoplasmic termini (Rubio and Wenthold 1997; Toth and McBain 1998; Gardner and others 2001; Kielland and others 2009; Zhu 2009). For example, in CA1 pyramidal neurons of the hippocampus, GluR1, GluR2L, and GluR4 all can mediate CA3→CA1 transmission, but their involvement is developmentally regulated; GluR4 is expressed only in the first postnatal week, GluR1 expression increases with increasing age and reaches the maximal, sustained expression level after the third postnatal week, and the expression of GluR2L peaks at the end of the second postnatal week and then slowly declines to about half in the adult (Zhu and others 2000; Kolleker and others 2003). Thus, GluR4 primarily mediates synaptic transmission in neonatal CA1 neurons, whereas GluR1 and GluR2L together mediate synaptic transmission in young and adult CA1 neurons. Interestingly, GluR1 differs from GluR4 and GluR2L in the PDZ motifs at its C-terminus. GluR1 has a canonical class I PDZ motif and mutations at this motif prevent synaptic trafficking of the receptor (Hayashi and others 2000; Passafaro and others 2001; Piccini and Malinow 2002), whereas GluR4 and GluR2L have an unconventional PDZ motif, which ends with one extra residue (i.e., Proline at GluR4 and Serine at GluR2L) and deletion of the extra residue blocks synaptic incorporation of the receptors (our unpublished data).
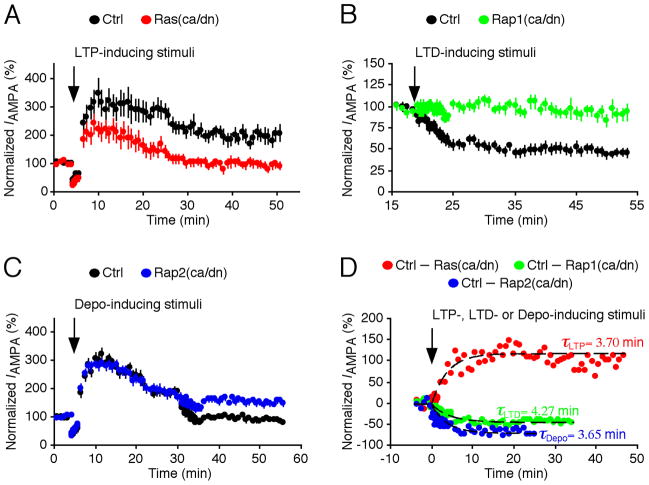
Fig. 2. Time course of AMPA-R trafficking during LTP, LTD and depotentiation.
A. Steady state synaptic AMPA-R-mediated response amplitudes in CA1 pyramidal neurons expressing Ras(ca)-GFP or Ras(dn)-GFP and neighboring non-expressing control neurons before and after LTP-inducing pairing.
B. Steady state synaptic AMPA-R-mediated response amplitudes in CA1 pyramidal neurons expressing Rap1(ca)-GFP or Rap1(dn)-GFP and neighboring non-expressing control neurons before and after LTD-inducing pairing.
C. Steady state synaptic AMPA-R-mediated response amplitudes in CA1 pyramidal neurons expressing Rap1(ca)-GFP or Rap1(dn)-GFP and neighboring non-expressing control neurons before and after LTP-and depotentiation-inducing pairings.
D. Subtracting synaptic AMPA-R-mediated responses in expressing neurons from neighboring non-expressing control neurons gives the time course of LTP-, LTD- and depotentiation-induced alterations of synaptic efficacy. Note that the data points are fitted with a single exponential function to estimate rate time constants of AMPA-R trafficking during LTP, LTD and depotentiation. The reanalysis uses the data collected in the previous studies (Zhu and others 2002; Zhu and others 2005).
In addition to the levels of protein expression, synaptic activity also regulates the relative contribution of AMPA-Rs with long cytoplasmic termini to synaptic transmission. In the intact brain, spontaneous activity is prevalent (Zhu and Connors 1999; Buzsaki and others 2002; Margrie and others 2002), and is sufficient to drive synaptic delivery of GluR2L and GluR4 (Zhu and others 2000; Kolleker and others 2003; Zhu 2009). Thus, both GluR2L and GluR4 mediate a significant portion of transmission even though their total protein expression levels are much lower than GluR2 and GluR1 (Kolleker and others 2003; Zhu 2009). On the other hand, strong synaptic activity, such as LTP-inducing stimuli, or experience-dependent activity and/or the presence of neuromodulatory factors (e.g., neuromodulators, hormones and neurotrophic factors) in vivo is required for synaptic delivery of GluR1-containing AMPA-Rs (Hayashi and others 2000; Takahashi and others 2003; Qin and others 2005; Kielland and others 2009; Zhu 2009). Consequently, although GluR1 expression is high in neurons, only a small percentage (~5–10%) of the receptors are involved in synaptic transmission and the majority of the receptors are located at extrasynaptic sites (Shi and others 1999; Zamanillo and others 1999; Andrasfalvy and others 2003; Kielland and others 2009). Supporting the idea of being differentially regulated, altering the expression level of GluR4, but not of GluR1 changes the relative contribution of these receptors to synaptic transmission (Zhu and others 2000; Qin and others 2005; Zhu 2009). Therefore, different synapses receiving different patterns of synaptic inputs may recruit specific AMPA-Rs with distinct subunit compositions (Kielland and others 2009; Zhu 2009). For example, in cortical spiny stellate and pyramidal neurons, GluR1 mediates transmission at intracortical synapses, but not at thalamocortical synapses, whereas GluR4 mediates transmission at both synapses (Zhu 2009). Similarly, GluR1 mediates transmission at retinogeniculate synapses, but not corticogeniculate synapses, whereas GluR4 mediates both retinogeniculate and corticogeniculate transmission in geniculate neurons of the thalamus (Kielland and others 2009). Selective incorporation of distinct AMPA-Rs allows different types of central synapses to participate in different forms of synaptic potentiation: spontaneous activity- and/or experience-dependent synaptic enhancements (Qin and others 2005; Kielland and others 2009; Zhu 2009). In addition, because GluR1-, GluR2L-, and GluR4-containing AMPA-Rs differ significantly in their gating properties (Jonas 2000), selective incorporation of distinct AMPA-Rs also allows different central synapses to generate transmission with very different kinetics and to compute various synaptic inputs arriving at the different subcellular compartments of neurons (Toth and McBain 1998; Zhu 2000; Gardner and others 2001; Froemke and others 2005; Kielland and others 2009; Zhu 2009).
GluR1- and GluR4-containing AMPA-Rs that lack GluR2 exhibit increased Ca2+-permeablity and enhanced rectification, which alter synaptic function and plasticity, and may cause or exacerbate some diseases (Cull-Candy and others 2006). GluR2-lacking AMPA-Rs usually contribute little transmission in adult principal neurons because the endoplasmic reticulum (ER) normally only allows the exit of properly assembled, GluR2-containing AMPA-Rs for synaptic delivery in these neurons (Greger and others 2003). However, neonatal pyramidal neurons display more rectified responses, indicative of GluR2-lacking AMPA-Rs, compared to nearby neurons with synaptic AMPA-R delivery blocked (Zhu and others 2000), or mature neurons (Kumar and others 2002). This presumably results from the low GluR2 expression level during early development, compelling neurons to use GluR2-lacking AMPA-Rs (Zhu and others 2000). Similar circumstances may occur when GluR2 trafficking is impaired, resulting in the same predominance of GluR2-lacking AMPA-Rs in synaptic transmission and perhaps aggravating pathological conditions, such as ischemia, amyotrophic lateral sclerosis and drug abuse (Liu and others 2004; Bellone and Luscher 2006; Clem and Barth 2006; Lai and others 2006; Liu and others 2006; Mameli and others 2007; Conrad and others 2008).
Synaptic AMPA-R removal during depotentiation
Some of the newly delivered AMPA-Rs with long cytoplasmic termini are removed from synapses during depotentiation (Fig. 1) (Zhu and others 2005), a form of synaptic depression that only occurs at recently (within a short window of ~0.5–2 hrs) potentiated synapses but not naïve synapses (Fujii and others 1991; O’Dell and Kandel 1994; Huang and others 1999; Zhou and others 2003). Synaptic removal of GluR2L-and GluR1-containing AMPA-Rs can occur rapidly, i.e., within ~15–25 min with a rate time constant of ~4 min (Fig. 2). The removed AMPA-Rs are recycled into the deliverable AMPA-R pool near the postsynaptic density (PSD), and the recycled AMPA-Rs are crucial for synaptic reinsertion in subsequent synaptic enhancements (Park and others 2004; Park and others 2006; Kielland and others 2009; Petrini and others 2009).
Synaptic AMPA-R exchange
The other synaptic AMPA-Rs with long cytoplasmic termini are exchanged with AMPA-Rs with short cytoplasmic termini (i.e., GluR2/3 AMPA-Rs) via an activity-independent process (Fig. 1) (Zhu and others 2000; Kolleker and others 2003; McCormack and others 2006). This exchange process includes two activity-independent anti-parallel AMPA-R trafficking events, both with slow rate time constants of ~16 hrs (McCormack and others 2006). As a consequence, following activity-dependent synaptic enhancement, synaptic strength initially appears lowered before returning to the potentiated level. This interesting phenomenon, originally observed (but not published) during the investigation of the long-term fate of newly delivered GluR4 after synaptic enhancement (Zhu and others 2000), provided the first clue that synaptic strength must be coded by molecules other than AMPA-Rs during synaptic exchange and inspired Malinow and colleagues to propose the “slot” theory. This model proposes that slot proteins or protein complexes (presumably consisting of certain PSD proteins), which are delivered or removed with AMPA-Rs during fast activity-dependent synaptic enhancement, depotentiation or depression, act as AMPA-R placeholders allowing a one-to-one replacement during the much slower activity-independent synaptic exchange (Hayashi and others 2000). The same pattern of initial lessening and then return to potentiated synaptic strength has also been observed during synaptic exchange of GluR1- and GluR2L-containing AMPA-Rs (McCormack and others 2006). These authors have further demonstrated that synaptic removal of GluR1, GluR2L or GluR4 and the addition of GluR2/3 replenish the synapses’ ability to produce new potentiation and depression, respectively, indicating the essential role of synaptic AMPA-R exchange in maintaining the capacity for bidirectional plasticity (McCormack and others 2006). It remains to be determined whether the exchanged GluR1-, GluR2L- or GluR4-containing AMPA-Rs are recycled into the deliverable pool for redelivery to the synapse.
Synaptic AMPA-R cycling
AMPA-Rs with only short cytoplasmic termini (e.g., GluR2/3 AMPA-Rs) constitutively cycle between synaptic and non-synaptic sites at a rapid rate time constant of ~15–20 min (Fig. 1). This process makes one-to-one replacements of AMPA-Rs, and requires no synaptic activity (Nishimune and others 1998; Osten and others 1998; Song and others 1998; Luscher and others 1999; Lee and others 2002). According to the slot hypothesis, the slot proteins, which can be added or removed with AMPA-Rs only during activity-dependent synaptic enhancement or depression, also play a central role in maintaining the constant transmission strength during activity-independent synaptic AMPA-R cycling. It has been speculated that the majority of AMPA-Rs in the cycling pool are synaptic with only a small number of them at non-synaptic sites and addition of GluR2/3 AMPA-Rs coming from the exchange is added into the same cycling pool at a slow rate of 15–18 hrs (McCormack and others 2006). This idea is supported by the findings that synaptic responses recover at a slow rate time constant of ~16 hrs after pharmacological inactivation of all surface AMPA-Rs (Adesnik and others 2005), as well as by direct immunogold labeling of GluR2/3 AMPA-Rs (our unpublished results). Together, synaptic exchange and cycling of AMPA-Rs play a key role in maintaining synaptic efficacy without being affected by continuous protein turnover.
Synaptic AMPA-R removal during LTD
AMPA-Rs with short cytoplasmic termini (i.e., GluR2/3 AMPA-Rs) can be removed from synapses during synaptic depression (e.g., long-term depression or LTD) (Fig. 1) (Zhu and others 2002; Chung and others 2003; Seidenman and others 2003). GluR2/3 can be removed within ~15–25 min with a rate time constant of ~4 min during LTD (Fig. 2). The removed receptors (presumably together with slot proteins) are diverted to the late endosomes/lysosomes via clathrin-dependent endocytosis (Beattie and others 2000; Wang and Linden 2000; Lee and others 2004; Brown and others 2005). Knockout mice deficient in the expression of both GluR2 and GluR3 show little absolute depression of synaptic transmission after LTD-inducing stimuli (Meng and others 2003), consistent with the fact that no GluR2/3 AMPA-Rs are available for removal from synapses of these mice. However, the baseline transmission in these mice is so severely reduced that any change in synaptic strength would appear as relatively large, thus the seemingly “normal” LTD (Meng and others 2003).
Whether AMPA-Rs travel into and out of synapses via direct exocytosis and endocytosis from and to intracellular compartments or indirectly via extrasynaptic membrane surface remains controversial (Passafaro and others 2001; Borgdorff and Choquet 2002; Ashby and others 2004; Park and others 2004; Adesnik and others 2005; Park and others 2006; Ehlers and others 2007; Yudowski and others 2007; Wang and others 2008). Recent physiology and imaging evidence shows that GluR1 traffics into and out of synapses via perisynaptic membrane sites in neurons in cultured and acute slices (Heine and others 2008; Yang and others 2008; Makino and Malinow 2009; Petrini and others 2009). This view is supported by immunogold labeling of GluR1 receptors at the perisynaptic membrane deliverable pool in intact brains (Kielland and others 2009).
Ras-MAPK Signaling and Synaptic Plasticity
Ras family small GTPases (i.e., Ras, Rap1 and Rap2) are guanine-nucleotide-binding proteins that serve as molecular “switches” by cycling between active (GTP-bound) and inactive (GDP-bound) states (Takai and others 2001). In neuronal cells, Ras, Rap1 and Rap2, are differentially stimulated by different forms of synaptic activity via activation of NMDA-Rs and influx of calcium to independently control three activity-dependent AMPA-R trafficking events (Thomas and Huganir 2004; Tada and Sheng 2006; Gu and Stornetta 2007). Particularly, LTP-inducing stimuli activate the Ras—extracellular signal-regulated kinase kinase (MEK)—extracellular signal-regulated kinase (ERK) and —phosphoinositide 3-kinase (PI3K)—protein kinase B (PKB/AKT) signaling pathways, which phosphorylate AMPA-Rs with long cytoplasmic termini and drive these receptors into synapses. LTD-inducing stimuli activate the Rap1—p38MAPK signaling pathway, which phosphorylates AMPA-Rs with only short cytoplasmic termini and leads to synaptic removal of the receptors. Depotentiation-inducing stimuli activate the Rap2—c-Jun amino-terminal kinase (JNK)—protein phosphatase 2B (PP2B, also known as calcineurin) signaling pathway, which dephosphorylates AMPA-Rs with long cytoplasmic termini and triggers synaptic removal of the receptors.
Ras signals synaptic AMPA-R delivery during LTP
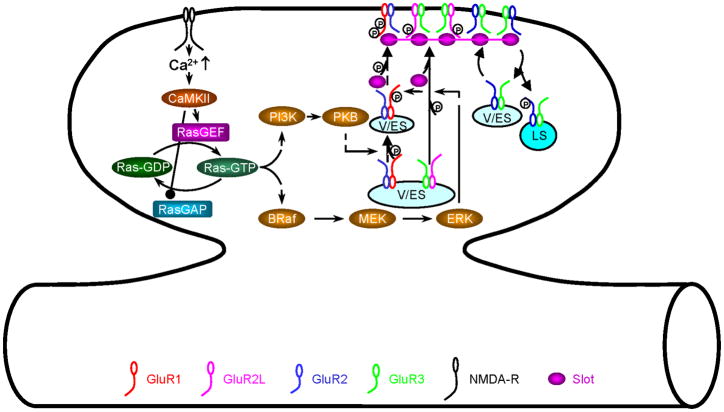
The small GTPase Ras controls synaptic delivery of AMPA-Rs with long cytoplasmic termini during LTP (Fig. 3) (Zhu and others 2002; Qin and others 2005; McCormack and others 2006; Hu and others 2008; Kielland and others 2009). Synaptic activity increases the level of active GTP-bound Ras, which binds its downstream effector, BRaf, to stimulate the MEK—ERK pathway. Activation of MEK—ERK signaling stimulates phosphorylation of GluR1 at S845 and GluR2L at S841. This single phosphorylation is sufficient to drive synaptic delivery of GluR2L but not GluR1 during LTP. Although ERK is unlikely to phosphorylate AMPA-Rs directly, other downstream serine/threonine kinases, such as p90 ribosomal S6 kinase (RSK) and/or mitogen- and stress-activated protein kinase (MSK), may serve this function (Waskiewicz and others 1997; Kyriakis and Avruch 2001). Strong synaptic activity, experience-dependent activity and/or the presence of neuromodulatory factors stimulate additional Ras signaling and activate the PI3K—PKB pathway. The signaling stimulates phosphorylation of GluR1 at S831 most likely through downstream serine/threonine kinases, such as the mammalian target of rapamycin (mTOR, also known as FRAP1) and/or glycogen synthase kinase (GSK, also known as tau protein kinase) (Datta and others 1999; Vivanco and Sawyers 2002). The phosphorylation of GluR1 at both S845 and S831 drives GluR1 into synapses during LTP. These results are consistent with the findings that LTP induces activation of MEK—ERK and PI3K—PKB signaling and that inhibiting these signaling pathways blocks LTP (English and Sweatt 1997; Kelly and Lynch 2000; Raymond and others 2002; Zhu and others 2002; Qin and others 2005; Horwood and others 2006). Together, these findings establish the foundation for the model that phosphorylation disrupts the retention of AMPA-Rs with long cytoplasmic termini at non-synaptic sites and initiates their synaptic trafficking and incorporation (Malinow and Malenka 2002; Thomas and Huganir 2004).
Fig. 3. Model for Ras signaling-regulated synaptic delivery of AMPA-Rs during LTP.
BRaf: V-raf murine sarcoma viral oncogene homolog B; CaMKII: calcium/calmodulin-dependent protein kinase II; ERK: extracellular signal-regulated kinase kinase; LS: lysosome; MEK: extracellular signal-regulated kinase; PI3K: phosphoinositide 3-kinase; PKB: protein kinase B; RasGEF: Ras activators; RasGAP: Ras inactivators; Slot: hypothesized protein(s) that are delivered or removed with AMPA-Rs; V/ES: vesicles/endosomes.
It is possible that phosphorylation of other cytoplasmic terminal sites of the receptors may also be involved; one study has reported that synaptic incorporation of GluR1 requires phosphorylation at S816 and S818 (Boehm and others 2006), while others have shown the phosphorylation of S816 and S818 of GluR1 facilitates its dendritic membrane surface delivery rather than synaptic incorporation in spines (Kessels and others 2009; Lin and others 2009). Importantly, recent studies have demonstrated that after opening of synaptic NMDA-R channels, calcium/calmodulin-dependent protein kinase II (CaMKII) activates briefly prior to Ras activation (Yasuda and others 2006; Lee and others 2009), and Ras is necessary and sufficient to mediate CaMKII-stimulated synaptic AMPA-R delivery during synaptic potentiation (Zhu and others 2002; Hu and others 2008), consistent with the suggestion that CaMKII regulates Ras signaling via RasGEFs and/or RasGAPs (Chen and others 1998; Kim and others 1998; Rumbaugh and others 2006). Because CaMKII is not involved in synaptic plasticity in neonatal neurons (Zhu and others 2000; Yasuda and others 2003), synaptic activity may directly stimulate Ras signaling via other Ras regulators, such as son of sevenless (SOS), during early development (Tian and others 2004). Thus, in the current model, synaptic activity stimulates the Ras—BRaf—MEK—ERK and Ras—PI3K—PKB pathways to control phosphorylation of AMPA-Rs with long cytoplasmic termini and their subsequent synaptic delivery during LTP.
Rap1 signals synaptic AMPA-R removal during LTD
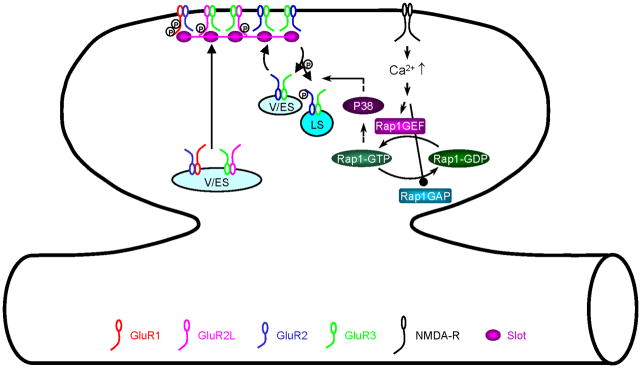
The small GTPase Rap1 controls synaptic removal of AMPA-Rs with short cytoplasmic termini during LTD (Fig. 4) (Zhu and others 2002; McCormack and others 2006). LTD-inducing stimuli (e.g., low frequency synaptic inputs) activate Rap1, which activates p38MAPK to remove synaptic GluR2/3 AMPA-Rs during LTD. Because phosphorylation of GluR2/3 is unlikely due directly to p38MAPK, other protein kinases, such as MAP kinase-interacting kinase (MNK) and/or p38-regulated/activated kinase (PRAK), may relay Rap1-p38MAPK signaling (Kyriakis and Avruch 2001; Gallo and Johnson 2002). The ultimate molecular target of Rap1—p38MAPK remains undefined, but S880 of GluR2 at its PDZ ligand domain is a good candidate. Phosphorylation of GluR2 at S880 disrupts its interactionwith glutamate receptor-interacting protein/AMPA-bindingprotein (GRIP/ABP) but not with PKC-interacting protein 1 (PICK1) (Chung and others 2000; Matsuda and others 2000), which reduces the recycling of GluR2/3 AMPA-Rs and thus depresses synaptic transmission (Daw and others 2000; Braithwaite and others 2002; Seidenman and others 2003; Lin and Huganir 2007). These studies are consistent with the model that Rap1—p38MAPK initiates phosphorylation of AMPA-Rs with short cytoplasmic termini to control synaptic removal of GluR2/3 AMPA-Rs during LTD.
Fig. 4. Model for Rap1 signaling-regulated synaptic removal of AMPA-Rs during LTD.
LS: lysosome; P38: p38 mitogen-activated protein kinases; Rap1GEF: Rap1 activators; Rap1GAP: Rap1 inactivators; Slot: hypothesized protein(s) that are delivered or removed with AMPA-Rs; V/ES: vesicles/endosomes.
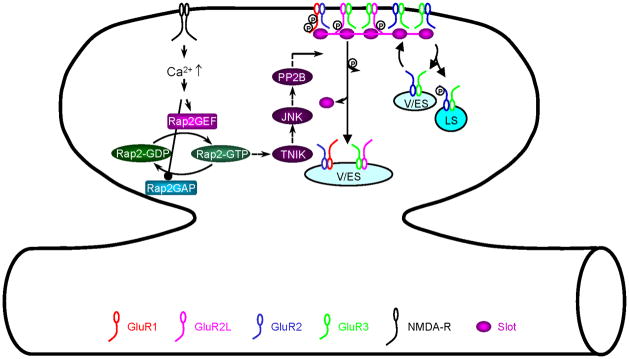
Rap2 signals synaptic AMPA-R removal during depotentiation
The small GTPase Rap2 controls synaptic removal of AMPA-Rs with long cytoplasmic termini during depotentiation (Fig. 5) (Zhu and others 2005; Kielland and others 2009). In response to depotentiation-inducing stimuli (e.g., low frequency synaptic inputs after LTP), Rap2 stimulates JNK activity via interaction of Traf2- and NCK-interacting kinase (TNIK) and MINK2 (a TNIK-related kinase). The Rap2—JNK-stimulated dephosphorylation and synaptic removal of GluR1 and GluR2L during depotentiation seems to be mediated by PP2B/calcineurin, consistent with the finding that PP2B is necessary and sufficient for depotentiation (Mulkey and others 1993; Zhuo and others 1999; Malleret and others 2001). This view is further supported by a recent report that PP2B is central for synaptic removal of GluR1 in cultured neurons (Bhattacharyya and others 2009), in which GluR1 is constitutively delivered to synapses and could thus be removed via a depotentiation process (Kessels and Malinow 2009). These studies support the model that Rap2—TNIK—JNK—PP2B signals dephosphorylation of AMPA-Rs with long cytoplasmic termini to control synaptic AMPA-R removal during depotentiation.
Fig. 5. Model for Rap2 signaling-regulated synaptic removal of AMPA-Rs during depotentiation.
JNK: c-Jun amino-terminal kinase; LS: lysosome; PP2B: protein phosphatase 2B; Rap2GEF: Rap2 activators; Rap2GAP: Rap2 inactivators; Slot: hypothesized protein(s) that are delivered or removed with AMPA-Rs; TNIK: Traf2- and NCK-interacting kinase; V/ES: vesicles/endosomes.
Ras and Rap signaling in synaptic and subcellular compartments
Ras proteins achieve their functional specificity by forming distinct signaling complexes with their various downstream effectors in different subcellular compartments (Mochizuki and others 2001; Mor and Philips 2006; Omerovic and Prior 2009; Zeke and others 2009). On the membrane, ~40% of activated Ras-GTP molecules form immobile nanoclusters that can recruit their downstream signaling effectors to achieve functional signal transduction, whereas the remaining ~60% of Ras-GTP molecules are freely diffusing monomers which are not engaged in signaling and therefore are non-functional (Murakoshi and others 2004; Plowman and others 2005; Tian and others 2007). In neuronal cells, recent in vivo results have shown that experience-dependent activity stimulates local Ras and Rap signaling to control synaptic plasticity and AMPA-R trafficking in individual synapses without affecting different types of synapses separated spatially by less than 1 μm (Kielland and others 2009; Zhu 2009), suggesting Ras and Rap signaling to be synapse-specific between different types of synapses. It is possible however, as recently shown by in vitro studies, that mobile, non-functional Ras-GTP molecules can spread up to 10 μm away and possibly integrate into the local signaling complexes of the same type of neighboring synapses to modulate their plasticity threshold (Harvey and Svoboda 2007; Harvey and others 2008). At single synapses, the Ras and Rap signaling pathways independently control synaptic trafficking of distinct AMPA-Rs during different forms of synaptic plasticity with little signaling cross-talk (Zhu and others 2002; Qin and others 2005; Zhu and others 2005; Kielland and others 2009), suggesting the existence of a signaling-specific mechanism within individual synapses as well. Forming distinct signaling complexes in the membrane of different cell surface subdomains (i.e., bulk plasma membrane and lipid raft) or intracellular organelles (i.e., ER, Golgi, endosomes and mitochondria) is a promising mechanism for Ras and Rap to independently signal distinct forms of synaptic AMPA-R trafficking and plasticity both between different synapses and within individual synapses.
Synaptic AMPA-R trafficking and learning and memory
Synaptic trafficking of AMPA-Rs mediates experience-dependent synaptic plasticity, as well as learning and memory. In particular, independent studies have demonstrated that sensory experience-dependent synaptic activity drives synaptic incorporation of GluR1-containing AMPA-Rs at cortical and thalamic synapses (Takahashi and others 2003; Clem and Barth 2006; McCormack and others 2006; Kielland and others 2009; Zhu 2009). Consistent with this idea, assuming neurons undergo new experience when animals are awake but not when they are asleep, physiological studies have demonstrated that GluR1-containing AMPA-Rs functionally incorporate into hippocampal and cortical synapses in awake, but not sleeping animals (Qin and others 2005; Hu and others 2008). This finding is further supported by biochemical assays showing enhanced PI3K—PKB signaling, elevated phosphorylation and synaptic expression of GluR1 in awake animals compared to sleeping animals (Qin and others 2005; Hu and others 2008; Vyazovskiy and others 2008). Interestingly, synaptic incorporation of GluR2L- and GluR4-containing AMPA-Rs occurs at hippocampal and cortical synapses in both awake and sleeping animals, requiring only spontaneous, experience-independent synaptic activity (Qin and others 2005; Hu and others 2008; Zhu 2009). Consistent with the notion that experience-dependent activity is essential for synaptic trafficking of GluR1, sensory deprivation blocks synaptic delivery and/or causes synaptic removal of GluR1 (Wright and others 2008; Kielland and others 2009). Importantly, active learning stimulates PI3K signaling and inhibiting PI3K signaling blocks associative learning but not spatial learning (Lin and others 2001; Chen and others 2005; Horwood and others 2006). Moreover, associative learning drives synaptic insertion of GluR1-containing AMPA-Rs and blocking synaptic delivery of GluR1 blocks this form of learning (Rumpel and others 2005; Hu and others 2007; Matsuo and others 2008). Finally, GluR1 knockout mice exhibit selective impairment of associative learning but not spatial learning (Zamanillo and others 1999; Schmitt and others 2004; Schmitt and others 2005; Sanderson and others 2007). Together, these results suggest that synaptic delivery of GluR1 plays a central role in consciousness-dependent associative learning.
The functional implications of other forms of synaptic AMPA-R trafficking in learning and memory are less clear. Whereas GluR1 knockout mice show normal spatial learning (Zamanillo and others 1999), GluR2 knockout mice have impaired spatial learning (Shimshek and others 2006), suggesting that synaptic trafficking of GluR2L and/or GluR2 may be important for this form of learning. In addition, recent studies have shown that hyperactivation of mGluR-mediated LTD, which removes synaptic GluR2/3 AMPA-Rs via stimulating p38MAPK signaling (Hsieh and others 2006), impairs inhibitory avoidance extinction (Dolen and others 2007). Thus, synaptic removal of GluR2/3 AMPA-Rs during LTD may correlate with eliminating learned experience and/or creating memory from new learning. Using a conditional knockout approach, a recent study has shown that long-term deletion of GluR1 or GluR1 and GluR2 severely reduces basal CA3→CA1 transmission by ~80% and ~95%, respectively (far more than the 50% share of synaptic GluR1- and GluR2L-containing AMPA-Rs determined in acute experiments, see also below) (Lu and others 2009), supporting the notion that ultimately synaptic delivery of GluR1/GluR2L and slot proteins plays a dominant role in determining synaptic strength and that synaptic AMPA-R exchange allows LTP to replenish the slot protein pool being continuously depleted by LTD (McCormack and others 2006). On the other hand, long-term deletion of GluR2 or GluR2 and GluR3 reduces the basal transmission by ~50% (Lu and others 2009), suggesting that complementary mechanisms may allow LTP to deliver fewer GluR1, depotentiation to remove more GluR1, and/or LTD to directly remove GluR1 if synaptic GluR1 fails to be exchanged with GluR2/3. Thus, the findings reported by Lu and colleagues underscore the subservient but imperative role of synaptic exchange with GluR2/3 AMPA-Rs in long-term preservation of newly gained synaptic efficacy (McCormack and others 2006). Together, these studies support the speculation that activity-independent synaptic AMPA-R exchange is vital for the long-term preservation and/or consolidation of learned experience (Matsuo and others 2008; Kessels and Malinow 2009). Obviously, much more work is needed to determine the functional role of each synaptic trafficking element in learning and memory.
“Happy-medium” dynamic regulation of synaptic signaling and trafficking
Based on the clinical observations and behavioral analysis of mouse models for mental diseases, it has been proposed that learning and memory require a “happy-medium” dynamic regulation of Ras signaling (Costa and Silva 2003; Thomas and Huganir 2004), namely learning ability will be impaired when the signaling dynamics are lessened due to the excessive up- or down-regulation of the activities of signaling molecules. Direct manipulation of Ras and Rap signaling has revealed that both hypo- and hyper-regulation of Ras/Rap signaling impair synaptic AMPA-R trafficking and synaptic plasticity, providing a cellular and molecular foundation for this theory (McCormack and others 2006). This study further illustrates that happy-medium dynamic regulation of Ras and Rap signaling sets an ideal ratio of AMPA-Rs with long and only short cytoplasmic termini at synapses, which is the key to maintaining an optimal capacity of bidirectional synaptic plasticity (i.e., synaptic potentiation and depression) (McCormack and others 2006). Indeed, the majority of studies have found the ratio of synaptic AMPA-Rs with long cytoplasmic termini and only short cytoplasmic termini to be ~1:1 on average, or each group of AMPA-Rs contributes ~50% to the basal transmission (e.g., (Wenthold and others 1996; Nishimune and others 1998; Luscher and others 1999; Luthi and others 1999; Xia and others 2000; Shi and others 2001; Zhu and others 2002; Kessels and others 2009; Kielland and others 2009; Zhu 2009)). In addition, many researchers have independently reported that the maximal amounts of LTP and LTD are ~100% and ~50%, respectively (e.g., (Zhu and others 2002; Chung and others 2003; Esteban and others 2003; Kamenetz and others 2003; Kolleker and others 2003; Seidenman and others 2003; Bagal and others 2005; Brown and others 2005; Qin and others 2005; Tomita and others 2005; Hu and others 2008)). The 50% reduction in transmission by maximal LTD is consistent with the proportion of AMPA-Rs with short cytoplasmic termini, or LTD-targeting GluR2/3 AMPA-Rs, in the total pool of synaptic AMPA-Rs. The 100% increase in transmission by maximal LTP is likely to be determined by the capacity of the PSD to hold additional AMPA-Rs and/or the availability of AMPA-Rs with long cytoplasmic termini (e.g., GluR1) in the deliverable pool in the nearby PSD (Kielland and others 2009; Petrini and others 2009). A recent report shows the number of GluR1 receptors in the deliverable pool to be roughly equal to those at synapses (Kielland and others 2009), providing an explanation for the size of maximal LTP. These experimental findings predict that genetic defects of molecules that disrupt the normal dynamic regulation of Ras and Rap signaling will reduce the capacity of synaptic plasticity and impair learning and memory, which is supported by clinical and genetic studies of a number of mental disorders with cognitive impairments (Eng 2003; Roberts and others 2006; Schubbert and others 2007; Ehninger and others 2008; Orloff and Eng 2008; Levitt and Campbell 2009).
Synaptic Dysfunction in Mental Disorders
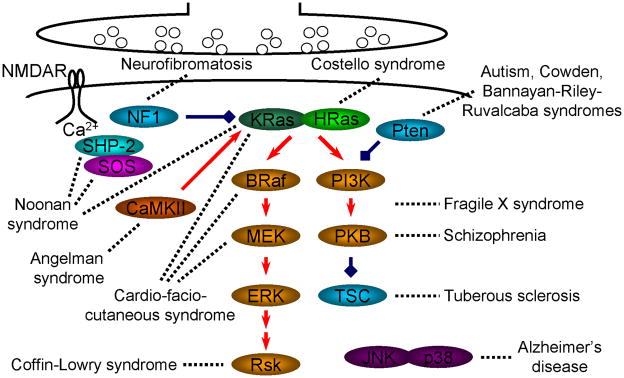
Mental disorders affect about 1 in 17 children and adults. Often the disorders are associated with intellectual impairments; close to half of the patients, representing 2–3% of the human population, have a severe learning disability with an IQ<70. Recent work, particularly genetic screening, has linked genetic defects of various molecules that cause aberrant Ras and Rap signaling with a number of mental disorders with deficits in cognitive functioning and adaptive behaviors (Fig. 6) (Eng 2003; Zhu and others 2004b; Roberts and others 2006; Schubbert and others 2007; Ehninger and others 2008; Orloff and Eng 2008; Levitt and Campbell 2009). These findings underscore the essential role of Ras and Rap signaling in controlling synaptic AMPA-R trafficking during synaptic plasticity (Thomas and Huganir 2004; Gu and Stornetta 2007), the foundation of learning and memory (Kessels and Malinow 2009). Here, we will further discuss the dysfunction of molecules involved in more than a dozen mental diseases associated with learning and memory impairment.
Fig. 6. Ras signaling molecules with mutations linked to mental disorders.
BRaf: V-raf murine sarcoma viral oncogene homolog B; CaMKII: calcium/calmodulin-dependent protein kinase II; ERK: extracellular signal-regulated kinase kinase; JNK: c-Jun amino-terminal kinase; LS: lysosome; MEK: extracellular signal-regulated kinase; NF1: neurofibromin 1, a RasGap; PI3K: phosphoinositide 3-kinase; PKB: protein kinase B; PP2B: protein phosphatase 2B; SHP-2: SH2 domain-containing protein-tyrosine phosphatase PTPN11; RSK: ribosomal S 6 serine/threonine kinase; SOS: son of sevenless, a RasGEF; TSC: tuberous sclerosis complex.
Alzheimer’s disease
Alzheimer’s disease is a progressive neurodegenerative disorder with its neuropathology first reported in 1906 by Alois Alzheimer, a Bavarian psychiatrist (Alzheimer 1907). This is the most common mental disorder affecting more than 30 million people worldwide, one in eight persons over 65 and nearly half of those over 85. The disorder is characterized by early deficits in learning and memory followed by loss of other higher cognitive functions, which correlate with synaptic depression and then frank neuronal degeneration (Price and Sisodia 1998; Haass and Selkoe 2007). During the early stages of cognitive decline, patients exhibit an interesting pathological hallmark, concurrent reduction in synaptic density and increase in synaptic size (DeKosky and Scheff 1990; Scheff and others 2007). Consistent with the enhanced synaptic depression, activities of p38MAPK, JNK, and PP2B, which stimulate synaptic removal of AMPA-Rs (Zhu and others 2002; Zhu and others 2005; Bhattacharyya and others 2009; Kielland and others 2009), are elevated in Alzheimer’s patients and aging animal models (Savage and others 2002; Zhu and others 2004a; Ferrer and others 2005; Norris and others 2005). Beta amyloid (Aβ), a peptide derivative of amyloid precursor protein, is excessively deposited in people with Alzheimer’s disease. Aβ activates p38MAPK and enhances synaptic removal of GluR2/3 AMPA-Rs during LTD (Kamenetz and others 2003; Hsieh and others 2006). Because Aβ does not affect synaptic JNK signaling (Hsieh and others 2006), it remains to be determined whether overproduction of other regulatory molecules, such as p25, a specific cyclin-dependent kinase 5 (Cdk5) activator, in Alzheimer’s patients (Patrick and others 1999; Cruz and Tsai 2004), stimulates JNK and PP2B signaling, and thus enhances synaptic removal of AMPA-Rs and depotentiation during the pathogenesis of the disease.
Angelman syndrome
Angelman syndrome is a neurodevelopmental disorder first identified by Harry Angelman, an English pediatrician in 1965 (Angelman 1965). The disorder, which occurs in one in every 15,000 – 20,000 births, is characterized by severe learning disability and epilepsy, and caused by a variety of genetic abnormalitiesinvolving the chromosome 15q11–13 region, due primarily to defects of the UBE3A gene in that region (Clayton-Smith and Laan 2003). Animal models with a null mutation for UBE3A show learning deficits due to an elevated level of phosphorylation of CaMKII at T286 and T305, which inhibits CaMKII activity (Jiang and others 1998; Weeber and others 2003; van Woerden and others 2007). These results are consistent with the findings of a postnatal onset of symptoms in humans and selective impairment of experience-dependent LTP in animal models (Weeber and others 2003; Yashiro and others 2009), given that CaMKII stimulates postnatal sensory experience-dependent Ras signaling and synaptic delivery of GluR1-containing AMPA-Rs during plasticity (Hayashi and others 2000; Zhu and others 2002; Qin and others 2005; Kielland and others 2009). Interestingly, LTD is also impaired in UBE3A-deficient mice. It remains unclear whether the defect is due directly to the impairment of other signaling molecules that regulate synaptic AMPA-R removal and depression (Yashiro and others 2009), and/or secondary to the impairment of synaptic delivery of GluR1 and slot proteins that may ultimately reduce available GluR2/3 AMPA-Rs for LTD (see above discussion and also (McCormack and others 2006; Lu and others 2009)).
Autism
Autism, first described by Leo Kanner in 1943 (Kanner 1943), encompasses a spectrum of highly heritable autism spectrum disorders with patients displaying impairments in social interaction, language, and repetitive or restrictive behaviors and interests (Geschwind 2009; Levitt and Campbell 2009; Vaccarino and others 2009). This is a very common neurodevelopmental disease with onset prior to the age of three affecting 1 out of every 150 individuals. Children with autism spectrum disorders often have a characteristic large head circumference and develop epilepsy; nearly half of them show severe intellectual disability. Either single gene mutations or a combination of moderate defects of multiple genes may cause autism. Genetic research has linked certain genes, such as receptor tyrosine kinases (RTKs), phosphatase and tensin homolog (PTEN), and SH3 and multiple ankyrin repeat domains protein 3 (Shank3) with a few autism spectrum disorders with high penetrance (Goffin and others 2001; Butler and others 2005; Campbell and others 2006; Durand and others 2007; Moessner and others 2007). The function of these autism-linked genes converge on PI3K and ERK signaling (Thomas and others 2005; McKay and Morrison 2007; Chalhoub and Baker 2009; Levitt and Campbell 2009), which is consistent with the central role of PI3K and ERK signaling in developmental regulation (Manning and Cantley 2007), social interaction (Kwon and others 2006), and synaptic AMPA-R delivery during synaptic plasticity (Zhu and others 2002; Qin and others 2005; Hu and others 2008; Kielland and others 2009).
Cardio-facio-cutaneous syndrome
Cardio-facio-cutaneous syndrome, first reported by Reynolds et al. in 1986 (Reynolds and others 1986), is a sporadic developmental disorder with characteristic craniofacial features, cardiac defects, ectodermal abnormalities and developmental delay (Roberts and others 2006). Although the exact prevalence remains unclear, the syndrome is likely to represent a very rare genetic condition (or an orphan disease) with only 100 patients reported in the literature so far. Genetic screening has associated more than 20 mutations in four different genes, BRaf (the majority of cases), KRas, MEK1, and MEK2 with the syndrome (Niihori and others 2006; Rodriguez-Viciana and others 2006; Makita and others 2007; Zenker and others 2007; Schulz and others 2008; Senawong and others 2008). These findings explain the clinical observations that while there is a wide spectrum of severity in CFC syndrome, all affected individuals have learning difficulties, consistent with the essential role of Ras—BRaf—MEK signaling for regulation of synaptic delivery of AMPA-Rs and synaptic plasticity (Zhu and Malinow 2002; Qin and others 2005; Chen and others 2006; McCormack and others 2006; Kielland and others 2009). Interestingly, the mutations of BRaf and MEK enhance or impair Braf and MEK kinase activity to various degrees (Davies and others 2002; Wan and others 2004; Rodriguez-Viciana and others 2006; Senawong and others 2008). It will be interesting to determine whether the amount of alternation in Ras signaling correlates with the degree of impairment in synaptic plasticity, learning and memory.
Coffin-Lowry syndrome
Coffin-Lowry syndrome, first reported by Coffin et al. and Lowry et al. (Coffin and others 1966; Lowry and others 1971), is a rare X-linked disorder characterized in patients by mental retardation as well as characteristic facial and skeletal abnormalities (Touraine and others 2002; Shalin and others 2006). The disease affects 1 in 40,000–50,000 births with the manifestations milder in females than in males. Genetic studies have associated p90 ribosomal S6 kinase-2 (RSK-2), one of four RSK family kinases, with the disease (Trivier and others 1996; Jacquot and others 1998; Delaunoy and others 2001). Because RSK is a serine-threonine protein kinase relaying the signaling downstream of the Ras—BRaf—MEK—ERK pathway (Thomas and Huganir 2004), it is likely that mutations of RSK impair the Ras-stimulated phosphorylation and synaptic incorporation of AMPA-Rs during plasticity. This notion is supported by recent findings that activation of NMDA-Rs stimulates RSK activity and impaired RSK signaling reduces synaptic AMPA responses (Thomas and others 2005).
Costello syndrome
Costello syndrome is a rare syndrome first described by Costello in 1971 (Costello 1977). This is another orphan disease with just over 100 patients now reported in the literature. Patients with Costello syndrome have mental retardation, cardiac defects, ectodermal and musculoskeletal anomalies (Rauen 2007). Genetic studies have correlated the clinical diagnosis of the syndrome with missense point mutations in the gene HRas with a majority of the alternations occurring in codons 12 and 13 (i.e., G12→S, G12→A, G13→C), implicating a possible gain of function of Ras signaling (Aoki and others 2005; Estep and others 2006; Kerr and others 2006). Given the key role of Ras signaling in controlling synaptic AMPA-R delivery during plasticity (Zhu and Malinow 2002; McCormack and others 2006; Kielland and others 2009), it will be interesting and important to determine whether and how these missense mutations impair synaptic AMPA-R trafficking and plasticity, as well as learning and memory.
Cowden and Bannayan-Riley-Ruvalcaba syndromes
Cowden syndrome is a multi-system disorder first described by Lloyd and Denis in 1963 (Lloyd and Denis 1963), and named after the family in which it was initially reported. The syndrome shows increased risks for malignancies and benign hamartomatous overgrowth and has an estimated prevalence of about one in 200,000 individuals (Hanssen and Fryns 1995; Gustafson and others 2007; Pilarski 2009). About 15% of patients also show developmental problems and mental retardation. Bannayan-Riley-Ruvalcaba syndrome (BRR) is a similar disorder named after the three people first reporting the syndrome: Bannayan, Riley, and Ruvalcaba (Riley and Smith 1960; Bannayan 1971; Zonana and others 1976; Ruvalcaba and others 1980). As with Cowden syndrome, some of BRR patients have learning disabilities (Lynch and others 2009). Whereas ~80% of patients with CS have an identifiable germline mutation in the PTEN gene (Liaw and others 1997; Nelen and others 1997), ~60% of patients with BRRS have detectable coding sequence mutations in PTEN (Marsh and others 1999; Perriard and others 2000; Lachlan and others 2007). BRR and Cowden syndrome may be one condition with BRR having a childhood onset and Cowden syndrome having an adulthood onset. Because PTEN codes a phosphatase that negatively regulates PI3K—AKT signaling, it is possible that loss-of-function mutations of PTEN may cause learning disability in the patients by excessively elevating synaptic PI3K—AKT signaling and enhancing GluR1 trafficking (Qin and others 2005; Hu and others 2008).
Fragile X syndrome
Fragile X syndrome is a common inherited form of mental retardation first linked to a fragile X chromosome by Lubs (Lubs 1969). The syndrome affects approximately one in every 4000 males and one in every 8000 females, and is caused by the loss of FMR1 gene function and fragile X mental retardation protein (FMRP) coded by FMR1, which modulates the function of a large number of other proteins (O’Donnell and Warren 2002; Bagni and Greenough 2005). Patients with fragile X syndrome typically have a moderate learning deficit, and the deficit is particularly pronounced in active, high-level associative learning (Lanfranchi and others 2009). Their IQ declines from ~80 at 5 years of age to ~50 through puberty due to suboptimal intellectual growth (Skinner and others 2005; Hall and others 2008).
Huber and colleagues have first investigated the cellular mechanism of the syndrome (Huber and others 2002). These authors reported that metabotropic glutamate receptor (mGluR)-dependent LTD is modestly up-regulated by ~10–15% in FMR1 knockout mice, a mouse model for fragile X syndrome (Bakker 1994), suggesting impaired mGluR signaling to be responsible for certain forms of impaired learning associated with the syndrome (Pfeiffer and Huber 2009). Recent studies have indicated that NMDA-R-dependent LTP is reduced in FMR1 knockout mice as well (Zhao and others 2005; Lauterborn and others 2007; Meredith and others 2007; Hu and others 2008), due to the selective impairment of signal transduction between Ras and PI3K that impairs GluR1-dependent (but not GluR2L- and GluR4-dependent) plasticity in FMR1 knockout mice (Hu and others 2008). The selective impairment of Ras—PI3K and GluR1-dependent plasticity explains the behavioral observations that FMR1 knockout mice have selective defects in associative learning but no defect in spatial learning (Bakker 1994; D’Hooge and others 1997; Paradee and others 1999; Dobkin and others 2000; Van Dam and others 2000; Frankland and others 2004; Zhao and others 2005), phenocopying GluR1 knockout mice (Zamanillo and others 1999; Schmitt and others 2005), or animals with PI3K signaling inhibited (Lin and others 2001; Chen and others 2005; Horwood and others 2006). The impairment of Ras—PI3K signaling also provides a mechanism for the predominant features associated with fragile X syndrome, including dendritic spine dysmorphogenesis (Comery and others 1997; Irwin and others 2001; Nimchinsky and others 2001; Grossman and others 2006), facial dysmorphism (O’Donnell and Warren 2002), as well as reduced risk of cancer (Schultz-Pedersen and others 2001), given the central role of Ras—PI3K signaling in development and cancer (Hanahan and Weinberg 2000; Govek and others 2005; Schubbert and others 2007).
Neurofibromatosis type 1
Neurofibromatosis type 1 (NF1), formerly known as von Recklinghausen disease (Reynolds and others 2003), is a neurocutaneous disorder with a prevalence of approximately 1 in 3500 people (Costa and Silva 2003; Williams and others 2009). Learning disability is the most commonly reported complication of NF1 in childhood and is present in 40–60% of the cases. The disorder is caused by mutations in the NF1 gene, which encodes neurofibromin. Because neurofibromin contains a GTPase-activating protein (GAP)-related domain and functions as a RasGAP, mutations of NF1 result in hyper-activation of Ras signaling. Genetic or pharmacological suppression of Ras signaling reverses the deficits in LTP and learning in mouse models of NF1 (Costa and others 2002; Li and others 2005; Cui and others 2008). One recent study also suggests that genetic reduction of NF1 expression in inhibitory interneurons, but not excitatory neurons, is responsible for the impaired LTP and learning associated with NF1 (Cui and others 2008). The unanswered question is whether NF1, which is expressed at postsynaptic sites of excitatory neurons (Husi and others 2000), is involved in the regulation of certain forms of synaptic plasticity and learning.
Noonan syndrome
Noonan syndrome, first described by Noonan and Ehmke (Noonan and Ehmke 1963), is a syndrome where patients typically have a short stature, congenital heart defects and distinctive craniofacial features. The incidence of Noonan syndrome has been estimated to be one in 1000–2500 live births and up to 35% of patients exhibit learning disability or mental retardation (Allanson 1987; Schubbert and others 2007; Tidyman and Rauen 2008). Genetic studies have associated multiple genes including PTPN11 (protein product SHP2, a nonreceptor tyrosine phosphatase), SOS1, RAF1, and KRas with Noonan syndrome (Tartaglia and others 2001; Schubbert and others 2006; Pandit and others 2007; Razzaque and others 2007; Roberts and others 2007; Tartaglia and others 2007). The mutations of these genes are likely to impair learning ability via dysregulation of Ras signaling (e.g., see (Pagani and others 2009)). While the majority of the mutations are likely to result in a gain of function of Ras signaling, some of them may reduce the signaling. Further functional studies are needed to determine whether and how mutations of PTPN11, SOS1, RAF1, and KRas impair synaptic AMPA-R trafficking and plasticity and cause the learning defects associated with Noonan syndrome.
Schizophrenia
Schizophrenia, as first characterized by Bleuler and Kraepelin (Bleuler 1911; Kraepelin 1919), tends to begin early in life and cause pervasive impairment in cognitive function, particularly associative learning. Although schizophrenia is a severe psychiatric disorder with a lifetime risk of ~1%, the understanding of the disease remains rudimentary due in part to its subtle, controversial, and diagnostically ineffective neuropathological correlates (Harrison and Weinberger 2005; Hall and others 2009; van Os and Kapur 2009). Clinical observations inspire speculation that schizophrenia may be caused by fine alterations of neuronal circuits and synaptic connections. This hypothesis is supported by the fact that many of the genes associated with susceptibility for schizophrenia, including Akt (encodes AKT, or PKB), COMT, Dysbindin, DAOA, GRM3, DISC1, RGS4, NRG1 (encodes Neuregulin 1, or NRG1) and ErbB4 (encodes ErbB4, an NRG1 receptor of the EGF family), may be involved in direct or indirect regulation of synaptic signaling and plasticity (Harrison and Weinberger 2005; Mei and Xiong 2008; Tan and others 2008; Hall and others 2009). In particular, NRG1—ErbB4 and PP2B signaling have been shown to depotentiate transmission by removing AMPA-Rs with long cytoplasmic termini (Huang and others 2000; Zhu and others 2005; Li and others 2007), whereas PKB signaling is required for synaptic delivery of GluR1-containing AMPA receptors during LTP (Qin and others 2005; Hu and others 2008). Because synaptic GluR1 trafficking is essential for associative learning (Zamanillo and others 1999; Rumpel and others 2005; Schmitt and others 2005; Hu and others 2007; Hu and others 2008; Matsuo and others 2008), these findings provide a potential molecular and cellular mechanism for the prominent defects of associative learning found in schizophrenia.
Tuberous sclerosis
Tuberous sclerosis, f irst described in depth by Bourneville (Bourneville 1880), is a genetic multisystem disorder characterized by widespread benign tumors (hamartomas and hamartias) in multiple organs, and disabling neurologic disorders, including epilepsy, mental retardation, and autism disorder (Kwiatkowski and Manning 2005; Crino and others 2006; Curatolo and others 2008). The disease, caused by mutations of TSC1 (encodes hamartin) and TSC2 (encodes tuberin), affects 1 in 6000 individuals (Kandt and others 1992; European Chromosome 16 Tuberous Sclerosis Consortium 1993; van Slegtenhorst and others 1997). Hamartin and tuberin form an intracellular complex that is inhibited by PKB signaling. The hamartin—tuberin complex, which has a functional GTPase activating protein (GAP) domain in hamartin, inactivates Ras homologue enriched in brain (Rheb), a small G protein of the Ras super family, and thus subsequently inhibits the downstream effector of Rheb, mammalian target of rapamycin (mTOR). The mechanism underlying the mental retardation associated with tuberous sclerosis remains unknown. However, because hamartin and tuberin mediate signal transduction from PKB to mTOR, which is required for phosphorylation and synaptic trafficking of GluR1-containing AMPA-Rs (Qin and others 2005), synaptic and spine plasticity (Tang and others 2002; Tavazoie and others 2005), and associative learning (Tischmeyer and others 2003; Horwood and others 2006), it is possible that mutations of TSC1 and TSC2 cause learning impairment by stimulating abnormally high PKB—mTOR signaling.
X-linked mental retardation
X-linked mental retardation (XLMR) is a common cause of moderate to severe intellectual disability with clearly X-linked pedigrees as first defined by Martin and Bell, and Renpenning et al. (Martin and Bell 1943; Renpenning and others 1962). XLMR is very heterogeneous with more than 60 XLMR genes now identified (Ropers 2006; Lisik and Sieron 2008; Humeau and others 2009). Mutations in some of these genes give rise to clinically distinguishable syndromes, such as fragile X syndrome, Coffin-Lowry syndrome and Rett syndrome, some of which have been discussed above. Many of the XLMR genes may be directly or indirectly (via regulation of the structure of synapses) involved in synaptic transmission and plasticity. For example, mutations of GRIA3, which encodes GluR3, alter the transmission kinetics of AMPA responses (Wu and others 2007); mutations of PAK3, which encodes p21-activated kinase 3, impair LTP and learning (Boda and others 2004; Meng and others 2005); and mutations of OPHN1, which encodes oligophrenin-1 (Billuart and others 1998), destabilize and remove synaptic AMPA receptors (Nadif Kasri and others 2009). Further studies are needed to determine whether mutations of other XLMR genes, such as RSK4 and ARHGEF6 (Yntema and others 1999; Kutsche and others 2000), also affect synaptic signaling and trafficking during synaptic plasticity and learning.
Conclusions and perspectives
Overwhelming evidence indicates that synaptic AMPA-R trafficking, mediated by Ras and Rap signaling, plays a key role in experience-dependent synaptic plasticity, as well as many forms of learning and memory. Happy-medium dynamic regulation of Ras and Rap signaling sustains the optimal ratio of distinct populations of AMPA-Rs (i.e., AMPA-Rs with long or only short cytoplasmic termini), vital for maintaining the highest capacity of plasticity, learning and memory. This notion is supported by genetic findings that disease-linked mutations of a large number of genes functionally converge on various signaling molecules along the Ras and Rap pathways, pushing the Ras and Rap signaling either too high or too low and impairing the signal transduction dynamics. This explains a few common characteristics often shared by mental diseases besides learning disability, including cardiac defects, facial dysmorphism and altered risk for cancer, given the central role of Ras—PI3K signaling in development and cancer (Hanahan and Weinberg 2000; Schubbert and others 2007). On the other hand, different signaling molecules in the Ras and Rap pathways differ in their convergences and divergences. This, together with the cell- and organism-specific mosaic expression patterns of mutations, may be responsible for the diversified syndromes observed in different mental disorders. Obviously, besides impairing synaptic AMPA-R trafficking and plasticity in excitatory neurons, our focus in this review, aberrant Ras and Rap signaling may also cause defects in synaptic plasticity and learning via impairments in inhibitory neurons (e.g., (Woo and others 2007; Cui and others 2008)), and/or even in non-neuronal cells.
This is an exciting time for the research of mental disorders; not only have genetic studies linked a large number of gene mutations with a number of mental diseases, but recent advances of electrophysiology, imaging and behavior analysis techniques allow the direct tests of whether and how these mutations may cause learning disability associated with the diseases (i.e., up- or down-regulating Ras and Rap signaling and synaptic AMPA-R trafficking). So far, traditional transgenic technologies have played a leading role in generating mouse models of disease-linked mutations useful for functional analysis. However, this approach is slow and cost prohibitive for a complete and organized investigation of the very large numbers of mental disorder-associated mutations, for example, more than 15 BRaf mutations associated with cardio-facio-cutaneous syndrome and more than 10 PTEN mutations associated with autism (Schubbert and others 2007; Orloff and Eng 2008). Thus, developing a rapid “transgenic” or gene modification technology becomes an urgent task. Obviously, once animal models are generated for these mutations, the physiology and behavioral analysis should immediately suggest potential pharmacological treatments for these diseases, given particularly that a large number of effective compounds stimulating or inhibiting Ras and Rap signaling are already FDA-approved or currently in clinical trials for treating depression and cancer (Beaulieu and others 2009; Hersey and others 2009; Liu and others 2009; Wagner and Nebreda 2009; Wong 2009). In addition, defining the role of mutations in mental diseases should also suggest effective genetic therapeutic treatments for the patients. Thus, we anticipate a significant improvement of patient welfare in the near future by using this line of translational research.
Acknowledgments
We thank Drs. Jim Casanova, Steve DeKosky, Thurl Harris, Lin Mei, Bettina Winckler, Jun Xia, Ryohei Yasuda, Xiaoxi Zhuan, and members of the Zhu laboratory for discussions and comments. We apologize to those whose work could not be cited owing to space limitations. Work in the laboratory of JJZ is supported by grants from the NIH, DOD and NSFC.
References
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–85. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Allanson JE. Noonan syndrome. J Med Genet. 1987;24:9–13. doi: 10.1136/jmg.24.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer A. über eine eigenartige Erkrankung der Hirnrinde. Allg Zeit Psychiatrie Psych-Gerichtliche Med. 1907;64:146–8. [Google Scholar]
- Andrasfalvy BK, Smith MA, Borchardt T, Sprengel R, Magee JC. Impaired regulation of synaptic strength in hippocampal neurons from GluR1-deficient mice. J Physiol. 2003;552:35–45. doi: 10.1113/jphysiol.2003.045575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelman H. ‘Puppet’ children: A report on three cases. Develop Med Child Neurol. 1965;7:681–8. doi: 10.1111/j.1469-8749.2008.03035.x. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, Tanaka Y, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37:1038–40. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- Ashby MC, De La Rue SA, Ralph GS, Uney J, Collingridge GL, Henley JM. Removal of AMPA receptors (AMPARs) from synapses is preceded by transient endocytosis of extrasynaptic AMPARs. J Neurosci. 2004;24:5172–6. doi: 10.1523/JNEUROSCI.1042-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagal AA, Kao JP, Tang CM, Thompson SM. Long-term potentiation of exogenous glutamate responses at single dendritic spines. Proc Natl Acad Sci U S A. 2005;102:14434–9. doi: 10.1073/pnas.0501956102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–87. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- Bakker C, et al. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Bannayan GA. Lipomatosis, angiomatosis, and macrencephalia. A previously undescribed congenital syndrome. Arch Pathol. 1971;92:1–5. [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–47. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–41. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Biou V, Xu W, Schluter O, Malenka RC. A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nat Neurosci. 2009;12:172–81. doi: 10.1038/nn.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billuart P, Bienvenu T, Ronce N, des Portes V, Vinet MC, Zemni R, et al. Oligophrenin-1 encodes a rhoGAP protein involved in X-linked mental retardation. Nature. 1998;392:923–6. doi: 10.1038/31940. [DOI] [PubMed] [Google Scholar]
- Bleuler E. In: Dementia praecox or the group of schizophrenias. Zinkin J, translator. International Universities Press; New York: 1911. 1950. [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 1973;232:331–56. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boda B, Alberi S, Nikonenko I, Node-Langlois R, Jourdain P, Moosmayer M, et al. The mental retardation protein PAK3 contributes to synapse formation and plasticity in hippocampus. J Neurosci. 2004;24:10816–25. doi: 10.1523/JNEUROSCI.2931-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–25. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Borgdorff AJ, Choquet D. Regulation of AMPA receptor lateral movements. Nature. 2002;417:649–53. doi: 10.1038/nature00780. [DOI] [PubMed] [Google Scholar]
- Bourneville DM. Sclerose tubereuse des circonvolutions cerebrales: idiotie et epilepsie hemiplegique. Arch Neurol (Paris) 1880;1:81–90. [Google Scholar]
- Braithwaite SP, Xia H, Malenka RC. Differential roles for NSF and GRIP/ABP in AMPA receptor cycling. Proc Natl Acad Sci U S A. 2002;99:7096–101. doi: 10.1073/pnas.102156099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TC, Tran IC, Backos DS, Esteban JA. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron. 2005;45:81–94. doi: 10.1016/j.neuron.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–21. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Csicsvari J, Dragoi G, Harris K, Henze D, Hirase H. Homeostatic maintenance of neuronal excitability by burst discharges in vivo. Cereb Cortex. 2002;12:893–9. doi: 10.1093/cercor/12.9.893. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103:16834–9. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–50. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AP, Ohno M, Giese KP, Kuhn R, Chen RL, Silva AJ. Forebrain-specific knockout of B-raf kinase leads to deficits in hippocampal long-term potentiation, learning, and memory. J Neurosci Res. 2006;83:28–38. doi: 10.1002/jnr.20703. [DOI] [PubMed] [Google Scholar]
- Chen HJ, Rojas-Soto M, Oguni A, Kennedy MB. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron. 1998;20:895–904. doi: 10.1016/s0896-6273(00)80471-7. [DOI] [PubMed] [Google Scholar]
- Chen X, Garelick MG, Wang H, Lil V, Athos J, Storm DR. PI3 kinase signaling is required for retrieval and extinction of contextual memory. Nat Neurosci. 2005;8:925–31. doi: 10.1038/nn1482. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–5. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–67. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton-Smith J, Laan L. Angelman syndrome: a review of the clinical and genetic aspects. J Med Genet. 2003;40:87–95. doi: 10.1136/jmg.40.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Barth A. Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron. 2006;49:663–70. doi: 10.1016/j.neuron.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Coffin GS, Siris E, Wegieka LC. Mental retardation with osteocartilaginous anomalies. Am J Dis Child. 1966;112:205–13. [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–62. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc Natl Acad Sci U S A. 1997;94:5401–4. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–21. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM, Federov NB, Kogan JH, Murphy GG, Stern J, Ohno M, et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–30. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- Costa RM, Silva AJ. Mouse models of neurofibromatosis type I: bridging the GAP. Trends Mol Med. 2003;9:19–23. doi: 10.1016/s1471-4914(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Costello JM. A new syndrome: mental subnormality and nasal papillomata. Aust Paediatr J. 1977;13:114–8. doi: 10.1111/j.1440-1754.1977.tb01135.x. [DOI] [PubMed] [Google Scholar]
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–56. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- Cruz JC, Tsai LH. Cdk5 deregulation in the pathogenesis of Alzheimer’s disease. Trends Mol Med. 2004;10:452–8. doi: 10.1016/j.molmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, et al. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–60. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–97. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–68. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Daw MI, Chittajallu R, Bortolotto ZA, Dev KK, Duprat F, Henley JM, et al. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron. 2000;28:873–86. doi: 10.1016/s0896-6273(00)00160-4. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Delaunoy J, Abidi F, Zeniou M, Jacquot S, Merienne K, Pannetier S, et al. Mutations in the X-linked RSK2 gene (RPS6KA3) in patients with Coffin-Lowry syndrome. Hum Mutat. 2001;17:103–16. doi: 10.1002/1098-1004(200102)17:2<103::AID-HUMU2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–13. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, Nagels G, Franck F, Bakker CE, Reyniers E, Storm K, et al. Mildly impaired water maze performance in male Fmr1 knockout mice. Neuroscience. 1997;76:367–76. doi: 10.1016/s0306-4522(96)00224-2. [DOI] [PubMed] [Google Scholar]
- Dobkin C, Rabe A, Dumas R, El Idrissi A, Haubenstock H, Brown WT. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. Neuroscience. 2000;100:423–9. doi: 10.1016/s0306-4522(00)00292-x. [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, et al. Correction of Fragile X Syndrome in Mice. Neuron. 2007;56:955–62. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–7. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–60. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Li W, Fox K, Stryker MP, Silva AJ. Reversing neurodevelopmental disorders in adults. Neuron. 2008;60:950–60. doi: 10.1016/j.neuron.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22:183–98. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;272:19103–6. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–43. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Estep AL, Tidyman WE, Teitell MA, Cotter PD, Rauen KA. HRAS mutations in Costello syndrome: detection of constitutional activating mutations in codon 12 and 13 and loss of wild-type allele in malignancy. Am J Med Genet A. 2006;140:8–16. doi: 10.1002/ajmg.a.31078. [DOI] [PubMed] [Google Scholar]
- European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell. 1993;75:1305–15. doi: 10.1016/0092-8674(93)90618-z. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Gomez-Isla T, Puig B, Freixes M, Ribe E, Dalfo E, et al. Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer’s disease and tauopathies. Curr Alzheimer Res. 2005;2:3–18. doi: 10.2174/1567205052772713. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, et al. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9:417–25. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Poo MM, Dan Y. Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature. 2005;434:221–5. doi: 10.1038/nature03366. [DOI] [PubMed] [Google Scholar]
- Fujii S, Saito K, Miyakawa H, Ito K, Kato H. Reversal of long-term potentiation (depotentiation) induced by tetanus stimulation of the input to CA1 neurons of guinea pig hippocampal slices. Brain Res. 1991;555:112–22. doi: 10.1016/0006-8993(91)90867-u. [DOI] [PubMed] [Google Scholar]
- Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol. 2002;3:663–72. doi: 10.1038/nrm906. [DOI] [PubMed] [Google Scholar]
- Gardner SM, Trussell LO, Oertel D. Correlation of AMPA receptor subunit composition with synaptic input in the mammalian cochlear nuclei. J Neurosci. 2001;21:7428–37. doi: 10.1523/JNEUROSCI.21-18-07428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH. Advances in autism. Annu Rev Med. 2009;60:367–80. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin A, Hoefsloot LH, Bosgoed E, Swillen A, Fryns JP. PTEN mutation in a family with Cowden syndrome and autism. Am J Med Genet. 2001;105:521–4. doi: 10.1002/ajmg.1477. [DOI] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Kong X, Ziff EB. AMPA receptor tetramerization is mediated by Q/R editing. Neuron. 2003;40:763–74. doi: 10.1016/s0896-6273(03)00668-8. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Elisseou NM, McKinney BC, Greenough WT. Hippocampal pyramidal cells in adult Fmr1 knockout mice exhibit an immature-appearing profile of dendritic spines. Brain Res. 2006;1084:158–64. doi: 10.1016/j.brainres.2006.02.044. [DOI] [PubMed] [Google Scholar]
- Gu Y, Stornetta RL. Synaptic plasticity, AMPA-R trafficking, and Ras-MAPK signaling. Acta Pharmacologica Sinica. 2007;28:928–36. doi: 10.1111/j.1745-7254.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Gustafson S, Zbuk KM, Scacheri C, Eng C. Cowden syndrome. Semin Oncol. 2007;34:428–34. doi: 10.1053/j.seminoncol.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hall J, Romaniuk L, McIntosh AM, Steele JD, Johnstone EC, Lawrie SM. Associative learning and the genetics of schizophrenia. Trends Neurosci. 2009;32:359–65. doi: 10.1016/j.tins.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Hall SS, Burns DD, Lightbody AA, Reiss AL. Longitudinal changes in intellectual development in children with Fragile X syndrome. J Abnorm Child Psychol. 2008;36:927–39. doi: 10.1007/s10802-008-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanssen AM, Fryns JP. Cowden syndrome. J Med Genet. 1995;32:117–9. doi: 10.1136/jmg.32.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 5. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450:1195–200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–40. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–7. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, et al. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–5. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersey P, Bastholt L, Chiarion-Sileni V, Cinat G, Dummer R, Eggermont AM, et al. Small molecules and targeted therapies in distant metastatic disease. Ann Oncol. 2009;20(Suppl 6):vi35–40. doi: 10.1093/annonc/mdp254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Horwood JM, Dufour F, Laroche S, Davis S. Signalling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci. 2006;23:3375–84. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–43. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Qin Y, Bochorishvili G, Zhu Y, van Aelst L, Zhu JJ. Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. J Neurosci. 2008;28:7847–62. doi: 10.1523/JNEUROSCI.1496-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–73. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Huang CC, Liang YC, Hsu KS. A role for extracellular adenosine in time-dependent reversal of long-term potentiation by low-frequency stimulation at hippocampal CA1 synapses. J Neurosci. 1999;19:9728–38. doi: 10.1523/JNEUROSCI.19-22-09728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–55. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau Y, Gambino F, Chelly J, Vitale N. X-linked mental retardation: focus on synaptic function and plasticity. J Neurochem. 2009;109:1–14. doi: 10.1111/j.1471-4159.2009.05881.x. [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–9. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am J Med Genet. 2001;98:161–7. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–71. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Jacquot S, Merienne K, De Cesare D, Pannetier S, Mandel JL, Sassone-Corsi P, et al. Mutation analysis of the RSK2 gene in Coffin-Lowry patients: extensive allelic heterogeneity and a high rate of de novo mutations. Am J Hum Genet. 1998;63:1631–40. doi: 10.1086/302153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, et al. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- Jonas P. The time course of signaling at central glutamatergic synapses. News Physiol Sci. 2000;15:83–9. doi: 10.1152/physiologyonline.2000.15.2.83. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, et al. APP processing and synaptic function. Neuron. 2003;37:925–37. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kandt RS, Haines JL, Smith M, Northrup H, Gardner RJ, Short MP, et al. Linkage of an important gene locus for tuberous sclerosis to a chromosome 16 marker for polycystic kidney disease. Nat Genet. 1992;2:37–41. doi: 10.1038/ng0992-37. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–50. [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelly A, Lynch MA. Long-term potentiation in dentate gyrus of the rat is inhibited by the phosphoinositide 3-kinase inhibitor, wortmannin. Neuropharmacology. 2000;39:643–51. doi: 10.1016/s0028-3908(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–25. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B, Delrue MA, Sigaudy S, Perveen R, Marche M, Burgelin I, et al. Genotype-phenotype correlation in Costello syndrome: HRAS mutation analysis in 43 cases. J Med Genet. 2006;43:401–5. doi: 10.1136/jmg.2005.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Kopec CD, Klein ME, Malinow R. Roles of stargazin and phosphorylation in the control of AMPA receptor subcellular distribution. Nat Neurosci. 2009;12:888–96. doi: 10.1038/nn.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–50. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielland A, Bochorishvili G, Corson J, Zhang L, Rosin DL, Heggelund P, et al. Activity patterns govern synapse-specific AMPA-R trafficking between deliverable and synaptic pools. Neuron. 2009;62:84–101. doi: 10.1016/j.neuron.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Liao D, Lau LF, Huganir RL. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron. 1998;20:683–91. doi: 10.1016/s0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- Kolleker A, Zhu JJ, Schupp BJ, Qin Y, Mack V, Borchardt T, et al. Glutamatergic plasticity by synaptic delivery of GluR-B(long)-containing AMPA receptors. Neuron. 2003;40:1199–212. doi: 10.1016/s0896-6273(03)00722-0. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. In: Dementia praecox and paraphrenia. Barclay RM, Robertson GM, translators and editors. R. E. Krieger Pub. Co; Huntington, N.Y: 1919. 1971. [Google Scholar]
- Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci. 2002;22:3005–15. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutsche K, Yntema H, Brandt A, Jantke I, Nothwang HG, Orth U, et al. Mutations in ARHGEF6, encoding a guanine nucleotide exchange factor for Rho GTPases, in patients with X-linked mental retardation. Nat Genet. 2000;26:247–50. doi: 10.1038/80002. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14(Spec No 2):R251–8. doi: 10.1093/hmg/ddi260. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–88. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lachlan KL, Lucassen AM, Bunyan D, Temple IK. Cowden syndrome and Bannayan Riley Ruvalcaba syndrome represent one condition with variable expression and age-related penetrance: results of a clinical study of PTEN mutation carriers. J Med Genet. 2007;44:579–85. doi: 10.1136/jmg.2007.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Xie C, McCormack SG, Chiang HC, Michalak MK, Lin X, et al. Amyotrophic lateral sclerosis 2-deficiency leads to neuronal degeneration in amyotrophic lateral sclerosis through altered AMPA receptor trafficking. J Neurosci. 2006;26:11798–806. doi: 10.1523/JNEUROSCI.2084-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranchi S, Cornoldi C, Drigo S, Vianello R. Working memory in individuals with fragile X syndrome. Child Neuropsychol. 2009;15:105–19. doi: 10.1080/09297040802112564. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Rex CS, Kramar E, Chen LY, Pandyarajan V, Lynch G, et al. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci. 2007;27:10685–94. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Liu L, Wang YT, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron. 2002;36:661–74. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004;43:221–36. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R. Activation of CaMKII in single dendritic spines during long-term potentiation. Nature. 2009;458:299–304. doi: 10.1038/nature07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P, Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest. 2009;119:747–54. doi: 10.1172/JCI37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Woo RS, Mei L, Malinow R. The Neuregulin-1 Receptor ErbB4 Controls Glutamatergic Synapse Maturation and Plasticity. Neuron. 2007;54:583–97. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Cui Y, Kushner SA, Brown RA, Jentsch JD, Frankland PW, et al. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr Biol. 2005;15:1961–7. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–7. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu KT, Leu TH, Chang WC, Gean PW. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron. 2001;31:841–51. doi: 10.1016/s0896-6273(01)00433-0. [DOI] [PubMed] [Google Scholar]
- Lin DT, Huganir RL. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27:13903–8. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, et al. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–87. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisik MZ, Sieron AL. X-linked mental retardation. Med Sci Monit. 2008;14:RA221–9. [PubMed] [Google Scholar]
- Liu B, Liao M, Mielke JG, Ning K, Chen Y, Li L, et al. Ischemic insults direct glutamate receptor subunit 2-lacking AMPA receptors to synaptic sites. J Neurosci. 2006;26:5309–19. doi: 10.1523/JNEUROSCI.0567-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–44. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Lau L, Wei J, Zhu D, Zou S, Sun HS, et al. Expression of Ca2+-permeable AMPA receptor channels primes cell death in transient forebrain ischemia. Neuron. 2004;43:43–55. doi: 10.1016/j.neuron.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Lloyd KM, Denis M. Cowden’s disease: A possible new symptom complex with multiple system involvement. Ann Intern Med. 1963;58:136–42. doi: 10.7326/0003-4819-58-1-136. [DOI] [PubMed] [Google Scholar]
- Lowry B, Miller JR, Fraser FC. A new dominant gene mental retardation syndrome. Association with small stature, tapering fingers, characteristic facies, and possible hydrocephalus. Am J Dis Child. 1971;121:496–500. [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, et al. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–68. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubs HA. A marker X chromosome. Am J Hum Genet. 1969;21:231–44. [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, et al. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–58. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Luthi A, Chittajallu R, Duprat F, Palmer MJ, Benke TA, Kidd FL, et al. Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron. 1999;24:389–99. doi: 10.1016/s0896-6273(00)80852-1. [DOI] [PubMed] [Google Scholar]
- Lynch NE, Lynch SA, McMenamin J, Webb D. Bannayan-Riley-Ruvalcaba syndrome: a cause of extreme macrocephaly and neurodevelopmental delay. Arch Dis Child. 2009;94:553–4. doi: 10.1136/adc.2008.155663. [DOI] [PubMed] [Google Scholar]
- Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–90. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita Y, Narumi Y, Yoshida M, Niihori T, Kure S, Fujieda K, et al. Leukemia in Cardio-facio-cutaneous (CFC) syndrome: a patient with a germline mutation in BRAF proto-oncogene. J Pediatr Hematol Oncol. 2007;29:287–90. doi: 10.1097/MPH.0b013e3180547136. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–4. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Malleret G, Haditsch U, Genoux D, Jones MW, Bliss TV, Vanhoose AM, et al. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell. 2001;104:675–86. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–3. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margrie TW, Brecht M, Sakmann B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch. 2002;444:491–8. doi: 10.1007/s00424-002-0831-z. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Kum JB, Lunetta KL, Bennett MJ, Gorlin RJ, Ahmed SF, et al. PTEN mutation spectrum and genotype-phenotype correlations in Bannayan-Riley-Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet. 1999;8:1461–72. doi: 10.1093/hmg/8.8.1461. [DOI] [PubMed] [Google Scholar]
- Martin JP, Bell J. A pedigree of mental defect showing sex linkage. J Neurol Psychiatry. 1943;6:154–7. doi: 10.1136/jnnp.6.3-4.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Launey T, Mikawa S, Hirai H. Disruption of AMPA receptor GluR2 clusters following long-term depression induction in cerebellar Purkinje neurons. Embo J. 2000;19:2765–74. doi: 10.1093/emboj/19.12.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–7. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack SG, Stornetta RL, Zhu JJ. Synaptic AMPA receptor exchange maintains bidirectional plasticity. Neuron. 2006;50:75–88. doi: 10.1016/j.neuron.2006.02.027. [DOI] [PubMed] [Google Scholar]
- McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26:3113–21. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–52. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Meng Y, Hanna A, Janus C, Jia Z. Abnormal long-lasting synaptic plasticity and cognition in mice lacking the mental retardation gene Pak3. J Neurosci. 2005;25:6641–50. doi: 10.1523/JNEUROSCI.0028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Jia Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron. 2003;39:163–76. doi: 10.1016/s0896-6273(03)00368-4. [DOI] [PubMed] [Google Scholar]
- Meredith RM, Holmgren CD, Weidum M, Burnashev N, Mansvelder HD. Increased threshold for spike-timing-dependent plasticity is caused by unreliable calcium signaling in mice lacking fragile x gene FMR1. Neuron. 2007;54:627–38. doi: 10.1016/j.neuron.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Yamashita S, Kurokawa K, Ohba Y, Nagai T, Miyawaki A, et al. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature. 2001;411:1065–8. doi: 10.1038/35082594. [DOI] [PubMed] [Google Scholar]
- Moessner R, Marshall CR, Sutcliffe JS, Skaug J, Pinto D, Vincent J, et al. Contribution of SHANK3 mutations to autism spectrum disorder. Am J Hum Genet. 2007;81:1289–97. doi: 10.1086/522590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor A, Philips MR. Compartmentalized Ras/MAPK signaling. Annu Rev Immunol. 2006;24:771–800. doi: 10.1146/annurev.immunol.24.021605.090723. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–5. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Iino R, Kobayashi T, Fujiwara T, Ohshima C, Yoshimura A, et al. Single-molecule imaging analysis of Ras activation in living cells. Proc Natl Acad Sci U S A. 2004;101:7317–22. doi: 10.1073/pnas.0401354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadif Kasri N, Nakano-Kobayashi A, Malinow R, Li B, Van Aelst L. The Rho-linked mental retardation protein oligophrenin-1 controls synapse maturation and plasticity by stabilizing AMPA receptors. Genes Dev. 2009;23:1289–302. doi: 10.1101/gad.1783809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelen MR, van Staveren WC, Peeters EA, Hassel MB, Gorlin RJ, Hamm H, et al. Germline mutations in the PTEN/MMAC1 gene in patients with Cowden disease. Hum Mol Genet. 1997;6:1383–7. doi: 10.1093/hmg/6.8.1383. [DOI] [PubMed] [Google Scholar]
- Niihori T, Aoki Y, Narumi Y, Neri G, Cave H, Verloes A, et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet. 2006;38:294–6. doi: 10.1038/ng1749. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci. 2001;21:5139–46. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, et al. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- Noonan JA, Ehmke DA. Associated noncardiac malformations in children with congenital heart disease. J Pediatr. 1963;63:468–70. [Google Scholar]
- Norris CM, Kadish I, Blalock EM, Chen KC, Thibault V, Porter NM, et al. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer’s models. J Neurosci. 2005;25:4649–58. doi: 10.1523/JNEUROSCI.0365-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell TJ, Kandel ER. Low-frequency stimulation erases LTP through an NMDA receptor-mediated activation of protein phosphatases. Learn Mem. 1994;1:129–39. [PubMed] [Google Scholar]
- O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. 2002;25:315–38. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- Omerovic J, Prior IA. Compartmentalized signalling: Ras proteins and signalling nanoclusters. Febs J. 2009;276:1817–25. doi: 10.1111/j.1742-4658.2009.06928.x. [DOI] [PubMed] [Google Scholar]
- Orloff MS, Eng C. Genetic and phenotypic heterogeneity in the PTEN hamartoma tumour syndrome. Oncogene. 2008;27:5387–97. doi: 10.1038/onc.2008.237. [DOI] [PubMed] [Google Scholar]
- Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, et al. The AMPA receptor GluR2 C terminus can mediate a reversible, ATP- dependent interaction with NSF and alpha- and beta-SNAPs. Neuron. 1998;21:99–110. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- Pagani MR, Oishi K, Gelb BD, Zhong Y. The phosphatase SHP2 regulates the spacing effect for long-term memory induction. Cell. 2009;139:186–98. doi: 10.1016/j.cell.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39:1007–12. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94:185–92. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–5. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, et al. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–30. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–26. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–22. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Perriard J, Saurat JH, Harms M. An overlap of Cowden’s disease and Bannayan-Riley-Ruvalcaba syndrome in the same family. J Am Acad Dermatol. 2000;42:348–50. doi: 10.1016/s0190-9622(00)90109-9. [DOI] [PubMed] [Google Scholar]
- Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63:92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Huber KM. The state of synapses in fragile X syndrome. Neuroscientist. 2009;15:549–67. doi: 10.1177/1073858409333075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini A, Malinow R. Critical postsynaptic density 95/disc large/zonula occludens-1 interactions by glutamate receptor 1 (GluR1) and GluR2 required at different subcellular sites. J Neurosci. 2002;22:5387–92. doi: 10.1523/JNEUROSCI.22-13-05387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilarski R. Cowden syndrome: a critical review of the clinical literature. J Genet Couns. 2009;18:13–27. doi: 10.1007/s10897-008-9187-7. [DOI] [PubMed] [Google Scholar]
- Plowman SJ, Muncke C, Parton RG, Hancock JF. H-ras, K-ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc Natl Acad Sci U S A. 2005;102:15500–5. doi: 10.1073/pnas.0504114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DL, Sisodia SS. Mutant genes in familial Alzheimer’s disease and transgenic models. Annu Rev Neurosci. 1998;21:479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, et al. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev. 2005;19:2000–15. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauen KA. HRAS and the Costello syndrome. Clin Genet. 2007;71:101–8. doi: 10.1111/j.1399-0004.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Redman SJ, Crouch MF. The phosphoinositide 3-kinase and p70 S6 kinase regulate long-term potentiation in hippocampal neurons. Neuroscience. 2002;109:531–6. doi: 10.1016/s0306-4522(01)00500-0. [DOI] [PubMed] [Google Scholar]
- Razzaque MA, Nishizawa T, Komoike Y, Yagi H, Furutani M, Amo R, et al. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat Genet. 2007;39:1013–7. doi: 10.1038/ng2078. [DOI] [PubMed] [Google Scholar]
- Renpenning H, Gerrard JW, Zaleski WA, Tabata T. Familial sex-linked mental retardation. Can Med Assoc J. 1962;87:954–6. [PMC free article] [PubMed] [Google Scholar]
- Reynolds JF, Webb MJ, Opitz JM. A new autosomal dominant acrofacial dysostosis syndrome. Am J Med Genet Suppl. 1986;2:143–50. doi: 10.1002/ajmg.1320250618. [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Browning GG, Nawroz I, Campbell IW. Von Recklinghausen’s neurofibromatosis: neurofibromatosis type 1. Lancet. 2003;361:1552–4. doi: 10.1016/s0140-6736(03)13166-2. [DOI] [PubMed] [Google Scholar]
- Riley HD, Smith WR. Macrocephaly, pseudopapilledema and multiple hemangiomata. Pediatrics. 1960;26:293–300. [Google Scholar]
- Roberts A, Allanson J, Jadico SK, Kavamura MI, Noonan J, Opitz JM, et al. The cardiofaciocutaneous syndrome. J Med Genet. 2006;43:833–42. doi: 10.1136/jmg.2006.042796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AE, Araki T, Swanson KD, Montgomery KT, Schiripo TA, Joshi VA, et al. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat Genet. 2007;39:70–4. doi: 10.1038/ng1926. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Tetsu O, Tidyman WE, Estep AL, Conger BA, Cruz MS, et al. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311:1287–90. doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- Ropers HH. X-linked mental retardation: many genes for a complex disorder. Curr Opin Genet Dev. 2006;16:260–9. doi: 10.1016/j.gde.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron. 1997;18:939–50. doi: 10.1016/s0896-6273(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Rumbaugh G, Adams JP, Kim JH, Huganir RL. SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons. Proc Natl Acad Sci U S A. 2006;103:4344–51. doi: 10.1073/pnas.0600084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–8. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Ruvalcaba RH, Myhre S, Smith DW. Sotos syndrome with intestinal polyposis and pigmentary changes of the genitalia. Clin Genet. 1980;18:413–6. doi: 10.1111/j.1399-0004.1980.tb01785.x. [DOI] [PubMed] [Google Scholar]
- Sanderson DJ, Gray A, Simon A, Taylor AM, Deacon RM, Seeburg PH, et al. Deletion of glutamate receptor-A (GluR-A) AMPA receptor subunits impairs one-trial spatial memory. Behav Neurosci. 2007;121:559–69. doi: 10.1037/0735-7044.121.3.559. [DOI] [PubMed] [Google Scholar]
- Savage MJ, Lin YG, Ciallella JR, Flood DG, Scott RW. Activation of c-Jun N-terminal kinase and p38 in an Alzheimer’s disease model is associated with amyloid deposition. J Neurosci. 2002;22:3376–85. doi: 10.1523/JNEUROSCI.22-09-03376.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–8. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- Schmitt WB, Arianpour R, Deacon RM, Seeburg PH, Sprengel R, Rawlins JN, et al. The role of hippocampal glutamate receptor-A-dependent synaptic plasticity in conditional learning: the importance of spatiotemporal discontiguity. J Neurosci. 2004;24:7277–82. doi: 10.1523/JNEUROSCI.1093-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt WB, Sprengel R, Mack V, Draft RW, Seeburg PH, Deacon RM, et al. Restoration of spatial working memory by genetic rescue of GluR-A-deficient mice. Nat Neurosci. 2005;8:270–2. doi: 10.1038/nn1412. [DOI] [PubMed] [Google Scholar]
- Schoepfer R, Monyer H, Sommer B, Wisden W, Sprengel R, Kuner T, et al. Molecular biology of glutamate receptors. Prog Neurobiol. 1994;42:353–7. doi: 10.1016/0301-0082(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Schubbert S, Bollag G, Shannon K. Deregulated Ras signaling in developmental disorders: new tricks for an old dog. Curr Opin Genet Dev. 2007;17:15–22. doi: 10.1016/j.gde.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Schubbert S, Zenker M, Rowe SL, Boll S, Klein C, Bollag G, et al. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38:331–6. doi: 10.1038/ng1748. [DOI] [PubMed] [Google Scholar]
- Schultz-Pedersen S, Hasle H, Olsen JH, Friedrich U. Evidence of decreased risk of cancer in individuals with fragile X. Am J Med Genet. 2001;103:226–30. [PubMed] [Google Scholar]
- Schulz AL, Albrecht B, Arici C, van der Burgt I, Buske A, Gillessen-Kaesbach G, et al. Mutation and phenotypic spectrum in patients with cardio-facio-cutaneous and Costello syndrome. Clin Genet. 2008;73:62–70. doi: 10.1111/j.1399-0004.2007.00931.x. [DOI] [PubMed] [Google Scholar]
- Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci. 2003;23:9220–8. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senawong T, Phuchareon J, Ohara O, McCormick F, Rauen KA, Tetsu O. Germline mutations of MEK in cardio-facio-cutaneous syndrome are sensitive to MEK and RAF inhibition: implications for therapeutic options. Hum Mol Genet. 2008;17:419–30. doi: 10.1093/hmg/ddm319. [DOI] [PubMed] [Google Scholar]
- Shalin SC, Egli R, Birnbaum SG, Roth TL, Levenson JM, Sweatt JD. Signal transduction mechanisms in memory disorders. Prog Brain Res. 2006;157:25–41. doi: 10.1016/s0079-6123(06)57003-7. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–43. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–43. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, et al. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–6. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Shimshek DR, Jensen V, Celikel T, Geng Y, Schupp B, Bus T, et al. Forebrain-specific glutamate receptor B deletion impairs spatial memory but not hippocampal field long-term potentiation. J Neurosci. 2006;26:8428–40. doi: 10.1523/JNEUROSCI.5410-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M, Hooper S, Hatton DD, Roberts J, Mirrett P, Schaaf J, et al. Mapping nonverbal IQ in young boys with fragile X syndrome. Am J Med Genet A. 2005;132:25–32. doi: 10.1002/ajmg.a.30353. [DOI] [PubMed] [Google Scholar]
- Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir RL. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- Tada T, Sheng M. Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol. 2006;16:95–101. doi: 10.1016/j.conb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–8. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- Tan HY, Nicodemus KK, Chen Q, Li Z, Brooke JK, Honea R, et al. Genetic variation in AKT1 is linked to dopamine-associated prefrontal cortical structure and function in humans. J Clin Invest. 2008;118:2200–8. doi: 10.1172/JCI34725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–72. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–8. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- Tartaglia M, Pennacchio LA, Zhao C, Yadav KK, Fodale V, Sarkozy A, et al. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat Genet. 2007;39:75–9. doi: 10.1038/ng1939. [DOI] [PubMed] [Google Scholar]
- Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 2005;8:1727–34. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–83. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Rumbaugh GR, Harrar DB, Huganir RL. Ribosomal S6 kinase 2 interacts with and phosphorylates PDZ domain-containing proteins and regulates AMPA receptor transmission. Proc Natl Acad Sci U S A. 2005;102:15006–11. doi: 10.1073/pnas.0507476102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T, Harding A, Inder K, Plowman S, Parton RG, Hancock JF. Plasma membrane nanoswitches generate high-fidelity Ras signal transduction. Nat Cell Biol. 2007;9:905–14. doi: 10.1038/ncb1615. [DOI] [PubMed] [Google Scholar]
- Tian X, Gotoh T, Tsuji K, Lo EH, Huang S, Feig LA. Developmentally regulated role for Ras-GRFs in coupling NMDA glutamate receptors to Ras, Erk and CREB. Embo J. 2004;23:1567–75. doi: 10.1038/sj.emboj.7600151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidyman WE, Rauen KA. Noonan, Costello and cardio-facio-cutaneous syndromes: dysregulation of the Ras-MAPK pathway. Expert Rev Mol Med. 2008;10:e37. doi: 10.1017/S1462399408000902. [DOI] [PubMed] [Google Scholar]
- Tischmeyer W, Schicknick H, Kraus M, Seidenbecher CI, Staak S, Scheich H, et al. Rapamycin-sensitive signalling in long-term consolidation of auditory cortex-dependent memory. Eur J Neurosci. 2003;18:942–50. doi: 10.1046/j.1460-9568.2003.02820.x. [DOI] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–77. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Toth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci. 1998;1:572–8. doi: 10.1038/2807. [DOI] [PubMed] [Google Scholar]
- Touraine RL, Zeniou M, Hanauer A. A syndromic form of X-linked mental retardation: the Coffin-Lowry syndrome. Eur J Pediatr. 2002;161:179–87. doi: 10.1007/s00431-001-0904-6. [DOI] [PubMed] [Google Scholar]
- Trivier E, De Cesare D, Jacquot S, Pannetier S, Zackai E, Young I, et al. Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature. 1996;384:567–70. doi: 10.1038/384567a0. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Grigorenko EL, Smith KM, Stevens HE. Regulation of cerebral cortical size and neuron number by fibroblast growth factors: implications for autism. J Autism Dev Disord. 2009;39:511–20. doi: 10.1007/s10803-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam D, D’Hooge R, Hauben E, Reyniers E, Gantois I, Bakker CE, et al. Spatial learning, contextual fear conditioning and conditioned emotional response in Fmr1 knockout mice. Behav Brain Res. 2000;117:127–36. doi: 10.1016/s0166-4328(00)00296-5. [DOI] [PubMed] [Google Scholar]
- van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–45. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science. 1997;277:805–8. doi: 10.1126/science.277.5327.805. [DOI] [PubMed] [Google Scholar]
- van Woerden GM, Harris KD, Hojjati MR, Gustin RM, Qiu S, de Avila Freire R, et al. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation. Nat Neurosci. 2007;10:280–2. doi: 10.1038/nn1845. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–8. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–47. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Edwards JG, Riley N, Provance DW, Jr, Karcher R, Li XD, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell. 2008;135:535–48. doi: 10.1016/j.cell.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. Embo J. 1997;16:1909–20. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeber EJ, Jiang YH, Elgersma Y, Varga AW, Carrasquillo Y, Brown SE, et al. Derangements of hippocampal calcium/calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. J Neurosci. 2003;23:2634–44. doi: 10.1523/JNEUROSCI.23-07-02634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–9. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123:124–33. doi: 10.1542/peds.2007-3204. [DOI] [PubMed] [Google Scholar]
- Wong KK. Recent developments in anti-cancer agents targeting the Ras/Raf/MEK/ERK pathway. Recent Pat Anticancer Drug Discov. 2009;4:28–35. doi: 10.2174/157489209787002461. [DOI] [PubMed] [Google Scholar]
- Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Wright N, Glazewski S, Hardingham N, Phillips K, Pervolaraki E, Fox K. Laminar analysis of the role of GluR1 in experience-dependent and synaptic depression in barrel cortex. Nat Neurosci. 2008:1140–2. doi: 10.1038/nn.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Arai AC, Rumbaugh G, Srivastava AK, Turner G, Hayashi T, et al. Mutations in ionotropic AMPA receptor 3 alter channel properties and are associated with moderate cognitive impairment in humans. Proc Natl Acad Sci U S A. 2007;104:18163–8. doi: 10.1073/pnas.0708699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Frerking M, Zhou Q. Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation. Proc Natl Acad Sci U S A. 2008;105:11388–93. doi: 10.1073/pnas.0802978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Riday TT, Condon KH, Roberts AC, Bernardo DR, Prakash R, et al. Ube3a is required for experience-dependent maturation of the neocortex. Nat Neurosci. 2009 doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–6. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]
- Yasuda R, Harvey CD, Zhong H, Sobczyk A, van Aelst L, Svoboda K. Supersensitive Ras activation in dendrites and spines revealed by two-photon fluorescence lifetime imaging. Nat Neurosci. 2006;9:283–91. doi: 10.1038/nn1635. [DOI] [PubMed] [Google Scholar]
- Yntema HG, van den Helm B, Kissing J, van Duijnhoven G, Poppelaars F, Chelly J, et al. A novel ribosomal S6-kinase (RSK4; RPS6KA6) is commonly deleted in patients with complex X-linked mental retardation. Genomics. 1999;62:332–43. doi: 10.1006/geno.1999.6004. [DOI] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, Beattie EC, et al. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J Neurosci. 2007;27:11112–21. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–11. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- Zeke A, Lukacs M, Lim WA, Remenyi A. Scaffolds: interaction platforms for cellular signalling circuits. Trends Cell Biol. 2009;19:364–74. doi: 10.1016/j.tcb.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker M, Lehmann K, Schulz AL, Barth H, Hansmann D, Koenig R, et al. Expansion of the genotypic and phenotypic spectrum in patients with KRAS germline mutations. J Med Genet. 2007;44:131–5. doi: 10.1136/jmg.2006.046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Ko SW, Ding HK, Wu LJ, Zhuo M. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci. 2005;25:7385–92. doi: 10.1523/JNEUROSCI.1520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Tao HW, Poo MM. Reversal and stabilization of synaptic modifications in a developing visual system. Science. 2003;300:1953–7. doi: 10.1126/science.1082212. [DOI] [PubMed] [Google Scholar]
- Zhu JJ. Maturation of layer 5 neocortical pyramidal neurons: amplifying salient layer 1 and layer 4 inputs by Ca2+ action potentials in adult rat tuft dendrites. J Physiol (Lond) 2000;526:571–87. doi: 10.1111/j.1469-7793.2000.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ. Activity level-dependent synapse-specific AMPA receptor trafficking regulates transmission kinetics. J Neurosci. 2009;29:6320–35. doi: 10.1523/JNEUROSCI.4630-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Connors BW. Intrinsic firing patterns and whisker-evoked synaptic responses of neurons in the rat barrel cortex. J Neurophysiol. 1999;81:1171–83. doi: 10.1152/jn.1999.81.3.1171. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Malinow R. Acute versus chronic NMDA receptor blockade and synaptic AMPA receptor delivery. Nat Neurosci. 2002;5:513–4. doi: 10.1038/nn0602-850. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–55. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Lee HG, Casadesus G, Smith MA, Perry G. Oxidative stress signalling in Alzheimer’s disease. Brain Res. 2004a;1000:32–9. doi: 10.1016/j.brainres.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Perry G, Smith MA. Alzheimer’s disease: the two-hit hypothesis. Lancet Neurol. 2004b;3:219–26. doi: 10.1016/S1474-4422(04)00707-0. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Pak D, Qin Y, McCormack SG, Kim MJ, Baumgart JP, et al. Rap2-JNK removes synaptic AMPA receptors during depotentiation. Neuron. 2005;46:905–16. doi: 10.1016/j.neuron.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Zhang W, Son H, Mansuy I, Sobel RA, Seidman J, et al. A selective role of calcineurin aalpha in synaptic depotentiation in hippocampus. Proc Natl Acad Sci U S A. 1999;96:4650–5. doi: 10.1073/pnas.96.8.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonana J, Rimoin DL, Davis DC. Macrocephaly with multiple lipomas and hemangiomas. J Pediatr. 1976;89:600–3. doi: 10.1016/s0022-3476(76)80397-6. [DOI] [PubMed] [Google Scholar]