Abstract
Background
Associations between parental depression and offspring affective and disruptive disorders are well documented. Few genetically informed studies have explored the processes underlying intergenerational associations.
Method
A semi-structured interview assessing DSM-III-R psychiatric disorders was administered to twins (n=1296) from the Australian Twin Register (ATR), their spouses (n=1046) and offspring (n=2555). We used the Children of Twins (CoT) design to delineate the extent to which intergenerational associations were consistent with a causal influence or due to genetic confounds.
Results
In between-family analyses, parental depression was associated significantly with offspring depression [hazard ratio (HR) 1.52, 95% confidence interval (CI) 1.20–1.93] and conduct disorder (CD; HR 2.27, CI 1.31–3.93). Survival analysis indicated that the intergenerational transmission of depression is consistent with a causal (environmental) inference, with a significant intergenerational association in offspring of discordant monozygotic (MZ) twin pairs (HR 1.39, CI 1.00–1.94). Logistic regression analysis suggested that the parental depression–offspring CD association was due to shared genetic liability in the parents and offspring. No intergenerational association was found when comparing the offspring of discordant MZ twins [odds ratio (OR) 1.41, CI 0.63–3.14], but offspring of discordant dizygotic (DZ) twins differed in their rates of CD (OR 2.53, CI 0.95–6.76). All findings remained after controlling for several measured covariates, including history of depression and CD in the twins’ spouses.
Conclusions
The mechanisms underlying associations between parental depression and offspring psychopathology seem to differ depending on the outcome. The results are consistent with a causal environmental role of parental depression in offspring depression whereas common genetic factors account for the association of parental depression and offspring CD.
Keywords: Children of twins, conduct disorder, environmental risk, genetic liability, parental depression
Introduction
Parental depression is associated with a range of adverse offspring outcomes (Connell & Goodman, 2002; Kane & Garber, 2004). In a meta-analysis of the effect of parental depression, Connell & Goodman (2002) reported weighted mean effect sizes (r) of 0.18 and 0.14 for maternal and paternal depression respectively for offspring internalizing problems and 0.17 and 0.16 respectively for offspring externalizing problems. Kane & Garber (2004) reported slightly higher mean effect sizes for paternal depression (0.24 and 0.19 respectively for internalizing and externalizing problems). Although traditional family studies included in these meta-analyses are important for highlighting the risks associated with parental depression, the studies cannot clarify the underlying causal processes.
Models of the transmission of depression (Goodman & Gotlib, 1999) identify environmental and biological factors (e.g. genetics) as important mechanisms. Historically, research has focused on environmental mediation through exposure to the depressed parent directly and/or indirectly (e.g. compromised parenting behaviors). Meta-analytic results suggest that depressed mothers exhibit greater disengagement and negative behavior and less positivity towards their children (Lovejoy et al. 2000). Moreover, many environmental factors have been shown to statistically mediate intergenerational associations of depression (see Goodman & Tully, 2006). Despite support for environmental mediation of intergenerational associations, these studies failed to account for the possibility of shared genetic liability (e.g. risk genes passed down from parent to offspring).
The failure to account for the possibility of genetic transmission across generations is a particularly salient omission given that depression is heritable in both adults (Bierut et al. 1999; Sullivan et al. 2000; Kendler et al. 2001) and children (Eaves et al. 1997; Eley & Stevenson, 1999; Rice et al. 2002). For example, Bierut et al. (1999) found moderate heritability for depression across DSM-III-R and DSM-IV diagnoses for adults. It is important to note that twin studies of depression merely raise the possibility that intergenerational transmission is due to shared genetic liability, but the studies do not prove genetic transmission (Rutter et al. 1999). Research designs equipped to evaluate both genetic and environmental transmission of risk are therefore necessary. There are several study designs, each with their own assumptions and limitations, that can examine both genetic and environmental processes (Rutter et al. 2001; D’Onofrio et al. 2003; Rutter, 2007).
Adoption studies have yielded mixed results. Using indirect measures of depression (e.g. clinical records), Wender et al. (1986) found evidence for genetic transmission whereas von Knorring et al. (1983) did not. A third adoption study yielded a significant genetic effect in the entire sample (Sullivan et al. 2000), but not when males and females were analyzed separately (Cadoret et al. 1985). The results suggest some environmental mediation of the transmission of depression, but the adoption design has several limitations and assumptions, including selective placement, lack of representativeness, and inability to control for prenatal environmental risks (Plomin et al. 2001; Rhee & Waldman, 2002). Using a study that addressed many assumptions of the previous adoption research, Tully et al. (2008) also found that maternal depression was an environmental liability for offspring depression and externalizing problems.
Using a different approach, Rice et al. (2005) conducted an extended twin analysis (the study of twin children and their parents) of the intergenerational transmission of depression and found an environmental effect for maternal report of depressive symptoms but not for cross-informant reports. The extended twin design assumes that the same genetic factors influence depression in both generations (Rutter et al. 2001; D’Onofrio, 2005). A recent Children of Twins (CoT) study (Silberg et al. 2010) indicated direct environmental transmission of depression whereas both genetic and environmental factors accounted for the increased risk of offspring conduct disorder (CD) associated with parental depression. Taken together, these studies generally support environmental mediation of the intergenerational transmission of depression. To gain a better understanding of the underlying mechanisms, however, converging evidence is necessary from a variety of study designs (Rutter et al. 2001). The current study aimed to examine the genetic and environmental mechanisms underlying associations between parental depression and offspring depression and CD in a large, high-risk sample drawn from a community-based twin study using the CoT design, a quasi-experimental design that can elucidate causal processes (Heath et al. 1985; Gottesman & Bertelsen, 1989).
This study is particularly well suited to exploring the processes underlying the intergenerational transmission of psychopathology for several reasons. The study (1) evaluated a high-risk sample with a large number of offspring exposed to parents with diagnosed major depressive disorder (MDD); (2) incorporated sampling weights to allow for approximation of population-based estimates; (3) included many measured characteristics of the adult twins, their spouses and offspring as covariates to rule out the possibility that these characteristics (e.g. assortative mating) explain the intergenerational associations; (4) used semi-structured, diagnostic interviews in both generations; and (5) assessed several offspring outcomes (D’Onofrio et al. 2005; Slutske et al. 2008).
Method
Participants were drawn from the Australian Twin Register (ATR), a volunteer registry recruited through the media, schools and other resources. In the first wave of data collection twins were mailed a questionnaire in 1980–1981 (n=8183, 69% response rate; Jardine & Martin, 1984). The second wave of data collection consisted of another mailed questionnaire in 1988–1989 (n=6327, 83% response rate; Heath & Martin, 1994). Participants were asked to provide the names and addresses of parents, sibling, spouses and biological children. Relatives of the twins (n=14421) were subsequently mailed a similar questionnaire resulting in responses from 60% of relatives, including 3318 spouses of twins (Lake et al. 2000). In the third wave of data collection (1992–1993) a semi-structured telephone interview was administered to twins/spouses (n=5889 individual twins, n=3844 twin spouses, 86% response rate; Heath et al. 1997).
The demographics of the ATR are largely consistent with the population from which they were drawn and several tests for self-selection bias have been conducted, with few detectable differences in risk for psychopathology (Heath et al. 1997; Slutske et al. 1997). The sample is predominantly Caucasian, which is consistent with the Australian population of the birth cohort. For a more detailed description of the ATR see Heath et al. (1997) or Slutske et al. (1997).
Offspring of the adult twins were recruited using a high-risk sampling strategy. First, offspring were selected if either the twin parent or co-twin had a history of (a) alcohol dependence and/or CD, (b) MDD and/or (c) divorce. Second, a control group was selected, consisting of the offspring of a random sample of twin pairs with offspring born between 1964 and 1983 and no history of alcohol dependence/CD, MDD or divorce. Interviews asking permission to contact the offspring were conducted with 1409 adult twins (85% response rate). The offspring were asked to complete a telephone interview and a mailed questionnaire, resulting in completed interview data from 2554 offspring (82% response rate) from 1286 nuclear families nested under 889 twin families. The age of the offspring ranged from 14 to 39 years (mean 25.1 years, S.D.=5.7) and 51% were female (see D’Onofrio et al. 2005; Slutske et al. 2008 for additional sample details).
Sampling weights were created for the offspring dataset, so that the results can generalize to all twins in the ATR (D’Onofrio et al. 2007b; Harden et al. 2007; Slutske et al. 2008). Following standard procedure for data-weighting in twin samples (Heath et al. 1998), multiple logistic regression was used to identify predictors of whether twins (i.e. at least one twin from a pair) from the ATR participated in the CoT study. Psychiatric and demographic characteristics were used as predictors (Harden et al. 2007). Previous studies have detailed the weighting variables and procedure (D’Onofrio et al. 2007b) and conducted sensitivity analyses (Harden et al. 2007; Slutske et al. 2008).
Measures of twin and spouse characteristics
The Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Bucholz et al. 1994) was administered to twin parents and their spouses during the third wave of data collection. The SSAGA assesses lifetime symptoms of alcohol abuse/dependence and related psychiatric disorders, including DSM-III-R MDD and CD. The SSAGA has demonstrated moderate to high inter-rater reliability in previous studies (Bucholz et al. 1994) and yields a diagnosis of DSM-III-R depression based on self-report. Among parents in the current study, the prevalence of MDD in the twins was 37% and in their spouses was 24%. The spousal (tetrachoric) correlation for MDD was relatively low in magnitude (r=0.18, p=0.04), consistent with other reports of assortative mating (Maes et al. 1998).
Additional family and parental characteristics were included in analyses to determine whether measured covariates confound the risks associated with parental depression. Parental history of divorce was coded as 0=no history of divorce/marital separation and 1=history of divorce during the offspring’s lifetime (biological parents). Age at first childbirth was coded continuously for mothers (range 12.1–41.6) and fathers (range 13.9–49.4). Education was coded on a seven-point scale (1=less than 7 years schooling, 2=8–10 years schooling, 3=11–12 years schooling, 4=apprenticeship, diploma, etc., 5=technical/teachers college, 6=university first degree, 7=university postgraduate training). Parental CD was coded as symptom counts of DSM-III-R diagnostic criteria. Parental substance use was coded as 0=no lifetime history and 1=lifetime history of any tobacco or illicit drug use. Alcohol dependence and abuse were coded as symptom counts of DSM-III-R diagnostic criteria for each disorder.
Offspring characteristics
In the CoT study, the SSAGA was administered by a telephone interview to assess lifetime diagnosis of DSM-III-R MDD and a diagnosis of DSM-III-R CD before the age of 18. If offspring did not endorse one of the two core symptoms of MDD (e.g. depressed mood or anhedonia) they were not administered the remaining MDD items. Offspring diagnosis of CD was based on symptoms before the age of 18. Offspring age, age squared, and sex (0=female, 1=male) were also included as covariates in all analyses.
CoT design
The CoT design, an extension of the traditional twin study, helps to separate the intergenerational associations between a predictor and outcomes into three processes: environmental risk factors specific to the risk factor measured, genetic factors that influence the parents and offspring, and environmental confounds that twins share. Most studies compare unrelated individuals (offspring exposed to parental depression to offspring in different families not exposed), which results in a between-family estimate. Between-family estimates include the effect of parental depression and everything else that correlates with depression. In the CoT design, by contrast, researchers compare cousins who are differentially exposed to parental depression by looking at the offspring of twins who are discordant (one co-twin was depressed and the other co-twin was not). This within-extended twin family effect accounts for all environmental factors that influence cousins similarly. The comparison of differentially exposed offspring of monozygotic (MZ) twins is the most rigorous test of causality in the CoT design because the design also controls for all genetic factors from the twin parents that may account for the intergenerational associations. Offspring of MZ twins share 25% of their genetic make-up with each other (socially they are twins, but genetically they are half siblings). The comparison of cousins of discordant dizygotic (DZ) twins controls for less genetic liability (12.5% of their genetic make-up is shared) than the offspring of MZ twins.
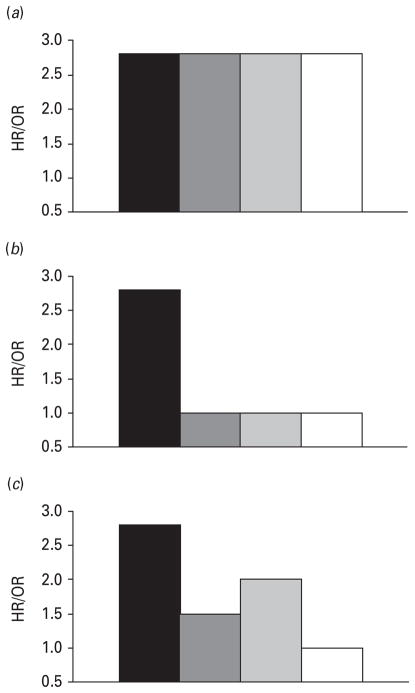
When using a quasi-experimental approach it is helpful to identify the predicted pattern of the results consistent with a causal association and alternative confounding hypotheses (Shadish et al. 2002). Fig. 1 illustrates the magnitude of the parent–offspring associations in various family designs that correspond to different causal processes. If the parent–offspring association remains strong in all comparisons (Fig. 1a), especially when accounting for genetic and environmental factors that influence both generations (by comparing offspring of discordant MZ twins), researchers can infer that the intergenerational association is causal, or environmentally mediated. If, however, the parent–offspring association is eliminated after controlling for background genetic and environmental factors when comparing differentially exposed offspring of MZ twins, then family background factors are implicated (Fig. 1b, c). Examining the association between parents and offspring using the offspring of both MZ and DZ twin pairs helps to identify whether the family background factors are genetic or environmental in origin. If the parent–offspring association is eliminated when comparing differentially exposed offspring of both MZ and DZ twin pairs (Fig. 1b), then environmental background factors are implicated. If the association is eliminated among the offspring of discordant MZ but not discordant DZ twin pairs (Fig. 1c), then genetic background factors are implicated. See D’Onofrio et al. (2005) and Slutske et al. (2008) for more details.
Fig. 1.
Predicted pattern of parent–offspring associations in various family comparisons corresponding to different models of intergenerational transmission. (a) Causal association. Under a causal mode of transmission the intergenerational association will be attenuated to the extent that between-family effects, age, prenatal factors, and twin confounds are controlled for. (b) Environmental confounds. Under a mode of transmission in which environmental confounds account for intergenerational associations, the intergenerational association will be attenuated in all discordant twins, discordant DZ twins and discordant MZ twins to the same extent because the confounding factors would be uncorrelated with genetic risk associated with parental depression. (c) Genetic confounds. Under a genetic mode of transmission the parent–offspring association would vary according to genetic relatedness. In discordant MZ twin pairs the association would be zero because the cousins share the same genetic risk associated with the twin parent’s depression. The parent–offspring association in discordant DZ twin families would be larger because the comparison accounts for less genetic confounds. ■, Unrelated controls;
 , all discordant twins;
, all discordant twins;
 , discordant DZ twins; □, discordant MZ twins.
, discordant DZ twins; □, discordant MZ twins.
Data analysis
Prevalence of MDD/CD among offspring of adult twins
To provide an initial glimpse of the processes underlying the intergenerational associations, the prevalence of offspring MDD and CD was calculated separately for several groups of families. The comparisons were based on the raw data, accounting for the nested, non-independent nature of the data but without using the sampling weights. First, the prevalence of MDD and CD was examined among the offspring of depressed and non-depressed parents in the entire sample (i.e. unrelated individuals) to establish an intergenerational association. Next, the prevalence among offspring of all discordant twins, without regard to zygosity, was estimated. This analysis compares cousins differentially exposed to a depressed parent. Finally, the prevalence among offspring of discordant DZ and MZ twins was calculated separately. Kaplan–Meier estimates were used to estimate the prevalence of offspring MDD given that not all offspring have passed through the risk period and, thus, the data are right censored. That is, some participants who had not yet received a diagnosis would receive a diagnosis sometime after the assessment. Simple percentages were computed for CD diagnosis before the age of 18.
Survival analysis/logistic regression analyses
Cox survival analyses provide statistical tests of the processes underlying the increased risk of depression in the offspring of depressed parents; to account for measured covariates of the adult twins, their spouses and the offspring; and to use the sampling weights. The analyses estimated the risk of offspring MDD associated with parental lifetime history of MDD, given that the assessment of offspring MDD was right censored. Continuous-time survival analysis using the proportional hazards model was used to estimate hazard ratios (HRs), which represent the increased risk of MDD among offspring of parents with MDD. Logistic regression was used to predict offspring CD diagnosis before age 18.
Several models examined the processes underlying the intergenerational associations between parental MDD and offspring MDD and CD. The first model estimated the phenotypic relationships between parental MDD and offspring MDD and CD in the entire sample (which compared unrelated offspring who are differentially exposed to parental depression), while controlling for offspring sex, age, age squared, and spousal MDD (which helped to account for assortative mating). Model 2 estimated the intergenerational association, while controlling statistically for family-level, parental, and offspring-measured covariates. The model therefore assesses the intergenerational association that is independent of the measured covariates. Model 3 broke down intergenerational associations into between- and within-extended twin family effects without regard to twin parent zygosity. The between-family effect was measured as the mean MDD diagnosis across twin parents in the extended family (1=both depressed, 0.5=one twin parent depressed, 0=neither depressed). The estimate therefore measures whether all cousins in an extended family with more twin depression have more problems than offspring in unrelated families where the twins had less depression. The within-family effect was measured as the deviation of each specific twin parent’s depression diagnosis from the mean of the extended family (0=concordant twins, 0.5=twin parent depressed, −0.5=twin aunt/uncle depressed) and assessed whether the offspring exposed to a twin parent with depression had more problems than their cousins whose twin parent did not have depression.
Model 4 further refined the within-extended family effects by examining associations in the offspring of discordant MZ and discordant DZ twins. This model yields two within-extended family estimates. The first is the association between parental depression and offspring functioning within MZ twin pairs. An attenuated parent–offspring association in discordant MZ twin families (the most rigorous test of causal environmental effect) would suggest that family background factors account for greater risk in the offspring of depressed parents. The second estimate is the difference between the within-DZ and within-MZ twin family comparisons, which is based on an interaction between the within-family parameter and zygosity. A greater within-twin family association in DZ compared to MZ twin families would implicate genetic factors because the comparison of offspring of MZ twin pairs controls for more genetic factors. That is, when controlling for more genetic factors (in discordant MZ twin families), the parent–offspring association would be attenuated to a greater degree than in discordant DZ twin pair families. If the difference between parent–offspring associations in MZ and DZ twin families is negligible, environmental selection factors would be suggested because the familial confounds would not be related to genetic risk. D’Onofrio et al. (2005) and Slutske et al. (2008) provide further details concerning the model fitting. Finally, model 5 estimated the between- and within-extended family effects while including the measured covariates.
Results
Prevalence of MDD/CD among offspring of adult twins
The prevalence of MDD and CD among the offspring of depressed and non-depressed parents is displayed in Table 1 for the entire sample and by twin–parent type. Given right censored data, Kaplan–Meier survival estimates at age 30 were used for MDD. It was not possible to use population weights in the Kaplan–Meier analysis. Thus, estimates may be slightly higher than expected in offspring of non-depressed parents because the twin parents in the ‘No diagnosis ’ group may have a history of divorce or antisocial behavior. The results indicate that the offspring of depressed parents have higher rates of MDD than offspring of non-depressed parents (37% and 30% respectively) when comparing unrelated offspring. This pattern remained when the sample was restricted to only comparing discordant twins (regardless of twin zygosity) with the prevalence among offspring of depressed (39%) and non-depressed twins (31%). When calculated separately by twin parent zygosity, the prevalence of offspring MDD did not vary across parental twin type. That is, there was greater prevalence in the offspring of depressed MZ and DZ twins (40% and 38%) as compared to the offspring of their non-depressed MZ and DZ co-twins (29% and 32%). These results provide support for a causal role of parental depression, such that an environmental process underlies the intergenerational association of depression.
Table 1.
Prevalence of offspring depression and conduct disorder (CD) by family type
| Twin parent depression | noffspring | Offspring depressiona (%) | Offspring CD (%) |
|---|---|---|---|
| Entire sample | 2555 | ||
| No diagnosis | 30 | 12 | |
| Diagnosis | 37 | 17 | |
| All discordant twins | 1043 | ||
| No diagnosisb | 31 | 14 | |
| Diagnosisc | 39 | 15 | |
| Discordant dizygotic (DZ) twins | 598 | ||
| No diagnosisb | 32 | 11 | |
| Diagnosisc | 38 | 16 | |
| Discordant monozygotic (MZ) twins | 445 | ||
| No diagnosisb | 29 | 19 | |
| Diagnosisc | 40 | 15 |
Prevalence based on Kaplan–Meier survival estimates at age 30.
Twin parent has a lifetime diagnosis of depression.
Twin parent does not have a lifetime diagnosis of depression.
In the entire sample, CD was also more prevalent among offspring of depressed than non-depressed parents (17% and 12% respectively). By contrast, there was almost no difference when the sample was restricted to offspring of discordant twins (15% among offspring of the depressed twins and 14% among offspring of non-depressed twins). There was greater prevalence among offspring of depressed DZ twins (16%) than those of their non-depressed co-twins (11%). By contrast, there was no difference in CD among the offspring of MZ twins with MDD (15%) and the offspring of the non-depressed co-twins (19%). The results for CD suggest that environmental processes related specifically to parental depression do not underlie the intergenerational association; rather, genetic factors seem to account for part of the relationship.
Survival analysis for depression in offspring
Survival models of offspring depression are presented in Table 2. Model 1 indicates a significant association between twin parent depression and offspring MDD [HR 1.52, 95% confidence interval (CI) 1.20–1.93] when controlling for spouse MDD, twin zygosity and offspring sex and age. The association remained in model 2 (HR 1.42, CI 1.14–1.76), which controlled for measured covariates. Several covariates were also statistically significant, indicating that being a female, family history of divorce, twin parent lower education, and spouse CD were also risk factors for offspring depression. In model 3 the intergenerational association was examined in differentially exposed cousins (regardless of twin parent zygosity), indicating a significant between-family (HR 1.63, CI 1.19–2.23) and within-extended family association (HR 1.39, CI 1.00–1.94). Model 4 specified that the magnitude of the parent–offspring association in discordant MZ twin pairs (HR 1.30, CI 0.80–2.12) was consistent with a specific influence of parental depression, and the difference between the within-extended family estimates among MZ and DZ twin families was small and not statistically significant (b=0.12 logits, p=0.719). Finally, model 5 examined the intergenerational association in discordant twins (regardless of twin parent zygosity) including measured covariates. The results replicate model 3, with slightly attenuated estimates of the between-family (HR 1.52, CI 1.13–2.04) and within-extended twin family (HR 1.31, CI 0.98–1.76) effects.
Table 2.
Parameter estimates of survival models predicting offspring depression
| Parameter | Model 1
|
Model 2
|
Model 3
|
Model 4
|
Model 5
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| b | S.E. | b | S.E. | b | S.E. | b | S.E. | b | S.E. | |
| Twin depression | ||||||||||
| Phenotypic | 0.42** | 0.12 | 0.21** | 0.12 | ||||||
| Between-twin family | 0.49* | 0.16 | 0.49* | 0.16 | 0.42* | 0.15 | ||||
| Within-twin family | 0.33* | 0.17 | 0.26 | 0.25 | 0.27** | 0.15 | ||||
| Difference between within-DZ and within-MZ estimates | 0.12 | 0.33 | ||||||||
| Spouse depression | 0.25* | 0.14 | 0.24** | 0.14 | 0.24** | 0.14 | 0.25** | 0.14 | 0.23** | 0.14 |
| Twin zygosity | 0.10 | 0.12 | 0.11 | 0.12 | 0.10 | 0.12 | 0.10 | 0.12 | 0.11 | 0.11 |
| Offspring sex | −0.65 | 0.14 | −0.55 | 0.15 | −0.65* | 0.14 | −0.65* | 0.14 | −0.67* | 0.14 |
| Offspring age | 0.02 | 0.11 | 0.07 | 0.10 | 0.02 | 0.11 | 0.02 | 0.11 | −0.007 | 0.11 |
| Offspring age squared | −0.001 | 0.002 | −0.002 | 0.002 | −0.001 | 0.002 | −0.001 | 0.002 | −0.001 | 0.002 |
| Parental divorce | 0.29* | 0.15 | 0.25* | 0.12 | ||||||
| Twin covariate | ||||||||||
| Education | 0.07 | 0.05 | 0.08** | 0.04 | ||||||
| Age at first child’s birth | −0.02 | 0.02 | −0.03 | 0.02 | ||||||
| Cigarette use | 0.20 | 0.13 | 0.13 | 0.11 | ||||||
| Alcohol abuse | 0.03 | 0.18 | 0.01 | 0.16 | ||||||
| Alcohol dependence | −0.09 | 0.24 | −0.06 | 0.21 | ||||||
| Illicit drug use | 0.16 | 0.20 | 0.24 | 0.18 | ||||||
| Conduct disorder | −0.003 | 0.06 | −0.01 | 0.06 | ||||||
| Spouse covariate | ||||||||||
| Education | −0.004 | 0.04 | 0.004 | 0.04 | ||||||
| Age at first child’s birth | 0.01 | 0.02 | 0.01 | 0.02 | ||||||
| Cigarette use | 0.13 | 0.16 | 0.14 | 0.16 | ||||||
| Alcohol abuse | −0.20 | 0.14 | −0.19 | 0.14 | ||||||
| Alcohol dependence | 0.06 | 0.19 | 0.05 | 0.18 | ||||||
| Illicit drug use | −0.27 | 0.17 | −0.25 | 0.16 | ||||||
| Conduct disorder | 0.16** | 0.09 | 0.15** | 0.09 | ||||||
DZ, Dizygotic; MZ, monozygotic; S.E., standard error. Analyses were weighted to estimate population-based parameters.
p<0.05,
p<0.10.
Logistic regression for CD in offspring
Estimates of survival models predicting offspring CD are presented in Table 3. Model 1 indicates a significant association between parental depression and offspring CD (HR 2.27, CI 1.31–3.93). Model 2 shows that this association remains (HR 1.80, CI 1.15–2.83), although slightly attenuated, when measured covariates were included. The inclusion of measured covariates indicated that female sex, twin parent alcohol dependence, and twin parent CD were significant risk factors. Model 3 further indicates a statistical trend for a within-family effect (HR 2.25, CI 0.88–5.76) in addition to the between-family effect (HR 2.27, CI 1.45–3.56). Model 4 indicates that the intergenerational association was negligible in discordant MZ families (HR 1.41, CI 0.63–3.14), implicating family background factors. The model also indicated that the intergenerational association varies by the genetic relatedness (i.e. twin zygosity) of the twin parents; the difference between the within-MZ and within-DZ twin family estimates (b=1.98 logits, p=0.070) implicates genetic factors. Model 5 revealed the same pattern of results when accounting for measured covariates, including a negligible association in discordant MZ twin families, and the difference in the within-MZ and within-DZ twin family estimates remained (b=1.83 logits, p=0.018). This pattern of results suggest that although there is a statistical association between parental depression and offspring CD in between-family analyses, genetic factors shared by the twin parent and child seem to account for the association.
Table 3.
Parameter estimates of survival models predicting offspring conduct disorder (CD)
| Parameter | Model 1
|
Model 2
|
Model 3
|
Model 4
|
Model 5
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| b | S.E. | b | S.E. | b | S.E. | b | S.E. | b | S.E. | |
| Twin depression | ||||||||||
| Phenotypic | 0.82* | 0.28 | 0.53* | 0.20 | ||||||
| Between twin family | 0.82* | 0.23 | 0.77* | 0.22 | 0.40** | 0.22 | ||||
| Within twin family | 0.81** | 0.48 | 0.34 | 0.41 | 0.23 | 0.39 | ||||
| Difference between within-DZ and within-MZ estimates | 1.98** | 1.09 | 1.64* | 0.64 | ||||||
| Spouse depression | −0.06 | 0.28 | −0.21 | 0.21 | −0.06 | 0.27 | −0.02 | 0.24 | −0.19 | 0.22 |
| Twin zygosity | 0.29 | 0.22 | 0.19 | 0.17 | 0.29 | 0.21 | 0.20 | 0.19 | 0.13 | 0.18 |
| Offspring sex | 0.39 | 0.28 | 0.53* | 0.21 | 0.39 | 0.28 | 0.43** | 0.25 | 0.55* | 0.21 |
| Offspring age | 0.38* | 0.18 | 0.42* | 0.16 | 0.38* | 0.17 | 0.39* | 0.17 | 0.43* | 0.16 |
| Offspring age squared | −0.01* | 0.003 | −0.008* | 0.003 | −0.01* | 0.003 | −0.008* | 0.003 | −0.008* | 0.003 |
| Parental divorce | 0.18 | 0.21 | 0.18 | 0.21 | ||||||
| Twin covariate | ||||||||||
| Education | −0.06 | 0.06 | −0.05 | 0.06 | ||||||
| Age at first child’s birth | 0.03 | 0.02 | 0.03 | 0.02 | ||||||
| Cigarette use | 0.10 | 0.18 | 0.06 | 0.18 | ||||||
| Alcohol abuse | −0.39 | 0.37 | −0.33 | 0.35 | ||||||
| Alcohol dependence | 1.16* | 0.43 | 1.07* | 0.38 | ||||||
| Illicit drug use | 0.33 | 0.34 | 0.37 | 0.23 | ||||||
| Conduct disorder | 0.19* | 0.08 | −0.06 | 0.11 | ||||||
| Spouse covariate | ||||||||||
| Education | −0.03 | 0.06 | −0.04 | 0.06 | ||||||
| Age at first child’s birth | −0.01 | 0.02 | −0.01 | 0.02 | ||||||
| Cigarette use | 0.28 | 0.25 | 0.27 | 0.25 | ||||||
| Alcohol abuse | 0.19 | 0.19 | 0.16 | 0.19 | ||||||
| Alcohol dependence | 0.33 | 0.24 | 0.38 | 0.24 | ||||||
| Illicit drug use | 0.44 | 0.24 | 0.37 | 0.23 | ||||||
| Conduct disorder | −0.09 | 0.16 | −0.06 | 0.11 | ||||||
DZ, Dizygotic; MZ, monozygotic; S.E., standard error. Analyses were weighted to estimate population-based parameters.
p<0.05,
p<0.10.
Discussion
Consistent with previous, genetically informative studies (Sullivan et al. 2000; Rice et al. 2005) and an adoption study of environmental liability of parental depression (Tully et al. 2008), the current study found support for a causal association between parental depression and offspring MDD. The causal inference is based on the comparisons of offspring of MZ twins discordant for MDD. These offspring, who are as genetically related as half siblings, differed in their own rates of MDD; offspring exposed to parental depression had more MDD than their cousins who were not exposed to parental depression. The association remained even when controlling for spousal depression and other measured covariates.
Some of the environmental processes hypothesized include parenting behaviors, disengagement, and negativity (Lovejoy et al. 2000; Cummings et al. 2005; Foster et al. 2008). Notably, the results of the current study are not contradictory of studies that document moderate genetic influences underlying depression (Sullivan et al. 2000). The CoT analyses in our study seek to explain only the covariation between parental and offspring depression rather than to explain all of the variance in offspring MDD. That is, although they are both genetically influenced, the genetic influences underlying parental depression may be somewhat independent of those underlying offspring depression. Results from genetically informative samples are particularly important to the study of the intergenerational transmission of depression, given the bulk of research relies on designs that cannot disentangle genetic and environmental processes. Only quasi-experimental studies are able to rule out alternative hypotheses that family background characteristics/genetic confounds may account for the intergenerational associations (Rutter, 2007).
In contrast to the results for offspring MDD, family background factors, including genetic confounds, seem to account for the parental MDD–offspring CD association because the magnitude of association varied by the genetic relatedness of the twin parents. That is, the intergenerational association was greater within DZ than MZ twin families. Moreover, the most rigorous test of the causal inference in the design, the comparison of the offspring from discordant MZ twins, did not yield a significant intergenerational association for offspring CD. These results are consistent with a recent CoT study (Silberg et al. 2010) indicating that parental depression–offspring CD associations are, at least partly, genetically mediated. By contrast, Tully et al. (2008) found evidence for environmental mediation of the association between parental depression and offspring externalizing disorders. As parental depression is associated with both affective and disruptive problems (Beardslee et al. 1998; Brennan et al. 2002; Cummings et al. 2005), this area of research would benefit from more studies examining associations between parental depression and a variety of offspring outcomes. Given the dearth of genetically informed studies and inconsistency across study designs and methods, future research is needed to clarify the nature of associations between parental depression and offspring externalizing problems.
Several characteristics of the study make it particularly well suited to investigating parental depression. First, the high-risk sample includes many participants, in both generations, with the diagnoses of interest. Second, many measured covariates were included to control for potential confounding factors. Third, the use of the sampling weights enabled us to approximate population-based estimates. Fourth, psychopathology in both generations was assessed by semi-structured interviews. Fifth, a range of offspring diagnoses were assessed, allowing us to examine the processes underlying the associations between parental MDD and both offspring MDD and CD.
Despite the study’s strengths, there are several limitations to consider. First, the ATR is a community sample and thus may not include individuals with extreme scores for MDD or CD. Second, the high-risk sampling strategy, which included discordant MZ twin pairs, may not be representative because of the high heritability of depression. That is, MZ twin pairs discordant for depression may not be representative of depressed individuals more generally. The use of sampling weights and advanced quantitative approaches for missing data were used to address these limitations as much as possible. The analyses rely on retrospective self-report and are limited to DSM-based criteria. The CoT design also does not take into account the genetic risk associated with depression in the spouse of the twins (Eaves et al. 2005). Although we controlled for measured covariates of the spouses (e.g. MDD and CD), it is impossible to determine whether every salient variable was measured accurately and included in the models. Given that family background factors seem to account for the association of parental MDD and offspring CD, spousal genetic risk factors may be important factors. Because we examined parental depression, in general, we are not able to detect differential processes underlying maternal–offspring and paternal–offspring associations. Finally, we did not explore heterogeneity in parental depression or co-morbid parental psychopathology (e.g. CD). It is well understood that depression is a heterogeneous condition in which many factors, including age at first onset, number of episodes, relapse rate, duration of longest episode, treatment response and demographic variables, contribute to variability (Harrington et al. 1996; Kendler et al. 1999; Carragher et al. 2009). Furthermore, our measure of twin discordance was simply lifetime history versus no lifetime history; thus our measurement was not sensitive to subthreshold levels of depression, which may be important to include in future studies.
The findings of our study will be strengthened by future research and replication in other samples using different designs and different measures of depression and CD. For example, depression may be better understood continuously rather than categorically. Furthermore, there is heterogeneity within both depression and CD that should be explored. It may be that the processes underlying intergenerational associations vary for early versus late-onset disorders (Goodman & Gotlib, 1999). Parental depression has important implications for offspring well-being, but it also often co-occurs with other forms of psychopathology (e.g. antisocial behavior, substance abuse); thus, it will be crucial for future research to explore how co-morbid parental psychopathology affects offspring development.
Our study is consistent with much of the previous research on the intergenerational transmission of depression. The literature regarding parental depression and offspring CD is inconsistent. The current results underscore the importance of identifying, more specifically, the environmental mechanisms responsible for the intergenerational transmission of depression, while highlighting the need for further research to clarify the genetic and environmental processes underlying intergenerational associations between parental MDD and offspring disruptive behavior.
Footnotes
Declaration of Interest
None.
References
- Beardslee WR, Versage EM, Gladstone T. Children of affectively ill parents: a review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:1134–1141. doi: 10.1097/00004583-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Heath AC, Bucholz KK, Dinwiddie SH, Madden PAF, Statham DJ, Dunne MP, Martin NG. Major depressive disorder in a community-based twin sample: are there different genetic and environmental contributions for men and women? Archives of General Psychiatry. 1999;56:557–563. doi: 10.1001/archpsyc.56.6.557. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Hammen C, Katz AR, Le Brocque RM. Maternal depression, paternal psychopathology, and adolescent diagnostic outcomes. Journal of Consulting and Clinical Psychology. 2002;70:1075–1085. doi: 10.1037//0022-006x.70.5.1075. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JIJ, Reich T, Schmidt I, Schuckit MA. A new semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, O’Gorman TW, Heywood E, Troughton E. Genetic and environmental factors in major depression. Journal of Affective Disorders. 1985;9:155–164. doi: 10.1016/0165-0327(85)90095-3. [DOI] [PubMed] [Google Scholar]
- Carragher N, Adamson G, Bunting B, McCann S. Subtypes of depression in a nationally representative sample. Journal of Affective Disorders. 2009;113:88–99. doi: 10.1016/j.jad.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Connell AM, Goodman SH. The association between psychopathology in fathers versus mothers and children’s internalizing and externalizing behavior problems: a meta-analysis. Psychological Bulletin. 2002;128:746–773. doi: 10.1037/0033-2909.128.5.746. [DOI] [PubMed] [Google Scholar]
- Cummings EM, Keller PS, Davies M. Towards a family process model of maternal and paternal depressive symptoms: exploring multiple relations with child and family functioning. Journal of Child Psychology and Psychiatry. 2005;46:479–489. doi: 10.1111/j.1469-7610.2004.00368.x. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM. Children of Twins design. In: Everitt BS, Howel DC, editors. Encyclopedia of Statistics in Behavioral Science. John Wiley & Sons, Ltd; Hoboken, N.J: 2005. pp. 256–258. [Google Scholar]
- D’Onofrio BM, Slutske W, Turkheimer E, Emery R, Heath A, Madden PAF, Martin NJ. The intergenerational transmission of childhood conduct problems: a Children of Twins study. Archives of General Psychiatry. 2007a;64:820–829. doi: 10.1001/archpsyc.64.7.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Turkheimer E, Eaves L, Corey L, Berg K, Solaas MH, Emery R. The role of the Children of Twins design in elucidating causal relations between parent characteristics and child outcomes. Journal of Child Psychology and Psychiatry. 2003;44:1130–1144. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Turkheimer E, Emery RE, Harden KP, Slutske WS, Heath AC, Madden PAF, Martin NG. A genetically informed study of the intergenerational transmission of marital instability. Journal of Marriage and Family. 2007b;69:793–809. doi: 10.1111/j.1741-3737.2007.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Turkheimer E, Emery R, Slutske W, Heath AC, Madden P, Martin N. A genetically informed study of marital instability and its association with offspring psychopathology. Journal of Abnormal Psychology. 2005;114:570–586. doi: 10.1037/0021-843X.114.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves L, Silberg J, Maes H. Revisiting the Children of Twins: can they be used to resolve the environmental effects of dyadic parental treatment on child behavior ? Twin Research and Human Genetics. 2005;8:283–290. doi: 10.1375/1832427054936736. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Silberg JL, Maes HH, Simonoff E, Pickles A, Rutter M, Neale MC, Reynolds CA, Erikson MT, Heath AC, Loeber R, Truett KR, Hewitt JK. Genetics and developmental psychopathology: 2. The main effects of genes and environment on behavioral problems in the Virginia Twin Study of Adolescent Behavioral Development. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1997;38:965–980. doi: 10.1111/j.1469-7610.1997.tb01614.x. [DOI] [PubMed] [Google Scholar]
- Eley TC, Stevenson J. Exploring the covariation between anxiety and depression symptoms: a genetic analysis of the effects of age and sex. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1999;40:1273–1282. [PubMed] [Google Scholar]
- Foster CE, Webster MC, Weissman MM, Pilowsky DJ, Wickramarante P, Rush AJ, Hughes CW, Garber J, Malloy E, Cerda G, Kornstein SG, Alpert JE, Wisniewski SR, Trivedi MH, Fava M, King CA. Course and severity of maternal depression: associations with family functioning and child adjustment. Journal of Youth and Adolescence. 2008;37:906–916. doi: 10.1007/s10964-007-9216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Tully E. Depression in women who are mothes: an integrative model of risk for the development of psychopathology in their sons and daughters. In: Keyes CLM, Goodman SH, editors. Women and Depression: A Handbook for the Social, Behavioral, and Biomedical Sciences. Cambridge University Press; New York: 2006. pp. 241–280. [Google Scholar]
- Gottesman II, Bertelsen A. Confirming unexpressed genotypes for schizophrenia. Archives of General Psychiatry. 1989;46:867–872. doi: 10.1001/archpsyc.1989.01810100009002. [DOI] [PubMed] [Google Scholar]
- Harden KP, Turkheimer E, Emery RE, D’Onofrio BM, Slutske WS, Heath AC, Martin NG. Marital conflict and conduct disorder in Children-of-Twins. Child Development. 2007;78:1–18. doi: 10.1111/j.1467-8624.2007.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington R, Rutter M, Fombonne E. Developmental pathways in depression: multiple meanings, antecedents, and endpoints. Development and Psychopathology. 1996;8:601–616. [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kendler KS, Eaves LJ, Markell D. The resolution of cultural and biological inheritance: informativeness of different relationships. Behavior Genetics. 1985;15:439–465. doi: 10.1007/BF01066238. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden PAF, Martin NG. Assessing the effects of cooperation bias and attrition in behavioral genetic research using data-weighting. Behavior Genetics. 1998;28:415–427. doi: 10.1023/a:1021633127604. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG. Genetic influences on alcohol consumption patterns and problem drinking: results from the Australian NHMRC Twin Panel Follow-Up Survey. Annals of the New York Academy of Sciences. 1994;708:72–85. doi: 10.1111/j.1749-6632.1994.tb24699.x. [DOI] [PubMed] [Google Scholar]
- Jardine R, Martin NG. Causes of variation in drinking habits in a large twin sample. Acta Geneticae Medicae et Gemellologiae. 1984;33:435–450. doi: 10.1017/s0001566000005882. [DOI] [PubMed] [Google Scholar]
- Kane P, Garber J. The relations among depression in fathers, children’s psychopathology, and father-child conflict: a meta-analysis. Clinical Psychology Review. 2004;24:339–360. doi: 10.1016/j.cpr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychological Medicine. 2001;31:605–616. doi: 10.1017/s0033291701003907. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Clinical characteristics of major depression that predict risk of depression in relatives. Archives of General Psychiatry. 1999;56:322–327. doi: 10.1001/archpsyc.56.4.322. [DOI] [PubMed] [Google Scholar]
- Lake RI, Eaves LJ, Maes HH, Heath AC, Martin NG. Further evidence against the environmental transmission of individual differences in neuroticism from a collaborative study of 45,850 twins and relatives on two continents. Behavior Genetics. 2000;30:223–233. doi: 10.1023/a:1001918408984. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behavior: a meta-analytic review. Clinical Psychology Review. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Maes HHM, Neale MC, Kendler KS, Hewitt JK, Silberg JL, Foley DL, Meyer JM, Rutter M, Simonoff E, Pickles A, Eaves LJ. Assortative mating for major psychiatric diagnoses in two population-based samples. Psychological Medicine. 1998;28:1389–1401. doi: 10.1017/s0033291798007326. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn Ge, McGuffin P. Behavior Genetics. 4. Worth Publishers; New York: 2001. [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128:490–529. [PubMed] [Google Scholar]
- Rice F, Harold GT, Thapar A. The genetic aetiology of childhood depression: a review. Journal of Child Psychology and Psychiatry. 2002;43:65–79. doi: 10.1111/1469-7610.00004. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Thapar A. The link between depression in mothers and offspring: an extended twin analysis. Behavior Genetics. 2005;35:565–577. doi: 10.1007/s10519-005-5432-0. [DOI] [PubMed] [Google Scholar]
- Rutter M. Proceeding from observed correlation to causal inference: the use of natural experiments. Perspectives on Psychological Science. 2007;2:377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Pickles A, Murray R, Eaves LJ. Testing hypotheses on specific environmental causal effects on behavior. Psychological Bulletin. 2001;127:291–324. doi: 10.1037/0033-2909.127.3.291. [DOI] [PubMed] [Google Scholar]
- Rutter M, Silberg J, O’Connor T, Simonoff E. Genetics and child psychiatry: I. Advances in quantitative and molecular genetics. Journal of Child Psychology and Psychiatry. 1999;40:3–18. [PubMed] [Google Scholar]
- Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. Houghton Mifflin; New York: 2002. [Google Scholar]
- Silberg JL, Maes H, Eaves LJ. Genetic and environmental influences on the transmission of parental depression to children’s depression and conduct disturbance: an extended Children of Twins study. Journal of Child Psychology and Psychiatry. 2010;51:734–744. doi: 10.1111/j.1469-7610.2010.02205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, D’Onofrio BM, Turkheimer E, Emery RE, Harden KP, Heath AC, Martin NG. Searching for an environmental effect of parental alcoholism on offspring alcohol use disorder: a genetically informed study of children of alcoholics. Journal of Abnormal Psychology. 2008;117:534–551. doi: 10.1037/a0012907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Heath AC, Dinwiddie SH, Madden PA, Bucholz KK, Dunne MP, Statham DJ, Martin NG. Modeling genetic and environmental influences in the etiology of conduct disorder: a study of 2,682 adult twin pairs. Journal of Abnormal Psychology. 1997;106:266–279. doi: 10.1037//0021-843x.106.2.266. [DOI] [PubMed] [Google Scholar]
- Sullivan P, Neale M, Kendler K. Genetic epidemiology of major depression: review and meta-analysis. American Journal Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Tully E, Iacono W, McGue M. An adoption study of parental depression as an environmental liability for adolescent depression and childhood disruptive disorders. American Journal of Psychiatry. 2008;165:1148–1154. doi: 10.1176/appi.ajp.2008.07091438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Knorring AL, Cloninger CR, Bohman M, Sigvardsson S. An adoption study of depressive disorders and substance abuse. Archives of General Psychiatry. 1983;40:943–950. doi: 10.1001/archpsyc.1983.01790080025003. [DOI] [PubMed] [Google Scholar]
- Wender PH, Kety SS, Rosenthal D, Schulsinger F, Ortman J, Lunde I. Psychiatric disorders in the biological and adoptive families of adopted individuals with affective disorders. Archives of General Psychiatry. 1986;43:923–929. doi: 10.1001/archpsyc.1986.01800100013003. [DOI] [PubMed] [Google Scholar]