Abstract
Background
While much is known about the effect of APOE alleles on fasting lipid concentrations, less is known about the effect of APOE alleles on postprandial triglyceridemia or the triglyceride response to fenofibrate.
Methods and Results
We evaluated the effects of the APOE locus on fasting and postprandial triglyceride concentrations as part of the Genetics of Lipid Lowering and Diet Network (GOLDN) study. Participants were evaluated following a high-fat meal challenge before (N=1072) and after 3 weeks of daily treatment with 160 mg of fenofibrate (N=738). Mixed models adjusted for gender, age, waist circumference and family relationship were used to examine the association of the ε4 carrier and ε2 carrier status versus ε3 homozygotes with fasting triglycerides and the area under the curve (AUC) for triglycerides during the high-fat meal challenge. Compared to the ε3/ε3 genotype, ε2 carriers had on average higher fasting triglyceride concentrations (130.5 mg/dL vs. 109.3 mg/dL, P<0.001). After fenofibrate treatment, the APOE genotype differences persisted in the fasting state (ε2 carriers: 85.1 mg/dL vs. ε3/ε3: 75.9 mg/dL, P<0.05). Carriers of the ε4 allele had significantly higher fasting triglyceride concentrations only pre-fenofibrate (120.9 mg/dL vs. 109.3 md/dL, P=0.008). APOE alleles did not have an effect on response to fenofibrate. Postprandial triglycerides were significantly higher for ε2 carriers versus ε3 homozygotes (but not ε4 carriers) both before and after fenofibrate treatment (P=0.01 and P=0.005, respectively).
Conclusions
APOE polymorphisms are important determinants of triglyceride concentrations, especially in the fasting state.
Keywords: apolipoproteins, lipids, genetics
Introduction
Elevated plasma triglyceride concentrations contribute to increased risk of cardiovascular disease (CVD) directly. Additionally, hypertriglyceridemia is often associated with other CVD risk factors such as obesity, metabolic syndrome, proinflammatory biomarkers, and type 2 diabetes mellitus 1. After a meal, over 90% of the circulating triglycerides are broken down and absorbed in intestinal cells where they are subsequently re-processed, packaged and secreted in chylomicrons 2. Additionally, fatty acids synthesized by the liver are converted to triglycerides and transported to the blood via very-low-density lipoprotein (VLDL) particles 2. The Apolipoprotein E (APOE) gene is a key mediator of both triglyceride and cholesterol metabolism. APOE binds to receptors on the liver to help mediate clearance of chylomicrons and VLDLs (triglyceride rich lipoproteins or TRLs) from the bloodstream 3–5. There have been over 30 APOE variants characterized of which many are associated with rare forms of hyperlipoproteinemia 6. In this study we focus on three common APOE protein isoforms (ε2, ε3, and ε4) caused by two functional polymorphisms that differ in physiological structure and affect the efficiency of triglyceride-rich lipoprotein clearance 3. The APOE ε2 allele is associated with higher fasting concentrations of triglycerides, whereas the ε4 allele has been linked to hypercholesterolemia and elevated levels of low-density lipoprotein (LDL)7;8. The APOE ε4 allele has also been associated with increased risk for myocardial infarction or other coronary risk in some 9–11 but not all studies12. Although much is known about the effect of APOE polymorphisms on fasting cholesterol and triglyceride concentrations 13, much less is known about the APOE gene effect in the postprandial state, particularly postprandial triglyceridemia. For example, one study found that among obese women the APOE ε4 polymorphism did not affect postprandial triglyceridemia, but the sample size was limited (N=93) 14.
A small number of studies have reported the association between APOE alleles and response to fenofibrate treatment, though with conflicting results 15–17. Whether APOE alleles would modify the effect of fenofibrate therapy on postprandial triglyceridemia has not been reported. In this large study we propose to test whether APOE genotypes are associated with postprandial triglyceridemia before and after treatment with fenofibrate. The effect of the interaction between APOE genotypes and fenofibrate concentration on postprandial triglyceridemia will be considered. We hypothesize APOE ε2 and ε4 carriers compared to ε3 homozygotes will have larger responses to fenofibrate and have higher postprandial triglyceridemia. A secondary hypothesis is postprandial triglyceride response to fenofibrate will be modified by APOE genotypes.
Methods
Study Population
The Genetics of Lipid Lowering and Diet Network (GOLDN) study was designed to identify genes that determine response of lipids to two interventions, one to raise lipids (ingestion of high-fat meal, referred to as postprandial lipemia challenge or PPL) and one to lower lipids (fenofibrate treatment 160 mg/day for 3 weeks). The GOLDN Study ascertained and recruited families from the NHLBI Family Heart Study from two genetically homogenous centers, Minneapolis, MN and Salt Lake City, UT who were self-reported to be white. In each case, only families with at least two siblings were recruited and only participants who did not take lipid-lowering agents (pharmaceuticals or nutraceuticals) for at least 4 weeks prior to the initial visit were included. To meet the sample size goals and increase relative pairs, participants were offered the option of doing either part of the protocol, high-fat meal and/or fenofibrate (TriCor, Abbott Laboratories) treatment. The fenofibrate treatment protocol included a second PPL challenge following treatment. For the pre- and post-fenofibrate analyses, the number of participants with complete data on triglyceride concentrations, APOE genotype and demographics were 1072 and 738, respectively. Detailed design and methodology of the study have been published 18.
Briefly, the PPL challenge protocol included an 8 hour fast and 24 hour abstinence from alcohol followed by ingestion of a high-fat meal formulated according to the protocol of Patsch et al 19. Specifically, participants were given 700 kilocalories/m2 of body surface area in the form of a high-fat milk shake with protein, carbohydrate, and fat content in the ratio of 3%, 14%, and 83% respectively. Participants were instructed to consume the shake within 15 minutes. Blood samples were drawn immediately before (fasting), 3.5, and 6 hours after ingestion of the high-fat meal. The study protocol was approved by the Institutional Review Board for human studies at the University of Minnesota, University of Utah, and Tufts University/New England Medical Center. Informed consent was obtained on all participants.
Biochemical Measurements
Triglycerides were measured using a glycerol blanked enzymatic method (Trig/GB, Roche Diagnositcs Corporation, Indianapolis In) on the Roche /Hitachi 911 Automatic analyzer. Fenofibric acid, the active moiety of fenofibrate, was measured by HPLC 20.
Genotyping
Genomic DNA was extracted from blood samples and purified using commercial Puregene reagents (Gentra System, Inc., Minneapolis, MN) following the manufacturer’s instructions. APOE genotyping was completed using the 5’ nuclease allelic discrimination TaqMan assay with ABI 7900HT system (Applied Biosystems, Foster City, CA) for the C130R (rs429358) and R176C (rs7412) polymorphisms under manufacturer suggested conditions. APOE genotypes were then called based on the guidelines of Hixson and Vernier 21.
Statistical Methods
Hardy-Weinberg equilibrium testing was completed for each SNP among a sample including 1 participant chosen at random from each of 200 families in GOLDN. One-way ANOVA and chi square tests were used to examine differences in characteristics of the study population for continuous and categorical traits by APOE genotype group. APOE was coded as a categorical variable with values ε3/ε3, ε2 carrier, and ε4 carrier. Persons with the ε2/ε4 genotype were excluded from the analysis in order to distinguish the distinct role of each allele and also because of the low frequency of this allele combination (N=48). Using the freely available software Quanto we had > 80% power (α=0.05) to see an effect size of at least R2=0.01 under a dominant model for either the ε4 or ε2 alleles (reference ε3/ε3) on postprandial triglycerides in our post-fenofibrate treatment analysis (N=738)22. Here R2 represents the marginal proportion of Y (postprandial triglycerides) explained by the genetic effect.
The difference in fasting triglyceride concentration (FTG) was calculated for all participants who completed both the pre- and post-fenofibrate PPL challenge and the average percent change in FTG was calculated and compared by genotype group at the univariate and multivariable level. All outcome variables were log transformed to meet the assumption of normality of residuals for linear modeling, and outliers (defined as > 4 standard deviations from the mean) were removed. A constant was added to percent change in FTG to make all values positive for the log transformation. Mixed models were used to test the association of APOE genotype group with FTG, percent change in FTG, and area under the curve (AUC) for triglycerides during the PPL challenge (measurements at 0, 3.5, and 6 hours) calculated using the trapezoidal rule. These models were adjusted for fixed effects including age, gender, and waist circumference with family ID included as a random variable. Including family as a random variable in the model induces a compound symmetry covariance structure. The method has been validated and has improved type 1 error rate compared to within cluster re-sampling methods 23. We felt adjustment for family was sufficient to control for potential allele frequency differences between groups and we did not adjust for population substructure in our analysis. The potential for gender differences in triglyceride response was examined by including a gender by genotype interaction term in the mixed models. We examined AUC triglycerides with and without adjustment for FTG concentration (0 hour measurement). The model for post-fenofibrate AUC triglycerides was additionally adjusted for area under the serum fenofibric acid concentration curve measured at 0, 3.5, and 6 hours following the dose of fenofibrate taken immediately before the second PPL challenge. Finally, an APOE genotype by AUC for serum fenofibric acid concentration interaction term was modeled to test if APOE alleles would modify the effect of fenofibrate therapy on postprandial triglyceridemia. Statistical analysis as carried out in SAS® (Version 9.1.2). The LSMEANS statement within the mixed model procedure was employed to estimate mean triglyceride concentrations and AUC triglycerides by genotype group adjusted for the covariates discussed above. These means are presented in the tables and figures.
Results
The two polymorphisms in APOE, C130R and R176C, that determined ε2, ε3, and ε4 allele genotypes were in Hardy-Weinberg equilibrium with P=0.15 and P=0.35, respectively. The genotyping rate for each variant was >99%. Among the GOLDN study population participating in the pre-fenofibrate treatment PPL challenge, age, gender, and waist circumference were not different by APOE genotype group after univariate comparisons while FTG concentrations were significantly different with P=0.02 (Table 1). The same comparisons yielded similar results for the participants in the post-fenofibrate treatment PPL challenge group; however, differences in FTG concentrations by genotype group were no longer present. Additionally, AUC for serum fenofibric acid concentration curve was not different by genotype group. FTG decreased on average −29.0%, −26.4%, and −34.4% after 3 weeks of fenofibrate treatment for the ε3/ε3 genotype, ε4, and ε2 carriers, respectively, with marginal significance (P=0.05) at the univariate level (Table 1). After adjusting for age, gender, waist circumference, baseline FTG and family relationship, the percent change in FTG after fenofibrate treatment was −35.2%, −33.0%, and −38.1% for ε3/ε3, ε4 carriers, and ε2 carriers, respectively. This difference was not statistically significant between any of the genotype groups.
Table 1.
Characteristics of the GOLDN study population that participated in the postprandial lipemia (PPL) challenge before and after treatment with fenofibrate160 mg/day by APOE genotype group.
| ε3/ε3 | ε4 Carrier | ε2 Carrier | P‡ | |
|---|---|---|---|---|
| Pre-Fenofibrate PPL | N=666 | N=289 | N=117 | |
| Age (y) | 49.2±17 | 46.9±15 | 48.4±16 | 0.12 |
| Gender (%women) | 52% | 54% | 41% | 0.06 |
| Waist Circumference (cm) | 96.1±16 | 96.6±18 | 98.8±13 | 0.24 |
| Fasting Triglycerides (FTG) (mg/dL) | 131.03±96 | 142.1±97 | 155.4±91 | 0.02 |
| Post-Fenofibrate PPL* | N=454 | N=203 | N=81 | |
| Age (y) | 49.7±17 | 47.5±15 | 49.0±16 | 0.27 |
| Gender (%women) | 50% | 55% | 40% | 0.06 |
| Waist Circumference (cm) | 96.8±16 | 97.2±17 | 99.7±13 | 0.31 |
| Fasting Triglycerides (FTG) (mg/dL) | 85.5±51 | 91.7±50 | 97.2±48 | 0.09 |
| Avg % change in FTG | −29.0% | −26.4% | −34.4% | 0.05 |
| Fenofibric Acid AUC† | 69.5±33 | 67.0±31 | 74.8±34 | 0.20 |
GOLDN participants were given the option to participate in the PPL challenge without fenofibrate or in the PPL challenge before and after 3-weeks daily treatment with 160 mg of fenofibrate. Thus samples in pre-fenofibrate PPL study overlap with those in the post-fenofibrate PPL study but are not exactly the same
Area under the serum fenofibric acid concentration curve measured at 0, 3.5 and 6 hours following the dose of fenofibrate taken immediately before the PPL challenge calculated using the trapezoidal rule, unit is arbitrary
P-values were derived from tests of the null hypothesis that no group is different using a one-way anova for continuous traits or the chi-square test for categorical variables
Estimated average FTG concentration by APOE genotype after adjustment for covariates both pre- and post-fenofibrate treatment is shown in Table 2. Compared to the ε3/ε3 genotype reference group, ε2 carriers had, on average, higher FTG concentrations both before and after fenofibrate treatment. The difference in FTG between ε2 carriers and ε3 homozygotes was more marked and more statistically significant before fenofibrate treatment (P=0.0008 vs. P=0.04), though fewer participants are included in the post-fenofibrate treatment estimates. Treatment with fenofibrate attenuated the APOE ε4 genotypic effects on FTG: carriers of the ε4 allele had significantly higher FTG only pre-fenofibrate (P=0.008, Table 2). The effect of APOE alleles on FTG before and after fenofibrate was not modified by gender.
Table 2.
Adjusted fasting triglyceride (FTG) concentrations by APOE genotype group before and after fenofibrate treatment (160 mg/day).
| ε3/ε3 | ε4 Carrier | ε2 Carrier | |
|---|---|---|---|
| Pre-Fenofibrate | N=666 | N=289 | N=117 |
| FTG * (mg/dL) | 109.3 (104, 115) | 120.9 (113, 129)‡b | 130.5 (118, 144)‡c |
| Post-Fenofibrate | N=454 | N=203 | N=81 |
| FTG*† (mg/dL) | 75.9 (72,80) | 81.9 (76,88) | 85.1 (77, 95) ‡a |
Values are least squares means (95% CIs) adjusted for age, gender, waist circumference, and family relationship
Additionally adjusted for area under the serum fenofibric acid curve
Significantly higher fasting triglyceride concentration when compared to the reference genotype (ε3/ε3). Superscripts a, b and c signify P<0.05, P<0.01and P<0.001, respectively
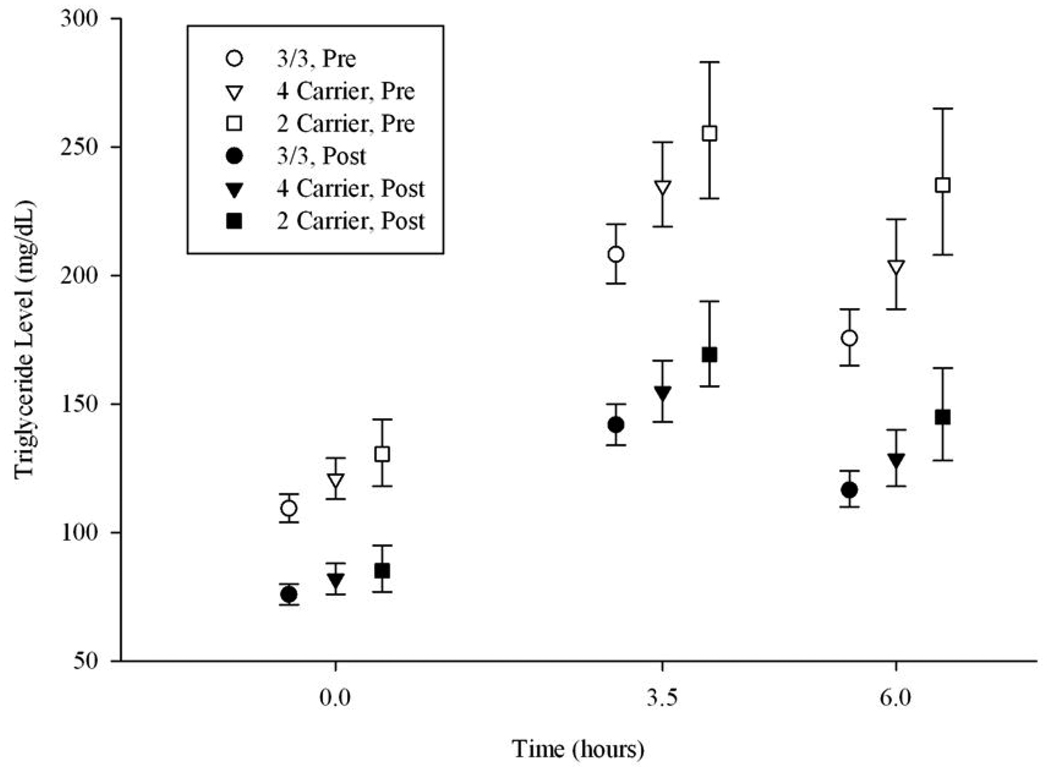
Average triglyceride concentrations estimated at 0, 3.5 and 6 hours after the high-fat meal by genotype group both before and after fenofibrate treatment are depicted in Figure 1. The 95% confidence intervals show the most variation for the ε2 carriers, a finding most likely due to the smaller sample size of that group (see Table 1). The figure depicts that postprandial triglyceride concentrations peak at the 3.5-hour measurement and start to decline by hour 6. At the sixth hour, on average, the concentrations remain greater than fasting concentrations. Estimated AUC for triglycerides during the PPL challenge before and after fenofibrate treatment by genotype group is presented in Table 3. We present models both adjusted and unadjusted for FTG. When our mixed models were unadjusted for FTG both the ε2 and ε4 allele carrier status was associated with higher AUC triglycerides before and after fenofibrate treatment with P<0.05. However, inclusion of FTG concentration in our models attenuated the effect of APOE alleles. Specifically, after adjustment for fasting triglycerides carrying the APOE ε2 allele was still associated with higher postprandial triglycerides (P=0.01 and P=0.0047 before and after fenofibrate treatment, respectively) but the effect of the APOE ε4 allele was removed. Like FTG concentration, the effect of APOE alleles on postprandial triglycerides before and after fenofibrate was not modified by gender. Finally, we report no interaction between APOE alleles and AUC serum fenofibric acid concentration on postprandial triglycerides when we include adjustment for fasting triglycerides.
Figure.
Average triglyceride concentrations by APOE genotype for the pre (N=1072) and post (N=738) fenofibrate treatment postprandial lipemia challenge
Table 3.
Area under the curve (AUC) for triglycerides measured at 0, 3.5 and 6 hours after the postprandial lipemia challenge by APOE genotype group before and after 3 weeks fenofibrate treatment (160 mg/day).
| ε3/ε3 | ε4 Carrier | ε2 Carrier | |
|---|---|---|---|
| Pre-Fenofibrate | N=666 | N=289 | N=117 |
| AUC* | 1077.7 (1022, 1136) | 1210.3 (1127, 1299) §e | 1340.5 (1211, 1484) §f |
| AUC*‡ | 1095.1 (1072, 1119) | 1127.7 (1095, 1162) | 1161.9 (1112, 1214)§d |
| Post-Fenofibrate | N=454 | N=203 | N=81 |
| AUC*† | 727.3 (689, 767) | 798.3 (742, 858) §d | 866.2 (778, 965)§e |
| AUC*†‡ | 738.7 (720, 757) | 754.6 (730, 779) | 793.9 (756, 832) §e |
Values are least squares means (95% CIs) with arbitrary unit adjusted for age, gender, waist circumference, and family relationship
additionally adjusted for area under the serum fenofibric acid curve
additionally adjusted for fasting (0 hour) triglyceride concentration
Significantly higher AUC when compared to the reference genoytype (ε3/ε3). Superscripts d, e and f signify d) P<0.05, e) P<0.005, f) P<0.0001
Discussion
It could be argued that studies of the non-fasting state are most relevant to human health as most humans spend more hours in the non-fasting state (up to 8 h after the last meal) than in the fasting state (more than 8 h after the last meal) 24. We report persons carrying the APOE ε2 allele are exposed to significantly higher triglyceride concentrations regardless of the metabolic state compared to persons with the most common ε3/ε3 genotype. Elevated triglyceride concentration is diagnosed in the fasting state and the Adult Treatment Panel III of the National Cholesterol Education Program has suggested four fasting triglyceride strata in the context of assessment of risk for cardiovascular disease: normal (< 150 mg/dL), borderline high (150–199 mg/dL), high (200–499 mg/dL) and very high (≥ 500 mg/dL). Treatment is suggested for fasting triglycerides ≥150 mg/dL 25. On average, before fenofibrate treatment ε2 carriers from GOLDN come the closest to having borderline high triglycerides with adjusted fasting mean 130.5 mg/dL (95% CI 118–144) and have a greater postprandial response to a high-fat meal (Figure 1). Exposure to elevated triglyceride concentrations is clinically important. Growing evidence links excess circulating remnant TRL particles (the products of lipolytic degradation of TRLs) to oxidative stress and subsequent atherosclerosis 26–28. These results suggest that screening for the APOE ε2 allele may provide useful information for targeting dietary fat restrictions for future prevention of CVD especially in high risk populations such as those with a family history of CVD.
Our results agree with other studies of fasting and non-fasting triglycerides. Several studies report increased triglyceride concentrations in the fasting state for ε2 and ε4 carriers 11;13;29;30, and another large study (N=9060) of a Danish population reports similar results for the non-fasting state 24. A meta-analysis of 45 populations in 17 countries reported the ε2/ε2, ε2/ε3, and ε3/ε4 genotypes, but not the ε4/ε4 genotype (in reference to the ε3/ε3 genotype) were associated with higher plasma triglyceride concentrations 13. Though the association of the ε2 allele with higher FTG concentrations is consistent in the literature, results vary more for ε4. In the Turkish Heart study, the ε2 allele, but not the ε4 allele, was associated with higher FTG concentrations in men, but no association of either allele was found in women 31. Another study reported lower FTG concentrations with the ε4/ε4 genotype 32.
There are fewer and mostly small studies (N<100) of postprandial triglycerides, especially among healthy individuals. In a study of 66 non-diabetic patients with the metabolic syndrome who consumed a 60 g fat load, carriers of ε2 or ε4 had a 6.2 greater odds (95% CI 1.4–16, P=0.01) of postprandial hypertriglyceridemia at 4 hours compared to ε3/ε3 homozygotes33. In two other studies of the postprandial state the ε4 allele was associated with higher triglyceride concentrations in patients with a form of hyperlipidemia 34;35, however the ε2 and ε4 alleles were not related to higher postprandial triglycerides in patients with non-insulin-dependent diabetes mellitus 36. Finally, in the largest study (N=407), carrying the ε2 allele and to a lesser extent carrying the ε4 allele was associated with 21.2% and 11.5% higher postprandial triglyceride concentrations at 6 hours (P<0.01 and p=0.05, respectively) in healthy young male adults from the European Atherosclerosis Research Study with and without a family history of myocardial infarction 37.
All of the studies of postprandial triglycerides we have mentioned adjusted for FTG, however results presented were not for AUC outcomes. In our study of AUC for triglycerides we present models both adjusted and unadjusted for FTG concentration (Table 3). While the postprandial AUC measurement incorporates the FTG in the calculation, the magnitude of the AUC is determined by both the FTG and the physiological response to the high fat meal. We and others have shown APOE alleles are associated with FTG measures and we wanted to focus on the postprandial state. Thus, to reach this end we concluded it necessary to adjust for the effects of baseline and focus on those results. We show the effect of APOE alleles on AUC for triglycerides is greatly attenuated after adjustment for FTG concentration and we conclude differences before adjustment are largely driven by baseline effects (Table 3).
The GOLDN study is different from other studies of fasting and postprandial triglycerides in that plasma was measured before and after three weeks of fenofibrate treatment. We report no difference in percent change in FTG concentration after fenofibrate treatment by APOE genotype group. This result has been reported previously in the GOLDN study 38. The effect of APOE allele status on response to fibrates varies in the literature. A study among hypertriglyceridemic patients (N=292) treated with fenofibrate for three months reported ε2 allele carriers have a better response to fenofibrate on all lipid parameters than ε3 or ε4 carriers15. Another study among patients with primary hypertriglyceridemia or mixed hyperlipidemia (N=136), reported the reduction in fasting triglyceride concentration after fenofibrate treatment (200 mg/day for 6 months) for ε3, ε4, and ε2, allele carriers was 36%, 33%, and 53%, respectively (P=0.033) 16. Conversely, the APOE genotype was not associated with plasma TG response to at least three years fenofibrate treatment (200 mg/day) among type 2 diabetics from the Diabetes Atherosclerosis Intervention Study (N=155) 17. The first study mentioned did not adjust for baseline measures while our study and the latter two did. We consider it important to adjust for baseline FTG in these models because it has been noted in the literature that change in triglyceride concentration after fibrates is dependent on baseline levels 39–41. Differing results even after adjustment for baseline measures may be due to variability in the underlying study populations and/or small sample size.
Finally, in the GOLDN population we were able to examine whether APOE genotypes modify the effect of fenofibrate treatment on postprandial triglyceridemia. There was no difference in the association of AUC serum fenofibric acid concentration with AUC postprandial triglycerides for ε2 carriers or ε4 carriers versus ε3 homozygotes when our model was adjusted for fasting triglyceride concentration. Future larger studies may consider the same interactive effect as our sample may have been small to identify the interaction (N=738).
The current study strengthens the evidence that the APOE ε2 allele is associated with higher triglyceride concentrations. This effect is consistent across different physiological states, i.e., fasting and after ingesting a high-fat meal. These observations are consistent with reports that the ε2 allele is associated with defective clearance of the ε2-containing remnant lipoproteins that are triglyceride-rich due to defective interaction with the VLDL/chylomicron cell receptor 5;42. The effect of the ε4 allele on triglyceride concentrations is not as strong as the ε2 allele, although we report it is associated with higher fasting triglyceride concentrations. In conclusion, APOE polymorphisms are important determinants of triglyceride concentrations in the fasting state. Because hypertriglyceridemia is an important risk factor for CVD and ε2 alleles are associated with higher triglyceride concentrations, future studies should determine if APOE genotype is useful for the prevention of this lipid disorder.
Elevated plasma triglyceride concentrations have been associated with an increased risk of cardiovascular disease (CVD). A key mediator of triglyceride metabolism is the apolipoprotein E (APOE) gene. While much is known about the association of APOE alleles (ε2, ε3, and ε4) with fasting triglyceride concentrations, much less is known about the association of these alleles with postprandial blood triglyceride concentrations. We evaluated the effects of the APOE locus on postprandial triglyceride concentrations as part of the Genetics of Lipid Lowering and Diet Network (GOLDN) study. This population is unique as participants were evaluated following a high-fat meal challenge before (N=1072) and after 3 weeks of daily treatment with 160 mg of fenofibrate (N=738). We report that APOE polymorphisms are important correlates of triglyceride concentrations. Importantly, APOE ε2 alleles are associated with higher triglycerides in the fasting and postprandial state before and after treatment with fenofibrate. Exposure to elevated triglyceride concentrations is clinically important, as growing evidence links excess circulating triglyceride rich lipoprotein remnant particles to oxidative stress and subsequent atherosclerosis. Future studies should determine if the APOE genotype is useful for the early prevention of hypertriglyceridemia especially among high risk groups such as those with a family history of CVD.
Acknowledgements
We are grateful to the staff of the GOLDN study for the assistance in data collection and management.
Grant/funding Support: This study was funded by National Heart Lung and Blood Institute grant number U01HL072524-04 and National Institute of Neurological Disorders and Stroke grant number T32NS054584.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Hodis HN, Mack WJ, Krauss RM, Alaupovic P. Pathophysiology of triglyceride-rich lipoproteins in atherothrombosis clinical aspects. Clin Cardiol. 1999;22:II15–II20. doi: 10.1002/clc.4960221404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan G, Al Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ. 2007;176:1113–1120. doi: 10.1503/cmaj.060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dergunov AD. Apolipoprotein E structure and substrate and receptor-binding activities of triglyceride-rich human plasma lipoproteins in normo- and hypertriglyceridemia. Biochemistry (Mosc) 2004;69:720–737. doi: 10.1023/b:biry.0000040195.34986.93. [DOI] [PubMed] [Google Scholar]
- 4.Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155:487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- 5.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 6.de Knijff P, van den Maagdenberg AM, Frants RR, Havekes LM. Genetic heterogeneity of apolipoprotein E and its influence on plasma lipid and lipoprotein levels. Hum Mutat. 1994;4:178–194. doi: 10.1002/humu.1380040303. [DOI] [PubMed] [Google Scholar]
- 7.Utermann G. Apolipoprotein E polymorphism in health and disease. Am Heart J. 1987;113:433–440. doi: 10.1016/0002-8703(87)90610-7. [DOI] [PubMed] [Google Scholar]
- 8.Utermann G, Kindermann I, Kaffarnik H, Steinmetz A. Apolipoprotein E phenotypes and hyperlipidemia. Hum Genet. 1984;65:232–236. doi: 10.1007/BF00286508. [DOI] [PubMed] [Google Scholar]
- 9.Song Y, Stampfer MJ, Liu S. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann Intern Med. 2004;141:137–147. doi: 10.7326/0003-4819-141-2-200407200-00013. [DOI] [PubMed] [Google Scholar]
- 10.Bennet AM, Di Angelantonio E, Ye Z, Wensley F, Dahlin A, Ahlbom A, Keavney B, Collins R, Wiman B, de Faire U, Danesh J. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298:1300–1311. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 11.Wilson PW, Myers RH, Larson MG, Ordovas JM, Wolf PA, Schaefer EJ. Apolipoprotein E alleles, dyslipidemia, and coronary heart disease. The Framingham Offspring Study. JAMA. 1994;272:1666–1671. [PubMed] [Google Scholar]
- 12.Ward H, Mitrou PN, Bowman R, Luben R, Wareham NJ, Khaw KT, Bingham S. APOE genotype, lipids, and coronary heart disease risk: a prospective population study. Arch Intern Med. 2009;169:1424–1429. doi: 10.1001/archinternmed.2009.234. [DOI] [PubMed] [Google Scholar]
- 13.Dallongeville J, Lussier-Cacan S, Davignon J. Modulation of plasma triglyceride levels by apoE phenotype: a meta-analysis. J Lipid Res. 1992;33:447–454. [PubMed] [Google Scholar]
- 14.Vansant G, Mertens A, Muls E. Determinants of postprandial lipemia in obese women. Int J Obes Relat Metab Disord. 1999;23 Suppl 1:14–21. doi: 10.1038/sj.ijo.0800790. [DOI] [PubMed] [Google Scholar]
- 15.Brisson D, Ledoux K, Bosse Y, St Pierre J, Julien P, Perron P, Hudson TJ, Vohl MC, Gaudet D. Effect of apolipoprotein E, peroxisome proliferator-activated receptor alpha and lipoprotein lipase gene mutations on the ability of fenofibrate to improve lipid profiles and reach clinical guideline targets among hypertriglyceridemic patients. Pharmacogenetics. 2002;12:313–320. doi: 10.1097/00008571-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Christidis DS, Liberopoulos EN, Kakafika AI, Miltiadous GA, Cariolou M, Ganotakis ES, Mikhailidis DP, Elisaf MS. The effect of apolipoprotein E polymorphism on the response to lipid-lowering treatment with atorvastatin or fenofibrate. J Cardiovasc Pharmacol Ther. 2006;11:211–221. doi: 10.1177/1074248406293732. [DOI] [PubMed] [Google Scholar]
- 17.Foucher C, Rattier S, Flavell DM, Talmud PJ, Humphries SE, Kastelein JJ, Ayyobi A, Pimstone S, Frohlich J, Ansquer JC, Steiner G. Response to micronized fenofibrate treatment is associated with the peroxisome-proliferator-activated receptors alpha G/C intron7 polymorphism in subjects with type 2 diabetes. Pharmacogenetics. 2004;14:823–829. doi: 10.1097/00008571-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Corella D, Arnett DK, Tsai MY, Kabagambe EK, Peacock JM, Hixson JE, Straka RJ, Province M, Lai CQ, Parnell LD, Borecki I, Ordovas JM. The -256T>C polymorphism in the apolipoprotein A-II gene promoter is associated with body mass index and food intake in the genetics of lipid lowering drugs and diet network study. Clin Chem. 2007;53:1144–1152. doi: 10.1373/clinchem.2006.084863. [DOI] [PubMed] [Google Scholar]
- 19.Patsch JR, Miesenbock G, Hopferwieser T, Muhlberger V, Knapp E, Dunn JK, Gotto AM, Jr, Patsch W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb. 1992;12:1336–1345. doi: 10.1161/01.atv.12.11.1336. [DOI] [PubMed] [Google Scholar]
- 20.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab. 2002;48:171–180. [PubMed] [Google Scholar]
- 21.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 22.Gauderman WJ, Morrison JM. QUANTO 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies. 2009 http://hydra.usc.edu/gxe.
- 23.Chung WK, Patki A, Matsuoka N, Boyer BB, Liu N, Musani SK, Goropashnaya AV, Tan PL, Katsanis N, Johnson SB, Gregersen PK, Allison DB, Leibel RL, Tiwari HK. Analysis of 30 genes (355 SNPS) related to energy homeostasis for association with adiposity in European-American and Yup'ik Eskimo populations. Hum Hered. 2009;67:193–205. doi: 10.1159/000181158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frikke-Schmidt R, Nordestgaard BG, Agerholm-Larsen B, Schnohr P, Tybjaerg-Hansen A. Context-dependent and invariant associations between lipids, lipoproteins, and apolipoproteins and apolipoprotein E genotype. J Lipid Res. 2000;41:1812–1822. [PubMed] [Google Scholar]
- 25.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 26.Kannel WB, Vasan RS. Triglycerides as vascular risk factors: new epidemiologic insights. Curr Opin Cardiol. 2009;24:345–350. doi: 10.1097/HCO.0b013e32832c1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace JP, Johnson B, Padilla J, Mather K. Postprandial lipaemia, oxidative stress and endothelial function: a review. Int J Clin Pract. 2010;64:389–403. doi: 10.1111/j.1742-1241.2009.02146.x. [DOI] [PubMed] [Google Scholar]
- 28.Havel RJ. Remnant lipoproteins as therapeutic targets. Curr Opin Lipidol. 2000;11:615–620. doi: 10.1097/00041433-200012000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Luc G, Bard JM, Arveiler D, Evans A, Cambou JP, Bingham A, Amouyel P, Schaffer P, Ruidavets JB, Cambien F. Impact of apolipoprotein E polymorphism on lipoproteins and risk of myocardial infarction. The ECTIM Study. Arterioscler Thromb. 1994;14:1412–1419. doi: 10.1161/01.atv.14.9.1412. [DOI] [PubMed] [Google Scholar]
- 30.Tiret L, de Knijff P, Menzel HJ, Ehnholm C, Nicaud V, Havekes LM. ApoE polymorphism and predisposition to coronary heart disease in youths of different European populations. The EARS Study. European Atherosclerosis Research Study. Arterioscler Thromb. 1994;14:1617–1624. doi: 10.1161/01.atv.14.10.1617. [DOI] [PubMed] [Google Scholar]
- 31.Mahley RW, Palaoglu KE, Atak Z, Dawson-Pepin J, Langlois AM, Cheung V, Onat H, Fulks P, Mahley LL, Vakar F. Turkish Heart Study: lipids, lipoproteins, and apolipoproteins. J Lipid Res. 1995;36:839–859. [PubMed] [Google Scholar]
- 32.Sing CF, Davignon J. Role of the apolipoprotein E polymorphism in determining normal plasma lipid and lipoprotein variation. Am J Hum Genet. 1985;37:268–285. [PMC free article] [PubMed] [Google Scholar]
- 33.Cardona F, Morcillo S, Gonzalo-Marin M, Tinahones FJ. The apolipoprotein E genotype predicts postprandial hypertriglyceridemia in patients with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90:2972–2975. doi: 10.1210/jc.2004-1912. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi J, Saito Y, Taira K, Hikita M, Takahashi K, Bujo H, Morisaki N, Saito Y. Effect of apolipoprotein E3/4 phenotype on postprandial triglycerides and retinyl palmitate metabolism in plasma from hyperlipidemic subjects in Japan. Atherosclerosis. 2001;154:539–546. doi: 10.1016/s0021-9150(00)00464-0. [DOI] [PubMed] [Google Scholar]
- 35.Reiber I, Mezo I, Kalina A, Palos G, Romics L, Csaszar A. Postprandial triglyceride levels in familial combined hyperlipidemia. The role of apolipoprotein E and lipoprotein lipase polymorphisms. J Nutr Biochem. 2003;14:394–400. doi: 10.1016/s0955-2863(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 36.Reznik Y, Morello R, Pousse P, Mahoudeau J, Fradin S. The effect of age, body mass index, and fasting triglyceride level on postprandial lipemia is dependent on apolipoprotein E polymorphism in subjects with non-insulin-dependent diabetes mellitus. Metabolism. 2002;51:1088–1092. doi: 10.1053/meta.2002.34696. [DOI] [PubMed] [Google Scholar]
- 37.Dallongeville J, Tiret L, Visvikis S, O'Reilly DS, Saava M, Tsitouris G, Rosseneu M, DeBacker G, Humphries SE, Beisiegel U. Effect of apo E phenotype on plasma postprandial triglyceride levels in young male adults with and without a familial history of myocardial infarction: the EARS II study. European Atherosclerosis Research Study. Atherosclerosis. 1999;145:381–388. doi: 10.1016/s0021-9150(99)00069-6. [DOI] [PubMed] [Google Scholar]
- 38.Smith JA, Arnett DK, Kelly RJ, Ordovas JM, Sun YV, Hopkins PN, Hixson JE, Straka RJ, Peacock JM, Kardia SL. The genetic architecture of fasting plasma triglyceride response to fenofibrate treatment. Eur J Hum Genet. 2008;16:603–613. doi: 10.1038/sj.ejhg.5202003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farnier M, Salko T, Isaacsohn JL, Troendle AJ, Dejager S, Gonasun L. Effects of baseline level of triglycerides on changes in lipid levels from combined fluvastatin + fibrate (bezafibrate, fenofibrate, or gemfibrozil) Am J Cardiol. 2003;92:794–797. doi: 10.1016/s0002-9149(03)00885-3. [DOI] [PubMed] [Google Scholar]
- 40.Kraja AT, Province MA, Straka RJ, Ordovas JM, Borecki IB, Arnett DK. Fenofibrate and Metabolic Syndrome. Endocr Metab Immune Disord Drug Targets. 2010 doi: 10.2174/187153010791213047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott R, O'Brien R, Fulcher G, Pardy C, D'Emden M, Tse D, Taskinen MR, Ehnholm C, Keech A. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–498. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisgraber KH, Innerarity TL, Mahley RW. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982;257:2518–2521. [PubMed] [Google Scholar]