Abstract
Background
Patient-reported measures include preferences and reports about care received, health behaviors, and outcomes of care (patient satisfaction and health-related quality of life). These measures are a core aspect of health care, but there is much to be learned about how to use them to improve clinical practice.
Method
We specify linkages among different patient-reported measures and focus upon the prospects and challenges for use of patient-reported outcomes in clinical practice.
Results
Patient-reported measures are important throughout the continuum of patient care. At the initial visit, patient-reported outcomes provide information about what is important to the patient, the patient's current behaviors, and the patient's baseline health-related quality of life. At subsequent visits, patient-reported outcomes help evaluate disease progression or regression as well as treatment effects.
Conclusions
Patient-reported measures can help clinicians target interventions that will improve patient outcomes of care. However, there are a number of challenges in using patient-reported outcomes in clinical practice.
Keywords: Quality of life, Quality indicators, Health care, Outcome assessment
Introduction
Patient-reported measures are so central to the practice of medicine that the standard medical evaluation is divided into two sections, patient-reported data (i.e., history of present illness, past history, social history, and review of systems) and data from other sources (i.e., physical examination findings and diagnostic results). Yet recently, much has been written about how clinicians (either because of a diversion from the roots of clinical medicine, the pressures of time, or the glow of a new technology) have become less effective and efficient at collecting patient-reported measures. Indeed, performance measures (e.g., Healthcare Effectiveness Data and Information Set [1], Veterans Health Administration's External Peer Review Program [2]) assess whether clinicians screen, diagnose, and treat specific conditions and achieve outcomes targeted in clinical trials (e.g., blood pressure levels), but not whether clinicians ask patients how illness affects the patient's ability to work or interact socially with others. Measures that focus on the provision of tests (e.g., hemoglobin A1c) or the achievement of intermediate outcomes (e.g., blood pressure less than 140/90) are important, but they provide limited information about patient experience with care and may encourage clinicians to focus more on the diagnostics studies than on the patient as a whole.
For this reason, there is a move to formalize the assessment of patient-reported measures in clinical practice. These formalized assessments might involve instruments that are either self-administered or administered verbally by a clinician and could include structured, semi-structured, and/or free-form items. In theory, a greater emphasis on collecting patient-reported measures could improve quality of care by encouraging clinicians to treat the whole patient rather than a conglomerate of diseases that co-exist within a single person [3].
This paper begins by examining the ways in which formalized assessments of patient-reported measures could improve health care and describes the challenges of using formalized assessments in clinical settings.
A broad and a narrow definition of patient-reported outcomes
The Food and Drug Administration (2006) refers to patient-reported outcomes as “any report coming from patients about a health condition and its treatment” [4]. In clinical practice, this encompasses a broad range of topics. It includes reports of symptoms such as pain, fatigue, and energy as well as functioning and well-being in physical, mental and social domains of life (i.e., health-related quality of life). It also includes health behaviors such as adherence to medicines, tobacco use, and participation in exercise programs. Moreover, it includes patients' expressions about their preferences for different types of care and their desire to participate (or not to participate) in care. Finally, this definition would include satisfaction with the care and reports about patient–clinician communication, coordination of care, and access to care. While we agree that a wide range of patient reports are important, we prefer to refer to the wider set as patient-reported measures and limit patient-reported outcomes to a smaller subset. Patient-reported measures are important in each step [5, 6] of a patient–clinician encounter. The steps include:
(1) Identify/elicit the problem(s); (2) Discuss with the patient about planned action(s); (3) Enact action(s)/cocreate plan(s); (4) Action(s); and (5) Learn about the effects (IDEAL). Assessing baseline health-related quality of life can help the provider identify the problem. A needs-assessment in combination with the skills, knowledge and resources available to the provider serve to help decide upon the appropriate action. The patient's report about care (e.g., communication) indicates how well the provider explained the course of action and engaged the patient in a discussion about the planned action. Patient reports of adherence can help a clinician understand whether the agreed-upon action has occurred. Finally, satisfaction with care and change in health-related quality of life can serve as indicators of treatment effectiveness.
Although ascertainment of patient-reported outcomes is an integral part of each of the IDEAL steps in the patient– clinician encounter, we propose that the central patient-reported “outcomes” are patient satisfaction with care and health-related quality of life. Satisfaction with care is already being formally assessed through efforts such as the Consumer Assessment of Healthcare Providers and Systems [4], but formalized assessments of health-related quality of life are not routinely collected and clinicians are not held accountable for collecting this information (e.g., in performance-measurement programs). Therefore, we focus on health-related quality of life in the remainder of this paper.
The position of health-related quality of life in the patient-reported measures framework
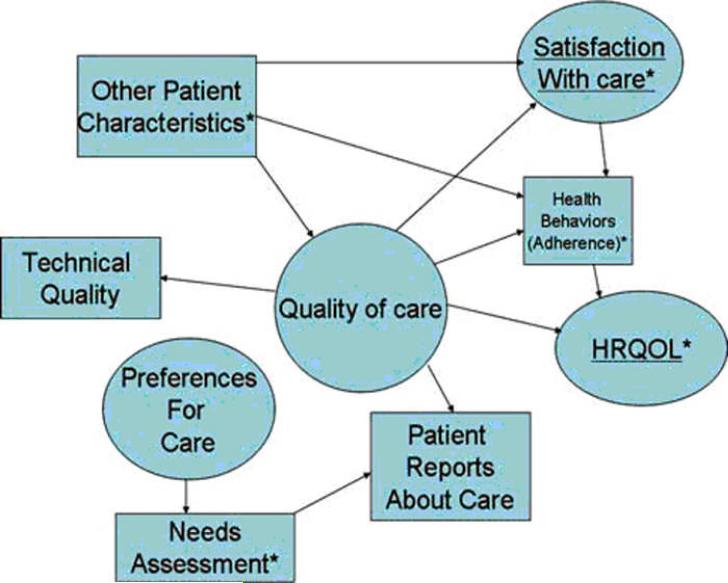
Hypothetical relationships between health-related quality of life and other types of information provided by patients are schematically displayed in Fig. 1. The diagram shows the ultimate outcome of care, health-related quality of life, at the far right. Health-related quality of life is influenced by the patient's health behaviors (e.g., adherence to care), and health behaviors are impacted by their satisfaction with care as well as patient characteristics such as values, beliefs (e.g., desire to participate in care), and social support. Quality of care, defined by technical quality indicators and patient reports about care, has a direct effect on satisfaction with care and on health-related quality of life. Preferences for care can be measured by a needs assessment. In turn, preference for care impact patient reports about care (if needs are met then patient reports about care are more positive).
Figure 1.
Fig. 1 Hypothetical relationships among different kinds of patient-reported measures. Circles denote latent constructs (i.e., multiple indicators define the construct) and boxes represent observed indicators (i.e., construct is manifest or measured directly). * Patient-reported measures
Evidence in favor of formalized assessments of health-related quality of life in clinical practice Formalized assessments of patient-reported outcomes may increase clinicians' attention to patient concerns, which are often overlooked [7]. An observational study conducted in both hospital-based and community practices found that physicians underestimated or did not detect 66% of functional limitations self-reported by patients [8]. Assessment of the patient's functioning and well-being in physical, mental, and social domains of health, may be useful for improving communication between patients and clinicians and screening for hidden disability or disease [9]. A randomized controlled trial (RCT) conducted in an oncology practice demonstrated that regular collection and feedback of health-related quality-of-life information to clinicians improved physician–patient communication [10]. A systematic review concluded that feeding back perceived mental health information to health care professionals improved the diagnosis of conditions (combined odds ratio = 1.91; 95% confidence interval: 1.28–2.83) [11].
Formalized assessments of patient-reported outcomes may be particularly helpful for improving patient-provider communication for patients with multiple complex comorbidities, whose frequent contacts with the health care system often center on acute conditions or issues related to known diagnoses [12, 13]. In a RCT, use of a health-related quality-of-life instrument in an oncology practice led to increased discussion of health-related quality-of-life issues (effect size = .38, P = .01) and greater identification of moderate-to-severe health problems in the intervention group [14]. In a RCT testing the impact of patient health status assessment by primary care clinicians in a health maintenance organization, assessment of patient health was associated with greater degree of clinician help with pain-related functional problems among male patients (33 vs. 47, P = .016, scale 0–100) [15]. A RCT conducted in community primary care practices demonstrated that use of a health-related quality-of-life instrument led to patients receiving more assistance with limitations in physical and daily activities (82% versus 70%, P \ 0.01) and greater patient understanding of fall prevention (39% vs. 23%; P \ 0.01) [16]. These studies suggest that a formalized assessment of health-related quality of life improves communication about incipient problems, although patients may be more willing than clinicians to discuss health-related quality-of-life issues [17].
Barriers to formalized assessments of health-reported quality of life in clinical practice A primary concern is whether clinicians have sufficient interest in using formalized assessments of patient-reported outcomes to alter their treatment plan.
Kaplan et al. argue that provision of medical care is likely to change a patient's health-related quality of life only modestly [18]. Factors beyond the immediate control of individual clinicians or institutions, such as the environment contribute to a patient's health-related quality of life [9]. If clinicians believe that collecting health-related quality-of-life information is unlikely to lead to a desired outcome, they may be less likely to use the information, even if, for example, incentives tied to performance measurement encourages them to assess patients' health-related quality of life [19]. Indeed, several studies suggest that physicians' treatment decisions are unaffected by the provision of health-related quality-of-life information [9, 11, 20]. A RCT found that intermittent provision of information to internists about physical, psychological, and social function of their patients coupled with a brief educational session to physicians regarding the interpretation and management of functional disability did not improve functional status in the intervention group [21]. However, in another RCT, computer-generated feedback of the patient's functional status, “chief complaint,” and problem-specific resource and management suggestions coupled with two brief educational sessions for physicians resulted in improvement in emotional well-being scores (P \ .03), reduction in reported social activity limitations among patients [70 (P \ .03), and increased rate of diagnosis of stress/anxiety (P \ .001) [22]. Finally, most health-related quality-of-life instruments have been developed for research studies rather than clinical practice, making interpretation of the evidence and applicability to a given patient challenging [3, 9, 23].
Formalized assessments of patient-reported outcomes should enhance, not detract from, the delivery of health care; given the time constraints of a typical clinical encounter, it seems unlikely that clinicians and patients would want to administer the patient-reported outcome instrument in the examination room. One way of collecting data would be through use of a health-related quality-oflife instrument administered in the clinic (outside the examination room). But the optimal location for patients may be at home because of the time constraints and potential bias associated with completing an assessment at the site of care. The ease of completing the questionnaire is also relevant [24]. Numerous practical questions remain unanswered.
A variety of health-related quality-of-life measures are available [25]. Disease-targeted measures are frequently noted to have the advantage of assessing the content that is most relevant for a given condition. But for helping clinicians integrate the effects of multiple conditions (e.g., primary care), generic health-related quality-of-life measures may be the more useful. These generic measures can assess health-related quality of life across different patient populations and conditions [25–27]. Enabling patients to prioritize the importance of problems within specific domains may be important [28]. Patient-generated, or individualized, measures have been proposed for use in clinical trial settings, although the practicality of measures such as the Schedule for the Evaluation of Individual Quality of Life (SEIQoL) have been questioned [29].
Cost is another consideration. Because many primary care clinics do not routinely use patient-reported outcome instruments, clinicians would need to use additional or reallocated resources to collect this information. Yet low financial margins in primary care practices pose barriers to changes [30] that would be necessary to administer the instruments in primary care clinics. Reimbursement for collecting this type of information would be necessary. Other implementation barriers are related to staff training and potential changes in clinic flow that might be necessary to use the instruments. Instruments such as the Dartmouth Primary Care Cooperative Information Project (COOP) chart system were designed to fit into the typical outpatient clinic's data collection routine, and clinicians and patients reported that the COOP instruments were easy to use, even in a busy practice [31, 32]. If administering instruments were routinely needed, training programs to help clinics and offices administer such instruments would be needed.
Additional barriers might also decrease the formalized collection of patient-reported outcomes in clinical practice. Lack of motivation to collect and use patient-reported outcomes driven by inertia is a significant concern, just as inertia is a barrier to widespread adherence to clinical guidelines [19]. Another barrier is the steep learning curve that some clinicians may face. There are many health-related quality-of-life instruments [20] and clinicians are generally unfamiliar with them. Even exposure to educational programs to improve knowledge of patient-reported outcomes may not be sufficient to change clinicians' behavior sufficiently [17]. One possible method of addressing this barrier would be for clinicians to select one particular instrument and build clinical decision-support tools to improve clinician knowledge and use of the instrument. More studies are needed to determine which types of tools and programs could adequately close gaps in clinicians' knowledge about patient-reported outcomes.
Health-related quality-of-life measures in performance-measurement sets
Measuring and feeding back the results of these measurements to clinicians may help them identify deficiencies in their care (e.g., insufficient monitoring of patients' overall trajectory or response to treatment) and may lead to increased quality-improvement activity (i.e., decrease inertia). Improvement may involve identifying mechanisms for increasing clinicians' formalized assessment of patient-reported outcomes and developing programs to improve their patients' patient-reported outcomes (i.e., patient satisfaction and health-related quality of life). Improvement may occur through a second pathway: Consumers (patient, purchaser, referring clinician), who want the best quality of care for the best price could select (or reward, recognize, punish, or pay) the health care clinicians who have higher scores. Consumers' selections could change market share and motivate care delivery teams or individual practitioners to improve [33].
Health-related quality-of-life information could help consumers differentiate between clinicians. For example, when patients have multiple complex conditions, determining how the treatments for the different conditions in the aggregate are affecting the patient in the different stages of treatment is as important as monitoring the treatment of individual conditions. Health-related qualityof-life measures would indicate whether and how much the clinician determines if treatments are affecting the patient's general health trajectory [34]—whether the clinician monitors if the effects of multiple treatments are causing the patient to drift from the patient's goals, and whether the clinician gauges when some therapies need to be modified or perhaps ceased, just as current measures assess if lab tests such as hemoglobin A1c are ordered to monitor glycemic control. Inclusion of these measures in performance measurement sets that are linked to quality improvement programs could encourage clinicians to elicit the patient's agenda and understand the patient's perspective, and could create a common language for clinicians of all specialties to communicate about the priorities of the patient [3]. However, more research is needed to identify the types of measures (e.g., generic versus disease-specific; process versus outcome) that should be included in performance-measurement sets and the impact on patient outcomes.
Conclusions
A greater emphasis on formalized assessment of patient-reported outcomes has the potential to improve clinical care. However, more work is needed to demonstrate if and how formalized assessments of patient-reported measures improve quality and outcomes of care. Future studies should also focus on addressing the challenges described above, including identifying methods of improving clinician knowledge and interest in using tools to formally assess health-related quality of life and improving methods of implementing collection and feedback of the data to clinicians.
Acknowledgments
We thank Theodore G. Ganiats, MD for his insightful comments on an earlier version of this manuscript. Dr. Fung was supported by funding from the Society of General Internal Medicine (through a grant from the American Board of Internal Medicine Foundation and the Commonwealth Fund). Dr. Fung is an employee of Zynx Health, Incorporated. Dr. Hays was supported by the a P01 grant (AG020679-01) from the National Institute on Aging and the UCLA Center for Health Improvement in Minority Elderly/Resource Centers for Minority Aging Research, NIH/NIA/NCMHD (P30-AG-021684-07). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of Zynx Health, which develops evidence-based tools for healthcare providers, or David Geffen School of Medicine at UCLA.
References
- 1.The Healthcare Effectiveness Data and Information Set (HEDIS) [Accessed 17 March 2008];National Committee for Quality Assurance. 2008 Available from: http://www.ncqa.org/tabid/59/Default.aspx.
- 2.Department of Veterans Affairs [Accessed 17 March 2008];External Peer Review Program. VHA Directive 2001-015. 2001 Available from: http://www1.va.gov/VHAPUBLICATIONS/ViewPublication.asp?pub_ID=103.
- 3.Higginson IJ, Carr AJ. Measuring quality of life: Using quality of life measures in the clinical setting. BMJ (Clinical Research Ed.) 2001;322(7297):1297–1300. doi: 10.1136/bmj.322.7297.1297. doi:10.1136/bmj.322.7297.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Food and Drug Administration [Accessed 17 March 2008];Guidance for industry. Patient-reported outcome measures: Use in medical product development to support labeling claims. 2006 doi: 10.1186/1477-7525-4-79. Available from: http:// www.fda.gov/OHRMS/DOCKETS/98fr/06d-0044-gdl0001.pdf. [DOI] [PMC free article] [PubMed]
- 5.Makoul G. Essential elements of communication in medical encounters: The Kalamazoo consensus statement. Academic Medicine. 2001;76(4):390–393. doi: 10.1097/00001888-200104000-00021. doi:10.1097/00001888-20010400000021. [DOI] [PubMed] [Google Scholar]
- 6.Mauksch LB, Dugdale DC, Dodson S, Epstein R. Relationship, communication, and efficiency in the medical encounter: Creating a clinical model from a literature review. Archives of Internal Medicine. 2008;168(13):1387–1395. doi: 10.1001/archinte.168.13.1387. doi: 10.1001/archinte.168.13.1387. [DOI] [PubMed] [Google Scholar]
- 7.Noel PH, Chris Frueh B, Larme AC, Pugh JA, Noel PH, Williams JW, Jr., et al. Collaborative care needs and preferences of primary care patients with multimorbidity. Health Expectations. 2005;8(1):54–63. doi: 10.1111/j.1369-7625.2004.00312.x. doi:10.1111/j.1369-7625.2004. 00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calkins DR, Rubenstein LV, Cleary PD, Davies AR, Jette AM, Fink A, et al. Failure of physicians to recognize functional disability in ambulatory patients. Annals of Internal Medicine. 1991;114(6):451–454. doi: 10.7326/0003-4819-114-6-451. [DOI] [PubMed] [Google Scholar]
- 9.McHorney CA. Health status assessment methods for adults: Past accomplishments and future challenges. Annual Review of Public Health. 1999;20:309–335. doi: 10.1146/annurev.publhealth.20.1.309. doi:10.1146/annurev. publhealth.20.1.309. [DOI] [PubMed] [Google Scholar]
- 10.Velikova G, Booth L, Smith AB, Brown PM, Lynch P, Brown JM, et al. Measuring quality of life in routine oncology practice improves communication and patient wellbeing: A randomized controlled trial. Journal of Clinical Oncology. 2004;22(4):714–724. doi: 10.1200/JCO.2004.06.078. doi:10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 11.Espallargues M, Valderas JM, Alonso J. Provision of feedback on perceived health status to health care professionals: A systematic review of its impact. Medical Care. 2000;38(2):175–186. doi: 10.1097/00005650-200002000-00007. doi:10.1097/00005650-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Jaen CR, Stange KC, Nutting PA. Competing demands of primary care: A model for the delivery of clinical preventive services. The Journal of Family Practice. 1994;38(2):166–171. [PubMed] [Google Scholar]
- 13.Nutting PA, Baier M, Werner JJ, Cutter G, Conry C, Stewart L. Competing demands in the office visit: What influences mammography recommendations? The Journal of the American Board of Family Practice. 2001;14(5):352. [PubMed] [Google Scholar]
- 14.Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: A randomized controlled trial. Journal of the American Medical Association. 2002;288(23):3027. doi: 10.1001/jama.288.23.3027. doi:10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]
- 15.Wasson J, Hays R, Rubenstein L, Nelson E, Leaning J, Johnson D, et al. The short-term effect of patient health status assessment in a health maintenance organization. Quality of Life Research. 1992;1(2):99–106. doi: 10.1007/BF00439717. doi:10.1007/BF00439717. [DOI] [PubMed] [Google Scholar]
- 16.Wasson JH, Stukel TA, Weiss JE, Hays RD, Jette AM, Nelson EC. A randomized trial of the use of patient self-assessment data to improve community practices. Effective Clinical Practice. 1999;2(1):1–10. [PubMed] [Google Scholar]
- 17.Greenhalgh J, Long AF, Flynn R. The use of patient reported outcome measures in routine clinical practice: Lack of impact or lack of theory? Social Science & Medicine. 2005;60(4):833–843. doi: 10.1016/j.socscimed.2004.06.022. doi:10.1016/j.socscimed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan SH, Kravitz RL, Greenfield S. A critique of current uses of health status for the assessment of treatment effectiveness and quality of care. Medical Care. 2000;38(9, Suppl):II184–II191. doi: 10.1097/00005650-200009002-00029. [DOI] [PubMed] [Google Scholar]
- 19.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. Journal of the American Medical Association. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. doi:10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 20.Fihn SD, McDonell MB, Diehr P, Anderson SM, Bradley KA, Au DH, et al. Effects of sustained audit/feedback on self-reported health status of primary care patients. The American Journal of Medicine. 2004;116(4):241–248. doi: 10.1016/j.amjmed.2003.10.026. doi:10.1016/j.amjmed.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Calkins DR, Rubenstein LV, Cleary PD, Davies AR, Jette AM, Fink A, et al. Functional disability screening of ambulatory patients: A randomized controlled trial in a hospital-based group practice. Journal of General Internal Medicine. 1994;9(10):590–592. doi: 10.1007/BF02599291. doi:10.1007/BF02599291. [DOI] [PubMed] [Google Scholar]
- 22.Rubenstein LV, McCoy JM, Cope DW, Barrett PA, Hirsch SH, Messer KS, et al. Improving patient quality of life with feedback to physicians about functional status. Journal of General Internal Medicine. 1995;10(11):607. doi: 10.1007/BF02602744. doi:10.1007/ BF02602744. [DOI] [PubMed] [Google Scholar]
- 23.Donaldson GW, Moinpour CM. Individual differences in quality-of-life treatment response. Medical Care. 2002;40(6, Suppl):III39–III53. doi: 10.1097/00005650-200206001-00007. doi:10.1097/00005650-200206001-00007. [DOI] [PubMed] [Google Scholar]
- 24.de Wit M, Delemarre-van de Waal HA, Pouwer F, Gemke RJ, Snoek FJ. Monitoring health related quality of life in adolescents with diabetes: A review of measures. Archives of Disease in Childhood. 2007;92(5):434–439. doi: 10.1136/adc.2006.102236. doi:10.1136/adc.2006.102236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coons SJ, Rao S, Keininger DL, Hays RD. A comparative review of generic quality-of-life instruments. PharmacoEconomics. 2000;17(1):13–35. doi: 10.2165/00019053-200017010-00002. doi:10.2165/00019053-200017010-00002. [DOI] [PubMed] [Google Scholar]
- 26.Garratt A, Schmidt L, Mackintosh A, Fitzpatrick R. Quality of life measurement: Bibliographic study of patient assessed health outcome measures. BMJ (Clinical Research Ed.) 2002;324(7351):1417. doi: 10.1136/bmj.324.7351.1417. doi:10.1136/bmj.324.7351.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haywood KL, Garratt AM, Fitzpatrick R. Quality of life in older people: A structured review of generic self-assessed health instruments. Quality of Life Research. 2005;14(7):1651–1668. doi: 10.1007/s11136-005-1743-0. doi:10.1007/s11136-005-1743-0. [DOI] [PubMed] [Google Scholar]
- 28.Hickey A, Barker M, McGee H, O'Boyle C. Measuring health-related quality of life in older patient populations: A review of current approaches. PharmacoEconomics. 2005;23(10):971–993. doi: 10.2165/00019053-200523100-00002. doi:10.2165/00019053-20052310000002. [DOI] [PubMed] [Google Scholar]
- 29.Patel KK, Veenstra DL, Patrick DL. A review of selected patient-generated outcome measures and their application in clinical trials. Value in Health. 2003;6(5):595–603. doi: 10.1046/j.1524-4733.2003.65236.x. doi: 10.1046/j.1524-4733.2003.65236.x. [DOI] [PubMed] [Google Scholar]
- 30.Phillips RL. Primary care in the United States: Problems and possibilities. BMJ (Clinical Research Ed.) 2005;331(7529):1400–1402. doi: 10.1136/bmj.331.7529.1400. doi:10.1136/bmj.331.7529.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson EC, Wasson JH, Johnson DJ, Hays RD. [Accessed 17 March 2008];Dartmouth COOP functional health assessment charts: Brief measures for clinical practice. 1996 Available from: http://www. dartmouth.edu/*coopproj/more_charts.html.
- 32.Nelson EC, Landgraf JM, Hays RD, Wasson JH, Kirk JW. The functional status of patients. How can it be measured in physicians' offices? Medical Care. 1990;28(12):1111–1126. doi:10.1097/00005650-199012000-00001. [PubMed] [Google Scholar]
- 33.Berwick DM, James B, Coye MJ. Connections between quality measurement and improvement. Medical Care. 2003;41(1, Suppl):I30–I38. doi: 10.1097/00005650-200301001-00004. doi:10.1097/00005650-200301001-00004. [DOI] [PubMed] [Google Scholar]
- 34.Hemingway H, Stafford M, Stansfeld S, Shipley M, Marmot M. Is the SF-36 a valid measure of change in population health? Results from the Whitehall II Study. BMJ (Clinical Research Ed.) 1997;315(7118):1273–1279. doi: 10.1136/bmj.315.7118.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]