Summary
The protein α-synuclein accumulates in the brain of patients with sporadic Parkinson’s disease (PD), and increased gene dosage causes a severe, dominantly inherited form of PD, but we know little about the effects of synuclein that precede degeneration. α-Synuclein localizes to the nerve terminal, but the knockout has little if any effect on synaptic transmission. In contrast, we now find that the modest over-expression of α-synuclein, in the range predicted for gene multiplication and in the absence of overt toxicity, markedly inhibits neurotransmitter release. The mechanism, elucidated by direct imaging of the synaptic vesicle cycle, involves a specific reduction in size of the synaptic vesicle recycling pool. Ultrastructural analysis demonstrates reduced synaptic vesicle density at the active zone, and imaging further reveals a defect in the reclustering of synaptic vesicles after endocytosis. Increased levels of α-synuclein thus produce a specific, physiological defect in synaptic vesicle recycling that precedes detectable neuropathology.
Introduction
Many neurodegenerative diseases are associated with the accumulation of a characteristic protein, and human genetics has linked mutations in several of them to familial forms of degeneration, indicating a causative role. However, the mechanism by which these proteins cause degeneration remains unknown. In particular, we do not know whether they produce disease through the gain of an abnormal function, such as multimerization (Haass and Selkoe, 2007), or an increase in their normal function. Indeed, we know remarkably little about the physiological role of most proteins that accumulate in neurodegenerative disease.
The protein α-synuclein (αsyn) accumulates in the Lewy bodies and dystrophic neurites characteristic of idiopathic Parkinson’s disease (PD) and Lewy body dementia (LBD) (Spillantini et al., 1998; Dickson, 2001). Point mutations in αsyn are also linked to an autosomal dominant form of PD (Polymeropoulos et al., 1997; Kruger et al., 1998; Zarranz et al., 2004). Taken together, these observations suggest a causative role for αsyn in sporadic as well as inherited PD and LBD. In addition, duplication and triplication of the αsyn gene suffice to cause a severe, highly penetrant form of PD (Singleton et al., 2003; Chartier-Harlin et al., 2004), and polymorphisms in regulatory elements of the αsyn gene predispose to PD (Maraganore et al., 2006), supporting a role for over-expression of the wild type protein in pathogenesis.
αSyn is a small (140 amino acid), peripheral membrane protein that localizes specifically to the axon terminal in neurons, suggesting a role in neurotransmitter release (Maroteaux et al., 1988; Iwai et al., 1995). Indeed, the protein has been implicated in the synaptic plasticity associated with song acquisition by birds (George et al., 1995). The N-terminus of αsyn contains 7 eleven residue repeats that form an amphipathic helix on membrane binding (Davidson et al., 1998; Bussell and Eliezer, 2003), and this membrane binding contributes to its presynaptic localization (Fortin et al., 2004). However, the role of αsyn in synaptic transmission has remained unclear. Mice lacking αsyn have been reported to show either no or very small (and opposing) effects on transmitter release (Abeliovich et al., 2000; Cabin et al., 2002; Chandra et al., 2004; Yavich et al., 2004).
In contrast to the minimal phenotype of synuclein knockout mice, recent work in model organisms has shown that the over-expression of αsyn produces considerable toxicity. Over-expression in yeast and Drosophila causes a defect in vesicular transport between the endoplasmic reticulum and Golgi complex that can be rescued by over-expression of rab proteins (Cooper et al., 2006; Gitler et al., 2008). However, these organisms lack a synuclein homologue, and wild type synuclein exerts much less if any toxicity in mammalian systems (Zhou et al., 2000; Petrucelli et al., 2002; Manning-Bog et al., 2003; Chandra et al., 2005; Cooper et al., 2006). More recently, over-expression of αsyn has been shown to inhibit catecholamine release from adrenal chromaffin cells and granule release from platelets (Park et al., 2002; Larsen et al., 2006), but we know little about the effects of over-expression on synaptic transmission.
Results
Since the loss of αsyn has been reported to have little effect on synaptic transmission, but over-expression of the wild type human protein causes PD and membrane trafficking defects in yeast, we examined the effects of over-expression on transmitter release. To study presynaptic effects directly, we used optical imaging with a fusion of vesicular glutamate transporter 1 (VGLUT1) to the modified GFP ecliptic pHluorin (VGLUT1-pHluorin) (Voglmaier et al., 2006). Expressed in primary neuronal culture as a fusion to VGLUT1 or other synaptic vesicle proteins, the fluorescence of lumenally oriented pHluorin is quenched by the low pH of resting synaptic vesicles, increases during exocytosis on exposure to the more alkaline extracellular medium, and decreases as a result of the acidification that accompanies endocytosis (Miesenbock et al., 1998). VGLUT1-pHluorin thus monitors synaptic vesicle exo- and endocytosis in real time. We cotransfected this reporter with either wild type human αsyn or empty vector into dissociated embryonic hippocampal neurons. Fixed at 2-3 weeks in vitro and stained with a human αsyn-specific antibody, the cultures show complete colocalization of human αsyn with VGLUT1-pHluorin at synaptic boutons (Fig. S1A). To assess the extent of over-expression, we used an antibody that recognizes both human and endogenous rat proteins (Fig. S1B). Quantitation of the immunofluorescence shows expression of αsyn at levels 2-3-fold over endogenous (Fig. S1C), very similar to the over-expression predicted for humans with a triplication of the αsyn gene locus (Miller et al., 2004). Consistent with previous results in vitro and in vivo (Matsuoka et al., 2001; Giasson et al., 2002; Lee et al., 2002; Fortin et al., 2005), we also observe no obvious toxicity related to the over-expression of αsyn, and no αsyn-immunoreactive deposits in the over-expressing cells (Fig. S1A,B).
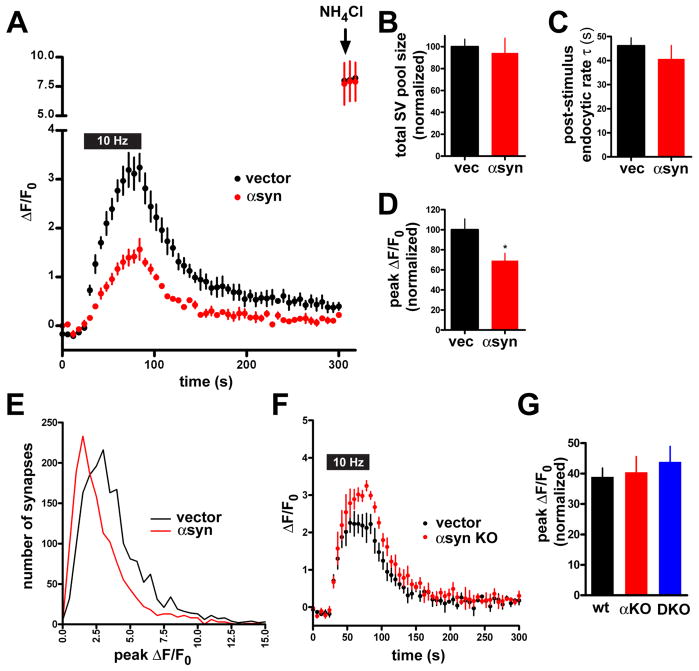
Over-expression of α-synuclein inhibits synaptic vesicle exocytosis
To determine whether the increased expression of αsyn affects synaptic vesicle cycling, we subjected the transfected neurons to field stimulation, and imaged VGLUT1-pHluorin. Relative to control, neurons over-expressing wild type αsyn show less increase in fluorescence due to stimulation (Figs. 1A, S1D,E). Since αsyn has been shown to disrupt membrane trafficking early in the secretory pathway of yeast (Cooper et al., 2006; Gitler et al., 2008), we used NH4Cl to alkalinize acidic intracellular compartments and reveal the intracellular pool of quenched VGLUT1-pHluorin at boutons. Figure 1B shows that the total amount of reporter does not differ between the two groups, and hence cannot account for the difference in response to stimulation. Plotting the frequency of all responses as a function of ΔF/F0, over-expression of αsyn shifts the entire peak to the left (Fig. 1E), indicating effects on exocytosis at all synapses, rather than a discrete subpopulation.
Figure 1. Over-expression of α-synuclein inhibits synaptic vesicle exocytosis.
(A) Time course of changes in the fluorescence of VGLUT1-pHluorin during and after 10 Hz stimulation for 60 s in neurons cotransfected with either wild type human αsyn or empty vector. Alkalinization with 50 mM NH4Cl reveals total VGLUT1-pHluorin (arrow). n=3 coverslips, 60 boutons for each condition. (B) Total fluorescence of VGLUT1-pHluorin revealed by the addition of NH4Cl shows that expression of the reporter on all synaptic vesicles is not altered by the over-expression of αsyn. Values are normalized to the fluorescence obtained in vector control. (C) The rate of endocytosis (τ) at the end of 10 Hz stimulation was determined by the fit to a single exponential, and shows no effect of αsyn over-expression. (D) Peak ΔF/F0 normalized to vector control shows a reduction in cells over-expressing wild type αsyn. *, p<0.05 for αsyn versus control, two-tailed, unpaired t-test. For panels B–D, n=9 coverslips, 180 nerve terminals from each condition and 3 independent transfections. (E) Frequency histogram of peak ΔF/F0 from a large number of synapses in response to 10 Hz stimulation for 60 s. n=9 coverslips from 3 independent transfections, 1809 boutons for vector, and 1428 boutons for αsyn (F) Time course of VGLUT1-pHluorin fluorescence change during and after 10 Hz stimulation for 60 s in hippocampal neurons from either wild type or α-synuclein knockout mice. n=3 coverslips, 60 boutons for each condition. (G) Peak ΔF/F0 after a 10 Hz 60 s stimulus normalized to total synaptic vesicle pool size (revealed by addition of NH4Cl) shows no significant difference between wild type and either αsyn KO or α/β double KO neurons. n=6 coverslips, 120 nerve terminals from each condition with 2 independent transfections. Values represent mean ± SEM.
We then used the VGLUT1-pHluorin reporter to image other aspects of the synaptic vesicle cycle. αSyn over-expression does not affect the time course of fluorescence decay after the stimulus (Fig. 1C), excluding an effect on compensatory endocytosis. However, αsyn also fails to alter the rate of fluorescence increase due to exocytosis. Rather, quantitation of peak ΔF/F0 shows a specific effect of αsyn on the extent rather than the rate of synaptic vesicle exocytosis (Fig. 1D). The effect of human αsyn over-expression also appears early in the stimulus, before VGLUT1-pHluorin has accumulated on the cell surface at levels high enough to detect its internalization (Voglmaier et al., 2006), further excluding effects on endocytosis that might occur specifically during the stimulus (Ferguson et al., 2007).
Since most neurons express substantial amounts of endogenous αsyn, we also over-expressed the human protein in postnatal hippocampal cultures from αsyn knockout (KO) mice (Abeliovich et al., 2000). Human αsyn has roughly the same effect on evoked synaptic vesicle exocytosis as in hippocampal cultures from rat (Fig. S1F). To determine more directly whether endogenous αsyn affects release, we also transfected VGLUT1-pHluorin alone into postnatal hippocampal cultures from αsyn KO mice and wild type littermates. The KO shows a trend toward increased evoked transmitter release, but the difference does not reach statistical significance. In the same experimental paradigm, the over-expression of αsyn thus has a greater effect on transmitter release than the loss of synuclein.
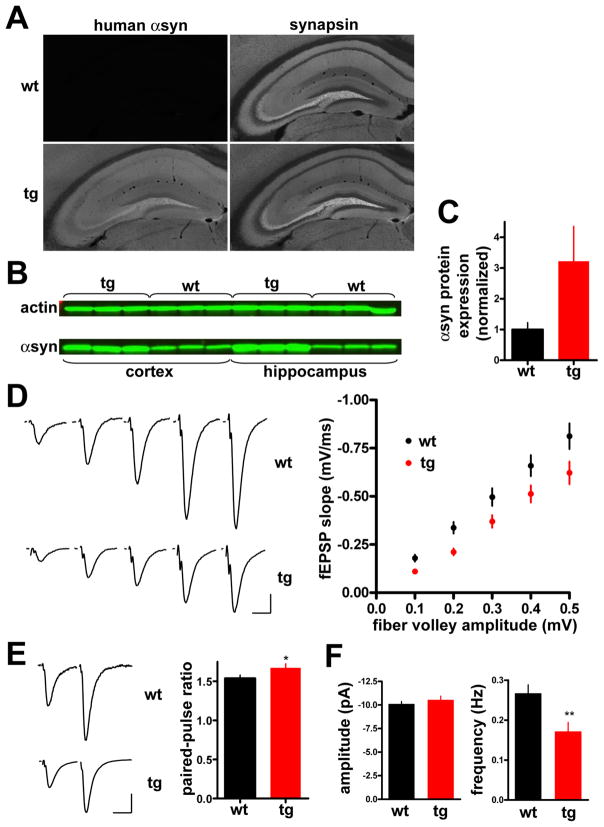
Inhibition of synaptic transmission in transgenic mice over-expressing α-synuclein
To determine whether αsyn influences synaptic transmission in vivo, we used electrophysiology to record the postsynaptic response in acute hippocampal slices from transgenic mice over-expressing wild type human αsyn. Produced using sequences from the prion promoter that drive widespread α syn expression in neurons (Fig. 2A), the transgenic mice express αsyn approximately 3-fold over endogenous by quantitative western analysis with the antibody that recognizes both rodent and human αsyn (Figs. 2B, C), very similar to the over-expression in culture. We also observed no obvious pathology or αsyn-immunoreactive deposits by light microscopy, or αsyn-immunoreactive aggregates by western analysis (Fig. S2). Using hippocampal slices from transgenic animals and wild type littermates, we recorded field excitatory postsynaptic potentials (fEPSPs) from synapses made by CA3 Schaffer collaterals onto dendrites in CA1 stratum radiatum. The transgenic mice show significantly less baseline transmission than wild type at a range of stimulus intensities (Fig. 2D), and the extent of inhibition resembles that observed for αsyn over-expression in culture. To determine whether the reduction reflects a presynaptic effect, we measured the paired pulse ratio (PPR). Schaffer collaterals characteristically show facilitation due to residual Ca2+ in the terminal, and manipulations that inhibit release generally increase PPR. Consistent with a presynaptic mechanism, we observe a small but significant increase in PPR (Fig. 2E). Further, the over-expression of synuclein reduces the frequency of spontaneous release without affecting quantal size (Fig. 2F). The analysis of synaptic transmission in brain slices thus strongly supports the physiological significance of impaired synaptic vesicle exocytosis observed in dissociated culture.
Figure 2. α–Synuclein over-expression in transgenic mice inhibits synaptic transmission.
(A) Brain sections from 3 week old transgenic mice (tg) and wildtype (wt) littermates were double stained for human αsyn using the 15G7 antibody, and for synapsins using an antibody that recognizes both synapsins I and II. (B) Extracts (10 μg) from cortex or hippocampus of αsyn transgenic mice and wt littermates were immunoblotted in triplicate using an antibody to actin and the syn-1 antibody to both mouse and human αsyn. (C) Quantitation of the western analysis shown in B indicates ~3-fold over-expression of α-synuclein in the transgenic mice. Samples from each animal were loaded in triplicate, and syn-1 immunoreactivity normalized to actin detected in the same blot. n=3 animals (D) Representative fEPSP traces from CA1 stratum radiatum of 3–5 week old transgenic mice and wild type littermates in response to increasing stimulation of Schaffer collaterals (left). An input-output curve of fiber volley amplitude versus fEPSP slope shows significantly less postsynaptic response by αsyn transgenic mice than wild type littermates (right). p<0.005 for all points by two-tailed t-test; n=44 slices for wt, 48 slices for transgenic mice from 11 mice for each (E) fEPSP response in CA1 stratum radiatum to paired pulse stimulation of Schaffer collaterals with a 40 ms interstimulus interval. The left panel shows representative fEPSPs recorded from slices of wild type (top) and transgenic animals (bottom). p<0.05 by unpaired, two-tailed t-test; n=45 slices for wt and 47 for transgenic mice. (F) Transgenic over-expression of αsyn reduces the frequency of spontaneous release (right) but not mEPSC amplitude (left). Values represent mean ± SEM.
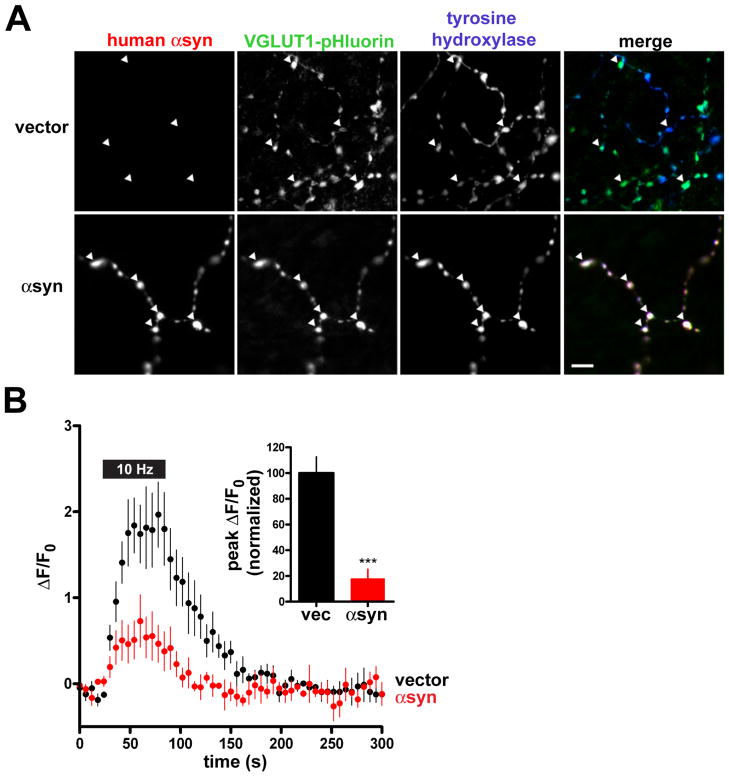
α– Synuclein inhibits synaptic vesicle exocytosis in midbrain dopamine neurons
Since the loss of dopamine neurons from the substantia nigra is a defining feature of PD, we also cotransfected human αsyn with VGLUT1-pHluorin into postnatal cultures from the rat ventral midbrain, which have indeed been shown to release glutamate as well as dopamine and express the related isoform VGLUT2 (Sulzer et al., 1998; Dal Bo et al., 2004). These cultures typically contain more than 90% dopamine neurons, but all the coverslips were fixed and immunostained for TH to select catecholamine boutons for analysis (Fig. 3A). Similar to hippocampal neurons, dopamine neurons that over-express αsyn show less synaptic vesicle exocytosis than control transfected dopamine neurons (Fig. 3B). The effect appears greater than in hippocampal neurons, but this may reflect differences in the properties of release by dopamine neurons rather than a different effect of αsyn. Indeed, the properties of synaptic vesicle recycling by dopamine neurons remain poorly understood, and since the overall effect of αsyn over-expression appears similar in both culture systems, we have further characterized the mechanism in hippocampal neurons, which normally express αsyn and accumulate the protein in both advanced PD and Lewy body dementia (Braak et al., 2003).
Figure 3. α–Synuclein inhibits synaptic vesicle exocytosis in midbrain dopamine neurons.
(A) Postnatal midbrain neurons cotransfected with VGLUT1-pHluorin and either human αsyn or empty vector were grown for 14–21 DIV and immunostained for human αsyn using the human-specific αsyn antibody 15G7, for VGLUT1-pHluorin using a monoclonal antibody to GFP, and for tyrosine hydroxylase (TH) using a polyclonal antibody. Arrowheads indicate TH+ boutons expressing VGLUT1-pHluorin with or without human αsyn. Scale bar = 5 μm. (B) Time course of response to 10 Hz stimulation for 60 s by TH+ neurons expressing VGLUT1-pHluorin and either wild type human αsyn or empty vector. Inset, peak ΔF/F0 values normalized to the response by cells transfected with empty vector. p<0.001, n=7 coverslips, 116 nerve terminals for vector, and 8 coverslips, 84 nerve terminals for αsyn from 2 independent transfections. Values indicate mean ± SEM.
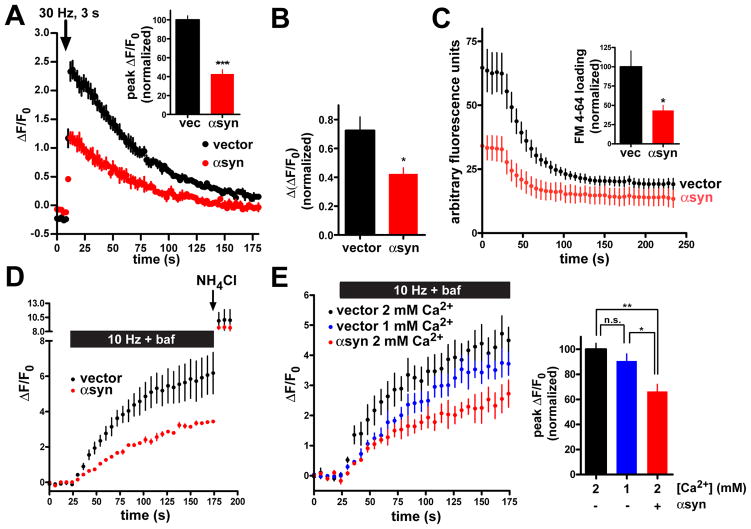
Over-expression of α– synuclein reduces the readily releasable and recycling synaptic vesicle pools
αSyn may interfere with synaptic vesicle mobilization, priming, docking or fusion. To assess an effect on fusion, we examined the release of vesicles docked and primed for exocytosis (the readily releasable pool, or RRP). Stimulating at 30 Hz for 3 s, which activates selectively the RRP (Pyle et al., 2000), αsyn over-expression inhibits release to the same extent as with more prolonged stimulation (Fig. 4A). The response to hypertonic sucrose has also been used to define the RRP (Rosenmund and Stevens, 1996), but changes in refractive index complicate the imaging. We therefore used the vacuolar H+-ATPase inhibitor bafilomycin to prevent reacidification of the vesicles that had undergone exocytosis, and imaged the cells just before the addition of hypertonic sucrose and after, when the cells had been returned to isotonic medium. Using this approach, αsyn also decreases peak fluorescence (Fig. 4B), supporting an effect of αsyn on the RRP. Since release by hypertonic sucrose does not require calcium (Rosenmund and Stevens, 1996), the effect of αsyn on synaptic vesicle exocytosis cannot involve a change in calcium entry or sensitivity.
Figure 4. α–Synuclein reduces the recycling pool of synaptic vesicles.
(A) Hippocampal neurons cotransfected with VGLUT1-pHluorin and either human αsyn or empty vector were stimulated at 30 Hz for 3 s to activate specifically the readily releasable pool of synaptic vesicles. n=3 coverslips, 60 boutons per condition. Inset, peak ΔF/F0 values normalized to the response in the vector control shows a substantial reduction in the cells over-expressing human αsyn. n=6 coverslips, 120 boutons per condition from 2 independent transfections. p<0.0001 by two-tailed, unpaired t-test. (B) Neurons expressing VGLUT1-pHluorin were stimulated with Tyrode’s solution containing 500 mM sucrose in the presence of 1μM bafilomycin to prevent reacidification of the internalized vesicles, and imaged in the absence of sucrose (to avoid distortion by changes in refractive index) both before and after stimulation. The change in ΔF/F0 normalized to vector control shows a reduction in neurons over-expressing human αsyn. n=9 coverslips, 180 boutons per condition from 3 independent transfections. p<0.05 by two-tailed, unpaired t-test. (C) Hippocampal neurons transfected with either αsyn-IRES2-GFP or IRES2-GFP were loaded with 15 μM FM 4-64 by 10 Hz stimulation for 60 s, maintained in the dye for 60 additional seconds to allow full endocytosis, and washed extensively before destaining at 10 Hz for 120 seconds. n = 3 coverslips, 71 boutons for αsyn-transfected, 73 nerve terminals for vector. αsyn over-expression reduces the amount of releasable dye uptake (inset). p < 0.05, n = 6 coverslips, 153 boutons for αsyn-over-expressing cells, and 208 boutons for vector control from 2 independent transfections. (D) Hippocampal neurons transfected with VGLUT1-pHluorin and either human αsyn or empty vector were stimulated at 10 Hz for 150 s in the presence of 1 μM bafilomycin to reveal the full recycling pool, followed by treatment with NH4Cl (arrow) to reveal the total synaptic vesicle pool. n = 60 boutons from 3 coverslips for each condition (E) Left panel - cultures were transfected and stimulated as in C but with controls stimulated in the presence of 1 mM as well as 2 mM Ca2+. n = 60 boutons from 3 coverslips per condition. Right panel - peak ΔF/F0 before addition of NH4Cl (normalized to 2 mM Ca2+ control) shows a reduction in the presence of over-expressed αsyn but no significant reduction of control boutons stimulated in 1 mM Ca2+. p<0.01 by one-way ANOVA with Tukey’s post hoc tests, p>0.05 for vector in 2 mM Ca2+ versus vector in 1 mM Ca2+, p<0.01 for vector in 2 mM Ca2+ versus αsyn in 2 mM Ca2+, and p<0.05 for vector in 1 mM Ca2+ versus αsyn in 2 mM Ca2+. n=120 boutons from 6 coverslips per condition and 2 independent transfections. Values represent mean ± SEM.
The reduction in RRP by αsyn suggests a defect in synaptic vesicle exocytosis at or close to the fusion event, but could also result from a decrease in the number of available vesicles, with no change in the rate of fusion. To distinguish between these possibilities, we examined uptake of the styryl dye FM 4-64 by neurons coexpressing αsyn and GFP (to identify the transfected cells), or GFP alone. After loading by stimulation at 10 Hz for 60 s, and incubation in dye for an additional 60 s to allow full endocytosis, the neurons were unloaded by 10 Hz stimulation for 120 s. The amount of dye released by the second stimulus provides a measure of the vesicle pool available for release (recycling pool). Boutons expressing αsyn show an ~50% decrease in the amount of specific FM dye uptake (Fig. 4C), supporting the results with VGLUT1-pHluorin. However, the rate of dye efflux shows no effect of αsyn over-expression (τ=49.8 ± 9.5 s for vector, τ=37.9 ± 5.9 s for αsyn, p=0.32). αSyn thus reduces the size of the synaptic vesicle recycling pool without affecting the kinetics of fusion. Since RRP size generally scales with the size of the recycling pool (Mozhayeva et al., 2002), the effect of αsyn on RRP presumably reflects the reduction in recycling pool size.
The size of the synaptic vesicle recycling pool can also be measured by stimulating neurons that express VGLUT1-pHluorin in the presence of the H+ pump inhibitor bafilomycin. Since bafilomycin blocks synaptic vesicle reacidification after endocytosis, prolonged stimulation results in the accumulation of unquenched reporter in all the synaptic vesicles that have undergone exocytosis, to reveal the entire recycling pool. Figure 4D shows that αsyn reduces the size of the recycling pool with no change in the total vesicle pool size revealed by NH4Cl, consistent with the results obtained using FM 4-64. However, slowed release might cause an apparent reduction in recycling pool size if stimulation does not persist long enough to release all the vesicles in the recycling pool. To address this possibility, we identified a concentration of external calcium that reduces the initial rate of fluorescence increase to that observed with αsyn over-expression. In 1 mM Ca2+, control cells (expressing VGLUT1-pHluorin but not human αsyn) show an initial rate of fluorescence increase very similar to that of cells expressing human αsyn, but with prolonged stimulation in bafilomycin, show a recycling pool size approaching that of control cells in 2 mM Ca2+ (Fig. 4E). In contrast, the recycling pool of cells expressing αsyn (in 2 mM Ca2+) never approaches that of controls. Thus, αsyn over-expression decreases specifically the size of the recycling pool, and not the rate of fusion.
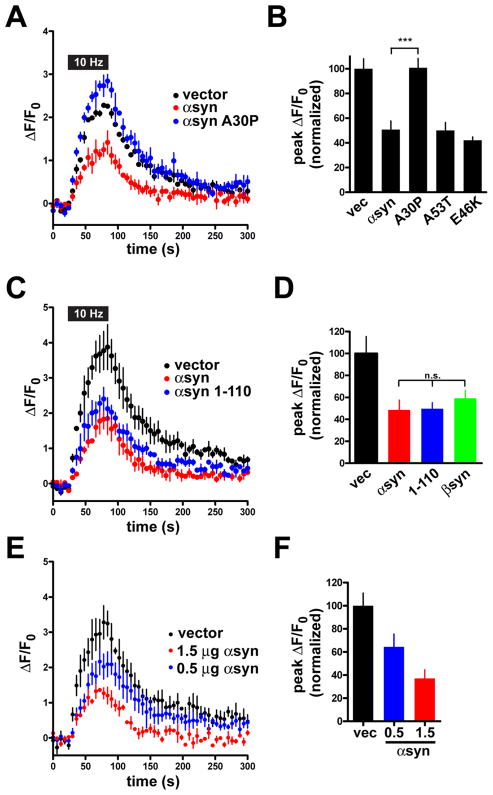
Inhibition of transmitter release requires the N-terminal membrane binding domain of synuclein and shows a linear dose-response to synuclein expression
The ability of transfected human αsyn to inhibit synaptic vesicle exocytosis provides an experimentally tractable system to identify the sequences responsible. first assessed the effect of three point mutations associated with familial PD (Polymeropoulos et al., 1997; Kruger et al., 1998; Zarranz et al., 2004), including the A30P mutation, which has previously been shown to disrupt the membrane association and synaptic localization of αsyn (Jo et al., 2002; Fortin et al., 2004). Transfected into hippocampal neurons with VGLUT1-pHluorin, A30P αsyn indeed shows no inhibition of synaptic vesicle exocytosis (Fig. 5A, B) despite levels of expression equivalent to the wild type protein by immunofluorescence with an antibody to the C-terminus that is not affected by the N-terminal A30P mutation (Fig. S3). Since the A30P mutation disrupts the membrane association of αsyn in vivo (Fortin et al., 2004), membrane association and the associated synaptic enrichment appear required for the inhibition of transmitter release. The lack of inhibition by A30P αsyn also serves as an additional control for wild type αsyn. In contrast to A30P αsyn, the other PD-associated mutants A53T and E46K retain membrane binding (Bussell and Eliezer, 2004; Fredenburg et al., 2007) and inhibit transmitter release (Fig. 5B).
Figure 5. The N-terminus, but not the C-terminus, of α-synuclein is required for the inhibition of synaptic vesicle exocytosis.
(A) Time course of VGLUT1-pHluorin fluorescence during a 10 Hz stimulus for 60 seconds in neurons expressing either vector, αsyn, or the PD-associated mutant A30P. (B) Peak ΔF/F0 normalized to the response in vector control shows that the A30P mutation abolishes the inhibition of neurotransmitter release by αsyn, but the A53T and E46K mutations have no effect. p<0.0001 by one-way ANOVA. p<0.001 for A30P versus wild type by Tukey’s multiple comparison test. n = 180 boutons from 9 coverslips per condition and 3 independent transfections. (C) Neurons transfected with wild type αsyn and the C-terminal truncation 1–110 also show a similar inhibition of synaptic vesicle exocytosis relative to vector control. (D) Peak ΔF/F0 normalized to the response in vector control shows that deletion of the C-terminus has no effect on the inhibition of neurotransmitter release by αsyn. The closely related isoform βsyn inhibits neurotransmitter release to a similar extent. p<0.01 by one-way ANOVA, but p>0.05 for 1–110 and βsyn versus wild type by Tukey’s multiple comparison test. n = 120 boutons from 6 coverslips per condition and 2 independent transfections. Values represent mean ± SEM. (E) Time course of changes in the fluorescence of VGLUT1-pHluorin during and after 10 Hz stimulation for 60 seconds in neurons transfected with 1.5 μg VGLUT1-pHluorin and either 1.5 μg vector control (vec), 1.5 μg αsyn, or 1.0 μg vector and 0.5 μg αsyn (0.5 μg αsyn). n = 3 coverslips, 60 boutons per condition. (F) Peak ΔF/F0 values at the end of the stimulus. Values are normalized to the response in the vector control condition. n=3 coverslips, 60 boutons per condition. The bars indicate mean ± SEM.
The highly charged C-terminus of αsyn has been suggested to interact with other proteins such as phospholipase D and the actin cytoskeleton (Payton et al., 2004), and contains a series of potential phosphorylation sites that may influence aggregation (Chen and Feany, 2005; Smith et al., 2005). However, deletion of the last 10, 20, and 30 residues has no effect on the inhibition of transmitter release by αsyn (Fig. 5C, D, data not shown). The N-terminus of αsyn thus suffices to inhibit synaptic vesicle exocytosis, supporting a primary role for the membrane-binding domain.
Consistent with a role for the more highly conserved N-terminus, we find that over-expression of β-synuclein (βsyn) also inhibits transmitter release (Fig. 5D). Since most neurons express βsyn as well as αsyn, redundancy may thus account for the minimal effect of the αsyn KO on synaptic vesicle exocytosis described above. To test this possibility, we analyzed α–/β-syn double KO mice, and observe a trend toward increased release that does not reach significance (Fig. 1G).
The similarity of KO and wild type mice in these and previous experiments raised the possibility that the effect of αsyn on synaptic transmission may require high levels of expression. Since the original titration showed expression proportionate to the amount of cDNA transfected (Fig. S1 and data not shown), we also transfected neurons with one third the usual amount. These cells show an inhibition of transmitter release, but to a lesser extent than neurons expressing more human αsyn (Fig. 5E, F). In a different set of cultures, we used twice as much αsyn cDNA as usual, and essentially eliminated the fluorescence response (Fig. S1D). The inhibition of synaptic vesicle exocytosis thus exhibits a roughly linear dose-response to over-expression of synuclein that extends well beyond baseline levels. In addition to arguing against a threshold effect that might suggest toxicity, this dose-response predicts that the knockout should have a small effect on transmitter release that would be difficult to detect above background variation.
Effects of α-synuclein on biochemical composition and ultrastructure of the nerve terminal
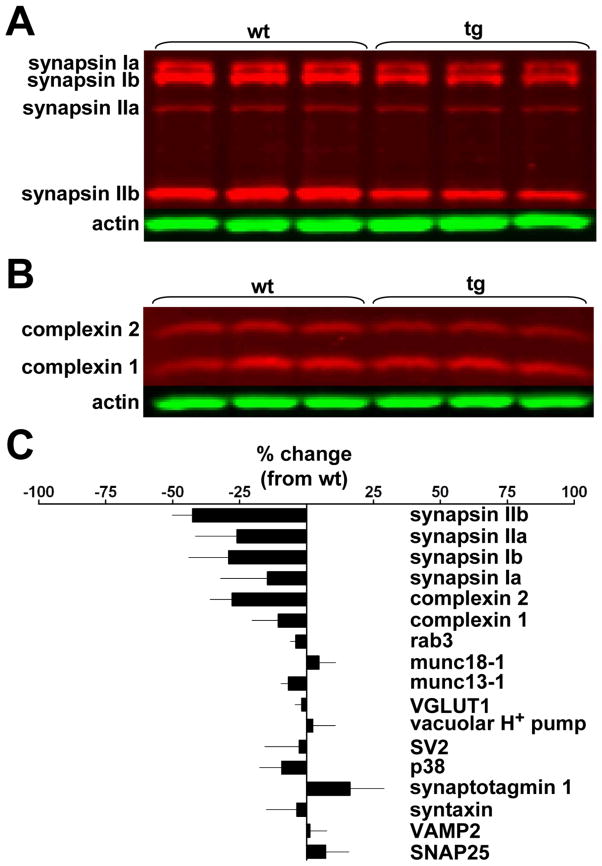
To determine whether the over-expression of αsyn affects the biochemical composition of the nerve terminal, we took advantage of the transgenic mice. In contrast to the expression of human αsyn by a small proportion of transfected neurons in culture, the widespread expression in transgenic mice should make any changes easier to detect. Using quantitative western analysis, we find no change in the amount of v- and t-SNARE fusion proteins, the calcium sensor synaptotagmin, SM proteins, rab3 and other proteins of unknown function such as synaptophysin and SV2 (Fig. 6). The preserved amount of integral synaptic vesicle membrane proteins further supports the results of imaging that suggest no effect of αsyn on the number of synaptic vesicles (Fig. 1A, B) (Rosahl et al., 1995). However, we do find a specific reduction in the synapsins (especially synapsin IIb) and complexin 2 (Fig. 6). The over-expression of αsyn thus appears to reduce the amount of two peripheral membrane proteins associated with synaptic vesicles.
Figure 6. α –Synuclein causes a selective decrease in the level of synapsins and complexins.
Western analysis of brain extracts from αsyn transgenic mice and wildtype littermates using fluorescent secondary antibodies show a reduction in synapsins (A) and complexins (B). (C) Quantitation shows no change in the level of many other synaptic proteins. n = 3 animals per genotype. Values represent mean ± SEM.
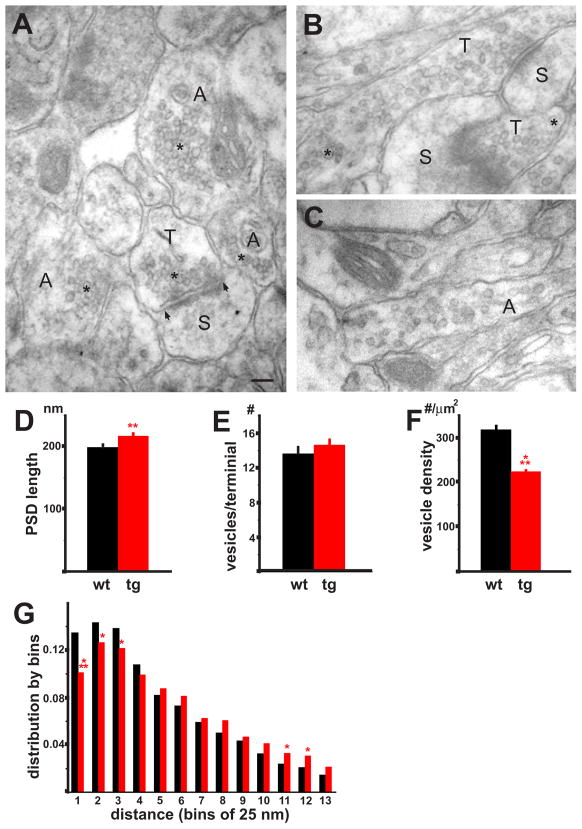
We used electron microscopy to assess further the effects of over-expressed human αsyn on synapse ultrastructure, again relying on the transgenic mice due to the relative uniformity of over-expression. Relative to the synaptic vesicles of wild type mice, which cluster at the active zone, the synaptic vesicles of transgenic animals show reduced clustering, both in boutons that contain an active zone in the same section, and in others that do not (Fig. 7B, C). The transgenic synapses appear normal in other respects, with no change in synapse density (0.75 ± 0.08 in the transgenic versus 0.77 ± 0.08 terminals/10 μm2 in wild type, p=0.79), no aggregates or evidence of toxicity, although quantitation reveals a slight enlargement of the postsynaptic density in transgenic mice (Fig. 7D). Consistent with the live imaging and the biochemistry, the total number of synaptic vesicles associated with an active zone does not differ between wild type and transgenic (Fig. 7E). However, vesicle density is substantially reduced (Fig. 7F). Quantitation of vesicle density as a function of distance from the active zone indeed shows that αsyn over-expression reduces the number of vesicles adjacent to the active zone, but increases those at greater distances (Fig. 7G).
Figure 7. Over-expression of α-synuclein disrupts synaptic vesicle clustering.
(A) An electron micrograph from stratum radiatum of hippocampal region CA1 in a wild type mouse shows clustered vesicles (*) in a terminal (T) closely apposed to the post-synaptic density of a dendritic spine (S). Although the nerve terminal and distal axons (A) are relatively large structures, their synaptic vesicles cluster (*), leaving most of the axoplasm unoccupied. (B) In a representative sex-matched transgenic sibling, synaptic vesicles show less clustering at the active zone, and occupy the full volume of the distal axon (*). (C) The distal axon of a transgenic mouse shows synaptic vesicles dispersed into the axon. Scale bar: 100 nm (A–C). (D) The post-synaptic density (PSD) is slightly longer in transgenic mice (p<0.05, n=217 for wild type and 199 for transgenic mice). Quantitation of synaptic vesicle number associated with a particular active zone (E) shows no significant differences between wild type and transgenic mice (p=0.37, n=199, 218). (F) In contrast, synaptic vesicle density in synaptic boutons of transgenic mice shows a substantial reduction relative to wild type (p<0.0001, n=217 for wild type and 201 for transgenic mice). (G) Histogram with bins indicating the following distances from the active zone: 1:25–50 nm, 2:50–75 nm, 3:75–100 nm, 4:100–125 nm, 5:125–150 nm, 6:150–175 nm, 7:175–200 nm, 8:200–225 nm, 9:225–250 nm, 10:250–275 nm, 11:275–300 nm, 12:300–325 nm, 13:325–350 nm. n = 2714 vesicles for wild type and 3193 for transgenic. A chi-square test shows p<0.05 for bins marked with * and p<0.0001 for bins marked with ***.
Over-expression of α-synuclein affects the reclustering of synaptic vesicles after endocytosis
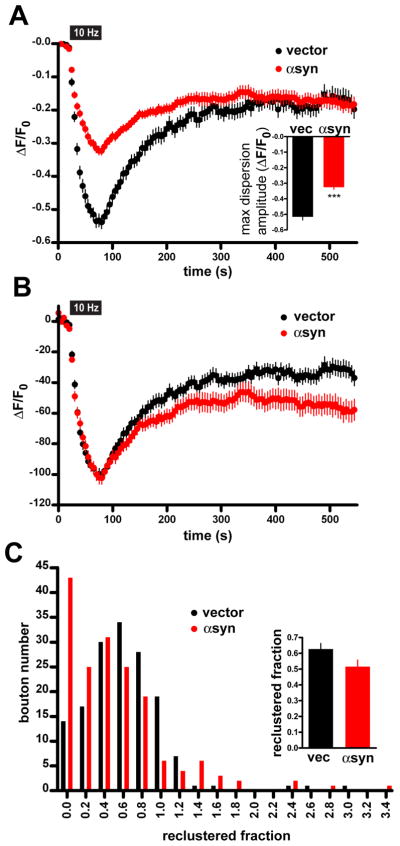
The effect of αsyn over-expression on synaptic vesicle density and recycling pool size, together with the lack of specific effect on endocytosis, suggest that αsyn may interfere with a step in the recycling pathway that follows endocytosis, such as the reclustering of newly formed synaptic vesicles. To visualize reclustering directly, we fused GFP to the N-terminus of VGLUT1: in this cytoplasmic location, GFP does not change in fluorescence with exo- or endocytosis, enabling it to serve as a reporter for the position of the protein along the axon. Indeed, previous work has shown that integral membrane proteins of the synaptic vesicle disperse with stimulation, consistent with their movement along the plasma membrane following exocytosis (Fortin et al., 2005). After dispersion, these proteins recluster with a time course (over 5–15 minutes) slower than endocytosis (<60 s). As shown in Figure 8A, GFP-VGLUT1 fluorescence at boutons declines with stimulation, and the over-expression of αsyn reduces the extent of this decline, consistent with the inhibition of release. However, normalization to the maximum decline in fluorescence with stimulation shows that VGLUT1 reclusters differently with over-expression of αsyn (Fig. 8B). Although the average time constant for fluorescence recovery in boutons that do recover appears no different from controls (τrecluster for αsyn 29.7 ± 2.7 s and for control 29.8 ± 2.6 s, p=0.98), the number of boutons that show no recovery increases substantially with over-expressed αsyn (Fig. 8C). αSyn thus affects the reclustering of synaptic vesicles after endocytosis.
Figure 8. Over-expression of α-synuclein inhibits synaptic vesicle reclustering after endocytosis.
(A) Time course of the synaptic GFP-VGLUT1 response to 10 Hz, 60 s stimulation in rat hippocampal neurons transfected with either human αsyn or vector control. Inset shows the amplitude of dispersion measured at the end of the electrical stimulus. ***, p<0.0001 by unpaired, two-tailed t-test. n=131 synapses for vector control and n=135 synapses from αsyn-transfected cells, 9 coverslips each, from 3 independent transfections. (B) Normalization of the data from (A) to the maximum extent of dispersion shows that boutons over-expressing αsyn exhibit a defect in the extent of reclustering. Inset shows, however, that there is no change in the time constant τ of reclustering. n=152 synapses for vector control and n=172 synapses from αsyn-transfected cells, 9 coverslips each from 3 independent transfections. (C) Distribution of boutons by the extent of reclustering shows that αsyn increases the proportion with reduced reclustering. p < 0.0001 by chi-square analysis. Inset shows the average reclustered fraction. n=154 synapses for vector control and n=168 synapses from αsyn-transfected cells, 9 coverslips each, from 3 independent transfections.
Discussion
The results show that over-expression of αsyn inhibits neurotransmitter release. Optical imaging of transfected neurons and electrophysiologic recording from the brain slices of transgenic mice both indicate a defect in release, consistent with the presynaptic location of αsyn. Using the same experimental paradigm, we find little or no difference between wild type and αsyn knockout. Relative to the KO, increased expression of αsyn thus appears to have a much greater effect on synaptic vesicle exocytosis.
Considering the minimal effect of the knockout, why does synuclein over-expression have a dramatic effect on transmitter release? First, over-expression could result in toxicity due to the gain of an abnormal function such as the impaired membrane trafficking observed in yeast, with indirect effects on transmitter release (Outeiro and Lindquist, 2003; Willingham et al., 2003; Cooper et al., 2006; Soper et al., 2008). However, we find no change in the level of VGLUT1-pHluorin reporter used for optical imaging of the synaptic vesicle cycle, excluding a major defect in the early secretory pathway. In addition, we deliberately over-expressed αsyn in the range predicted for patients with a duplication or triplication of the gene, reducing the likelihood of toxicity due to massive accumulation. The similar effects of αsyn over-expression in transgenic mice also exclude a role for the toxicity of acute transfection, and support the physiological significance of these observations for synaptic transmission in vivo. In addition, we did not detect any inclusions by immunofluorescence or oligomers by western analysis. The ability of a point mutation (A30P) to block the inhibition of synaptic vesicle exocytosis by αsyn further supports the specificity of the effect by wild type. The lack of change in total synaptic vesicle pool size, endocytosis and the kinetics of exocytosis also argue against non-specific toxicity.
Second, functional redundancy with βsyn and γsyn may account for the minimal phenotype of the αsyn KO. Indeed, the three isoforms are very similar in sequence, overlap in distribution, and double KO mice lacking γsyn as well as αsyn show a substantial increase in dopamine release not observed in single knockout mice (Senior et al., 2008). Consistent with potential redundancy, we now find that over-expression of βsyn also inhibits transmitter release. However, the minimal effect of the α/βsyn double KO on synaptic vesicle exocytosis and synaptic transmission (Chandra et al., 2004) makes redundancy unlikely to account for the lack of detectable effect in the αsyn single KO. Since βsyn has been suggested to protect against the aggregation of αsyn (Hashimoto et al., 2001; Uversky et al., 2002; Park and Lansbury, 2003), does not produce toxicity and is not associated with PD, the ability of βsyn to inhibit transmitter release argues further against a role for toxicity.
Third, the roughly linear inhibition of transmitter release suggests that the effect of the KO may simply be difficult to detect. Based on the response to over-expression 2-3-fold above endogenous, the effect of losing synuclein is in fact predicted to be small, and possibly within the noise associated with these measurements. Synuclein may thus have an important role at baseline. However, the effects of over-expression also suggest that the endogenous protein may have a particularly important role when up-regulated. Indeed, synuclein up-regulates under a variety of conditions (Vila et al., 2000; Quilty et al., 2006) including sporadic PD (Chiba-Falek et al., 2006), and our observations predict dramatic effects on synaptic transmission.
The hydrophilic C-terminus of αsyn has been suggested to interact with a number of proteins (Payton et al., 2004; McFarland et al., 2008), and to undergo phosphorylation at multiple sites (Okochi et al., 2000; Ellis et al., 2001; Fujiwara et al., 2002; Chen and Feany, 2005). However, we find that deletion of the C-terminus does not affect the ability of αsyn to inhibit synaptic vesicle exocytosis, excluding a requirement for the C-terminus in this function of αsyn, although it might still have a regulatory role.
Consistent with a crucial role for the N-terminal membrane-binding domain, the PD-associated A30P mutation abolishes the inhibition of transmitter release by αsyn. Since this mutation prevents the membrane association of αsyn in a number of experimental systems (Jo et al., 2002; Outeiro and Lindquist, 2003; Kubo et al., 2005), and prevents the synaptic enrichment of αsyn in neurons (Fortin et al., 2004), a high concentration of the protein at these structures appears essential for the effect on transmitter release. In adrenal medullary cells, the A30P mutant still inhibits the exocytosis of chromaffin granules (Larsen et al., 2006), but this may reflect the compact round shape of these cells that does not require specific targeting of synuclein to the release site. The A30P may thus retain some intrinsic ability to inhibit release. The inability of PD-associated A30P αsyn to inhibit transmitter release in neurons raises questions about the relevance of this activity to the pathogenesis of PD, but the reduced effect of the A30P mutant may in fact account for the late onset of disease and incomplete penetrance observed in families with the A30P mutation relative to those with A53T (Kruger et al., 2001).
How does αsyn influence synaptic transmission? Since hypertonic sucrose elicits release independent of calcium, the ability of synuclein to inhibit release stimulated by hypertonic sucrose excludes an effect of synuclein on calcium entry or the calcium-dependent triggering of release. In addition, αsyn does not alter the kinetics of either synaptic vesicle exo- or endocytosis. Rather, αsyn reduces the size of the synaptic vesicle recycling pool assessed either with FM 4-64 or by stimulation in the presence of the H+ pump inhibitor bafilomycin. Since the readily releasable pool shows a proportionate reduction in size, we infer that this reduction simply reflects the change in recycling pool. Further, the analysis of VGLUT1-pHluorin in the presence of NH4Cl, the quantitative analysis of synaptic vesicle proteins, and the electron microscopy show that αsyn does not reduce the total number of synaptic vesicles. Thus, synuclein affects specifically the size of the synaptic vesicle recycling pool.
Transgenic over-expression of αsyn also increases the paired-pulse ratio. Although changes in PPR are generally considered to reflect changes in calcium accumulation, the manipulation of release probability independent of calcium has also been shown to influence PPR (Rosahl et al., 1993; Augustin et al., 2001). In addition, the changes in PPR produced by synuclein expression are much smaller than those produced by manipulating calcium, presumably because synuclein reduces the entire pool of recycling vesicles as well as the readily releasable pool, limiting the availability of vesicles for the second pulse.
The analysis of transgenic mice over-expressing αsyn shows a reduced number of synaptic vesicles adjacent to the active zone, consistent with a defect in vesicle mobilization rather than fusion. The transgenic mice indeed show a general reduction in synaptic vesicle clustering, suggesting a physical basis for the defect in mobilization. How does αsyn control synaptic vesicle clustering and the size of the recycling pool? The biochemical analysis suggests several possibilities.
The transgenic mice over-expressing αsyn show a 20–45% reduction in the amount of multiple synapsins, and previous work has implicated the synapsins in synaptic vesicle mobilization (Hilfiker et al., 1999). The knockout of synapsin I reduces recycling pool size by 30–40% (Ryan et al., 1996).However, the ultrastructural analyses of synapses from mice lacking either synapsin I or synapsin II show a reduction in the total number of synaptic vesicles, distinct from the primary defect in recycling pool size observed with αsyn over-expression (Rosahl et al., 1995). In addition, the over-expression of αsyn appears to cause a more severe defect in the recycling pool than the complete loss of synapsins, and the transgenic mice show only a partial reduction in synapsins. The effect of αsyn over-expression is thus unlikely to reflect simply the loss of synapsins. Rather, αsyn and the synapsins both seem to influence related processes involved in vesicle mobilization.
Over-expression of αsyn also reduces the amount of complexin 2 in the transgenic mice. A change in the level of complexins, which are implicated in a step at or close to fusion with the plasma membrane (Sudhof and Rothman, 2009), might thus be consistent with a proposed role for synuclein as chaperone for SNARE proteins (Chandra et al., 2005). However, a change in SNARE protein function seems unlikely to account for the observed defect in synaptic vesicle mobilization, or the reduced clustering of synaptic vesicles near the active zone. On the other hand, α-/β-synuclein double KO mice show a 30% increase in complexins (particularly complexin 2) (Chandra et al., 2004), suggesting that the reduction in complexin 2 observed in the transgenic mice may reflect a gain in the normal function of αsyn.
The imaging of VGLUT1-pHluorin provides indirect evidence that synuclein impairs the reclustering of synaptic vesicles after endocytosis. To visualize this directly, we used a cytoplasmic fusion of VGLUT1 to GFP rather than the lumenal fusion to ecliptic pHluorin. Although the steady-state dispersion of synaptic vesicles observed by electron microscopy might have resulted from a subtle defect in reclustering that becomes evident only over time, we observe a clear defect in reclustering after only a single period of stimulation. The imaging of GFP-VGLUT1 thus provides information complementary to the pHluorin fusion, and direct evidence for a defect in synaptic vesicle reclustering that presumably accounts for the synaptic vesicle dispersion observed by electron microscopy, and the reduction in recycling pool size observed by imaging.
Conclusion
These experiments demonstrate that increased expression of αsyn causes a specific physiological impairment of neurotransmitter release in the absence of overt toxicity. It remains unclear whether this functional disturbance leads eventually to anatomical degeneration. However, considerable evidence implicates increased expression of αsyn in the pathogenesis of sporadic as well as familial PD, indicating that the changes observed must represent some of the earliest events in the progression toward PD. The results also suggest an explanation for the ability of αsyn to protect against degeneration caused by loss of the presynaptic chaperone cysteine string protein alpha (CSPα) (Chandra et al., 2005). CSPα appears required for the maintenance of presynaptic function but not for transmitter release itself: synaptic transmission in the CSPα KO appears normal early in development, but the terminals eventually degenerate (Fernandez-Chacon et al., 2004). CSPα thus has an activity-dependent role in maintenance of the nerve terminal, and the inhibition of release caused by αsyn over-expression may simply decrease the requirement for CSPα. Importantly, over-expression of A30P αsyn was unable to rescue the loss of CSPα (Chandra et al., 2005), presumably because the A30P mutant cannot inhibit transmitter release.
Since αsyn is widely expressed, the results predict that individuals carrying a duplication or triplication of the gene will show changes from an early age in the function of many neural circuits. In sporadic PD, where up-regulation of αsyn may first occur in only a subset of cells that normally express the protein (Braak et al., 2003), the deficit may be more restricted. Nonetheless, physiological defects may prove more sensitive than anatomic or even biochemical changes as early markers for PD, and serve as targets for early, therapeutic intervention.
Experimental Procedures
Neuronal Culture and Transfection
Primary hippocampal cultures were prepared as previously described (Li et al., 2005). For cotransfection, 1.5 μg VGLUT1-pHluorin DNA and 1.5 μg pCAGGS or pCAGGS-αsyn DNA were used per 3 × 106 cells. For experiments using FM dyes, neurons were grown as above but plated at 177–283 cells/mm2 and grown inverted over a monolayer of glial cells (Banker and Goslin, 1998).
To prepare hippocampal cultures from mice, hippocampi from P0 P1 αsyn KO mice or wildtype littermates were dissociated in 0.25% trypsin, washed several times in HBSS containing 10 mM HEPES and 20 mM glucose, triturated, and electroporated with 0.6 μg total DNA per 500,000 cells (Amaxa). In cotransfection experiments, 0.3 μg VGLUT1-pHluorin DNA and 0.3 μg pCAGGS or pCAGGS-αsyn DNA were used. After transfection, cells were allowed to recover for 10 minutes and plated in MEM containing 21 mM glucose, 5% FBS, 2% B27, 1% Glutamax, and Mito+ serum extender (BD Biosciences) at a density of 1768 cells/mm2 onto coverslips coated with poly-L-lysine (Sigma). Cultures were maintained with 5-FU and uridine containing media as described above for rat-derived cultures.
Postnatal rat midbrain cultures were prepared from the ventral mesencephalon of P0–P1 rats as described (Mena et al., 1997) and electroporated with 0.6 μg total DNA per 500,000 cells (Amaxa) before plating onto astrocyte feeder layers at a density of 1415 – 2830 cells/mm2.
Live Cell Imaging
Transfected neurons were imaged between 14–21 DIV at room temperature (24°C) as previously described (Voglmaier et al., 2006) unless otherwise stated. For imaging at a more physiological temperature, the stage was warmed to 35°C using the TC-344B Dual Automatic Stage Temperature Controller (Warner Instrument). Images were obtained under epifluorescence illumination with a 63x 1.2NA water objective and an ORCA ER CCD camera (Hamamatsu), using 2×2 on-chip pixel binning. The fluorescence of individual synaptic boutons was quantified by placing 4×4 ROIs manually over the center of fluorescent puncta. The fluorescence in these regions was averaged, subtracted by the average of 3 ROIs over regions of the field without cell bodies or processes, and the fractional change in fluorescence over time normalized to the initial fluorescence (ΔF/F0). Traces represent a single experiment in which 20 boutons were selected per coverslip, and the data from 3 coverslips averaged. To quantify peak ΔF/F0, 20 boutons were selected per coverslip, and 9 coverslips from 3 independent transfections were averaged. All imaging and quantitation was performed blind to the construct transfected.
To measure recycling pool size using FM 4-64, neurons transfected with either pCAGGS-IRES2-GFP or pCAGGS-αsyn-IRES2-GFP were incubated in Tyrode’s buffer containing 15 μM FM 4-64 and stimulated at 10 Hz for 60 seconds. The dye was washed out 1 minute after the end of stimulation to allow full internalization and the cells washed in Tyrode’s buffer for an additional 10 – 15 minutes before unloading at 10 Hz for 120 seconds. The pool size was determined by quantifying the amount of FM fluorescence released by the second stimulus at boutons expressing GFP.
To quantify recycling and total pool size using VGLUT1-pHluorin, neurons transfected with either VGLUT1-pHluorin + vector control or VGLUT1-pHluorin + αsyn were stimulated at 10 Hz for 150 seconds in the presence of 1 μM bafilomycin (Calbiochem) to reveal total recycling pool size. At the end of the stimulus, Tyrode’s containing 50 mM NH4Cl was added to reveal the total size of the synaptic vesicle pool.
To assess synaptic vesicle dispersion, neurons transfected with VGLUT1-GFP and either vector control or αsyn were stimulated at 10 Hz for 60 seconds in Tyrode’s solution containing 25 mM HEPES and 119 mM NaCl. Images were collected as above for 10 min at 0.2 Hz under epifluorescence illumination with a 100x 1.49 NA oil objective. The fluorescence at nerve terminals was quantified by outlining the perimeter of synaptic boutons and calculating the average fluorescence intensity. The extent of dispersion (D) was determined by averaging five ΔF/F0 values surrounding the peak of fluorescence change. The extent of reclustering (R) was determined by measuring the fluorescence plateau after stimulus cessation. The reclustering fraction (Fr) was calculated as . The kinetics of reclustering were analyzed only for those boutons where the fluorescence recovery was fit by a single exponential A–B (1–e−kt). Boutons that did not display fluorescence recovery or whose recovery did not fit a single exponential were grouped in bin 100 (Fig. 8C).
Electrophysiology
Transverse 300–400 μm hippocampal slices were prepared from 24- to 36-day-old animals hemizygous for the human αsyn transgene and from wild type littermates, and field EPSPs evoked in CA1 stratum as previously described (Lu et al., 2009). Slices from transgenic mice and wild type littermates were interleaved, and the experimenter was blind to genotype. mEPSCs were monitored by whole cell recording, also as previously described (Lu et al., 2009).
Supplementary Material
Acknowledgments
We thank E. Wallender and B. Mukherjee for technical support, K. Thorn and the UCSF Nikon Center for assistance with the imaging, M. Larsson for help with the electron microscopy, A. Frigessi for guidance in the statistical analysis, and R. Tsien for thoughtful discussions. This work was supported by the UCSF MSTP and a predoctoral fellowship from the Hillblom Foundation (to V.M.N.), a postdoctoral fellowship from the Hillblom Foundation (to K.N.), the American Heart Association (W.L.), the University of Oslo (V.B.), the Norwegian Research Council (F.A.C.), the National Institutes of Mental Health (R.A.N. and R.H.E.), the National Parkinson Foundation, and the Michael J. Fox foundation (R.H.E.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Augustin I, Korte S, Rickmann M, Kretzschmar HA, Sudhof TC, Herms JW, Brose N. The cerebellum-specific Munc13 isoform Munc13-3 regulates cerebellar synaptic transmission and motor learning in mice. J Neurosci. 2001;21:10–17. doi: 10.1523/JNEUROSCI.21-01-00010.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G, Goslin K. Culturing nerve cells. 2. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Bussell R, Jr, Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329:763–778. doi: 10.1016/s0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- Bussell R, Jr, Eliezer D. Effects of Parkinson’s disease-linked mutations on the structure of lipid-associated alpha-synuclein. Biochemistry. 2004;43:4810–4818. doi: 10.1021/bi036135+. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, et al. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci U S A. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- Chiba-Falek O, Lopez GJ, Nussbaum RL. Levels of alpha-synuclein mRNA in sporadic Parkinson disease patients. Mov Disord. 2006;21:1703–1708. doi: 10.1002/mds.21007. [DOI] [PubMed] [Google Scholar]
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science (New York, NY. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Bo G, St-Gelais F, Danik M, Williams S, Cotton M, Trudeau LE. Dopamine neurons in culture express VGLUT2 explaining their capacity to release glutamate at synapses in addition to dopamine. J Neurochem. 2004;88:1398–1405. doi: 10.1046/j.1471-4159.2003.02277.x. [DOI] [PubMed] [Google Scholar]
- Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- Dickson DW. Alpha-synuclein and the Lewy body disorders. Current opinion in neurology. 2001;14:423–432. doi: 10.1097/00019052-200108000-00001. [DOI] [PubMed] [Google Scholar]
- Ellis CE, Schwartzberg PL, Grider TL, Fink DW, Nussbaum RL. alpha-synuclein is phosphorylated by members of the Src family of protein-tyrosine kinases. J Biol Chem. 2001;276:3879–3884. doi: 10.1074/jbc.M010316200. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Brasnjo G, Hayashi M, Wolfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, et al. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science (New York, NY. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Wolfel M, Nishimune H, Tabares L, Schmitz F, Castellano-Munoz M, Rosenmund C, Montesinos ML, Sanes JR, Schneggenburger R, et al. The synaptic vesicle protein CSP alpha prevents presynaptic degeneration. Neuron. 2004;42:237–251. doi: 10.1016/s0896-6273(04)00190-4. [DOI] [PubMed] [Google Scholar]
- Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of alpha-synuclein. J Neurosci. 2005;25:10913–10921. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredenburg RA, Rospigliosi C, Meray RK, Kessler JC, Lashuel HA, Eliezer D, Lansbury PT., Jr The impact of the E46K mutation on the properties of alpha-synuclein in its monomeric and oligomeric states. Biochemistry. 2007;46:7107–7118. doi: 10.1021/bi7000246. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, et al. The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci U S A. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Mante M, Mallory M, Masliah E. beta-Synuclein inhibits alpha-synuclein aggregation: a possible role as an anti-parkinsonian factor. Neuron. 2001;32:213–223. doi: 10.1016/s0896-6273(01)00462-7. [DOI] [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:269–279. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Jo E, Fuller N, Rand RP, St George-Hyslop P, Fraser PE. Defective membrane interactions of familial Parkinson’s disease mutant A30P alpha-synuclein. J Mol Biol. 2002;315:799–807. doi: 10.1006/jmbi.2001.5269. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Leenders KL, Sprengelmeyer R, Muller T, Woitalla D, Portman AT, Maguire RP, Veenma L, Schroder U, et al. Familial parkinsonism with synuclein pathology: clinical and PET studies of A30P mutation carriers. Neurology. 2001;56:1355–1362. doi: 10.1212/wnl.56.10.1355. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kubo S, Nemani VM, Chalkley RJ, Anthony MD, Hattori N, Mizuno Y, Edwards RH, Fortin DL. A combinatorial code for the interaction of alpha-synuclein with membranes. J Biol Chem. 2005;280:31664–31672. doi: 10.1074/jbc.M504894200. [DOI] [PubMed] [Google Scholar]
- Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, et al. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Waites CL, Staal RG, Dobryy Y, Park J, Sulzer DL, Edwards RH. Sorting of vesicular monoamine transporter 2 to the regulated secretory pathway confers the somatodendritic exocytosis of monoamines. Neuron. 2005;48:619–633. doi: 10.1016/j.neuron.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Purisai MG, Bolin LM, Di Monte DA. Alpha-synuclein overexpression protects against paraquat-induced neurodegeneration. J Neurosci. 2003;23:3095–3099. doi: 10.1523/JNEUROSCI.23-08-03095.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraganore DM, de Andrade M, Elbaz A, Farrer MJ, Ioannidis JP, Kruger R, Rocca WA, Schneider NK, Lesnick TG, Lincoln SJ, et al. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006;296:661–670. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Vila M, Lincoln S, McCormack A, Picciano M, LaFrancois J, Yu X, Dickson D, Langston WJ, McGowan E, et al. Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol Dis. 2001;8:535–539. doi: 10.1006/nbdi.2001.0392. [DOI] [PubMed] [Google Scholar]
- McFarland MA, Ellis CE, Markey SP, Nussbaum RL. Proteomic analysis identifies phosphorylation-dependent a-synuclein protein interactions. Mol Cell Proteomics. 2008 doi: 10.1074/mcp.M800116-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena MA, Khan U, Togasaki DM, Sulzer D, Epstein CJ, Przedborski S. Effects of wild-type and mutated copper/zinc superoxide dismutase on neuronal survival and L-DOPA-induced toxicity in postnatal midbrain culture. J Neurochem. 1997;69:21–33. doi: 10.1046/j.1471-4159.1997.69010021.x. [DOI] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Miller DW, Hague SM, Clarimon J, Baptista M, Gwinn-Hardy K, Cookson MR, Singleton AB. Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology. 2004;62:1835–1838. doi: 10.1212/01.wnl.0000127517.33208.f4. [DOI] [PubMed] [Google Scholar]
- Mozhayeva MG, Sara Y, Liu X, Kavalali ET. Development of vesicle pools during maturation of hippocampal synapses. J Neurosci. 2002;22:654–665. doi: 10.1523/JNEUROSCI.22-03-00654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi M, Walter J, Koyama A, Nakajo S, Baba M, Iwatsubo T, Meijer L, Kahle PJ, Haass C. Constitutive phosphorylation of the Parkinson’s disease associated alpha-synuclein. J Biol Chem. 2000;275:390–397. doi: 10.1074/jbc.275.1.390. [DOI] [PubMed] [Google Scholar]
- Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science (New York, NY. 2003;302:1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Lansbury PT., Jr Beta-synuclein inhibits formation of alpha-synuclein protofibrils: a possible therapeutic strategy against Parkinson’s disease. Biochemistry. 2003;42:3696–3700. doi: 10.1021/bi020604a. [DOI] [PubMed] [Google Scholar]
- Park SM, Jung HY, Kim HO, Rhim H, Paik SR, Chung KC, Park JH, Kim J. Evidence that alpha-synuclein functions as a negative regulator of Ca(++)-dependent alpha-granule release from human platelets. Blood. 2002;100:2506–2514. doi: 10.1182/blood.V100.7.2506. [DOI] [PubMed] [Google Scholar]
- Payton JE, Perrin RJ, Woods WS, George JM. Structural determinants of PLD2 inhibition by alpha-synuclein. J Mol Biol. 2004;337:1001–1009. doi: 10.1016/j.jmb.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Petrucelli L, O’Farrell C, Lockhart PJ, Baptista M, Kehoe K, Vink L, Choi P, Wolozin B, Farrer M, Hardy J, et al. Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36:1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science (New York, NY. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Pyle JL, Kavalali ET, Piedras-Renteria ES, Tsien RW. Rapid reuse of readily releasable pool vesicles at hippocampal synapses. Neuron. 2000;28:221–231. doi: 10.1016/s0896-6273(00)00098-2. [DOI] [PubMed] [Google Scholar]
- Quilty MC, King AE, Gai WP, Pountney DL, West AK, Vickers JC, Dickson TC. Alpha-synuclein is upregulated in neurones in response to chronic oxidative stress and is associated with neuroprotection. Experimental neurology. 2006;199:249–256. doi: 10.1016/j.expneurol.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Geppert M, Spillane D, Herz J, Hammer RE, Malenka RC, Sudhof TC. Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell. 1993;75:661–670. doi: 10.1016/0092-8674(93)90487-b. [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Sudhof TC. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Li L, Chin LS, Greengard P, Smith SJ. Synaptic vesicle recycling in synapsin I knock-out mice. J Cell Biol. 1996;134:1219–1227. doi: 10.1083/jcb.134.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior SL, Ninkina N, Deacon R, Bannerman D, Buchman VL, Cragg SJ, Wade-Martins R. Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. Eur J Neurosci. 2008;27:947–957. doi: 10.1111/j.1460-9568.2008.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science (New York, NY. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Smith WW, Margolis RL, Li X, Troncoso JC, Lee MK, Dawson VL, Dawson TM, Iwatsubo T, Ross CA. Alpha-synuclein phosphorylation enhances eosinophilic cytoplasmic inclusion formation in SH-SY5Y cells. J Neurosci. 2005;25:5544–5552. doi: 10.1523/JNEUROSCI.0482-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper JH, Roy S, Stieber A, Lee E, Wilson RB, Trojanowski JQ, Burd CG, Lee VM. Alpha-synuclein-induced aggregation of cytoplasmic vesicles in Saccharomyces cerevisiae. Mol Biol Cell. 2008;19:1093–1103. doi: 10.1091/mbc.E07-08-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science (New York, NY. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, Rayport S. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Li J, Souillac P, Millett IS, Doniach S, Jakes R, Goedert M, Fink AL. Biophysical properties of the synucleins and their propensities to fibrillate: inhibition of alpha-synuclein assembly by beta- and gamma-synucleins. The Journal of biological chemistry. 2002;277:11970–11978. doi: 10.1074/jbc.M109541200. [DOI] [PubMed] [Google Scholar]
- Vila M, Vukosavic S, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S. Alpha-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. Journal of neurochemistry. 2000;74:721–729. doi: 10.1046/j.1471-4159.2000.740721.x. [DOI] [PubMed] [Google Scholar]
- Voglmaier SM, Kam K, Yang H, Fortin DL, Hua Z, Nicoll RA, Edwards RH. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51:71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Willingham S, Outeiro TF, DeVit MJ, Lindquist SL, Muchowski PJ. Yeast genes that enhance the toxicity of a mutant huntingtin fragment or alpha-synuclein. Science (New York, NY. 2003;302:1769–1772. doi: 10.1126/science.1090389. [DOI] [PubMed] [Google Scholar]
- Yavich L, Tanila H, Vepsalainen S, Jakala P. Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhou W, Hurlbert MS, Schaack J, Prasad KN, Freed CR. Overexpression of human alpha-synuclein causes dopamine neuron death in rat primary culture and immortalized mesencephalon-derived cells. Brain Res. 2000;866:33–43. doi: 10.1016/s0006-8993(00)02215-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.