Abstract
Echogenic liposomes (ELIP) have additional promise, beyond diagnostic agents, as vehicles for delivering oligonucleotides (ODN), especially if the release of the agent can be triggered and its uptake can be enhanced by ultrasound application at a specific site. The purpose of this study was to co-encapsulate air and NF-κB decoy ODN within ELIP allowing ultrasound to release encapsulated ODN from ELIP, and to accurately quantify release of encapsulated ODN from ELIP upon ultrasound application. FITC-labeled sense ODN (2 mM) was incorporated within ELIP using freeze/thaw method. Encapsulation efficiency of FITC-ODN was spectrofluorometrically analyzed by quenching fluorescence of unencapsulated FITC-ODN using a complementary strand tagged with Iowa Black FQ-ODN. Quenching of FITC-ODN (0.05 μM) with Iowa Black FQ-ODN (0.1 μM) was found to be efficient (92.4 ± 0.2 %), allowing accurate determination of encapsulated ODN. Encapsulation efficiency of ODN was 14.2 ± 2.5 % in DPPC/DOPC/DPPG/CH liposomes and 29.6 ± 1.5 % in DPPC/DOPE/DPPG/CH liposomes. Application of ultrasound (1 MHz continuous wave, 0.26 MPa peak-to-peak pressure amplitude, 60 seconds.) to the latter formulation triggered 41.6 ± 4.3 % release of ODN from ODN-containing ELIP. We have thus demonstrated that ODN can be encapsulated into ELIP and released efficiently upon ultrasound application. These findings suggest potential applications for gene therapy in atherosclerosis treatment.
Keywords: Oligonucleotide, liposome, ultrasound, controlled release
1. INTRODUCTION
Cis-element ds-oligonucleotides are short DNA fragments that attenuate a cis-trans interaction, leading to the removal of trans factors from the endogenous cis-element with subsequent modulation of specific gene expression [1–3]. Recent studies in cell culture and animal models have demonstrated that the decoy can be beneficial for treatment of cancer and cardiovascular diseases. Several decoys are currently undergoing evaluation in clinical trials [4].
Despite these important applications, therapeutics development using oligonucleotides (ODN) has been hampered by several problems. Of particular concern are their short half-life [5, 6] and limited cellular or tissue uptake [7]. A number of vectors have been developed to prevent ODN from degradation by encapsulating ODN with some form of protective coating, including liposomes [8], hemagglutinating virus of Japan (HVJ)-liposomes [9], polymeric microspheres [10], and a variety of nanoparticles [11]. Also, several methodologies have been developed to enhance cellular uptake of the decoys, including pressure-mediated transfer [12], electrically enhanced transfer, and biolistic bombardment [13].
We have previously developed a novel drug delivery method employing echogenic liposomes (ELIP) that co-encapsulate a gas and a drug such that the latter can be released upon application of ultrasound [14]. In the present study, we demonstrate that ELIP can be utilized to encapsulate and release ODN. This method has several advantages. First, packaging protects the ODN from nucleases. Second, release of contents can be controlled by modulating ultrasound parameters. A single high amplitude ultrasound pulse can be used for bolus release, a series of low amplitude pulses for sustained release, or a combination of these for therapeutic release. This is particularly valuable when the local concentration of an agent must be increased to a therapeutic level. Thirdly, ultrasound-driven cavitation of the air bubbles in ELIP can increase drug or gene transport into the arterial wall. The endothelium presents the primary barrier for transmural drug transport; the larger the agent, the higher the barrier. Cavitation caused by ultrasound energy increases membrane permeability and facilitates cellular transfection of large molecules such as gene-containing plasmids [15]. In the presence of ultrasound contrast agents, however, such cavitation effects can be considerably amplified. Studies have demonstrated that destruction of gas-filled microbubbles by ultrasound leads to pericapillary extravasation of red blood cells with concomitant transport of microbubble fragments and colloid particles deep into the pericapillary tissues [16, 17]. ELIP nucleate cavitation events upon ultrasound exposure, and hence have the capability to deliver their payload beyond the endothelium and into the arterial bed. A delivery strategy for serum-sensitive therapeutic agent is thus feasible; ODN or similarly large molecules are sequestered in ELIP vehicles and released by application of ultrasound after arrival at the target site.
In order to determine the encapsulation efficiency of therapeutic agents within liposomes, separation procedures are generally required to remove the unencapsulated agents so that the encapsulated agents can be assayed. A variety of separation procedures have been developed, including column chromatography, density centrifugation, and dialysis. These are, however, time-consuming and may introduce errors due to the manipulations involved. An alternative and more efficient technique with sufficient sensitivity is desirable to accurately quantify encapsulation efficiency without separation procedures. We utilize “molecular beacons” to determine efficiency of ODN encapsulation into delivery vehicles such as liposomes. Molecular beacons are ODN probes that can report the presence of specific nucleic acids in homogenous solutions [18]. A fluorescent moiety is attached to an ODN strand used in conjunction with a complementary strand to which a quenching moiety is attached. When the two moieties hybridize, the fluorophore and quencher are brought close enough for energy transfer, with the consequence that the fluorescence is eliminated or markedly reduced. Using this molecular beacon, we demonstrate the efficient encapsulation of NF-κB decoy ODN within ELIP and quantitate the release of encapsulated ODN from ELIP upon ultrasound application.
2. MATERIALS AND METHODS
2.1. Materials
L-α-phosphatidylcholine (chicken egg, EPC), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine (EDOPC), 1,2-dipalmitoyl-sn-glycero-3-[phospho-rac-1-glycerol] (DPPG), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE), and cholesterol (CH) were purchased from Avanti Polar Lipids (Alabaster, AL) and stored at −20 °C in chloroform. FITC-NF-κB decoy sense ODN (5′-AGT TGA GGG GAC TTT CCC AGG C/36-FAM/ - 3′) and Iowa BlackTM FQ labeled NF-κB decoy antisense ODN (5′ - /5IAbFQ/GCC TGG GAA AGT CCC CTC AAC T – 3′) from Integrated DNA Technologies, Inc. (Coralville, IA) were utilized. Mannitol, TritonX-100, and 3-morpholinepropanesulfonic acid (MOPS) were purchased from Sigma Chemical Co. (St. Louis, MO).
2.2. Iowa Black FQ-ODN Quenching Method
Fluorescence measurements at single wavelengths were performed using a spectrofluorometer (Alphascan, Photon Technologies Inc., South Brunswick, NJ), and data were analyzed with software supplied with the instrument. Excitation and emission monochromators were set at 490 and 520 nm, respectively. The self-quenching concentration of NF-κB FITC-ODN was experimentally determined by measuring the change in fluorescence intensity as a function of increasing concentrations of FITC-ODN.
The amount of Iowa Black FQ-ODN for optimal quenching of FITC-ODN fluorescence under our conditions was determined by titration of 0.05 μM of FITC-ODN (in 50 mM MOPS buffer containing 110 mM NaCl, pH 7.2) with Iowa Black FQ-ODN (2 μM) to a final molar ratio of 2:1 (Iowa Black FQ-ODN:FITC-ODN). A control titration was performed using MOPS buffer in place of Iowa Black FQ-ODN.
After the optimal amount of Iowa Black FQ-ODN was established, the fluorescence of FITC-ODN and its quenching by Iowa Black FQ-ODN was determined in 5 % TritonX-100 and in the presence of three different liposome samples, chosen to reveal potential influence of the electrostatic charge of the liposome. Cationic liposomes were prepared with EPC/EDOPC/CH (60:10:30, mol ratio), anionic liposomes with EPC/DPPE/DPPG/CH (69:8:8:15, mol ratio), and neutral liposomes with EPC/CH (80:20, mol ratio). The samples were added to FITC-ODN to yield a final lipid concentration of 0.5 mg/ml. In addition, the Iowa Black FQ method was tested using buffers at differing pH condition.
2.3. Preparation of Acoustically Active ODN-Containing Liposomes
Two different lipid mixtures were used for ELIP preparation. These differed in phosphatidylcholine-phosphatidylethanolamine ratios and had the compositions; DPPC/DOPC/DPPG/CH (36:36:8:20, mol ratio) and DPPC/DOPE/DPPG/CH (36:36:8:20, mol ratio). The lipids (1 mg total weight for each sample) were mixed in chloroform in a small glass vial. The solvent was removed by evaporation under argon in a 50 °C water bath with constant spinning of the vial until a thin film of lipids had formed on the vial wall. The lipid film was placed under high vacuum (<100 mTorr) for 4 hours for complete removal of solvent. The dried lipid film was hydrated with FITC-ODN at 2 μM in 0.32 M mannitol. For double-stranded ODN studies, untagged double-stranded ODN were added at 1 mM (6.4 μg of ODN) in addition to the FITC-ODN. The resultant liposome dispersion (20 mg/ml) was heated shortly using a hair dryer, and sonicated in a water bath for 10 seconds to disperse the lipid and promote its hydration. The samples were subjected to three freeze/thaw cycles for optimal ODN encapsulation using dry ice [14]. After the third freeze/thaw cycle, the samples were again frozen in dry ice and lyophilized for 24 hours. The lyophilized dry cake was stored at 4 °C; as needed, it was resuspended at a concentration of 10 mg/ml in deionized water and used immediately.
2.4. Measurement of Encapsulation
The encapsulation efficiency and ultrasound-triggered release of FITC-ODN were determined fluorometrically using a procedure analogous to a previously published method that takes advantage of quenching of the calcein fluorophore by cobalt ion [19, 20]. In the present study, Iowa Black FQ-ODN, an ODN commonly used in real-time PCR, was the quencher and a complementary FITC-ODN was the fluorophore. Resuspended liposomes (10 mg/ml) encapsulating FITC-ODN were diluted to 5 mg/ml with 50 mM MOPS/110 mM NaCl. Thirty μl of this sample was then diluted to 300 μl with MOPS buffer resulting in a final lipid concentration of 0.5 mg/ml and FITC-ODN concentration of 0.05 μM during fluorescence measurements. Iowa Black FQ-ODN was used to quench the fluorescence of external FITC-ODN so that the residual fluorescence represented the entrapped FITC-ODN. The liposomes were then lysed with detergent to determine the background fluorescence at zero encapsulated volume. Fluorescence intensity was measured before (Fb) and after (Fa) the addition of 15 μl of 2 μM Iowa Black FQ-ODN, and measured again after the addition of 15 μl of 10% Triton X-100 (Ftotq). The fluorometer was set at 490 nm excitation and 520 nm emission with 3 nm slit widths. Encapsulation efficiency of the entrapped FITC-ODN was calculated as:
2.5. Ultrasound-Triggered Release
Ultrasound-triggered release experiments were performed using cell culture inserts (12 mm diameter, Costar Corning Inc., Corning, NY) which had a membrane-lined bottom. A cell culture insert was placed on the surface of a thin layer of deionized water on a Rho-C rubber pad, an anechoic material, such that the insert and the rubber would not contact each other. A calibrated 1-MHz continuous wave ultrasound transducer which has a 10 mm diameter transducer (Sonitron 1000, Rich-Mar Corp, Inola, OK) was mounted 5 mm above the membrane of the cell culture insert. The membrane was subjected to 0.26 MPa peak-to-peak pressure amplitude for 60 seconds.
The ultrasound-triggered release of ODN was investigated using two different formulations of liposomes (DPPC/DOPC/DPPG/CH and DPPC/DOPE/DPPG/CH). For each liposomal formulation, both echogenic and non-echogenic liposomes were evaluated. Non-echogenic liposomes were prepared by incubating echogenic liposomes at room temperature for over 48 hours after resuspension and then slowly subjecting them to vacuum for 5 minutes to release the residual air that had not diffused out during the incubation period.
The release of encapsulated FITC-ODN was determined by a procedure similar to that used to measure encapsulation. Resuspended liposome samples (10 mg/ml) were diluted to 5 mg/ml with 50 mM MOPS/110 mM NaCl. Thirty μl of this dispersion was added to glass culture tubes containing 270 μl MOPS/NaCl buffer for fluorescence measurements. The fluorescence intensity (Fin) of the suspension was measured after the addition of 15 μl of 2 μM Iowa Black FQ-ODN. The sample was removed from the fluorometer, transferred to cell culture inserts, and exposed to the ultrasound (1 MHz, 0.26 MPa peak-to-peak pressure amplitude) for 60 seconds. The sample was transferred to fluorometer tubes, and fluorescence intensity (Fultrasound) was measured. Finally, 15 μl of 10% Triton X-100 was added and fluorescence intensity (Ftotq) was measured. In each case sufficient time was allowed for the FITC-ODN and Iowa Black FQ-ODN to completely hybridize determined by assessing the lack of signal changes. The amount of release was calculated as:
2.6. Determination of Echogenicity
Liposomes were imaged using a 20-MHz intravascular ultrasound catheter (3.2 F, Boston Scientific Scimed Inc, Maple Grove, MN) in a 5 ml glass vial. Relative echogenicity (apparent brightness) of the liposomal formulations was objectively assessed using computer-assisted videodensitometry with white indicating complete reflection and black, zero reflection. This process involves image acquisition, pre-processing, and gray scale quantification. Images were digitized to 640 × 480-pixel spatial resolution (approximately 0.045 mm/pixel) and 8-bit (256 level) amplitude resolution. Image processing and analysis were performed using Image Pro-Plus Software (Media Cybernetics, Silver Spring, MD). Data are reported as mean gray scale values.
2.7. Statistics
Multiple groups were compared using the analysis of variance (ANOVA) with pairwise multiple comparison performed using the Tukey Test (SigmaStat, Systat Software Inc, Chicago, IL). A p-value less than 0.05 was considered significant.
3. RESULTS
3.1. Iowa Black FQ-ODN Quenching Method
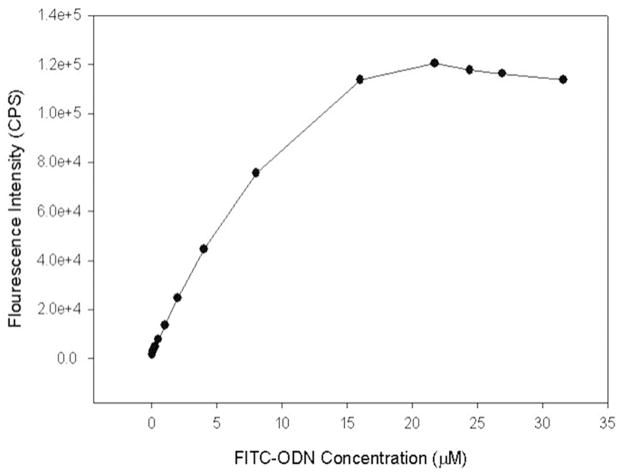
Fig. 1 demonstrates that, under baseline conditions (6 mm diameter fluorometer cuvettes, right angle detection), there is a linear relationship between the FITC-ODN fluorescence intensity and concentration between zero to 8 μM. The fluorescence intensity was sublinear at FITC-ODN concentrations above 8 μM, and started to decrease at 20–24 μM (presumably primarily as a consequence of self-quenching, but the inner filter effect may also contribute). The FITC-ODN concentration of 2 μM was utilized for other experiments.
Figure 1.
Concentration dependence of FITC-ODN fluorescence (6 mm diameter cuvette). FITC-ODN fluorescence is linear up to 8 μM. Above 8 μM, the relationship becomes nonlinear, and intensity begins to decrease at high concentration starts self-quenching.
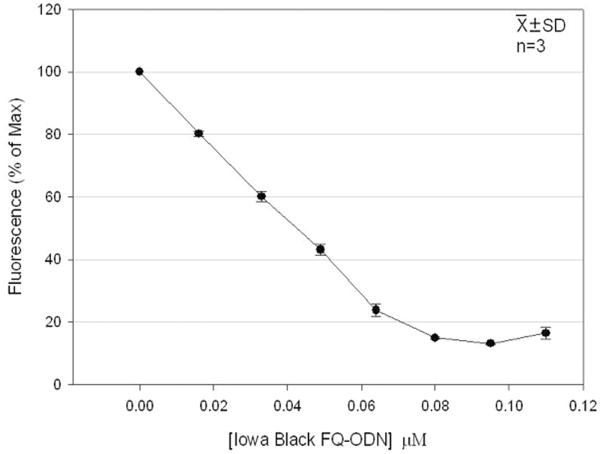
In neutral solution, Iowa Black FQ-ODN quenches FITC-ODN fluorescence efficiently. At a molar concentration of 0.098, Iowa Black FQ-ODN quenches 92.4 ± 0.2 % of the FITC-ODN fluorescence at 0.05 μM. Fig. 2 demonstrates the adjusted quenching efficiency, taking into account the change in fluorescence intensity due to the change in sample volume upon Iowa Black FQ-ODN addition. At a molar ratio of 1:1 (0.05 μM Iowa Black FQ-ODN:0.05 μM FITC-ODN), Iowa Black FQ-ODN quenches 70 % of the FITC-ODN fluorescence. Since the OD500 of 2 μM Iowa Black FQ-ODN is 0.0025, effects due to Iowa Black absorption are insignificant in these experiments. Our preliminary experiments to investigate potential non-specific quenching effects demonstrated that 2 μM Iowa Black FQ-ODN added to 0.05 μM of calcein decreased fluorescence intensity by only 4 % after correction for dilution (data not published). These data confirm that significant quenching of FITC-ODN fluorescence occurs only when the two complementary ODNs hybridize.
Figure 2.
Quenching of FITC-ODN fluorescence upon hybridization with Iowa Black FQ-ODN. The fluorescence of FITC-ODN alone at 0.05 μM was taken as 100 %.
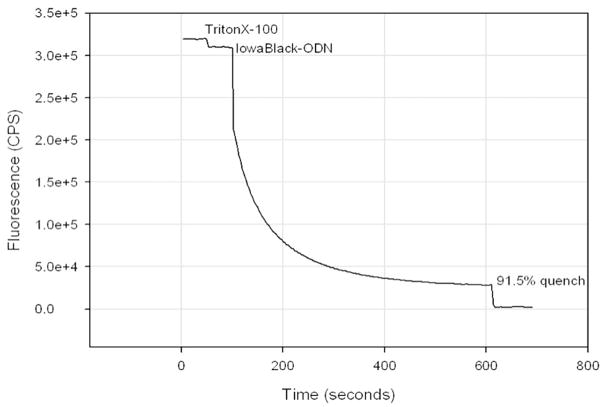
The detergent Triton X-100 has little effect on either the initial fluorescence of FITC-ODN or the quenching capacity of Iowa Black FQ-ODN (Fig. 3). The fluorescence of FITC-ODN and Triton X-100 was 96 ± 2 % that of FITC-ODN alone, and the change in volume may account for this slight disparity. Quenching of the initial FITC-ODN fluorescence was only slightly reduced (87 ± 0.2 %) in the presence of Triton X-100. Fig. 3 demonstrates that approximately 8 minutes are needed for the two ODNs to completely hybridize at the concentrations used. Higher concentrations result in more rapid and more complete quenching.
Figure 3.
Time course of quenching by Iowa Black FQ-ODN in the presence of Triton X-100. The fluorescence of FITC-ODN and its quenching by Iowa Black FQ-ODN was determined in the presence of 5 % TritonX-100. The time necessary for the completely hybridize is approximately 8 minutes.
A neutral pH has been found to be optimal for this procedure (Table 1). A low pH is undesirable due to reduced fluorescence of FITC. At pH 8.5, Iowa Black FQ-ODN quenches 85.0 ± 0.4 % of FITC-ODN fluorescence, albeit more slowly than in neutral buffer.
Table 1.
The fluorescence of FITC-ODN and FITC-ODN/Iowa Black FQ-ODN at differing pH’s. FITC-ODN was present at 0.05 μM in the absence or presence of 0.10 μM Iowa Black FQ-ODN. The fluorescence of FITC-ODN or FITC-ODN/Iowa Black FQ-ODN at neutral pH (control) was taken as 1.0 for determining relative fluorescence at other pH values. “Time” (last column) refers to the time to attain a stable fluorescence signal demonstrating how long it takes to complete hybridization of ODN.
| pH | Fraction of Initial FITC-ODN Fluorescence | Fraction of FITC-ODN/Iowa Black FQ-ODN Fluorescence | % Quench | Time (mins) |
|---|---|---|---|---|
| 7.2 (control) | 1.0 | 1.0 | 87±0.2 | 8 |
| 8.5 | 1.20±0.02 | 2.0±0.09 | 85±0.4 | 17 |
| 12.2 | 1.40±0.08 | 19.1±0.50 | 3.0±2.6 | 25 |
Anionic, neutral, and the DOPE liposome encapsulation preparations (DPPC/DOPE/DPPG/CH) had little effect on the initial FITC-ODN fluorescence intensity or on the quenching efficiency of Iowa Black FQ-ODN (Table 2). In the presence of highly cationic liposomes (at 0.5 mg/ml) the initial FITC-ODN fluorescence is reduced by half and quenching is reduced to only 36.0 ± 2.9 % of that found in the absence of the lipid dispersion (Table 2). These data suggest that modifications in the procedure may be necessary when cationic lipids are used to take advantage of electrostatic attractive forces between DNA and cationic lipids for increased association. The initial decrease in fluorescence may be due to the formation of a lipoplex, and poor quenching may occur because most of the FITC-ODN is not accessible to Iowa Black FQ-ODN. It may not matter, however, since a tight, electrostatically-bound complex such as a lipoplex may not be susceptible to ultrasound-induced dissociation. At present there are few data on this question.
Table 2.
The fluorescence of FITC-ODN and FITC-ODN/Iowa Black FQ-ODN in the presence of differing liposome preparations. FITC-ODN was present at 0.05 μM in the absence or presence of 0.10 μM Iowa Black FQ-ODN. The fluorescence of FITC-ODN or FITC-ODN/Iowa Black FQ-ODN in the absence of liposomes (control) was taken as 1.0 for determining relative fluorescence in the presence of various liposome preparations. The time necessary for hybridization of ODN and subsequent florescent quenching are tabulated.
| Liposomes | Fraction of Initial FITC-ODN Fluorescence | Fraction of FITC-ODN/Iowa Black FQ-ODN Fluorescence | % Quench | Time (mins) |
|---|---|---|---|---|
| None (control) | 1.0 | 1.0 | 87±0.2 | 8 |
| Anionic A500=0.024 | 0.99±0.02 | 1.40±0.08 | 85±0.7 | 8 |
| Cationic A500=0.026 | 0.49±0.01 | 2.90±0.27 | 36±2.9 | 8 |
| Neutral A500=0.529 | 0.97±0.05 | 1.22±0.21 | 86±0.1 | 8 |
| Encap. prep A500=1.080 | 1.00±0.03 | 1.80±0.00 | 83±0.3 | 8 |
3.2. Quantitation of Acoustic Reflectivity
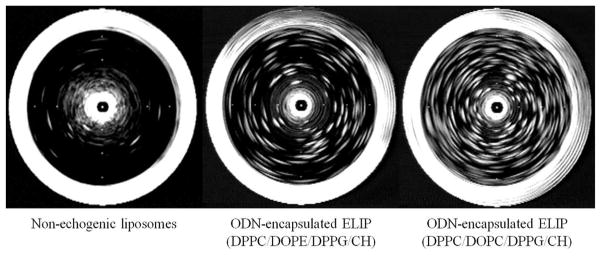
An ultrasound-sensitive liposomal ODN delivery system requires liposomes capable of co-encapsulating ODN and air. As air reflects ultrasound, we evaluated the air encapsulation by measuring acoustic reflectivity (echogenicity) expressed as a mean gray scale value after ODN entrapment. As a control, we used non-echogenic liposomes, which was prepared by degas the echogenic liposomes through vacumn. Both ODN-entrapping formulations of liposomes (those containing DOPC and those containing DOPE) clearly demonstrated echogenicity, as shown by the abundance of white spots in two images at the right of Fig. 4. The white perimeter of each image is due to high ultrasound reflectivity of the wall of the glass vial. The ODN-ELIP (DPPC/DOPC/DPPG/CH) at 0.25 mg/ml showed high echogenicity with a mean gray scale value of 74 ± 15, while the samples containing DOPE had a mean gray scale value of 41 ± 11. These data indicate that we successfully encapsulated air (i.e. cavitation nuclei) in the ODN-ELIP thus establishing that these agents possess the minimum requirement for ultrasound-triggered delivery of ODN to the arterial bed.
Figure 4.
Ultrasound images of non-echogenic liposomes (left) and two different ELIP formulations that encapsulated ODN, DPPC/DOPE/DPPG/CH (middle) DPPC/DOPC/DPPG/CH (right). All preparations were at 0.25 mg lipid/ml). Liposomes were imaged with a 20-MHz intravascular ultrasound catheter in a 5 ml glass vial. Acoustic reflectivity of ODN-ELIP is clearly observed whereas the liposomes without entrapped air demonstrate no echogenicity (white ring at periphery represents reflection from the wall of the vial).
3.3. Encapsulation and Ultrasound-Triggered Release of ODN
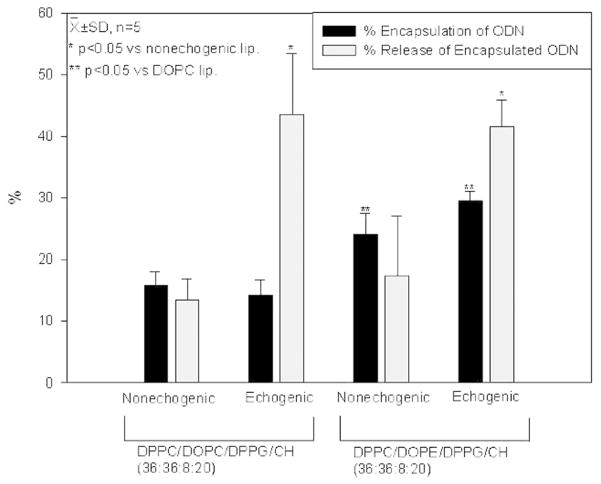
FITC-ODN was encapsulated (14.2 ± 2.5 %) by DPPC/DOPC/DPPG/CH ELIP, whereas liposomes composed of DPPC/DOPE/DPPG/CH encapsulated twice as much (29.6 ± 1.5 %) (Fig. 5). With ultrasound application for 60 seconds, the release of the encapsulated FITC-ODN reached 43.6 ± 9.9 % with the all-PC composition and 41.6 ± 4.3 % with the PC/PE composition. Release from non-echogenic liposomes was measurable, but considerably less than that from echogenic vesicles (15 % of the original FITC-ODN for each composition).
Figure 5.
Encapsulation and ultrasound-triggered release of FITC-ODN with two types of liposomal compositions demonstrating enhanced release of ODN from ELIP. Although both compositions released similar percentages of ODN upon ultrasound application, DOPE liposomes contained twice as many ODNs as did DOPC liposomes (p < 0.05).
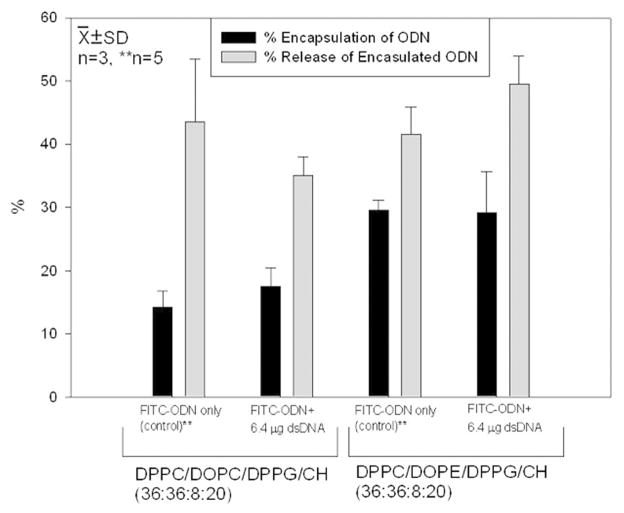
We hypothesized that trace amount of FITC-ODN added into ODN solution could represent the ODN encapsulation and release. Fig. 6 illustrates encapsulation and release efficiencies of FITC-ODN alone and those combined with untagged, trace amont of double-stranded ODNs (6.4 μg of dsDNA and 2 μg of FITC-ODN). There was little difference in encapsulation or release efficiency between the two formulations.
Figure 6.
Encapsulation and release efficiencies of FITC-ODN using two types of liposomal formulation. There is no significant difference between data for preparations containing FITC-ODN only and those in which FITC-ODN was combined with untagged double-stranded ODN.
4. DISCUSSION
The aims of this study were threefold. One was to develop a methodology to accurately measure ODN encapsulation within conventional liposomes. The second objective was to use this method to determine the amount of encapsulation of NF-κB decoy ODN within ELIP. The third aim was to develop ELIP that co-encapsulate gas and ODN that exhibit ultrasound-triggered release and to quantify release of the encapsulated ODN from these ELIP upon ultrasound application.
We have previously demonstrated encapsulation and release of calcein, a small hydrophilic molecule, from ELIP [14]. ODN are much larger molecules than calcein and the present study is the first to demonstrate that such large molecules can be encapsulated within echogenic liposomes and released upon ultrasound application. A fluorescence quenching method was developed as an easy and accurate technique to assess ODN encapsulation and release efficiency. In this study, we have successfully modified our previous methods co-encapsulating a gas and calcein to create liposomes that can entrap a gas and ODN. Thirty % of ODN encapsulation efficiency was obtained with retention of echogenicity. Ultrasound application (1 MHz continues wave, 0.26 MPa peak-to-peak pressure amplitude, 60 seconds) enhanced ODN release up to 42 %. In this experiment, the power choice was dictated by the instrument and 2 W/cm2 is simply the maximum it is capable of. A higher release rate might be able to obtain at higher power.
Ultrasound-triggered release of payloads from ELIP is affected by the co-encapsulated gas [14] and the parameters of the applied ultrasound. Simultaneously with the rarefaction phase of the ultrasound wave, the gas pocket in the liposome must undergo expansion, stressing the lipid monolayers that stabilize the microscopic gas bubble and stretching the attached bilayers of the liposome. If the pressure drop induced by ultrasound is large enough, the stress will exceed the elastic limit of the weakest surface, and at some point either the bilayer or monolayer will rend (Fig. 7). When the integrity of the vesicle is lost, some or all of the content will be released. Our data demonstrated similar release efficiencies with two different lipid formulations (Fig. 5) so that there is a possibility that these two formulations (both at 10 μl/mg lipids) may contain similar amounts of encapsulated gas. The average size of our ODN ELIP is about 800 nm. The threshold intensity of ultrasound to produce cavitation effect depends on the ultrasound characteristics and the bubble size.
Figure 7.
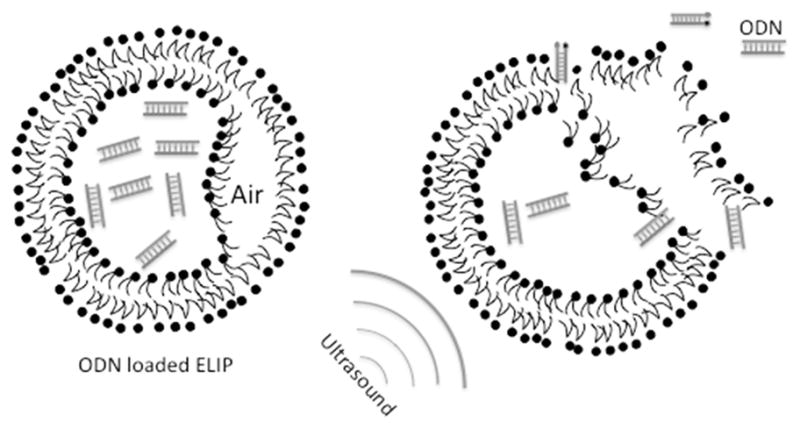
A schematic of co-encapsulation of air and ODN into ELIP allowing ultrasound-triggered release of ODN from ODN-containing ELIP.
In our encapsulation procedure, the ODN was encapsulated through freeze/thawing in the present of mannitol. Unlike some other sugars such as trehalose, mannitol is a poor cryoprotectant. Liposome disruption and fusion occur during freezing and thawing and this has profound effects on encapsulation efficiency. Although we cannot know in detail what happens, it is likely that fusion of small vesicles not only generates larger vesicles (with correspondingly larger internal volumes), but also creates openings that allowing encapsulation of the external phase. Liposome compositions have long been known to influence vesicle-vesicle fusion and the PE content in particular is known to favor fusion in a host of different circumstance [21]. Given this general influence of PE and its known influence on fusion, it is not surprising that the PE-containing composition encapsulated ODN more efficiently than did the composition lacking PE.
Several methods have been described for the quantification of encapsulated payloads within liposomes. Most of these require separation of the liposomes from the bulk solution [11], which is not only time-consuming but also leads to losses, dilution and inaccuracies. The need for separation procedure was obviated by using a fluorescence quenching method similar to one previously described for quantifying ultrasound-triggered release of calcein, as an internal content marker, from ELIP [19, 20]. To retain the advantage of the quenching methodology, we have employed a dual-labeled ODN probe, widely used in assays for genetic analysis such as real-time polymerase chain reactions [22]. Complementary ODN are labeled with both reporter and quencher dyes. The fluorescence from the reporter dye is quenched through fluorescence resonance energy transfer when the two dyes become physically close to one another by hybridization.
In the case of the particular ODN used in this manuscript, the NF-κB decoy sense strand was labeled with FITC and the antisense strand was labeled with an efficient FITC quencher, Iowa-Black. The FITC-labeled ODN was encapsulated into ELIP. Addition of the complementary Iowa-Black strand to the dispersion brought about quenching of the unencapsulated NF-κB. Because the ODN cannot cross the lipid membrane, the residual fluorescence provides an accurate measurement of encapsulated ODN.
Hybridization is affected by temperature, buffer and incubation time. In the case of using a hybridization procedure to assay liposome encapsulation, these variables are readily optimized and maintained constant under common conditions of encapsulating ODN. Lipid composition also has an influence in that cationic liposomes interact strongly with ODN and, therefore, additional research will be necessary to utilize this method with cationic lipoplexes. Under our conditions and with neutral and anionic lipids, the Iowa Black FQ-ODN quenching method was optimal at neutral pH.
Although the upper limit on the concentration of FITC-ODN is 4 μM due to the nonlinearity of the concentration dependence of its fluorescence, the total amount of measurable encapsulated DNA can be increased by adding untagged, double-stranded ODN. That is, the method provides a valid measure of total encapsulated ODN even when most of the ODN is unlabeled and double-stranded. The lack of any interference of double-stranded ODN is important because ODN used for therapeutic purpose are double-stranded. Of course, the procedure can also be used to quantify encapsulation of single-stranded ODN, provided that it is not complementary to either of the assay components, FITC-ODN and Iowa Black FQ-ODN.
5. CONCLUSION
We have developed an easy and efficient fluorescence quenching method for quantitation of ODN encapsulation. Liposomes previously developed as contrast agents for ultrasound image enhancement have been demonstrated to permit efficient encapsulation of ODN while retaining their echogenicity. Application of this ODN encapsulation assay revealed that these ODN-ELIP more efficiently release their payloads upon ultrasound application than do their non-echogenic counterparts. This novel strategy of ultrasound-triggered ODN delivery has many potential applications for gene therapy in a variety of cardiovascular pathologies.
Acknowledgments
This work was in part supported by the National Institutes of Health (GM57305, GM52329 - MacDonald, HL59586, HL74002 - McPherson) and the American Heart Association (0535512Z - Huang). The authors thank Christy Holland and Jonathan Kopechek of the University of Cincinnati for ultrasound probe calibration. We thank Melvin Klegerman, Susan Laing and Patrick Kee for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morishita R, Higaki J, Tomita N, Ogihara T. Application of transcription factor “decoy” strategy as means of gene therapy and study of gene expression in cardiovascular disease. Circ Res. 1998;82(10):1023–1028. doi: 10.1161/01.res.82.10.1023. [DOI] [PubMed] [Google Scholar]
- 2.Morishita R, Nakagami H, Taniyama Y, Matsushita H, Yamamoto K, Tomita N, Moriguchi A, Matsumoto K, Higaki J, Ogihara T. Oligonucleotide-based gene therapy for cardiovascular disease. Clin Chem Lab Med. 1998;36(8):529–534. doi: 10.1515/CCLM.1998.090. [DOI] [PubMed] [Google Scholar]
- 3.Tomita N, Morishita R, Tomita S, Yamamoto K, Aoki M, Matsushita H, Hayashi S, Higaki J, Ogihara T. Transcription factor decoy for nuclear factor-kappaB inhibits tumor necrosis factor-alpha-induced expression of interleukin-6 and intracellular adhesion molecule-1 in endothelial cells. J Hypertens. 1998;16(7):993–1000. doi: 10.1097/00004872-199816070-00013. [DOI] [PubMed] [Google Scholar]
- 4.Nimjee SM, Rusconi CP, Sullenger BA. Aptamers: an emerging class of therapeutics. Annu Rev Med. 2005;56:555–583. doi: 10.1146/annurev.med.56.062904.144915. [DOI] [PubMed] [Google Scholar]
- 5.Akhtar S, Kole R, Juliano RL. Stability of antisense DNA oligodeoxynucleotide analogs in cellular extracts and sera. Life Sci. 1991;49(24):1793–1801. doi: 10.1016/0024-3205(91)90480-y. [DOI] [PubMed] [Google Scholar]
- 6.Temsamani J, Tang JY, Padmapriya A, Kubert M, Agrawal S. Pharmacokinetics, biodistribution, and stability of capped oligodeoxynucleotide phosphorothioates in mice. Antisense Res Dev. 1993;3(3):277–284. doi: 10.1089/ard.1993.3.277. [DOI] [PubMed] [Google Scholar]
- 7.Akhtar S, Juliano RL. Cellular uptake and intracellular fate of antisense oligonucleotides. Trends Cell Biol. 1992;2(5):139–144. doi: 10.1016/0962-8924(92)90100-2. [DOI] [PubMed] [Google Scholar]
- 8.Juliano RL, Akhtar S. Liposomes as a drug delivery system for antisense oligonucleotides. Antisense Res Dev. 1992;2(2):165–176. doi: 10.1089/ard.1992.2.165. [DOI] [PubMed] [Google Scholar]
- 9.Kaneda Y, Yamamoto S, Nakajima T. Development of HVJ envelope vector and its application to gene therapy. Adv Genet. 2005;53:307–332. [PubMed] [Google Scholar]
- 10.Kovacs JR, Zheng Y, Shen H, Meng WS. Polymeric microspheres as stabilizing anchors for oligonucleotide delivery to dendritic cells. Biomaterials. 2005;26(33):6754–6761. doi: 10.1016/j.biomaterials.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Yamada Y, Kogure K, Nakamura Y, Inoue K, Akita H, Nagatsugi F, Sasaki S, Suhara T, Harashima H. Development of efficient packaging method of oligodeoxynucleotides by a condensed nano particle in lipid envelope structure. Biol Pharm Bull. 2005;28(10):1939–1942. doi: 10.1248/bpb.28.1939. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Lei J, Vollmer R, Huang L. Mechanism of liver gene transfer by mechanical massage. Mol Ther. 2004;9(3):452–457. doi: 10.1016/j.ymthe.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Nunamaker EA, Zhang HY, Shirasawa Y, Benoit JN, Dean DA. Electroporation-mediated delivery of catalytic oligodeoxynucleotides for manipulation of vascular gene expression. Am J Physiol Heart Circ Physiol. 2003;285(5):H2240–2247. doi: 10.1152/ajpheart.00350.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang SL, MacDonald RC. Acoustically active liposomes for drug encapsulation and ultrasound-triggered release. Biochim Biophys Acta. 2004;1665(1–2):134–141. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Newman CM, Lawrie A, Brisken AF, Cumberland DC. Ultrasound gene therapy: on the road from concept to reality. Echocardiography. 2001;18(4):339–347. doi: 10.1046/j.1540-8175.2001.00339.x. [DOI] [PubMed] [Google Scholar]
- 16.Price RJ, Skyba DM, Kaul S, Skalak TC. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998;98(13):1264–1267. doi: 10.1161/01.cir.98.13.1264. [DOI] [PubMed] [Google Scholar]
- 17.Skyba DM, Price RJ, Linka AZ, Skalak TC, Kaul S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation. 1998;98(4):290–293. doi: 10.1161/01.cir.98.4.290. [DOI] [PubMed] [Google Scholar]
- 18.Tyagi S, Kramer FR. Molecular beacons: probes that fluoresce upon hybridization. Nat Biotechnol. 1996;14(3):303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 19.Kendall DA, MacDonald RC. A fluorescence assay to monitor vesicle fusion and lysis. J Biol Chem. 1982;257(23):13892–13895. [PubMed] [Google Scholar]
- 20.Kendall DA, MacDonald RC. Characterization of a fluorescence assay to monitor changes in the aqueous volume of lipid vesicles. Anal Biochem. 1983;134(1):26–33. doi: 10.1016/0003-2697(83)90258-0. [DOI] [PubMed] [Google Scholar]
- 21.Siegel DP. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases. II Implications for membrane-membrane interactions and membrane fusion. Biophys J. 1986;49(6):1171–1183. doi: 10.1016/S0006-3495(86)83745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson MK. Choosing reporter-quencher pairs for efficient quenching through formation of intramolecular dimers. Methods Mol Biol. 2006;335:17–29. doi: 10.1385/1-59745-069-3:17. [DOI] [PubMed] [Google Scholar]