Abstract
BACKGROUND
Retinopathy of prematurity is a leading cause of childhood blindness worldwide. Peripheral retinal ablation with conventional (confluent) laser therapy is destructive, causes complications, and does not prevent all vision loss, especially in cases of retinopathy of prematurity affecting zone I of the eye. Case series in which patients were treated with vascular endothelial growth factor inhibitors suggest that these agents may be useful in treating retinopathy of prematurity.
METHODS
We conducted a prospective, controlled, randomized, stratified, multicenter trial to assess intravitreal bevacizumab monotherapy for zone I or zone II posterior stage 3+ (i.e., stage 3 with plus disease) retinopathy of prematurity. Infants were randomly assigned to receive intravitreal bevacizumab (0.625 mg in 0.025 ml of solution) or conventional laser therapy, bilaterally. The primary ocular outcome was recurrence of retinopathy of prematurity in one or both eyes requiring retreatment before 54 weeks’ postmenstrual age.
RESULTS
We enrolled 150 infants (total sample of 300 eyes); 143 infants survived to 54 weeks’ postmenstrual age, and the 7 infants who died were not included in the primary-outcome analyses. Retinopathy of prematurity recurred in 4 infants in the bevacizumab group (6 of 140 eyes [4%]) and 19 infants in the laser-therapy group (32 of 146 eyes [22%], P = 0.002). A significant treatment effect was found for zone I retinopathy of prematurity (P = 0.003) but not for zone II disease (P = 0.27).
CONCLUSIONS
Intravitreal bevacizumab monotherapy, as compared with conventional laser therapy, in infants with stage 3+ retinopathy of prematurity showed a significant benefit for zone I but not zone II disease. Development of peripheral retinal vessels continued after treatment with intravitreal bevacizumab, but conventional laser therapy led to permanent destruction of the peripheral retina. This trial was too small to assess safety.
Retinopathy of prematurity is a neovascular retinal disorder of childhood that causes loss of vision by means of macular dragging and retinal detachment. It is a leading cause of childhood blindness in the United States and other highly industrialized nations, occurring primarily in infants of low birth weight (≤1250 g; mean, 700 g).1 The incidence of blindness in infants due to retinopathy of prematurity is relatively low, about 1 case in 820 infants,2 because of good neonatal care and appropriate screening and treatment.1 The disorder is a major cause of childhood blindness in developing countries, manifesting in larger premature infants (birth weight ≤2000 g; mean, 1400 g). The worldwide prevalence of blindness due to retinopathy of prematurity is 50,000.1
Retinal vascularization on the internal retinal surface begins at the optic nerve at 16 weeks’ gestation and proceeds anteriorly, reaching the edge of the temporal retina at 40 weeks’ gestation. The zone indicates the area of vascularization, with zone I referring to a circle whose radius extends from the optic disk and is twice the distance between the center of the disk and the center of the macula. Zone II resembles an annulus and encircles zone I. It extends from zone I to the nasal extent of the retina. Zone II posterior surrounds zone I and has an external circumference based on a radius (originating at the center of the optic disk) that is three times the distance between the center of the disk and the center of the macula. Zone III is the remaining crescent of retina, primarily on the temporal side. Retinopathy of prematurity in zone I is the most difficult to treat and has a high incidence of recurrence warranting additional treatment.3-9
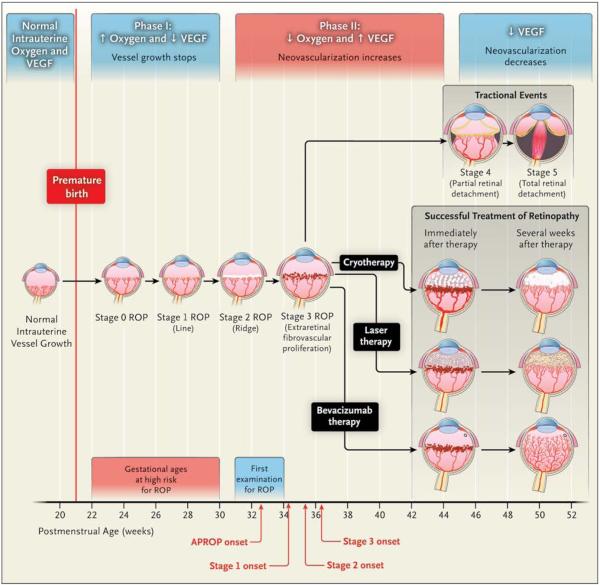
Stages of retinopathy of prematurity are defined by vessel appearance at the interface between the vascular and avascular retinal areas (Fig. 1). This interface resembles a line for stage 1, a three-dimensional ridge for stage 2, and a ridge with neovascularization extending into the vitreous gel for stage 3, which is the ideal time for treatment. (Plus disease — as in stage 3+ — indicates that two or more quadrants of the eye have dilated veins and tortuous arteries near the optic disk.) The neovascularization can progress to form fibrous bands that cause partial retinal detachment (stage 4), and ultimately, total retinal detachment (stage 5).
Figure 1. Pathogenesis and Therapy of Retinopathy of Prematurity (ROP).
Phases 1 and 2 of ROP are associated with different levels of vascular endothelial growth factor (VEGF), oxygen, and neovascular activity. ROP stages 0 to 5 are shown, as are the outcomes, when therapy is successful, of cryotherapy (established in a clinical trial in 1988),10 laser therapy (established in clinical trial in 2003),12 and intravitreal bevacizumab (this study). Cryotherapy involves scarring of the full ocular thickness, laser therapy scarring of the retinal thickness, and intravitreal bevacizumab scarring with a needle near the limbus. Also shown are the postmenstrual ages at which infants are at high risk for ROP, the appropriate postmenstrual age for the first ocular screening examination for ROP, and the mean postmenstrual ages at the onset of aggressive posterior ROP (APROP) (type 2 ROP) and stages 1, 2, and 3 (type 1 ROP).
In 1988, cryotherapy (freezing from the external ocular surface, affecting the sclera, choroid, and the full thickness of the retina) was recommended for stage 3+ retinopathy of prematurity extending for 5 consecutive “hours” (150 degrees) or 8 cumulative “hours” (240 degrees, as measured on a clock face) (Fig. 1).10 In the 1990s, treatment of stage 3+ disease underwent a slow transition from cryotherapy to laser therapy (in which a laser is applied through the dilated pupil to the internal retinal surface). Both these treatments destroy the majority of the cells that produce vascular endothelial growth factor (VEGF) in the retina. VEGF is a key factor in the progression of retinopathy of prematurity.11 In 2003, earlier use of conventional laser therapy (called confluent laser therapy by ophthalmologists) was recommended to treat stage 3+ retinopathy of prematurity, which results in better outcomes.12 Conventional laser therapy for zone I retinopathy of prematurity is successful in approximately 50% of cases but inevitably causes permanent loss of the peripheral visual field and often induces clinically significant myopia. When multiple applications of conventional laser therapy fail to induce regression of retinopathy of prematurity, vitrectomy is required.13,14
The pathogenesis of retinopathy of prematurity (Fig. 1) involves two discrete phases: phase 1 occurs from roughly 22 to 30 weeks’ postmenstrual age, and phase 2 from roughly 31 to 44 weeks’ postmenstrual age. It is now understood that phase 1 involves relative hyperoxia and decreased VEGF levels, whereas phase 2 involves relative hypoxia and increased VEGF levels. The understanding of these relationships between oxygen and VEGF has allowed for an improved strategy for managing retinopathy of prematurity, including both prevention or early treatment (i.e., in phase 1) and later treatment (i.e., in phase 2).11
The use of anti-VEGF agents, primarily intravitreal bevacizumab, is an emerging treatment for acute retinopathy of prematurity.11 The Food and Drug Administration approved intravenous bevacizumab therapy in 2004 for the treatment of metastatic colon cancer; the drug works by reducing the size and number of new vessels feeding metastases. Off-label use of intravitreal bevacizumab therapy for ophthalmologic neovascular disorders began shortly thereafter.11 Cases and series of cases of stage 3+, 4, and 5 retinopathy of prematurity have been treated with intravitreal bevacizumab, both as monotherapy and in combination with conventional laser therapy or vitrectomy or both.15-29 Contraction of membranes, with resulting acceleration of retinal detachment, can occur when intravitreal bevacizumab is administered too late (at stages 4 and 5).18,22
In our prospective, randomized, stratified, controlled, multicenter clinical trial, BEAT-ROP (Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity),30 we compared intravitreal bevacizumab monotherapy and conventional laser therapy with regard to the ocular outcomes among infants with stage 3+ retinopathy of prematurity who had zone I or II posterior disease.
METHODS
STUDY OVERSIGHT
The institutional review boards at the University of Texas and at the 14 other participating hospitals approved the study protocol (available with the full text of this article at NEJM.org). Parents or guardians of the infants provided written informed consent before enrollment, and the study had an independent data and safety monitoring board (see the Supplementary Appendix, available at NEJM.org). The study was conducted in accordance with the protocol.
STUDY DESIGN
The study, which was controlled but not masked, owing to the marks made by laser therapy, compared the efficacy of intravitreal bevacizumab monotherapy (0.625 mg in 0.025 ml of solution) with the efficacy of conventional laser therapy in reducing the incidence of recurrent retinopathy of prematurity. The study was designed by the planning committee (see the Supplementary Appendix). The data for each infant were recorded on a case-report form, sent to the study headquarters (University of Texas Health Science Center at Houston), entered into a password-secured data system by a research assistant, and analyzed by one of the authors.
STUDY PARTICIPANTS
Infants with a birth weight of 1500 g or less and a gestational age of 30 weeks or less were examined, beginning at 4 weeks’ chronologic age or 31 weeks’ postmenstrual age, whichever was later.31 Serial eye examinations were performed according to a standard schedule suggested by the American Academy of Ophthalmology, the American Academy of Pediatrics, and the American Association for Pediatric Ophthalmology and Strabismus.31 The International Classification for Retinopathy of Prematurity (revised) was used to classify retinopathy of prematurity.32
ENROLLMENT
Enrollment occurred between March 13, 2008, and August 4, 2010. Infants with stage 3+ retinopathy of prematurity in zone I or zone II posterior in each eye were eligible. Infants with stage 4 or 5 retinopathy of prematurity in either eye were not eligible. We used RetCam photography (Clarity Medical Systems) to document retinopathy of prematurity before randomization; eligibility was established by the treating ophthalmologist and was confirmed by a second ophthalmologist on the basis of electronically transmitted RetCam images.
RANDOMIZATION
A secure, computer-generated randomization schedule stratified on the basis of zone was maintained by a study-group member who did not participate in enrollment. Treatment assignments were revealed to the investigators only after eligibility for enrollment had been confirmed. Infants (both eyes) were randomly assigned to undergo either conventional laser therapy or intravitreal bevacizumab monotherapy. We randomly assigned infants, rather than eyes, to the study groups because VEGF circulates in the blood, and recurrence in one eye could therefore affect the contralateral eye, and because substantial inflammation after conventional laser therapy in one eye and little inflammation after intravitreal bevacizumab therapy in the other eye could result in irreversible deprivation amblyopia in the eye that underwent conventional laser therapy.
OCULAR OUTCOMES
Data from the Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) trial (ClinicalTrials.gov number, NCT00000133)10 and the Early Treatment for Retinopathy of Prematurity (ETROP) trial (NCT00027222)12 suggest that recurrence after peripheral retinal ablation for stage 3+ retinopathy of prematurity generally occurs before 55 weeks’ postmenstrual age. In our initial submission to ClinicalTrials.gov, we defined the primary outcome in terms of treatment success: an absence of recurrence of stage 3+ retinopathy of prematurity in one or both eyes in zone I or posterior zone II by 54 weeks’ postmenstrual age. Before the date on which the data were first analyzed, we changed the primary outcome to treatment failure: the recurrence of neovascularization in one or both eyes arising from the retinal vessels and requiring retreatment by 54 weeks’ postmenstrual age (with ascertainment performed between 50 and 70 weeks). We took RetCam photographs to document recurrence; the treating and confirming ophthalmologists viewed these photographs, without masking of treatment assignments, before deciding on additional treatment. Cases requiring additional treatment consisting of a few laser applications to inadvertently skipped areas within 1 week after initial treatment were not considered to be recurrences.
In addition, we took RetCam photographs of all infants 1 week and 1 month after the initial and any additional treatments and at 54 weeks’ postmenstrual age. The photographs taken at 54 weeks were assessed post hoc by six independent experts on retinopathy of prematurity who made up the BEAT-ROP Reading Center (see the Supplementary Appendix). Treatment assignments were concealed from these ophthalmologists by cropping the photographs so that they included only the optic disk and macula (without laser marks), to allow for evaluation of the ocular outcomes of macular dragging and retinal detachment. A consensus was reached by the reading center for every case of macular dragging or retinal detachment. Recurrence of disease and the need for retreatment were validated by the reading center on the basis of the full, uncropped photographs.
STATISTICAL ANALYSIS
Findings from the CRYO-ROP trial10 and ETROP trial12 suggested that the recurrence rates with conventional laser therapy would be up to 50% for zone I disease and up to 20% for zone II posterior disease. The clinical data available at the time that we designed our study suggested that the recurrence rates after intravitreal bevacizumab monotherapy would be 10% for zone I disease and 1% for zone II posterior disease. Assuming a rate of death or loss to follow-up of 10%, we calculated that a sample of 24 participants per treatment group for zone I disease and 50 participants per treatment group for zone II posterior disease would be required to detect a significant difference in the rate of the primary outcome between the two treatment groups. Thus, to draw an overall conclusion regarding the efficacy of intravitreal bevacizumab in both zones, the study was designed to enroll a total of 150 infants, at least 50 of them with zone I retinopathy of prematurity.
Descriptive statistics from a two-sample t-test or Fisher’s exact test were used to evaluate whether the group of infants assigned to intravitreal bevacizumab and the group assigned to conventional laser therapy were similar before treatment with respect to demographic and clinical characteristics, including risk factors for retinopathy of prematurity. The primary ocular outcome, recurrence of retinopathy of prematurity, is a variable with three possible values: 0 for recurrence in neither eye, 1 for recurrence in one eye, and 2 for recurrence in both eyes. This outcome was evaluated by means of an ordered logistic-regression model. The primary analysis was an intention-to-treat analysis of all infants who were randomly assigned to a treatment group. Infants who died or were lost to follow-up were considered to have missing data in the primary analysis. We also carried out a post hoc sensitivity analysis in which deaths were considered to be recurrences. SAS for Windows software, version 9.1 (SAS Institute), was used to perform all statistical analyses. A P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
INFANTS
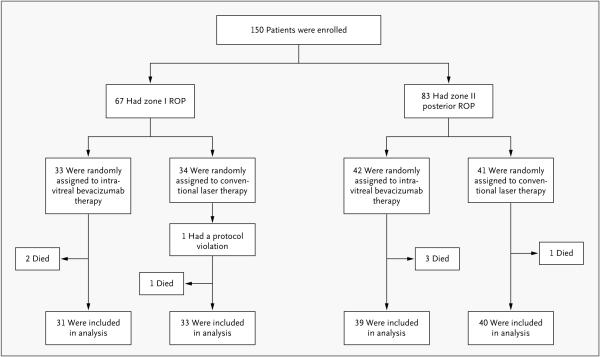
We enrolled a total of 150 infants with stage 3+ retinopathy of prematurity — 67 infants with zone I disease and 83 infants with zone II posterior disease (Fig. 2). A total of 75 infants were randomly assigned to undergo intravitreal bevacizumab monotherapy, and 75 to conventional laser therapy. Table 1 lists clinical characteristics of infants and risk factors for retinopathy of prematurity. One protocol violation occurred, involving an infant with zone I disease who was randomly assigned to the conventional laser therapy group but was instead treated with intravitreal bevacizumab. Table 1 shows that the number of infants intubated as part of treatment was significantly greater in the conventional laser therapy group than in the intravitreal bevacizumab group (P<0.001).
Figure 2.
Enrollment, Randomization, and Follow-up of the 150 Study Infants.
Table 1.
Risk Factors and Other Characteristics of Infants with Zone I Retinopathy of Prematurity (ROP) or Zone II Posterior ROP, According to Treatment Group.*
| Characteristic | Zone I ROP (N = 67) | Zone II Posterior ROP (N = 83) | ||||
|---|---|---|---|---|---|---|
| Intravitreal Bevacizumab (N = 33) |
Conventional Laser Therapy (N = 34) |
P Value | Intravitreal Bevacizumab (N = 42) |
Conventional Laser Therapy (N = 41) |
P Value | |
| Birth weight — g | 615.2±139.5 | 657.9±159.5 | 0.25 | 689.2±111.3 | 680.7±156.8 | 0.78 |
| Gestational age — wk | 24.2±1.3 | 24.3±1.6 | 0.69 | 24.5±1.2 | 24.5±1.4 | 0.84 |
| Male sex — no. (%) | 20 (61) | 21 (62) | 1.00 | 27 (64) | 29 (71) | 0.64 |
| Mother’s race or ethnic group — no. (%)† | 0.17 | 0.48 | ||||
| White | 7 (21) | 4 (12) | 6 (14) | 11 (27) | ||
| Black | 4 (12) | 10 (29) | 8 (19) | 9 (22) | ||
| Hispanic | 22 (67) | 19 (56) | 25 (60) | 19 (46) | ||
| Other | 0 | 1 (3) | 3 (7) | 2 (5) | ||
| No. of infants delivered in current pregnancy — no. (%) | 0.28 | 1.00 | ||||
| 1 | 25 (76) | 31 (91) | 33 (79) | 32 (78) | ||
| 2 | 6 (18) | 2 (6) | 8 (19) | 8 (20) | ||
| 3 | 2 (6) | 1 (3) | 1 (2) | 1 (2) | ||
| Apgar score‡ | ||||||
| At 1 min | 4.0±2.2‡ | 3.4±2.5 | 0.35 | 4.7±2.4 | 4.4±2.1‡ | 0.47 |
| At 5 min | 6.4±1.8‡ | 5.2±3.0 | 0.051 | 6.8±1.8 | 6.5±1.9‡ | 0.34 |
| Intubation before randomization — no. (%) | 12 (36) | 8 (24) | 0.29 | 6 (14) | 7 (17) | 0.77 |
| Postmenstrual age — wk | ||||||
| At randomization | 34.3±1.5 | 33.6±1.6 | 0.06 | 35.5±2.1 | 35.7±1.9 | 0.64 |
| At treatment | 34.5±1.4 | 33.7±1.6 | 0.03 | 35.7±2.1 | 35.8±2.0 | 0.77 |
| Duration of hospitalizations from birth to treatment — wk | 10.3±1.3 | 9.4±1.9 | 0.01 | 11.2±2.0 | 11.3±2.2 | 0.88 |
| Patent ductus arteriosus ligation — no. (%) | 13 (39) | 14 (41) | 1.00 | 10 (24) | 18 (44) | 0.07 |
| Culture-proven sepsis — no. (%) | 10 (30) | 11 (32) | 1.00 | 14 (33) | 19 (46) | 0.27 |
| Surgery for necrotizing enterocolitis — no. (%) | 7 (21) | 5 (15) | 0.54 | 10 (24) | 10 (24) | 1.00 |
| Intraventricular hemorrhage grade — no. (%)§ | 0.63 | 0.09 | ||||
| Grade I | 2 (6) | 2 (6) | 6 (14) | 1 (2) | ||
| Grade II | 4 (12) | 5 (15) | 7 (17) | 3 (7) | ||
| Grade III | 3 (9) | 2 (6) | 1 (2) | 5 (12) | ||
| Grade IV | 4 (12) | 9 (26) | 8 (19) | 11 (27) | ||
| Periventricular leukomalacia — no. (%) | 0.28 | 0.92 | ||||
| Mild | 3 (9) | 2 (6) | 1 (2) | 1 (2) | ||
| Moderate | 1 (3) | 0 | 2 (5) | 3 (7) | ||
| Severe | 0 | 3 (9) | 1 (2) | 1 (2) | ||
| Intubated as part of treatment — no. (%) | 0 | 24 (71) | <0.001 | 0 | 30 (73) | <0.001 |
Plus–minus values are means ±SD. Numbers in bold face were significantly different between the two treatment groups.
Mother’s race or ethnic group was self-reported.
Apgar scores were not available for one infant receiving intravitreal bevacizumab for zone I ROP and one infant receiving conventional laser therapy for zone II posterior ROP, because the infants were born at home.
Intraventricular hemorrhage can range from grade I to grade IV, with higher grades indicating more severe bleeding.
RECURRENT RETINOPATHY
Table 2 shows the ocular outcomes for the 143 surviving infants who were evaluated for recurrent retinopathy of prematurity at 54 weeks’ postmenstrual age, including 1 infant who received intravitreal bevacizumab in violation of the protocol; the outcome for this infant was included with the data for the laser-therapy group (i.e., we adhered to an intention-to-treat analysis). The rate of recurrence (primary outcome) for zone I and posterior zone II combined was significantly higher with conventional laser therapy than with intravitreal bevacizumab (26% [19 of 73 infants]) vs. 6% [4 of 70 infants]; odds ratio with bevacizumab, 0.17; 95% confidence interval [CI], 0.05 to 0.53; P = 0.002). The absolute difference between the two groups in the risk of recurrence was 20 percentage points (95% CI, 9 to 32). A post hoc sensitivity analysis with deaths considered as bilateral recurrences (i.e., recurrence in both eyes) yielded similar results (odds ratio for recurrence with bevacizumab vs. laser therapy, 0.36; 95% CI, 0.15 to 0.84; P = 0.02). The four reported cases of complications — one case of corneal opacity and three of lens opacity — occurred in association with conventional laser therapy for zone II posterior disease.
Table 2.
Ocular Outcomes in the 143 Survivors at 54 Weeks’ Postmenstrual Age.*
| Variable | Zone I ROP (N = 64) | Zone II Posterior ROP (N = 79) | ||
|---|---|---|---|---|
| Intravitreal Bevacizumab (N = 31) |
Conventional Laser Therapy (N = 33)† |
Intravitreal Bevacizumab (N = 39) |
Conventional Laser Therapy (N = 40) |
|
| Recurrence of ROP (primary outcome) — no. of patients (%) |
||||
| None | 29 (94) | 19 (58) | 37 (95) | 35 (88) |
| In one eye | 2 (6) | 5 (15) | 0 | 1 (2) |
| In both eyes | 0 | 9 (27) | 2 (5) | 4 (10) |
| Eyes affected — no. | 2 | 23 | 4 | 9 |
| Odds ratio for recurrence with bevacizumab (95% CI) [P value] |
||||
| Per zone | 0.09 (0.02–0.43) [0.003] | 0.39 (0.07–2.11) [0.27] | ||
| For zones I and II combined | 0.17 (0.05–0.53) [0.002] | |||
| Interval from treatment to recurrence — wk‡ | 19.2±8.6 | 6.4±6.7 | 14.4±0.8 | 6.8±4.2 |
| Vitrectomy — no. of eyes | 0 | 13 | 2 | 0 |
| Structural outcomes of recurrence — no. of eyes | ||||
| Macular dragging | 1 | 16 | 0 | 6 |
| Retinal detachment | 0 | 2 | 2 | 0 |
| Complications requiring intraocular surgery — no. of eyes | ||||
| Cornea opacity requiring corneal transplant | 0 | 0 | 0 | 1 |
| Lens opacity requiring cataract removal | 0 | 0 | 0 | 3 |
Plus–minus values are means ±SD. Of the 150 infants enrolled, 7 died before 54 weeks: 5 before hospital discharge and 2 after discharge; 2 had been receiving intravitreal bevacizumab for zone I retinopathy of prematurity (ROP), 1 conventional laser therapy for zone I ROP, 3 intravitreal bevacizumab for zone II posterior ROP, and 1 conventional laser therapy for zone II posterior ROP.
One infant who had been randomly assigned to undergo conventional laser therapy for zone I ROP was instead given intravitreal bevacizumab (a protocol violation). In analyses, we followed the intention-to-treat principle and assumed that treatment was successful in both eyes (i.e., no recurrent disease with conventional laser therapy).
The interval from treatment to recurrence for both zones considered together was 16.0±4.6 weeks for 6 eyes after intravitreal bevacizumab therapy versus 6.2±5.7 weeks for 32 eyes after conventional laser therapy.
The rate of recurrence with zone I disease alone was significantly higher with conventional laser therapy than with intravitreal bevacizumab (42% [14 of 33 infants] vs. 6% [2 of 31 infants]; odds ratio with bevacizumab, 0.09; 95% CI, 0.02 to 0.43; P = 0.003) (Table 2). We observed recurrence after intravitreal bevacizumab in a total of two eyes (unilateral), with macular dragging in one of the two eyes. We observed recurrence after conventional laser therapy in 23 eyes (5 unilateral and 9 bilateral), with macular dragging in 16 eyes and retinal detachment in 2 eyes.
The rate of recurrence with zone II posterior disease alone did not differ significantly between the laser-therapy group and the bevacizumab group (12% [5 of 40 infants] and 5% [2 of 39 infants]; odds ratio with bevacizumab, 0.39; 95% CI, 0.07 to 2.11; P = 0.27) (Table 2). Retinopathy recurred in four eyes (bilateral in two infants) after intravitreal bevacizumab therapy, with no instances of macular dragging but with retinal detachment in two eyes (bilateral). After conventional laser therapy, retinopathy recurred in nine eyes (one unilateral and four bilateral), with macular dragging in six eyes but with no instances of retinal detachment.
REVASCULARIZATION
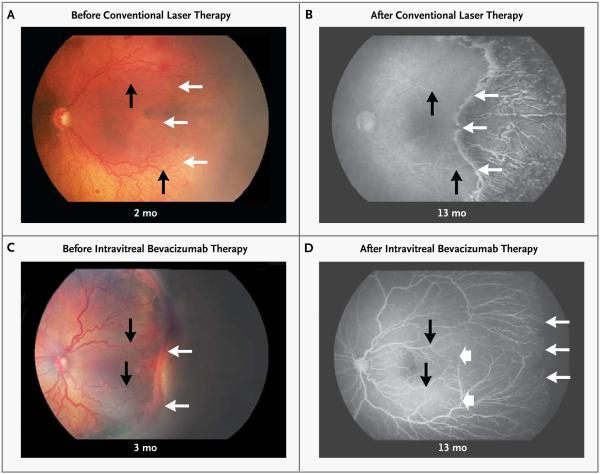
Conventional laser therapy resulted in permanent destruction of the vessels in the peripheral retina, whereas intravitreal bevacizumab allowed for continued vessel growth into the peripheral retina, a finding that is consistent with previous studies of case series.21,26,29 This is evident in Figure 3, which shows photographs of retinas before treatment and fluorescein angiograms of the retinas 1 year after treatment in two infants with zone I retinopathy of prematurity, one treated with conventional laser therapy and the other treated with intravitreal bevacizumab.
Figure 3. Fundus Photographs and Fluorescein Angiograms of Retinas in Study Infants with Stage 3+ Retinopathy of Prematurity in Zone I, before and after Treatment.
Panels A and B show the left retina of an infant before conventional laser therapy (at approximately 2 months of age, or 33.1 weeks’ postmenstrual age) and after therapy (at 13 months’ postmenstrual age), respectively. The infant was born at 24 weeks’ gestational age, with a birth weight of 760 g. The post-treatment photograph shows destruction of the full thickness of the peripheral retina, with only choroidal vessels (not retinal vessels) visible in the lasered area. Panels C and D show the left retina in another infant before intravitreal bevacizumab therapy (at approximately 3 months of age, or 35.6 weeks’ postmenstrual age) and after therapy (at 13 months’ postmenstrual age), respectively. The infant was born at 23 weeks’ gestational age, with a birth weight of 495 g. The post-treatment photograph shows continued vascularization of the peripheral retina. In all four panels, black arrows indicate identical retinal points for comparison before and after treatment, and thin white arrows indicate the extent of vascularization at each time point; the wide white arrows in Panel D indicate the extent of vascularization at the time of treatment with bevacizumab.
INFANTS WHO DIED
Seven infants (five who underwent intravitreal bevacizumab therapy and two who underwent conventional laser therapy) died before 54 weeks’ postmenstrual age and without having reached the primary outcome. Table 3 lists the baseline clinical characteristics of these infants, their risk factors for retinopathy of prematurity, and the circumstances of their deaths.
Table 3.
Characteristics of Infants Who Died.*
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
| Intravitreal Bevacizumab for Zone I ROP |
Intravitreal Bevacizumab for Zone I ROP |
Conventional Laser Therapy for Zone I ROP |
Intravitreal Bevacizumab for Zone II ROP |
Intravitreal Bevacizumab for Zone II ROP |
Intravitreal Bevacizumab for Zone II ROP |
Conventional Laser Therapy for Zone II ROP |
|
| Randomization assignment | |||||||
| Birth weight (g) | 595 | 450 | 650 | 470 | 575 | 550 | 665 |
| Gestational age (wk) | 24 | 23 | 28 | 23 | 24 | 24 | 24 |
| Sex | M | F | M | F | F | M | M |
| Mother’s race or ethnic group† | Hispanic | White | Hispanic | White | Black | Black | Hispanic |
| Infants delivered in current pregnancy (no.) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Intubation before randomization | No | Yes | Yes | Yes | Yes | No | No |
| Postmenstrual age at treatment (wk) | 33.6 | 33.3 | 33.6 | 32.6 | 35.7 | 36.4 | 35.1 |
| Patent ductus arteriosus ligation | No | Yes | No | No | No | Yes | Yes |
| Culture-proven sepsis | Yes | No | Yes | Yes | No | Yes | Yes |
| Surgery for necrotizing enterocolitis | No | No | No | No | Yes | No | Yes |
| Intraventricular hemorrhage‡ | No | No | Yes, grade IV | Yes, grade II | Yes, grade IV | Yes, grade II | Yes, grade IV |
| Periventricular leukomalacia | No | No | No | No | No | No | No |
| Intubated as part of treatment | No | No | No | No | No | No | No |
| Duration of hospitalization from treatment to discharge (days) |
47 | NA | NA | NA | NA | 83 | NA |
| Duration of mechanical ventilation from treatment to discharge (days) |
0 | NA | NA | NA | NA | 1 | NA |
| Duration of oxygen administration from treatment to discharge (days) |
47 | NA | NA | NA | NA | 83 | NA |
| Discharged home while receiving oxygen | Yes | NA | NA | NA | NA | Yes | NA |
| Postmenstrual age at death (wk) | 49.9 | 33.7 | 37.4 | 37.4 | 44.7 | 48.7 | 39.3 |
| Cause of death | Low oxygen | Adherence to do- not-resuscitate order |
Sepsis (coagulase- negative staphy- lococci) |
Respiratory failure | Respiratory failure | Low oxygen | Respiratory failure |
| Location at death | At home (without oxygen monitoring) |
In hospital | In hospital | In hospital | In hospital | At home (without oxygen monitoring) |
In hospital |
Data regarding hospital discharge are not given for some infants because they were not discharged. NA denotes not applicable, and ROP retinopathy of prematurity.
Mother’s race or ethnic group was self-reported.
Intraventricular hemorrhage can range from grade I to grade IV, with higher grades indicating more severe bleeding.
DISCUSSION
Our clinical study showed increased efficacy of intravitreal bevacizumab as compared with conventional laser therapy for stage 3+ retinopathy of prematurity when both zones I and II were considered (absolute difference in the risk of recurrence, 20 percentage points; 95% CI, 9 to 32). Our sample was sufficiently large to show significant efficacy of intravitreal bevacizumab for zone I disease but not for zone II posterior disease. Bevacizumab is an inexpensive drug that can be rapidly administered at the bedside by any ophthalmologist. In contrast, conventional laser therapy is laborious and requires special training to administer, as well as expensive equipment, endotracheal intubation, and a location designated for the use of lasers.
Safety is the primary reason for exercising caution when considering the use of intravitreal bevacizumab in the treatment of neonates. Both mortality and morbidity must be considered. For an assessment of mortality at a 5% significance level and with 80% power, a sample of 2800 infants would be required to determine whether intravitreal bevacizumab, as compared with conventional laser therapy, is associated with a significantly increased death rate (i.e., a rate 1.5 times as high as the 5.4% death rate at less than 9 months in the ETROP clinical trial). An assessment of local or systemic toxicity would require an even larger sample.
Ocular inflammation has been observed after seven or more injections of bevacizumab.33 As compared with other currently available anti-VEGF agents, bevacizumab has the advantage of being a large molecule (150 kD; it is a full antibody) that cannot penetrate the intact retina or escape the eye except in very small amounts, as shown in animal models (unless laser therapy has destroyed the natural retinal barrier).34 Taken together, the relatively long half-life of intravitreal bevacizumab (5 to 10 days),35,36 the highly viscous preterm vitreous gel, and a definitive end point (completion of retinal vascularization) suggest that a single injection of bevacizumab (half the dose typically administered intravitreally in adults for ocular neovascular diseases such as age-related macular degeneration and diabetic retinopathy) would be adequate to treat retinopathy of prematurity.34 A pathological study of the eyes of a very-low-birth-weight infant (350 g) born at 22 weeks’ gestational age who was treated with intravitreal bevacizumab showed no local toxic effects.37 The infant received two injections of intravitreal bevacizumab (0.50 mg in 0.02 ml of solution) in each eye, with continuation of retinal differentiation and vascularization to the point at which retinal precursors would have migrated before preterm birth.37
A major ocular side effect of ablative conventional laser therapy, especially in infants with zone I retinopathy of prematurity, is clinically significant loss of visual field.34 At the time of preterm birth in infants of 24 weeks’ gestational age or younger, the retinal vessels (which have already formed) and the vascular precursors (which would normally develop into retinal vessels) stop advancing. After intravitreal bevacizumab monotherapy, the retinal vessels advance to the point at which the vascular precursors have ceased migration, with differentiation of the underlying retina. However, the vascular precursors do not advance; thus, the far peripheral retina never fully vascularizes and does not differentiate (Fig. 3D).38 Treatment with intravitreal bevacizumab also averts the complications of conventional laser therapy in very mild stage 3+ retinopathy of prematurity that might regress spontaneously.12 Furthermore, intravitreal bevacizumab allows for the successful treatment of very extensive stage 3+ retinopathy of prematurity, both standard (type 1) and aggressive posterior (type 2) retinopathy, because intravitreal bevacizumab, which is available immediately after administration, decreases the high levels of VEGF in the vitreous gel, which are not reduced by conventional laser therapy.34
Although the literature suggests that intravitreal bevacizumab monotherapy is uniformly successful for stage 3+ retinopathy of prematurity,21,26,29 we observed that disease recurred in 2 of 62 eyes in infants with zone I disease and in 4 of 78 eyes in infants with zone II posterior disease. Moreover, for both zones considered together, the mean (±SD) time to recurrence was 16.0±4.6 weeks for 6 eyes after intravitreal bevacizumab therapy, as compared with 6.2±5.7 weeks for 32 eyes after conventional laser therapy (Table 2). This serves as a warning to clinicians that careful follow-up is needed in infants treated with intravitreal bevacizumab, who cannot be considered to be successfully treated until there is completion of vascularization with no active disease or clinically significant tractional elements. We observed no systemic or local toxic effects attributable to the administration of bevacizumab, but our study was too small to address the question of whether intravitreal bevacizumab is safe.
Additional research is needed to determine the dose of intravitreal bevacizumab that should be used for retinopathy of prematurity at any stage versus advanced disease and for standard (type 1) versus aggressive posterior (type 2) disease. Criteria should be developed regarding the duration and frequency of follow-up and the management of recurrence.
Supplementary Material
Acknowledgments
Supported by grants from Research to Prevent Blindness, the National Eye Institute (Core Grant P30EY10608), and the Hermann Eye Fund (all to Drs. Mintz-Hittner and Chuang), as well as research funds from the Alfred W. Lasher III Professorship and a grant from the University of Texas Health Science Center at Houston–Center for Clinical and Translational Science–Clinical Research Center (Clinical and Translational Science Award Grant UL1 RR024148) (both to Dr. Mintz-Hittner).
Footnotes
Dr. Mintz-Hittner reports receiving honoraria from the Bascom Palmer Eye Institute and Clarity Medical Systems, testifying as an expert witness in a retinopathy of prematurity suit, and having travel expenses paid by Pediatrix. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Lad EM, Nguyen TC, Morton JM, Moshfeghi DM. Retinopathy of prematurity in the United States. Br J Ophthalmol. 2008;92:320–5. doi: 10.1136/bjo.2007.126201. [DOI] [PubMed] [Google Scholar]; Br J Ophthalmol. 2010;94:1268. doi: 10.1136/bjo.2009.169326. Erratum. [DOI] [PubMed] [Google Scholar]
- 3.Axer-Siegel R, Snir M, Cotlear D, et al. Diode laser treatment of posterior retinopathy of prematurity. Br J Ophthalmol. 2000;84:1383–6. doi: 10.1136/bjo.84.12.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz X, Kychenthal A, Dorta P. Zone I retinopathy of prematurity. J AAPOS. 2000;4:373–6. doi: 10.1067/mpa.2000.107904. [DOI] [PubMed] [Google Scholar]
- 5.O’Keefe M, Lanigan B, Long VW. Outcomes of zone I retinopathy of prematurity. Acta Ophthalmol Scand. 2003;81:614–6. doi: 10.1111/j.1395-3907.2003.00171.x. [DOI] [PubMed] [Google Scholar]
- 6.Récsán Z, Vámos R, Salacz G. Laser treatment of zone I prethreshold and stage 3 threshold retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2003;40:204–7. doi: 10.3928/0191-3913-20030701-06. [DOI] [PubMed] [Google Scholar]
- 7.Kychenthal A, Dorta P, Katz X. Zone I retinopathy of prematurity: clinical characteristics and treatment outcomes. Retina. 2006;26(Suppl):S11–S15. doi: 10.1097/01.iae.0000244285.79004.e6. [DOI] [PubMed] [Google Scholar]
- 8.Spencer R. Long-term visual outcomes in extremely low-birth-weight children (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:493–516. [PMC free article] [PubMed] [Google Scholar]
- 9.Drenser KA, Trese MT, Capone A., Jr. Aggressive posterior retinopathy of prematurity. Retina. 2010;30(Suppl):S37–S40. doi: 10.1097/IAE.0b013e3181cb6151. [DOI] [PubMed] [Google Scholar]
- 10.Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Arch Ophthalmol. 1988;106:471–9. doi: 10.1001/archopht.1988.01060130517027. [DOI] [PubMed] [Google Scholar]
- 11.Smith LEH. Through the eyes of a child: understanding retinopathy through ROP: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2008;49:5177–82. doi: 10.1167/iovs.08-2584. [DOI] [PubMed] [Google Scholar]
- 12.Early Treatment for Retinopathy of Prematurity Cooperative Group Revised indications for the treatment of retinopathy of prematurity: results of the Early Treatment for Retinopathy of Prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 13.Quinn GE, Dobson V, Barr CC, et al. Visual acuity of eyes after vitrectomy for retinopathy of prematurity: follow-up at 5 1/2 years. Ophthalmology. 1996;103:595–600. doi: 10.1016/s0161-6420(96)30647-7. [DOI] [PubMed] [Google Scholar]
- 14.Repka MX, Tung B, Good WV, et al. Outcome of eyes developing retinal detachment during the Early Treatment for Retinopathy of Prematurity Study (ETROP) Arch Ophthalmol. 2006;124:24–30. doi: 10.1001/archopht.124.1.24. [DOI] [PubMed] [Google Scholar]
- 15.Chung EJ, Kim JH, Ahn HS, Koh HJ. Combination of laser photocoagulation and intravitreal bevacizumab (Avastin) for aggressive zone I retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2007;245:1727–30. doi: 10.1007/s00417-007-0661-y. [DOI] [PubMed] [Google Scholar]
- 16.Travassos A, Teixeira S, Ferreira P, et al. Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging. 2007;38:233–7. doi: 10.3928/15428877-20070501-09. [DOI] [PubMed] [Google Scholar]
- 17.Quiroz-Mercado H, Martinez-Castellanos MA, Hernandez-Rojas ML, Salazar-Teran N, Chan RV. Antiangiogenic therapy with intravitreal bevacizumab for retinopathy of prematurity. Retina. 2008;28(Suppl):S19–S25. doi: 10.1097/IAE.0b013e318159ec6b. [DOI] [PubMed] [Google Scholar]
- 18.Honda S, Hirabayashi H, Tsukahara Y, Negi A. Acute contraction of the proliferative membrane after intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2008;246:1061–3. doi: 10.1007/s00417-008-0786-7. [DOI] [PubMed] [Google Scholar]
- 19.Lalwani GA, Berrocal AM, Murray TG, et al. Off-label use of intravitreal bevacizumab (Avastin) for salvage treatment in progressive threshold retinopathy of prematurity. Retina. 2008;28(Suppl):S13–S18. doi: 10.1097/IAE.0b013e3181644ad2. [DOI] [PubMed] [Google Scholar]; Retina. 2009;29:127. Erratum. [Google Scholar]
- 20.Kusaka S, Shima C, Wada K, et al. Efficacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study. Br J Ophthalmol. 2008;92:1450–5. doi: 10.1136/bjo.2008.140657. [DOI] [PubMed] [Google Scholar]
- 21.Mintz-Hittner HA, Kuffel RR., Jr. Intravitreal injection of bevacizumab (Avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina. 2008;28:831–8. doi: 10.1097/IAE.0b013e318177f934. [DOI] [PubMed] [Google Scholar]; Retina. 2008;28:1374. Erratum. [Google Scholar]
- 22.Zepeda-Romero LC, Liera-Garcia JA, Gutiérrez-Padilla JA, Valtierra-Santiago CI, Cardenas-Lamas LJ. Paradoxical vascular-fibrotic reaction after intravitreal bevacizumab for retinopathy of prematurity. Eye (Lond) 2010;24:931–3. doi: 10.1038/eye.2009.156. [DOI] [PubMed] [Google Scholar]
- 23.Law JC, Recchia FM, Morrison DG, Donahue SP, Estes RL. Intravitreal bevacizumab as adjunctive treatment for retinopathy of prematurity. J AAPOS. 2010;14:6–10. doi: 10.1016/j.jaapos.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Lee JY, Chae JB, Yang SJ, Yoon YH, Kim J-G. Effects of intravitreal bevacizumab and laser in retinopathy of prematurity therapy on the development of peripheral retinal vessels. Graefes Arch Clin Exp Ophthalmol. 2010;248:1257–62. doi: 10.1007/s00417-010-1375-0. [DOI] [PubMed] [Google Scholar]
- 25.Altinsoy HI, Mutlu FM, Güngör R, Sarici SU. Combination of laser photocoagulation and intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging. 2010 March 9; doi: 10.3928/15428877-20100215-03. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 26.Dorta P, Kychenthal A. Treatment of type 1 retinopathy of prematurity with intravitreal bevacizumab (Avastin) Retina. 2010;30(Suppl):S24–S31. doi: 10.1097/IAE.0b013e3181ca1457. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed AE, Channa R, Durrani J, Ali A, Ahmad K. Early experience with intravitreal bevacizumab combined with laser treatment for retinopathy of prematurity. Middle East Afr J Ophthalmol. 2010;17:264–7. doi: 10.4103/0974-9233.65500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nazari H, Modarres M, Parvaresh MM, Falavarjani KG. Intravitreal bevacizumab in combination with laser therapy for the treatment of severe retinopathy of prematurity (ROP) associated with vitreous or retinal hemorrhage. Graefes Arch Clin Exp Ophthalmol. 2010;248:1713–8. doi: 10.1007/s00417-010-1430-x. [DOI] [PubMed] [Google Scholar]
- 29.Wu W-C, Yeh P-T, Chen S-N, Yang C-M, Lai C-C, Kuo H-K. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: a multicenter study in Taiwan. Ophthalmology. 2011;118:176–83. doi: 10.1016/j.ophtha.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Mintz-Hittner HA. Bevacizumab eliminates the angiogenic threat of retinopathy of prematurity (BEAT-ROP) 2010 ClinicalTrials.gov. http://clinicaltrials.gov/ct2/show/NCT00622726?term=Bevacizumab+eliminates+the+angiogenic+threat+of+retinopathy+of+prematurity+%28BEAT-ROP%29&rank=1.
- 31.Section of Ophthalmology American Association of Pediatrics. American Academy of Ophthalmology. American Association for Pediatric Ophthalmology and Strabismus Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117:572–6. doi: 10.1542/peds.2005-2749. [DOI] [PubMed] [Google Scholar]; Pediatrics. 2006;118:1324. Erratum. [Google Scholar]
- 32.International Committee for the Classification of Retinopathy of Prematurity The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]; Arch Ophthalmol. 2006;124:1669–70. doi: 10.1001/archopht.124.11.1669-c. Erratum. [DOI] [PubMed] [Google Scholar]
- 33.Bakri SJ, Larson TA, Edwards AO. Intraocular inflammation following intravitreal injection of bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2008;246:779–81. doi: 10.1007/s00417-007-0754-7. [DOI] [PubMed] [Google Scholar]
- 34.Mintz-Hittner HA. Avastin as monotherapy for retinopathy of prematurity. J AAPOS. 2010;14:2–3. doi: 10.1016/j.jaapos.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin) Ophthalmology. 2007;114:855–9. doi: 10.1016/j.ophtha.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol. 2008;146:508–12. doi: 10.1016/j.ajo.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 37.Kong L, Mintz-Hittner HA, Penland RL, Kretzer FL, Chévez-Barrios P. Intravitreal bevacizumab as anti-vascular endothelial growth factor therapy for retinopathy of prematurity: a morphologic study. Arch Ophthalmol. 2008;126:1161–3. doi: 10.1001/archophthalmol.2008.1. [DOI] [PubMed] [Google Scholar]
- 38.Mintz-Hittner HA, Kretzer FL. Postnatal retinal vascularization in former preterm infants with retinopathy of prematurity. Ophthalmology. 1994;101:548–58. doi: 10.1016/s0161-6420(94)31301-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.