Abstract
The possible inclusion complexes of Cp2NbCl2 into calixarenes hosts have been investigated. The existence of a true inclusion complex in the solid state was confirmed by a combination of NMR, ab-initio calculations, thermogravimetric analysis, FTIR, Raman and PXRD. Ab-initio calculations, 1H NMR solution and solid state 13C CP MAS NMR results demonstrated that p-sulfonic calix[6]arene does form an inclusion complex with Cp2NbCl2. Raman spectroscopy showed, for the inclusion compound of p-sulfonic calix[6]arene-Cp2NbCl2, a band between 500–850 cm−1 characteristic of Nb-O vibration. This result suggests that Nb(V) may engage in coordination with the oxygen of the sulfonate group, as part of the host-guest interaction. However, it is important to mention that the niobocene dichloride (Cp2NbCl2) dissolves in water and undergoes oxidation and hydrolysis processes to yield Cp2NbCl2(OH) species. For that reason this band does not exclude that the Nb-O band belongs to Cp2NbCl2(OH). Solid State 13C CP MAS NMR and solution 1H NMR spectroscopies together with ab-initio results showed that Cp2NbCl2 is included in the p-sulfonic calix[6]arene cavity, with both Cp rings inside the cavity. In contrast, the solution 1H NMR results demonstrated that calix[6]arene does not form inclusion complex with Cp2NbCl2 in CDCl3 solution. Cp2NbCl2 is not included in the calix[6]arene cavity, possibly due to the lack of sulfonate heads which promote Nb-O interactions and assist the inclusion of Cp2NbCl2 into the cavity.
Keywords: Cp2NbCl2, p-sulfonic calix[n]arene, calix[6]arene, host-guest, inclusion, antitumor, 13C CP MAS
1. Introduction
Calixarenes are cup-like organic macrocyclic molecules formed by the ortho-condensation of para-substituted phenols by formaldehyde which can be functionalized at either the rim, R or R′, Scheme I. Water soluble and in particular p-sulfonic calix[n]arene, have been the subject of growing interest in the biological domain. The inclusion complexes have found pharmaceutical applications due to the increased aqueous solubility of the drugs, better oral absorption and their improved stability towards heat, light, oxidizing reagents and acidic conditions [1]. Moreover, water soluble calix[n]arene (n = 4–8) derivatives have received considerable attention in recent years because of their selective metal ion binding properties in aqueous solution, the formation of clay-like bilayer structures in the solid state, and the observation of H2O···π aromatic hydrogen bonding [2–7].
Scheme I.
There are many advantages to use calixarenes as host molecules. Among these are weak London Forces, hydrogen bonding, π-π interaction, and dipole-dipole moments, which play important roles in complex formation and drug release. p-Sulfonic calix[n]arenes provided not only a hydrophobic environment (benzene rings or alkyl chains) with the above favorable properties, but also hydrophilic heads (sulfonates) and form water soluble encapsulated complexes [2–7].
Our research involves many aspects of the metallocene antitumor agents, from cyclopentadienyl functionalization, structure modification with biologically active ancillary ligands, spectroscopic studies and development of analytical methodology to preparation and characterization of the inclusion complexes of metallocene antitumor agents, in order to afford thermally and hydrolytically stable species with enhanced anticancer properties [8–22]. Herein we report the interaction of Cp2NbCl2 with calix[n]arenes in aqueous and chloroform solutions and their characterization in solution and solid state.
2. Experimental
2.1 Materials and Methods
p-Sulfonic calix[n]arenes, n = 4,6,8, (Acros) and calix[6]arene (Sigma-Aldrich) were obtained commercially and used as received. Cp2NbCl2 was purchased from Aldrich and used as received.
The thermal analysis experiments were performed using a TAQ100 (DSC) and TAQ500 (TGA) instrument. The heating rate was 3° C/min for DSC analysis and 10° C/min for TGA analysis. A differential scanning calorimeter interfaced to a PC was used to measure the thermal properties of the inclusion compounds. The calorimeter operated with a nitrogen flow of 50 mL/min. The temperature of the calorimeter was calibrated from the observed melting points of indium. FTIR data were collected on a Nexus 670 spectrometer using Thunderdome ATR. Raman data were collected using Jobin Yvon LabRam (DU/20 DE-320) in the 200–3000 cm−1 range, with an excitation wavelength of 532 nm and a resolution of 2 cm−1. Powder XRD data were collected on a Siemens D5000 diffractometer using Cu Kα radiation, λ = 1.5418 Å.
NMR data were collected in a 400 MHz WB instrument with a 4 mm probe in double resonance mode and 13 kHz magic angle sample spinning at room temperature. Cross polarization from protons to carbon with 2 ms contact time, 34 ms acquisition time and 5s recycle delay. The number of scans varies according to the sample amount, linewidth, and available spectrometer time. 1H NMR spectra were recorded on a Bruker Avance 500 MHz and chemical shifts are referenced to HOD/H2O or CDCl3 as internal reference.
2.2 Ab-initio calculations
Ab-initio calculations were performed using the Gaussian, G03W program package running on a personal computer [23]. The initial geometry of Cp2NbCl2 was obtained using the X-ray structure available at the Cambridge Crystallographic Data Centre (CIF CPCLNB). For p-sulfonic calix[6]arene and calix[6]arene we used the X-ray structure of calix[6]arene tris-carbolyxic acid (CCDC 299787), removing the carboxy groups for OH and the ter-butyl for H or SO3H. The fully optimized geometry for the free Cp2NbCl2 and p-sulfonic calix[6]arene or calix[6]arene were obtained at the B3LYP level, using the standard LanL2DZ basis set. In order to obtain the best p-sulfonic calix[6]arene- or calix[6]arene- Cp2NbCl2 host-guest interactions, several geometries were calculated by a set of single point calculations at the DFT level
2.3 Syntheses of calix[n]arene derivatives-Cp2NbCl2 inclusion complexes
2.3.1
0.2 mmol of p-sulfonic calix[n]arene (n= 4, 6 and 8), corrected for water content, was dissolved in 30 mL of deionized water and the resulting pH was 2.1. 0.2 mmol (58.8 mg) of Cp2NbCl2 was added to the p-sulfonic calix[n]arene solution, and the resulting pH was 2.2. After stirring for 30 minutes under aerobic conditions at room temperature, the reaction mixture was filtered in a fritted funnel of fine porosity (type F 4.0–5.5 microns) to remove any particle suspended and lyophilized to obtain amorphous voluminous solid product. For the p-sulfonic calix[4]arene:niobocene, re-dissolving the solid, heating to 60°C and cooling to 10°C induces precipitation and the solid is collected by filtration and dried in vacuo. For p-sulfonic calix[n]arene (n=6, 8) there is no need to recrystallize in water. Only the p-sulfonic calix[n]arene-niobocene (n =4, 6, 8) in a 1:1 ratio is isolated in this procedure and fully characterized. Yield: p-sulfonic calix[4]arene-niobocene 80%, p-sulfonic calix[6]arene-niobocene (96%), p-sulfonic calix[8]arene-niobocene (96%).
2.3.2
0.2 mmol of calix[6]arene was dissolved in 30 mL of chloroform and 0.2 mmol of Cp2NbCl2 was added. After stirring for 30 minutes under aerobic conditions at room temperature, the reaction mixture was lyophilized to obtain amorphous voluminous solid product. Yield: 90%.
Anal. Calc. for p-sulfonic calix[4]arene-Cp2NbCl2(OH)·XH2O [(C28H24O16S4)-(C10H10NbOHCl2)·7H2O]
Calculated anal. C, 38.61; H, 4.17. Found: C, 38.93; H, 4.25. IR (cm−1): 3119(w), 1695(w), 1457(m), 1117(m), 1031(m), 845(s), 812(s). TGA data: dehydration temperature range (°C): 25–100, Mass loss (%): 13.77. DSC Endothermic max (°C), D = dehydration, M = melting, MR = molecular reorganization: (126.32 D), (209.08 MR), 250.79 M (254.55 M). PXRD major characteristic peaks at 2θ°: 10.54, 12.94, 15.44, 16.82, 17.48 (strongest), 18.58 (secondly), 23.80, 26.14. 1H NMR, D2O: δ 7.51(s), 6.30(s), 4.05(s). The second species in a 2:1 p-SO4-calix[4]arene:niobocene ratio has a Cp signal at 5.3(s).
Anal. Calc. for p-sulfonic calix[6]arene-Cp2NbCl2(OH)·XH2O [(C42H36O24S6)-(C10H10NbOHCl2)·11H2O]
Calculated anal.C, 38.40; H, 4.28. Found: C, 38.28; H, 4.26. IR (cm−1): 3236(w), 1593(w), 1445(m), 1270(m), 1032(m), 887(s), 848(s). Raman (cm−1): 500–700 (b), 1250–1750 (2 peaks). TGA data: dehydration temperature range (°C): 25–100, Mass loss(%): 16.24. DSC Endothermic max (°C): 95.50 D (140.36 D), 234.00 M (254.10 M). PXRD major characteristic peaks at 2θ°: 15.72, 18.10, 21.30 (strongest), 22.80, 25.50, 27.24, 29.14. 13C- CP spectrum: δ 156.5, 152.9, 152.0, 136.5, 135.6, 134.8, 130.2, 128.9, 128.0, 127.6, 126.9, 123.3, 122.1, 121.7, 120.7, 119.9, 118.5, 33.1, 32.8, 31.7, 30.0. 1H NMR, D2O: δ 7.23(s), 5.63(s), 3.68(s).
Anal. Calc. for p-sulfonic calix[8]arene-Cp2NbCl2(OH)·XH2O [(C56H48O32S8)-(C10H10NbOHCl2)·20H2O]
Calculated anal. C, 36.69; H, 4.61. Found: C, 36.48; H, 4.60. IR (cm−1): 3192(w), 1647(w), 1447(m), 1271(m), 1033(m), 885(s), 849(w). TGA data: dehydration temperature range (°C): 25–100, Mass loss(%): 17.27. DSC Endothermic max (°C): 115.39 D (141.54 MR) 138.36 MR (215.35 MR), 235 M. PXRD major characteristic peaks at 2θ°: 16.62, 22.08 (strongest), 29.44. 1H NMR, D2O: δ 7.49(s), 6.10(s), 4.00(s)
3. Results and discussion
3.1 Ab-initio calculations
Niobocene dichloride (Cp2NbCl2) dissolves in water and undergoes an oxidation and hydrolysis processes to yield Cp2NbCl2(OH) species [16,24]. This species has been characterized by NMR spectroscopy and has been attributed to be the active species in aqueous solution. Therefore in aqueous solution, it is very likely that the predominant species is Cp2NbCl2(OH). The methodology used to prepare the p-sulfonic calix[n]arene–Cp2NbCl2 inclusion complex is the addition of solid Cp2NbCl2 into the aqueous solution containing p-sulfonic calix[n]arene. Thus, the species encapsulated in solution into the p-sulfonic calix[n]arene may involve Cp2NbCl2(OH) but, Cp2NbCl2 as initial species encapsulated into the calix[n]arene cavity cannot be ruled out. Under aerobic conditions, eventually Cp2NbCl2 oxidizes into Cp2NbCl2(OH). Thus, after 30 minutes in aqueous solution under aerobic environment, we expect that Cp2NbCl2(OH) is the major species present. Therefore, the species encapsulated in the solid state should be diamagnetic. Throughout the text, we will use Cp2NbCl2 as the species to be included into the calixarene cavity. p-Sulfonic calix[n]arenes when dissolved in water have a pH of 2.1. However, even at this acidic pH one or more sulfonic groups might be deprotonated [3]. Nevertheless, our analytical data suggests that all of the -SO3 groups are protonated.
The inclusion complexes were characterized by a series of physical techniques, including thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), FTIR, Raman, powder x-ray diffraction (PXRD), solid state 13C CP-MAS and 1H NMR spectroscopies combined with ab-initio calculations.
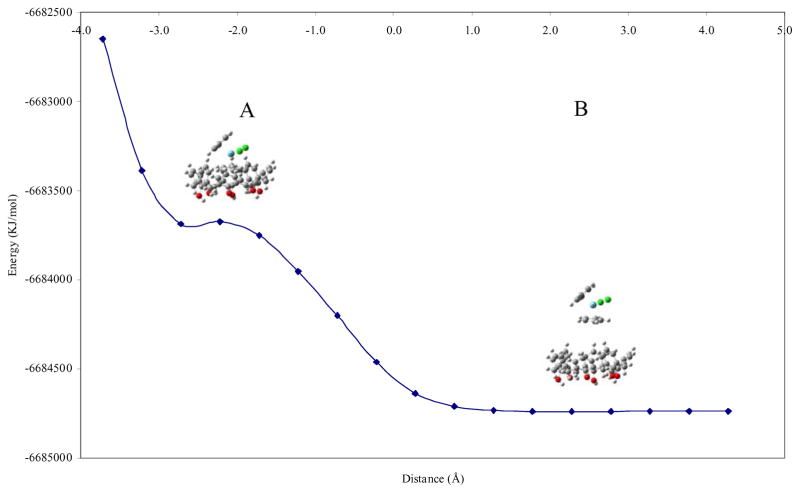
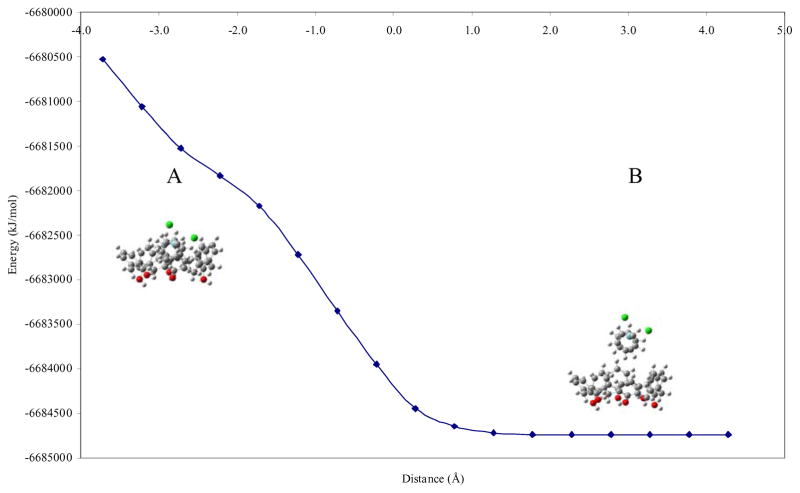
Our initial strategy involved theoretical calculations to explore the possibility of niobocene inclusion into the calix[n]arene cavity and then perform the experiments to corroborate our predictions. The calix[n]arene–organometallic inclusion geometries were investigated by ab-initio calculations, using a rigid-body approach to explore the possibility of host-guest formation and its most likely inclusion geometry. This approach does not account for the calix[n]arene nonrigidity, but it has shown to give valuable information concerning preferential inclusion geometries and guest mobility [25,26]. Several different inclusion geometries were calculated with calix[6]arene and p-sulfonic calix[6]arene as the hosts in order to obtain the possible interaction geometries. Figure 1 and 2 show the results obtained for two representative inclusion geometries for calix[6]arene-Cp2NbCl2 inclusion: one Cp and two Cp inclusion geometries respectively. In the plot, the distance (abscisa) is defined by the plane of the outer hydrogen atoms of the lower rim (phenol protons) of the host and the nearest hydrogen atom of the approaching Cp2NbCl2 molecule. Negative distance values refer to inclusion of the organometallic molecule into the hydrophobic cavity. Positive distance values indicate no inclusion.
Figure 1.
Plot of energy versus host-guest distance for one cyclopentadienyl (Cp) geometry for calix[6]arene-Cp2NbCl2.
Figure 2.
Plot of energy versus host-guest distance for two cyclopentadienyl (Cp) geometry for calix[6]arene-Cp2NbCl2. A represents inclusion and B represent non-inclusion.
The energy profiles obtained for the inclusion of Cp2NbCl2 into calix[6]arene cavity show that the lowest energy structure was found to be structure B. For one Cp inclusion geometry, the lowest energy was at a distance of 2.3 Å with −6.684738 × 106 kJ/mol whereas for two Cp inclusions geometry the lowest energy was at 2.3 Å with −6.684740 × 106 kJ/mol, indicating no inclusion of the Cp rings into the host. These results demonstrated that the inclusion of Cp2NbCl2 inside the hydrophobic cavity (structure A) is not the lowest energy conformation, being energetically disfavored. The inclusion interaction does not reach the lowest energy perhaps due the lack of hydrophilic component in the upper rim (sulfonate heads) that promotes some sort of Nb-O interactions which may assist the inclusion. In addition, the energy found for the inclusion of Cp2NbCl2 in calix[6]arene in the lower rim (phenol group) was least stable (−6.681667 × 106 kJ/mol) than the inclusion which occurs through the upper rim (−6.683687 × 106 kJ/mol), with an energy difference of 2.020 × 103 kJ/mol.
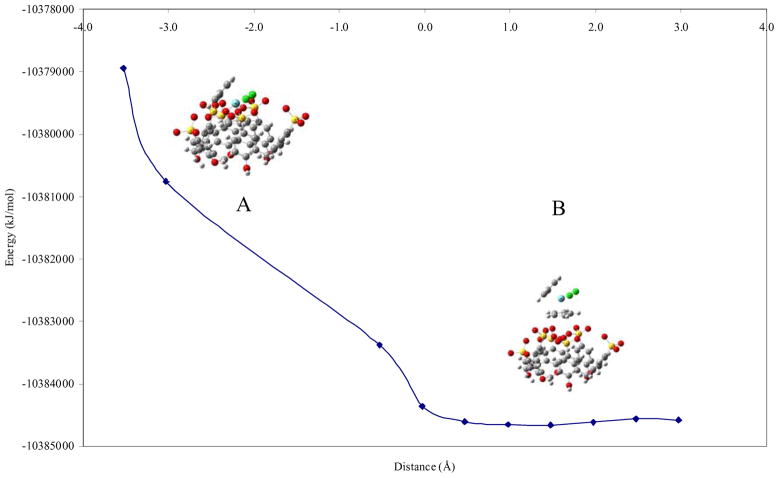
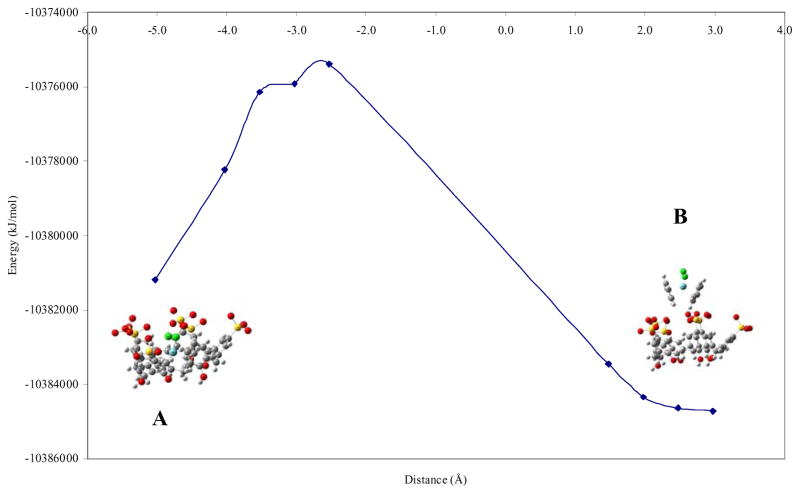
Figures 3 and 4 show the results obtained for two representative inclusion geometries for p-sulfonic calix[6]arene and Cp2NbCl2: one or two cyclopentadienyl rings at different distances inside the cavity. As mentioned previously, negative distance values refer to inclusion of the organometallic molecule into the hydrophobic cavity.
Figure 3.
Plot of energy versus host-guest distance for one cyclopentadienyl (Cp) geometry for p-sulfonic calix[6]arene-Cp2NbCl2.
Figure 4.
Plot of energy versus host-guest distance for two cyclopentadienyl (Cp) geometry for p-sulfonic calix[6]arene-Cp2NbCl2.
The ab-initio calculations for one Cp inclusion geometry, Figure 3, showed that the inclusion of Cp2NbCl2 in p-sulfonic calix[6]arene (structure A) is energetically costly and that the lowest energy is reached when Cp2NbCl2 in p-sulfonic calix[6]arene adopt structure B with an energy of −1.0384659 × 107 kJ/mol at a distance of 1.5 Å, indicating no inclusion. This result suggests that the inclusion of just one Cp ring inside the hydrophobic cavity is not favorable energetically. One possible explanation is that the other Cp is oriented toward the hydrophilic environment which destabilizes this inclusion conformation. Additionally, the energy found for the inclusion of Cp2NbCl2 in p-sulfonic calix[6]arene in the lower rim (phenol oxygen) was least stable (−1.0384430 × 107 kJ/mol) than when the inclusion occurs through the upper rim (−1.0384659 × 107 kJ/mol), with a energy difference of 2.29 × 102 kJ/mol.
On the other hand, Figure 4 has an energy profile pattern different to Figures 1–3. Figure 4 has two structures, A and B, separated from other highly energetic intermediate structures.
From Figure 4 we observe that the lowest energy conformation is structure B at a distance of 3.0 Å and energy of −1.0384714 × 107 kJ/mol, suggesting no inclusion. At this distance the two molecules are separated without any type of binding interaction. But this result must be taken with caution. The fact that the two molecules are separated, not in contact, will decrease the energy. Furthermore, the fact that this curve has different shape than the other ones (Figures 1–3) with two minimum in energy separated by steep curve suggests that the energy minimum at 3.0 Å separation is just the consequence of the two molecules that do not feel perturbation by any type of binding interactions (albeit weak) and they are in their corresponding ground states. That presumption was confirmed with the energy values for each molecule without binding interaction, for Cp2NbCl2 is −1.242785 × 106 kJ/mol and for p-sulfonic calix[6]arene is −9.141375 × 106 kJ/mol. The addition of their energies corresponds to the energy determined for the lowest conformation for structure B. Therefore, the second lowest minimum (structure A) is significant.
The second lowest energy point in Figure 4 is structure A at a distance of −5.0 Å (−1.0381182 × 107 kJ/mol). This structure corresponds to a geometry where both Cp rings are included inside the p-sulfonic calix[6]arene cavity. This second lowest energy conformation (A) was favored for the inclusion of the two cyclopentadienyl into the hydrophobic environment, which promotes their stabilization by π-π interactions between the aromatic rings of the p-sulfonic calix[6]arene and the π-electrons of the Cp rings. Thus, the second energy minimum at −5 Å strongly suggests that the inclusion of both Cp rings is in fact feasible. This will be later verified by NMR spectroscopic methods.
The ab-initio calculations showed in addition of the inclusion, the possibility of coordination of the metal center with the oxygen of the sulfonate groups as part of the interaction with the organometallic molecule. In one Cp inclusion geometry, the energy for coordination of niobium by one sulfonate is −1.0378948 × 107 kJ/mol whereas for two Cp inclusion geometry is −1.0375921 × 107 kJ/mol. Both geometries have higher energies than structure A. Raman spectrum for the inclusion compound of p-sulfonic calix[6]arene-Cp2NbCl2 showed a broad band around 700 cm−1 which could to belong Nb-O vibration but, it is overlapped by the -SO3− vibration mode (see Supplementary Materia,l Figure 1S). However, is important to mention that the niobocene dichloride dissolves in water and undergoes an oxidation to yield Cp2NbCl2(OH) species [16,24]. For that reason, this band does not exclude that Nb-O vibration belongs to Cp2NbCl2(OH). Nevertheless, under the conditions the Raman spectrum was obtained (excitation wavelength of 532 nm), the compound has fluorescence, making the vibration bands assignment difficult. But based on the coordination properties of the sulfonic calix[6]arene, Nb(V)-O(sulfonate) coordination cannot be ruled out [2–5].
3.2 NMR Spectroscopic Studies
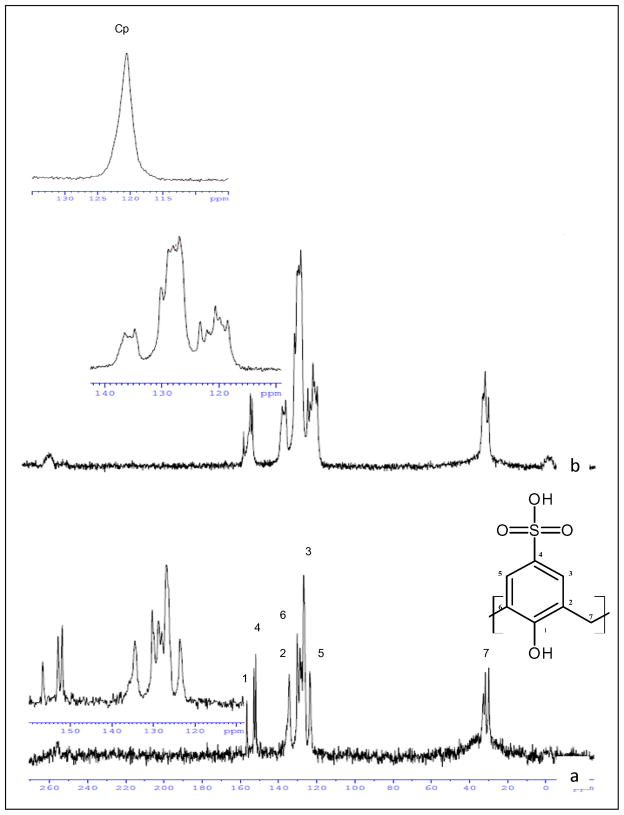
The inclusion compounds were further investigated by solid state 13C CP-MAS NMR spectroscopy. This technique has allowed us to distinguish between the free host and the inclusion complexes, since the chemical environment between these two species are different and as a result the 13C chemical shift patterns are different. Figure 5 presents the solid state 13C CP-MAS NMR spectra of Cp2NbCl2, p-sulfonic calix[6]arene and the inclusion complex for p-sulfonic calix[6]arene-Cp2NbCl2. According to previous reports, the carbon resonances are assigned as followed: C-1,4 (150–160 ppm), C-2,6,3,5 (140-120 ppm), C-7 (35-25 ppm) [27]. Upon analysis of Figure 5, it is evident that the inclusion complex shows broad peaks corresponding to the C-1.4, C-2, 6, 3, 5 and C-7. The Cp peak corresponding to the Cp2NbCl2 apparently is overlapped by the C-5 signal in the region of 120 ppm and might be more than one signal corresponding to the Cp rings, but this region has many signal to make a more convincing assignment (see Supplementary Material). Additionally, the peak corresponding to C-4 broadened whereas C-3 and C-5 signals are overlapped. Signals for C-2 and C-6 are less overlapped and they begin to separate. This evidence strongly suggests that Cp2NbCl2 (Cp2NbCl2(OH)) is included in the p-sulfonic calix[6]arene and the chemical environment of the carbon atoms has changed and becomes more unsymmetrical. The shallow inclusion of niobocene into the p-sulfonic calix[6]arene may explain why C-3, C-4 and C-5 experience more distortion on the signals than C-2 and C-6.
Figure 5.
13C CP-MAS spectra of (a) p-sulfonic calix[6]arene, (b) p-sulfonic calix[6]arene-Cp2NbCl2 lyophilized. Top inset: Cp signal of Cp2NbCl2 after dissolved in water and lyophilized.
In order to investigate in more details the nature of the p-sulfonic calix[n]arene-niobocene interaction, 1H NMR spectroscopic studies in D2O for p-sulfonic calix[4]arene-Cp2NbCl2 and p-sulfonic calix[6]arene-Cp2NbCl2 were performed. The p-sulfonic calix[n]arene-Cp2NbCl2 complexes have the advantage that they are soluble in water. We performed a Job plot to determine the stoichiometry of p-sulfonic calix[4]arene-niobocene inclusion compound. The Cp signals for hydrolysis of Cp2NbCl2 in D2O are at 6.689 ppm (major product) and 6.785 ppm (minor product) while the p-sulfonic calix[4]arene has two signals at 7.50 and 3.95 ppm. At the molar fraction (χniobocene) of 0.10, the spectrum shows two Cp signals at 6.13 and 5.57 ppm in a ratio of 1:1.1 corresponding to two different niobocene species. As we increase the molar ratio of niobocene dichloride, the intensity of the peak at 5.57 ppm initially increases slightly up to a molar fraction (χniobocene) of 0.33, whereas the intensity of the signal at 6.13 increases as we increase the molar fraction reaching a maximum at χniobocene of 0.5 (see Supplementary Material, Figure 4S). Thus, these two Cp signals do not belong to the free species, Cp2NbCl2(OH). Both signals initially move up field and eventually shift down field. In all cases the signals show broadening suggesting these two inclusion processes are dynamic.
Using the aforementioned NMR data, the stoichiometry of the p-sulfonic calix[4]arene-niobocene inclusion compounds were determined by monitoring the Cp signals as we increase the χniobocene. Two Job plots for the two Cp signals, ΔδCp = δfreeCp − δinclusionCp vs. χniobocene, were obtained (Supplementary Material). Upon analysis of the Job plots we determined that the species at 5.57 ppm (albeit in lower quantity at the end of the titration) belongs to p-sulfonic calix[4]arene-niobocene in a 2:1 ratio. The major species at 6.13 ppm belongs to the p-sulfonic calix[4]arene-:niobocene in a 1:1 ratio.
To investigate in more details the dynamic nature of the the p-sulfonic calix[4]arene-niobocene inclusion systems, Variable temperature (VT) 1H NMR experiments were performed. VT 1H NMR experiments of p-sulfonic calix[4]arene-Cp2NbCl2 in D2O showed that as we lower the temperature from room temperature (25°C), the signal at 3.90 ppm corresponding to the CH2 of the p-sulfonic calix[4]arene broadens and the low field Cp peak begins to split in two signals. At 25°C the half-height line width of the CH2, Δν1/2, is 1.13 Hz while at 5°C is 3.69 Hz indicating dynamic process where at least two p-sulfonic calix[4]arene-Cp2NbCl2 species are present and chemical exchange between the species exist. In addition, the low field Cp signal at 6.13 is split in two singlets which again suggest that there are two magnetically unequivalent Cp rings in chemical exchange in the NMR time scale. Due to the solvent limitations, we could not reach the separation of the two proton’s CH2 peaks.
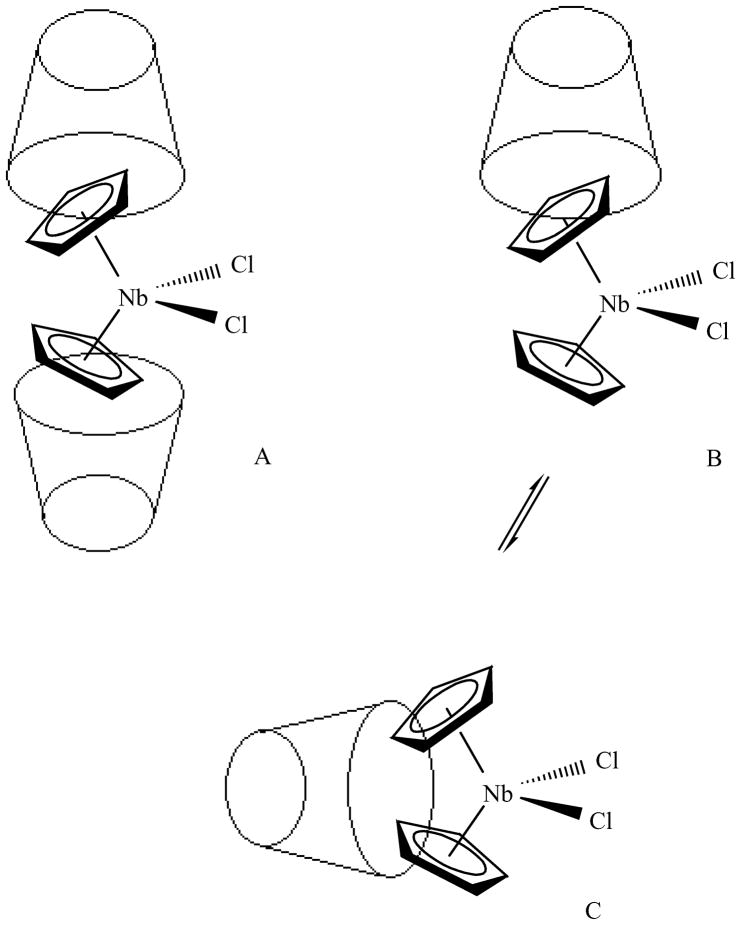
We are aware that p-sulfonic calix[4]arene may exist in four stable conformations (cone, partial cone, 1,2-alternate and 1,3-alternate), the cone conformation (bowl shape) being favored by stabilization by intramolecular hydrogen-bonding interactions among OH groups [28]. Thus, we believe that p-sulfonic calix[4]arene-niobocene inclusion compounds contain the p-sulfonic calix[4]arene in the cone conformation. Since the inclusions are dynamic as stated before, we envision that three species could exist in solution, according to Figure 6. Figure 6 depicts the possible inclusion geometries present in solution for p-sulfonic calix[4]arene-niobocene inclusion system. It is difficult, due to the dynamic process, under the NMR time scale to completely rule out inclusion geometry C as one of the geometry coexisting with geometry B, although geometry B explains better the two Cp signals in the 1H NMR spectrum at low temperature.
Figure 6.
Inclusion geometries present in solution for p-sulfonic calix[4]arene:niobocene inclusion system.
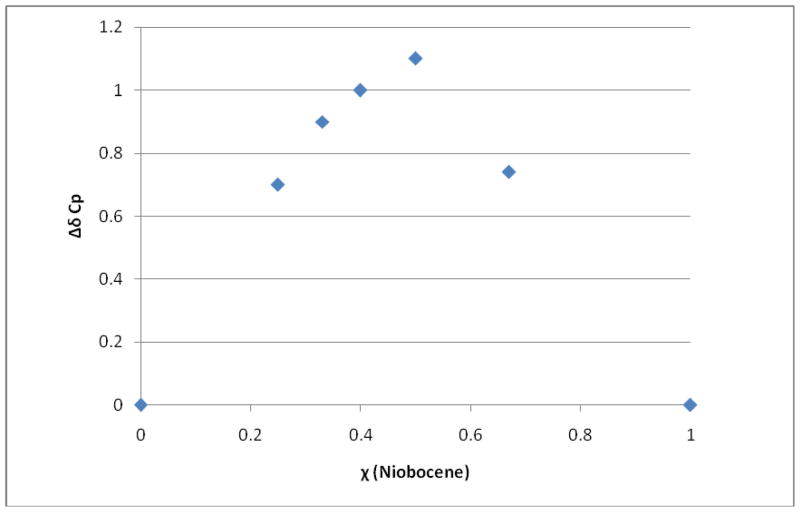
Likewise, the p-sulfonic calix[6]arene-niobocene system was investigated in solution by 1H NMR spectroscopy in D2O and the Cp signal monitored. The Job plot (ΔδCp = δfreeCp − δinclusionCp vs. χ (Cp2NbCl2) (Figure 7) shows that the stoichiometry for the p-sulfonic calix[6]arene-niobocene inclusion complex is 1:1, see Supplementary Material. This suggests that, different to the p-sulfonic calix[4]arene-niobocene, niobocene has the two Cp rings inside the p-sulfonic calix[6]arene cavity, similar to structure A in Figure 4, as predicted by ab-initio calculations and it is the only species present in solution. As a consequence, just one Cp signal was observed in the 1H NMR spectrum. Identical results were obtained with p-sulfonic calix[8]arene-niobocene inclusion. This is the logical result of p-sulfonic calix[8]arene and p-sulfonic calix[6]arene having larger cavity diameters than the p-sulfonic calix[4]arene and can accommodate the niobocene inside the cavity, in a cone (bowl shape) conformation.
Figure 7.
Job plot of p-sulfonic calix[6]arene and Cp2NbCl2, in D2O at 25 °C.
Meanwhile, 1H NMR spectroscopic studies in CDCl3 for calix[6]arene-niobocene showed Cp and the calix[6]arene proton signals corresponding to the free species. These results suggest no inclusion of niobocene into the cavity of calix[6]arene. Apparently, the lack of a hydrophilic environment (sulfonate heads) on the calix[6]arene does not promote the inclusion of niobocene into the hydrophobic cavity to reach a lowest energy conformation. Based on this solution information, we confirm the ab-initio results that the lowest energy conformation is outside the cavity of calix[6]arene without inclusion.
3.3 Thermal Properties and Powder X-ray diffraction
The thermal properties of the niobocene, p-sulfonic calixarenes and the inclusion complexes were studied using TGA and DSC, see Table 1. In general, the p-sulfonic-p-sulfonic calix[n]arenes loose 18–25 % of mass in the range of 25–100° C, indicating loss of water in the calixarenes. The TGA and DSC thermograms of the p-sulfonic calix[n]arenes inclusion complexes demonstrated mass loss of 13–17 % between 25–100 ° C, also indicating loss of water from the p-sulfonic-calix[n]arene inclusion complexes. But the amount of water in the inclusion complexes is smaller than the free p-sulfonic calix[n]arenes, indicating that some of the water molecules inside the cavity have been replaced by niobocene. Additionally, the percentage of water present in the inclusion complexes is close to the amount of water calculated by elemental analysis.
Table 1.
Thermoanalytical data of inclusion complexes and precursors. Note: D= Dehydration, M = Melting, Molecular reorganization = MR and TG =Glass transition.
| Dehydration | DSC Endothermic max (°C) | |||
|---|---|---|---|---|
| Temperature range (°C) | Mass loss (%) | Onset point (°C) | Onset point (peak max) (°C) | |
| p-sulfonic calix[4]arene | 25–100 | 25.27 | 259.32 | 162.99 D (172.35 D) 274.50 M (285.83 M) |
| p-sulfonic calix[6]arene | 25–100 | 23.57 | 250.74 | 140.40 D (147.46 D), (199.60 MR),(252.33 M) |
| p-sulfonic calix[8]arene | 25–100 | 18.25 | 251.66 | 125.34 D, 168.79 MR, 244.32 M (264.53 M) |
| Cp2NbCl2 | 25–100 | 4.42 | 232.78 400.22 |
TG 278.98 106.93 D, 222.13 MR, 285.76 M, 307.87 –exo Decomposition |
| p-sulfonic calix[4]arene-Cp2NbCl2 inclusion | 25–100 | 13.77 | 249.44 | (126.32 D), (209.08 MR) 250.79 M (254.55 M) |
| p-sulfonic calix[6]arene-Cp2NbCl2 inclusion | 25–100 | 16.24 | 241.50 | 95.50 D (140.36 D) 234.00 M (254.10 M) |
| p-sulfonic calix[8]arene-Cp2NbCl2 inclusion | 25–100 | 17.27 | 240.38 | 115.39 D (141.54 MR) 138.36 MR (215.35 MR), 235 M |
Powder x-ray powder diffraction (PXRD) analysis was undertaken on the inclusion complexes. Table 2 shows the PXRD peaks of the hosts, guests, physical mixtures and inclusion complexes. Upon analysis of Table 2, we can observe that niobocene has a very simple diffraction pattern and the p-sulfonic calix[n]arenes have well defined diffraction patterns at 2θ < 30°. The physical mixtures are only overlapping spectra of the free p-sulfonic calix[n]arene and niobocene dichloride. For instance, the XRD diffractogram of p-sulfonic calix[6]arene-Cp2NbCl2 physical mixture (PM) contains a combination of free sulfonic calix[6]arene and niobocene diffraction peaks. On the other hand, the diffraction pattern of the p-sulfonic calix[6]arene-Cp2NbCl2 inclusion complex is different to the physical mixture as well as to the free p-sulfonic calix[6]arene and niobocene species. The p-sulfonic calix[6]arene-Cp2NbCl2 inclusion XRD diffractogram showed that this solid is more amorphous and has a broad peak at higher angles (2θ > 30°), demonstrating that this compound is different to a physical mixture or the free species. Similar behaviors were observed for the p-sulfonic calix[4]arene-Cp2NbCl2 and p-sulfonic calix[8]arene-Cp2NbCl2 inclusion complexes. Therefore, based on the XRD data, we further corroborate that p-sulfonic calix[n]arene can form inclusion complexes with niobocene, see Supplementary Material.
Table 2.
Powder x-ray diffraction diffractogram analysis of inclusion complexes, physical mixtures (PM) and precursors. PM = physical mixtures.
| Compound | Major characteristic peaks at 2θ |
|---|---|
| p-sulfonic calix[4]arene | 17.56 (strongest), 24.28 (secondly) |
| p-sulfonic calix[6]arene | 7.74, 9.12, 14.72, 15.50 (strongest), 17.88, 20.16, 21.44 (secondly), 22.76, 25.20, 29.16 |
| p-sulfonic calix[8]arene | 12.00, 12.90, 16.30 (secondly), 17.12, 19.68 (strongest), 22.14, 23.32, 25.58, 31.28, 36.72 |
| Cp2NbCl2 | 13.7 (strongest), 15.3, 33.7 (secondly), 19.2, 22.3 |
| p-sulfonic calix[4]arene-Cp2NbCl2 inclusion | 10.54, 12.94, 15.44, 16.82, 17.48 (strongest), 18.58 (secondly), 23.80, 26.14 |
| p-sulfonic calix[6]arene-Cp2NbCl2 inclusion | 15.72, 18.10, 21.30 (strongest), 22.80, 25.50, 27.24, 29.14 |
| p-sulfonic calix[8]arene-Cp2NbCl2 inclusion | 16.62, 22.08 (strongest), 29.44 |
| p-sulfonic calix[4]arene-Cp2NbCl2 (PM) | 13.7 (weak), 17.66 (strongest), 23.92 |
| p-sulfonic alix[6]arene-Cp2NbCl2 (PM) | 13.7 (second strongest), 17.68 (strongest), 21.88, 23.08, 25.26 |
| p-sulfonic calix[8]arene-Cp2NbCl2 (PM) | 11.92, 13.7, 16.24 (secondly), 19.48 (strongest), 22.24, 23.16, 25.38 |
In light of our theoretical and experimental studies we determined that a calix[n]arene without sulfonate groups does not have the capabilities to include Cp2NbCl2 into the calixarene hydrophobic cavity. In addition, the phenol groups are not good Lewis bases to engage in bonding with Nb(V) center. On the other hand, water soluble p-sulfonic calix[n]arenes enclose niobocene into the hydrophobic cavity, perhaps assisted by Nb(V)-O(sulfonate) interactions. We have not been able to obtained single crystal for X-ray diffraction but based on previous precedents we will discuss the host-guest interactions.
There are several precedents on p-sulfonic calix[n]arenes enclosing and coordinating metal complexes. The hydrophobic cavity typically incorporates the organic moiety of the complex while the sulfonates coordinate the metal center. For instance, in [Cu(py)2(H2O)3·p-sulfonic calix[4]arene]2, the pyridine is inside the hyprophobic cavity while Cu(II) is coordinated by the sulfonate groups (Cu-O(sulfonate)) forming a dimeric species [2]. Furthermore, for Na[Eu(H20)9]2[Eu(py-N-O)(H20)5(p-sulfonic calix[5]arene)2], the Eu(III) is bridging two calixarenes through the Eu(III)-O(sulfonate) bonds and the py-N-O ligand (in [Eu(py-N-O)(H20)5(p-sulfonic calix[5]arene)2]) is inside the hydrophobic cavity [3]. Likewise, lanthanide ions such as Gd, Yb, and Tb bind the accessible sulfonate groups (from the p-sulfonic calix[5]arene) instead of the phenols and the py-N-O ligand is inside the hydrophobic cavity [3]. In [Y(H2O)7Na(H2O)4(p-sulfonatocalix[4]arene)] and [Eu(H2O)7Na(H2O)4(p-sulfonic calix[4]arene), the metal ions are coordinated by the sulfonate groups [29]. Therefore, sulfonate groups are better ligands to oxyphilic metal ions than phenols. The inner cavity diameters of the calix[n]arenes are also important in the inclusion compounds. The inner cavity diameters of calix[4]arene, calix[6]arene and calix[8]arene are 3.0, 7.6 and 11.7 Ǻ respectively [1].
Based on these precedents and our experimentation, we can explain our findings as follow. First, the sulfonate groups are important not only for water solubility but there is a potential coordination site for oxyphilic metals such as Nb(V) metal center. This is supported by our ab-initio calculations. It was shown that niobocene prefers to enter into the cavity by the upper rim (sulfonate head) rather than the lower rim (phenols). But our ab-initio calculations also showed that the inclusion of the Cp rings into the hydrophobic cavity as structure A, in Figure 4, is favored over the inclusion of the Cp rings accompanied by sulfonate coordination. Thus, coordination of Nb(V) by the sulfonate group could be envisioned as an initial step to assist the inclusion of the Cp rings into the hydrophobic cavity. Our predictions were further corroborated by other physical techniques. Solid state CP C-13 NMR showed that the p-sulfonic calix[6]arene adopts an unsymmetrical conformation in the inclusion compound. Solution 1H NMR spectroscopy showed that the proton signals of the Cp rings inside the cavity experience up-field shifts as a result of shielding by the aromatic ring current of the calix[n]arenes. This strongly suggests that the Cp rings are inside the hydrophobic cavity. Also, while p-sulfonic calix[6]arene and p-sulfonic calix[8]arene have cavities that can incorporate niobocene dichloride, the smaller size of the p-sulfonic calix[4]arene cavity presents steric constraints to form inclusion compounds.
4. Concluding remarks
We have explored the host-guest interactions between water soluble p-sulfonic calix[n]arenes and water insoluble calix[n]arenes and niobocene dichloride by theoretical and experimental methods. The ab-initio calculations clearly showed that only p-sulfonic calix[6]arene is able to form host-guest complexes with Cp2NbCl2, however the geometry of the encapsulation strongly depends on the size of the p-sulfonic calix[n]arene cavity. This was further verified by wide variety of physical methods, but in particular solid state 13C CP-MAS and solution 1H NMR spectroscopies allowed us to corroborate the theoretical calculations. While p-sulfonic calix[8]arene and p-sulfonic calix[6]arene can hold both Cp rings inside the cavity, the smaller calixarene, p-sulfonic calix[4]arene exhibits different behavior. It seems that p-sulfonic calix[4]arene can adopt two inclusion geometries, see Figure 6. In the 1:1 p-sulfonic calix[4]arene-niobocene inclusion compound, one Cp ring approaches the sulfonate heads of the cavity with a shallow penetration (Cp located at the mouth of the cavity). In the 2:1 p-sulfonic calix[4]arene:niobocene inclusion compound, the Cp rings are capped by two p-sulfonic calix[4]arenes, leading to more shielded chemical shifts for the Cp signals. In contrast, water insoluble calix[6]arene cannot form host-guest complex with niobocene perhaps due to the lack of hydrophilicity (sulfonate heads), and possibly coordination capability of the calixarene.
Finally, we found that water soluble p-sulfonic calix[n]arenes provide a good alternative to prepare water soluble metallocene anticancer agents with potential pharmaceutical applications. p-Sulfonic calix[n]arenes have been used for pharmaceutical applications providing more thermal stability to the encapsulated drugs. In our case, p-sulfonic calix[n]arenes provide higher hydrolytic stability which is one of the major drawbacks of metallocene anticancer agents.
Supplementary Material
Acknowledgments
E.M. acknowledges the NIH-MBRS SCORE Program at the University of Puerto Rico Mayagüez, grant #S06 GM008103-37. In addition, EM thanks NSF-MRI Program for providing funds for the purchase of the 500 MHz NMR instrument.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang W, de Villiers MM. Am Pharm Rev. 2006;9:70. [Google Scholar]
- 2.Atwood JL, Orr GW, Juneja RK, Bott SG, Hamada F. Pure Appl Chem. 1993;65:1471. [Google Scholar]
- 3.Steed JW, Johnson CP, Barnes CL, Juneja RK, Atwood JL, Reilly S, Hollis RL, Smith PH, Clark DL. J Am Chem Soc. 1995;117:11426. [Google Scholar]
- 4.Kaifer AE. Acc Chem Res. 1999;32:62. doi: 10.1021/ar5001204. [DOI] [PubMed] [Google Scholar]
- 5.Bukhaltsev E, Goldberg I, Vigalok A. Organometallics. 2005;24:5732. [Google Scholar]
- 6.Atwood JL, Clark DL, Juneja RK, Orr GW, Robinson KD, Vincent RL. J Am Chem Soc. 1992;114:7558. [Google Scholar]
- 7.Jose P, Menon S. Bioinorg Chem Appl. 2007:Article ID 65815. doi: 10.1155/2007/65815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao LM, Vera JL, Matta J, Meléndez E. J Biol Inorg Chem. 2010;15:851. doi: 10.1007/s00775-010-0649-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao LM, Meléndez E. Metal-Based Drugs. 2010:Article ID 286298. doi: 10.1155/2010/286298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernández R, Méndez J, Lamboy J, Torres M, Román FR, Meléndez E. Toxicol in Vitro. 2010;24:178. doi: 10.1016/j.tiv.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao LM, Matta J, Rheingold AL. E Meléndez J Organometal Chem. 2009;694:4134. doi: 10.1016/j.jorganchem.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feliciano I, Matta J, Meléndez E. J Biol Inorg Chem. 2009;14:1109. doi: 10.1007/s00775-009-0554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao LM, Hernández R, Matta J, Meléndez E. Metal Based Drugs. 2009:Article ID 420784. doi: 10.1155/2009/420784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernández R, Lamboy J, Gao LM, Matta J, Román FR, Meléndez E. J Biol Inorg Chem. 2008;13:685. doi: 10.1007/s00775-008-0353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morales A, Weber RT, Meléndez E. App Organometal Chem. 2008;22:440. doi: 10.1002/aoc.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morales A, Struppe J, Melendez E. J Inclusion Phenomena and Macrocyclic Chemistry. 2008;60:263. [Google Scholar]
- 17.Gao LM, Hernández R, Matta J, Meléndez E. J Biol Inorg Chem. 2007;12:959. doi: 10.1007/s00775-007-0268-0. [DOI] [PubMed] [Google Scholar]
- 18.Vera JL, Román FR, Meléndez E. Biorg Med Chem. 2006;14:8683. doi: 10.1016/j.bmc.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Cardona A. E Meléndez Anal Bioanal Chem. 2000;386:1689. doi: 10.1007/s00216-006-0740-7. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez MI, Chavez-Gil TE, Colon Y, Diaz N, Meléndez E. J Electroanal Chem. 2005;576:315. [Google Scholar]
- 21.Perez Y, Lopez V, Rivera-Rivera L, Cardona A, Melendez E. J Biol Inorg Chem. 2005;10:94. doi: 10.1007/s00775-004-0614-4. [DOI] [PubMed] [Google Scholar]
- 22.Vera JL, Román FR, Meléndez E. Anal Bioanal Chem. 2004;379:399. doi: 10.1007/s00216-004-2596-z. [DOI] [PubMed] [Google Scholar]
- 23.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision B.04. Gaussian, Inc; Pittsburgh PA: 2003. [Google Scholar]
- 24.Harding MM, Prodigalidad M, Lynch MJ. J Med Chem. 1996;39:5012. doi: 10.1021/jm9603678. [DOI] [PubMed] [Google Scholar]
- 25.Pereira CCL, Nolasco M, Braga SS, Almeida Paz FA, Ribeiro-Claro P, Pillinger M, Goncalves IS. Organometallics. 2007;26:4220. [Google Scholar]
- 26.Braga SS, Gonclaves IS, Pillinger M, Ribeiro-Claro P, Teixeira-Dias JJC. J Organomet Chem. 2001;11:632. [Google Scholar]
- 27.Brouwer EB, Gougeon RDM, Hirschinger J, Udachin K, Harris R, Ripmeester RJA. Phys Chem Chem Phys. 1999;1:4043. [Google Scholar]
- 28.Gutsche CD, Baeur LJ. J Am Chem Soc. 1985;107:6052. [Google Scholar]
- 29.Atwood JL, Barbour LJ, Hardie MJ, Raston CL, Statton MN, Webb HR. Cryst Eng Comm. 2001;4:1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.