Abstract
Objective
Periadventitial delivery of the nitric oxide (NO) donor PROLI/NO following arterial injury effectively inhibits neointimal hyperplasia. Given the short half-life of NO release from PROLI/NO, our goal was to determine if inhibition of neointimal hyperplasia by PROLI/NO was due to NO, or its metabolites nitrite and nitrate.
Methods and Results
In vitro, the NO donor DETA/NO inhibited proliferation of rat aortic vascular smooth muscle cells (RASMC), but neither nitrite nor nitrate did. In vivo, following rat carotid artery balloon injury or injury plus the molar equivalents of PROLI/NO, nitrite, or nitrate (n=8–11/group), PROLI/NO was found to provide superior inhibition of neointimal hyperplasia (82% inhibition of intimal area, and 44% inhibition of medial area, p<0.001). Only modest inhibition was noted with nitrite or nitrate (45% and 41% inhibition of intimal area, and 31% and 29% inhibition of medial area, respectively, p<0.001). No effects on blood pressure were noted with any treatment groups. In vivo, only PROLI/NO inhibited cellular proliferation and increased arterial lumen area compared to injury alone (p<0.001). However, all three treatments inhibited inflammation (p<0.001).
Conclusions
PROLI/NO was more effective at inhibiting neointimal hyperplasia following arterial injury than nitrite or nitrate. However, modest inhibition of neointimal hyperplasia was observed with nitrite and nitrate, likely secondary to anti-inflammatory actions. In conclusion, we have demonstrated that the efficacy of NO donors is primarily due to NO production and not its metabolites, nitrite and nitrate.
Keywords: Peripheral Vascular Disease, Neointimal Hyperplasia, Nitric Oxide, Nitrite/Nitrate
INTRODUCTION
Peripheral arterial disease (PAD) is a major cause of morbidity and mortality in the United States and although both open and endovascular therapies are available to treat PAD, these treatment modalities are limited by high rates of restenosis secondary to neointimal hyperplasia.[1] Our lab and others have shown that periadventitial application of NO donors inhibits the development of neointimal hyperplasia in animal models of arterial injury.[2–7] Of the NO donors our laboratory has evaluated, the diazeniumdiolate disodium 1-[2-(carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate (PROLI/NO) has been the most effective.[2; 4] As a diazeniumdiolate, PROLI/NO provides a predictable release of 2 moles of NO per mole of compound and in powder form can be easily applied to the periadventitial surface of the artery. However, compared to other diazeniumdiolates, PROLI/NO has the shortest half-life (2 seconds) in vitro at a constant pH of 7.4 and temperature of 37°C.[8] Following the quick, high burst release from PROLI/NO, NO also has a very short half-life in vivo and is rapidly scavenged by hemoglobin or metabolized to the inorganic ions nitrite and nitrate, which have longer respective half-lives of approximately 45 minutes and up to 6 hours.[9] Given the very short half-life of NO release from PROLI/NO as well as NO itself, we considered if the inhibition in neointimal hyperplasia observed with PROLI/NO may be because of the metabolites nitrite and nitrate and not just to the initial release of NO from the donor PROLI/NO.
Inorganic nitrite and nitrate are no longer considered inert end products of NO metabolism but have been shown to be biologically active in the vasculature. Nitrite has been shown to have vasoprotective properties. For example, intravenous administration of nitrite in vivo has been shown to stimulate vasodilation in human and non-human primates and nitrite has also been shown to inhibit proliferation in smooth muscle cells from rat pulmonary arteries.[9; 10] However, these potentially therapeutic properties of nitrite and nitrate have largely been attributed to the ability of inorganic nitrite and nitrate to function as a reservoir for further NO production. Nitrate is reduced to nitrite, mostly by bacterial nitrate reductase, and then nitrite is converted back to NO through a variety of enzymatic and nonenzymatic mechanisms both in the circulation and in the tissues.[11] Therefore, it has been suggested that nitrite and nitrate may be potential therapeutic agents for pathology such as neointimal hyperplasia through the reconversion to NO which has many vasoprotective properties that inhibit neointima formation.
Thus, the aim of this study was to determine whether periadventitial application of the NO metabolites nitrite and nitrate following balloon angioplasty in the rat carotid injury model would be as efficacious as the molar equivalent of the NO donor PROL/INO for the prevention of neointimal hyperplasia. Our hypothesis is that the NO release from PROLI/NO will provide greater inhibition of neointimal formation than either inorganic nitrite or nitrate.
MATERIALS AND METHODS
NO donors
The NO donors used in this study are diazeniumdiolates that were generously supplied by Drs. Joseph Hrabie and Joseph Saavedra of NCI-Frederick. The diazeniumdiolates used in this study were chosen based on their NO release rates, their safety profiles, and for their efficacy in previously published experiments.[4; 8; 12] PROLI/NO was chosen for our in vivo experiments based on prior demonstration of its superior efficacy in the prevention of neointimal hyperplasia when compared to other diazeniumdiolates.[2; 4] For in vitro experiments, 1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA/NO) was used because of its longer half-life of 20 hours, making this a more appropriate NO donor to use for 24-hour in vitro assays.[8; 12] Sodium nitrite [NaNO2] and sodium nitrate [NaNO3] were purchased from Sigma Aldrich (St. Louis, MO, USA).
Cell culture
Rat aortic smooth muscle cells (RASMC) and rat aortic endothelial cells (RAEC) were isolated and cultured from the aortas of 8-week male Sprague Dawley rats (Harlan, Indianapolis, IN) using the collagenase method and maintained as previously described.[13–15] RASMC and RAEC were characterized by anti-smooth muscle α-actin monoclonal antibodies (Sigma; St. Louis, MO) and von Willebrand factor (Dako Cytomation; Carpenteria, CA), respectively, and by cell-specific morphology. Cells were maintained in medium containing equal volumes of Dulbecco’s Modified Eagle’s Medium-low glucose (JRH; Lenexa, KS) and Ham’s F12 (JRH; Lenexa, KS) supplemented with 10% fetal bovine serum (FBS, Invitrogen; Carlsbad, CA), 100 U/mL penicillin (Invitrogen), 100 μg/mL streptomycin (Invitrogen) and 4 mM L-glutamine (VWR; West Chester, PA) and incubated at 37°C, 95% air and 5% CO2. RAEC were also supplemented with endothelial cell growth supplement (Millipore, Billerica, MA) and MEM non-essential amino acids (Invitrogen). For all experiments, cells used were between passages three and eight. After plating, cells were growth-arrested with media containing no FBS for 24 hours and then exposed to media containing DETA/NO or the molar equivalents of either nitrite or nitrate for an additional 24 hours before collection.
Proliferation assay
Tritiated (3H) thymidine incorporation was assessed as a surrogate for cellular proliferation. Male RASMC and RAEC were plated in 12-well plates at 1 × 104 and 0.75 × 104 cells/well, respectively. In addition to DETA/NO, nitrite or nitrate, treatment media also contained 3H-thymidine (5 μCi/mL, PerkinElmer, Wellesley, MA). 3H-thymidine incorporation into trichloroacetic acid–precipitated DNA was quantified by scintillation counting.
Cell death assay
A trypan blue exclusion protocol was performed to assess overall cell death. RASMC and RAEC were plated in 6-well plates at 6 × 104 and 5 × 104 cells/well, respectively. Following starvation and treatment, cells were trypsinized, collected, and placed in suspension. Aliquots of cell suspension were exposed to an equal volume of 4% trypan blue and the percentage of blue (nonviable) cells was determined by counting on a hemocytometer.
Rat carotid artery injury model
All animal procedures were performed in accordance with the principles outlined in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication; National Academy Press, 1996) and approved by the Northwestern University Animal Care and Use Committee. Ten-week-old male Sprague-Dawley rats (Charles River, Wilmington, MA, USA) weighing 300–400g were anesthetized with inhaled isoflurane (1.0–5.0%). Treatment groups included injury alone (n=11), injury+20 mg PROLI/NO (n=8), injury+11 mg sodium nitrite (n=8), or injury+13 mg sodium nitrate (n=8). Prior to the procedure, atropine (0.1 mg/kg) and carprofen (Rimadyl TM) (0.15 mg/kg) were administered subcutaneously to decrease airway secretions and for pain control, respectively. Equaline sterile lubricant (Boise, ID) was applied to the animal’s eyes. Following a midline nick incision, the left common, internal and external carotid arteries were identified. After distal ligation of the external carotid artery, the internal and common carotid arteries were occluded with atraumatic clamps and a No. 2-French arterial embolectomy catheter (Edwards Lifesciences, Irvine, CA, USA) was inserted into the external carotid artery and advanced into the common carotid artery. Uniform injury was created by inflating the balloon to 5 atmospheres of pressure for 5 minutes. After removal of the balloon, the external carotid artery was ligated and blood flow restored. PROLI/NO, nitrite, or nitrate were immediately applied evenly to the external surface of the injured common carotid artery in their dry powder form. The neck incision was closed in two layers. Rats were sacrificed at 2 weeks for morphometric and immunohistochemical analysis.
Tissue processing
Carotid arteries were harvested following in-situ perfusion-fixation with phosphate buffered saline (PBS) and 2% paraformaldehyde. Vessels were placed in paraformaldehyde at 4°C for 1 hour, then overnight in 30% sucrose in PBS at 4°C for cryo-protection. The tissue was quick-frozen in Optimal Cutting Temperature compound (Tissue Tek, Hatfield, PA) and 5-μm sections were cut throughout the entire injured segment of the common carotid artery using a Microm HM 550 cryostat.
Morphometric analysis
Carotid arteries harvested at 2 weeks were examined histologically for evidence of neointimal hyperplasia using routine hematoxylin-eosin (H&E) staining. Digital images were collected with light microscopy using an Olympus BHT microscope (Melville, NY) with 4X, 10X and 40X objectives. Six evenly-spaced sections through each injured carotid artery were analyzed. Intima area, media area, and luminal area were measured. These values were obtained (arbitrary units) using ImageJ software and all analysis was performed by a single individual.
Immunohistochemistry
From each animal, three evenly spaced carotid sections from the area of injury were stained and examined for evidence of proliferation and inflammation using immunohistochemical staining. For proliferation, rats received an intraperitoneal injection of bromodeoxyuridine (BrdU, 100 mg/kg) at 24 and 1 hour prior to sacrifice. For all immunohistochemistry, frozen sections were fixed in acetone for 5 minutes, rinsed in PBS-Tween 20 for 2 minutes, and blocked with horse serum (Sigma, St. Louis, MO, USA) in 0.5% bovine serum albumin (BSA) for 30 minutes. Primary antibody in BSA was applied for 1 hour: anti-ED1 (monocyte/macrophage, 1:200, Santa Cruz Biotechnology; Santa Cruz, CA), anti-CD45 (leukocyte, 1:200, AbD Serotec; Raleigh, NC) or anti-BrdU (1:200, Abcam; Cambridge, MA). Secondary antibody in BSA was applied for 30 minutes (horse anti-mouse biotinylated, affinity-purified anti-immunoglobulin, 1:500, Vector Labs; Burlingame, CA). The sections were incubated in Vectastain ABC reagent for 30 minutes, the chromagen/substrate (DAB peroxidase substrate kit, Vector Labs, Burlingame, CA) for 2 minutes, counterstained with Gill’s hematoxylin solution (Fisher Scientific, Pittsburgh, PA), dehydrated, and coverslipped with mounting medium (Permount, Fisher Scientific, Pittsburgh, PA). For negative controls, PBS was substituted for the primary antibody. Digital images were taken with light microscopy using an Olympus BHT microscope (Melville, NY) and SPOT camera basic Software (Diagnostic Instruments, Inc, Sterling Heights, MI). For nuclear stains (i.e., CD45 and BrdU), cells with positive staining were counted by a blinded investigator in four high power fields per arterial section and expressed as an average. For cytoplasmic stains (i.e., ED1), staining was quantified by a blinded investigator using a scale of 0–4.
Blood pressure and heart rate measurements
Blood pressure and heart rate measurements were collected using the CODA non-invasive blood pressure system (Kent Scientific, Torrington, CT). Measurements were taken both prior to and following induction of anesthesia, and both 5 and 10 minutes following injury. For measurements taken prior to induction, animals were restrained in a Broome rodent restrainer (Harvard Apparatus; Holliston, MA) and blood pressure and heart rate measured with a large rat tail cuff. For each time point, a maximum of 20 measurement cycles were taken at 5 second intervals and all valid measurements were subsequently included in analysis. Results are reported as an average of the mean blood pressure and heart rate per animal in each treatment group.
Statistical analysis
Results are expressed as mean ± standard error of the mean. Differences between multiple groups were analyzed using one-way analysis of variance (ANOVA) with the Student-Newman-Keuls post hoc test for all pair-wise comparisons (SigmaStat; SPSS, Chicago, IL). Statistical significance was assumed when p<0.05.
RESULTS
VSMC proliferation was inhibited by NO but not by nitrite or nitrate in vitro
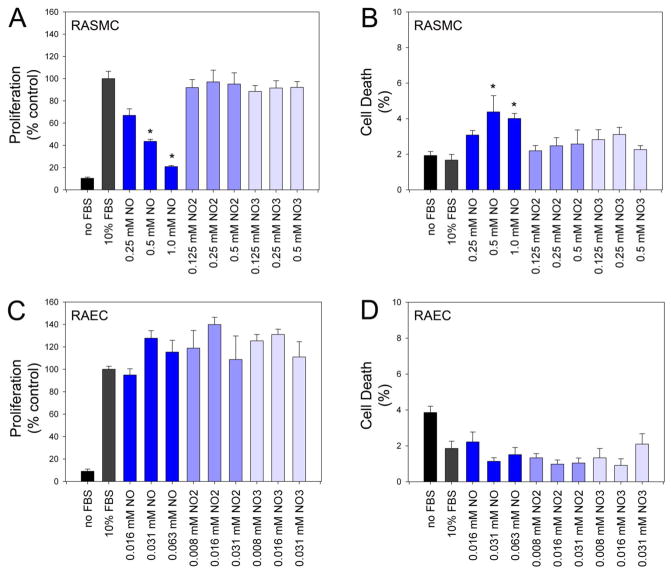
To determine the effect of the metabolites nitrite and nitrate on proliferation and cell death in vitro, RASMC and RAEC were exposed to increasing concentrations of DETA/NO and molar equivalent concentrations of nitrite or nitrate (Figure 1A and 1C). Results were expressed as a percentage of the control group counts (i.e., proliferation) or total cells (i.e., cell death). For RASMC exposed to DETA/NO, proliferation was inhibited in a dose-dependent fashion and reached significance at the two highest concentrations, with 80% inhibition of proliferation observed with 1.0 mM DETA/NO (p<0.001; Figure 1A). RASMC exposed to the molar equivalent concentrations of nitrite or nitrate proliferated at rates similar to controls. We also evaluated the ability of nitrite and nitrate to inhibit proliferation of RASMC at very low concentrations, specifically at 50 nM, 125 nM, 250 nM, 500 nM, 1 μM, 5 μM, 10 μM, 25 μM, and 50 μM, and still observed no inhibition of proliferation. Last, we evaluated the effect of the parent compound, proline, on inhibition of RASMC proliferation and observed no inhibition over a range of concentrations (25–1000 μM).
Figure 1.
Proliferation (A & C) and cell death (B & D) were assessed in rat aortic smooth muscle cells (RASMC) and rat aortic endothelial cells (RAEC) following exposure to increasing concentrations of DETA/NO or molar equivalent concentrations of nitrite and nitrate. Data representative of 3 separate experiments. n=3/treatment group. *p<0.05 compared to control; NO=nitric oxide, NO2=nitrite, NO3=nitrate.
Overall, there was less than 10% cell death in all treatment groups. As expected, there was a small increase in RASMC cell death following exposure to 0.5 and 1.0 mM DETA/NO, with a 3% and 2% increase in death compared to control respectively (p<0.02, Figure 1B). RASMC exposed to the molar equivalent concentrations of nitrite or nitrate experienced cell death similar to control.
Because RAEC in vitro have increased sensitivity to DETA/NO as compared to RASMC, lower concentrations of the NO donor and molar equivalent concentrations of nitrite and nitrate were evaluated. Although there was an overall increase in proliferation of RAEC exposed to DETA/NO, nitrite and nitrate, these results were not statistically significant (Figure 1C). Similar to RASMC, overall cell death in all treatment groups was less than 10% and although there was a trend toward decreased death in cells exposed to DETA/NO, nitrite or nitrate, these results did not reach statistical significance (Figure 1D).
Periadventitial delivery of PROLI/NO, nitrite, or nitrate does not cause hypotension
One limitation of NO-based therapies administered systemically is the development of unwanted side effects such as hypotension. Although NO is diffusible and can penetrate all layers of the artery wall when applied to the adventitia, it is our assertion that local periadventitial delivery does not produce enough systemic NO absorption to produce systemic side effects. To evaluate this definitively, we measured the blood pressure and heart rate at 4 time points before and after exposure to NO: 1) prior to anesthesia, 2) after induction of anesthesia, 3) 5 minutes after injury and NO exposure, and 4) 10 minutes after injury and NO exposure (Table 1). There were no significant changes in heart rate among any of the treatment groups for all time points measured. With regards to blood pressure, as anticipated, animals in all treatment groups experienced significant hypotension with induction of anesthesia (mean Δ −41 mmHg for all treatment groups, p≤0.006). At 10 minutes following injury, mean blood pressure in the injury alone group decreased significantly compared to the post-anesthetic induction blood pressure (Δ −18 mmHg, p<0.001). However, treatment with PROLI/NO, nitrite or nitrate was associated with an increase in blood pressure (PROLI/NO Δ +17 mmHg, p<0.001; nitrite Δ +31 mmHg, p=0.218; nitrate Δ +7 mmHg, p<0.001). These data demonstrate that NO and its metabolic end products nitrite and nitrate do not cause hypotension when administered locally.
Table 1.
Mean Rat Blood Pressure (BP) and Heart Rate (HR)
| Mean Systolic BP | Before Anesthesia | After Anesthesia | 5 min after Injury/Treatment | 10 min after Injury/Treatment |
|---|---|---|---|---|
| Injury Alone | 115 ± 2 | 86 ± 1* | 70 ± 2*# | 68 ± 1*# |
| Injury + PROLI/NO | 114 ± 9 | 64 ± 1* | 85 ± 2# | 81 ± 1# |
| Injury + Sodium Nitrite | 94 ± 4 | 66 ± 2* | 70 ± 3* | 97 ± 4* |
| Injury + Sodium Nitrate | 126 ± 5 | 68 ± 1 | 69 ± 2 | 75 ± 2 |
|
| ||||
| Mean HR | Before Anesthesia | After Anesthesia | 5 min after Injury/Treatment | 10 min after Injury/Treatment |
|
| ||||
| Injury Alone | 553 ± 14 | 530 ± 32 | 524 ± 44 | 469 ± 6 |
| Injury + PROLI/NO | 706 ± 127 | 607 ± 37 | 570 ± 31 | 489 ± 2 |
| Injury + Sodium Nitrite | 638 ± 45 | 603 ± 52 | 528 ± 12 | 515 ± 7 |
| Injury + Sodium Nitrate | 632 ± 48 | 405 ± 21 | 467 ± 14 | 492 ± 12 |
p<0.05 compared to Before Anesthesia
p<0.05 compared to After Anesthesia
PROLI/NO inhibits neointimal hyperplasia more effectively than either nitrite or nitrate
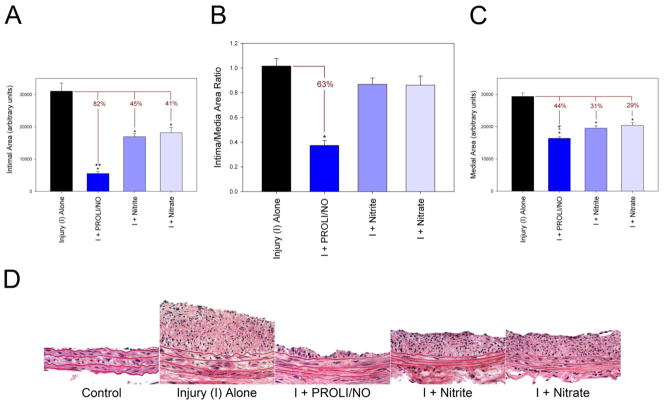
The aim of our study was to compare the effect of PROLI/NO to the NO metabolites nitrite or nitrate in vivo on neointimal hyperplasia using the rat carotid injury model. A dose of 20 mg of PROLI/NO was chosen based on our previous studies.[2] Molar equivalent doses of nitrite and nitrate were calculated based on the predicted moles of NO that would be released from PROLI/NO. Analysis of our results indicates that although there was some inhibition of neointima formation following application of nitrite and nitrate, PROLI/NO was the most effective at inhibiting neointimal hyperplasia (Table 2 and Figure 2A–D). Compared to injury alone, PROLI/NO inhibited initmal area by 82% compared to only 45% and 41% observed with nitrite and nitrate, respectively (p<0.001 for PROLI/NO, nitrite, and nitrate vs. injury alone; p<0.05 for PROLI/NO vs. nitrite and nitrate, Figure 2A). However, only the PROLI/NO treated group resulted in a statistically significant decrease in the intima/media and intima/(intima+media) area ratios (63% and 48% reduction, respectively, p<0.001, Figure 2B, Table 2).
Table 2.
Morphometric analysis of the carotid arteries 14 days after balloon injury
| Treatment Group | Lumen Area | Intima Area | Media Area | I/M | I/(I+M) |
|---|---|---|---|---|---|
| Injury alone | 111387.3 ± 3622 | 34909.2 ± 3270 | 29652.0 ± 1099 | 1.11 ± 0.08 | 0.48 ± 0.01 |
| Injury + PROLI/NO | 129116.2 ± 2856* | 8428.5 ± 1371* | 16715.3 ± 577* | 0.48 ± 0.06* | 0.25 ± 0.02* |
| Injury + Sodium Nitrite | 111530.3 ± 3756 | 17631.3 ± 1051* | 20481.4 ± 973* | 0.90 ± 0.60 | 0.45 ± 0.02 |
| Injury + Sodium Nitrate | 114674.1 ± 4032 | 18177.3 ± 1653* | 21015.0 ± 935* | 0.89 ± 0.07 | 0.43 ± 0.03 |
Abbreviations: I/M = intima/media area ratio; I/(I+M) = intima/(intima+media) area ratio.
p<0.001 vs. injury alone
Figure 2.
Quantification of neointimal hyperplasia via morphometric analysis of rat carotid artery sections at 14 days: (A) intimal area, (B) intima/media area ratio and (C) medial area. Units are arbitrary (n=8–11). *p<0.05 vs. injury alone. **p<0.05 vs. nitrite or nitrate. τp<0.05 vs. nitrate. (D) H&E stained rat carotid artery sections from each treatment group (400x). I=injury.
In addition to inhibition of neointimal formation, we have previously demonstrated that PROLI/NO affects arterial remodeling following injury.[2] Similar to previous results, PROLI/NO inhibited medial area in this study by 44% compared to injury alone (p<0.001, Figure 2C). Nitrite and nitrate also inhibited medial area but to a lesser extent with a 31% and 29% reduction compared to injury alone, respectively (p<0.001). The reduction in medial area induced by PROLI/NO was statistically greater than the reduction induced by nitrate (p<0.05) but not nitrite. With respect to lumen area, only the PROLI/NO-treated group resulted in a statistically significant increase in lumen area compared to injury alone (16% increase, p<0.001, Table 2).
PROLI/NO, but not nitrite or nitrate, inhibited cellular proliferation in vitro
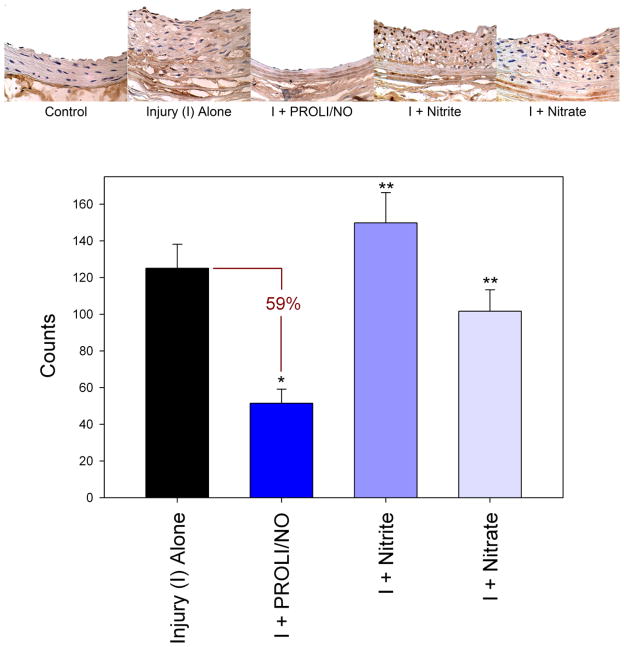
Given that DETA/NO inhibited proliferation of RASMC but nitrite and nitrate did not, we wanted to evaluate the effect of PROLI/NO, nitrite, and nitrate on cellular proliferation following arterial injury in vivo. Interestingly, we observed a pattern similar to the observed in vitro results. PROLI/NO inhibited cellular proliferation by 59% compared to injury alone (p<0.001, Figure 3) whereas nitrite and nitrate did not affect cellular proliferation following arterial injury in vivo. In fact, compared to treatment with PROLI/NO, arteries exposed to nitrite or nitrate had significantly increased BrdU incorporation (3-fold, p<0.001 and 2-fold, p=0.012, respectively). This indicates that the efficacy of nitrite and nitrate in vivo is not secondary to effects on cellular proliferation.
Figure 3.
Graphical and histologic representations of cellular proliferation in rat carotid artery sections at 14 days as indicated by immunohistochemical staining for bromodeoxyuridine (BrdU) DNA incorporation. *p<0.05 as compared to injury alone. **p<0.05 as compared to injury + PROLI/NO. I=injury, Counts=counts per high power field.
PROLI/NO, nitrite, and nitrate inhibit the inflammatory response following arterial injury
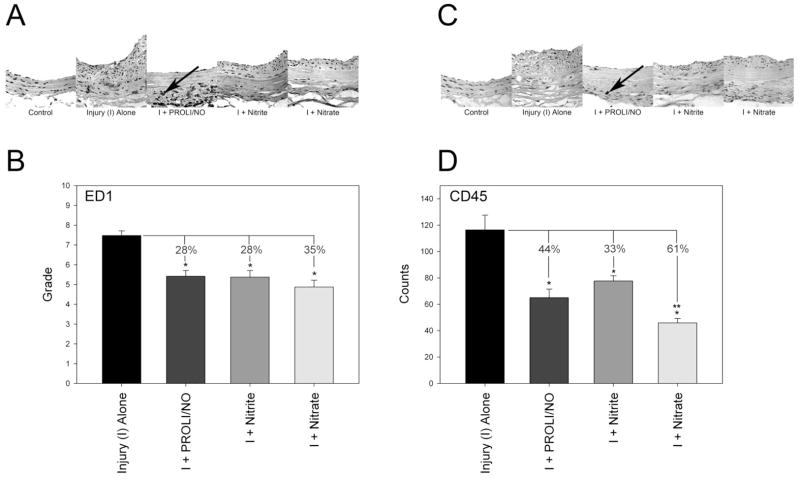
We have previously demonstrated that NO inhibits monocyte/macrophage infiltrate following injury.[2] In this study, monocyte/macrophage and leukocyte infiltration were assessed with immunohistochemical staining for ED1 and CD45, respectively. PROLI/NO significantly inhibited monocyte/macrophage infiltrate by 28% compared to injury alone (p<0.001, Figure 4A and 4B). Monocyte/macrophage infiltration was also inhibited with nitrite and nitrate by 28% and 35%, respectively, compared to injury alone (p<0.001). The reduction in monocyte/macrophage infiltration was not statistically different among the three groups. NO inhibited leukocyte infiltration by 44% compared to injury alone (p<0.001, Figure 4C and 4D). Nitrite inhibited leukocyte infiltration by 33% following injury (p=0.001), while nitrate inhibited leukocyte infiltration to an even greater extent following injury (61%, p<0.001). The reduction in CD45 infiltration by nitrate was significantly different than the reduction caused by nitrite (p=0.035), but not PROLI/NO. These data, together with the cellular proliferation data, provide a potential explanation for the differences observed in efficacy between PROLI/NO, nitrite, and nitrate at preventing neointimal formation. The efficacy of nitrite and nitrate may be due to their anti-inflammatory properties since they appear to have no antiproliferative properties in this animal model.
Figure 4.
Inflammation following rat carotid artery injury at 14 days: Representative sections and quantification of (A and B) monocyte/macrophage (ED1) and (C and D) leukocyte (CD45) infiltration, respectively. ED1 staining was quantified on a scale of 0–4 for the intima, media, and adventitia, and the sum of these grades is reported. CD45 staining was quantified as the number of positive staining cells (e.g., at arrow) per high power field. *p<0.05 vs. injury alone. **p=0.035 vs. nitrite. I=injury.
Discussion
In this paper we have shown that the NO donor PROLI/NO provides superior inhibition of neointimal hyperplasia when compared to either of its metabolites, nitrite or nitrate. Although less efficacious, nitrite and nitrate did provide modest inhibition of neointima formation and did affect arterial remodeling. Our data indicate that the efficacy of nitrite and nitrate at preventing neointimal hyperplasia appears to be related to its anti-inflammatory effects on monocyte/macrophage and CD45 leukocyte infiltration, since neither nitrite or nitrate inhibited proliferation in vitro or in vivo. Inflammation plays an important role in neointimal formation. Any therapy that has anti-inflammatory properties will affect the arterial injury response. Thus, our data suggest that although NO donors may provide superior efficacy for the prevention of neointimal hyperplasia in vivo, there is some therapeutic potential for nitrite and nitrate in the vasculature.
Nitrite and nitrate were once thought of as inert end products of NO metabolism. However, we now know that nitrite and nitrate serve as a storage reservoir for production of NO. Nitrate, either produced from NO metabolism or through dietary sources, can be converted to nitrite via nitrate reductase. Although this process is catalyzed primarily by bacterial nitrate reductase in the gut where nitrate serves as an alternative electron acceptor to oxygen, the enzyme xanthine oxidoreductase has also been shown to have nitrate reductase activity in rodents and humans, primarily in the liver and intestine, under acidic conditions.[16–19] Nitrite can be reduced to NO through several enzymatic and nonenzymatic mechanisms including interaction with deoxyhemoglobin, deoxymyoglobin, protons, and xanthine oxidoreductase.[11; 16; 18–22] In fact, in the vasculature conversion of nitrite to NO by deoxyhemoglobin is an important pathway for NO-mediated vasodilation under hypoxic conditions.[11] Nitrate reductase activity has even been identified in vascular smooth muscle cells, from homogenized rings of arterial wall, and even from mouse macrophage RAW264.7 cells.[23] Thus, these pathways serve as an important supplement to the production of NO from L-arginine by NO synthases (NOS).
Because the reduction of nitrite leads to the direct production of NO, in comparison with nitrate which must first be reduced to nitrite, most studies have focused on the therapeutic potential of nitrite. Several investigators have shown that nitrite causes vasodilation and a decrease in blood pressure in human and non-human primates.[9; 24–26] Furthermore, conversion of nitrite to NO has been proposed as a mechanism by which vasodilation occurs under hypoxic/acidic conditions.[26] In fact, the conversion of nitrite to NO by deoxyhemoglobin/myoglobin, the mitochondrial electron transport chain, and xanthine oxidoreductase are all dependent upon low oxygen tension. The increased reduction of nitrite to NO in hypoxic environments is important to the potential efficacy of nitrite for the prevention of tissue damage in ischemia reperfusion injury seen with myocardial infarction and post-subarachnoid hemorrhage vasospasm.[11; 27; 28] Nitrite has also been shown to affect cGMP production, cytochrome P450 activity, and heat shock protein 70 and heme oxygenase-1 expression in several different tissues.[29] Thus, it is clear that nitrite has both direct and indirect biological activity in vivo.
In a recent study by Zuckerbraun et al, nitrite treatment inhibited proliferation in rat pulmonary artery vascular smooth muscle cells (VSMC) in vitro through the cyclin dependent kinase inhibitor p21.[10] Since NO-mediated inhibition of neointimal hyperplasia is mediated in part through its antiproliferative effects on VSMC,[30; 31] these findings support the assertion that nitrite and nitrate may be useful for the prevention of neointimal hyperplasia. Interestingly, our study did not reproduce the inhibitory effect of nitrite on proliferation reported by Zuckerbraun et al, even though the doses used in our study were significantly higher than those used to inhibit proliferation in the Zuckerbraun study. However, a distinct difference between our study and that of Zuckerbraun et al is that we studied the effects of nitrite and nitrate in a normoxic environment while Zuckerbraun et al studied the effects of nitrite and nitrate in a hypoxic environment. Since nitrite reduction to NO occurs at significantly higher rates under hypoxic conditions as discussed above,[32] it is possible that this preconditioning increased reduction of nitrite to NO, thereby accounting for the antiproliferative effects observed in the Zuckerbraun et al study. The modest inhibition in neointimal hyperplasia and anti-inflammatory effects of nitrite seen in our study may therefore be secondary to direct effects of the metabolites and not through conversion to NO.
A direct mechanism that may account for the modest efficacy of nitrite and nitrate at inhibiting neointimal hyperplasia in our study are their anti-inflammatory effects. It is well-known that inflammation plays an important role in stimulating proliferation following arterial injury.[33; 34] It is also known that NO inhibits inflammation following arterial injury.[2; 4; 35] Thus, our finding that both nitrite and nitrate inhibit infiltration of monocytes/macrophases and CD45 leukocytes in a normoxic environment is interesting and important. Stokes et al administered nitrite in the drinking water of mice on a hypercholesterolemic diet and found a decrease in leukocyte adhesion and improved vasorelaxation in the microvasculature.[36] This study was conducted in a normoxic environment, similar to our study. Thus, while our data support that of Stokes et al, we also report that nitrate has distinct biological activity against inflammatory cells, in fact greater than nitrite. Since our study as well as Stokes et al was conducted in a normoxic environment, these data suggest that nitrite and nitrate may inhibit inflammation through a direct mechanism, independent of conversion back to nitrite or NO.
Nitrite and nitrate may not have been as effective at inhibiting neointimal hyperplasia compared to PROLI/NO because of the delivery method. In our study, the NO donors and metabolites were applied periadventitially following arterial injury. We have found this method of delivery to result in consistent and reproducible effects of PROLI/NO on neointimal hyperplasia.[2; 37–39] Other studies that have examined the efficacy of nitrite or nitrate have focused on the intravenous, intraperitoneal or inhaled routes. Since NO is a highly diffusible molecule, it is possible that the efficacy of PROLI/NO is due to diffusion of NO throughout the arterial wall. Less is known about the diffusible nature of nitrite and nitrate. While nitrite and nitrate are small molecules, they are anions and may not be able to diffuse through all layers of the arterial wall to have direct effects on the cells in the media and intima when applied to the adventitia. However, since the adventitia is now recognized as a predominate source of cells that repopulates the media and contributes to neointimal development, application of any therapy to the adventitia is likely to be more effective than luminal delivery. Moreover, since many of the known pathways that convert nitrate to nitrite, or nitrite to NO exist in the vasculature,[11] and given the existence of the vaso vasorum in the adventitia, it is possible for these alternative routes of NO production to take place in the adventitia. Thus, while our delivery method may have been a limitation of this study, it may also represent an advantageous method of delivery.
In conclusion, periadventitial delivery of PROLI/NO provides superior inhibition of neointimal hyperplasia following rat carotid artery injury when compared to the NO metabolites nitrite and nitrate. Although nitrite and nitrate did not demonstrate antiproliferative effects in vitro or in vivo, a modest inhibition of neointima formation was seen following treatment with these anions. This effect may be related to inhibition of the inflammatory response following injury observed with these anions. Overall, NO release from PROLI/NO is very effective at inhibiting neointimal hyperplasia by affecting multiple aspects of the arterial injury response (i.e., proliferation, inflammation, etc). The efficacy of PROLI/NO at inhibiting neointimal hyperplasia deserves to be studied in a preclinical large animal model.
Acknowledgments
The authors would like to express their thanks to the Institute for BioNanotechnology in Medicine, the Feinberg Cardiovascular Research Institute, and to Edwards Lifesciences for generously providing the Fogarty balloon catheters. The authors would also like to express their thanks to Lynnette Dangerfield for her “tireless” effort.
Sources of Funding
This work was supported in part by funding from the National Institutes of Health (K08HL084203; MRK), (T32 HL094293-01, GEH), and the American Vascular Association (MRK). It was also supported in part by the generosity of Mrs. Hilda Rosenbloom and Ms. Eleanor Baldwin. Lastly, this work was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abbreviations
- PROLI/NO
the diazeniumdiolate disodium 1-[2-(carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate
- DETA/NO
1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate
- RASMC
rat aortic smooth muscle cells
- RAEC
rat aortic endothelial cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM, Jr, White CJ, White J, White RA, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Pearce CG, Najjar SF, Kapadia MR, Murar J, Eng J, Lyle B, Aalami OO, Jiang Q, Hrabie JA, Saavedra JE, Keefer LK, Kibbe MR. Beneficial effect of a short-acting NO donor for the prevention of neointimal hyperplasia. Free Radic Biol Med. 2008;44:73–81. doi: 10.1016/j.freeradbiomed.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masters KS, Lipke EA, Rice EE, Liel MS, Myler HA, Zygourakis C, Tulis DA, West JL. Nitric oxide-generating hydrogels inhibit neointima formation. J Biomater Sci Polym Ed. 2005;16:659–672. doi: 10.1163/1568562053783722. [DOI] [PubMed] [Google Scholar]
- 4.Kapadia MR, Chow LW, Tsihlis ND, Ahanchi SS, Eng JW, Murar J, Martinez J, Popowich DA, Jiang Q, Hrabie JA, Saavedra JE, Keefer LK, Hulvat JF, Stupp SI, Kibbe MR. Nitric oxide and nanotechnology: a novel approach to inhibit neointimal hyperplasia. J Vasc Surg. 2008;47:173–182. doi: 10.1016/j.jvs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulton GJ, Davies MG, Barber L, Gray JL, Svendsen E, Hagen PO. Local effects of nitric oxide supplementation and suppression in the development of intimal hyperplasia in experimental vein grafts. Eur J Vasc Endovasc Surg. 1998;15:279–289. doi: 10.1016/s1078-5884(98)80030-0. [DOI] [PubMed] [Google Scholar]
- 6.Kaul S, Cercek B, Rengstrom J, Xu XP, Molloy MD, Dimayuga P, Parikh AK, Fishbein MC, Nilsson J, Rajavashisth TB, Shah PK. Polymeric-based perivascular delivery of a nitric oxide donor inhibits intimal thickening after balloon denudation arterial injury: role of nuclear factor-kappaB. J Am Coll Cardiol. 2000;35:493–501. doi: 10.1016/s0735-1097(99)00543-4. [DOI] [PubMed] [Google Scholar]
- 7.Chaux A, Ruan XM, Fishbein MC, Ouyang Y, Kaul S, Pass JA, Matloff JM. Perivascular delivery of a nitric oxide donor inhibits neointimal hyperplasia in vein grafts implanted in the arterial circulation. J Thorac Cardiovasc Surg. 1998;115:604–612. doi: 10.1016/S0022-5223(98)70325-3. [DOI] [PubMed] [Google Scholar]
- 8.Hrabie JA, Keefer LK. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chem Rev. 2002;102:1135–1154. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- 9.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, III, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 10.Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, Bauer PM, Choi JJ, Curtis E, Choi AM, Gladwin MT. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase-dependent nitric oxide generation. Circulation. 2010;121:98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 11.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 12.Keefer LK. Progress toward clinical application of the nitric oxide-releasing diazeniumdiolates. Annu Rev Pharmacol Toxicol. 2003;43:585–607. doi: 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]
- 13.Nan YS, Feng GG, Hotta Y, Nishiwaki K, Shimada Y, Ishikawa A, Kurimoto N, Shigei T, Ishikawa N. Neuropeptide Y enhances permeability across a rat aortic endothelial cell monolayer. Am J Physiol Heart Circ Physiol. 2004;286:H1027–H1033. doi: 10.1152/ajpheart.00630.2003. [DOI] [PubMed] [Google Scholar]
- 14.Suh SH, Vennekens R, Manolopoulos VG, Freichel M, Schweig U, Prenen J, Flockerzi V, Droogmans G, Nilius B. Characterisation of explanted endothelial cells from mouse aorta: electrophysiology and Ca2+ signalling. Pflugers Arch. 1999;438:612–620. doi: 10.1007/s004249900085. [DOI] [PubMed] [Google Scholar]
- 15.Ding Y, Vaziri ND. Calcium channel blockade enhances nitric oxide synthase expression by cultured endothelial cells. Hypertension. 1998;32:718–723. doi: 10.1161/01.hyp.32.4.718. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 17.Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4:411–417. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 18.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1:546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 19.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 20.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millar TM, Stevens CR, Blake DR. Xanthine oxidase can generate nitric oxide from nitrate in ischaemia. Biochem Soc Trans. 1997;25:528S. doi: 10.1042/bst025528s. [DOI] [PubMed] [Google Scholar]
- 22.Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem. 2000;275:7757–7763. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, III, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 25.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 26.Modin A, Bjorne H, Herulf M, Alving K, Weitzberg E, Lundberg JO. Nitrite-derived nitric oxide: a possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol Scand. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 27.Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, Kim JM, Song EC, Kim M, Roh JK. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 28.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1:290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 30.Kibbe MR, Li J, Nie S, Watkins SC, Lizonova A, Kovesdi I, Simmons RL, Billiar TR, Tzeng E. Inducible nitric oxide synthase (iNOS) expression upregulates p21 and inhibits vascular smooth muscle cell proliferation through p42/44 mitogen-activated protein kinase activation and independent of p53 and cyclic guanosine monophosphate. J Vasc Surg. 2000;31:1214–1228. doi: 10.1067/mva.2000.105006. [DOI] [PubMed] [Google Scholar]
- 31.Kibbe MR, Nie S, Seol DW, Kovesdi I, Lizonova A, Makaroun M, Billiar TR, Tzeng E. Nitric oxide prevents p21 degradation with the ubiquitin-proteasome pathway in vascular smooth muscle cells. J Vasc Surg. 2000;31:364–374. doi: 10.1016/s0741-5214(00)90166-6. [DOI] [PubMed] [Google Scholar]
- 32.Feelisch M, Fernandez BO, Bryan NS, Garcia-Saura MF, Bauer S, Whitlock DR, Ford PC, Janero DR, Rodriguez J, Ashrafian H. Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide-generating and -scavenging systems. J Biol Chem. 2008;283:33927–33934. doi: 10.1074/jbc.M806654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. Br J Surg. 1994;81:1254–1269. doi: 10.1002/bjs.1800810904. [DOI] [PubMed] [Google Scholar]
- 34.Libby P, Schwartz D, Brogi E, Tanaka H, Clinton SK. A cascade model for restenosis. A special case of atherosclerosis progression. Circulation. 1992;86:III47–III52. [PubMed] [Google Scholar]
- 35.Kibbe MR, Tzeng E, Gleixner SL, Watkins SC, Kovesdi I, Lizonova A, Makaroun MS, Billiar TR, Rhee RY. Adenovirus-mediated gene transfer of human inducible nitric oxide synthase in porcine vein grafts inhibits intimal hyperplasia. J Vasc Surg. 2001;34:156–165. doi: 10.1067/mva.2001.113983. [DOI] [PubMed] [Google Scholar]
- 36.Stokes KY, Dugas TR, Tang Y, Garg H, Guidry E, Bryan NS. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296:H1281–H1288. doi: 10.1152/ajpheart.01291.2008. [DOI] [PubMed] [Google Scholar]
- 37.Ahanchi SS, Varu VN, Tsihlis ND, Martinez J, Pearce CG, Kapadia MR, Jiang Q, Saavedra JE, Keefer LK, Hrabie JA, Kibbe MR. Heightened efficacy of nitric oxide-based therapies in type II diabetes mellitus and metabolic syndrome. Am J Physiol Heart Circ Physiol. 2008;295:H2388–H2398. doi: 10.1152/ajpheart.00185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varu VN, Ahanchi SS, Hogg ME, Bhikhapurwala HA, Chen A, Popowich DA, Vavra AK, Martinez J, Jiang Q, Saavedra JE, Hrabie JA, Keefer LK, Kibbe MR. Insulin enhances the effect of nitric oxide at inhibiting neointimal hyperplasia in a rat model of type 1 diabetes. Am J Physiol Heart Circ Physiol. 2010;299:H772–H779. doi: 10.1152/ajpheart.01234.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogg ME, Varu VN, Vavra AK, Popowich DA, Banerjee MN, Martinez J, Jiang Q, Saavedra JE, Keefer LK, Kibbe MR. Effect of nitric oxide on neointimal hyperplasia based on sex and hormone status. Free Radic Biol Med. 2011 doi: 10.1016/j.freeradbiomed.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]