INTRODUCTION
Eclampsia is currently defined in the obstetrical literature as the occurrence of unexplained seizure during pregnancy in a woman with preeclampsia.1,2 In the Western world, the incidence of eclampsia is ~1 in 2000 to 1 in 3000 pregnancies3–5, but the incidence is 10-fold higher than that in tertiary referral centers and undeveloped countries where there is poor prenatal care, and in multi-fetal gestations.6,7 Eclampsia is associated with high maternal and fetal mortality and morbidity.3,8,9 Nearly 1 in 50 women with eclampsia die as do 1 in 14 of their offspring, and mortality rates are considerably higher in undeveloped countries.3,8,9 Eclampsia is also associated with significant life-threatening complications, including neurological events. In the brain, seizure can cause stroke, hemorrhage, edema and brain herniation acutely,10–13 but also predisposes to epilepsy and cognitive impairment later in life.13,14
Preeclampsia by definition is a prodrome for eclampsia, making hypertension and proteinuria prerequisite for seizure during pregnancy. However, women who develop eclampsia exhibit a wide spectrum of signs and symptoms ranging from severe hypertension and proteinuria to mild or absent hypertension with no proteinuria.6,9,15 In a study of 53 pregnancies complicated by eclampsia, only 7 women (13%) could be considered to have severe preeclampsia prior to seizure.15 A similar result was found in a study in the United Kingdom in which high blood pressure (≥120 mmHg diastolic) was recorded in only 20% of patients with eclampsia.3 The findings that a fair number of women with eclampsia do not have the clinical definition of hypertension or proteinuria suggests that eclampsia is not always a progression from severe preeclamptic disease to seizure (eclampsia). While this alternative view of the eclamptic seizure was presented over 10 years ago, there has been little progress in understanding the underlying cause of eclampsia.3
Eclampsia remains a significant life-threatening complication of pregnancy, yet there are no reliable tests or symptoms for predicting the development of seizure. In addition, while magnesium sulfate (MgSO4) is the primary treatment of preeclamptic women for prevention of eclampsia, its use is controversial because of potential serious side effects including areflexia and respiratory distress.16–19 Thus, eclampsia is difficult to predict and treat likely because of our lack of understanding of its underlying cause. This review will highlight our current understanding of how pregnancy and preeclampsia affect the brain and cerebral circulation that could promote neuronal excitability (seizure) and ways in which to manage seizure in preeclamptic women during pregnancy and preeclampsia.
MECHANISMS OF SEIZURE DURING PREECLAMPSIA
Role of cerebral circulation and autoregulation of cerebral blood flow in eclampsia
The mechanism by which women undergo seizure during preeclampsia is not known. Adding to the difficultly of understanding eclampsia is that clinical findings of eclampsia have shown varying degrees of hemorrhage, cerebral edema and vasculopathy.20–23 Two hypothesis regarding the cause of eclampsia have received the most attention, both of which are focused on the function of the cerebral vasculature and autoregulation of cerebral blood flow (CBF) during elevations in blood pressure. First, the concept that the cerebral circulation is in a state of “overautoregulation” in response to elevated cerebral perfusion pressure during preeclampsia that causes ischemia has been put forth.22 This hypothesis is based on brain imaging that has shown areas of vasospasm in eclamptic women.23 Both cytotoxic and vasogenic edema have been shown in eclampsia21,24,25 and thus ischemic brain injury that causes seizure is possible. However, the reversibility of neurological symptoms and neuroradiologic lesions within a few days or weeks postpartum in most cases argues against true cerebral ischemic necrosis. In fact, clinical and neuroimaging findings are more consistent with vasogenic edema.25–27 For example, the neuroradiologic hallmarks of eclampsia are reversible abnormalities that appear hypodense on computed tomography (CT) and hyperintense on T2-weighted magnetic resonance (MR) images,25,27,28–31 both suggestive of edema. Further studies using diffusion-weighted MR images found that these hyperintense areas on MR had a high apparent diffusion coefficient value, indicative of vasogenic and not cytotoxic edema.25,31
The second hypothesis regarding the underlying mechanism for the neurological symptoms and edema formation during eclampsia is that it represents a form of hypertensive encephalopathy in which a rapid rise in blood pressure overcomes the myogenic vasoconstriction of cerebral arteries and arterioles causing loss of autoregulatory capacity and blood-brain barrier (BBB) disruption that promotes vasogenic edema.21,25–29 Hypertensive encephalopathy has been more recently termed posterior reversible encephalopathy syndrome (PRES) in order to highlight the propensity for edema formation to occur in the posterior cerebral cortex.30,32,33 The propensity for hyperperfusion and edema to form posteriorly is not known, but many have suggested that decreased sympathetic innervation of the posterior cerebral arteries for lack of a better explanation. However, there are a number of reasons why edema formation would be increased posteriorly other than sympathetic innervation. For example, capillary density is increased in the posterior brain region,34 an effect that would increase transcapillary filtration and promote greater edema in that region of the brain during acute hypertension. In any case, the diagnosis of eclampsia as a form of hypertensive encephalopathy or PRES has arisen from numerous similarities in clinical presentation including comparable imaging findings on CT and MR,25,28,30,35 the same neurological features, including severe and persistent headache, uncontrolled vomiting, cortical blindness and seizures,35–38 and the prompt reversibility of symptoms after blood pressure has been restored.36–38
As stated above, clinical studies have consistently found that there is little correlation between blood pressure and the occurrence of seizure in pregnancy.2,3,9,15,39 In fact, studies have found that ~40% of women with eclampsia have seizure at normal blood pressure and without proteinuria.3,9,15 These findings suggest that preeclampsia may not necessarily be a prodrome for eclampsia and imply that factors or processes associated with normal pregnancy may promote the eclamptic seizure. Because of this, our own studies have focused on the adaptation of the brain and cerebral circulation to pregnancy and how that might contribute to the neurological complications of preeclampsia and eclampsia, discussed below.
Effect of pregnancy on cerebral hemodynamics
In normotensive adults, CBF is maintained at approximately 50 mL per 100 g of brain tissue per minute, provided cerebral perfusion pressure is in the range of ~60 to 150 mmHg.40 Above and below this limit, autoregulation is lost and there is a linear relationship between CBF and mean arterial pressure.41,42 The importance of understanding how pregnancy and preeclampsia affect autoregulation of CBF is that significant brain tissue damage occurs, including BBB disruption and edema formation, when autoregulatory mechanisms are lost.43–46 At pressures above the autoregulatory limit, the myogenic vasoconstriction of vascular muscle is overcome by the excessive intravascular pressure and forced dilatation of cerebral vessels occurs.43–46 The loss of myogenic tone during forced dilatation decreases cerebrovascular resistance and increases CBF, a result that produces hyperperfusion, BBB disruption and vasogenic edema formation.43–46 This sequence of events is considered the underlying cause of the neurological complications of hypertensive encephalopathy, PRES, and in cases of eclampsia in which there is elevated arterial pressure.30,32–36
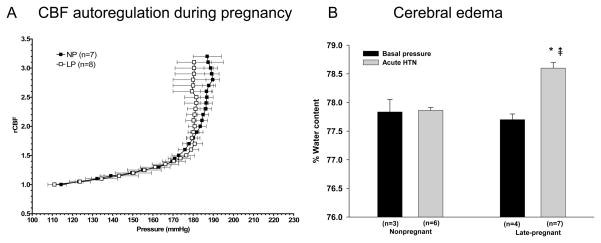
Because numerous studies have shown that eclampsia can occur at normal blood pressure has led to the suggestion that autoregulation of CBF is shifted to the lower range of pressures during pregnancy. Thus, forced dilatation of cerebral arteries and arterioles would occur at lower pressures, causing vasogenic edema at pressures that would be considered within the autoregulatory range. However, studies in humans and animals have shown that autoregulation is intact during normal pregnancy and not different from the nonpregnant state.26,41,47 We used a rat model of pregnancy to measure autoregulation of CBF and found that there was no difference in autoregulatory capacity or the pressure at which breakthrough occurred between nonpregnant and late-pregnant animals (Figure 1A).41 Importantly, when brain water content was measured in these same animals, it was found that only pregnant animals developed significant edema formation (Figure 1B). Thus, it appears that at least in animals, pregnancy predisposes the brain to edema when blood pressure is acutely elevated to the point of breakthrough of autoregulation. However, the autoregulatory curve does not appear to be shifted to lower pressures during pregnancy.
Figure 1. Cerebral blood flow autoregulation during pregnancy and subsequent edema formation.
A, Cerebral blood flow (CBF) autoregulatory curves from nonpregnant (NP) and late-pregnant (LP) anesthetized rats determined using laser Doppler and acute phenylephrine infusion. Notice that autoregulation was intact in both groups of animals from ~110–180mmHg as demonstrated by little change in CBF with pressure. However, at pressures above ~180mmHg, breakthrough occurred, substantially increasing CBF. There was no difference in the pressure of breakthrough between NP and LP animals. B, Cerebral edema formation in response to autoregulatory breakthrough in the same NP and LP rats shown in A. Notice that only the LP animals developed edema formation at this time point (10 minutes) after breakthrough. Partially published in Hypertension 2007;49:334–340.
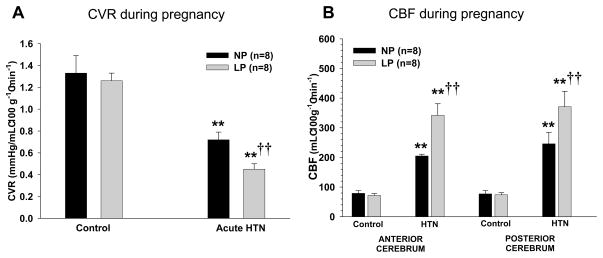
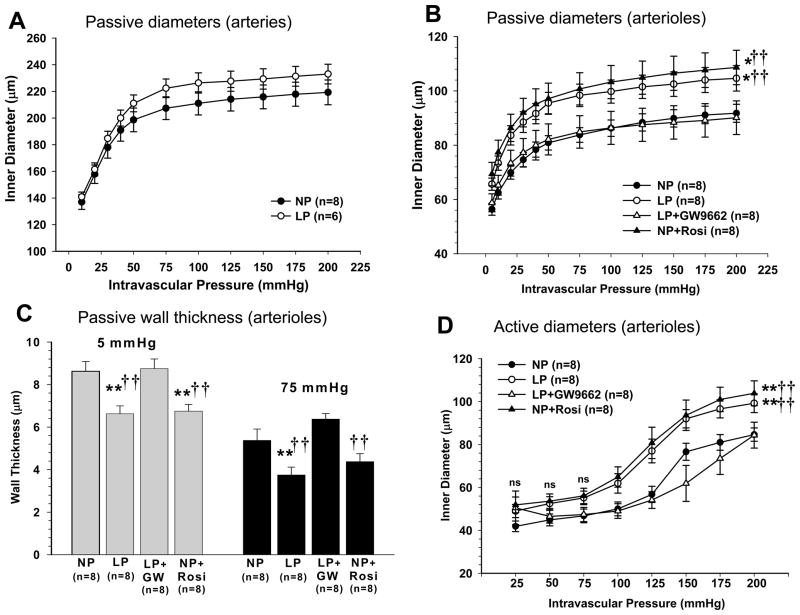
In a recent study, we explored mechanisms by which pregnancy causes an increase in edema formation in response to acute hypertension.34 We used microspheres to measure absolute changes in CBF under basal conditions and in response to acute hypertension. We found that while CBF was not different during pregnancy basally, it was associated with an ~40% increase in CBF in response to autoregulatory breakthrough (Figure 2). The increase in CBF in the pregnant animals was accompanied by selective outward remodeling of penetrating brain arterioles due to pregnancy-induced activation of peroxisome proliferator-activated receptor gamma (PPARγ; Figure 3). Thus, it appears that pregnancy promotes a decrease in small vessel resistance in the brain when blood pressure was acutely elevated, an effect that would be expected to increase BBB permeability and edema formation. These findings are in agreement with what has been suggested in studies using transcranial Doppler and MR imaging in humans during normal pregnancy.48,49 Thus, pregnancy appears to alter the structure of cerebral parenchymal vessels to increase vascular space. However, because of the prominent role of large arteries to vascular resistance in the brain, the increase in vascular space is inconsequential until pressures are elevated to the point of forced dilatation. Once large artery resistance is decreased and hydrostatic pressure is transmitted to the capillary bed, there is greater BBB disruption and edema formation (Figure 4).
Figure 2. Effect of pregnancy on cerebral vascular resistance and cerebral blood flow.
Microspheres were used to measure CBF in nonpregnant (NP) and late-pregnant (LP) animals under basal normotensive conditions and in response to acute hypertension induced by phenylephrine. Pregnancy did not alter CVR (A) or CBF (B) under normotensive conditions, but significantly decreased CVR and increased CBF in response to acute hypertension. **p<0.01 vs. control; †† p<0.01 vs. NP. Published as Cipolla MJ, et al. J Appl Physiol. 2010 Am Physiol Soc., used with permission.
Figure 3. Effect of pregnancy and PPARγ activation on remodeling of brain penetrating arterioles.
Penetrating brain arterioles isolated from nonpregnant control (NP), late-pregnant control (LP), NP treated with the PPARγ agonist rosiglitazone for 3 weeks to mimic pregnancy (NP+Rosi) or LP and treated with the PPARγ inhibitor GW9662 (LP+GW9662) for the last half of pregnancy were used to measure lumen diameter and wall thickness under pressurized conditions. (A) Passive pressure vs. diameter curves of cerebral (pial) arteries from NP and LP rats. Pregnancy did not affect the luminal size of cerebral arteries. (B) Penetrating brain arterioles from LP and NP rosiglitazone-treated animals had significantly larger lumen diameters compared to NP control and LP GW9662-treated animal; **p<0.01 vs.NP; †† p<0.01 vs. LP-GW. (C) Wall thickness was significantly decreased in penetrating arterioles during pregnancy and PPARγ activation; **p<0.01 vs.NP; †† p<0.01 vs. LP-GW. Thus, pregnancy and PPARγ activation cause outward hypotrophic remodeling of brain penetrating arterioles. (D) Active pressure vs. diameter curves of penetrating arterioles shows that all vessels had myogenic reactivity within the autoregulatory pressure range from 25 to 100 mmHg then underwent forced dilatation. Arterioles from LP and rosiglitazone-treated NP animals had larger lumens than NP and GW9662-treated LP animals; **p<0.01 vs.NP; †† p<0.01 vs. LP-GW. Published as Cipolla MJ, et al. J Appl Physiol. 2010 Am Physiol Soc., used with permission.
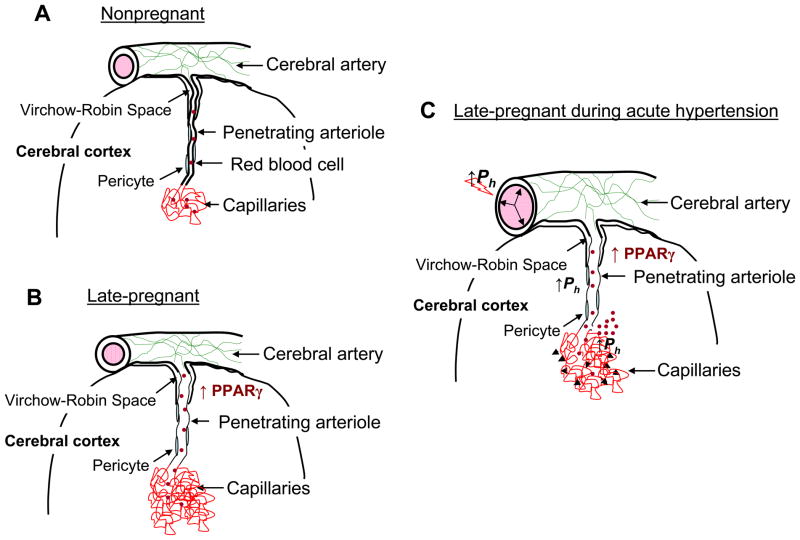
Figure 4. Summary diagram of cerebral vascular adaptation to pregnancy and the effect of acute hypertension.
(A) Cerebral arteries and arterioles that lie on top of the brain give rise to smaller arterioles that penetrate into the brain tissue, passing first through the Virchow-Robin Space. After passing through this space, arterioles are closely associated with multiple cell types, including pericytes, astrocytes and neurons. Penetrating arterioles are long and largely unbranched segments of the vasculature that connect upstream vessels to the capillary microcirculation and as such contribute significantly to cerebrovascular resistance in the brain. (B) During pregnancy, penetrating arterioles undergo outward hypotrophic remodeling under the influence of PPARγ activation that is increased during pregnancy. In addition to outward remodeling of arterioles in the brain, capillary density increases. These alterations in structure appear to occur only in the vasculature associated with brain parenchyma, a segment of the vasculature in which expression of PPARγ is relatively low. Thus, we speculate that PPARγ-dependent mechanisms in brain parenchyma have a paracrine influence on the associated vasculature. We further speculate that upstream cerebral arteries, that appear unaffected by pregnancy and PPARγ activation, maintain vascular resistance that is protective in downstream microvessels in relation to hydrostatic pressure. (C) During acute hypertension, similar to what occurs during severe preeclampsia and eclampsia, forced dilatation of large cerebral arteries occurs, decreasing vascular resistance and allowing greater transmission of hydrostatic pressure to downstream arterioles and capillaries. Because the arterioles have undergone outward hypotrophic remodeling, wall stress is significantly elevated, an effect that could promote increases in permeability as well as rupture and hemorrhage (denoted in the figure by the black arrows). Increases in hydrostatic pressure also affect the capillary bed to increase transcapillary filtration and promote edema formation that is greater during pregnancy due to decreased vascular resistance and increased vascular volume and capillary density. Published as Cipolla MJ, et al. J Appl Physiol. 2010 Am Physiol Soc., used with permission.
Effect of preeclampsia on cerebral hemodynamics
Impaired cerebral blood flow autoregulation is thought to be a major influence in the development of eclampsia due to decreased vascular resistance and increased pressure on the microcirculation that promotes vasogenic edema.27,50,32–35 Numerous studies have attempted to measure cerebral perfusion pressure and autoregulation of CBF during preeclampsia and eclampsia.26,47,51–54 Most studies have found that preeclampsia is associated with elevated cerebral perfusion pressure, a result that is not surprising given the appearance of hypertension in those patients.47,53 Whether or not autoregulation is intact under these conditions is not clear as some studies have found that increased perfusion pressure was associated with increased cerebrovascular resistance, suggesting autoregulation is intact.47 In contrast, other studies have found that preeclampsia and eclampsia were associated with decreased vascular resistance and hyperperfusion.26,51 Measurement of dynamic CBF autoregulation, a noninvasive technique that uses physiological changes in blood pressure to assess autoregulation, was performed on patients with eclampsia and found a substantial disturbance in CBF autoregulation.53,54
Whether or not autoregulation is intact in women with preeclampsia and eclampsia may be related to the occurrence of seizure. Seizure causes excessive neuronal excitation and significant increases in sympathetic outflow that releases large amounts of epinephrine and norepinephrine into the circulation.55,56 The resulting increased peripheral vascular resistance during seizure causes acute hypertension and PRES-like symptoms including autoregulatory breakthrough and edema formation.57 It is therefore possible that seizure actually precedes and is the cause of elevated blood pressure and loss of autoregulation in the brain in women with eclampsia.
The role of the BBB in eclampsia
If the primary explanation for the eclamptic seizure is that is represents a form of hypertensive encephalopathy or PRES in which severely elevated blood pressure secondary to the preeclamptic state causes autoregulatory breakthrough, BBB disruption and vasogenc brain edema, how these events develop and proceed to seizure should be considered. Increased cerebrovascular permeability is considered the most important factor for development of cerebral edema and is determined by the cerebral endothelial cells that form the BBB.58 The unique morphological features of cerebral endothelial cells that prevent the passage of solutes and ions from the blood into the brain tissue are the presence of high electrical resistance tight junctions that limit paracellular transport and the low rate of pinocytotic vesicle formation that reduces transcellular transport.59,60 In addition, the cerebral endothelial cells have a very low rate of hydraulic conductivity, making the passage of water into the brain in response to hydrostatic pressure limited.61 These unique features of the BBB make the cerebral endothelium more similar to epithelial cells than to endothelium in the periphery and are a protective feature that limits brain edema formation.
When there is an increase in permeability of the BBB, either via transceullar or paracellular routes, vasogenic edema can occur. The expansion of the extracellular space during vasogenic edema occurs within the closed space of the skull, causing progressive brain compression and the classic neurological symptoms of headache, nausea, vomiting, cortical blindness, and convulsions.62,63 BBB disruption can also pass damaging proteins and serum constituents into the brain that can cause seizure.64–66 Studies have found that increased BBB permeability alone is sufficient to provoke seizure activity.65 Passage of other damaging proteins, most notably albumin, into the brain parenchymal is also seizure provoking.66 Thus, the BBB has a central role in the development of neurological complications, including seizure.
BBB permeability during preeclampsia and eclampsia
Increased BBB permeability during severe preeclampsia and eclampsia can be caused by several factors. First, severely elevated microvascular pressure, secondary to the preeclamptic state, increases transcapillary filtration in the brain and vasogenic edema formation. Studies have shown that forced dilatation of cerebral artery and arteriole myogenic tone causes a substantial increase in pinocytotic vesicle formation in cerebral endothelium.67,68 Thus, factors or events that cause increased hydrostatic pressure on the cerebral microcirculation, such as autoregulatory breakthrough that has been shown to occur in PRES and eclampsia, have the potential to increase vasogenic edema formation and passage of damaging proteins into the brain parenchyma.
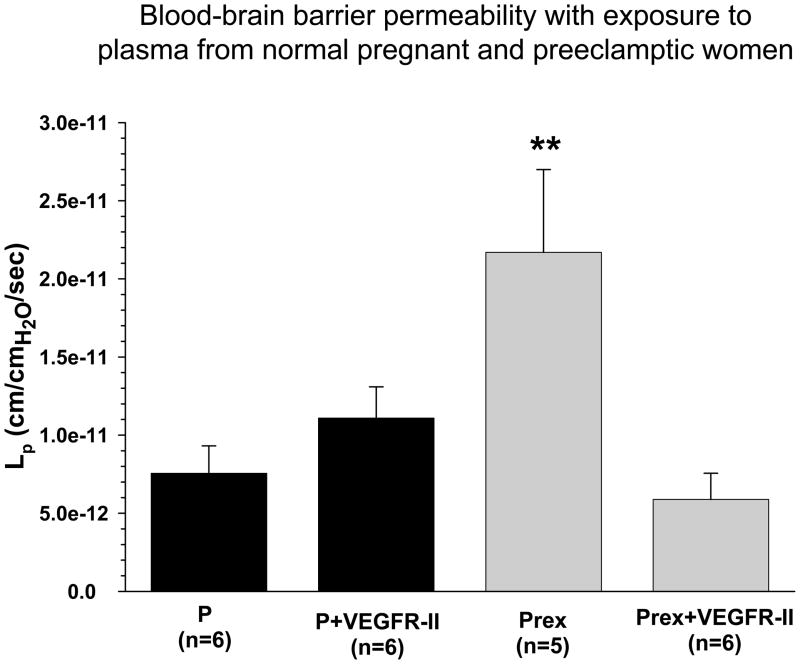
Second, there could be pregnancy- or preeclampsia-induced increases in BBB permeability without an increase in hydrostatic pressure, such as an effect on tight junctional protein expression. Tight junctions are not passive structures, but can be rapidly modulated by signaling pathways that affect the structure of the tight junction and the organization of the endothelial cell actin cytoskeleton.69–71 Similarly, transcellular transport has been shown to be regulated by mediators such as vascular endothelial growth factor (VEGF) and other inflammatory mediators.60,63,72,73 Recently, we found that plasma from severely preeclamptic women significantly increased BBB permeability compared to normal pregnant women, an effect that was completely prevented by a non-selective VEGF receptor tyrosine kinase inhibitor (Figure 5).74 Because permeability in response to plasma was assessed in cerebral veins from normal pregnant animals, suggests that there are circulating factors during preeclampsia that increase BBB permeability independent of inherent changes in barrier properties.
Figure 5. Increased blood-brain barrier permeability in response to plasma from preeclamptic women.
Graph showing hydraulic conductivity (Lp) at 36 minutes of cerebral veins exposed to normal pregnant (P) or preeclamptic (Prex) plasma with and without the addition of VEGFR-II to inhibit VEGF receptor tyrosine kinase activity in the plasma perfusate, or 60 pg/mL VEGF without plasma. Veins exposed to P plasma were unaffected by VEFGR-II. However, VEGFR-II prevented an increase of Lp in veins exposed to Prex plasma, suggesting VEGF receptor tyrosine kinase activity is involved in increased BBB permeability in response to Prex plasma. VEGF alone produced modest permeability that was significantly decreased from PREX plasma only. ** p<0.01 vs. all. Published in Hypertension 2010;56(5):1003–1008.
Role of elevated TNF-α during preeclampsia on the BBB and seizure
Another important feature of the BBB that is important for seizure induction is that it regulates passage of cytokines from the blood into the brain. Most cytokines do not readily cross into the brain but are regulated by transporters on the BBB.75 This may be of particular importance during preeclampsia because it has been shown that this condition is associated with a large increase in proinflammatory cytokines in the blood, most notably tumor necrosis factor alpha (TNF-α).76–78 Elevated TNF-α during preeclampsia could have significant effects on the brain. Unlike most cytokines, circulating TNF-α can cross the BBB through receptor-mediated endocytosis.79 The binding of TNF-α to its receptors on the BBB increases paracellular permeability that can then promote vasogenic edema.80 In addition, TNF-α is a recognized innate cytokine that initiates peripheral and vascular inflammatory responses.81 Peripheral inflammation has been shown to cause TNF-α dependent microglial activation that increases neuronal excitability in the brain.82,83 TNF-α upregulates endothelial cell adhesion molecules such as E-selectin, ICAM-1 and VCAM-1 that facilitate passage of leukocytes into the brain.80 Leukocyte infiltration of the BBB has been shown be seizure provoking by activating microglia that can then produce TNF-α.84 TNF-α production in the brain can both lower the seizure threshold and cause seizure itself,82,83 via effects on AMPA and GABAA receptors.85 The importance of TNF-α in seizure induction is underscored by the findings that TNF-α infused into rats increases seizure susceptibility and decreases seizure threshold.82,83 How TNF-α is regulated during pregnancy, both peripherally through induction of soluble receptors and at the BBB where there could be an effect of receptor expression, is not known. However, because TNF-α crosses the BBB by receptor-mediated endocytosis suggests there can be regulation of its effects. In fact, one study found that while TNF-α was significantly elevated in preeclamptic women, the fractional excretion was less despite proteinuria.77 The decreased clearance and renal excretion of TNF-α during preeclampsia could represent regulation by soluble receptors. How pregnancy or preeclampsia affect TNF-α receptors on the BBB is currently unknown but seems like an important area of investigation given the central role this cytokine has in seizure induction.
Role of aquaporins (AQPs) in seizure and eclampsia
The AQPs are a family of channel forming transmembrane proteins that facilitate the movement of water, glycerol, and other solutes across the plasma membrane of cells.86–89 Aquaporin 4 (AQP4), the predominant aquaporin in the brain, has been localized in the astrocytic endfeet surrounding blood vessels, but is not in endothelium.91–93 One role of astrocytic AQP4 may be regulation of water transport once it has crossed the BBB, mainly because of the highly polarized localization of AQP4 in the perivascular endfeet of astrocytes.89,94 AQP4 in astrocytes is thought to contribute to BBB properties by taking up excess water brought into the brain by disruption of the BBB.94 Numerous studies that have shown increased AQP4 expression during conditions that cause vasogenic brain edema, including brain tumors, focal ischemia, and brain injury.94–98 Interestingly, AQP4 is increased in the brain during pregnancy.99.100 The effect of increased expression of AQP4 in the brain during pregnancy is not clear, but could affect seizure threshold as AQP4 knockout mice were shown to be less susceptible to seizure, suggesting elevated AQP4 lowers the seizure threshold.101
Animal models of preeclampsia and eclampsia
Because of the difficulty in predicting and studying neurological complications associated with severe preeclampsia and eclampsia, there is a need for animal models to help us understand these conditions. Numerous animal models of preeclampsia exist and many have been useful for understanding particular aspects of this condition. For example, reduced uteroplacental perfusion (RUPP) and high soluble fms-related tyrosine kinase 1 (sflt-1) have been used extensively as models of preeclampsia, however, how they mimic other pathological changes that occur during preeclampsia, such as changes at the BBB or neuronal excitability, are not known. In humans, endothelial dysfunction and oxidative stress have been measured in other vascular beds during preeclampsia.102–104 It is thus reasonable to assume that similar effects occur in the cerebral circulation, although this is not known for sure. If so, these events could have a significant influence on brain excitability as upregulation of adhesion molecules could cause passage of leukocytes into the brain parenchyma and promote seizure.83,84 However, because of the unique nature of the cerebral endothelium it cannot be assumed that events that happen peripherally are mimicked by the BBB. In addition, neurological complications to our knowledge have yet to be assessed in any model of preeclampsia. Thus, animal models of preeclampsia may be useful for understanding the development of hypertension, proteinuria and placental ischemia during pregnancy, they have yet to be applied to understanding how neurological complications arise.
MANAGEMENT OF ECLAMPSIA
Consequences of seizure
Although the complications of seizure during pregnancy are rare, prolonged generalized tonic-clonic seizure activity during pregnancy has been shown to cause maternal acidosis, hypoxia and brain trauma, including hemorrhage. Abruptio placenta is common after prolonged seizure, occurring in 20–50% of women.105 Convulsive seizures are also dangerous for the fetus. Fetal heart rate slows during and for up to 20 minutes after a maternal convulsion, suggestive of fetal hypoxia. In addition to acute events, recent studies have found that women with prior eclampsia have increased white matter lesions and lasting psychological and cognitive effects.106–108 Thus, maternal convulsions can cause trauma to both the mother and fetus acutely and have long-lasting negative consequences.
Clinical presentation, diagnostic evaluation and differential diagnosis
Headache and visual disturbances with occipital lobe edema are common symptoms of eclamptic encephalopathy. Hyperperfusion and edema occur most often and more pronounced posteriorly in the subcortical white matter of the occipital lobes, although gray matter and anterior circulation is involved in the most severe cases. Seizures may be focal or secondary generalized. Some women have neurological complications without seizures, including confusion, aphasia, cognitive deficits, and depressed level of consciousness. Although hypertension and preeclampsia occur most often in eclamptic women, it is not uncommon for women to present with seizures in late-pregnancy and early postpartum with mild or absent hypertension, as discussed above.
The diagnosis of eclampsia is based on the findings of encephalopathy or seizures in late-pregnancy or postpartum with imaging. Computed tomography or MR imaging should be used to show evidence of edema and rule out alternative brain lesions or hemorrhage. MR imaging with diffusion-weighted imaging can be useful to distinguish reversible edema from ischemic stroke. Patients may have intracerebral or cerebellar hemorrhage that can be due to vascular anomalies or can result from trauma associated with seizure. Other common causes of seizure during pregnancy, other than eclampsia, include idopathic epilepsy, trauma, congenital defects, neoplasms, meningitis, intracerebral hemorrhage, and drug and alcohol toxicity.
Management of the eclamptic patient
The mainstays of management of the neurological symptoms of severe preeclamptic and eclamptic patients are the rapid lowering of blood pressure, administration of MgSO4 and delivery of the fetus. Mean arterial blood pressure should be lowered in patients with severe hypertension by 15–20%. Intravenous labetalol, hydralazine, or nicardipine work rapidly and safely. Nitroprusside and nitroglyercine contain cyanide and thus can cause fetal toxicity. Angiotenisin-converting enzyme (ACE) inhibitors are contraindicated during pregnancy (category D). Fluid therapy can be beneficial to eclamptic patients and those with severe preeclampsia because of the associated volume contraction (or lack of expansion of plasma volume associated with normal pregnancy), however, this should be monitored closely in women with severe and persistent hypertension, oliguria and pulmonary edema.
Prevention of the eclamptic seizure in a woman with preeclampsia is obvious and justified. Traditionally, obstetricians have favored the use of MgSO4 for the prevention of the eclamptic convulsion whereas neurologists have favored anti-epileptic agents such as phenytoin. A single first-onset seizure that occurs during pregnancy and resolves within minutes can usually be managed without anti-convulsant agents. MgSO4 therapy has been shown to be more effective than anti-convulsant agents, including phenytoin, and is now considered the treatment of choice for preventing eclampsia in preeclamptic women.109–111 Therapy can start with a loading dose of 4 to 6g MgSO4 followed by 2g/hr infusion. A supplemental dose of 2g can be given if seizure recurs.
There is concern over the use of high doses of MgSO4. Dose-related depression of neuromuscular transmission has been shown in preeclamptic women receiving traditional MgSO4 therapy.112 Studies have also shown that there is little to no change in electroencephalograms (EEGs) of eclamptic women obtained during MgSO4 treatment. 112,113 At levels of 8 to 10 mEq/L, tendon reflexes are depressed. At levels of 10 to 12 mEq/L, there is a high risk of respiratory depression. Renal insufficiency can also occur with high MgSO4. Thus, tendon reflexes, respiratory function, and urine output should be followed closely. It is more important to follow women for signs of neurological depression rather than serum levels and adjust therapy accordingly. When petallar reflexes are lost, MgSO4 should be discontinued. If respirations are depressed, calcium gluconate should be given. Although it has been shown that MgSO4 is more effective at preventing eclamptic convulsions than phenytoin or diazepam, refractory seizures should be aggressively treated with traditional anti-convulsant agents. In addition, the cause of the seizure should be determined and whether further seizures are likely, and thus the need for anti-epileptic medication. If needed, monotherapy with pentobarbital or phenytoin are effective.
Mechanisms of MgSO4 for seizure prevention
MgSO4 is the therapy of choice for preventing eclamptic convulsions. The mechanisms by which MgSO4 is effective at preventing eclamptic convulsions are likely multifactorial and have been recently reviewed in 16 and will only be summarized here. MgSO4 is a calcium antagonist and as such could inhibit vascular smooth muscle contraction.114 MgSO4 is a potent vasodilator, however, its effects in the cerebral circulation are considerably less effective than systemic vasculature.115 In addition, the sensitivity to MgSO4 is decreased in cerebral arteries from late-pregnant and postpartum animals, suggesting MgSO4 is not acting as a vasodilator in the cerebral circulation.115 MgSO4 has been shown to protect the BBB, likely through its calcium antagonistic effects in the cerebral endothelium.116 When pregnant rats were treated with clinically relevant doses of MgSO4, they had significantly less BBB permeability during acute hypertension.116 Thus, MgSO4 could prevent recurrent seizure by protecting the BBB. Lastly, MgSO4 is an NMDA receptor antagonist and thus would act as an anti-convulsant if it were in high enough concentration in the brain.117
Acknowledgments
We gratefully acknowledge the continued support of the NIH grants NS19108 to RPK, and NS045940 to MJC as well as the NINDS Neural Environment Cluster supplement (NS045940-06S1) and ARRA Supplement NS045940-05S1 to MJC. We also gratefully acknowledge the Totman Medical Research Trust, the Georgio Pardi Foundation and the Preeclampsia Foundation.
References
- 1.Roberts JM, Redman CWG. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341:1447–1454. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- 2.Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–410. doi: 10.1097/01.AOG.0000152351.13671.99. [DOI] [PubMed] [Google Scholar]
- 3.Douglas KA, Redman CWG. Eclampsia in the United Kingdom. BMJ. 1994;309:1395–1400. doi: 10.1136/bmj.309.6966.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R. Epidemiology of preeclampsia and eclampsia in the United States, 1979–1986. Am J Obstet Gynecol. 1990;163(2):460–465. doi: 10.1016/0002-9378(90)91176-d. [DOI] [PubMed] [Google Scholar]
- 5.Möller B, Lindmark G. Eclampsia in Sweden, 1976–1980. Acta Obstet Gynecol Scand. 1986;65(4):307–314. doi: 10.3109/00016348609157350. [DOI] [PubMed] [Google Scholar]
- 6.Mattar F, Sibai BM. Eclampsia. VIII. Risk factors for maternal morbidity. Am J Obstet Gynecol. 2000;182(2):307–312. doi: 10.1016/s0002-9378(00)70216-x. [DOI] [PubMed] [Google Scholar]
- 7.Makhseed M, Musini VM. Eclampsia in Kuwait 1981–1993. Aust N Z J Obstet Gynaecol. 1996;36(3):258–263. doi: 10.1111/j.1479-828x.1996.tb02706.x. [DOI] [PubMed] [Google Scholar]
- 8.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33(3):130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Katz VL, Farmer R, Kuller JA. Preeclampsia into eclampsia: toward a new paradigm. Am J Obstet Gynecol. 2000;182:1389–1396. doi: 10.1067/mob.2000.106178. [DOI] [PubMed] [Google Scholar]
- 10.Servillo G, Striano P, Striano S. Posterior reversible encephalopathy syndrome (PRES) in obstetric critically ill patients. Intensive Care Med. 2003;29:2323–2326. doi: 10.1007/s00134-003-1901-1. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore E, Choi HA, Hirsch LJ, Claassen J. Seizures and CNS hemorrhage: spontaneous intracerebral and aneurysmal subarachnoid hemorrhage. Neurologist. 2010;16(3):165–75. doi: 10.1097/NRL.0b013e3181c7cd0b. [DOI] [PubMed] [Google Scholar]
- 12.Menon B, Shorvon SD. Ischaemic stroke in adults and epilepsy. Epilepsy Res. 2009;87(1):1–11. doi: 10.1016/j.eplepsyres.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46(11):1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 14.Holmes GL, Lenck-Santini PP. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 2006;8(3):504–515. doi: 10.1016/j.yebeh.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Sabai BM. Eclampsia. VI. Maternal-perinatal outcome in 254 consecutive cases. Am J Obstet Gynecol. 1990;163:1049–1055. doi: 10.1016/0002-9378(90)91123-t. [DOI] [PubMed] [Google Scholar]
- 16.Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke. 2009;40(4):1169–1175. doi: 10.1161/STROKEAHA.108.527788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pritchard JA, Cunningham FG, Pritchard SA. The Parkland Memorial Hospital protocol for treatment of eclampsia: Evalauation of 235 cases. Am J Obstet Gynecol. 1984;148:951–960. doi: 10.1016/0002-9378(84)90538-6. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson JO. Does magnesium sulfate treat eclamptic convulsions? Clinical Neuropharmacology. 1986;9:37–45. doi: 10.1097/00002826-198602000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan PW, Lesser RP, Fisher RS, Repke JT, Hanley DF. No, magnesium sulfate should not be used in treating eclamptic seizures. Arch Neurol. 1988;45:1361–1364. doi: 10.1001/archneur.1988.00520360079017. [DOI] [PubMed] [Google Scholar]
- 20.Zunker P, Happe S, Georgiadis AL, Louwen F, Georgiadis D, Ringelstein EB, Holgreve W. Maternal cerebral hemodynamics in pregnancy-related hypertension. A prospective transcranial Doppler study. Ultrasound Obstet Gynecol. 2000;16:179–187. doi: 10.1046/j.1469-0705.2000.00194.x. [DOI] [PubMed] [Google Scholar]
- 21.Thomas SV. Neurologic aspects of eclampsia. J Neurol Sci. 1998;155:37–43. doi: 10.1016/s0022-510x(97)00274-8. [DOI] [PubMed] [Google Scholar]
- 22.Easton DJ. Severe preeclampsia/eclampsia hypertensive encephalopathy of pregnancy? Cerebrovasc Dis. 1998;8:53–58. doi: 10.1159/000015818. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher JJ, Kramer AH, Bleck TP, Solenski NJ. Overlapping features of eclampsia and postpartum angiopathy. Neurocrit Care. 2009;11(2):199–209. doi: 10.1007/s12028-009-9221-0. [DOI] [PubMed] [Google Scholar]
- 24.Koch S, Rabinstein A, Falcone S, Forteza A. Diffusion-weighted imaging shows cytotoxic and vasogenic edema in eclampsia. Am J Neurorad. 2001;22:1068–1070. [PMC free article] [PubMed] [Google Scholar]
- 25.Kanki T, Tsukimori K, Mihara F, Nakano H. Diffusion-weighted images and vasogenic edema in eclampsia. Obstet Gynecol. 1999;93:821–823. doi: 10.1016/s0029-7844(98)00575-4. [DOI] [PubMed] [Google Scholar]
- 26.Williams KP, Wilson S. Persistence of cerebral hemodynamic changes in patients with eclampsia: A report of three cases. Am J Obstet Gynecol. 1999;181:1162–1165. doi: 10.1016/s0002-9378(99)70101-8. [DOI] [PubMed] [Google Scholar]
- 27.Manfredi M, Beltramello A, Bongiovanni LG, Polo A, Pistoia L, Rizzuto N. Eclamptic encephalopathy: imaging and pathogenetic considerations. Acta Neurol Scand. 1997;96:277–282. doi: 10.1111/j.1600-0404.1997.tb00284.x. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz RB, Jones KM, Kalina P, Gajakian RL, Mantello MT, Garada B, Holman BL. Hypertensive encephalopathy: findings on CT, MR-Imaging, and SPECT-Imaging in 14 cases. Am J Radiol. 1992;159:379–383. doi: 10.2214/ajr.159.2.1632361. [DOI] [PubMed] [Google Scholar]
- 29.Port JD, Beauchamp NJ. Reversible intracerebral pathologic entities mediated by vascular autoregulatory dysfunction. Radiographics. 1998;18:253–267. doi: 10.1148/radiographics.18.2.9536483. [DOI] [PubMed] [Google Scholar]
- 30.Servillo G, Striano P, Striano S. Posterior reversible encephalopathy syndrome (PRES) in obstetric critically ill patients. Intensive Care Med. 2003;29:2323–2326. doi: 10.1007/s00134-003-1901-1. [DOI] [PubMed] [Google Scholar]
- 31.Engelter ST, Provenzale JM, Petrella JR. Assessment of vasogenic edema in eclampsia using diffusion imaging. Neuroradiology. 2000;42:818–820. doi: 10.1007/s002340000439. [DOI] [PubMed] [Google Scholar]
- 32.Wityk RJ, Pessin MS. Hypertensive Encephalopathy. In: Hunt Batjer H, editor. Cerebrovascular Disease. Vol. 8. Lippincott Raven Publishers; Philadelphia: 1997. pp. 97–102. [Google Scholar]
- 33.Mirza A. Posterior reversible encephalopathy syndrome: A variant of hypertensive encephalopathy. J Clin Neurosci. 2006:590–595. doi: 10.1016/j.jocn.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 34.Cipolla MJ, Sweet JG, Chan SL. Cerebral Vascular Adaptation to Pregnancy and its Role in the Neurological Complications of Eclampsia. J Appl Physiol. 2010 doi: 10.1152/japplphysiol.01159.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demirtaş Ö, Gelal F, Dirim V, Demirtaş LO, Uluç E, Baloğlu A. Cranial MR imaging with clinical correlation in preeclampsia and eclampsia. Neuroradiology. 2005;11:189–194. [PubMed] [Google Scholar]
- 36.Donaldson JO. The brain in eclampsia. Hypertens Pregnancy. 1994;13:115–133. [Google Scholar]
- 37.Richards AM, Moodley J, Graham DI, Bullock MR. Active management of the unconscious eclamptic patient. Br J Obstet Gynecol. 1986;93:554–562. doi: 10.1111/j.1471-0528.1986.tb07953.x. [DOI] [PubMed] [Google Scholar]
- 38.Richards AM, Graham DI, Bullock MR. Clinical pathological study of neurological complications due to hypertensive disorders of pregnancy. J Neurol Neurosurg Psychiatr. 1988;51:416–421. doi: 10.1136/jnnp.51.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sibai BM, Abdella TN, Spinnato JA, Anderson GD. Eclampsia. V. The incidence of nonpreventable eclampsia. Am J Obstet Gynecol. 1986;154(3):581–586. doi: 10.1016/0002-9378(86)90605-8. [DOI] [PubMed] [Google Scholar]
- 40.Phillips SJ, Whisnant JP. Hypertension and the brain. Arch Intern Med. 1992;152:938–945. [PubMed] [Google Scholar]
- 41.Euser AG, Cipolla MJ. Cerebral blood flow autoregulation and edema formation during pregnancy in anesthetized rats. Hypertension. 2007;49(2):334–340. doi: 10.1161/01.HYP.0000255791.54655.29. [DOI] [PubMed] [Google Scholar]
- 42.Heistad DD, Kontos HA. The Cardiovascular System III. In: Berne RM, Sperelakis N, editors. Handbook of Physiology. Vol. 5. American Physiological Society; Bethesda, MD: 1979. pp. 137–182. [Google Scholar]
- 43.Busija DW, Heistad DD. Factors involved in the physiological regulation of the cerebral circulation. Rev Physiol Biochem Pharmacol. 1984;101:161–211. doi: 10.1007/BFb0027696. [DOI] [PubMed] [Google Scholar]
- 44.Johansson B, Li C-L, Olsson Y, Klatzo I. Effect of acute arterial hypertension on the blood-brain barrier to protein tracers. Acta Neuropath. 1970;16:117–124. doi: 10.1007/BF00687666. [DOI] [PubMed] [Google Scholar]
- 45.Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–H383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- 46.Kontos HA, Wei EP, Dietrich WE, Navari RM, Povlishock JT, Ghatak NR, Ellis EF, Patterson JL., Jr Mechanism of cerebral arteriolar abnormalities after acute hypertension. Am J Physiol. 1981;240:H511–H527. doi: 10.1152/ajpheart.1981.240.4.H511. [DOI] [PubMed] [Google Scholar]
- 47.Zatik J, Major R, Aranyozi J, Molnar C, Limburg M, Fulesdi B. Assessment of cerebral hemodynamics during roll over test in healthy, pregnant women and those with pre-eclampsia. Br J Obstet Gynaecol. 2001;108:353–358. doi: 10.1111/j.1471-0528.2001.00095.x. [DOI] [PubMed] [Google Scholar]
- 48.Zeeman GG, Hatab M, Twickler DM. Maternal cerebral blood flow changes in pregnancy. Am J Obstet Gynecol. 2003;189:968–972. doi: 10.1067/s0002-9378(03)00820-2. [DOI] [PubMed] [Google Scholar]
- 49.Nevo O, Soustiel JF, Thaler I. Maternal cerebral blood flow during normal pregnancy: a cross-sectional study. Am J Obstet Gynecol. doi: 10.1016/j.ajog.2010.05.031. [DOI] [PubMed]
- 50.Donaldson JO. Eclamptic hypertensive encephalopathy. Semin Neurol. 1988;8:230–233. doi: 10.1055/s-2008-1041383. [DOI] [PubMed] [Google Scholar]
- 51.Zeeman GG, Hatab M, Twickler DM. Increased cerebral blood flow in preeclampsia with magnetic resonance imaging. Am J Obstet Gynecol. 2004;191:1425–1429. doi: 10.1016/j.ajog.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, Bravo SM, Klufas RA, Chai RY, Repke JT. Pre-eclampsia-eclampsia: clinical and neuroradiologic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology. 2000;217:371–376. doi: 10.1148/radiology.217.2.r00nv44371. [DOI] [PubMed] [Google Scholar]
- 53.Oehm E, Hetzel A, Els T, Berlis A, Keck C, Will H-G, Reinhard M. Cerebral hemodynamics and autoregulation in reversible posterior leukoencephalopathy syndrome caused by pre-/eclampsia. Cerebrovasc Dis. 2006;22:204–208. doi: 10.1159/000093810. [DOI] [PubMed] [Google Scholar]
- 54.Oehm E, Reinhard M, Keck C, Els T, Spreer J, Hetzel A. Impaired dynamic cerebral autoregulation in eclampsia. Ultrasound Obstet Gynecol. 2003;22:395–398. doi: 10.1002/uog.183. [DOI] [PubMed] [Google Scholar]
- 55.Sakamoto K, Saito T, Orman R, Koizumi K, Lazar J, Salciccioli L, Stewart M. Autonomic consequences of kainic acid-induced limbic cortical seizures in rats: peripheral autonomic nerve activity, acute cardiovascular changes, and death. Epilepsia. 2008;49(6):982–996. doi: 10.1111/j.1528-1167.2008.01545.x. [DOI] [PubMed] [Google Scholar]
- 56.Simon RP, Aminoff MJ, Benowitz NL. Changes in plasma catecholamines after tonic-clonic seizures. Neurology. 1984;34(2):255–257. doi: 10.1212/wnl.34.2.255. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen-Lam J, Kiernan MC. Acute cortical blindness due to posterior reversible encephalopathy. J Clin Neurosci. 2008;15(10):1182–1185. doi: 10.1016/j.jocn.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 58.Wagner K, Klienholz M, de Courten-Myers G, Myers R. Hyperglycemic versus normoglycemic stroke: topography of brain metabolites, intracellular pH, and infarct size. J Cereb Blood Flow Metab. 1992;12:213–222. doi: 10.1038/jcbfm.1992.31. [DOI] [PubMed] [Google Scholar]
- 59.Betz AL, Dietrich WD. Blood-brain barrier dysfunction in cerebral ischemia. In: Ginsberg MD, Bogousslavsky J, editors. Cerebrovascular Disease: Pathophysiology, Diagnosis and Management. 25. I. Blackwell Science; Malden, MA: 1998. [Google Scholar]
- 60.Wahl M, Unterberg A, Baethmann A, Schilling L. Mediators of blood-brain barrier dysfunction and formation of vasogenic brain edema. J Cereb Blood Flow Metab. 1988;8:621–634. doi: 10.1038/jcbfm.1988.109. [DOI] [PubMed] [Google Scholar]
- 61.Roberts TJ, Chapman AC, Cipolla MJ. PPAR-gamma agonist rosiglitazone reverses increased cerebral venous hydraulic conductivity during hypertension. Am J Physiol Heart Circ Physiol. 2009;297(4):H1347–H1353. doi: 10.1152/ajpheart.00630.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dinsdale HB, Mohr JP. Hypertensive Encephalopathy. In: Barnett Henry JM, Mohr JP, Stein Bennet M, Yatsu Frank M., editors. Stroke Pathophysiology, Diagnosis and Management. 3. Vol. 34 Churchill Livingston; New York, NY: [Google Scholar]
- 63.Cipolla MJ. Stroke and the Blood-Brain Interface. In: Spray D, Dermietzel R, editors. Blood-brain Barrier Interfaces. Wiley Press; 2006. [Google Scholar]
- 64.Pavlovsky L, Seiffert E, Heinemann U, Korn A, Golan H, Friedman A. Persistent BBB disruption may underlie alpha interferon-induced seizures. J Neurol. 2005;252(1):42–46. doi: 10.1007/s00415-005-0596-3. [DOI] [PubMed] [Google Scholar]
- 65.Marchi N, Teng Q, Ghosh C, Fan Q, Nguyen MT, Desai NK, Bawa H, Rasmussen P, Masaryk TK, Janigro D. Blood-brain barrier damage, but not parenchymal white blood cells, is a hallmark of seizure activity. Brain Res. 2010;1353:176–186. doi: 10.1016/j.brainres.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Vliet EA, da Costa Araújo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130(Pt 2):521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 67.Nag S, Robertson DM, Dinsdale HB. Quantitative estimate of pinocytosis in experimental acute hypertension. Acta Neuropathol (Berl) 1979;46:107–116. doi: 10.1007/BF00684811. [DOI] [PubMed] [Google Scholar]
- 68.Cipolla MJ, Crete R, Vitullo L, Rix RD. Transcellular transport as a mechanism of blood-brain barrier disruption during stroke. Front Biosci. 2004;9:777–785. doi: 10.2741/1282. [DOI] [PubMed] [Google Scholar]
- 69.Blum MS, Toninelli E, Anderson JM, Balda MS, Zhou J, O’Donnel L, Pardi R, Bender JR. Cytoskeletal rearrangement mediates human microvascular endothelial tight junction modulation by cytokines. Am J Physiol. 1997;273:H286–H294. doi: 10.1152/ajpheart.1997.273.1.H286. [DOI] [PubMed] [Google Scholar]
- 70.Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 71.Kniesel U, Wolburg H. Tight junctions of the blood-brain barrier. Cell Mol Neurobiol. 2000;20:57–76. doi: 10.1023/A:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hofman P, Blaauwgeers HG, Tolentino MJ, Adamis AP, Nunes Cardozo BJ, Vrensen GF, Schlingemann RO. VEGF-A induced hyperpermeability of blood-retinal barrier endothelium in vivo is predominantly associated with pinocytotic vesicular transport and not with formation of fenstrations. Curr Eye Res. 2000;21:637–645. [PubMed] [Google Scholar]
- 73.Fischer S, Wiesnet M, Marti HH, Renz D, Schaper W. Simultaneous activation of several second messengers in hypoxia-induced hyperpermeability of brain derived endothelial cells. J Cell Physiol. 2004;198:359–69. doi: 10.1002/jcp.10417. [DOI] [PubMed] [Google Scholar]
- 74.Amburgey OA, Chapman AC, May V, Bernstein IM, Cipolla MJ. Plasma from preeclamptic women increases blood-brain barrier permeability: role of vascular endothelial growth factor signaling. Hypertension. 2010;56(5):1003–1008. doi: 10.1161/HYPERTENSIONAHA.110.158931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cipolla MJ. Integrated Systems Physiology – from Molecule to Function. Morgan & Claypool Life Sciences Publishers; San Rafael, CA: 2009. The Cerebral Circulation. [PubMed] [Google Scholar]
- 76.Matthiesen L, Berg G, Ernerudh J, Ekerfelt C, Jonsson Y, Sharma S. Immunology of preeclampsia. Chem Immunol Allergy. 2005;89:49–61. doi: 10.1159/000087912. [DOI] [PubMed] [Google Scholar]
- 77.Cackovic M, Buhimschi CS, Zhao G, Funai EF, Norwitz ER, Kuczynski E, Lockwood CJ, Buhimschi IA. Fractional excretion of tumor necrosis factor-alpha in women with severe preeclampsia. Obstet Gynecol. 2008;112(1):93–100. doi: 10.1097/AOG.0b013e31817c4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jonsson Y, Rubèr M, Matthiesen L, Berg G, Nieminen K, Sharma S, Ernerudh J, Ekerfelt C. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70(1–2):83–91. doi: 10.1016/j.jri.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 79.Pan W, Csernus B, Kastin AJ. Upregulation of p55 and p75 receptors mediating TNF-alpha transport across the injured blood-spinal cord barrier. J Mol Neurosci. 2003;21(2):173–184. doi: 10.1385/JMN:21:2:173. [DOI] [PubMed] [Google Scholar]
- 80.Pan W, Kastin AJ. Tumor necrosis factor and stroke: role of the blood-brain barrier. Prog Neurobiol. 2007;83(6):363–374. doi: 10.1016/j.pneurobio.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oppenheim JJ, Feldmann M. Introduction to the role of cytokines in innate host defense and adaptive immunity. In: Oppenheim JJ, Feldmann M, editors. Cytokine Reference. Vol. 1. New York: Academic Press; 2001. pp. 3–20. [Google Scholar]
- 82.Riazi K, Galic MA, Kuzmiski JB, Ho W, Sharkey KA, Pittman QJ. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc Natl Acad Sci U S A. 2008;105(44):17151–17156. doi: 10.1073/pnas.0806682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factor-alpha signaling during peripheral organ inflammation. J Neurosci. 2009;29(7):2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rossi B, Angiari S, Zenaro E, Budui SL, Constantin G. Vascular inflammation in central nervous system diseases: adhesion receptors controlling leukocyte-endothelial interactions. J Leukoc Biol. 2010 Dec 17; doi: 10.1189/jlb.0710432. [DOI] [PubMed] [Google Scholar]
- 85.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;23;25(12):3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ishibashi K, Kuwahara M, Sasaki S. Molecular biology of aquaporins. Rev Physiol Biochem Pharmacolol. 2000;141:1–32. doi: 10.1007/BFb0119576. [DOI] [PubMed] [Google Scholar]
- 87.Agre P, Bonhivers M, Borgnia MJ. The aquaporins, blueprints for cellular plumbing systems. J Biol Chem. 1998;273:14659–14662. doi: 10.1074/jbc.273.24.14659. [DOI] [PubMed] [Google Scholar]
- 88.Verkman AS. Aquaporin water channels and endothelial cell function. J Anat. 2002;200:617–627. doi: 10.1046/j.1469-7580.2002.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nature Reviews Neuroscience. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- 90.Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem. 1994;269:5497–5500. [PubMed] [Google Scholar]
- 91.Jung JS, Bhat RV, Preston GM, Guggino WB, Baraban JM, Agre P. Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci USA. 1994;91:13052–13056. doi: 10.1073/pnas.91.26.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amiry-Moghaddam M, Xue R, Haug F-M, Neely JD, Bhardwaj A, Agre P, Adams ME, Froehner SC, Mori S, Ottersen OP. Alpha syntrophin deletion removes the perivascular but not the endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 2004;18(3):542–544. doi: 10.1096/fj.03-0869fje. [DOI] [PubMed] [Google Scholar]
- 94.Amiry-Moghaddam M, Otuska T, Hurn PD, Traystman RJ, Haug F-M, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci USA. 2003;100:2106–2111. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumors. J Neurol Neurosurg Psychiat. 2002;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taniguchi M, Yamashita T, Kumura E, Tamatani M, Kobayashi A, Yokawa T, Maruno M, Kato A, Ohnishi T, Kohmura E, Tohyama M, Yoshimine T. Induction of aquaporin-4 water channel mRNA after focal cerebral ischemia in rat. Mol Brain Res. 2000;78:131–137. doi: 10.1016/s0169-328x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 97.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nature Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 98.Vizuete ML, Venero JL, Vargas C, Ilundain AA, Echevarria M, Machado A, Cano J. Differential upregulation of aquaporin-4 mRNA expression in reactive astrocytes after brain injury: potential role in brain edema. Neurobiol Dis. 1999;6:245–258. doi: 10.1006/nbdi.1999.0246. [DOI] [PubMed] [Google Scholar]
- 99.Quick AM, Cipolla MJ. Pregnancy-induced upregulation of aquaporin-4 protein in brain and its role in eclampsia. FASEB J. 2005;19(2):170–175. doi: 10.1096/fj.04-1901hyp. [DOI] [PubMed] [Google Scholar]
- 100.Wiegman MJ, Bullinger LV, Kohlmeyer MM, Hunter TC, Cipolla MJ. Regional expression of aquaporin 1, 4, and 9 in the brain during pregnancy. Reprod Sci. 2008;15(5):506–516. doi: 10.1177/1933719107311783. [DOI] [PubMed] [Google Scholar]
- 101.Binder DK, Yao X, Verkman AS, Manley GT. Increased seizure duration in mice lacking aquaporin-4 water channels. Acta Neurochir Suppl. 2006;96:389–392. doi: 10.1007/3-211-30714-1_80. [DOI] [PubMed] [Google Scholar]
- 102.Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost. 2009;7(3):375–384. doi: 10.1111/j.1538-7836.2008.03259.x. [DOI] [PubMed] [Google Scholar]
- 103.Sankaralingam S, Xu Y, Sawamura T, Davidge ST. Increased lectin-like oxidized low-density lipoprotein receptor-1 expression in the maternal vasculature of women with preeclampsia: role for peroxynitrite. Hypertension. 2009;53(2):270–277. doi: 10.1161/HYPERTENSIONAHA.108.122630. [DOI] [PubMed] [Google Scholar]
- 104.Roberts JM, Hubel CA. Oxidative stress in preeclampsia. Am J Obstet Gynecol. 2004;190(5):1177–1178. doi: 10.1016/j.ajog.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 105.Shaner MD. Neurological Problems of Pregnancy. In: Bradley WG, Daroff RB, Fenichel GM, Marsden CD, editors. Neurology in Clinical Practice. 85. II. Butterworth-Heinemann; 2000. pp. 2257–2267. [Google Scholar]
- 106.Aukes AM, de Groot JC, Aarnoudse JG, Zeeman GG. Brain lesions several years after eclampsia. Am J Obstet Gynecol. 2009;200(5):504.e1–5. doi: 10.1016/j.ajog.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 107.Aukes AM, Wessel I, Dubois AM, Aarnoudse JG, Zeeman GG. Self-reported cognitive functioning in formerly eclamptic women. Am J Obstet Gynecol. 2007;197(4):365.e1–6. doi: 10.1016/j.ajog.2007.06.044. [DOI] [PubMed]
- 108.Postma IR, Wessel I, Aarnoudse JG, Zeeman GG. Neurocognitive functioning in women with a history of eclampsia: executive functioning and sustained attention. Am J Perinatol. 2010;27(9):685–690. doi: 10.1055/s-0030-1253099. [DOI] [PubMed] [Google Scholar]
- 109.Lucas MJ, Leveno KJ, Cunningham FG. A comparison of magnesium sulfate with phenytoin for the prevention of eclampsia. N Engl J Med. 1995;333:201–205. doi: 10.1056/NEJM199507273330401. [DOI] [PubMed] [Google Scholar]
- 110.Chien PFW, Khan KS, Arnott N. Magnesium sulphate in the treatment of eclampsia and pre-eclampsia: An overview of the evidence from randomised trials. Br J Obstet Gynaecol. 1996;103:1085–1091. doi: 10.1111/j.1471-0528.1996.tb09587.x. [DOI] [PubMed] [Google Scholar]
- 111.Duley L, Henderson-Smart D. Magnesium sulphate versus diazepam for eclampsia. Cochrane Database Syst Rev. 2003:4. doi: 10.1002/14651858.CD000127. [DOI] [PubMed] [Google Scholar]
- 112.Ramanathan J, Sibai BM, Pillai R, Angel JJ. Neuromuscular transmission studies in preeclamptic women receiving magnesium sulfate. Am J Obstet Gynecol. 1988;158:40–46. doi: 10.1016/0002-9378(88)90772-7. [DOI] [PubMed] [Google Scholar]
- 113.Pritchard JA, Cunningham FG, Pritchard SA. The Parkland Memorial Hospital protocol for treatment of eclampsia: Evalauation of 235 cases. Am J Obstet Gynecol. 1984;148:951–960. doi: 10.1016/0002-9378(84)90538-6. [DOI] [PubMed] [Google Scholar]
- 114.Altura BM, Altura BT, Carella A, Gebrewold A, Murakawa T, Nishio A. Mg2+ - Ca2+ interaction in contractility of vascular smooth muscle: Mg2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can J Physiol Pharmacol. 1987;65:729–745. doi: 10.1139/y87-120. [DOI] [PubMed] [Google Scholar]
- 115.Euser AG, Cipolla MJ. Resistance artery vasodilation to magnesium sulfate during pregnancy and the postpartum state. Am J Physiol Heart Circ Physiol. 2005;288:H1521–H1525. doi: 10.1152/ajpheart.00994.2004. [DOI] [PubMed] [Google Scholar]
- 116.Euser AG, Bullinger L, Cipolla MJ. Magnesium sulphate treatment decreases blood brain barrier permeability during acute hypertension in pregnant rats. Exp Physiol. 2008;93:254–261. doi: 10.1113/expphysiol.2007.039966. [DOI] [PubMed] [Google Scholar]
- 117.Hallak M, Berman RF, Irtenkauf SM, Janusz C, Cotton DB. Magnesium sulfate treatment decreases N-methyl-D-aspartate receptor binding in the rat brain: An autoradiographic study. J Soc Gynecol Invest. 1994;1:25–30. doi: 10.1177/107155769400100106. [DOI] [PubMed] [Google Scholar]