Summary
Background
Chromosome instability is thought to be a major contributor to cancer malignancy and birth defects. For balanced chromosome segregation in mitosis, kinetochores on sister chromatids bind and pull on microtubules emanating from opposite spindle poles. This tension contributes to the correction of improper kinetochore attachments and is opposed by the cohesin complex that holds the sister chromatids together. Normally, within minutes of alignment at the metaphase plate, chromatid cohesion is released, allowing each cohort of chromatids to move synchronously to opposite poles in anaphase, an event closely coordinated with mitotic exit.
Results
Here we show that during experimentally induced metaphase delay spindle pulling forces can cause asynchronous chromatid separation, a phenomenon we term “cohesion fatigue.” Cohesion fatigue is not blocked by inhibition of Plk1, a kinase essential for the “prophase pathway” of cohesin release from chromosomes or by depletion of separase, the protease that normally drives chromatid separation at anaphase. Cohesion fatigue is inhibited by drug-induced depolymerization of mitotic spindle microtubules and by experimentally increasing the levels of cohesin on mitotic chromosomes. In cells undergoing cohesion fatigue, cohesin proteins remain associated with the separated chromatids.
Conclusion
In cells arrested at metaphase, pulling forces originating from kinetochore-microtubule interactions can, with time, rupture normal sister chromatid cohesion. This cohesion fatigue, resulting in unscheduled chromatid separation in cells delayed at metaphase, constitutes a previously overlooked source for chromosome instability in mitosis and meiosis.
Introduction
In mitosis, before anaphase, sister chromatid cohesion is maintained by the cohesin complex. In vertebrates most cohesin is released early from chromosomes via the kinase-dependent “prophase pathway” [1–3]. Some cohesin is retained on chromosomes to keep sister chromatids attached during alignment at the metaphase plate. At metaphase, degradation of securin and cyclin B occurs, allowing activation of the protease separase [4]. Separase cleaves the RAD21/SCC1 component of the residual chromosome-associated cohesin allowing chromatid separation [5]. The drop in Cdk1 activity triggers the other events of mitotic exit [6].
Recently we reported that depletion of SKA3, a component of the Spindle and Kinetochore-Associated complex, induced cells to arrest at metaphase; thereafter chromatids began to separate asynchronously [7]. This phenotype superficially resembled studies where cohesin loading or stability was compromised [8–10]. However, unlike studies of cohesin defects, chromatid separation in SKA3-depleted cells required the intact mitotic spindle. Here we show that metaphase arrest, induced by several distinct approaches, results in unscheduled chromatid separation that is dependent on pulling forces from spindle microtubules. Thus delay or arrest at metaphase may be an unrecognized source for chromosome instability.
Results
Metaphase arrest results in chromosome scattering that is a consequence of unscheduled chromatid separation
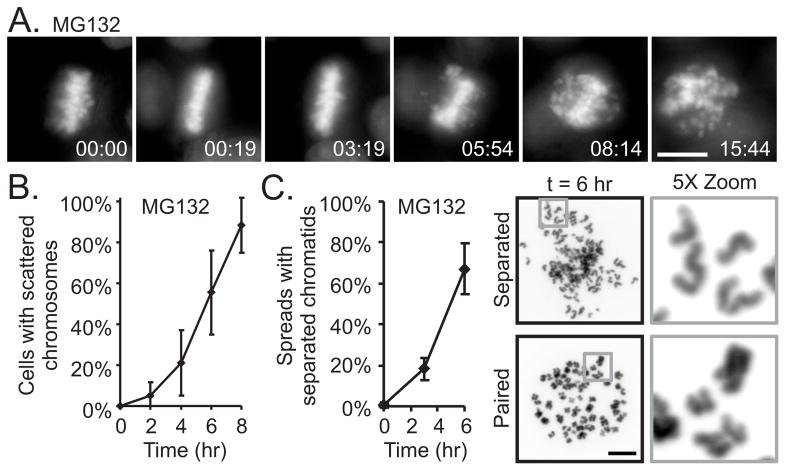
We treated HeLa cells expressing histone H2B-GFP with the proteasome inhibitor, MG132 and used video microscopy. As previously demonstrated, proteasome inhibitors induce metaphase arrest [11, 12]. However, we found that, subsequent to metaphase arrest, cells began to exhibit scattering of their chromosomes along the spindle. The timing of this scattering varied among cells with some exhibiting scattering after a few tens of minutes while others remained aligned at metaphase for hours (Figure 1A, 1B and Movie S1). Within individual cells, chromosome scattering occurred asynchronously and often was accompanied by continuous rotation of the entire spindle. (We defined a cell as exhibiting the scattered phenotype when approximately 10% of the chromosomes had moved irreversibly off the metaphase plate.)
Figure 1.
Metaphase arrest of HeLa H2B-GFP cells results in chromosome scattering that is a consequence of unscheduled chromatid separation. (A) Live cell imaging shows that a cell arrested at metaphase by proteasome inhibition (MG132) scatters chromosomes. (B) Analysis of chromosome movements from 8 live cell imaging experiments indicates that scattering after metaphase arrest induced by proteasome inhibition is asynchronous within the culture. (C) Chromosome spreads reveal that scattered chromosomes are individual chromatids. The appearance after MG132-mediated metaphase arrest of individual chromatids within chromosome spreads correlate with the time-dependent scattering of chromosomes observed by live cell imaging (3 experiments, n > 167 for each time point within each experiment). Bars = 10 μm, Time = hr:min, Error bars = standard deviation (see also Figure S1, S2A and Movie S1)
To determine the nature of the scattered chromosomes, we examined chromosome spreads from cells treated with MG132 and found a time-dependent increase in the percentage of cells with separated chromatids (Figure 1C). Thus the phenotype of chromosome scattering seen during metaphase arrest is a consequence of unscheduled chromatid separation, a phenomenon we term “cohesion fatigue.” As we previously found [7], cells depleted of SKA3 also arrest at metaphase and then scatter their chromatids (Figure S1A and Movie S1). The same outcome resulted after depletion of any SKA complex components either alone or in combination (data not shown). RPE1 cells, which are immortal but not transformed, showed the same response to MG132 treatment or SKA3 depletion (Figure S1B). As reported previously by others [13], we found that MG132 induced the formation of multipolar spindles. Even within multipolar spindles, chromatids could still scatter (Figure S1C). As an additional test we induced metaphase arrest by depleting the APC/C-activator, Cdc20 by RNAi. During arrest at metaphase, chromosomes in these Cdc20-depleted cells experienced cohesion fatigue (Figure S2A).
Separase depletion does not block cohesion fatigue
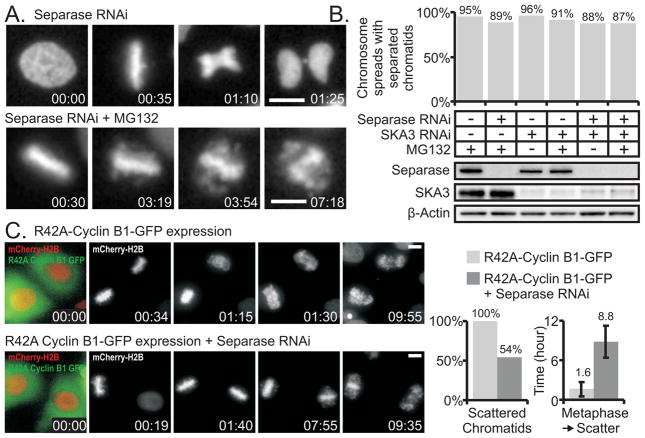
We depleted separase with RNAi and arrested cells in metaphase by treatment with MG132 or by SKA3 depletion. Greater than 95% of cells in cultures transfected only with siRNA to separase displayed abortive anaphases with nearly all cells exhibiting a cut phenotype or lagging chromosomes during anaphase (Figure 2A and Movie S2). Immunoblotting also indicated that separase depletion by RNAi was effective. However, separase depletion did not diminish chromatid scattering in cells arrested at metaphase by proteasome inhibition or by SKA3 depletion as assessed by chromosome spreads prepared 6 hours post mitotic arrest and by video microscopy (Figure 2B and Movie S3). Additionally we co-depleted the APC/C-activator, Cdc20, and separase by RNAi to both induce metaphase arrest and suppress separase activity. During arrest at metaphase, chromosomes underwent scattering that was not blocked by co-depletion of separase (Figure S2B and S2C).
Figure 2.
Depletion of separase does not block cohesion fatigue. (A) Upper Panels: Live cell imaging confirms loss of separase activity mediated by RNAi; cells fail to appropriately segregate sister chromatids during anaphase and cytokinesis results in a cut phenotype. Lower Panels: Separase depletion does not prevent scattering in cells arrested at metaphase by proteasome inhibition (MG132). (B) Chromosome spreads and cell extracts were prepared from arrested mitotic cells that had been transfected with non-targeting, separase-targeting, or SKA3-targeting siRNA duplexes. These cells had arrested in mitosis due to proteasome inhibition and/or SKA3 depletion. Graphically represented are the percentages of chromosome spreads classified as separated. Chromosome spreads reveal that separase depletion does not prevent cohesion fatigue in cells arrested by proteasome inhibition or SKA3 depletion. The number of spreads tallied for each condition ranged between 164 and 270. Western blotting indicates successful depletion of separase and SKA3 by RNAi. (C) Separase is required for early but not late chromatid separation induced by overexpression of degradation-resistant cyclin B. Upper panels: HeLa H2B-mCherry/R42A-CyclinB-GFP cells delay at metaphase for durations less than 1.6 hours, separate sister chromatids, and remain arrested in mitosis. Lower panels: HeLa H2B-mCherry/R42A-CyclinB-GFP cells lacking separase delay for several hours at metaphase and subsequently experience cohesion fatigue. HeLa H2B-mCherry/R42A-CyclinB-GFP cells separated sister chromatids with an average metaphase to scatter duration of 1.6 ± 1.1 hours. In contrast, 46% of HeLa H2B-mCherry/R42A-CyclinB-GFP cells lacking separase maintained metaphase alignment of chromosomes; the remaining 54% separated sister chromatids after an extended metaphase arrest. Bars = 10 μm, Time = hr:min, Error bars = standard deviation (see also Figure S2B, S2C and Movies S2, S3, and S4)
Separase is required for early but not late chromatid separation induced by overexpression of degradation-resistant cyclin B1
Moderate expression of degradation-resistant forms of cyclin B1 was previously shown to induce a mitotic delay at metaphase [14, 15]. However, expression of degradation-resistant cyclin B1 allows proteolysis of the separase inhibitor, securin and may thus activate separase [14]. We found that expression of degradation-resistant cyclin B1 generated a metaphase delay of 1.6 ± 1.1 hours, which was followed by chromatid scattering. However, when degradation-resistant cyclin B1 was expressed in cells where separase was depleted, the metaphase delay was greatly prolonged, and scattering typically occurred after several hours (Figure 2C and Movie S4). Thus in cells delayed at metaphase by degradation-resistant cyclin B1, early chromatid scattering was separase dependent; in contrast, cohesion fatigue (later chromatid scattering) was not separase dependent.
Inhibition of Plk1 in metaphase-arrested cells does not block cohesion fatigue
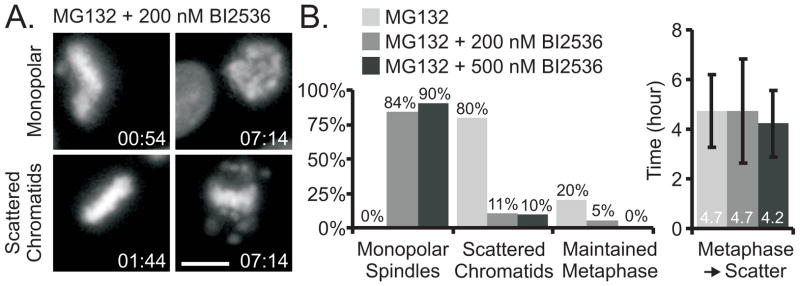
In early mitosis most cohesin is released from chromosome arms through a mechanism that requires Plk1. Cohesion fatigue might depend on continued activity of this Plk1-mediated release of cohesin. To address this possibility, we arrested cells at metaphase with MG132 and treated them with BI2536, a small molecule inhibitor of Plk1. As previously reported, in most cells BI2536 caused collapse of metaphase spindles forming monopoles (Figure 3A upper row, Movie S5) [16]. However, some metaphase spindles remained bipolar, and most of these exhibited scattering (Figure 3A, lower row, Figure 3B and Movie S5). We also treated MG132-arrested, metaphase cells with a structurally distinct Plk1 inhibitor, DAP81 [17]. Again monopolar spindles formed in most cells but some retained bipolarity and underwent scattering (Figure S3A and Movie S5). To test whether Plk1 kinase activity was equally inhibited in monopolar and bipolar cells, we treated cells with the Plk1 inhibitors, then fixed and labeled them with antibody to phospho-serine189 of Cdc25C, a known mitotic Plk1 target site [18]. Quantification of labeling revealed no significant differences between monopolar and bipolar cells in labeling with the phospho-specific antibody (Figure S3B). Thus cohesion fatigue in bipolar spindles does not appear to require continued activity of the Plk1-mediated prophase pathway.
Figure 3.
Inhibition of Plk1 in metaphase-arrested cells does not block cohesion fatigue. (A) Live cell imaging indicated that HeLa H2B-GFP cells arrested in mitosis by proteasome inhibition (MG132) and treated with the Plk1 inhibitor, BI2536 at 200 or 500 nM, either formed monopolar spindles, or they maintained metaphase arrest that typically led to cohesion fatigue. (B) Quantification of HeLa H2B-GFP cells treated with MG132 and BI2536: Cells treated with only MG132 maintained bipolar spindles; 80% of these cells experienced cohesion fatigue with an average metaphase to scatter duration of 4.7 ± 1.4 hours. While the majority of cells treated with MG132 and BI2536 formed monopolar spindles, some maintained bipolar spindles, most of which experienced cohesion fatigue with an average metaphase-to-scatter duration similar to that of cells in MG132 alone. Bars = 10 μm, Time = hr:min, Error bars = standard deviation (see also Figure S3 and Movie S5)
Manipulating cohesin levels on chromosomes affects the timing of chromatid separation
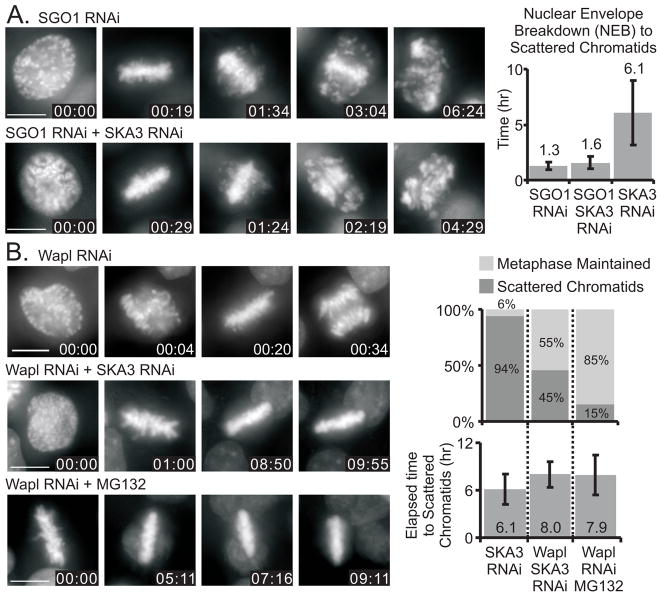
Shugoshin 1 (SGO1) is a protein that protects centromeric cohesin from premature release during early mitosis [8, 10, 19]. Cells depleted of SGO1 form transient metaphase plates but then undergo asynchronous chromatid separation and arrest in M phase [8]. We found that cells depleted of SGO1 or depleted of both SGO1 and SKA3 aligned chromosomes at the metaphase plate, but subsequently underwent scattering after a relatively short time compared to cells depleted of only SKA3 (Figure 4A, Figure S1A). Thus SGO1-dependent protection of cohesin extends the time that chromatids are able to maintain cohesion at metaphase.
Figure 4.
Manipulating cohesin levels on chromosomes affects the timing of chromosome scattering. (A) Upper panels: HeLa H2B-GFP cells depleted of SGO1 to reduce cohesin levels on mitotic chromosomes achieve metaphase alignment and then separate chromatids with an average duration from nuclear envelope breakdown (NEB) to scatter of 1.3 ± 0.3 hours. Lower panels: HeLa H2B-GFP cells depleted of both SGO1 and SKA3 separate chromatids in an average duration from NEB to scatter of 1.6 ± 0.6 hours. For comparison the graph also shows that cells depleted only of SKA3 separate chromatids after metaphase arrest with an average duration from NEB to scatter of 6.1 ± 2.9 hours. (B) Upper panels: HeLa H2B-GFP cells depleted of Wapl proceed through mitosis. Middle and lower panels: Depletion of Wapl inhibits chromatid scattering in SKA3-depleted or MG132-treated cells. In Ska3-depleted cells 94% underwent chromatid scattering. The percentage was reduced to 45% by co-depletion of Wapl, and depletion of Wapl reduced chromatid scattering to just 15% in MG132-treated cells. 6% of cells depleted only of SKA3 maintain metaphase alignment. For Wapl-depleted cells that did undergo scattering, the average time from metaphase to scattering was also increased. Bars = 10 μm, Time = hr:min, Error bars = standard deviation
Depletion of the protein Wapl increases cohesin levels on sister chromatids [20, 21]. As shown previously [21], RNAi-mediated depletion of Wapl does not block progression through mitosis (Figure 4B). While nearly all cells depleted of SKA3 alone underwent scattering, over 50% of the cells co-depleted of Wapl and SKA3 remained arrested at metaphase for the duration of observation (Figure 4B). Similar results were obtained when Wapl depletion was analyzed in cells arrested at metaphase with MG132 (Figure 4B). Thus increasing chromosome cohesin by Wapl depletion inhibits or delays chromatid scattering in cells arrested at metaphase.
Spindle microtubules are required for cohesion fatigue
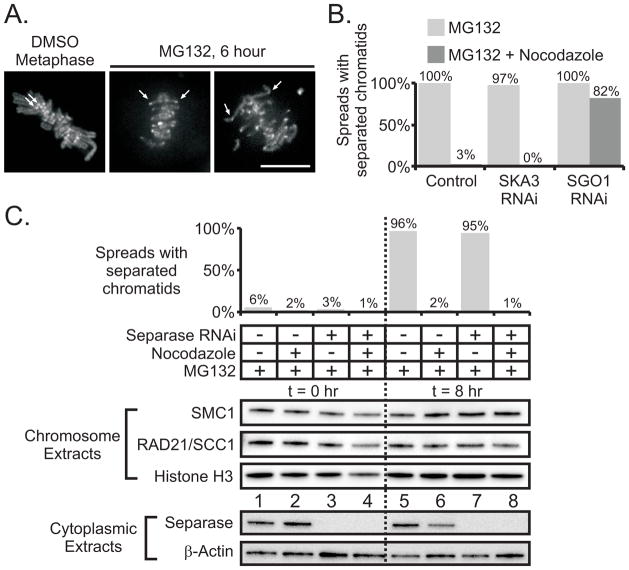
To study the dynamics of chromatid separation we imaged LLC-PK cells constitutively expressing topoisomerase IIα-GFP that serves to mark kinetochores and chromosome arms within living cells. Upon treatment with MG132 for several hours, many cells revealed intermediate stages of scattering in which the sister centromeres were far apart while distal parts of the arms remained coupled (Figure 5A and Movie S6). These intermediate stages of cohesion fatigue could also be detected after MG132 treatment in fixed Hela cells, labeled to detect kinetochores (data not shown). These results suggested that pulling forces from spindle microtubules might be required for cohesion fatigue. Consistent with this notion, the microtubule destabilizer, nocodazole, blocked chromatid separation assayed by chromosome spreads in cells treated with MG132 or depleted of SKA3 but not in cells depleted of SGO1 (Figure 5B).
Figure 5.
Spindle microtubules are required for cohesion fatigue and cohesin is retained on separated chromatids. (A) Live cell imaging of LLC-PK cells stably expressing GFP-topoisomerase II: Sister centromeres (arrows) remain close together at metaphase within untreated cells. In contrast within cells experiencing cohesion fatigue, sister centromeres are widely separated while regions of the chromosome arms away from centromeres remain joined. Bar = 10 μm. (B) Quantified results of chromosome spreads prepared from mitotic HeLa cells treated for 17 hours with MG132 or MG132 + Nocodazole. Cultures were transfected with non-targeting siRNA duplexes, siRNA duplexes targeting SKA3, or siRNA duplexes targeting SGO1. Under all RNAi conditions, nocodazole-free conditions resulted in greater than 97% of spreads containing separated chromatids. Nocodazole reduced chromatid separation to 3% or less in MG132-treated or MG132-treated/SKA3-depleted cells. In contrast, nocodazole caused only a slight reduction in chromatid separation for MG132-treated/SGO1-depleted cells. (C) Chromosome spreads and extracts were prepared from mitotic cells transfected with non-targeting or separase-targeting siRNA duplexes and held in mitosis by proteasome inhibition (MG132) in the presence or absence of intact microtubules (± nocodazole). The graphs shows the percentages of chromosome spreads classified as separated at t = 0 hr and t = 8 hr post proteasome inhibition. The number of spreads tallied for each condition ranged between 207 and 312. Greater than 95% of cells with intact spindle microtubules underwent cohesion fatigue regardless of separase depletion. Isolated chromosomes or chromatids were blotted for SMC1 and RAD21/SCC1; histone H3 serves as a loading control. Chromatid separation due to cohesion fatigue (t = 8 hr, MG132 only, lane 5) does not result in reduction of chromatin-associated SMC1 and RAD21/SCC1 in comparison to t = 0 (lane 1) or in comparison to chromosomes that remain paired (t = 8 hr, MG132 + nocodazole, lane 6). Depletion of separase did not affect the levels of chromatin-associated SMC1 or RAD21/SCC1. Western blotting of cytoplasmic extracts indicated successful depletion of separase by RNAi; β-actin serves as a loading control. (see also Figure S4 and Movie S6)
Formally the failure of chromatids to separate in nocodazole might be due to activation of the spindle checkpoint. The proteasome functions downstream of spindle checkpoint inactivation, hence cells lacking the checkpoint and treated with MG132 remain arrested at metaphase. In cells treated with MG132, the depletion of essential checkpoint protein, MAD2, did not block chromatid scattering (Figure S4). Thus the spindle checkpoint is not responsible for blocking chromatid separation in nocodazole. Chromatid separation during metaphase arrest requires intact pulling forces from spindle microtubules.
Cohesin complex is retained on separated chromatids after cohesion fatigue
HeLa cells transfected with non-targeting or separase-targeting siRNA duplexes were arrested in mitosis by proteasome inhibition (MG132) in the presence or absence of microtubule pulling forces (± nocodazole). We isolated chromatin fractions at time 0 and at 8 hours. As expected chromosome spreads revealed that, in the absence of nocodazole, most chromatids had separated at 8 hours (Figure 5C). However, western blotting of chromatin fractions revealed that the levels of SMC1 and RAD21/SCC1, two core components of the cohesin complex, were indistinguishable before and after cohesion fatigue. The depletion of separase had no effect on separation measured in chromosome spreads or on association of cohesin proteins with chromatin extracts. Thus, cohesin proteins remain associated with chromatids induced to separate due to pulling forces exerted at metaphase.
Discussion
Under normal circumstances in mitosis, metaphase is relatively brief. In cells arrested at metaphase by various treatments, asynchronous chromatid separation is a frequent, shared fate. This separation, which we term cohesion fatigue, appears to be gradual with the centromeres leading the separation followed by the arms. The cohesin complex appears to be the major molecular mechanism that resists the substantial pulling forces caused by kinetochore-microtubule interaction thus preventing premature chromatid separation. The strength of this resistance is dependent on the sum of the contributions of pathways that load, stabilize, and remove chromosome-associated cohesin. For example, when cohesin stability on chromosomes is compromised by depletion of SGO1, cells delay only briefly at metaphase before chromatids scatter. In contrast, when cohesin loading and stability is enhanced by depletion of Wapl, chromatids become more resistant than normal to separation. Other proteins or mechanisms such as DNA catenation may play auxiliary roles in sister chromatid cohesion.
Treatment of metaphase-arrested cells with chemical Plk1 inhibitors does not block scattering suggesting that continued activity of the prophase cohesin release pathway is not required. Similarly, siRNA-mediated depletion of separase protein does not block cohesion fatigue suggesting that separase is not essential. After undergoing cohesion fatigue, separated chromatids retain cohesin complex proteins. These findings are consistent with the idea that the canonical prophase and separase pathways are not required for cohesion fatigue. However, it remains possible that when active, prophase and separase pathways may augment chromatid separation in cells at metaphase. However, our evidence suggests that given sufficient time, spindle pulling forces alone are sufficient to overcome chromatid cohesion without wide scale release of cohesin proteins from chromosomes.
Why does sister chromatid cohesion eventually fail during metaphase arrest? Most simply the strong pulling forces of the kinetochores on the metaphase spindle microtubules may over time partially rupture the molecular linkages of the cohesin complex beginning at the kinetochores and progressing to the chromosome arms. Alternatively, over extended periods at metaphase, the poleward forces acting on the kinetochores may exploit cohesin protein dynamics, locking in momentary releases of the cohesion complex to drive chromatid separation. In this manner pulling forces from the kinetochores may ratchet chromatids apart. Eventually when the last elements of cohesion on chromosome arms are lost, chromosomes become irrevocably separated. This stochastic model for progressive chromatid separation is consistent with the asynchrony of chromatid scattering and with images showing intermediate stages where kinetochores are distantly separated while arm cohesion persists. These mechanisms may permit the uncoupling of sister chromatids while cohesin complex remains bound on the chromatids. Alternatively, the cohesin complex might briefly dissociate as chromatids disjoin but then reload on the separated sister chromatids. Finally it also possible that only a minor fraction of chromosome-associated cohesin is responsible for sister chromatid cohesion at metaphase, and loss of this minor fraction from cohesion-fatigued chromatids may have eluded our detection by western blotting.
Arrest of cells at metaphase with aligned chromosomes is not unprecedented as a mitotic perturbation. However, that metaphase arrest on its own, independent of its molecular cause can generate unscheduled chromatid separation and thus contribute to chromosome instability has not been previously explored. While we have used methods to impose strong metaphase arrest and cause massive chromatid separation, more subtle defects leading to short metaphase delays and chromatid separation in one or a few chromosomes may be more dangerous since the aneuploid cells that result may be more likely to survive. Recent evidence suggests that merotelic attachments (one kinetochore attached to both spindle poles) may cause both the numerical and structural chromosome deficiencies characteristically found in many human cancers [22, 23]. We find that single kinetochores on chromatids often become merotelically attached after fatigue-induced chromatid separation. Progression of cells containing single chromatids through further cell cycles may underlie the numerical and structural chromosome instability found in cancer.
Because many pathways affect the deposition, stabilization and timely release of cohesin from chromosomes, it is possible that disruption of normal pathways that regulate chromosome cohesin may contribute to chromosome instability in tumor cells. Accumulated defects in cohesin mechanics may accelerate tumor aneuploidy [24, 25]. An inability to extinguish the spindle checkpoint once chromosomes have reached metaphase alignment may induce a metaphase delay or arrest. Recently overexpression of HEC1 (Highly Expressed in Cancer 1), a key kinetochore component, has been found to hyperactivate the spindle checkpoint and induce tumor formation in mice [12]. Overexpression of the checkpoint protein MAD2 leads to mitotic arrest in both yeast and metazoans and has been reported to contribute to aneuploidy in mouse models and in human tumors [26–31].
Lastly cohesion fatigue may also have implications for chromosome instability in meiosis, particularly in oocytes. In humans and other mammals the first meiotic division takes place over several hours, oocytes then arrest at metaphase of the second meiotic division to await fertilization, often for many hours. Mouse oocytes show increased chromatid separation with time after ovulation [32]. Decreased cohesin levels in mouse oocytes were recently implicated in age-related aneuploidy in mouse oocytes [33, 34]. Thus spindle forces acting over long periods on kinetochores in both meiotic divisions coupled with defects in cohesin physiology may contribute to age-related increases of aneuploidy seen in human oocytes.
Supplementary Material
Highlights.
Metaphase delay can cause unscheduled, asynchronous chromatid separation termed “Cohesion Fatigue.”
Cohesion fatigue does not require Plk1 or separase activity but does require intact microtubules.
Cohesin remains associated with chromatids separated by cohesion fatigue.
Cohesion fatigue during metaphase delay may contribute to chromosome instability.
Acknowledgments
We thank Dorothea Rudolph of Boehringer Ingelheim, Tarun Kapoor, and Ulf Peters for BI2536, Tarun Kapoor and Ulf Peters for DAP81, Robert Benezra and Addgene for plasmid 20972 H2B-mCherry, Paul Clute and Jonathon Pines for Cyclin B-GFP and (R42A) Cyclin B-GFP plasmids, and Shang Cai and Claire Walczak for the HeLa H2B-mCherry cell line. We also thank Dean Dawson for discussion and Arshad Desai for sharing unpublished information. This work was supported by the National Institute of General Medical Sciences (R01GM50412) and by the McCasland Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sumara I, Vorlaufer E, Stukenberg PT, Kelm O, Redemann N, Nigg EA, Peters JM. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol Cell. 2002;9:515–525. doi: 10.1016/s1097-2765(02)00473-2. [DOI] [PubMed] [Google Scholar]
- 2.Hauf S, Roitinger E, Koch B, Dittrich CM, Mechtler K, Peters JM. Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol. 2005;3:419–432. doi: 10.1371/journal.pbio.0030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gimenez-Abian JF, Sumara I, Hirota T, Hauf S, Gerlich D, de la Torre C, Ellenberg J, Peters JM. Regulation of sister chromatid cohesion between chromosome arms. Curr Biol. 2004;14:1187–1193. doi: 10.1016/j.cub.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 4.Stemmann O, Zou H, Gerber SA, Gygi SP, Kirschner MW. Dual inhibition of sister chromatid separation at metaphase. Cell. 2001;107:715–726. doi: 10.1016/s0092-8674(01)00603-1. [DOI] [PubMed] [Google Scholar]
- 5.Hauf S, Waizenegger IC, Peters JM. Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science. 2001;293:1320–1323. doi: 10.1126/science.1061376. [DOI] [PubMed] [Google Scholar]
- 6.Potapova TA, Daum JR, Pittman BD, Hudson JR, Jones TN, Satinover DL, Stukenberg PT, Gorbsky GJ. The reversibility of mitotic exit in vertebrate cells. Nature. 2006;440:954–958. doi: 10.1038/nature04652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daum JR, Wren JD, Daniel JJ, Sivakumar S, McAvoy JN, Potapova TA, Gorbsky GJ. Ska3 is required for spindle checkpoint silencing and the maintenance of chromosome cohesion in mitosis. Curr Biol. 2009;19:1467–1472. doi: 10.1016/j.cub.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuinness BE, Hirota T, Kudo NR, Peters JM, Nasmyth K. Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 2005;3:434–449. doi: 10.1371/journal.pbio.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rankin S, Ayad NG, Kirschner MW. Sororin, a substrate of the anaphase-promoting complex, is required for sister chromatid cohesion in vertebrates. Mol Cell. 2005;18:185–200. doi: 10.1016/j.molcel.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Salic A, Waters JC, Mitchison TJ. Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell. 2004;118:567–578. doi: 10.1016/j.cell.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Genschik P, Criqui MC, Parmentier Y, Derevier A, Fleck J. Cell cycle -dependent proteolysis in plants. Identification Of the destruction box pathway and metaphase arrest produced by the proteasome inhibitor mg132. Plant Cell. 1998;10:2063–2076. doi: 10.1105/tpc.10.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojcik C, Schroeter D, Stoehr M, Wilk S, Paweletz N. An inhibitor of the chymotrypsin-like activity of the multicatalytic proteinase complex (20S proteasome) induces arrest in G2-phase and metaphase in HeLa cells. Eur J Cell Biol. 1996;70:172–178. [PubMed] [Google Scholar]
- 13.Ehrhardt AG, Sluder G. Spindle pole fragmentation due to proteasome inhibition. J Cell Physiol. 2005;204:808–818. doi: 10.1002/jcp.20335. [DOI] [PubMed] [Google Scholar]
- 14.Chang DC, Xu N, Luo KQ. Degradation of cyclin B is required for the onset of anaphase in Mammalian cells. J Biol Chem. 2003;278:37865–37873. doi: 10.1074/jbc.M306376200. [DOI] [PubMed] [Google Scholar]
- 15.Wolf F, Wandke C, Isenberg N, Geley S. Dose-dependent effects of stable cyclin B1 on progression through mitosis in human cells. EMBO J. 2006;25:2802–2813. doi: 10.1038/sj.emboj.7601163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenart P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters JM. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 17.Peters U, Cherian J, Kim JH, Kwok BH, Kapoor TM. Probing cell-division phenotype space and Polo-like kinase function using small molecules. Nat Chem Biol. 2006;2:618–626. doi: 10.1038/nchembio826. [DOI] [PubMed] [Google Scholar]
- 18.Toyoshima-Morimoto F, Taniguchi E, Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 2002;3:341–348. doi: 10.1093/embo-reports/kvf069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitajima TS, Hauf S, Ohsugi M, Yamamoto T, Watanabe Y. Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting Shugoshin localization. Curr Biol. 2005;15:353–359. doi: 10.1016/j.cub.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 20.Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, Mechtler K, Peters JM. Wapl controls the dynamic association of cohesin with chromatin. Cell. 2006;127:955–967. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 21.Gandhi R, Gillespie PJ, Hirano T. Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr Biol. 2006;16:2406–2417. doi: 10.1016/j.cub.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerrero AA, Gamero MC, Trachana V, Futterer A, Pacios-Bras C, Diaz-Concha NP, Cigudosa JC, Martinez AC, van Wely KH. Centromere-localized breaks indicate the generation of DNA damage by the mitotic spindle. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4159–4164. doi: 10.1073/pnas.0912143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez AC, van Wely KH. Centromere fission, not telomere erosion, triggers chromosomal instability in human carcinomas. Carcinogenesis. 2011 doi: 10.1093/carcin/bgr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barber TD, McManus K, Yuen KW, Reis M, Parmigiani G, Shen D, Barrett I, Nouhi Y, Spencer F, Markowitz S, et al. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci U S A. 2008;105:3443–3448. doi: 10.1073/pnas.0712384105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer R, Fofanov V, Panigrahi A, Merchant F, Zhang N, Pati D. Overexpression and mislocalization of the chromosomal segregation protein separase in multiple human cancers. Clin Cancer Res. 2009;15:2703–2710. doi: 10.1158/1078-0432.CCR-08-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He X, Patterson TE, Sazer S. The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex. Proc Natl Acad Sci U S A. 1997;94:7965–7970. doi: 10.1073/pnas.94.15.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sotillo R, Hernando E, Diaz-Rodriguez E, Teruya-Feldstein J, Cordon-Cardo C, Lowe SW, Benezra R. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell. 2007;11:9–23. doi: 10.1016/j.ccr.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sotillo R, Schvartzman JM, Socci ND, Benezra R. Mad2-induced chromosome instability leads to lung tumour relapse after oncogene withdrawal. Nature. 2010;464:436–440. doi: 10.1038/nature08803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Yin F, Du Y, Du W, Chen B, Zhang Y, Wu K, Ding J, Liu J, Fan D. MAD2 as a key component of mitotic checkpoint: A probable prognostic factor for gastric cancer. Am J Clin Pathol. 2009;131:793–801. doi: 10.1309/AJCPBMHHD0HFCY8W. [DOI] [PubMed] [Google Scholar]
- 30.Yu L, Guo WC, Zhao SH, Tang J, Chen JL. Mitotic arrest defective protein 2 expression abnormality and its clinicopathologic significance in human osteosarcoma. APMIS. 2010;118:222–229. doi: 10.1111/j.1600-0463.2009.02583.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang SH, Xu AM, Chen XF, Li DH, Sun MP, Wang YJ. Clinicopathologic significance of mitotic arrest defective protein 2 overexpression in hepatocellular carcinoma. Hum Pathol. 2008;39:1827–1834. doi: 10.1016/j.humpath.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Mailhes JB, Young D, London SN. Postovulatory ageing of mouse oocytes in vivo and premature centromere separation and aneuploidy. Biol Reprod. 1998;58:1206–1210. doi: 10.1095/biolreprod58.5.1206. [DOI] [PubMed] [Google Scholar]
- 33.Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol. 2010;20:1522–1528. doi: 10.1016/j.cub.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lister LM, Kouznetsova A, Hyslop LA, Kalleas D, Pace SL, Barel JC, Nathan A, Floros V, Adelfalk C, Watanabe Y, et al. Age-related meiotic segregation errors in Mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol. 2010;20:1511–1521. doi: 10.1016/j.cub.2010.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.