Abstract
A 63-year-old woman, who presented with severe jaundice and elevated serum conjugated bilirubin level, denied alcohol and drug use and showed no evidence of viral hepatitis. Based on clinical and laboratory features, she was diagnosed with autoimmune hepatitis with primary biliary cirrhosis. Hematological and immunochemical assays, radiographic imaging, clinical examination, and liver biopsy were conducted. Laboratory results were the following: negative for fluorescence antinuclear antibody, negative for antismooth muscle antibodies but positive for antinuclear antibody (enzyme-linked immunosorbent assay) and antimitochondrial M2 antibody, high titers of serum globulin, and positive for cytomegalovirus IgM. Liver biopsy showed submassive lobular necrosis, inflammation with broad areas of parenchymal collapse, and chronic nonsuppurative destructive cholangitis. The patient responded well to corticosteroid therapy. This case might illustrate an association between cytomegalovirus infection and the occurrence of autoimmune hepatitis.
Keywords: autoimmune hepatitis, fluorescence antinuclear antibody, enzyme linked immuno sorbent assay, primary biliary cirrhosis, cytomegalovirus infection
Introduction
Autoimmune hepatitis (AIH) is characterized by chronic necroinflammation, manifesting as severe lobular necrosis and inflammation with broad areas of parenchymal collapse with no known cause. Immune reactions against host antigens are found to be the major pathologic mechanism. In fact, AIH might be associated with a number of other autoimmune diseases,1 but the exact mechanisms of onset remain unknown. In AIH, the immune system produces one or more types of autoantibodies. The most common are antinuclear antibodies (ANA), smooth muscle antibodies (SMA), and antibodies to liver and kidney microsomes (anti-LKM).1 Evidence suggests that AIH is induced by antibody-dependent cell-mediated cytotoxicity, which involves both antibody-mediated and cellular immunity against specific liver antigens on hepatocyte membranes.2 Several studies have documented the involvement of genetic factors including human leukocyte antigen (HLA) types DR3 and DR4.3 Some reports have described AIH cases in which a virus such as hepatitis A,4 hepatitis B,5 or Epstein–Barr6 was the causative agent.
Sera from patients with multiple autoimmune diseases have been found to have elevated cytomegalovirus (CMV) IgM titers, perhaps implying an as yet unidentified association between CMV and autoimmune diseases.7
We present a case of fluorescence ANA-negative AIH-associated primary biliary cirrhosis (PBC) that appears to have been triggered by reactivated CMV infection.
Case report
In 2010, a 63-year-old Japanese woman was admitted to a local hospital, after which she was transferred to our hospital for investigation of liver dysfunction. One month prior, she had experienced fever. She had no history of medicine or drug use. The findings of physical examinations of admission to our hospital were the following: body temperature, 36.8°C; pulse rate, 69 beats/minute and regular; blood pressure, 122/77 mmHg; respiratory rate, 16/minute; no sign of anemia at the conjunctiva palpebra, but an icteric finding was made at the conjunctiva bulbi. She had no skin rash or erythema. No flapping tremor or palmar erythema was observed. Superficial lymph nodes were not palpable. The lungs were normal on auscultation. A chest radiograph and an electrocardiogram were normal. The results of urinalysis were normal, with the exception of a positive result for urobilinogen. The results of biochemical analysis of the blood were the following: total bilirubin, 6.6 mg/dL (normal range: 0.2–1.2); direct bilirubin, 4.4 mg/dL (normal range: 0.0–0.2); aspartate aminotransferase, 344 IU/L (normal range: 10–40); alanine aminotransferase, 238 IU/L (normal range: 5–45); alkaline phosphatase, 572 IU/L (normal range: 100–325); γ-guanosine triphosphate, 129 IU/L (normal value: <30); IgG, 2,498 mg/dL (normal range: 870–1,700); and IgM, 279 mg/dL (normal range: 46–260). CMVIgM antibody was high; IgG antibody was above the normal ranges at admission (Table 1).
Table 1.
Laboratory data
| Erythrocyte sedimentation rate | 2 | /l hr |
| Blood count | ||
| White blood cells | 3.70 | 3.30–9.00 mL |
| Red blood cells | 3.84 | 3.38–500 × 104/mL |
| Hemoglobin | 11.4 | 11.5–15.0 g/dL |
| Hematocrit | 34.2 | 34.8%–45.0% |
| Platelets | 10.1 | 14–34 × 104/μL |
| Blood coagulation | ||
| Prothrombin time and international normalized ratio | 1.93 | 0.85–1.15 |
| Hepaplastin test | 26 | 70%–130% |
| Antithrombin III | 36 | 81–12 |
| Urinalysis | ||
| Gravity | 1.021 | 1.006–1.0 |
| pH | 6 | 4.5–8.0 |
| Glucose | (−) | (−) mg/dL |
| Protein | (−) | (−) mg/dL |
| Blood | (−) | (−) |
| Ketone | (−) | (−) |
| Bilirubin | (−) | (−) |
| Urobilinogen | (−) | (−) |
| Blood chemistry | ||
| Total bilirubin | 6.6 | 0.2–1.2 mg/dL |
| Direct bilirubin | 4.4 | 0.0–0.2 mg/dL |
| Aspartate aminotransferase | 344 | 10–40 IU/L |
| Alanine aminotransferase | 238 | 5–45 IU/L |
| γ-glutamyl transpeptidase | 129 | 30 IU/dL |
| Alkaline phosphatase | 572 | 100–325 IU/l |
| Cholinesterase | 112 | 200–452 IU/I |
| Total protein | 7.3 | 6.7–8.3 g/dL |
| Albumin | 3.3 | 3.8–5.3 g/dL |
| Total cholesterol | 133 | 120–219 mg/dL |
| Blood urea nitrogen | 6.3 | 18.0–20.0 |
| Creatinine | 0.62 | 0.47–0.71 mg/dL |
| Fasting blood sugar | 143 | 70–109 mg/dL |
| Immunological and serological data | ||
| Hepatitis B surface antigen | (−) | |
| Hepatitis C surface antigen | (−) | |
| Hepatitis A antibody | (−) | <0.71 |
| IgM Hbc antibody | (−) | <1.0 |
| Hepatitis C virus RNA | <1.2 | <1.2 loglU/mL |
| CMV-IgM | 2.127 (+) | <0.8 |
| CMV-IgG | 10 (+) | <0.8 |
| EBV-VCA IgM | <10 | <×10 |
| EBV-VCA IgG | 1280 | <×10 |
| EBV-DNA PCR | (−) | |
| IgG | 2496 | 870–1700 mg/dL |
| IgM | 272 | 46–260 mg/dL |
| Fluorescent antinuclear antibody test | <×40 | <×40 |
| Antinuclear antibody (ELISA) | 44.1 (+) | <20 index |
| Anti-smooth muscle antibody | <×20 (−) | <×20 |
| Liver kidney microsomal type 1 antibody | <1.7 (−) | <5.0 |
| Anti-mitochondria M2 antibody | 18.4 (+) | <7.0 |
| Thyroid stimulating hormone | 1.254 | 0.436–3.8 μlU/mL |
| Free thyroxine | 1.14 | 1.0–1.7 ng/dL |
| Thyroglobulin | 0.7 (+) | <0.3 U/mL |
| Rheumatoid factor | 25 (+) | <20 |
| Rheumatoid arthritis hemagglutinin <×40 | 20 (−) | <40 |
| Cyclic citrullinated peptide | 8 (−) | <4.5 U/mL |
| Lupus anticoagulant: dilute | 1.1 (−) | <1.3 |
| Russell viper venom time | ||
| DNA | 5 (−) | <6 |
| Classicla antineutrophil cytoplasmic antibody | <3.5 (−) | <3.5 U/mL |
| Protoplasmic-straining antineutrophil cytoplasmic antibodies | <1.3 (−) | <9.0 U/mL |
| Total complement activity | 37.1 (−) | 29.0–48.0 U/mL |
| Anti-Jo-1 antibody | <1.0 (−) | <1.0 |
| Anti-ribonucleoprotein antibody | 20.3 (+) | <17.0 |
| Anti-SS-A antibody | <5.0 (−) | <15 |
| Anti-SS-B antibody | <5.0 (−) | <15.0 |
| Anti-centromere antibody | 6.5 (−) | <10.0 |
| Anti-Scl-70 antibody | <5.0 (−) | <16 |
| HLA-DR haplotypes | DR-4 |
Abbreviations: CMV, cytomegalovirus; DNA, deoxyribonucleic acid; EBV, Epstein-Barr virus; Ig Immunoglobulin; PCR, polymerase chain reaction; RNA, ribonucleic acid; VCA, virus capsid antigen.
Tests for hepatitis A IgM antibody, hepatitis B surface antigen, and anti-hepatitis C virus antibody were negative. ANA was negative by fluorescence assay (<×40), but it was positive as measured using enzyme-linked immunosorbent assay (ELISA) (44.1 index; normal range <20), anti-mitochondria M2 antibody level was 18.4 (normal value: <7.0), anti-LKM antibody was negative, anti-SMA was <×20, and anti-dsDNA antibody (<7.0 IU/mL; normal value: <20 IU/mL) was negative. The test result for HLA-DR4 was positive. The level of anti-ribonucleoproteins antibody was 20.3 (normal value: <17) (Table 1).
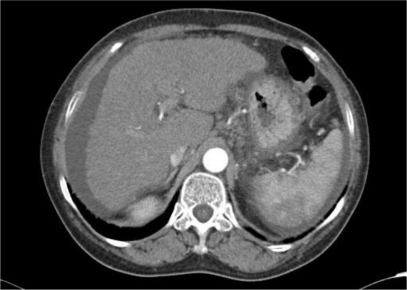
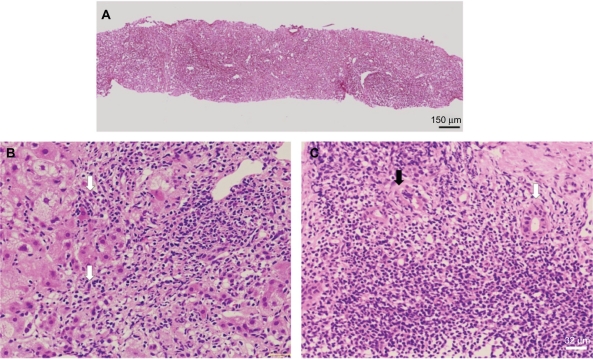
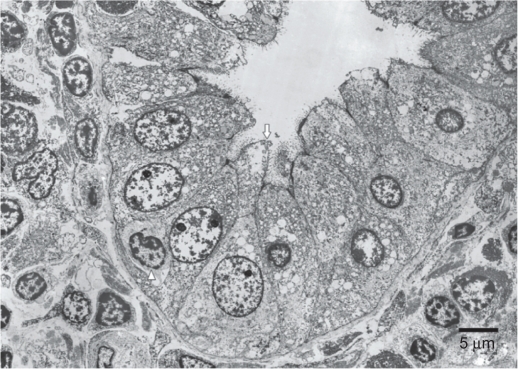
Abdominal ultrasonography and computed tomography were conducted to determine the cause of hepatic dysfunction. Ascites, but no abdominal mass, were detected (Figure 1). A needle liver biopsy was performed on the third hospital day. Histological examination showed severe lobular necrosis, inflammation with broad areas of parenchymal collapse, and both nondestructive and destructive cholangitis surrounded by many lymphocytes and plasma cells (Figure 2). Regarding results of electron microscopy, migrating lymphocytes were found more frequently in a medium-sized interlobular bile duct surrounded by many inflammatory cells. One lymphocyte had just migrated through the bile duct epithelial cells. Complete absence or partial loss of microvilli and microvillous bleb formation was visible in the same bile duct epithelial cell (Figure 3).
Figure 1.
Abdominal computed topography finding. Arterial phase computed tomography shows no abdominal mass, but ascites.
Figure 2.
Needle biopsy specimen of the liver.
Notes: A) Low magnification of silver impregnation staining for reticulin showing broad areas of parenchymal collapse. B) High magnification of a hematoxylin and eosin stained section showing interfacial hepatitis (arrow). C) High magnification of a hematoxylin and eosin stained section showing non-destructive cholangitis (black arrowhead) and destructive cholangitis (white arrowhead) surrounded by numerous plasma lymphocytic cells.
Figure 3.
Electron microscopy findings.
Notes: Migrating lymphocytes were found more frequently in a medium-sized interlobular bile duct surrounded by many inflammatory cells. One lymphocyte had just migrated through the bile duct epithelial cells. Complete absence or partial loss of microvilli and microvillous bleb formation was visible in the same bile duct epithelial cell. The arrow indicates bleb formation. The arrowhead denotes migrating lymphocytes through the bile duct epithelial cells.
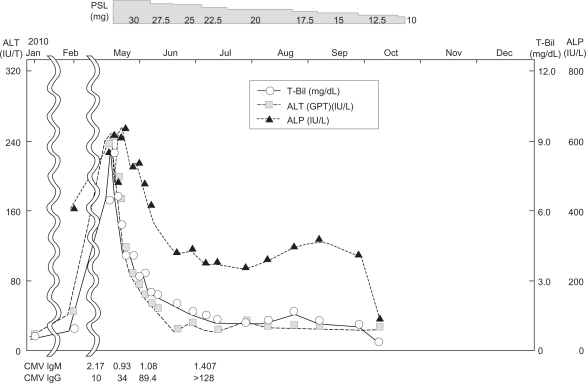
The clinical and pathological findings were highly suggestive of AIH, the score of which was calculated according to the system proposed by the revised International AIH Group.8 The negative ANA result assessed by fluorescent ANA was used in the calculation. The AIH score was 9, corresponding to nondiagnosis of AIH. The clinical/pathological findings of anti-mitochondria M2 antibody and chronic nonsuppurative destructive cholangitis indicated PBC. The final diagnosis might be overlapping syndrome of AIH and PBC preceding CMV infection. Her severe jaundice was improved, and her bilirubin and transaminase levels began to decline after administration of prednisolone for 3 days. They were normalized after 1 month (Figure 4).
Figure 4.
Clinical course.
Discussion
The role of viruses in AIH has been proposed repeatedly, but convincing evidence has linked two viruses – hepatitis A and Epstein–Barr virus – to type 1 of the disease, only in rare cases where a genetic predisposition exists and the viral infection occurs at the right time. In spite of an impressive amount of information conclusively showing molecular mimicry between cytochrome P4502D6 sequences (the target autoantigen of autoantibodies characteristic of AIH type 2) and viral (hepatitis C virus, herpes simplex virus 1, CMV, human T lymphotropic viruses 1 and 2) or bacterial (Salmonella typhimurium) antigens, no infectious agent has been clearly implicated for causing type 2 of the disease.9 At admission and at 2, 4, and 8 weeks after admission, the respective CMV IgM indices (normal value: <0.8) were 2.17, 0.93, 1.08, and 1.407; and IgG indices (normal value: <2.0) were 10, 34, 89.4, >128, and >128. Consequently, serial CMV Ig measurements demonstrated a slight increase of IgM at admission which declined gradually over time, whereas IgG increased with time to very high levels. These findings suggested CMV infection, consistent with a previous report that some patients with secondary CMV infection had long-lasting IgM antibodies, and that all had IgG antibodies to CMV.10
The present case is a rare case of negative fluorescence-ANA and low-titer ELISA-ANA AIH type 1 with coincident CMV infection. Furthermore, LKM antibodies were also negative. Although the clinical features strongly suggest AIH, calculation of AIH score using the result did not recommend a diagnosis of AIH from negative fluorescence-ANA. Immunofluorescence assay is the ANA detection method used in the calculation of AIH score.8 Although immunofluorescence is a sensitive test, it is laborious when testing large quantities of patient samples and is subject to errors of human interpretation and variation in fluorescence microscopes.11 The enzyme immunoassay test system is an excellent alternative to the immunofluorescence assay system for screening patient serum for the presence of clinically significant ANAs. The enzyme immunoassay efficiently screens large quantities of patient samples and reduces risks of human error.12 Both methods have different issues with respect to precision.
Our patient was positive for AMA, and liver biopsy showed a mixed portal inflammatory infiltrate with destructive and nondestructive cholangitis (Figure 3C). The reported frequency of AMA in patients with definitive AIH was 18 in 206 (7.5%) patients as assessed by immunoblotting.13 Therefore, AMA positivity is not a typical AIH finding. In the present case, the findings of AMA positivity and destructive biliary injury resembling chronic nonsuppurative destructive cholangitis in the biopsy strongly suggest a diagnosis of PBC.14 Although AIH and PBC might share some common histological features, the patterns seen on liver biopsy typically differ. Interface hepatitis or lobular hepatitis with infiltration of plasma cells is a characteristic of acute or chronic AIH.15 Nondestructive ductular reaction is detected more frequently in acute AIH than in chronic disease, but the destructive cholangitis lesions that are typical of PBC are not present.15,16 Moreover, for electron microscopy, the bile duct epithelial cell membrane facing the lymphocyte was disrupted. Lymphocyte – bile duct epithelial cell detachment is an important ultrastructural lesion associated with extensive bile duct destruction in PBC livers.17 Cellular immune mechanisms involving T cell reaction were thought to be significantly involved in the formation of chronic nonsuppurative destructive cholangitis and bile duct loss.18
In our case, the presence of portal inflammation and mixed destructive and nondestructive cholangitis changes, supported by other biochemical changes, led to a final diagnosis of overlapping syndrome of acute exacerbation AIH and PBC. This case is rare because these changes coincide or even precede CMV infection.
In the present case, ELISA assay for ANA and liver biopsy led to AIH and early treatment with prednisolone, which produced a good clinical outcome.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354(1):54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 2.Eggink HF, Houthoff HJ, Huitema S, Gips CH, Poppema S. Cellular and humoral immune reactions in chronic liver disease. I. Lymphocyte subsets in liver biopsies of patients with untreated idiopathic autoimmune hepatitis, chronic active hepatitis B and primary biliary cirrhosis. Clin Exp Immunol. 1982;50(1):17–24. [PMC free article] [PubMed] [Google Scholar]
- 3.Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. DR human leukocyte antigens and disease severity in chronic hepatitis C. J Hepatol. 1996;24(6):666–673. doi: 10.1016/s0168-8278(96)80261-3. [DOI] [PubMed] [Google Scholar]
- 4.Vento S, Garofano T, Di Perri G, Dolci L, Concia E, Bassetti D. Identification of hepatitis A virus as a trigger for autoimmune chronic hepatitis type I in susceptible individuals. Lancet. 1991;337(8751):1183–1187. doi: 10.1016/0140-6736(91)92858-y. [DOI] [PubMed] [Google Scholar]
- 5.Laskus T, Slusarczyk J. Autoimmune chronic active hepatitis developing after acute type B hepatitis. Dig Dis Sci. 1989;34(8):1294–1297. doi: 10.1007/BF01537282. [DOI] [PubMed] [Google Scholar]
- 6.Vento S, Guella L, Mirandola F, et al. Epstein–Barr virus as a trigger for autoimmune hepatitis in susceptible individuals. Lancet. 1995;346(8975):608–609. doi: 10.1016/s0140-6736(95)91438-2. [DOI] [PubMed] [Google Scholar]
- 7.Barzilai O, Sherer Y, Ram M, Izhaky D, Anaya JM, Shoenfeld Y. Epstein–Barr virus and cytomegalovirus in autoimmune diseases: are they truly notorious? A preliminary report. Ann N Y Acad Sci. 2007;1108:567–577. doi: 10.1196/annals.1422.059. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31(5):929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 9.Vento S, Cainelli F. Is there a role for viruses in triggering autoimmune hepatitis? Autoimmun Rev. 2004;3(1):61–69. doi: 10.1016/S1568-9972(03)00053-3. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen SL, Sørensen I, Andersen HK. Kinetics of specific immunoglobulins M, E, A, and G in congenital, primary, and secondary cytomegalovirus infection studied by antibody-capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1988;26(4):654–661. doi: 10.1128/jcm.26.4.654-661.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bridges AJ, Anderson JD, McKay J, Wang G, Johnson J, Sharp GC. Antinuclear antibody testing in a referral laboratory. Lab Med. 1993;24(6):345–349. [Google Scholar]
- 12.Jaskowski TD, Schroder C, Martins TB, Mouritsen CL, Litwin CM, Hill HR. Screening for antinuclear antibodies by enzyme immunoassay. Am J Clin Pathol. 1996;105(4):468–473. doi: 10.1093/ajcp/105.4.468. [DOI] [PubMed] [Google Scholar]
- 13.Farias AQ, Goncalves LL, Bittencourt PL, et al. Applicability of the IAIHG scoring system to the diagnosis of antimitochondrial/anti-M2 seropositive variant form of autoimmune hepatitis. J Gastroenterol Hepatol. 2006;21(5):887–893. doi: 10.1111/j.1440-1746.2006.04130.x. [DOI] [PubMed] [Google Scholar]
- 14.Scheuer P. Primary biliary cirrhosis. Proc R Soc Med. 1967;60(12):1257–1260. doi: 10.1177/003591576706001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czaja AJ, Carpenter HA. Autoimmune hepatitis with incidental histologic features of bile duct injury. Hepatology. 2001;34(4 Pt 1):659–665. doi: 10.1053/jhep.2001.27562. [DOI] [PubMed] [Google Scholar]
- 16.Iwai M, Jo M, Ishii M, Mori T, Harada Y. Comparison of clinical features and liver histology in acute and chronic autoimmune hepatitis. Hepatol Res. 2008;38(8):784–789. doi: 10.1111/j.1872-034X.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 17.Tobe K. Electron microscopy of liver lesions in primary biliary cirrhosis. II. A bile duct with chronic non-suppurative destructive cholangitis. Acta Pathol Jpn. 1982;32(2):345–357. [PubMed] [Google Scholar]
- 18.Harada K, Nakanuma Y. Molecular mechanisms of cholangiopathy in primary biliary cirrhosis. Med Mol Morphol. 2006;39(2):55–61. doi: 10.1007/s00795-006-0321-z. [DOI] [PubMed] [Google Scholar]