Highlights
► We mapped influenza HA antibodies using a robust yeast display system. ► The full-length H5 subtype HA was expressed on yeast in the proper conformation. ► Mutant library of yeast-displayed HA1 was used to fine map anti-HA mAbs epitopes. ► The potent neutralizing NR2728 mAb interacts with the receptor binding site. ► H5-2A mAb mapped specifically to a highly conserved epitope only found on H5 HA.
Keywords: Yeast surface display, Fine epitope mapping, Influenza, H5N1
Abstract
Highly pathogenic H5N1 avian influenza viruses pose a debilitating pandemic threat. Thus, understanding mechanisms of antibody-mediated viral inhibition and neutralization escape is critical. Here, a robust yeast display system for fine epitope mapping of viral surface hemagglutinin (HA)-specific antibodies is demonstrated. The full-length H5 subtype HA (HA0) was expressed on the yeast surface in a correctly folded conformation, determined by binding of a panel of extensively characterized neutralizing human monoclonal antibodies (mAbs). These mAbs target conformationally-dependent epitopes of influenza A HA, which are highly conserved across H5 clades and group 1 serotypes. By separately displaying HA1 and HA2 subunits on yeast, domain mapping of two anti-H5 mAbs, NR2728 and H5-2A, localized their epitopes to HA1. These anti-H5 mAb epitopes were further fine mapped by using a library of yeast-displayed HA1 mutants and selecting for loss of binding without prior knowledge of potential contact residues. By overlaying key mutant residues that impacted binding onto a crystal structure of HA, the NR2728 mAb was found to interact with a fully surface-exposed contiguous patch of residues at the receptor binding site (RBS), giving insight into the mechanism underlying its potent inhibition of virus binding. The non-neutralizing H5-2A mAb was similarly mapped to a highly conserved H5 strain-specific but poorly accessible location on a loop at the trimer HA interface. These data further augment our toolchest for studying HA antigenicity, epitope diversity and accessibility in response to natural and experimental influenza infection and vaccines.
1. Introduction
Rapid worldwide dissemination of highly pathogenic H5N1 avian influenza viruses among poultry and ongoing viral evolution through genetic drift and reassortment raise concerns of a potential influenza pandemic, which occurs when a new virus emerges globally and infects individuals who have little or no immunity [1]. Humoral immunity is the mainstay of protection during the course of influenza virus infection. Antibodies also provide a major contribution to vaccine-induced protection against influenza through multiple mechanisms [2]. The ability to map the putative binding sites of virus-specific monoclonal antibodies (mAbs) can improve our understanding of anti-viral immunity by providing precise insight into the variable or conserved nature of their epitopes, as well as their neutralization activity and neutralization escape potential. Influenza hemagglutinin (HA) is the major viral surface glycoprotein that mediates binding and entry of the virus to host cells and is a primary target of neutralizing antibody responses [2]. HA-specific antibodies can inhibit infection by blocking viral attachment to sialic acid residues of surface proteins on host cells, interfering with the structural transition of HA that triggers fusion activity in the endosome, or by simultaneous inhibition of attachment and virus-cell fusion [3]. Precise mapping of the HA epitopes targeted by neutralizing mAbs can define the structural requirements for protective anti-viral function and shed light on the mechanisms of antigenic drift in HA [2].
Despite the many important epitope mapping studies and various mapping methods that have been reported, a pressing need remains to expand strategies for accurately determining the regions recognized by newly discovered anti-influenza neutralizing antibodies. A strategy that can be implemented without prior knowledge of binding sites or the binding stoichiometry of these antibodies would be highly valuable. Recently, yeast has been shown to be a simple and feasible platform for display of various surface proteins for engineering and library screening applications [4]. Yeast can be easily grown on a large scale with simple nutritional demands and offers the advantage of providing eukaryotic post-translational modifications lacking in bacterial phage display [4], [5]. It has also proven possible on yeast to identify both linear and conformational antibody epitopes of complex proteins and to map them down to the energetically important amino acid residues [4], [6], [7].
The precursor/full-length HA protein (HA0) is post-translationally cleaved into two subunits, HA1 and HA2. In this study, a yeast surface display system for expression of HA0 (H5 subtype) is described. The proper folding of HA0 was confirmed by binding to a panel of human neutralizing mAbs that target a conformation-dependent stem region of HA containing a highly conserved epitope common to Group 1 influenza A viruses [3]. Domain mapping using separately displayed HA1 and HA2 subunits located the unknown epitopes of two anti-H5 mAbs (NR2728 and H5-2A) on HA1. This system was further used to fine epitope map these two anti-H5 mAbs by screening a random mutagenesis library of HA1 mutants by fluorescence-activated cell sorting (FACS). Clones with selective loss of binding to one of the two tested anti-HA mAbs were isolated and analyzed to identify specific residues that negatively impacted binding. When analyzed in the context of the HA crystal structure, clustering of amino acids led to the identification of the mAb epitopes.
2. Materials and methods
2.1. Cells, antibodies and viruses
The yeast cell strain Saccharomyces cerevisiae EBY100 was provided by Pacific Northwest National Laboratory (Richland, WA). The mAbs D7, D8, F10, G17, H40, A66, D80, E90, H5-2A, 11A and 80R were isolated from a human phage display library [3]. H5-2A was raised against monomeric HA0, and the other anti-H5 mAbs were obtained by panning with trimeric HA0 (highly pathogenic avian subtype H5N1 A/Vietnam/1203/04). NR2728, an HA-specific (A/Vietnam/1203/2004) mouse mAb, was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository (Manassas, VA). The reassortant H5N1 avian influenza virus VNH5N1-PR8/CDC-RG, which contains HA and NA genes derived from A/Vietnam/1203/2004 was obtained from the Center for Disease Control (CDC, Atanta, GA) and propagated in Madin Darby Canine Kidney (MDCK) cells at The New England Regional Center of Excellence for Biodefense and Emerging Infectious Diseases (NERCE) at Harvard Medical School (Boston, MA).
2.2. Construction of yeast surface display vectors
The full-length protein (HA0) and its subunits (HA1 and HA2) were cloned into a yeast display vector, pCTCON2 (from Dane Wittrup, Massachusetts Institute of Technology, Cambridge, MA). Briefly, HA0, HA1 or HA2 gene fragments of A/Vietnam/1203/2004 were PCR-amplified using the pAcGP67A-HA vector [3] as template and specific primers and cloned into the pCTCON2 yeast vector in-frame with the endogenous yeast Aga2p signal peptide and aga2 gene at the N-terminal end and cMyc at the C-terminal end (Fig. 1 A). The resulting plasmids were transformed into competent yeast using the Frozen-EZ Yeast Transformation Kit (Zymo Research, Irvine, CA), which were then grown on synthetic dextrose plus casein amino acids (SD-CAA) agar plates under dual selection (Ura- and Trp-) at 30 °C for 3 days. Single colonies were grown overnight in 5 ml SD-CAA medium (20 g/L glucose, 6.7 g/L yeast nitrogen base without amino acids, 5.4 g/L Na2HPO4, 8.6 g/L NaH2PO4·H2O and 5 g/L casamino acids) at 30 °C with shaking. Expressions of HA proteins were induced with galactose in SG-CAA medium (similar to SD-CAA medium except dextrose was replaced by galactose) at 20 °C for 3 days.
Fig. 1.

Display of full-length HA0 and its subunits on yeast cell surface. (A) Schematic of HA proteins displayed on the yeast surface (upper) and the gene construct expressing HA (full-length or each subunit) as a fusion protein with the Aga2 signal peptide (aga2SP), Aga2p and cMyc tag (lower). (B) Display of full-length proteins was confirmed by FACS after indirect immunofluorescent labeling of the C-terminal tag with an anti-cMyc mAb or of HA with a polyclonal anti-H5 antibody. (C) Western blot analysis of total yeast cells showing HA0 or its subunits expressed as a fusion to Aga2p at expected sizes. Lanes 1–3: HA0, HA1 and HA2, respectively.
2.3. FACS analysis of HA expression on yeast surface
Surface expression of the HA0 and its subunits were confirmed by FACS (BD FacsCalibur, BD Biosciences, San Jose, CA; FlowJo software, Tree Star, Ashland, OR) using anti-cMyc directed towards the C-terminal tag. After induction (Section 2.2), cells were washed with PBS 0.5% BSA (PBS-B), probed with anti-cMyc (1:200 in PBS-B; 1 h, 25 °C) and then stained with anti-chicken Alexa 488-conjugated antibody (Invitrogen, Carlsbad, CA; 30 min, 4 °C). For binding specificity of surface-expressed HAs, the induced cells were incubated with anti-HA antibodies (1 h, 25 °C), followed by a FITC-conjugated goat anti-human or anti-mouse IgG (30 min, 4 °C).
2.4. Competitive binding of H5-2A and NR-2728
Yeast cells expressing the HA1 subunit were probed with unlabeled H5-2A or NR-2728 mAb for 1 h at 4 °C. Unbound antibodies were removed after three washes with PBS-B. H5-2A and NR2728 were conjugated using an Alexa Fluor 647 mAb labeling kit (Invitrogen). Conjugated H5-2A or NR2728 was then added to the cells for 1 h at 4 °C. Yeast cells were washed three times with PBS-B and analyzed by FACS.
2.5. Western blotting
Yeast surface-expressed HA proteins were resolved using SDS–polyacrylamide gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane. After blocking with 5% skim milk overnight, the blot was probed with anti-H5 polyclonal antibody (1 h, 25 °C), followed by horseradish peroxidase (HRP)-conjugated anti-mouse IgG (1 h, 25 °C). Proteins were detected using the West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) and exposure to autoradiography film.
2.6. Construction of the epitope mapping library in yeast
A library of HA1 mutants was generated by an error-prone PCR method using the GeneMorph II random mutagenesis kit (Invitrogen). The HA1 subunit was amplified from pCTCON2-HA1 using the primers: pctcon2-MUT-F, 5′-CGACGATTGAAGGTAGATACCCATACGACGTTCCAGACTACGCTCTGCAG-3′; pctcon2-MUT-R, 5′-CAGATCTCGAGCTATTACAAGTCCTCTTCAGAAATAAGCTTTTGTTC-3′. Five micrograms of each gel-purified construct containing the mutant HA1 sequence were co-transformed with a NheI/BamHI-digested pCTCON2 vector into competent yeast cells and cultured on SD-CAA plates. The transformed cells were serially titrated on SD-CAA agar plates to determine the size of the library. The yeast library was grown in SD-CAA medium and induced to display proteins as described in Section 2.2.
2.7. Selection and fine epitope mapping by FACS
Surface expression of the HA1 mutant library was induced (Section 2.2) before each round of FACS selection for mutants binding only by mAb H5-2A or NR2728 mAbs after double staining with both antibodies (1 h, 25 °C), followed by secondary PE-conjugated anti-human IgG and FITC-conjugated anti-mouse IgG (30 min, 4 °C). Yeast cell populations binding exclusively to H5-2A or NR2728 were sorted on a DakoCytomation High Speed MoFloSorter (DaKoCytomation, Fort Collins, CO) and enriched in SD-CAA medium. After a second round of selection and sorting, the cells were plated to isolate individual clones and binding specificity confirmed by FACS. Plasmids from yeast clones binding exclusively to H5-2A or NR2728 mAbs were recovered (Zymoprep Yeast Plasmid Miniprep Kit, Zymo Research) and DNA sequenced using the primers pCTCON2-F, 5′-GTTCCAGACTACGCTCTGCAGG and pCTCON2-R, 5′-GATTTTGTTACATCTACACTGTTG.
2.8. Hemagglutination inhibition (HI) assay
The inhibition of hemagglutination by mAb H5-2A or NR2728 was performed to assess antibody response to the H5N1 virus. Twofold dilutions of mAb samples were mixed with reassortant H5N1 virus at a concentration of 4 HA units per well and incubated for 30 min at RT. Fifty microliters of a 0.5% suspension of turkey RBCs were added to each well, and hemagglutination was assessed visually after 1 h.
2.9. Determination of linear versus conformational epitopes
For determination of linear versus conformational epitopes, the yeast surface-expressed HA1 subunit was denatured by heating the yeast cells at 80 °C for 30 min. After chilling on ice for 20 min, cells were labeled with H5-2A or NR-2728 mAb, followed by a FITC-conjugated IgG for FACS analysis.
3. Results
3.1. Cloning and expression of HA proteins on yeast cell surface
The yeast surface display system utilizes the α-agglutinin adhesion receptor composed of the Aga1p and Aga2p proteins to display recombinant proteins. The cDNA sequences of HA0 or its subunits without the associated signal peptide and transmembrane domain were separately cloned in-frame with the endogenous yeast Aga2p signal peptide and aga2 gene at the N-terminal end and cMyc at the C-terminal end (Fig. 1A). When transformed in yeast expressing endogenous Aga1, the heterologous HA fused to Aga2 is displayed on the surface via disulfide bonds between Aga2p and Aga1p.
The HA0 and each of the subunits were determined to be successfully displayed on the yeast surface at comparable levels by FACS staining of the C-terminal cMyc tag or HA protein itself (Fig. 1B). Western blotting with anti-HA polyclonal antibody showed bands at expected sizes for HA0 (105 kDa), HA1 (80 kDa) and HA2 (60 kDa), confirming that the surface-displayed HA proteins were expressed as fusions to Aga2p (∼28 to 30 kDa) (Fig. 1C).
3.2. Binding specificity of surface-displayed HA0 to anti-HA mAbs with pre-defined epitopes
To determine immunoreactivity and specificity of the displayed HA0 protein, a panel of anti-HA mAbs previously shown to bind to a discontinuous and conformationally sensitive epitope was assayed for binding with the displayed HA proteins using FACS (Fig. 2 A). The HA0 reacted with eight different anti-HA mAbs (D7, D8, F10, G17, H40, A66, D80 and E90) that each were previously reported to have broad-spectrum and extremely potent neutralization activity and to bind to a highly conserved conformational epitope on the HA membrane-proximal stem region [3]. The X-ray co-crystal structure of the HA-F10 mAb complex showed that the F10 mAb is directed toward a conformation-dependent region shared by both HA1 and HA2 [3]. Here, it was further demonstrated by binding of the F10 mAb that monomeric HA0 was expressed in its correctly folded conformation on yeast cells, and this interaction was lost after denaturation of the target protein (Fig. 2B). As expected, no immunoreactivity was detected to the negative controls 11A and 80R (mAbs against the S1 domain the SARS-CoV spike protein) (Fig. 2A).
Fig. 2.
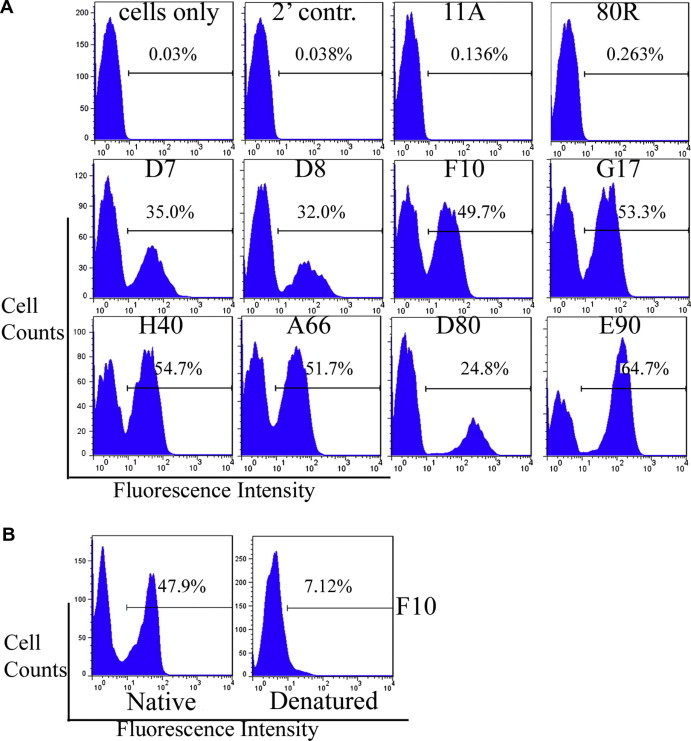
FACS analysis of anti-HA mAbs binding to yeast surface-displayed HA0. (A) HA0 is bound by conformationally sensitive mAbs that target the highly conserved stem region of HA, which mediates viral membrane fusion with host cells. The 11A and 80R mAbs were used as negative controls. (B) The F10 mAb was shown to be conformationally sensitive by loss of binding to heat-denatured HA on yeast.
3.3. Analysis of binding specificity of yeast-displayed HA to anti-HA antibodies with undefined epitopes
After demonstrating that yeast-displayed HA0 could bind to antibodies with known specificities, this system was tested by mapping two HA-reactive antibodies for which the specific epitopes were not previously known. The binding specificities to the yeast surface-displayed HA protein or its subunits of the human H5-2A and the mouse NR2728 mAbs were analyzed by FACS. Both H5-2A and NR2728 mAbs bound to surface-displayed HA0 and HA1 but not HA2, suggesting that they have contact residues mapping specifically to the HA1 subunit (Fig. 3 A). Further analyses revealed that both H5-2A and NR2728 retained their binding reactivities to the yeast surface-displayed HA1 after heat denaturation, suggesting that they bind to linear or continuous epitopes (Fig. 3B).
Fig. 3.
Binding properties and epitope specificities of H5-2A and NR2728 mAbs. (A) Both H5-2A and NR2728 bound to yeast-displayed HA0 and HA1 but not to HA2. (B) Bindings of H5-2A and NR2728 to heat-denatured HA1 on yeast were both retained, indicating that both mAbs recognize linear HA epitopes.
3.4. Fine epitope mapping by yeast display
In order to use this yeast system for future influenza antibody studies, it was important to demonstrate its broad capability for fine mapping HA-specific antibodies. Therefore, low rate mutagenesis by an error-prone PCR method was used to generate a mutant HA1 gene library of about 2 × 105 clones as determined by plating serial dilutions of the transformed yeast cells. The H5-2A and NR2728 mAbs were further characterized in these fine mapping studies. The library was screened by two rounds of FACS to select yeast clones with selective loss of binding to H5-2A or NR2728 but that retained surface expression of HA and binding to other HA-specific antibodies. Fifty individual clones isolated by sorting were sequenced and evaluated to identify the binding epitope on the HA protein. Multiple mutants containing a single amino acid alteration in HA1 were identified by DNA sequencing, and several of these mutations conferred considerable and selective loss of binding to one of the two anti-HA mAbs. Five mutations (T206, N210, Q211, R212 and K238) abolished binding of the H5-2A mAb, while binding of anti-cMyc and NR2728 were unchanged (Fig. 4 A top). The binding epitope of H5-2A was mapped to a conserved loop on HA1, which is distant from the receptor binding sites. The sequence of the H5-2A epitope located in the loop of HA1 was highly H5-strain specific and not found in any other subtypes among the total of 10,815 unique HA sequences available in a public influenza database (http://www.ncbi.nlm.nih.gov/genomes/FLU/FLU.html) as of Feb. 24, 2011 (Sup. Table 1). Five of the HA mutations (S125, H129, Y161, I164 and R166) caused total loss of NR2728 binding, while binding of H5-2A as well as anti-cMyc were unchanged (Fig. 4A bottom). In the competitive binding assay, unlabeled H5-2A did not cross-compete with labeled NR2728 (Alexa Flour 647) but did self-compete with labeled H5-2A (Alexa Flour 647) for binding to the HA1 subunit (Fig. 4C). Analysis of the location of mutations in the selected clones indicated that NR2728 binds to HA1 at an interface overlapping with the receptor binding site by forming a contiguous patch on the solvent-exposed surface of the receptor binding site, whereas H5-2A binds to a loop on HA1 away from the receptor binding site (RBS) (Fig. 4B, left and middle). Within the trimeric HA crystal structure, the NR2728 epitope is fully exposed, while the H5-2A epitope is cryptic at the interface of the HA monomers (Fig. 4B, right). The results of both the competitive binding assay (Fig. 4C) and the mapping of the loss-of-binding mutations (Fig. 4B) indicated that these two mAbs had non-identical/non-overlapping epitopes (Fig. 4B). The binding epitopes on HA for H5-2A and NR2728 were also tested for functionality in a HI assay. In contrast to H5-2A which did not inhibit the agglutination of turkey RBCs mediated by the influenza strain H5N1, NR2728 showed strong inhibitory activity in this assay (Fig. 4D). Of note, the potently neutralizing F10 mAb which targets the HA stem region had virtually no activity in the HI assay (Fig. 4D), which is consistent with our previous work showing that this antibody inhibits influenza by a mechanism other than blocking virus binding to host cells [3]. Taken together, these results suggest that the specific interaction of NR2728 with the HA RBS resulted in its potent inhibition of virus attachment to sialic acid receptors.
Fig. 4.
Fine epitope mapping of H5-2A and NR2728 mAbs. (A) FACS profiles demonstrating immunoreactivity of HA1 mutant clones displayed on the yeast surface. Clones with single-point mutations that selectively abolished binding of either H5-2A or NR2728 are shown. (B) Crystal structure of H5 HA0 (PDB 3FKU) with indicated epitopes recognized by H5-2A or NR-2728 mAbs to the HA1 monomer on the yeast surface (left) and a close-up view (middle). Top-down view of the HA trimer 3-fold axis shows the surface-exposed NR2728 epitope (yellow) and cryptic H5-2A epitope (gray) at the trimer interface (right). (C) Competition assay between H5-2A and NR-2728 mAbs. FACS histograms show that pre-incubation of HA with unlabeled HA-2A resulted in self-competition with the Alexa Flour 647-labeled H5-2A, while no competition was detected with the labeled NR2728 for the binding of surface-displayed HA1. (D) HI assay showing strong inhibition ability of NR2728, which was demonstrated above to have a epitope adjacent to the RBS, by blocking virus-mediated hemagglutination. Meanwhile, H5-2A showed no HI activity.
4. Discussion
In this study, an accurate method was developed for epitope mapping of antibodies against the HA protein of the highly pathogenic H5N1 influenza virus. The monomeric HA0 proteins were demonstrated to be correctly folded and expressed on the yeast surface, and this display system was further validated with a mutagenized library of the HA1 as a practical approach for fine epitope mapping. Analysis of HA1 mutants on the yeast surface which had selectively lost binding to a given mAb can allow localization of epitopes on HA without prior knowledge of potential contact residues. The yeast display system has been effectively utilized to characterize antibody-binding epitopes of various host and microbial surface proteins [6], [7], [8], but it has not been used previously to fine map influenza antibodies which is a critical component of understanding the pandemic potential of newly emerging influenza viruses.
Although a few reports have shown that full-length influenza HA0 can be expressed on the yeast surface, detailed information was not provided as to whether the yeast-displayed HA was folded into its correct native conformation [9], [10], [11]. In this study, the full-length extracellular HA0 protein and HA1 and HA2 subunits were cloned into a yeast surface expression vector (Fig. 1A). These constructs produced recombinant HA proteins that were effectively displayed at high levels (Fig. 1A and B). The expression of surface-displayed HA proteins and their corresponding sizes were further confirmed by Western blotting (Fig. 1C). Notably, the yeast surface-expressed HA variants reacted properly with anti-HA mAbs that recognize linear or conformation-dependent epitopes, suggesting that the proteins were processed through the secretory pathway with intact antigenic determinants and conformational integrity (Fig. 2, Fig. 3). In particular, the proper conformation of the yeast-displayed HA0 protein was demonstrated by binding of a panel of eight broadly neutralizing antibodies against antigenically diverse influenza A Group 1 subtypes, including H1, H2, H5, H6, H8, H9, H11, H12, H13 and H16 [3].
To determine whether the displayed HA proteins could be used to define critical residues for anti-HA mAb recognition, the H5-2A and NR2728 mAbs were mapped using an error-prone PCR-derived library of H5 HA1 on the surface of yeast. The yeast clones were screened for selective loss of binding to the specific mAb but retention of binding to both the cMyc tag and other H5-specific antibodies. The H5-2A mAb epitope is located mainly on a single loop that is buried in the HA1 trimer interface, away from the receptor binding site and its surrounding hypervariable dominant loops (Fig. 4B). This location and its putative cryptic location within the HA trimer may result in the epitope being placed under weak evolutionary selection pressure for immune escape, hence resulting in a relatively conserved sequence among the H5 strains. In fact, 80% of the H5 sequences (1895/2374) aligned with the H5-2A epitope “TSTLNQR”, and 99% of these (1881/1895) contained the exact binding sequence (Sup. Table 2). Although the H5-2A mAb lacked neutralizing activity ([3] and unpublished data) or agglutination of erythrocytes in the HI assay (Fig. 4D), the H5-2A mAb may have diagnostic value due to its conserved and serotype-specific epitope. The NR2728-binding epitope spans a larger surface area that overlaps with the RBS on HA. The critical residues of the NR2728-specific epitope are located in two distinct turns where the residues are surface-accessible and contiguous to the receptor binding residues. Thus, this epitope mapping information corroborates the observation that NR2728 can strongly inhibit virus binding to cells in our HI assay (Fig. 4D) and is consistent with its previously determined viral neutralization activity [12]. NR2728 and H5-2A both retained binding to denatured HA, suggesting that they may both recognize linear or continuous epitopes. However, the findings of involvement of discontinuous amino acids for both mAbs also leave open the possibility of a more complex epitope that shows partial resistance to denaturation/renaturation under the experimental conditions used here.
In summary, our results demonstrate that yeast is a viable host for expression of HA proteins in correctly folded conformational forms and can be utilized for domain mapping and fine epitope mapping of residues in contact with neutralizing anti-HA mAbs. The recent influenza pandemic of 2009 highlighted the need to have better tools for quickly identifying viral targets for prophylactic vaccines and generating passive therapeutic antibody candidates against multiple subtypes of influenza A virus. Thus, HA-specific mAb epitope mapping can be used to identify the location of neutralizing epitopes which can further corroborate the structural characteristics of the epitope as well as mechanisms of mAbs-mediated virus neutralization and neutralization escape. In addition, it is conceivable that the standard serological method used to subtype the HA of newly isolated influenza A viruses using polyclonal anti-HA sera could be replaced with a panel of subtype-specific mAbs similar to H5-2A. The detailed epitope mapping of anti-HA antibodies presented here could potentially aid in efforts to better understand antibody epitopes of highly pathogenic H5N1 avian influenza virus or other emerging influenza strains as we remain on guard for the next influenza pandemic.
Acknowledgments
This work was supported by Grants from the National Institutes of Health: U01-AI074518-01 to W.A.M. and 1K01AI073861 to T.H. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agency.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbrc.2011.04.139.
Appendix A. Supplementary data
References
- 1.Subbarao K., Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nat. Rev. Immunol. 2007;7:267–278. doi: 10.1038/nri2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han T., Marasco W.A. Structural basis of influenza virus neutralization. Ann. N. Y. Acad. Sci. 2011 doi: 10.1111/j.1749-6632.2010.05829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sui J., Hwang W.C., Perez S., Wei G., Aird D., Chen L.M., Santelli E., Stec B., Cadwell G., Ali M., Wan H., Murakami A., Yammanuru A., Han T., Cox N.J., Bankston L.A., Donis R.O., Liddington R.C., Marasco W.A. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009;16:265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepper L.R., Cho Y.K., Boder E.T., Shusta E.V. A decade of yeast surface display technology: where are we now? Comb Chem. High Throughput Screen. 2008;11:127–134. doi: 10.2174/138620708783744516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altmann M., Linder P. Power of yeast for analysis of eukaryotic translation initiation. J. Biol. Chem. 2010;285:31907–31912. doi: 10.1074/jbc.R110.144196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cochran J.R., Kim Y.S., Olsen M.J., Bhandari R., Wittrup K.D. Domain-level antibody epitope mapping through yeast surface display of epidermal growth factor receptor fragments. J. Immunol. Methods. 2004;287:147–158. doi: 10.1016/j.jim.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Levy R., Forsyth C.M., LaPorte S.L., Geren I.N., Smith L.A., Marks J.D. Fine and domain-level epitope mapping of botulinum neurotoxin type A neutralizing antibodies by yeast surface display. J. Mol. Biol. 2007;365:196–210. doi: 10.1016/j.jmb.2006.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliphant T., Engle M., Nybakken G.E., Doane C., Johnson S., Huang L., Gorlatov S., Mehlhop E., Marri A., Chung K.M., Ebel G.D., Kramer L.D., Fremont D.H., Diamond M.S. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Wang Y., Liang Y., Ni B., Wan Y., Liao Z., Chan K.H., Yuen K.Y., Fu X., Shang X., Wang S., Yi D., Guo B., Di B., Wang M., Che X., Wu Y. Fine antigenic variation within H5N1 influenza virus hemagglutinin’s antigenic sites defined by yeast cell surface display. Eur. J. Immunol. 2009;39:3498–3510. doi: 10.1002/eji.200939532. [DOI] [PubMed] [Google Scholar]
- 10.Wang C.Y., Luo Y.L., Chen Y.T., Li S.K., Lin C.H., Hsieh Y.C., Liu H.J. The cleavage of the hemagglutinin protein of H5N2 avian influenza virus in yeast. J. Virol. Methods. 2007;146:293–297. doi: 10.1016/j.jviromet.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Wasilenko J.L., Sarmento L., Spatz S., Pantin-Jackwood M. Cell surface display of highly pathogenic avian influenza virus hemagglutinin on the surface of Pichia pastoris cells using alpha-agglutinin for production of oral vaccines. Biotechnol. Prog. 2010;26:542–547. doi: 10.1002/btpr.343. [DOI] [PubMed] [Google Scholar]
- 12.Benne C.A., Kroon F.P., Harmsen M., Tavares L., Kraaijeveld C.A., De Jong J.C. Comparison of neutralizing and hemagglutination-inhibiting antibody responses to influenza A virus vaccination of human immunodeficiency virus-infected individuals. Clin. Diagn. Lab. Immunol. 1998;5:114–117. doi: 10.1128/cdli.5.1.114-117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


