Abstract
In postnatal tissues, angiogenesis occurs in nontumoral conditions on appropriate stimuli. In the nervous tissue, hypoxia, neural graft, increased neural function, and synaptic activity are associated with neoangiogenesis. We have investigated the occurrence of neoangiogenesis in the superior cervical ganglia (scg) of newborn rats treated for 8–21 days with 6-hydroxy-dopamine (6-OHDA), nerve growth factor (NGF), or 6-OHDA + NGF. The two latter treatments induced a significant increase in scg size. However, the increase after combined treatment far exceeded that of NGF alone. Similarly, histological and histochemical analysis revealed neuronal hypertrophy and endothelial cell hyperplasia associated with stromal hypertrophy (as described by laminin immunostaining) and increased vascular bed (as revealed by platelet/endothelial cell adhesion molecule-1 immunostaining) in 6-OHDA + NGF-treated pups. NGF, either alone or associated with 6-OHDA, also induced a significant up-regulation of NADPH diaphorase, neuronal nitric oxide synthase, and vascular endothelial growth factor expression in scg neurons. The present investigation suggests that the increase of scg size induced by NGF and 6-OHDA + NGF is associated with neoangiogenesis, and that the induction of vasoactive and angiogenic factors in neurons represents a further and previously undisclosed effect of NGF.
Nerve growth factor (NGF) is a neurotrophin that plays a crucial role in promoting growth, differentiation, and function in sympathetic nerve cells (1). In recent years, NGF has been described as a “pleiotropic” molecule (2), involved in a variety of peripheral actions such as tissue inflammation (3), neuropeptide expression regulation (4), skin physiology (5), and peripheral tissue regeneration (6, 7). Furthermore, target-derived NGF, which binds to its low- and high-affinity receptors on nerve terminals and is retrogradely transported to neural sensory and sympathetic perikarya, is known to prevent natural cell death and to promote and modulate peripheral innervation (8–10).
Previous findings by Levi-Montalcini et al. (11) showed that NGF administration prevents nerve cell degeneration induced by 6-hydroxy-dopamine (6-OHDA), a neurotoxic compound that causes a selective but reversible destruction of sympathetic nerve endings in adult animals and widespread lesions in the cell body and neuronal cell death in newborn animals (12). The combined administration of 6-OHDA and NGF treatment in developing rodents results in an impressive growth of sympathetic ganglia because of an increase in the number and size of postganglionic neurites as well as glial and satellite cells (11, 13). These experiments led also to the still untested hypothesis that NGF might influence endothelial cell proliferation and that combined treatment would magnify this effect.
Recent evidence indeed suggests that NGF alone or in combination with other biologically active endogenous molecules can exert its action on endothelial cells and most probably on angiogenic activity (14, 15). The known effects of NGF (16) on cells of the immune system [macrophages, mast cells (3)], on connective tissue [fibroblast (17, 18) and osteoblasts (19)], on endothelial cells (20), and in tissue homeostasis and the widespread distribution as well of NGF receptors in peripheral tissues (21) support this hypothesis. It has also been suggested that in peripheral sensory neurons, NGF stimulates the production of vascular endothelial growth factor (VEGF), the most powerful mitogen for endothelial cells (15).
Despite this extensive research, the role of NGF in the regulation of endothelial cells remains hypothetical. The aim of this research was to explore the influence of NGF on angiogenic mechanisms; we have therefore analyzed the effect of treatment with NGF and NGF + 6-OHDA on the distribution of the biological markers differentially involved in neoangiogenesis regulation in newborn rats. Here we report that vessel density increases in superior cervical ganglia of newborn rats treated with 6-OHDA + NGF, associated with increased expression of the angiogenic peptide VEGF and neuronal nitric oxide synthase (nNOS) in neurons. The result suggests that angiogenesis may be regulated through activation of angiogenic and vasodilatation agents directly produced by neurons on request, and NGF indirectly stimulates this process.
Materials and Methods
NGF Preparation and Animal Treatment.
NGF was prepared from mouse submaxillary glands according to the method of Bocchini and Angeletti (22). Fresh 6-OHDA (Sigma) solution in distilled water containing 0.1 mg/ml of ascorbic acid to prevent oxidation was prepared shortly before use. Pregnant rats were purchased from Charles River Italia (Calco, Varese, Italy) and housed in single cages on arrival. A total of 40 newborn rats were treated. As soon as delivered, each litter was culled to eight pups, which were distributed into four groups of two rats each. The groups were injected daily as follows: group 1 with NGF 10 μg/g of body weight (b.w.) in the morning and 6-OHDA 100 μg/g of b.w. in the evening; group 2 with NGF 10 μg/g b.w. in the morning; group 3 with 6-OHDA 100 μg/g of b.w. in the evening; and group 4 with saline 0.1 ml in the morning.
Animals from the four experimental groups were killed 8 or 21 days after the beginning of treatment. According to procedures for light microscopy studies, the animals were killed by cervical translocation; superior cervical ganglia (scg) was either dissected under a stereomicroscope, fixed in alcoholic Bouin and mounted in toto, or embedded in Paraplast (Carlo Erba, Milano, Italy), cut into 8-μm thick sections, and stained with toluidine blue. Few animals received a single administration of the above-mentioned treatments.
Immunocytochemistry.
At the 8th postnatal day, pups from all experimental groups were anaesthetized and perfused via the ascending aorta with saline solution followed by 4% paraformaldehyde. scg was dissected under a stereomicroscope, immersed in ice-cold 4% paraformaldehyde fixative for 24 h, and then rinsed in ice-cold 0.1 M Sorensen's buffer 0.2 M, pH 7.5 containing 5% sucrose for 48 h. After rinsing, scg was quickly frozen in CO2 and cut by using a Leitz cryostat 1720 (sections 14-μm thick; −20°C). Sections were then collected on glass slides coated with chrome alum and gelatin and immediately processed for the immunofluorescence procedure. Slides from all animals were run in the same assay. The sections were first incubated in 0.1 M PBS at room temperature for 10–30 min, followed by incubation at 4°C overnight in humid atmosphere with the primary antisera [mouse anti-NGF low-affinity receptor p75 antibody, clone IgG192, generously supplied by E. M. Johnson (Washington University, St. Louis), diluted 1:250; rabbit antilaminin (Sigma), 1:25; goat antiplatelet/endothelial cell adhesion molecule-1 (PECAM-1) against a peptide mapping at the carboxy terminus of mouse PECAM-1 (Santa Cruz Biotechnology), 1:300]. The staining specificity was assessed by overnight preincubation in vitro of the antiserum with the respective antigen (100 μg of antigen/ml diluted antiserum). This treatment prevented staining. The primary antibodies were diluted in PBS containing 0.3% Triton X-100, vol/vol. The sections were then rinsed in PBS for 30 min (3 × 10 min) and incubated at 37°C for 120 min in a humid atmosphere with fluorescein isothiocyanate-conjugated anti-mouse, anti-rabbit, or anti-goat Ig (Dako) containing 0.3% Triton X-100. They were again rinsed in PBS as above, mounted in glycerol and PBS (3:1, vol/vol) containing 0.1% 1,4-phenylenediamine, and finally examined by using a Nikon Microphot FXA microscope equipped with a 100-W mercury lamp and B-2A filter combination.
Histopathology.
Staining techniques for histological studies included toluidine blue, cresyl violet for Nissl staining, Mallory–Azan reaction, and hematoxylin-eosin staining. Visualization of NADPH–diaphorase activity was obtained by incubation of cryostat sections in 0.1 M phosphate buffer, pH 8.0, containing 1 mM NADPH (Sigma), 2 mM nitro blue tetrazolium (Sigma), and 0.3% Triton X-100 at 37°C (17). Duration of the incubation (60–120 min) was determined by staining intensity. The reaction was terminated by washing in 0.1 M PBS solution. The sections were then thoroughly washed in distilled water, air-dried, and finally coverslipped with Eukitt (Kindler, Freiburg, Germany).
Image Analysis.
The AIS Analytical Imaging Station Imaging Computer Research system (St. Catherines, ON, Canada) was used for image analysis. Images of the processed sections were loaded via a VarioCam PCO Computer Optics, Kelheim, Germany, charge-coupled device (CCD) camera. For quantitative analysis of the histochemical composition of ganglia (laminin and PECAM-1 immunoreactivity (IR) and Nissl-stained neurons) ×20 objective was applied; digitalized images were then contrast-enhanced to clearly differentiate positivity from background, and a thresholding procedure was established to determine the proportion of immunoreactive area in a constant area of each section (512 × 512 pixels). Sections with anti-VEGF and NADPH-diaphorase staining were also loaded via a VarioCam CCD camera. The system was calibrated for density values, with density level 255 corresponding to 0.017 density relative optical density (ROD) and density level 0 corresponding to 2.4 density ROD.
Statistical Methods.
The animal sample was divided into four experimental groups, into which newborn rats from all litters were randomly allocated and evenly distributed. Caring, housing, and feeding as well as treatment and experimental procedures were identical for all animals studied. Allocation to either category of treatment duration also occurred randomly. Scg size was expressed as neuronal area. The quantitative histochemical analysis (laminin and PECAM-1 IR and Nissl-stained neurons) was carried out on three nonconsecutive sections from five areas of each ganglia from at least five pups in each experimental group. Mean values of the proportion of immunoreactive area in a constant area of each section were used for statistical analysis. Twenty to twenty-five cells were measured in each ganglion for staining intensity evaluation.
Descriptive analysis (mean values and standard error) of all relevant variables was performed. Statistical analysis for comparison of treatments was performed by one-way ANOVA followed by Dunnett's test.
Results
The effect of treatment on growth of scg is illustrated in Fig. 1. In accordance with what is already described (11, 13), chemical sympathectomy by 6-OHDA dramatically reduced scg size, whereas NGF treatment prevented reduction. Moreover, sgc was found to be larger in NGF-treated rats compared with control animals. scg grew even larger when combined treatment was administered. Mean neuronal area (μm2) measured 202.5 + 38.2 (SD) in control rats and 247.1 + 47.1 in NGF-treated animals (P < 0.01). Coadministration of NGF and 6-OHDA not only prevented shrinkage of neurons but also further induced hyperplasia (mean + SD, μm2: 6-OHDA 134.6 + 47.4; 6-OHDA + NGF 280.4 + 40.3, P < 0.001). Neuron size, but not general morphology, was affected by treatment, as indicated by form factor measurements (data not shown).
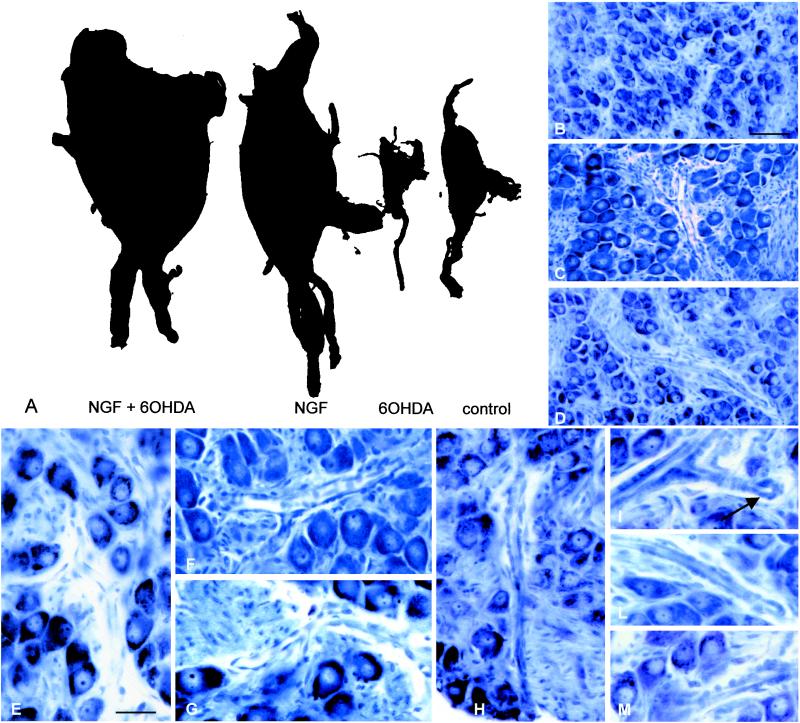
Figure 1.
(A) Whole-mount preparation of superior cervical ganglia of littermate rats (26 days) treated with NGF and 6-OHDA, NGF, 6-OHDA, and saline. 6-OHDA administration induces a well known degeneration of the sympathetic neurons, which, on the contrary, is prevented by NGF treatment. scg are larger in NGF-treated pups than in control pups; however, growth induced by double treatment is impressive. Toluidine-blue micrographs show general morphology in control (B), NGF (C), and 6-OHDA + NGF- (D) treated pups. High-magnification micrographs show intragangliar blood vessels in controls (E), NGF-treated (F and G), and 6-OHDA + NGF-treated (H–M) pups. In the latter group, within the vessel and capillary wall, high cellularity can be observed, and buds of newly formed vessels are clearly detectable (arrow, H). (Bars = B–D, 200 μm; E–M, 100 μm.)
Gross Histology.
Coadministration of NGF and 6-OHDA determined an impressive growth of the stromal component in scg. Large nests of connective tissue were observed in pups treated with 6-OHDA + NGF for 21 days (Fig. 1D), whereas only very fine trabeculae of connective tissue intermingled with neurons were observed in control rats (Fig. 1B). The vascular component of double-treated rats included mature elements and also vessels characterized by very high cellularity of the wall, with round nuclei and buds indicating growth of neo-formed vessels (Fig. 1 D and H–M). In pups treated with NGF, numerous mast cells were observed around vessels. It is important to point out that, as already reported (11, 13), all cellular elements had a completely normal individual morphology, and no signs of malignant proliferation were apparent.
Quantitative Immunohistochemistry.
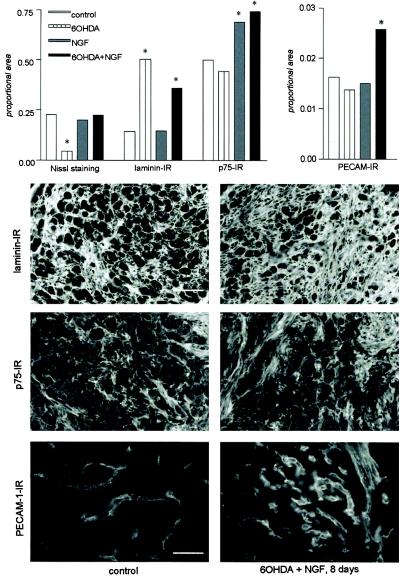
To investigate histological composition of scg after NGF and NGF + 6-OHDA treatment, proteins of the extracellular matrix were analyzed. The experiments were performed after 8 days treatment, when the histological effects were less pronounced but already present. Laminin immunostaining, which identifies basal membrane in fibroblasts, neurons, and endothelial cells, labeled all areas of the ganglia not occupied by neurons (Fig. 2). Quantitative evaluation of Nissl staining compared with laminin immunoreactivity (IR) confirmed that the cellular component did not completely account for the ganglionic hypertrophy observed (Fig. 2, histograms), suggesting therefore an increase of the stromal component too. The vascular bed was also investigated by using PECAM-1 as a marker (Fig. 2). In animals undergoing double treatment and in discrete areas of the scg, the percent area covered by the PECAM-IR signal increased, indicating a higher density of the vascular component (Fig. 2, histograms). Labeling for the NGF low-affinity receptor p75 was also up-regulated by NGF and double treatment as well, as shown in Fig. 2.
Figure 2.
Histochemical composition of scg after treatment. The figure shows laminin, p75, and PECAM-1 IR in scg of controls (Left) and pups treated for 8 days with 6-OHDA associated with NGF (Right). The graph shows the quantitative histochemical composition. Data are expressed as area covered by the immunoreactive signal inside a standard sampling window (proportional area). As also seen in micrographs, in the 6-OHDA-treated group laminin IR occupies the largest proportional area because of the very low presence of neuronal component. In double treatment preparations, a true increase of the stromal component can be measured. p75 IR can be detected intra- and extracellularly: the percentage related to its proportional area increases in NGF and 6-OHDA + NGF-treated pups. PECAM-1 IR depicts nets of newly formed vessels in double-treated pups. An overall increase of the percent of area covered by PECAM-1 IR can be measured. Statistical evaluation: ANOVA and Dunnett's test; *, P < 0.05. [Bars = 100 (laminin also for p75) and 50 (PECAM-1) μm.]
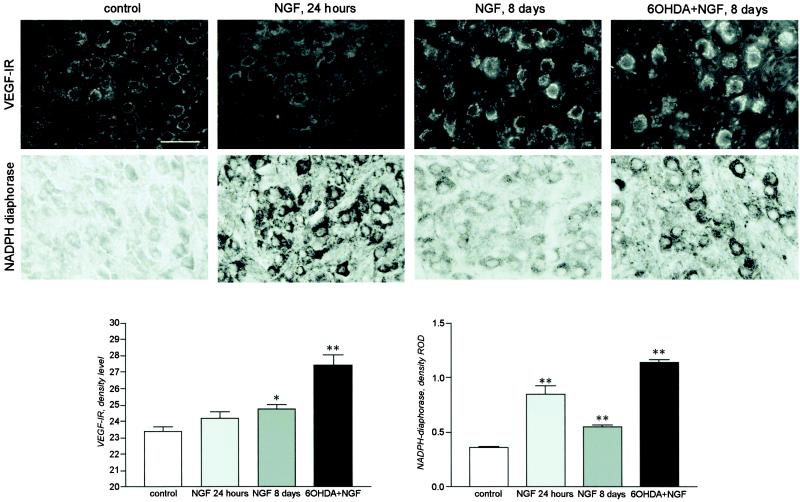
We investigated also the expression of angiogenetic and vasodilator agents to test for a regulatory effect on neoangiogenesis; in particular, the expression of VEGF and the neural isoform of NO synthase were analyzed. We observed that neural production of VEGF was up-regulated by NGF and 6-OHDA + NGF after 8 days of treatment, whereas one single NGF administration produced no detectable change (Fig. 3 Upper and graph bars). The intracellular level of enzymes associated with NO synthesis was also strongly up-regulated by NGF, as indicated by the level of nNOS expression (data not shown) and NADPH–diaphorase staining (Fig. 3 Lower and graph bars). A single NGF administration exerted a more powerful effect than a protracted 8-day administration; 6-OHDA coadministration further potentiated NGF effect.
Figure 3.
VEGF IR and NADPH–diaphorase staining in neurons in scg of controls and newborn rats treated with NGF (1 and 8 days) and 6-OHDA + NGF (8 days) and density evaluation. Eight days of NGF treatment induces a significant increase in VEGF expression; a greater increase occurs after concomitant 6-OHDA and NGF administration. Single NGF administration strongly up-regulates NADPH–diaphorase staining, but the increase induced by 8 days of combined 6-OHDA and NGF treatment far exceeds that of NGF single administration. Statistical analysis: ANOVA and Dunnett's test; *, P < 0.05. (Bar = 100 μm.)
Discussion
It has been previously shown that combined NGF and 6-OHDA treatment in neonatal rats induces a paradoxical increase of scg size as a result of enormous growth of postganglionic neurites and, to a much lesser extent, of glial and satellite cells. In the present study, we investigated the effect of NGF, alone or in combination with the neurotoxin 6-OHDA, on neoangiogenesis markers. The results of this study show that NGF treatment induces impressive growth of the stromal component and determines vascular neoformation in sympathetic ganglia. These effects are reinforced by 6-OHDA coadministration, thus suggesting that NGF effects are activated by lesioning. Concomitantly, NGF increases VEGF and nNOS expression and NADPH–diaphorase staining in neurons within the scg.
NGF and Histological Components.
The increase of the stromal component of sympathetic ganglia on NGF and NGF + 6-OHDA administration is well illustrated by laminin immunostaining, a marker for the basal membrane in most cell types (23). NGF receptors have been described in stroma in different organs, such as human prostate tissue (24), cornea (25, 26), synovial tissue (17), and bone marrow (27). However, laminin also stimulates growth of nerve process and favors NGF action in vivo and in vitro. Indeed, the addition of laminin to in vitro systems significantly enhances neurite extension and strongly stimulates NGF action in cultured dorsal root ganglia (28) and superior cervical ganglia (29). The finding that the expression of p75 increased in both neurons and extracellular elements after NGF administration, alone or in association with 6-OHDA, is particularly meaningful. This increase is not associated with neuronal death; on the contrary, neurons appear to be numerous and hypertrophic, with no histological marks of degeneration.
NGF and Neuronal Component of scg.
The effect of NGF alone and as double treatment on regulation of vasoactive and angiogenetic factors was investigated by the assessment of VEGF and NO production in scg. VEGF is a secreted mitogen specific for the vascular endothelium; it is known to exert a pivotal role in normal and pathological angiogenesis (30). NO is considered the link between neural activity and activity-dependent regional vasodilatation (31). We have observed that, whereas VEGF production by sympathetic neurons is very low in control animals, it is strongly stimulated by NGF administration. Moreover, this effect is enhanced by the concomitant administration of 6-OHDA. It has been shown that neural production of VEGF in the developing nervous tissue correlates with vasculogenesis (32) and that at birth, VEGF production switches from neurons to astrocytes (33). VEGF expression in the peripheral nervous system is also stimulated by some pathological conditions, such as diabetic neuropathy (15) and, in mature central nervous systems, by hypoxia and ischemia (34, 35). Moreover, when exogenously applied, VEGF proves to exert a neurotrophic effect on cultured adult mouse scg, measured as axonal outgrowth (36). NO production is also up-regulated by NGF, the NGF effect being extremely powerful and fast of onset.
NGF and Neoangiogenesis.
The demonstration of induction of postnatal angiogenesis by NGF represents the most interesting result of this research. This effect is well documented in preparations featuring toluidine blue staining and immunostaining for PECAM-1. Angiogenesis, the sprouting of new capillaries from preexisting blood vessels, is complete around postnatal day 20 (37) and is inhibited in mature tissues. However, several nontumoral conditions can trigger neoangiogenesis in nervous tissue by tilting the balance between angiogenic and angiostatic factors toward activation. Adaptation of brain circulation to severe chronic hypoxia is achieved by both angiogenesis and microvascular hypertrophy (38, 39). Angiogenesis has been shown to occur also in nerve cell grafts (40) and on spinal cord lesion (41), during peripheral nerve regeneration (42). Angiogenesis is also triggered by physiological tasks that increase neural function and synaptic activity (43). Overall, these data indicate that migration and proliferation of endothelial cells, remodeling of extracellular matrix, and functional maturation of newly assembled vessels can be triggered also in postnatal nontumoral nervous tissue.
This study suggests that angiogenesis is triggered by the increase of scg size because of NGF and NGF + 6-OHDA treatment. We also suggest that in our experimental conditions a “neural drive” for angiogenesis might occur in postnatal nervous tissue, correlated with increased metabolic request, and that survival factors, like NGF, act to promote it. We are of the opinion that NGF promotes neuron-induced angiogenesis by stimulating VEGF production.
A number of stimuli may induce VEGF expression, including several growth factors and cytokines, hormones, phorbol esters, oncogenes, NO, and hypoxia through hypoxia-inducible factor (44). In addition, neuronal VEGF expression has been found to correlate with angiogenesis (45). Moreover, brain-derived neurotrophic factor, a member of the neurotrophin family, also seems to exert a direct angiogenic action on endothelial cells in the heart (46), whereas local NGF administration has proved to increase angiogenesis during sciatic nerve regeneration (47).
The effect of NGF on NO production also supports our hypothesis, because a single NGF injection induces a strong up-regulation of the neuronal isoform of NO synthesis enzyme and of the associated histochemical NAPDH–diaphorase reaction. This is followed by VEGF-induced angiogenesis and consequently by a reduction of NO synthesis enzyme production. We acknowledge that early in hypertrophy induction, a NO-mediated vasodilation allows metabolic supply to occur, whereas later, VEGF-mediated angiogenesis can provide adequate metabolic supply. This pattern of coordinated actions between NO and VEGF has been demonstrated in different tissues (48, 49), under different physiological (50) and pathological nontumoral (51) conditions.
In conclusion, our data show an increased vessel density in superior cervical ganglia of newborn rats treated with 6-OHDA + NGF; this effect is associated with increased expression of the angiogenic peptide VEGF and nNOS in neurons. The results suggest that angiogenesis may be regulated through activation of angiogenic and vasodilation agents directly produced by neurons on request, and that NGF indirectly stimulates this process.
Acknowledgments
The technical assistance of Nadia De Sordi (Department of Veterinary Morphophysiology and Animal Production, University of Bologna) is gratefully acknowledged. This work was supported by the Pathophysiology Center for the Nervous System, Hesperia Hospital, Modena, Italy. The contribution of L.A. was supported by PF5 subproject 3 Biotecnologie, Consiglio Nazionale delle Ricerche, to R.L.-M. and L.A. NGF was prepared by Luigi Manni, a recipient of a grant from the Levi-Montalcini Foundation.
Abbreviations
- NGF
nerve growth factor
- 6-OHDA
6-hydroxy-dopamine
- VEGF
vascular endothelial growth factor
- nNOS
neuronal nitric oxide synthase
- scg
superior cervical ganglia
- PECAM-1
platelet/endothelial cell adhesion molecule-1
- IR
immunoreactivity
References
- 1.Levi-Montalcini R, Booker B. Proc Natl Acad Sci USA. 1960;46:373–384. doi: 10.1073/pnas.46.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ransohoff R M, Trebst C. Exp Med. 2000;191:1625–1630. doi: 10.1084/jem.191.10.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloe L. Microsc Res Tech. 1999;45:285–291. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<285::AID-JEMT12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Patel T D, Jackman A, Rice F L, Kucera J, Snider W D. Neuron. 2000;25:345–357. doi: 10.1016/s0896-6273(00)80899-5. [DOI] [PubMed] [Google Scholar]
- 5.Pincelli C, Marconi A. J Dermatol Sci. 2000;22:71–79. doi: 10.1016/s0923-1811(99)00065-1. [DOI] [PubMed] [Google Scholar]
- 6.Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. N Engl J Med. 1998;338:1174–1180. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- 7.Bernabei R, Landi F, Bonini S, Onder G, Lambiase A, Pola R, Aloe L. Lancet. 1999;354:307. doi: 10.1016/S0140-6736(99)02784-1. [DOI] [PubMed] [Google Scholar]
- 8.Meakin S O, Shooter E M. Trends Neurosci. 1992;15:323–331. doi: 10.1016/0166-2236(92)90047-c. [DOI] [PubMed] [Google Scholar]
- 9.Barbacid M. Curr Opin Cell Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 10.Rush R A, Chie E, Liu D, Tafreschi A, Zettler C, Zhou X F. Clin Exp Pharmacol Physiol. 1997;24:549–555. doi: 10.1111/j.1440-1681.1997.tb02089.x. [DOI] [PubMed] [Google Scholar]
- 11.Levi-Montalcini R, Aloe L, Mugnaini E, Oesch F, Thoenen H. Proc Natl Acad Sci USA. 1975;72:595–599. doi: 10.1073/pnas.72.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angeletti P, Levi-Montalcini R. Proc Natl Acad Sci USA. 1970;65:114–121. doi: 10.1073/pnas.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aloe L, Mugnaini E, Levi-Montalcini R. Arch Ital Biol. 1975;113:326–353. [PubMed] [Google Scholar]
- 14.Sondell M, Lundborg G, Kanje M. Brain Res. 1999;846:219–228. doi: 10.1016/s0006-8993(99)02056-9. [DOI] [PubMed] [Google Scholar]
- 15.Samii A, Unger J, Lange W. Neurosci Lett. 1999;262:159–162. doi: 10.1016/s0304-3940(99)00064-6. [DOI] [PubMed] [Google Scholar]
- 16.Levi-Montalcini R. NeuroReport. 1998;9:R71–R83. [PubMed] [Google Scholar]
- 17.Pozza M, Guerra M, Manzini E, Calzà L. J Rheumatol. 2000;27:1121–1127. [PubMed] [Google Scholar]
- 18.Tuveri M, Generini S, Matucci-Cerinic M, Aloe L. Lancet. 2000;356:1739–1740. doi: 10.1016/S0140-6736(00)03212-8. [DOI] [PubMed] [Google Scholar]
- 19.Mogi M, Kondo A, Kinpara K, Togari A. Life Sci. 2000;67:1197–1206. doi: 10.1016/s0024-3205(00)00705-0. [DOI] [PubMed] [Google Scholar]
- 20.Hammes H P, Federoff H J, Brownlee M. Mol Med. 1995;1:527–534. [PMC free article] [PubMed] [Google Scholar]
- 21.Lome,-Hoeth C, Shooter E M. J Neurochem. 1998;64:1780–1790. doi: 10.1046/j.1471-4159.1995.64041780.x. [DOI] [PubMed] [Google Scholar]
- 22.Bocchini V, Angeletti P U. Proc Natl Acad Sci USA. 1969;64:787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erickson A C, Couchman J R. J Histochem Cytochem. 2000;48:1291–1306. doi: 10.1177/002215540004801001. [DOI] [PubMed] [Google Scholar]
- 24.Paul A, Habib F. Urol Res. 1998;26:111–116. doi: 10.1007/s002400050032. [DOI] [PubMed] [Google Scholar]
- 25.Pflug B R, Dionne C, Kaolan D R, Lynch J, Djakiew D. Endocrinology. 1995;136:262–268. doi: 10.1210/endo.136.1.7828539. [DOI] [PubMed] [Google Scholar]
- 26.You L, Kruse F E, Volcker H E. Invest Ophtalmol Visual Sci. 2000;41:692–702. [PubMed] [Google Scholar]
- 27.Caneva L, Soligo D, Cattoretti G, De Harven E, Deliliers G L. Blood Cells Mol Dis. 1995;21:73–85. doi: 10.1006/bcmd.1995.0011. [DOI] [PubMed] [Google Scholar]
- 28.Yu X, Dillon G P, Bellamkonda R B. Tissue Eng. 1999;5:291–304. doi: 10.1089/ten.1999.5.291. [DOI] [PubMed] [Google Scholar]
- 29.Cowen T, Jenner C, Song G X, Santoso A W, Gavazzi I. Neurochem Res. 1997;22:1003–1011. doi: 10.1023/a:1022478926949. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara N, Davis-Smyth T. Endocrinol Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 31.Iadecola C. Trends Neurosci. 1993;16:206–214. doi: 10.1016/0166-2236(93)90156-g. [DOI] [PubMed] [Google Scholar]
- 32.Breier G, Albrecht S, Sterrer S, Risau W. Development (Cambridge, UK) 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- 33.Marti H H, Risua W. Proc Natl Acad Sci USA. 1998;95:15809–15814. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lennmyr F, Ata K A, Funa K, Olsson Y, Terent A. J Neuropathol Exp Neurol. 1998;57:874–882. doi: 10.1097/00005072-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Kovacs Z, Ikezaki K, Samoto K, Inamura T, Fukui M. Stroke. 1996;27:1865–1872. doi: 10.1161/01.str.27.10.1865. [DOI] [PubMed] [Google Scholar]
- 36.Sondell M, Lundborg G, Kanje M. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plate K H. J Neuropathol Exp Neurol. 1999;58:313–320. doi: 10.1097/00005072-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Harik S I, Hritz M A, LaManna J C. J Physiol. 1995;485:525–530. doi: 10.1113/jphysiol.1995.sp020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patt S, Sampaolo S, Theallier-Janko A, Tschairkin I, Cervos-Navarro J. J Cereb Blood Flow Metab. 1997;17:801–806. doi: 10.1097/00004647-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Krum J M, Rosenstein J M. Exp Neurol. 1998;154:57–65. doi: 10.1006/exnr.1998.6930. [DOI] [PubMed] [Google Scholar]
- 41.Bartholdi D, Rubin B P, Schwab M E. Eur J Neurosci. 1997;9:2549–2560. doi: 10.1111/j.1460-9568.1997.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 42.Hobson M I, Brown R, Green C J, Terenghi G. Br J Plast Surg. 1997;50:125–131. doi: 10.1016/s0007-1226(97)91325-4. [DOI] [PubMed] [Google Scholar]
- 43.Black J E, Isaacs K R, Anderson B J, Alcantara A A, Greenough W T. Proc Natl Acad Sci USA. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klagsbrun M, D'Amore P A. Cytokine Growth Factor Rev. 1996;7:249–257. doi: 10.1016/s1359-6101(96)00027-5. [DOI] [PubMed] [Google Scholar]
- 45.Ogunshola O O, Stewart W B, Mihalcik V, Solli T, Madri J A, Ment L R. Dev Brain Res. 2000;119:139–153. doi: 10.1016/s0165-3806(99)00125-x. [DOI] [PubMed] [Google Scholar]
- 46.Donovan M J, Lin M I, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibanez C F, Rafii S, Hempstead B L. Development (Cambridge, UK) 2000;127:4531–4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- 47.Santos P M, Winterowd J G, Allen G G, Bothwell M A, Rubel E W. Otolaryngol Head Neck Surg. 1991;105:12–25. doi: 10.1177/019459989110500103. [DOI] [PubMed] [Google Scholar]
- 48.Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek G F. Am J Physiol. 1999;276:C812–C820. doi: 10.1152/ajpcell.1999.276.4.C812. [DOI] [PubMed] [Google Scholar]
- 49.Papapetropoulos A, Garcia-Cardena G, Madri J A, Sessa W C. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen B-Q, Lee D, Zioncheck T F. J Biol Chem. 1999;274:33057–33063. doi: 10.1074/jbc.274.46.33057. [DOI] [PubMed] [Google Scholar]
- 51.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Chen D, Symes J F, et al. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]