Abstract
Background and Aims
Gluten proteins are the major storage protein fraction in the mature wheat grain. They are restricted to the starchy endosperm, which forms white flour on milling, and interact during grain development to form large polymers which form a continuous proteinaceous network when flour is mixed with water to give dough. This network confers viscosity and elasticity to the dough, enabling the production of leavened products. The starchy endosperm is not a homogeneous tissue and quantitative and qualitative gradients exist for the major components: protein, starch and cell wall polysaccharides. Gradients in protein content and composition are the most evident and are of particular interest because of the major role played by the gluten proteins in determining grain processing quality.
Methods
Protein gradients in the starchy endosperm were investigated using antibodies for specific gluten protein types for immunolocalization in developing grains and for western blot analysis of protein extracts from flour fractions obtained by sequential abrasion (pearling) to prepare tissue layers.
Key Results
Differential patterns of distribution were found for the high-molecular-weight subunits of glutenin (HMW-GS) and γ-gliadins when compared with the low-molecular-weight subunits of glutenin (LMW-GS), ω- and α-gliadins. The first two types of gluten protein are more abundant in the inner endosperm layers and the latter more abundant in the subaleurone. Immunolocalization also showed that segregation of gluten proteins occurs both between and within protein bodies during protein deposition and may still be retained in the mature grain.
Conclusions
Quantitative and qualitative gradients in gluten protein composition are established during grain development. These gradients may be due to the origin of subaleurone cells, which unlike other starchy endosperm cells derive from the re-differentiation of aleurone cells, but could also result from the action of specific regulatory signals produced by the maternal tissue on specific domains of the gluten protein gene promoters.
Keywords: Triticum aestivum, wheat grain, gluten proteins, bread wheat, immunolocalization, protein bodies, pearling
INTRODUCTION
Wheat is one of the most important and widely consumed food crops, with flour produced from the starchy endosperm storage tissue being used to produce bread, noodles, pasta and a wide range of other food products.
The wheat grain is a single-seeded fruit, or caryopsis, and consists of three distinct parts: the embryo, which forms the next generation; the starchy endosperm, a storage tissue which supports germination and early seedling growth; and the bran, comprising the pericarp (fruit coat), testa (seed coat) and outer endosperm (aleurone layer). The starchy endosperm cells are packed with insoluble storage components, mainly starch and protein, which account for over 80 % of the grain mass. During milling the endosperm is usually separated from the germ and the bran in order to obtain the white flour, which is the most suitable raw material for modern breadmaking.
The endosperm is formed by a double fertilization event within the embryo sac, with two female polar nuclei and one pollen reproductive nucleus fusing to give a triploid constitution (Olsen, 2004). Following fertilization, numerous mitotic divisions of the nuclei which are not accompanied by cell division lead to the formation, after 1 or 2 d, of a single multinucleate cell with a peripheral zone of cytoplasm (syncitium) and a large central vacuole. Cellularization then occurs, initiated by the appearance of anticlinal cell walls which separate the nuclei in the endosperm syncitium and form the so-called ‘alveoli’. The walls are first observed in the syncitium adjacent to the ventral crease (Fineran et al., 1982), and then spread laterally along the endosperm lobes to the dorsal side. Periclinal and anticlinal walls then form between the dividing nuclei, resulting in the formation of several layers of cells. Finally, the cell walls growing from the dorsal and ventral sides of the grain meet, completing the cellularization.
Little is known about the specification of cell fate in the wheat endosperm but it has been suggested to result from positional signalling as observed in maize (Hueros et al., 1995, 1999; Opsahl-Ferstad et al., 1997; Becraft and Asuncion-Crabb, 2000) and barley (Olsen et al., 1990; Doan et al., 1996). Differentiation starts at the beginning of the cellularization stage (Olsen, 2001) when the peripheral layer of cells derived from the first periclinal division of the nuclei in the alveoli begin to differentiate into the aleurone, consisting in wheat of a single layer of cuboid-shaped cells with thick cell walls, large nuclei and dense cytoplasm. Cells of the inner layer differentiate instead into highly vacuolated, thin-walled cells which will constitute the starchy endosperm. Soon after the cellularization phase is completed, cell division resumes in the inner cell files, but unlike the alveolar divisions, which are strictly periclinal, the division planes are orientated randomly so that after one or two rounds of cell division the pattern of cell files is lost. The whole process is completed about 2 weeks after anthesis and subsequent endosperm growth occurs almost exclusively by cell expansion. An exception is presented by the aleurone layer, in which the cells continue to divide periclinally, behaving like a cambium up to 19 d post-anthesis (dpa) (Sandstedt, 1946; Jennings and Morton, 1963). The cell layers immediately below the aleurone, called the subaleurone cells, are therefore considered to result from the division and re-differentiation of aleurone cells. Nevertheless, these cells remain distinct from both the aleurone and the central starchy endosperm cells, as discussed below.
At grain maturity, only the embryo and the aleurone cells remain viable, the subaleurone and central starchy endosperm cells having undergone a process of programmed cell death (PCD), which includes the degeneration of nuclei and cytoplasm (Young and Gallie, 1999). The mature starchy endosperm cells therefore contain starch granules embedded in a protein matrix. The starch is distributed fairly evenly throughout this tissue, particularly in the dorsal part of the grain, with the exception of the sub-aleurone cells where the content of starch is significantly lower (Ugalde and Jenner, 1990a). No starch is present in the cells of the aleurone layer, which are instead rich in protein and triacylglycerols.
The second major cell-specific components of the starchy endosperm cells, and the most important in terms of flour functional properties, are the gluten proteins. These belong to the prolamin family of cereal grain storage proteins and are characterized by their high content of the amino acids proline and glutamine. They are classically divided into monomeric gliadins and polymeric glutenins, with the latter comprising subunits which are further divided on the basis of their molecular masses into high-molecular-weight subunits of glutenin (HMW-GS) and low-molecular-weight subunits of glutenin (LMW-GS). Clear gradients exist in protein concentration across the starchy endosperm, being high in the sub-aleurone cells and lower in the central starchy endosperm cells. Consequently, the sub-aleurone cells in the dorsal region of the grain contain nearly twice the amount of protein (mass per unit fresh volume) compared with the cells adjacent to the endosperm cavity (Ugalde and Jenner, 1990b). Recent studies have also shown that the protein gradient is not only quantitative but also qualitative, in that different sub-classes of gluten proteins are differentially expressed in different regions of the endosperm (Stoger et al., 2001; Lamacchia et al., 2001; Mills et al., 2005; Pistón et al., 2009; Tosi et al., 2009).
The present paper explores these protein gradients in detail by using antibodies recognizing specific gluten protein types.
MATERIALS AND METHODS
Protein extraction and western blot analysis
Wholemeal flour was prepared from field-grown grain of wheat Triticum aestivum ‘Cadenza’ using a ball mill. Total grain protein was extracted with 25 µL mg−1 of dry weight extraction/loading buffer: 50 mm Tris–HCl (pH 6·8), 2 % (w/v) sodium dodecylsulphate, 10 % (v/v) glycerol, 0·1 % (w/v) bromophenol blue and 200 mm dithiothreitol (DTT). Gluten proteins were extracted by sequential extraction: monomeric gliadins were extracted using 16 µL mg−1 dry weight of 70 % (v/v) ethanol; the remaining pellet was dried in a Speedy Vac and then re-extracted with the same volume of 50 % (v/v) propan-1-ol + 2 % DTT. Extracts were dried in a Speedy Vac and resuspended in loading buffer (see above). Samples were denatured at 80 °C for 3 min, and then separated on a precast 4–12 % Bis-Tris Nu-Page gel (Invitrogen, Paisley, UK). For western blotting, replicate 15-μL aliquots of total protein extracts were separated in adjacent lanes on the same gel and blotted onto nitrocellulose paper (HybondN + ; Amersham, Bucks., UK) following the manufacturer's instructions. The membranes were stained with Ponceau S solution (Sigma P7170, Gillingham, UK) and strips corresponding to individual lanes were probed separately with the different antibodies.
Strips were blocked with 5 % (w/v) skimmed dried milk in Tris-buffered saline (TBS) (20 mm Tris, 500 mm NaCl, pH adjusted to 7 with HCl) at room temperature for 1 h, and then incubated in primary antibody solution [1 % (w/v) bovine serum albumin (BSA) in 0·05 % (v/v) Tween in TBS] for a further 1 h. Antibodies used and their dilutions were as follows:
IFRN 0610 mouse monoclonal antibody which recognizes an epitope (QQSF) common to many gliadins and LMW-GS but not to HMW-GS (Brett et al., 1999), 1 : 2000.
Glia-α-9, 9-68 mouse monoclonal, specific for an epitope present on α-gliadins (Mitea et al., 2008), 1 : 10 000.
R2-HMG rabbit polyclonal, raised against an HMW subunit peptide (GYYPTSPQQPGC) and specifically recognizes HMW-GS (Denery-Papini et al., 1996), 1 : 5000.
Anti-NT2-ω, rabbit polyclonal antibody, raised against the N-terminal sequence (SRLLSPRGKELGC) of ω5-gliadins (Denery-Papini et al., 2000), 1 : 2000.
S3B512 mouse monoclonal antibody, raised against a peptide from the γ-gliadin repetitive domain (PEQPFPQGC) specific for γ-gliadins (INRA, Nantes, France), 1 : 2000.
Membranes were washed three times in 0·05 % (v/v) Tween in TBS and incubated in secondary antibody solution (either anti-mouse or anti-rabbit alkaline phosphatase conjugated at a 1 : 6250 dilution). After 1 h of incubation, the membranes were rinsed three times, for 5 min each in 0·05 % (v/v) Tween in TBS, then once more in TBS and finally developed using a ready-made NBT/BCIP developing solution (Sigma-Aldrich).
Pearling of wheat seeds
A test run was carried out on 50-g seeds of wheat ‘Cadenza’ in a pearling mill (Streckel & Schrader, Hamburg, Germany) to determine the proportions of the grain removed (shell rates) after 1, 2, 3, 4, 5 and 10 min. A cubic interpolative curve was then used to calculate the times required to remove 7, 13, 20, 30, 40 and 50 % of the total seed weight.
Six pearling fractions were therefore prepared by sequential pearling using the predicted pearling times, corresponding to 7, 6, 7, 10, 10 and 10 % of the grain weight. These are called fractions 1–6, and correspond to flours enriched in pericarp tissue, aleurone layer, sub-aleurone layer and three progressively more central areas of the starchy endosperm, respectively. The grain remaining after pearling, which corresponded to about 50 % of the original weight, was milled in a ball-mill and called fraction 7.
SDS–PAGE gel scanning and protein quantification
Protein extracts were separated by SDS–PAGE as described above. Gels were stained overnight with standard gel stain for total proteins [40 % (v/v) methanol, 10 % (v/v) trichloroacetic acid (TCA), 0·1 % (w/v) Coomassie Brilliant Blue R-250] and destained for 24 h in 10 % (w/v) TCA, followed by 2 h in distilled water.
Gels were scanned with an HP scanner and groups of proteins were quantified using Phoretix™ 1D software (Nonlinear Dynamycs, Durham, NC, USA) based on band intensity and area. For analysis, bands were divided into two main groups corresponding to HMW-GS and LMW-GS + gliadins.
Plant material for microscopy
Plants of wheat ‘Cadenza’ were grown under glasshouse conditions at Rothamsted Research as previously described by Tosi et al. (2004). Developing caryopses were harvested from the middles of ears at 8, 14, 21 and 28 dpa and immediately prepared for microscopy.
Sample preparation for microscopy
Transverse sections (approx. 1 mm thick) were cut in fixative from the middle of the grain. Sections were fixed for 5 h at room temperature in 4 % (w/v) paraformaldehyde and 2·5 % (w/v) glutaraldehyde in 0·1 m Sorenson's phosphate buffer (prepared with NaH2PO4.2H2O and Na2HPO4.12H2O), pH 7·2. After three rinses in buffer the specimens were dehydrated in an ethanol series, infiltrated with LR White Resin (medium grade, TAAB L012) for several days and polymerized at 55 °C.
Semi-thin (1 µm) sections were cut using a Reichert-Jung Ultracut ultramicrotome, collected on drops of distilled water on multi-well slides coated with poly-l-lysine hydrobromide (Sigma P1399) and dried on a hot plate at 40 °C. Sections for general morphology and observation of protein bodies were stained with 0·01 % (w/v) Toluidine Blue in 1 % (w/v) sodium tetraborate, pH 9, and examined with bright-field optics on a Zeiss Axiophot microscope. Some sections were also stained with 1 % Naphtol Blue Black (also called Amido Black, C.I. 20170) in 7 % (v/v) acetic acid, which stains proteins specifically (Fisher, 1968; Dwarte and Ashford, 1982).
Immunofluorescence
Fixed and resin-embedded grain sections were briefly rinsed with PBST [PBS, Sigma A4417, 0·1 % (v/v) Tween 20, pH 7·4] and incubated in blocking solution [5 % (w/v) BSA (Sigma A7638) diluted in PBST] for 40 min at room temperature. This was followed by incubation in the primary antibodies diluted in 1 % BSA (w/v) in PBST for 2 h at room temperature. Antibodies were used singly or in combination (dilutions are shown in parentheses). The rabbit polyclonal anti-HMW-R2 (1 : 100) was combined with the mouse monoclonal anti-IFRN 0610 (1 : 100), the mouse monoclonal anti-α-gliadin (glia-α-9, 9-68) (1 : 1000) or the mouse monoclonal anti-γ-gliadin (1 : 100). The rabbit polyclonal anti-ω-gliadin-5 (1 : 200) was combined with the mouse monoclonal anti-IFRN 0610 (1 : 100) or the mouse monoclonal anti-α-gliadin (glia-α-9, 9-68) (1 : 1000). The unbound primary antibodies were removed by several rinses with PBST for a period of 20 min. The sections were then incubated for 1 h at room temperature in the dark with the secondary antibodies (Alexa Fluor 488 or 568 goat anti-rabbit or anti-mouse IgG; Invitrogen A-11001, A-11004) diluted 1 : 250 in 1 % BSA in PBST. Finally, the slides were rinsed twice with PBST and three times with PBS.
Sections were examined with a Zeiss Axiophot epifluorescence microscope. A Retiga Exi CCD digital camera (Qimaging, Surrey, BC, Canada) and MetaMorph software version 7·5·5 (Molecular Devices, Sunnyvale, CA, USA) were used to acquire the images.
RESULTS
Specificity of antibodies
The specificity of a library of antibodies raised against purified gluten fractions or synthetic peptides based on gluten protein sequences was determined by western blotting of a total protein extract from wholemeal flour of wheat ‘Cadenza’ after separation by SDS–PAGE.
This showed that that the library of antibodies recognized almost the entire range of gluten proteins (Supplementary Data S1, available online). The polyclonal antibodies R2-HMW and anti-NT2-W were raised in rabbit against synthetic peptides and recognize, respectively, HMW-GS (both x- and y-type) and ω-5-type gliadins encoded by the B genome of hexaploid bread wheat (Tatham and Shewry, 1985; Dupont et al., 2000). IFRN 0610 is a monoclonal antibody raised against a total glutenin fraction and recognizes the sequences QQSF and QQSY common to most wheat prolamins but not to HMW-GS. This antibody binds to several gliadins and LMW-GS bands. The α-gli-9 antibody is also monoclonal and recognizes a coeliac-toxic motif present in α-gliadins. It binds to several bands in the α-gliadin size range but also, weakly, to ω-gliadins. Finally, the monoclonal γ-gliadin anti-peptide antibody binds specifically to γ-gliadin repeats.
All the antibodies described above were used for immunomicroscopy while the ω-, α- and γ-gliadin antibodies were also tested on SDS–PAGE separations of the pearling fractions.
Protein composition of pearling fractions
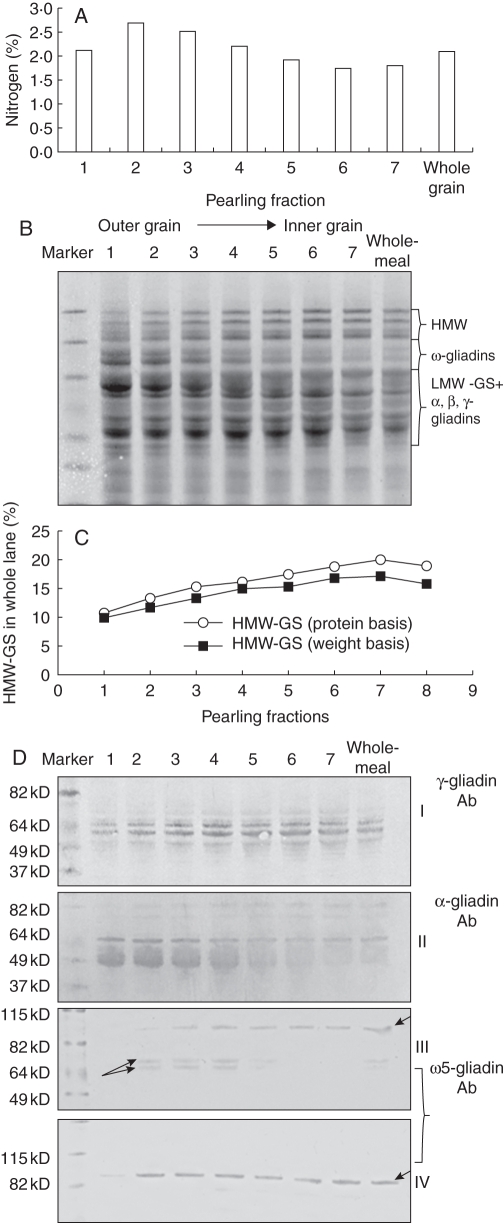
A series of fractions, each corresponding to between 6 and 10 % of the grain dry weight, was prepared by sequential pearling of wheat grain (Supplementary Data S2). These fractions showed a clear gradient in N content, with fraction 2, corresponding to the outermost part of the grain endosperm, containing about 50 % more nitrogen on a dry weight basis than fraction 6 corresponding to the inner endosperm cells (Fig. 1A). SDS–PAGE (Fig. 1B) and western blotting analysis was carried out on these fractions with antibodies against specific gluten protein types (Fig. 1D), loading either equal amounts of protein or protein from equal amounts of the pearling fractions, to take account of the quantitative protein gradient. Figure 1C shows the quantification of HMW-GS in the various pearling fractions using densitometric scanning of SDS–PAGE gels (Supplementary Data S3) while Fig. 1D shows results of western blotting with the ω-, γ- and α-gliadin antibodies. These analyses show gradients in all types of gluten protein. The HMW-GS are readily identified and quantified on SDS–PAGE and clearly increased in proportion from fraction 1 (corresponding to the outer layers, aleurone and sub-aleurone) to fraction 6 (inner starchy endosperm). Changes in the intensity and number of bands corresponding to LMW-GS and gliadins are also evident across the fractions. However, as the α-gliadins, γ-gliadins and LMW-GS bands overlap in the SDS–PAGE separations the differences were not readily quantified by gel scanning. It was also not possible to quantify the relative amount of ω-gliadins by gel scanning due the presence of bands corresponding to non-gluten proteins which overlapped with the ω-gliadins in the total SDS extract separations. Western blotting with gliadin-specific antibodies was therefore used, revealing contrasting gradients in distribution for the α-gliadins which were more abundant in the outer layers and the γ-gliadins which were more abundant in the inner part (similar in distribution to the HMW-GS). The distribution of ω-gliadins was determined using an antibody specific for ω-5 gliadins. This recognized several bands which differed in their distribution, with two bands of Mr of approx. 70 kDa (see arrows in Fig 1D, III) being abundant in the outer layers and practically absent from the inner ones and a major band of Mr of approx. 90k Da (see arrowhead in Fig 1D, III and IV) being more evenly distributed through the endosperm. The two ω-gliadin bands that were unevenly distributed across the grain were not extracted by 70 % (v/v) ethanol but were extracted by 50 % (v/v) propan-1ol + DTT. An antibody against LMW-GS was also used which recognized four bands which were equally abundant in all six pearling fractions (data not shown).
Fig. 1.
Analyses of grain nitrogen and protein content in pearling fractions. (A) Percentage nitrogen in pearling fractions. Pearling fractions correspond to 1st = 7 %, 2nd = 6 %, 3rd = 7 %, 4th = 10 %, 5th = 10 %, 6th = 10 %, 7th = 50 % grain weight. Fraction 7 was obtained by ball milling of pearled grains. (B) SDS–PAGE of total protein extracts from pearling fractions and wholemeal flour. The same amount of protein was loaded in each lane. The position on the gel of the main gluten protein types are indicated on the right. (C) Percentage of HMW-GS in total protein extracts of pearling fractions calculated using scanning gel analysis. The diagram shows the results acquired from both gels loaded on a same-weight basis (extracts from the same amount of flour were loaded for each pearling fraction) and gels loaded on a same-protein basis (the same amount of protein was loaded for each pearling fraction), to take in account the quantitative protein gradient. (D) Western blot analysis of protein extracts from pearling fractions. Western blots I and II correspond to total protein extracts probed with antibodies against γ-gliadin and α-gliadin, respectively. Western blots III and IV correspond to glutenin and gliadin fractions of gluten, respectively, probed with the antibody specific for ω-5 gliadins. Arrows with different orientations indicate proteins having opposite gradients of distribution across the grain.
Spatial patterns of gluten protein distribution
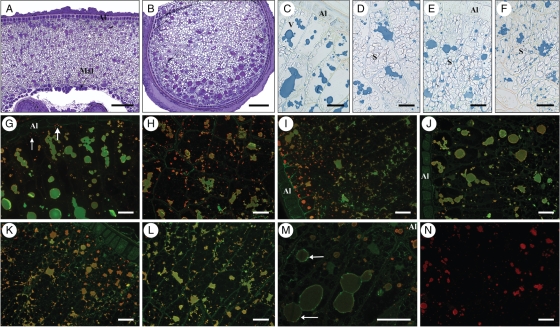
Analysis of pearling fractions revealed clear gradients in the total protein content and in the distributions of different groups of gluten proteins within the starchy endosperm. However, this approach does not provide detailed information on the composition of different cell layers and types. The patterns of distribution of the different groups of gluten proteins at the tissue and cell levels were therefore determined by indirect fluorescence microscopy, using combinations of specific primary antibodies in double labelling experiments and detection with secondary antibodies conjugated to either AlexaFluor 488 or 568. These fluorophores fluoresce in the green and red regions of the spectra, respectively, with no overlapping of their excitation or emission spectra. Five different primary antibodies were used on cross-sections of wheat grain at four different stages of development: 8, 14, 21 and 28 dpa. Toluidine Blue and Naphthol Blue stains were also used at each stage to determine grain tissue structure and development.
8 dpa
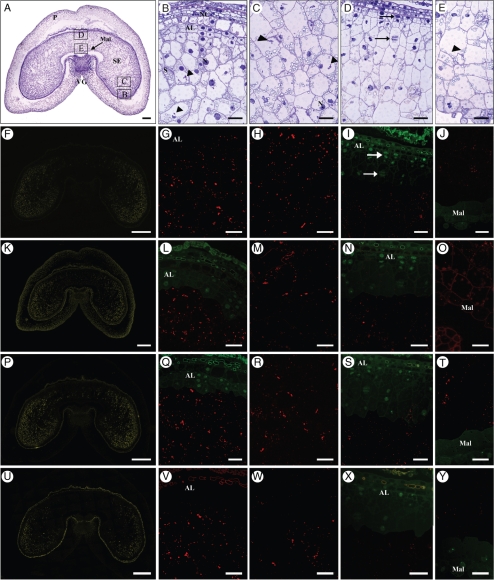
Toluidine Blue staining of the sections at 8 dpa (Fig. 2A–E) shows a fully cellularized endosperm with a clearly defined endosperm cavity, and the cell files produced by periclinal division of the outer layer of endosperm cells are still recognizable both in the lobes (Fig. 2B) and in the dorsal region (Fig. 2D), opposite the endosperm cavity. The aleurone layer is still not fully differentiated although the layer of cells closest to the pericarp appears to have a denser cytoplasm and no starch granules. Starch granules are present in cells two to three layers in from the pericarp and are usually located at the periphery of the cells, as most of the centre of the cell is occupied by a large vacuole. Cell division can be observed in the outer layers, up to two or three layers from the pericarp (see arrows in Fig. 2D).
Fig. 2.
Transverse sections of 8–10-dpa grain. (A–E) Toluidine Blue stain. (A) Low-magnification view: scale bar = 250 µm. The areas labelled B–E show the regions of the grain that were studied in detail and correspond to parts (B)–(E) of the figure. Scale bars in (B–E) = 50 µm. (B) Lobe outer region. Small protein bodies (arrowheads) are already present in the vacuoles of developing starchy endosperm cells. (C) Lobe central area. Small protein bodies can be seen in endosperm cells, but most of them are smaller than the one observed in (B). (D) Dorsal region of the grain. Arrows show actively dividing cells. (E) Ventral region of the grain opposite the ventral groove and comprising modified aleurone cells. Protein bodies are very small. (F–J) Single labelling showing the distribution of LMW-GS and gliadins when probed with the IFRN 0610 antibody and detected with the goat anti-mouse AlexaFluor 568 secondary antibody. (F) Low-magnification view: scale bar = 500 µm. Labelling is strongest in the lobes and decreases toward the dorsal and ventral areas of the grain. Scale bars in (G–J) = 50 µm. (G) Lobe region. Protein bodies are strongly labelled as shown by the red fluorescence. (H) Central area of the lobe. (I) Dorsal region of the grain. Protein bodies are much smaller than in the lobes and no protein bodies are present in cell layers still undergoing division (arrows). (J) Ventral region of the grain. Protein bodies are very small, but already clearly labelled. (K–O) Single labelling showing the distribution of HMW-GS when probed with the polyclonal antibody R2-HMG specific for HMW glutenin subunits and detected with the goat anti-rabbit AlexaFluor 568 secondary antibody. (K) Low-magnification view: scale bar = 500 µm. Labelling is strongest in the lobes, where protein bodies are larger. Scale bars in (L–O) = 50 µm. (L) Lobe region. Clear labelling of protein bodies is observed below the differentiating aleurone layer. (M) Central part of the lobe. (N) Dorsal part of the grain. Protein bodies are very small and labelling is very weak. (O) Ventral region. (P–T) Single labelling showing the distribution of α-gliadins when probed with the monoclonal glia-α-9, 9-68 antibody and detected with the goat anti-mouse AlexaFluor 568 secondary antibody. (P) Low-magnification view: scale bar = 500 µm. Labelling is stronger in the lobes. Scale bars in (Q–T) = 50 µm. (Q) Lobe region. Protein bodies are strongly labelled in cells just below the layer of still dividing outer endosperm cells. (R) Central part of the lobe. Protein bodies are clearly labelled. (S) Dorsal region of the grain. Protein bodies are small but clearly labelled. (T) Ventral region. Protein bodies are small but clearly labelled. (U–Y) Single labelling showing the distribution of γ-gliadin when probed with the monoclonal S3-B512 monoclonal antibody and detected with the goat anti-mouse AlexaFluor 568 secondary antibody. (U) Low-magnification view: scale bar = 500 µm. Labelling is stronger in the lobes. Scale bars in (V–Y) = 50 µm. (V) Lobe region. Protein bodies are strongly labelled in cells below the layer of still dividing outer endosperm cells. (W) Central area of the lobe. (X) Dorsal region. Protein bodies are very small and very weakly labelled below the layer of dividing cells. (Y) Ventral region. Labelling is extremely weak in this area of the grain. Note: in (F), (K), (P) and (U) the colour has been changed from red to yellow to facilitate visualization. In (I), (J), (L), (N), (Q), (S), (T), (X) and (Y), in order to help visualization of the cells' content, immunolabelling images (red) were partly overlaid with images from the green channel showing autofluorescence. Abbreviations: AL, aleurone; Mal, modified aleurone; N, nucleus; NC, nucellus; S, starch; SE, starchy endosperm; P, pericarp; VG, ventral groove. Arrows indicate dividing nuclei. Arrowheads indicate protein bodies in vacuoles.
At this stage the protein bodies are very small and can usually be identified inside the vacuoles (arrowheads in Fig. 2B, C, E). They are strongly stained by Toluidine Blue at this magnification (20×) and, unlike the starch granules, are clearly visible only in the lobe regions and from three cell layers in from the pericarp; their size appears to be similar across the different cells in the region. Immunostaining for gluten proteins with the IFRN 0610 antibody (Fig. 2F–J) shows clear labelling of the lobes with little labelling of the dorsal region opposite the endosperm cavity. However, higher magnification shows that protein bodies are in fact present in this dorsal region, but only in the cells several layers beneath the pericarp, and are much smaller than those in the lobes. In general, cells that are still actively dividing (see Fig. 2D, arrows) do not appear to accumulate proteins although starch granules are clearly visible.
Immunostaining with the HMW-GS antibody (Fig. 2K–O) gives a weaker signal than with the IFRN 0610 antibody but also shows that the signal is concentrated in the lobe regions (Fig. 2L), although labelling of very small protein bodies in the region proximal to the endosperm cavity is also observed (Fig. 2O).
The labelling pattern obtained with the glia-α-9 antibody (Fig. 2P–T) is very similar to that observed with IFRN 0610 except that some labelling is also visible in the dorsal region although restricted to cells four to five layers from the pericarp.
Immunostaining with the γ-gliadin-specific monoclonal antibody (Fig. 2U–Z) gives a similar pattern of labelling to that with the HMW-glutenin antibody, in being almost absent from the dorsal region and strongest in the most distal region of the lobes.
14 dpa
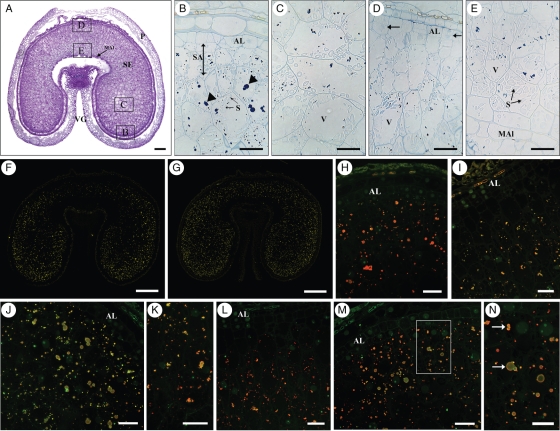
In grains at 14 dpa the aleurone layer is almost fully differentiated (Fig. 3A, B, D) but periclinal divisions in this layer are still clearly visible (Fig. 3D, arrows), especially in the upper dorsal region, where cell files are also present. The cells in the dorsal region have maintained a prismatic shape while those in the lobes are more rounded.
Fig. 3.
Transverse sections of 14-dpa grain. (A) Toluidine Blue stain. Scale bar = 250 µm. The areas labelled B–E show the regions of the grain that were studied in detail, and correspond to the figure parts (B) to (E). (B–E) Naphthol Blue Black stain; scale bars = 50 µm. (B) Lobe region. Protein bodies have enlarged, but are smaller in the sub-aleurone layer. (C) Lobe centre area. Protein bodies are small. (D) Dorsal region. Evidence of periclinal divisions can still be seen (arrows). Protein bodies are very small and more evident towards the inner area of the grain. (E) Ventral area next to modified aleurone. Starch is very abundant. (F) Single labelling showing the distribution of γ-gliadin when probed with the S3-B512 monoclonal antibody and detected with the goat anti-mouse AlexaFluor 568 secondary antibody. Labelling is stronger in the lobes. (G) Single labelling showing the distribution of HMW-GS when probed with the polyclonal anti-HMW-R2 antibody and detected with the goat anti-rabbit AlexaFluor 568 secondary antibody. Labelling is fairly even across the grain. (H, I) Double labelling showing the distribution of HMW-GS, in green, and γ-gliadins, in red. The two types of gluten proteins are co-localized in protein bodies throughout the endosperm. (J, K) Double labelling showing the distribution of HMW-GS, in green and α-gliadins, in red (monoclonal glia-α-9, 9-68 antibody detected with the goat anti-mouse AlexaFluor 568 secondary antibody). Small protein bodies appear enriched in α-gliadins. (L–N) Double labelling with antibody specific for HMW-GS (green) and antibody IFR 0610, recognizing LMW-GS and gliadins (red): (L) dorsal region; (M) sub-aleurone cells in the lobe; (N) enlargement of area within the square in panel (M). Arrows show protein bodies differentially enriched in HMW-GS (green) or LMWGS and gliadins (orange). Scale bars: (F, G) = 500 µm, (H–J, L,M) = 25 µm, (K, N) = 50 µm. Note: In (F) and (G) the colour has been changed from red to yellow to facilitate visualization. Abbreviations: AL, aleurone; MAl, modified groove aleurone; S, starch; SA, sub-aleurone layer; SE, starchy endosperm; P, pericarp; VG, ventral groove. Arrowheads indicate protein bodies in vacuoles.
The amount of starch is greater compared with 8 dpa, particularly in the area just adjacent to the endosperm cavity (Fig. 3E), but vacuoles still form a significant proportion of the total volume of the endosperm cells. The protein bodies present in the cells two and three layers below the newly differentiated aleurone layer in the lobe regions are much larger and more rounded (Fig. 3B, arrowheads) than those in the cells directly adjacent to the aleurone, and than those in the cells of the inner endosperm, which are smaller and form irregularly shaped clusters.
Immunolabelling with the γ-gliadin antibody (Fig. 3F) shows labelling in both the lobes and the upper dorsal region, although the labelling remains stronger in the lobes, particularly in the more distal parts. By contrast, the HMW antibody (Fig. 3G) shows a more even pattern of labelling across the seed with the intensity being similar in the lobes and the dorsal region. Double labelling was also carried out with the HMW antibody and the γ-gliadin antibody (Fig. 3H, I), showing that these two types of gluten protein are co-localized in protein bodies throughout the endosperm. However, the protein bodies in the dorsal region (Fig. 3I) appear to contain a lower ratio of γ-gliadin/HMW-GS compared with the protein bodies in the lobes (Fig. 3H), staining yellow compared with orange (due to a stronger green component from the fluorophore attached to the HMW subunit antibody). Neither of the antibodies shows significant labelling of the sub-aleurone cells, and labelling is absent from the dorsal region of the seed (Fig. 3I).
Co-location is also observed for HMW-GS and α-gliadins (Fig. 3J, K) but it is clear that many of the protein bodies, and in particular the smaller ones, are differentially enriched in one or other of these two types of gluten protein. In particular, the α-gliadins appear to be more abundant than the HMW-GS in the cells near the endosperm cavity, adjacent to the modified aleurone layer (Fig. 3K).
Differences are observed in the patterns of labelling with the IFRN 0610 and the HMW-GS antibodies (Fig. 3L–N) indicating differences in the distributions of the types of proteins that they recognize. The heaviest labelling by the IFRN 0610 antibody is observed in the dorsal region (Fig. 3L) and in the sub-aleurone cells in the lobes (Fig. 3M). The fusion of small protein bodies differentially enriched in HMW-GS or in the gliadins and LMW-GS recognized by IFRN 0610 is also observed in several cells. It can also be noted that the IFRN 0610 antibody labelled the small protein bodies more heavily than the large protein bodies (arrows in Fig. 3N) throughout the endosperm, while the HMW-GS antibody showed the opposite pattern.
The antibody specific for LMW-GS could not be used on sections for microscopy, probably due to denaturation of the corresponding epitope during fixation and embedding.
21 dpa
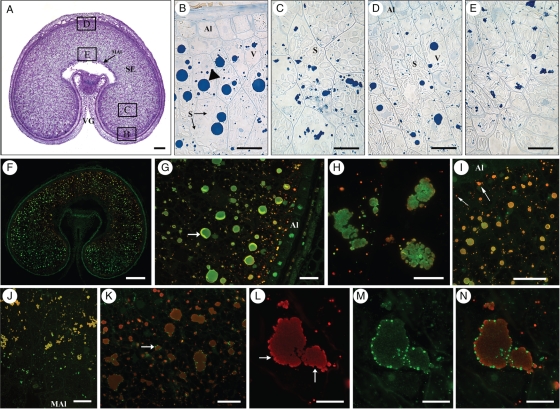
At 21 dpa the aleurone layer is completely differentiated and the cells have dense cytoplasm which stains intensely with toluidine blue (Fig. 4A). Periclinal divisions of aleurone cells are still visible in the dorsal region but are infrequent (data not shown). The starchy endosperm cells have accumulated more starch, which now fills most of the cell volume, while the vacuoles, especially in the dorsal region, are much smaller and contain large protein bodies.
Fig. 4.
Transverse sections of 21-dpa grain. (A) Toluidine Blue stain: scale bar = 250 µm. The areas B–E show the regions of the grain that were studied in detail and correspond to the figure parts (B)–(E). (B–E) Naphtol blue black stain. Scale bars = 50 µm. (B) Lobe region. Arrowhead shows a large protein body in the sub-aleurone. (C) Lobe centre area. Protein bodies are smaller than in the sub-aleurone. (D) Dorsal region. The aleurone layer is well differentiated (AL). (E) Ventral area next to modified aleurone. Protein bodies are smaller and are often in clusters. (F–H) Double labelling showing the distribution of HMW-GS in green (probed with the anti-HMW-R2 polyclonal antibody and detected with the goat anti-rabbit AlexaFluor 488 secondary antibody) and gliadins and LMW-GS (probed with the IFRN 0610 monoclonal antibody and detected with the goat anti-mouse AlexaFluor 568 secondary antibody). (F) Whole grain. The more pronounced red fluorescence in the outermost cell layers suggests enrichment in LMW-GS and gliadins, particularly in the smaller protein bodies. Scale bar = 500 µm. (G) Lobe, sub-aleurone region. Larger protein bodies appear rich in HMW-GS as indicated by the stronger green fluorescence, while smaller protein bodies in the subaleurone layers exhibit more red fluorescence, suggesting a higher LMW-GS and gliadin content. The arrow shows a fold in the sample due to incomplete stretching of the resin. Scale bar = 50 µm. (H) Protein bodies from the centre of the lobe showing aggregation and fusion of protein bodies. Scale bar = 25 µm. (I) Lobe area. Double labelling with antibodies specific for HMW-GS, in green, and α-gliadins, in red. α-gliadins are more abundant than HMW-GS in the protein bodies of sub-aleurone cells (arrows). Scale bar = 50 µm. (J) Double labelling showing the distribution of HMW-GS in green and γ-gliadin in red, in cells adjacent to the endosperm cavity (modified aleurone). The orange colour of the protein bodies suggests their enrichment in α-gliadins. Scale bar = 50 µm. (K–N) Double labelling showing the distribution of ω5-gliadins in green (anti-NT2-ω, rabbit polyclonal antibody detected with the goat anti-rabbit AlexaFluor 488 secondary antibody) and the α-gliadins in red (monoclonal glia-α-9, 9-68 detected with the goat anti-mouse AlexaFluor 568 secondary antibody). ω5-gliadins are concentrated in small deposits on the surface of protein bodies (arrows). (L, M) Single channel images showing the distribution of α-gliadins (L) and ω5-gliadins (M) in protein bodies of a sub-aleurone cell. (N) Double channel image obtained by combining (L) and (M). Scale bar: (K) = 50 µm, (L–N) = 25 µm. Abbreviations: Al, aleurone; MAl, modified groove aleurone; S, starch; V, vacuole; SE, starchy endosperm; VG, ventral groove. Arrowheads indicate protein bodies in vacuoles.
In general, larger protein bodies are observed in the sub-aleurone cells in the lobe regions (Fig. 4B), but large protein bodies can also be observed at this stage in the dorsal region of the grain (Fig. 4D). The sub-aleurone protein bodies retain a round, regular shape while the protein bodies in the central endosperm appear to be clusters of smaller protein bodies and have irregular shapes (compare Fig. 4B, D with Fig. 4E). It is also clear from several micrographs that the enlargement of the large protein bodies occurs at this stage by fusion of smaller protein bodies (Fig. 4B–D, arrows), as the outlines of the individual small protein bodies are distinguishable.
Double labelling with the IFRN 0610 and the HMW-GS antibodies (Fig. 4F–H) shows clear segregation in the deposition of the gluten proteins recognized by these antibodies.
The gliadins and LMW-GS recognized by IFRN 0610 appear more abundant in the outermost cell layers (Fig. 4F) and enriched in the smaller protein bodies while the larger protein bodies, both in the sub-aleurone layer and in the inner endosperm cells, are enriched in HMW-GS (Fig. 4G, H). Overall, gliadins and LMW-GS are more abundant in the dorsal regions of the grain and around the endosperm cavity (see Fig. 4F).
As observed at 14 dpa, double labelling with the antibodies specific for HMW-GS and α-gliadins (Fig. 4I) indicates that the deposition of these two types of proteins is initially segregated, with the α-gliadins being more abundant in small protein bodies. Furthermore, at this stage, α-gliadins appeared to be more abundant than HMW-GS in the protein bodies of sub-aleurone cells (Fig. 4I, arrows).
Double labelling with the antibodies for HMW-GS and γ-gliadins indicates that these two types of protein are co-located in the protein bodies of the central endosperm, both in the lobes and in the dorsal region (data not shown). However, very little γ-gliadin appears to be present in the outer layers of the endosperm and in the cells adjacent to the endosperm cavity (Fig. 4J).
Very clear segregation is observed when the antibody specific for ω-5 gliadins is used in double labelling experiments with the α-gliadin antibody (Fig. 4K–N), with the ω-5 gliadin antibody strongly labelling small deposits on the periphery of the large protein bodies labelled by the α-gliadin antibody. However, whereas the small peripheral deposits are not labelled by the α-gliadin antibody (Fig. 4L, arrows), weak uniform labelling of the large protein bodies is observed with the ω-5 gliadin antibody (Fig. 4M, N).
28 dpa
At 28 dpa nuclei are no longer recognizable in the starchy endosperm cells which, particularly in the dorsal region of the grain and in the inner layer of the lobes, are packed with starch and protein (Fig. 5D–F). Furthermore, in some cells the protein bodies have merged to form a continuous matrix surrounding the starch granules (Fig. 5D). However, the presence of a continuous protein matrix is not observed in the sub-aleurone cells of the lobe regions (Fig. 5B, C) where it is possible to observe fusion of large protein bodies inside still clearly recognizable vacuoles. Particularly notable at this stage is that the protein bodies of the sub-aleurone layer differ in shape from those in the inner endosperm cells, being rounded rather than irregular.
Fig. 5.
Transverse sections of 28-dpa grain. (A, B) Toluidine Blue stain. Scale bars = 250 µm. (A) Dorsal region of the grain. (B) Lobe. (C–F) Naphtol Blue Black stain. Scale bars = 50 µm. (C) Lobe region. Larger protein bodies have formed by fusion of smaller protein bodies. (D) Lobe centre area. Cells are packed with small and large starch granules and protein bodies are fusing. (E) Dorsal region. (F) Ventral area next to modified aleurone. (G–I) Double labelling showing the distribution of HMW-GS in green (HMW-R2 polyclonal antibody detected with the goat anti-rabbit AlexaFluor 488 secondary antibody) and LMW-GS and gliadins in red (IFRN 0610 monoclonal antibody detected with the goat anti-mouse AlexaFluor 568 secondary antibody). Scale bars = 50 µm. (G) Lobe region. Large protein bodies are rich in HMW-GS whereas the small protein bodies in the subaleurone remain rich in LMW-GS and gliadins, as shown by the red and dark orange fluorescence (arrows). (H) Centre of the lobe. Small protein bodies appear enriched in LMW-GS (red fluorescence). (I) Dorsal area. The subaleurone layer is rich in protein bodies, which fluoresce red or dark orange, indicating enrichment in LMW-GS and gliadins. (J–L) Double labelling showing the distribution of HMW subunits in green (as above) and α-gliadin in red (monoclonal glia-α-9, 9-68 antibody detected with the goat anti-mouse AlexaFluor 568 secondary antibody). (J) Lobe region. (K) Dorsal region. (L) Dorsal area close to the endosperm cavity (Mal). (M) Lobe region. Double labelling showing the distribution of ω5-gliadins in green (anti-NT2ω polyclonal antibody detected with the goat anti-rabbit AlexaFluor 488 secondary antibody) and LMW-GS and gliadins in red (as above ). The small deposits on the periphery of protein bodies are still clearly labelled with the antibody specific for ω5-gliadin (arrows); the protein bodies in the subaleurone layers are rich in LMW-GS, as indicated by their red/dark orange fluorescence. Scale bar = 50 µm. (N) Central part of the lobe. Single labelling showing the distribution of γ-gliadin (S3-B512 monoclonal antibody detected with the goat anti-mouse AlexaFluor 568 secondary antibody). Scale bar = 50 µm. Abbreviations: Al, aleurone; Mal, modified groove aleurone; S, starch; V, vacuole.
Immunolabelling (Fig. 5G–N) shows that the large protein bodies in the cells below the sub-aleurone (comprising two cell layers) and throughout the inner endosperm are enriched in HMW-GS whereas the smaller protein bodies, and in particular those in the sub-aleurone adjacent to the aleurone, are more heavily labelled with the α-gliadin antibody and by IFRN 0610 antibody. Heavier labelling of the small protein bodies in the inner endosperm of the lobes is observed with IFRN 0610 than with the antibodies specific for α- and γ-gliadins, suggesting that these protein bodies are enriched in the LMW-GS recognized by IFRN 0610.
DISCUSSION
The functional properties of the wheat grain are determined mainly by the gluten protein fraction, in terms of both protein content and protein quality, and wheat flours from different mill-streams, which derive from different regions of the endosperm, have different breadmaking properties, due to the presence of compositional gradients. Typically, the protein percentage in the mature endosperm is low in the cells near the endosperm cavity and increases in an outward radial direction (Morris et al., 1945; Normand et al., 1965; Farrand, 1974; Farrand and Hinton, 1974) while the percentage of starch follows an inverse gradient. Consequently, it is possible to isolate high protein flour fractions derived from cells of the outer (i.e. sub-aleurone) layers of the starchy endosperm and low protein flour fractions from cells of the central part of the grain. However, the protein gradient is also qualitative as demonstrated by the fact that the flour fractions derived from the central endosperm, although lower in total protein content, generally have better functional properties than flour derived from the outer layers (Yahata et al., 2006; Okrajková et al., 2007).
In this paper immunostaining microscopy was used to study the in vivo distribution of gluten proteins in bread wheat, revealing qualitative gradients in the various types of gluten proteins across the grain. This confirms our previous study of durum wheat (Tosi et al., 2009) and provides novel information based on the use of a library of antibodies specific for different types of gluten proteins. Thus, an antibody for HMW-GS was used either alone or in combination with antibodies recognizing specific gliadins and LMW-GS, to demonstrate the presence of a higher proportion of HMW-GS in the central part of the endosperm while gliadins and LMW-GS were generally more abundant in the sub-aleurone layer and the cells immediately adjacent to it. This gradient was first apparent at 14 dpa, became more evident at 21 dpa and was still clearly present at physiological maturity when some of the larger protein bodies had fused to form a protein matrix. The spatial patterns of accumulation that we observed for HMW-GS and LMW-GS/gliadins using immunostaining are broadly in agreement with expression patterns of HMW-GS and LMW-GS gene promoter : GUS constructs reported in transgenic wheat (Lamacchia et al., 2001; Stoger et al., 2001; Jones et al., 2008).
The qualitative and quantitative protein gradients shown by microscopy were also consistent with analyses of flour fractions obtained by pearling of mature wheat grains. The total protein content increased between the outermost fraction and the second fraction and then steadily decreased in the fractions corresponding to inner parts of the endosperm. A gradual increase in the relative intensity of the bands corresponding to HMW-GS was also observed in Coomassie Blue-stained SDS–PAGE gel separations of the pearling fractions from the outer to inner parts of the endosperm, while an opposite trend was observed for ω-gliadins and α-gliadins when specific antibodies were used in western blotting experiments to detect these protein types in the pearling fractions. However, it was surprising to note that the distribution of γ-gliadins (also determined using a γ-gliadin-specific antibody) was more similar to that of the HMW-GS than those of the LMW-GS and α-gliadins, to which they are most closely related in protein and gene promoter sequences (Shewry and Halford, 2002).
However, not all the proteins belonging to a given subgroup showed an identical trend in expression across the grain and some bands vary in staining intensity in the different endosperm fractions (see Fig. 1B, D).
Differences in protein distribution throughout the endosperm have also been observed in barley (Shewry et al., 1993; Tecsi et al., 2000) and in rice (Ellis et al., 1987), the sub-aleurone cells of both species being rich in proteins. Immunocytochemical and pearling studies of barley have also shown that the protein-rich sub-aleurone cells are enriched in S-rich and S-poor prolamins (B and C hordeins), while the HMW prolamin of barley (D hordein) is only present in significant amounts in the inner part of the starchy endosperm (Shewry et al., 1996).
Although it is well documented that gradients in protein distribution are present in cereal grains, their biological significance is not known. Similarly, we do not know the precise mechanism by which these gradients are established during grain development. Ugalde and Jenner (1990a) carried out quantitative compositional analysis of microsections cut along the radial axis of wheat grains and showed that the increase in protein concentration from the endosperm cavity to the periphery of the endosperm resulted mainly from the pattern of protein deposition, given that starch was deposited fairly evenly along the endosperm. The same authors also established that amino acids were exclusively supplied to the grain via the endosperm cavity and although there were gradients in the concentration of total soluble amino acids across the endosperm, they did not match the patterns of protein deposition. Furthermore, they found little change in the amino acid composition across the starchy endosperm, with the exception of free aspartate, which was higher in the outer layers. They concluded that the transport of amino acids across the endosperm did not impose any limits to the pattern of protein deposition as deposition was greater in the regions of endosperm that were furthest from the source of amino acid substrate.
Both the quantitative and the qualitative gradients of protein distribution appear to follow the radial pattern of cell development of the endosperm and it may therefore be hypothesized that the gradients have a developmental basis. Cellularization of the wheat endosperm follows a centripetal mode and is concluded by about 6–8 dpa (Mares et al., 1975, 1977; Fineran et al., 1982) which according to transcriptomic studies coincides with the initiation of starch and storage protein synthesis (Shewry et al., 2009). The same transcriptomic data also show that the timing of expression of most gluten protein genes is similar, the only exception being a short (approx. 2 d) time lag in the accumulation of transcripts for HMW-GS compared with those encoding other gluten proteins. Further cell division of the endosperm may continue until 20 dpa (Jennings and Morton, 1963), due to periclinal divisions of the cells from the outermost layer of the endosperm, so that the oldest cells occur in the centre and the youngest at the perimeter of the endosperm. Although only limited accumulation of storage compounds occurs in cells that are still actively dividing (see Figs 2I, L, N, Q and 3H, S), the most recently formed sub-aleurone cells contain the highest proportion of protein at maturity. Furthermore, PCD is initiated in the wheat endosperm at about 16 dpa and progresses stochastically during development (Young and Gallie, 1999), which contrasts with other cereals such as maize where cell death is initiated within the upper central endosperm and then expands outward in an ordered manner. In wheat, therefore, there seems to be no relationship between the relative position of a cell (or a cell layer) within the endosperm and the duration of accumulation of storage compounds in that cell. This may suggest that the higher amount of protein accumulated in the sub-aleurone cells compared with those in the inner endosperm is determined by a higher rate of protein synthesis in the outer layers of the endosperm, not by a longer duration of deposition. Genes encoding gluten proteins are primarily regulated at the level of gene transcription (Giese and Hopp, 1984; Bartel and Thompson, 1986; Sorensen et al., 1989) and it can be hypothesized that gradients in the concentrations of specific transcription factors or other regulatory signals from the maternal tissues and acting on specific domains of the gluten protein gene promoters could modulate/regulate the rates of transcription of gluten protein genes in the different endosperm regions. The endosperm cells in the sub-aleurone produced by the divisions in the ‘cambium-like’ layer would therefore become progressively less exposed to these signals from the maternal tissue as they move further from the aleurone during development. These regulatory signals would have to display different specificity for the promoters of the various classes of gluten proteins, in order to determine a qualitative protein gradient.
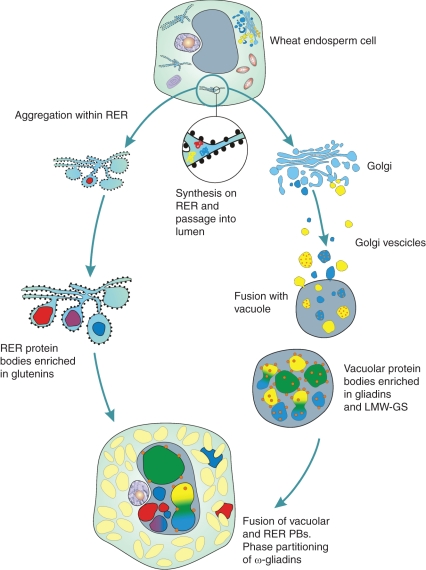
A degree of segregation between different gluten proteins also exists at the intracellular level. Double labelling experiments with combinations of antibodies recognizing specific types of gluten proteins revealed that, similarly to what has been reported for durum wheat (Tosi et al., 2009), protein bodies within the same cell may be differentially enriched in the various protein types. Several previous studies have provided convincing evidence for the existence of two types of protein bodies (Parker, 1980, 1982; Bechtel et al., 1991; Rubin et al., 1992; Tosi et al., 2009) which accumulate simultaneously and independently in wheat starchy endosperm cells. One type appears to be formed by aggregation of storage proteins within the endoplasmic reticulum (ER) and is enriched in HMW-GS (Rubin et al., 1992) while the other type results from aggregation at a post-ER location (the vacuole) and is enriched in gliadins, especially in the earliest stages of development. The unevenness in labelling of protein bodies that we observed in these studies could result from a different ontogeny of the protein bodies (i.e. vacuolar or ER-derived protein bodies) and therefore reflect the different trafficking mechanisms of the gluten protein types, or could result from segregation of the protein types into different domains within the same cell organelle. Fusion of smaller protein bodies which are differentially enriched in specific types of gluten protein would then lead to the formation of larger protein bodies with a more complex structure and composition. However, in most cases double labelling of the larger protein bodies gave fairly even patterns, suggesting that different gluten protein types become homogeneously distributed following the fusion of the smaller protein bodies. An exception was the ω-5 gliadins, which partition separately from the other classes of gluten proteins to form small deposits on the surface of the large protein bodies. This is, to our knowledge, the first time that such clear segregation has been reported for gluten proteins, although it has been described for gluten proteins and globulins in protein bodies of wheat (Bechtel et al., 1991) and oat (Lending et al., 1989). A diagram summarizing the pathway of formation of wheat protein bodies is shown in Fig. 6.
Fig. 6.
Diagram summarizing the pathway of formation of wheat protein bodies. RER = rough endoplasmic reticulum.
It is clear from our data that more studies are required o better understand the precise mechanism regulating protein gradients in cereals, their possible biological meaning (i.e. role in grain development and seed germination) and how they are influenced by the environment and nutritional factors.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank colleagues in the Bioimaging Unit at Rothamsted, in particular Dr Allison van de Meene and Mrs Jean Devonshire, for their help and support with the microscopy studies. We also thank Lynda Castle, at Rothamsted ‘Visual Communication Unit’, for drawing the diagram in Fig. 6. We are extremely grateful to Dr Sandra Denery-Papini, Dr Oliver Tranquet (INRA, Nantes, France) and Professor Frits Koning (Leiden University Medical Center) for providing some of the antibodies used for this study. J.H. is a PhD student supported by BBSRC and Campden BRI. Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC) of the UK.
LITERATURE CITED
- Bartel D, Thompson RD. Synthesis of messenger-RNAs coding for abundant endosperm proteins during wheat grain development. Plant Science. 1986;46:117–125. [Google Scholar]
- Bechtel DB, Wilson JD, Shewry PR. Immunocytochemical localisation of the wheat storage protein triticin in developing endosperm tissue. Cereal Chemistry. 1991;68:573–577. [Google Scholar]
- Becraft PW, Asuncion-Crabb YT. Positional cues specify and maintain aleurone cell fate during maize endosperm development. Development. 2000;127:4039–4048. doi: 10.1242/dev.127.18.4039. [DOI] [PubMed] [Google Scholar]
- Brett GM, Mills ENC, Goodfellow BJ, Fido RJ, Tatham AS, Shewry PR, Morgan MRA. Epitope mapping studies of broad specificity monoclonal antibodies to cereal prolamins. Journal of Cereal Science. 1999;29:117–128. [Google Scholar]
- Denery-Papini S, Popineau Y, Quillien L, Van Regenmortel MHV. Specificity of antisera raised against synthetic peptide fragments of high Mr glutenin subunits. Journal of Cereal Science. 1996;23:133–144. [Google Scholar]
- Denery-Papini S, Samson MF, Autran JC. Anti-peptide antibodies directed against omega-gliadins for the detection of sequences from bread and durum wheats. Food and Agricultural Immunology. 2000;12:67–75. [Google Scholar]
- Doan DNP, Linnestad C, Olsen O-A. Isolation of molecular markers from the barley endosperm coenocyte and the surrounding nucellus cell layers. Plant Molecular Biology. 1996;31:877–886. doi: 10.1007/BF00019474. [DOI] [PubMed] [Google Scholar]
- Dupont FM, Vensel WH, Chan R, Kasarda DD. Characterisation of the 1B-type ω-gliadins form Triticum aestivum cultivar Butte. Cereal Chemistry. 2000;77:607–614. [Google Scholar]
- Dwarte D, Ashford AE. The chemistry and microstructure of protein bodies in celery endosperm. Botanical Gazette. 1982;143:164–175. [Google Scholar]
- Ellis JR, Gates PJ, Boulter D. Storage-protein deposition in the developing rice caryopsis in relation to the transport tissues. Annals of Botany. 1987;60(663–670) [Google Scholar]
- Farrand EA. Study of relationships between wheat protein contents of two U.K varieties and derived flour protein contents at varying extraction rates. I. Studies on an experimental commercial mill and a laboratory Buhler mill. Cereal Chemistry. 1974;51:56–65. [Google Scholar]
- Farrand EA, Hinton JJC. Study of relationships between wheat protein contents of two U.K. varieties and derived flour protein contents at varying extraction rates. II. Studies by hand-dissection of individual grains. Cereal Chemistry. 1974;51:66–73. [Google Scholar]
- Fineran BA, Wild DJC, Ingerfeld M. Initial wall formation in the endosperm of wheat, Triticum aestivum: a reevaluation. Canadian Journal of Botany. 1982;60:1776–1795. [Google Scholar]
- Fisher DB. Protein staining of ribboned epon sections for light microscopy. Histochemistry and Cell Biology Journal. 1968;16:92–96. doi: 10.1007/BF00306214. [DOI] [PubMed] [Google Scholar]
- Giese H, Hopp E. Influence of nitrogen nutrition on the amount of hordein, protein Z and β-amylase messenger RNA in developing endosperms of barley. Carlsberg Research Communication. 1984;49:365–383. [Google Scholar]
- Hueros G, Varotto S, Salamini F, Thompson RD. Molecular characterization of BET1, a gene expressed in the endosperm transfer cells of maize. Plant Cell. 1995;7:747–757. doi: 10.1105/tpc.7.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueros G, Gómez E, Cheikh N, et al. Identification of a promoter sequence from the BETL-1 gene cluster able to confer transfer cell-specific expression in transgenic maize. Plant Physiology. 1999;121:1143–1152. doi: 10.1104/pp.121.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings AC, Morton RK. Changes in nucleic acids and other phosphorus-containing compounds of developing wheat grain. Australian Journal of Biological Science. 1963;16:342–351. [Google Scholar]
- Jones HD, Freeman JA, Sparks CA. A transgenic approach to understanding gene expression in cereals. In: Molina Cano JL, Christou P, Graner A, et al., editors. Cereal science and technology for feeding ten billion people: genomics era and beyond. 2008. (Options méditerranéennes série A. Séminaires Méditerranéens, No. 81). EUCARPIA/CIHEAM. [Google Scholar]
- Lamacchia C, Shewry PR, Di Fonzo N, et al. Endosperm specific activity of a storage protein gene promoter in transgenic wheat seed. Journal of Experimental Botany. 2001;52:243–250. [PubMed] [Google Scholar]
- Lending CR, Chesnut RS, Shaw KL, Larkins BA. Immunolocalization of avenin and globulin storage proteins in developing endosperm of Avena sativa L. Planta. 1989;178:315–324. doi: 10.1007/BF00391859. [DOI] [PubMed] [Google Scholar]
- Mares DJ, Norstog K, Stone BA. Early stages in development of wheat endosperm. I. The change from free nuclear to cellular endosperm. Australian Journal of Botany. 1975;23:311–326. [Google Scholar]
- Mares DJ, Stone BA, Jeffery C, Norstog K. Early stages in development of wheat endosperm. II. Ultrastructural observationson cell wall formation. Australian Journal of Botany. 1977;25:599–613. [Google Scholar]
- Mills ENC, Parker NL, Wellner N, Toole G, Feeney K, Shewry PR. Chemical imaging: the distribution of ions and molecules in developing wheat grains. Journal of Cereal Science. 2005;43:193–201. [Google Scholar]
- Mitea C, Havenaar R, Drijfhout JW, Edens L, Dekking L, Koning F. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for coeliac disease. Gut. 2008;57:25–32. doi: 10.1136/gut.2006.111609. [DOI] [PubMed] [Google Scholar]
- Morris VH, Alexander TL, Pascoe ED. Studies of the composition of the wheat kernel. I. Distribution of ash and protein in center sections. Cereal Chemistry. 1945;22:351–361. [Google Scholar]
- Normand FL, Hogan JT, Deobald HJ. Protein content of successive peripheral layers milled from wheat, barley, grain sorghum, and glutinous rice by tangential abrasion. Cereal Chemistry. 1965;42:359. [Google Scholar]
- Okrajková A, Prieto-Linde ML, Muchová Z, Johansson E. Protein concentration and composition in wheat flour mills streams Cereal Research Communications. 2007;35:119–128. [Google Scholar]
- Olsen OA. Endosperm development: cellularization and cell fate specification. Annual Review of Plant Molecular Biology. 2001;52:233–267. doi: 10.1146/annurev.arplant.52.1.233. [DOI] [PubMed] [Google Scholar]
- Olsen O. Nuclear endosperm development in cereals and Arabidopsis thaliana. The Plant Cell. 2004;16:s214–s227. doi: 10.1105/tpc.017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen OA, Jakobsen KS, Schmelzer E. Development of barley aleurone cells: temporal and spatial patterns of accumulation of cell-specific mRNAs. Planta. 1990;181:462–466. doi: 10.1007/BF00192998. [DOI] [PubMed] [Google Scholar]
- Opsahl-Ferstad HG, Le Deunff E, Dumas C, Rogowsky PM. ZmEsr, a novel endosperm-specific gene expressed in a restricted region around the maize embryo. Plant Journal. 1997;12:235–246. doi: 10.1046/j.1365-313x.1997.12010235.x. [DOI] [PubMed] [Google Scholar]
- Parker ML. Protein body inclusions in developing wheat endosperm. Annals of Botany. 1980;46:29–36. [Google Scholar]
- Parker ML. Protein accumulation in the developing endosperm of a high protein line of Triticum dicoccoides. Plant Cell and Environment. 1982;5:37–43. [Google Scholar]
- Pistón F, Marin S, Hernando A, Barro F. Analysis of the activity of a γ-gliadin promoter in transgenic wheat and characterization of gliadin synthesis in wheat by MALDI-TOF during grain development. Molecular Breeding. 2009;23:655–667. [Google Scholar]
- Rubin R, Levanony H, Galili G. Evidence for the presence of two different types of protein bodies in wheat endosperm. Plant Physiology. 1992;99:718–724. doi: 10.1104/pp.99.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstedt RM. Photomicrographic studies of wheat starch. 1. Development of the starch granules. Cereal Chemistry. 1946;23:337–359. [Google Scholar]
- Shewry PR, Halford NG. Cereal seed storage proteins: structures, properties and role in grain utilization. Journal of Experimental Botany. 2002;53:947–958. doi: 10.1093/jexbot/53.370.947. [DOI] [PubMed] [Google Scholar]
- Shewry PR, Price J, Tatham AS, et al. Spatial patterns of hordein distribution in relation to barley malting quality. Aspects of Applied Biology. 1993;36:43–49. [Google Scholar]
- Shewry PR, Brennan C, Tatham AS, et al. The development, structure and composition of the barley grain in relation to its end use properties. Cereals 96. Proceedings of the 46th Australian Cereal Chemistry Conference. 1996:158–162. Sydney, September 1996. [Google Scholar]
- Shewry PR, Underwood C, Wan Y, et al. Storage product synthesis and accumulation in developing grains of wheat. Journal Cereal Science. 2009;50:106–112. [Google Scholar]
- Sorensen MB, Cameron-Mills V, Brandt A. Transcriptional and post-transcriptional regulation of gene expression in developing barley endosperm. Molecular and General Genetics. 1989;217:195–201. [Google Scholar]
- Stoger E, Parker M, Christou P, Casey R. Pea legumin overexpressed in wheat endosperm assembles into an ordered paracrystalline matrix. Plant Physiology. 2001;125:1732–1742. doi: 10.1104/pp.125.4.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatham AS, Shewry PR. The conformation of wheat gluten proteins. The secondary structures and thermal stabilities of α, β, γ and ω gliadins. Journal of Cereal Science. 1985;3:103–113. [Google Scholar]
- Tecsi L, Darlington HF, Harris N, Shewry PR. Patterns of protein deposition and distribution in developing and mature barley grain. Barley Genetics, VIII. Proceedings of the 8th International Barley Genetic Symposium. 2000;2:226–268. Adelaide, Australia. [Google Scholar]
- Tosi P, D'Ovidio R, Napier J, Bekes F, Shewry PR. Expression of epitope-tagged LMW glutenin subunits in the starchy endosperm of transgenic wheat and their incorporation into glutenin polymers. Theoretical and Applied Genetics. 2004;108:468–476. doi: 10.1007/s00122-003-1459-x. [DOI] [PubMed] [Google Scholar]
- Tosi P, Parker M, Carzaniga R, Martin B, Gritsch CS, Shewry PR. Trafficking of storage proteins in developing grain of wheat. Journal of Experimental Botany. 2009;60:979–991. doi: 10.1093/jxb/ern346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugalde TD, Jenner CF. Substrate gradients and regional patterns of dry matter deposition within developing wheat endosperm. I. Carbohydrates. Australian Journal of Plant Physiology. 1990a;17:693–704. [Google Scholar]
- Ugalde TD, Jenner CF. Substrate gradients and regional patterns of dry matter deposition within developing wheat endosperm. II. Amino acids and protein. Australian Journal of Plant Physiology. 1990b;17:693–704. [Google Scholar]
- Yahata E, Maruyama-Funatsuki W, Nishio Z, et al. Relationship between the dough quality and content of specific glutenin proteins in wheat mill streams and its application to making flour suitable for instant chinese noodles. Bioscience Biotechnology and Biochemistry. 2006;70:788–797. doi: 10.1271/bbb.70.788. [DOI] [PubMed] [Google Scholar]
- Young TE, Gallie DR. Analysis of programmed cell death in wheat endosperm reveals differences in endosperm development between cereals. Plant Molecular Biology. 1999;39:915–926. doi: 10.1023/a:1006134027834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.