Abstract
Background and Aims
Sex allocation has been studied mainly in small herbaceous plants but much less in monoecious wind-pollinated trees. The aim of this study was to explore changes in gender segregation and sex allocation by Pinus halepensis, a Mediterranean lowland pine tree, within tree crowns and between trees differing in their size or crown shape.
Methods
The production of new male and female cones and sex allocation of biomass, nitrogen and phosphorus were studied. The relationship between branch location, its reproductive status and proxies of branch vigour was also studied.
Key Results
Small trees produced only female cones, but, as trees grew, they produced both male and female cones. Female cones were produced mainly in the upper part of the crown, and male cones in its middle and lower parts. Lateral branch density was correlated with the number of male but not female cones; lateral branches were more dense in large than in small trees and even denser in hemispherical trees. Apical branches grew faster, were thicker and their phosphorus concentration was higher than in lateral shoots. Nitrogen concentration was higher in cone-bearing apical branches than in apical vegetative branches and in lateral branches with or without cones. Allocation to male relative to female function increased with tree size as predicted by sex allocation theory.
Conclusions
The adaptive values of sex allocation and gender segregation patterns in P. halepensis, in relation to its unique life history, are demonstrated and discussed. Small trees produce only female cones that have a higher probability of being pollinated than the probability of male cones pollinating; the female-first strategy enhances population spread. Hemispherical old trees are loaded with serotinous cones that supply enough seeds for post-fire germination; thus, allocation to males is more beneficial than to females.
Keywords: Allocation, biomass, branch vigour, crown shape, gender segregation, nitrogen, Pinus halepensis, phosphorus, sex allocation, tree size
INTRODUCTION
Sex allocation in monoecious plants has been the focus of many studies (e.g. Hamilton, 1967; Charlesworth and Charlesworth, 1981; Charnov, 1982; Lovett Doust, 1989; Wolfe and Shmida, 1997; Sato, 2000; Sarkissian et al., 2001). Resource allocation to male or female functions in bisexual organisms reflects its relative contribution to total plant fitness (Charnov, 1982; de Jong et al., 2008). Sex allocation theory predicts that individual plants of a given size adjust their sex allocation by increasing allocation to the gender with higher marginal fitness (Charnov, 1982). Many sex-ratio-related studies focused on dioecious (Barret et al., 1999; Bram and Quinn, 2000; Correia and Diaz Barradas, 2000) or monoecious herbaceous plants (Mendez, 1998; Sarkissian et al., 2001); far fewer have focused on trees (Ross and Pharis, 1987; Fox, 1993; de Jong and Klinkhamer, 1994). Fox (1993) proposed that monoecious trees are most suitable for size-dependent sex ratio studies because they flexibly adjust their sex ratio in space and time in response to changes in their size (Bell, 1985). Here we examine sex allocation by Pinus halepensis (Mirov, 1967; Panetsos, 1981), which can serve as a model for sex allocation studies in monoecious wind-pollinated trees.
According to sex allocation theory, wind-pollinated plants should increase their male reproductive effort with increasing plant size (Burd and Allen, 1988; Klinkhamer et al., 1997). When seed dispersal distances are short and mate competition is locally intense, wind-pollinated trees can increase their fitness through long-distance dispersal of pollen (Lloyd and Bawa, 1984). Several studies on the size-dependent sex ratio in wind-pollinated plants have supported this hypothesis (Freeman et al., 1981; Schoen and Stewart, 1986; Bickel and Freeman, 1993; Allison, 1991), but others have not (Primack and McCall, 1986; Smith, 1981).
According to sex allocation theory (Charnov, 1982; Freeman et al., 1981; de Jong et al., 2008) monoecious plants have the advantage of being flexible in their sex allocation; individuals can alter their sex allocation to female or male according to changes in their vigour due to spatial differences in microhabitat quality or temporal changes among years (Wolfe and Shmida, 1997). Accordingly, Freeman et al. (1981) found that Atriplex individuals allocate more to maleness in harsh microhabitats and more to femaleness in better habitats. In general, young or weak monoecious plants will allocate relatively more to maleness while mature or strong individuals will allocate more to femaleness. However, this prediction is in contrast to the common sexual pattern in most pine species where young trees are females and allocation to male is delayed (Floyd, 1983; Shmida et al., 2000). This study tries to challenge this enigma.
Plant architecture is also known to influence reproductive effort (Veillon, 1978; Lechowitcz, 1984; Schmitt, 1993) and can function as a constraint on inflorescence production by limiting the number of shoots and thus the number of reproductive meristems (Geber, 1990; Arista and Talavera, 1997; Preston, 1999). For example, Bickel and Freeman (1993) showed that crown shape (cylindrical or hemispherical) plays a role in determining the size-dependent sex ratio in wind-pollinated monoecious trees. Several other authors suggested that environmental influences, allocation constraints and physiological constraints may affect plant architecture and thus may indirectly affect sex allocation in a non-adaptive way (Burd and Allen, 1988; Solomon, 1989; Travest, 1992; Bickel and Freeman, 1993). Forestry manipulation of crown pruning, application of plant growth regulators and manipulation of photoperiod can change sex expression in many conifers including pines (Ross and Pharis, 1987). To the best of our knowledge, there are only a limited number of studies on changes in gender segregation and sex allocation with plant size and shape in monoecious trees, which can be explained by plant life history traits.
Choosing the right currency for sex allocation studies has been the subject of debate. Some authors prefer biomass (Abrahamson and Caswell, 1982; Reekie and Bazzaz, 1987) as it reflects carbon allocation, whereas others prefer limiting nutrients, such as nitrogen (N) or phosphorus (P) (Thompson and Stewart, 1981; Chapin, 1989). Ashman and Baker (1992) found differences in N and P demand by female or male function. Moreover, different currencies may be allocated differently to sexual functions (Ashman, 1994; Witkowski and Lamont, 1996). Thus, different currencies may result in different conclusions regarding patterns of female or male sexual allocation.
Pines, like many other conifers, produce separate male (microsporangiate) and female (macrosporangiate) cones. In many pines, male and female cones are located on different branch types; females mainly on vertical apical branches and males on lateral branches located in the middle and lower parts of the crown. Most conifers have a juvenile vegetative growing phase lasting from several years to several decades followed by a longer bisexual phase (Mirov, 1967; Ross and Pharis, 1987; Gifford and Foster, 1989). Due to reduction in apical dominance and increase in lateral branch development, some pines change their crown shape with age from conical to cylindrical and finally to hemispherical (Wilson, 1986, 2000; Ross and Pharis, 1987; Shmida et al., 2000). The development of female cones on vigorous apical branches and male cones on lower lateral branches, that often grow in shade, led to the hypothesis that a high photosynthetic rate favours female cone production and, conversely, that low carbohydrate status favours male cone production (Ross and Pharis, 1987).
Gender segregation and sex allocation were studied in some boreal pines, most of which are tall trees growing in dense forests (e.g. Ross and Pharis, 1987; Savolainen et al. 1993; Cobb et al., 2002). In contrast, P. halepensis is a short-lived pine (80–90 years in nature) of Mediterranean lowlands with a unique life history. Pinus halepensis grows in fire-prone areas and is killed by canopy fires, but recruits only from seeds released after fire from its serotinous, canopy-stored seed bank. Pinus halepensis is also a successful pioneer species in abandoned fields and other disturbed areas. Therefore, it is an effective invasive species outside its natural range and a rapidly expanding species throughout the Mediterranean basin (Richardson, 1998; Ne'eman and Trabaud, 2000).
Gender segregation and sexual allocation patterns in P. halepensis were examined. Our specific objectives were to: (a) examine the changes in relative sex allocation (cone number, biomass, N and P) to male and female functions in relation to changes in tree size and crown shape (cylindrical or hemispherical); (b) study the changes in gender segregation within the crown and among trees in relation to size and crown shape; (c) explore the relationship between: branch location (low, medium or high), proxies of its vigour (annual growth, diameter, specific weight, concentrations of P and N in the branch and needles) and its reproductive status (with or without cones); and (d) identify possible adaptive values of these gender segregation and sex allocation patterns in relation to the unique life history of P. halepensis.
MATERIALS AND METHODS
Gender segregation
A total of 213 Pinus halepensis Mill. trees that represented a wide range of size classes were selected from four natural populations (Haifa University 73, Etzba 60, Beit Oren 40 and Hreibe 40), growing 1–5 km apart, in a typical Mediterranean climate on Mount Carmel, northern Israel. Tree height was measured using a telescopic pole, and the average of two perpendicular diameters was used to calculate tree canopy area, using the formulae of cylinder and cone surfaces in accordance with canopy shape. Male and female cone production was estimated in all the trees; to do this, the number of cones in four 1 m2 squares was counted and its average was multiplied by crown area that was calculated from crown radius and height. The trees were classified as non-reproductive (without any cones), males (with only male cones), females (with only female cones) or bisexual (with both male and female cones). In addition, the functional gender (Lloyd, 1980) of each tree was calculated, and it was classified as male when femaleness was <0·2, as female when femaleness was >0·8 and as bisexual when femaleness was 0·2–0·8. Finally, the percentage of non-reproductive, male, female and bisexual trees by size class was calculated.
For the sex segregation study, we haphazardly selected 79 bisexual trees (University of Haifa populations) with a conical or cylindrical crown. Large bisexual trees with a hemispherical crown were relatively rare and all were selected. Thus, to avoid uneven sample sizes and minimize the effect of tree size, for the comparison between cylindrical hemispherical trees, the ten largest trees with cylindrical crowns that did not differ in their average diameter at 1·3 m [diameter at breast height (DBH)] from the hemispherical trees (t19 = 0·366, P = 0·553) were selected. For all trees, DBH was measured, and the crowns were divided into three (low, medium and high) equal height classes. At each crown level, the number of new female cones was counted and the number of male cone clusters multiplied by the average number of cones per cluster was estimated. For a sub-sample of 40 trees with cylindrical crowns and for all ten trees with hemispherical crowns lateral branch density was also determined. Lateral branch density was determined by counting the number of lateral branch ends in four 1 m2 squares of crown area and their average was used to represent each tree.
In February, the male cone clusters on all 213 trees, and the number of male cones in a random sample of ten clusters, were counted to calculate the number of male cones per tree. In June, on the same trees, the number of new female cones of the current year (after pollination), 1-year-old green female cones and 2-year-old brown cones that included ripe seeds ready for dispersal were counted.
Sex allocation
To determine biomass allocation, 25 male cones, 25 new female cones, 25 one-year-old green cones and 25 two-year-old brown cones were collected from various trees. They were all dried for 4 d at 70 °C and each individual cone was then weighed. To determine allocation of N and P, we haphazardly sampled 20 dried cones from each type, and cut, ground and sieved (0·5 mm) them. Concentrations of N and P were determined by a standard acid digestion procedure; 150 mg of powder of each cone type was digested in 5 mL of 30 n sulfuric acid, sodium sulfate, copper sulfate and selenium. Nitrogen and P concentrations were analysed calorimetrically, in diluted digestions, using a continuous-flow analyser. The concentrations were multiplied by the sample dry weight to calculate N and P content (mg) of each cone type. The annual biomass, N and P allocation to male function was calculated as the product of the number of male cones per tree, and the average dry weight, and N or P content of a single male cone. The annual allocation to female function consists of simultaneous allocation to three cone cohorts. Therefore, female allocation is the sum of allocation to: (1) new cones; (2) 2-year-old green; and (3) 2-year-old brown cone cohorts. It was calculated using the following formula:
where Fcy is the number of new female cones of the current year per tree; F1y is the number of 1-year-old green cones per tree; F2y is the number of 2-year-old brown cones per tree; and C is the calculated currency [biomass as dry weight, N or P content (mg) in a single cone].
The number of brown cones in some trees was larger than that of green cones, reflecting annual variation in cone yield. The content of N and P in brown cones was lower than that of green cones. Consequently, in some instances, calculated allocation of N and P to female function was negative. To avoid this unrealistic situation, we simulated the situation of no annual variation in cone number by capping the number of brown cones to be not more than the number of green cones, namely number of brown cones ≤ number of green cones. This was applied to annual allocation to female biomass, N and P. It should be noted that this may cause some overestimation in N and P and biomass allocation to female cones in some trees.
Vigour proxies
As proxies for branch vigour, we measured: annual length growth rate (cm year−1); branch diameter (cm); specific dry weight (g cm−3); and N and P concentration of the branch and its needles (mg g−1 dry weight).
To test the effect of branch location and reproductive status on vigour parameters, ten independent branches (from different trees) were selected for each of the following categories: (a) lateral reproductive branch with a male cone; (b) lateral vegetative branch without a cone; (c) apical reproductive branch with a female cone; and (d) apical vegetative branch without a cone. The growth of 40 branches carrying new female cones, 40 carrying 1-year-old green female cones and 40 carrying 2-year-old mature brown female cones was also measured. Growth was measured over a 4 month period (February–June), which is the growing season.
Data analyses
Relationships between tree height and DBH were analysed by linear regression. The numbers of male and female (various ages) cones were counted on the same tree, and were compared using repeated measures analysis of variance (ANOVA) and Bonferroni correction for post-hoc comparisons. Annual allocation to male and female functions was compared using paired t-tests, and their relationship to tree size was tested by linear regression.
Following Fox (1993), to test relative sex allocation to male and female function, we plotted log(male) vs. log(female); a slope equal to 1 indicates an equal isometric increase in allocation to male and female, a slope >1 indicates relatively higher allocation to male function and a slope <1 indicates relatively higher allocation to female function. To test the changes in sex allocation with tree size, log(male/female) vs. log(DBH) was plotted; a slope >0 indicates a relative increase in allocation to male compared with female function with increasing tree size. To avoid log(0), which is undefined, and negative values of log(proportion), log(x + 1) transformation was performed.
T-tests were used to compare relative female with total [female × (male + female)−1] sex allocation of biomass, N and, P between trees with a hemispherical crown shape and equal-sized trees with a cylindrical crown shape.
Lateral branch density in trees differing in their crown shape was compared with t-test, and its relationship to tree size and number of male cones was examined by linear regression.
The Friedman test was used to compare the distribution of cone types among crown levels, as the data could not be transformed to have a normal distribution.
The effect of branch location (apical or lateral) and its status (reproductive or vegetative) on branch growth, diameter, specific weight, N and P concentration in the branch and in its needles was analysed by two-way ANOVA.
Proportions were arcsin[sqrt(x)] transformed and densities were log(x + 1) transformed for normal distribution and to increase homogeneity of the variances, before analyses.
RESULTS
Gender segregation
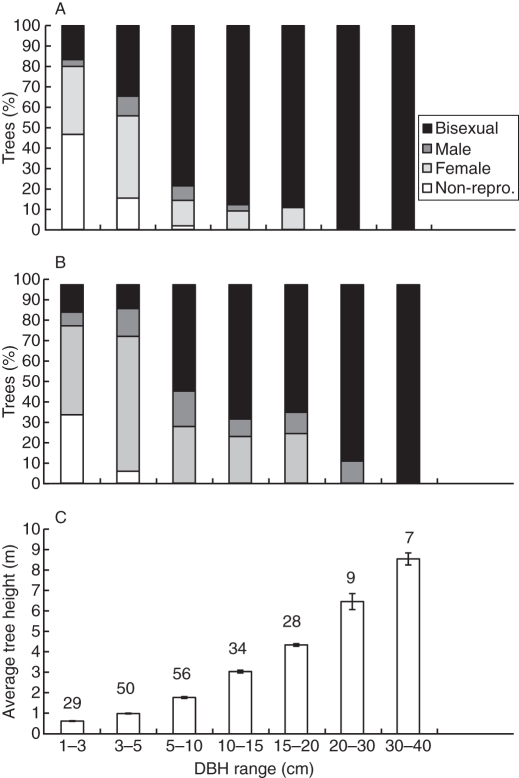
Trees demonstrated a clear gender segregation pattern among tree DBH classes, which was similar when using qualitative morphological criteria for tree gender (Fig. 1A) or quantitative functional gender (Lloyd, 1980) (n = 213; Fig. 1B). The smallest trees were mainly non-reproductive or produced only female cones, with only a few small bisexual trees. Larger trees were all reproductive, with a decreasing percentage of females and an increasing percentage of bisexual trees with increasing DBH. Trees with a DBH >20 cm were all bisexual. The percentage of male trees was low, with no clear trend along the DBH gradient (Fig. 1A, B). In a preliminary study, P. halepensis tree height was positively and significantly correlated with DBH (F1,49 = 300·1, P < 0·01, r2 = 0·883, n = 50); therefore, we used DBH as a proxy for tree size. Trees lower than 1 m were mainly females and those above 2 m were mainly bisexual (Fig 1C).
Fig. 1.
(A) Relative segregation of qualitative tree gender (non-reproductive, female, male and bisexual trees) determined by the presence or absence of male and female cones. (B) Quantitative ‘functional gender’ (Lloyd, 1980). (C) Average tree height (± s.e.) per DBH (diameter at breast height) class of 213 P. halepensis trees of all genders, growing in four natural populations on Mount Carmel. Israel. The numbers of trees in each DBH class are given on the figure.
Female and male cones were not evenly distributed among the low, medium and high levels of tree crowns (Fridman test: χ2 = 98·6, d.f. = 2, P < 0·001 and χ2 = 64·3, d.f. = 2, P < 0·001 for female and male cones, respectively). Almost all new female cones (94 %) were produced in the highest level tree crowns, only a few (6 %) at the medium level and none at the lowest level. Most male cones were produced at the medium (55 %) and low (35 %) levels, with only a few (10 %) at the highest level of the crowns.
Lateral branch density and the number of male cones were significantly higher (t19 = 4·282, P < 0·001 and t19 = 3·858, P = 0·002, respectively) in trees with hemispherical crowns than in equal-sized trees with cylindrical crowns, whereas the number of new female cones was not different (t19 = 0·463, P = 0·649) between trees differing in crown shapes (Fig. 2A–C). Biomass, N and P allocation to female cones relative to total sex allocation [(female × (male + female)−1] tended to be lower in trees with hemispherical crowns than in equal-sized trees with cylindrical crowns (Fig. 2D–F), but the differences were not significant (t19 = 0·832, P = 0·416, t19 = 1·763, P = 0·095 and t19 = 1·438, P = 0·168, respectively).
Fig. 2.
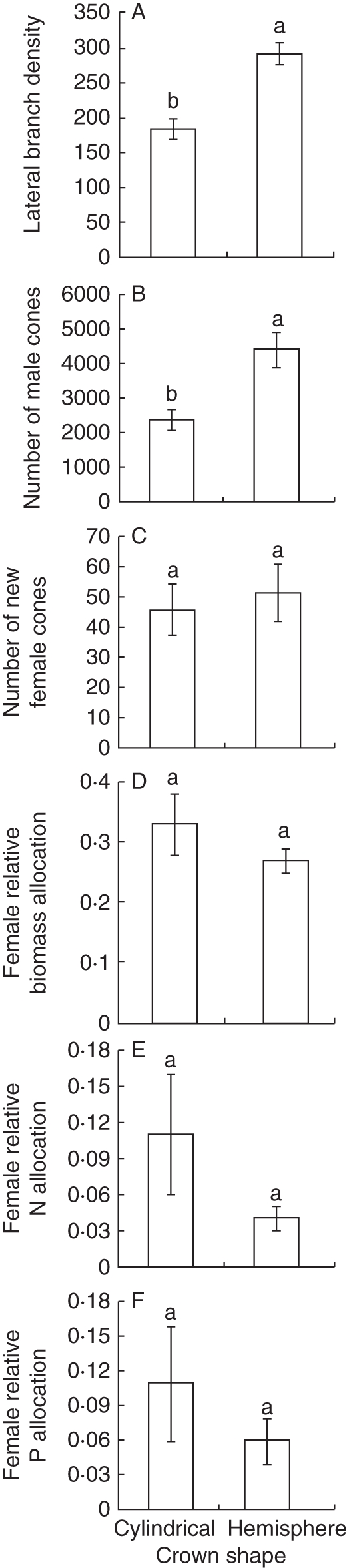
(A) Average (± s.e.) lateral branch density (per m2 of crown area); (B) number of male cones per tree; (C) number of new female cones per tree; (D) biomass allocation to female cones relative to total sex allocation [female/(male + female)]; (E) N allocation to female cones relative to total sex allocation [female/(male + female)]; (F) P allocation to female cones relative to total sex allocation [female/(male + female)], for trees with cylindrical and hemispherical crown shapes. Bars represent the s.e., and different letters indicate significant differences (t-test P < 0·05, n = 10 for each crown shape category).
Lateral branch density was positively correlated with the number of male cones in trees with cylindrical crowns (F1,19 = 9·092, P = 0·017, r2 = 0·532) and among equal-sized trees with hemispherical crowns (F1,19 = 6·018, P = 0·031, r2 = 0·259). However, the number of new female cones was not correlated with lateral shoot density in trees with a cylindrical crown (F1,19 = 1·789, P = 0·198) or in trees with a hemispherical crown (F1,19 = 1·018, P = 0·331).
Sex allocation
The number of male cones was much higher than the number of new female cones; the number of new female cones was much higher than the number of 1-year-old green female cones; the number of 2-year-old brown cones was larger than the number of 1-year-old green cones; and all differences were significant (F3,212 = 202·3, n = 213, P < 0·001) (Table 1). Allocation of biomass (dry weight), N and P to single male cones, a new female cone, single 1- and 2-year-old female cones was significantly different (for biomass, F3,212 = 43·2, P < 0·001; for N, F3,212 = 98·3, P < 0·001; and for P, F3,212 = 49·5, P < 0·001). Allocation to male cones (in all three currencies) was smaller than to new female cones, and allocation to female cones increased during the first year of their growth. During their second year, female cone growth in biomass was not significantly different from zero, and there was a significant decrease in N and P content (Table 1).
Table 1.
Average (± s.e.) number of cones per tree (n = 213), biomass (n = 25), N (n = 20) and P content (n = 20) per male cone, per new female cone, and per 1- and 2-year-old female cones
| Cone type and age | Cones per tree | Biomass (g d. wt cone−1) | N (mg cone−1) | P (mg cone−1) |
|---|---|---|---|---|
| Male | 3111·5 ± 417·3a | 0·02 ± 0·01c | 0·21 ± 0·08d | 0·03 ± 0·01d |
| New female | 13·7 ± 1·8b | 0·32 ± 0·01b | 5·83 ± 0·69c | 0·87 ± 0·35c |
| One-year-old female | 2·9 ± 0·4d | 21·87± 0·07a | 260·25 ± 0·41a | 37·40 ± 5·61a |
| Two-year-old female | 5·2 ± 0·8c | 24·87± 0·71a | 122·58 ± 1·23b | 21·96 ± 3·80b |
Different letters (within a column) indicate significant differences (Bonferroni post-hoc test, P < 0·0083).
There was no significant difference (paired t212 = 0·695, P = 0·488) in annual biomass allocation (of an average sized tree) to male or female (including new) cones. Annual allocation of N and P by trees was significantly larger (for N, t212 = 2·813, P = 0·005; and for P, t212 = 2·213, P = 0·028) to male than to female cones (Table 2). Annual allocation of biomass, N and P to male and female cones increased significantly with DBH (Table 2).
Table 2.
Average (± s.e.) DBH (cm) and annual allocation, of an average-sized tree, to male and female (including new cones and the annual growth of 1- and 2-year-old cones) cones of: biomass (g d. wt), N (mg) and P (mg). Results of linear regression analyses (slope, F, P and R2) for each of the log-transformed variables against log DBH as dependent variable (n = 213)
| Regression |
||||||
|---|---|---|---|---|---|---|
| Variable | Average | ± s.e. | Slope | F | P | R2 |
| DBH | 9·4 | 0·5 | ||||
| Male biomass | 102·7 | 15·6 | 2·6 | 306·6 | <0·001 | 0·592 |
| Female biomass | 71·2 | 8·3 | 2·2 | 178·0 | <0·001 | 0·676 |
| Male N | 45·5 | 4·7 | 1. 7 | 106·7 | <0·001 | 0·336 |
| Female N | 31·1 | 4·4 | 0·8 | 25·1 | <0·001 | 0·106 |
| Male P | 5·4 | 0·5 | 0·9 | 100.9 | <0·001 | 0·324 |
| Female P | 4·8 | 0·7 | 0·4 | 11·4 | 0·001 | 0·051 |
The regressions between the relative male to female allocation [log(M F−1)] for cone number, allocation of biomass, N and P vs. tree size [log(DBH)] as predictor yield significant positive slopes (Table 3). The slope was higher for cone number and biomass than for N and P, indicating a higher male-biased allocation for cone number and biomass than for N and P (Table 3).
Table 3.
Linear regression between the relative male to female annual allocation of: cone number [log(M cones/F cones)], allocation to biomass [log(M BM/F BM)], allocation to N [log(M N/F N)], allocation to P [log(M P/F P)] and tree size log(DBH) as predictor
| ANOVA |
Coefficient |
|||||
|---|---|---|---|---|---|---|
| Variable | n | F | P | Slope | t | P |
| Log(M cones/F cones) | 160 | 45·8 | <0·001 | 1·161 | 6·768 | <0·001 |
| Log(M BM/F BM) | 155 | 180·4 | <0·001 | 2·238 | 13·433 | <0·001 |
| Log(M N/F N) | 163 | 15·2 | <0·001 | 0·591 | 3·9 | <0·001 |
| Log(M P/F P) | 163 | 11·5 | 0·001 | 0·486 | 3·4 | 0·001 |
Vigour proxies
Apical branches grew faster and were thicker than lateral shoots, but they did not differ in their specific dry weight (Fig. 3A–C). Branch growth and specific weight did not differ between vegetative (without cones) and reproductive (with cones) apical shoots, and there was no interaction between the effects of branch location and cone bearing (Table 4). Phosphorus concentration was higher in apical than in lateral branches, with no difference between vegetative and reproductive branches and their needles, and there were no interactions between the effects of branch location and cone bearing (Fig. 3D, Table 4). Nitrogen concentration was higher in cone-bearing apical branches than in apical vegetative branches and in lateral branches with or without cones; the effect of branch location was significant, the effect of cones was not significant (Fig. 3E, Table 4). Phosphorus and nitrogen concentrations in needles were affected by branch location but not by its reproductive status (Fig. 3E–F, Table 4). In addition, there was no difference in growth rate of branches carrying new, 1- or 2-year-old female cones (F2,38 = 0·203, P = 0·817).
Fig. 3.
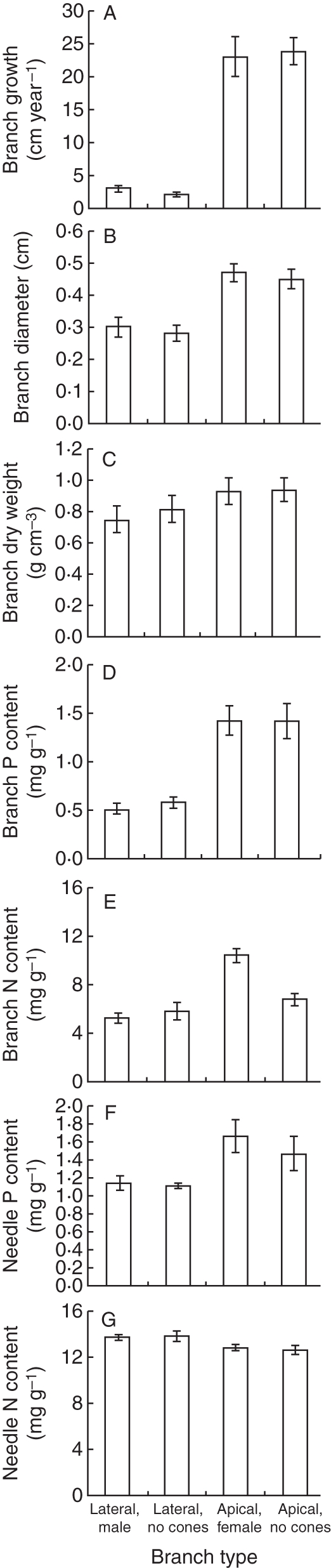
(A) Average (± s.e.) branch annual growth rate (cm year−1); (B) branch diameter (cm); (C) branch specific dry weight (g cm−3); (D) branch P concentration (mg g−1 d. wt); (E) branch N concentration (mg g−1 d. wt); (F) needle P concentration (mg g−1 d. wt); and (G) needle N concentration (mg g−1 d. wt) of lateral branches with and without male cones and of apical branches with and without female cones (n = 40 for each branch type category).
Table 4.
Two-way ANOVAs for the effect of branch location (lateral or apical) and its reproductive status (with or without cone) on branch growth, diameter, specific dry weight, P and N concentration of the branch, and P and N concentration of the needles (n = 40)
| Source | Sum of squares | d.f. | Mean squares | F | P |
|---|---|---|---|---|---|
| Branch growth | |||||
| Corrected model | 4385·369 | 3 | 1461·790 | 42·846 | <0·001 |
| Location | 4378·556 | 1 | 4378·556 | 128·338 | <0·001 |
| Cone | 0·006 | 1 | 0·006 | 0·000 | 0·989 |
| Location × cone | 6·806 | 1 | 6·806 | 0·199 | 0·658 |
| R2 = 0·78 | |||||
| Branch diameter | |||||
| Corrected model | 0·293 | 3 | 0·10 | 13·42 | <0·001 |
| Location | 0·289 | 1 | 0·29 | 39·71 | <0·001 |
| Cone | 0·004 | 1 | 0·00 | 0·55 | 0·463 |
| Location × cone | 0 | 1 | 0·00 | 0·00 | 1·000 |
| R2 = 0·53 | |||||
| Branch specific dry weight | |||||
| Corrected model | 0·252 | 3 | 0·08 | 1·25 | 0·306 |
| Location | 0·229 | 1 | 0·23 | 3·42 | 0·073 |
| Cone | 0·01 | 1 | 0·01 | 0·22 | 0·643 |
| Location × cone | <0·001 | 1 | 0·01 | 0·12 | 0·733 |
| R2 = 0·09 | |||||
| Branch P | |||||
| Corrected model | 5·337 | 3 | 1·779 | 11·771 | <0·001 |
| Location | 4·452 | 1 | 4·452 | 29·460 | <0·001 |
| Cone | 0·305 | 1 | 0·305 | 2·018 | 0·164 |
| Location × cone | 0·580 | 1 | 0·580 | 3·835 | 0·058 |
| R2 = 0·50 | |||||
| Branch N | |||||
| Corrected model | 158·746 | 3 | 52·915 | 16·989 | <0·001 |
| Location | 92·879 | 1 | 92·879 | 29·820 | <0·001 |
| Cone | 22·181 | 1 | 22·181 | 7·121 | 0·011 |
| Location × cone | 43·686 | 1 | 43·686 | 14·026 | 0·001 |
| R2 = 0·59 | |||||
| Needle P | |||||
| Corrected model | 2·103 | 3 | 0·701 | 3·898 | 0·016 |
| Location | 1·908 | 1 | 1·908 | 10·610 | 0·002 |
| Cone | 0·124 | 1 | 0·124 | 0·691 | 0·411 |
| Location × cone | 0·071 | 1 | 0·071 | 0·393 | 0·535 |
| R2 = 0·25 | |||||
| Needle N | |||||
| Corrected model | 11·588 | 3 | 3·863 | 5·628 | 0·003 |
| Location | 11·213 | 1 | 11·213 | 16·339 | <0·001 |
| Cone | 0·013 | 1 | 0·013 | 0·018 | 0·893 |
| Location × cone | 0·362 | 1 | 0·362 | 0·528 | 0·472 |
| R2 = 0·32 | |||||
DISCUSSION
Here new data are provided on gender segregation and sex allocation to add to the few studied cases of monoecious wind-pollinated trees (Jordano, 1991; Arista and Talavera, 1997; Shmida et al., 2000), and the adaptive value of the results is examined in the light of the special life history of P. halepensis, a post-fire obligate seeder and an invasive plant.
Gender segregation
Pinus halepensis trees begin their reproduction at the age of 5 years (Shmida et al., 2000), which is very young relative to other pines (Richardson, 1998). Pinus halepensis trees start reproduction as females and turn bisexual when they also start producing male cones (Fig. 1), as do many other pines (Panetsos, 1981; Ross and Pharis, 1987; Matziris, 1997). This pattern, however, contrasts with the reproduction pattern of many other monoecious species, where small individuals are usually males and produce female flowers or cones only later (Freeman et al., 1981; Willson, 1983). In Israel, the majority of small P. halepensis trees (DBH <5 cm) function as females, whereas larger trees (DBH >5 cm) are bisexual (Fig. 1). Both female-first and early age of first reproduction have clear adaptive values to P. halepensis, which is an expanding and invasive species as well as a post-fire obligate seeder. If small pines begun their reproduction as males, their male success would have been extremely low because of the small amounts of pollen they produce and the short distance their pollen can disperse due to their low height. In contrast, female cones on young and small trees have considerable chances to be pollinated by pollen dispersed from distant high trees. This is an advantage for pines in expanding populations as well as for post-fire regenerating populations. Producing seeds at a young age turns a young newly established tree into a new dispersal kernel that further enhances population expansion; this trait has an adaptive value mainly in the no-fire scenario. The early production of cones, which are mostly serotinous (Ne'eman and Trabaud, 2000), helps the population escape the immaturity risk (Keely et al., 1999), which is the risk of being burned again before the establishment of a large enough canopy-stored seed bank that secures its post-fire establishment.
Most conifers, including pines, have an apparent gender segregation pattern within their crown. Female cones are located on the main vertical branches in the upper part of the crown, whereas male cones are produced mostly in the middle and lower parts. This segregation pattern is more pronounced in younger than in older trees (Ross and Pharis, 1987; Shmida et al., 2000) and is dependent on both density and architecture (Arista and Talavera, 1997). Our results confirm this pattern for P. halepensis (Fig. 2). Several explanations have been proposed for this pattern, the main ones being the ‘pollination’ and ‘vigour’ hypotheses. The pollination hypothesis points to the disadvantage of male above female flowers or cones as this may enhance self-pollination; male below female will thus decrease selfing risk (Faegri and Van der Pijl, 1971). Further support is provided by the maturation of male cones earlier than female cones (on the same individual) and consequent earlier pollen dispersal than ovule receptivity typical of most conifers (Ross and Pharis, 1987). Small pollen loads and low pollination rates are a major cause of cone abortion in P. halepensis (Goubitz et al., 2002). During their second year, when most cone growth occurs, female cones are green and they have photosynthetic activity that could partially palliate their carbon cost. However, our results also support the ‘vigour’ hypothesis, which explains the location of female cones on apical fast growing branches by the fact that they serve as efficient sinks for photosynthetic resources (Wareing and Phillipis, 1970; Lev-Yadun and Liphschitz, 1987; Ross and Pharis, 1987; Owens, 1991). As the production of a single female cone is more costly than that of a male cone in terms of biomass, N and P (Table 1), female cone maturation could be more assured by developing on vigorous branches. Our results support the vigour hypothesis as we show that lateral branches grow more slowly, have a smaller diameter, contain less N and P (Fig. 3, Table 4), and thus can be considered less vigorous than apical branches. Branch-specific dry weight did not differ between branch types, indicating similar wood structure, but main branches have a larger diameter than lateral ones and consequently more vascular tissue, which enables improved water and nutrient transport from the root and carbon transport from neighbouring branches to the developing female cones. Increased diameter growth also provides more mechanical support for the female cones (Wilson, 2000). Our results demonstrate that ‘pollination’ and ‘vigour’ hypotheses are not exclusive or contradictory. Indeed, for P. halepensis, locating female cones on top of young trees is advantageous from both the pollination and vigour aspects: increasing the probability of pollination by foreign pollen, maturation and seed production in young trees, which are advantageous in both fire and non-fire scenarios.
Most pine species have cylindrical or cone-shaped crowns, but several change their crown shape from cylindrical to hemispherical at maturation (Ross and Pharis, 1987; Shmida et al., 2000). Old, lone-standing P. halepensis trees change their crown to hemispherical at maturation but they do not grow in height (Shmida et al., 2000). This gives a unique opportunity to disentangle the effect of growth and shape, which often occur simultaneously. Trees with hemispherical crowns produced more male cones than did equal-sized trees with cylindrical crown, but there was no difference in the production of new female cones and in allocation of biomass, N and P to female function (Fig. 2). We found also that trees with hemispherical crowns had higher lateral branch density (Fig. 2A) and a significant correlation between these two variables. We can thus suggest that the difference in lateral shoot density is the proximate cause of the higher production of male cones in hemispherical than in cylindrical trees. Sex allocation theory predicts an increase in allocation to males that originates from higher local mate competition and lower probability for a seedling to be established under or near larger mother trees, which also holds for the comparison between trees with cylindrical and hemispherical crowns. Pinus halepensis is a relatively short-lived tree that rarely grows older than approx. 90 years in nature, and most hemispherical trees are old. These trees are commonly loaded with serotinous cones, dead branches and old empty cones, and a thick dead-needle layer covers the ground underneath. Under such conditions, the probability of seedling establishment under its mother tree is very low, regardless of tree crown shape. Therefore, with low chances of seedling recruitment, such trees should increase allocation to male relative to female function. Moreover, post-dispersal seed predation is very high in such pine forests, depleting the soil seed bank (Ne'eman and Izhaki, 1999) and only the canopy-stored serotinous seed bank that consists of several cone cohorts is safe. In contrast to the low regeneration probability under a living mother tree, this site becomes the favourable post-fire regeneration site for seeds of serotinous cones. All these life history-related traits can explain the higher allocation to male than to female cones in old hemispherical trees relative to younger similar-sized cylindrical trees.
Sex allocation
Green female cones were the highest in biomass, N and P content; brown cones were not different from green cones in their biomass but their N and P content was lower (Table 1). The decrease in N and P probably reflects biodegradation and mobilization of organic compounds from the cone prior to its death. Mature and serotinous cones comprise dead scales and viable seeds that will be dispersed as a reaction to drought (regular cones) and a combination of heat and drought (serotinous cones).
The relative allocation to male or female functions may vary with the different currencies (Ashman, 1994; Witkowski and Lamont, 1996; Ishida et al., 2005). Biomass allocation, in mature P. halepensis trees, was balanced between male and female functions, whereas N and P were allocated relatively more to male function (Table 2). This difference may reflect the structural differences between male and female cones. Pine male cones are constructed only of thin microsporophylls full of pollen, while mature female cones are constructed mainly of dead woody tissue (Gifford and Foster, 1989) to protect the seeds from pre-dispersal predation in regular cones (Janzen, 1971; Richardson 1998), and from the heat of fire in serotinous cones (Habrouk et al., 1999). These structural differences explain the differences in N and P contents of 1-year-old green female cones and 2-year-old mature brown cones, while there was no difference in biomass allocation to a single cone (Table 1).
The allocation to all functions increased linearly with tree size, but the rates differed among currencies (Table 2). According to sex allocation theory, the relative male to female allocation ratio should increase with tree size. The relative male to female allocation increased with tree size in all currencies (cone number, biomass, N and P) (Table 3), since all the regression lines were significant with positive slopes (Fox, 1993), and as expected from sex allocation theory (Charnov, 1982). However, the slopes for N and P were moderate, and r2 extremely low, indicating that there were other, probably more important factors explaining the increase in relative male to female allocation with increase in tree size; we have no suggestion as to what these factors could be. In general, our results provided support for the relatively higher increase in male than female allocation with increase in tree size, as expected from sex allocation theory (Freeman et al., 1981; Schoen and Stewart, 1986; Allison, 1991; Bickel and Freeman, 1993).
This study indicates that there are no trade-offs between reproduction and vegetative growth at the shoot level. Shoot vigour did not differ between reproductive branches (male or female) and their corresponding non-reproductive counterparts; moreover, shoot growth was not affected by the developmental state of the female cone attached to it (Fig. 3, Table 4). Such a lack of trade-off was also found for a dioecious shrub (Delph, 1990), but in other studies a vegetative–reproductive trade-off was found (Hoffman and Alliende, 1984; Lovett Doust and Lovett Doust, 1988; Chippolini and Whigham, 1994; Obeso, 1998). Thus, the differences in shoot vigour between male and female shoots are the result of their location within the crown and not because of their cone gender.
Conclusions
As P. halepensis trees grow and get older, their apical dominance decreases, lateral shoot density increases, and their crowns change from a conical to a cylindrical and later to a hemispherical shape. In young trees, female cones are produced first, at a young age, and they are restricted to apical, more vigorous branches. These traits seem to be of adaptive value to this pioneer and post-fire obligate seeder species. When trees grow, lateral shoot density increases, leading to increased allocation to male function in accordance with sex allocation theory. As a trees get older, the change in their canopies from a cylindrical to a hemispherical crown, with no increase in tree height, is accompanied by a further increase in male-biased sex allocation. This change can also be explained by the natural history of P. halepensis as a short-lived tree whose population regeneration is fire or disturbance dependent. We conclude that plant natural history traits should be carefully examined when considering sex allocation and gender segregation as they supply complementary explanations.
ACKNOWLEDGEMENTS
The authors thank J. van Rheenen and M. van Staalduinen for the N and P analyses, and two anonymous referees for their useful comments.
LITERATURE CITED
- Abrahamson W, Caswell H. On the comparative allocation of biomass, energy and nutrients in plants. Ecology. 1982;63:982–991. [Google Scholar]
- Allison TD. Variation in sex expression in Canada yew (Taxus canadensis) American Journal of Botany. 1991;78:569–578. [Google Scholar]
- Arista M, Talavera S. Gender expression in Abies pinsapo Boiss., a Mediterranean fir. Annals of Botany. 1997;79:337–342. [Google Scholar]
- Ashman TL. Reproductive allocation in hermaphrodite and female plants of Sidalcea oregana spp. spicata (Malvaceae) using four currencies. American Journal of Botany. 1994;81:433–438. [Google Scholar]
- Ashman TL, Baker I. Variation in floral sex allocation with time of season and currency. Ecology. 1992;73:1237–1243. [Google Scholar]
- Barrett SCH, Case AL, Peters GB. Gender modification and resource allocation in subdioecious Wurmbea dioica (Colchicaceae) Journal of Ecology. 1999;87:123–137. [Google Scholar]
- Bell G. On the function of flowers. Proceedings of the Royal Society B: Biological Sciences. 1985;224:223–265. [Google Scholar]
- Bickel AM, Freeman DC. Effects of pollen vector and plant geometry on floral sex ratio in monoecious plants. American Midland Naturalist. 1993;130:239–247. [Google Scholar]
- Bram MR, Quinn JA. Sex expression, sex-specific traits and the effects of salinity on growth and reproduction of Amaranthus cannabins (Amaranthaceae), a dioecious annual. American Journal of Botany. 2000;87:1609–1618. [PubMed] [Google Scholar]
- Burd M, Allen TFH. Sexual allocation strategy of wind-pollinated plants. Evolution. 1988;42:403–407. doi: 10.1111/j.1558-5646.1988.tb04145.x. [DOI] [PubMed] [Google Scholar]
- Chapin FS, Van Cleve K. Approaches to studying nutrient uptake, use and loss in plants. In: Pearcy RW, Ehrlinger J, Mooney HA, Rundel PW, editors. Plant physiological ecology. New York: Chapman and Hall; 1989. pp. 185–208. [Google Scholar]
- Charlesworth D, Charlesworth B. Allocation of resources to male and female functions in hermaphrodites. Biological Journal of the Linnean Society. 1981;15:57–74. [Google Scholar]
- Charnov EL. The theory of sex-allocation. 1982 Princeton University Press, Princeton, NJ. [PubMed] [Google Scholar]
- Charnov EL. On sex allocation and selfing in higher plants. Evolutionary Ecology. 1987;1:30–36. [Google Scholar]
- Chippolini ML, Whigham DF. Sexual dimorphism and cost of reproduction in the dioecious shrub Lindera benzoin (Lauraceae) American Journal of Botany. 1994;81:65–75. [Google Scholar]
- Cobb NS, Trotter RT, Whitham TG. Long-term sexual allocation in herbivore resistant and susceptible pinion pine (Pinus edulis) Oecologia. 2002;130:78–87. doi: 10.1007/s004420100785. [DOI] [PubMed] [Google Scholar]
- Correia O, Diaz-Barradas MC. Ecophysiological differences between male and female plants of Pistacia lentiscus L. Plant Ecology. 2000;149:131–142. [Google Scholar]
- Delph LF. Sex-differential resource allocation patterns in the subdioecious shrub Hebe subalpina. Ecology. 1990;71:1342–1351. [Google Scholar]
- Faegri I, Van der Pijl L. The principles of pollination ecology. 2nd edn. London: Pergamon; 1971. [Google Scholar]
- Floyd ME. Dioecy in five P. edulis populations in the Southwestern United States. American Midland Naturalist. 1983;110:405–411. [Google Scholar]
- Fox JF. Size and sex allocation in monoecious woody plants. Oecologia. 1993;94:110–113. doi: 10.1007/BF00317310. [DOI] [PubMed] [Google Scholar]
- Freeman DC, Drant McArthur E, Harper KT Blauer AC. Influence of environment on the floral sex ratio of monoecious plants. Evolution. 1981;35:194–197. doi: 10.1111/j.1558-5646.1981.tb04875.x. [DOI] [PubMed] [Google Scholar]
- Geber MA. The cost of meristem limitation in Polygonum arenastrum: negative genetic correlations between fecundity and growth. Evolution. 1990;44:799–819. doi: 10.1111/j.1558-5646.1990.tb03806.x. [DOI] [PubMed] [Google Scholar]
- Gifford EM, Foster AS. Morphology and evolution of vascular plants. 3rd edn. New York: W. H. Freeman and Company; 1989. [Google Scholar]
- Goubitz S, Werger MJA, Shmida A, Ne'eman G. Cone abortion in Pinus halepensis: the role of pollen quantity, tree size and cone location. Oikos. 2002;97:125–133. [Google Scholar]
- Hamilton WD. Extraordinary sex ratios. Science. 1967;256:477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- Habrouk A, Retana J, Espelta JM. Role of heat tolerance and cone protection of seeds in the response of three pine species to wildfires. Plant Ecology. 1999;145:91–99. [Google Scholar]
- Hoffman AJ, Alliende MC. Interactions in the patterns of vegetative growth and reproduction in woody dioecious plants. Oecologia. 1984;61:109–114. doi: 10.1007/BF00379095. [DOI] [PubMed] [Google Scholar]
- Ishida TA, Hattori K, Shibata S, Suzuki M, Kimura MT. Sex allocation of a cosexual wind-pollinated tree, Quercus dentata, in terms of four currencies. Journal of Plant Research. 2005;118:193–197. doi: 10.1007/s10265-005-0206-6. [DOI] [PubMed] [Google Scholar]
- Janzen DH. Seed predation by animals. Annual Review of Ecology and Systematics. 1971;2:465–492. [Google Scholar]
- de Jong TJ, Klinkhamer PGL. Plant size and reproductive success through female and male function. Journal of Ecology. 1994;82:399–402. [Google Scholar]
- de Jong T, Shmida A, Thuijsman F. Sex allocation in plants and the evolution of monoecy. Evolutionary Ecology Research. 2008;10:1082–1109. [Google Scholar]
- Jordano P. Gender variation and expression of monoecy in Juniperus pheonica (L.) (Cupressaceae) Botanical Gazete. 1991;152:476–485. [Google Scholar]
- Keeley JE, Fotheringham CJ, Ne'eman G. Immaturity risk in a fire dependent pine. Journal of Mediterranean Ecology. 1999;1:41–48. [Google Scholar]
- Klinkhamer PGL, de Jong TJ, Metz H. Sex and size in cosexual plants. Trends in Evolution and Ecology. 1997;12:260–265. doi: 10.1016/s0169-5347(97)01078-1. [DOI] [PubMed] [Google Scholar]
- Lechowicz MJ. The effects of individual variation in physiological and morphological traits on the reproductive capacity of the common cocklebur, Xanthium strumarium L. Evolution. 1984;37:833–844. doi: 10.1111/j.1558-5646.1984.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Lev-Yadun S, Liphschitz N. The ontogeny of gender of Cupressus sempervirens L. Botanical Gazette. 1987;148:407–412. [Google Scholar]
- Lloyd DG. Sexual segregation in plants III. A quantitative method for describing the gender of plants. New Zealand Journal of Botany. 1980;18:103–108. [Google Scholar]
- Lloyd DG, Bawa KS. Modification of the gender of seed plants in varying conditions. Evolutionary Biology. 1984;17:255–338. [Google Scholar]
- Lovett-Doust J. Plant reproductive strategies and resource allocation. Trends in Evolution and Ecology. 1989;4:230–234. doi: 10.1016/0169-5347(89)90166-3. [DOI] [PubMed] [Google Scholar]
- Lovett-Doust J, Lovett-Doust L. Modules of production and reproduction in a dioecious clonal shrub. Rhus typhina. Ecology. 1988;69:741–750. [Google Scholar]
- Matziris D. Variation in growth, flowering and cone production in a clonal seed orchard of aleppo pine grown in Greece. Silvae Genetica. 1997;46:224–228. [Google Scholar]
- Mendez M. Modification of phenotypic and functional gender in the monoecious Arum italicum (Araceae) American Journal of Botany. 1998;85:225–234. [PubMed] [Google Scholar]
- Mirov NT. The genus Pinus. New York: Ronald Press; 1967. [Google Scholar]
- Ne'eman G, Izhaki I. The effect of stand age and microhabitat on soil seed banks in Mediterranean aleppo pine forests after fire. Plant Ecology. 1999;144:115–125. [Google Scholar]
- Ne'eman G, Trabaud L. Ecology, biogeography and management of Pinus halepensis and P. brutia Mediterranean pine forest ecosystems. Leiden: Buckhuys Publishers; 2000. [Google Scholar]
- Obeso JR. Sex ratios, size distributions and sexual dimorphism in the dioecious tree Ilex aquifolium (Aquifoliaceae) American Journal of Botany. 1998;85:1602–1608. [PubMed] [Google Scholar]
- Owens JN. Flowering and seed set. In: Raghavendra AS, editor. Physiology of trees. New York: John Wiley; 1991. pp. 247–271. [Google Scholar]
- Panetsos KP. Monograph of Pinus halepensis (Mill.) and P. brutia (Ten.) Annales Forestales. 1981;9:39–77. [Google Scholar]
- Preston KA. Can plasticity compensate for architectural constraints on reproduction? Patterns of seed production and carbohydrate translocation in Perilla frutescens. Journal of Ecology. 1999;87:697–712. [Google Scholar]
- Primack RB, McCall C. Gender variation in a red maple population (Acer rubrum: Aceraceace): a seven-year study of a polygamodioecious species. American Journal of Botany. 1986;73:1239–1248. [Google Scholar]
- Reekie EG, Bazzaz FA. Reproductive effort in plants II. Does carbon reflect the allocation of other resources? American Naturalist. 1987;129:897–906. [Google Scholar]
- Richardson DM. Ecology and biogeography of Pinus. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Ross SD, Pharis RP. Control of sex expression in conifers. Plant Growth Regulation. 1987;6:37–60. [Google Scholar]
- Sarkissian TS, Barrett SCH, Harder LD. Gender variation in Sagittaria latifolia (Alismataceae): is size all that matters? Ecology. 2001;82:360–373. [Google Scholar]
- Sato T. Effects of phenological constraints on sex allocation in cosexual monocarpic plants. Oikos. 2000;88:309–318. [Google Scholar]
- Savolainen O, Kärkkäinen K, Harju A, Nikkanen T, Rusanen M. Fertility variation in Pinus sylvestris: a test of sexual allocation theory. American Journal of Botany. 1993;80:1016–1020. [Google Scholar]
- Schmitt J. Reaction norms of morphological and life history traits to light availability in Impatiens capensis. Evolution. 1993;47:1554–1568. doi: 10.1111/j.1558-5646.1993.tb01258.x. [DOI] [PubMed] [Google Scholar]
- Schoen DJ, Stewart SC. Variation in male reproductive investment and male reproductive success in white spruce. Evolution. 1986;40:1109–1120. doi: 10.1111/j.1558-5646.1986.tb05737.x. [DOI] [PubMed] [Google Scholar]
- Shmida A, Lev-Yadun S, Goubitz S, Ne'eman G. Sexual allocation and gender segregation in Pinus halepensis, P. brutia and P. pinea. In: Ne'eman G, Trabaud L, editors. Ecology, biogeography and management of Pinus halepensis and P. brutia Mediterranean pine forest ecosystems. Leiden: Buckhuys Publishers; 2000. pp. 9–104. [Google Scholar]
- Smith CC. The facultative adjustment of sex ratio in lodgepole pine. American Naturalist. 1981;188:297–305. [Google Scholar]
- Solomon BP. Size dependent sex ratio in monoecious wind pollinated annual Xanthium strumarium. American Midland Naturalist. 1989;121:209–218. [Google Scholar]
- Thompson K, Stewart AJA. The measurement and meaning of reproductive effort in plants. American Naturalist. 1981;117:205–211. [Google Scholar]
- Travest A. Sex expression in a natural population of the monoecious annual, Ambrosia artemisiifolia. American Midland Naturalist. 1992;127:309–315. [Google Scholar]
- Veillon JM. Architecture of the New Caledonian species of Araucaria. In: Tomlinson PB, Zimmerman MH, editors. Tropical trees as living systems. Cambridge: Cambridge University Press; 1978. pp. 233–245. [Google Scholar]
- Wareing PF, Phillipis LD. The control of growth and differentiation in plants. Oxford: Pergamon Press; 1970. [Google Scholar]
- Willson MF. Plant Reproductive Ecology. New York: John Wiley and Sons; 1983. [Google Scholar]
- Wilson BF. Apical control of compression wood action in white pine branches. Wood Science and Technology. 1986;20:111–117. [Google Scholar]
- Wilson B. Apical control of branch growth and angle in woody plants. American Journal of Botany. 2000;87:60–607. [PubMed] [Google Scholar]
- Witkowski ETF, Lamont BB. Disproportionate allocation of mineral nutrients and carbon between vegetative and reproductive structures in Banksia hookeriana. Oecologia. 1996;105:38–42. doi: 10.1007/BF00328789. [DOI] [PubMed] [Google Scholar]
- Wolfe LM, Shmida A. The ecology of sex expression in a gynodioecious Israeli desert shrub (Ochradenus baccatus) Ecology. 1997;78:101–110. [Google Scholar]