Abstract
Background and Aims
The bacterium Xylella fastidiosa (Xf), responsible for Pierce's disease (PD) of grapevine, colonizes the xylem conduits of vines, ultimately killing the plant. However, Vitis vinifera grapevine varieties differ in their susceptibility to Xf and numerous other plant species tolerate Xf populations without showing symptoms. The aim of this study was to examine the xylem structure of grapevines with different susceptibilities to Xf infection, as well as the xylem structure of non-grape plant species that support or limit movement of Xf to determine if anatomical differences might explain some of the differences in susceptibility to Xf.
Methods
Air and paint were introduced into leaves and stems to examine the connectivity between stem and leaves and the length distribution of their vessels. Leaf petiole and stem anatomies were studied to determine the basis for the free or restricted movement of Xf into the plant.
Key Results
There were no obvious differences in stem or petiole vascular anatomy among the grape varieties examined, nor among the other plant species that would explain differences in resistance to Xf. Among grape varieties, the more tolerant ‘Sylvaner’ had smaller stem vessel diameters and 20 % more parenchyma rays than the other three varieties. Alternative hosts supporting Xf movement had slightly longer open xylem conduits within leaves, and more connection between stem and leaves, when compared with alternative hosts that limit Xf movement.
Conclusions
Stem–leaf connectivity via open xylem conduits and vessel length is not responsible for differences in PD tolerance among grape varieties, or for limiting bacterial movement in the tolerant plant species. However, it was found that tolerant host plants had narrower vessels and more parenchyma rays, possibly restricting bacterial movement at the level of the vessels. The implications of xylem structure and connectivity for the means and regulation of bacterial movement are discussed.
Keywords: Grape, grapevine, Vitis vinifera, host, leaf, stem, xylem, Pierce's disease, Xylella fastidiosa
INTRODUCTION
Xylella fastidiosa (Xf) is a xylem-limited bacterium that lives as a harmless endophyte in most plants species, but various subspecies and strains of Xf are differentially pathogenic in several agriculturally important crops such as coffee, citrus and grapevine (Hopkins and Purcell, 2002). The bacterium is transmitted by xylem sap-feeding sharpshooter leafhoppers (Redak et al., 2004), which acquire Xf while feeding on the xylem of infected plants (Houston et al., 1947). In susceptible cultivars of grapevine, Xylella fastidiosa subsp. Piercei infection results in leaf scorch, premature leaf senescence, petiole ‘matchsticks’, incomplete periderm development and eventually death (Stevenson et al., 2005); a suite of symptoms collectively referred to as Pierce's disease (PD).
PD symptoms were traditionally thought to result from the accumulation of bacteria and its associated gum within the xylem vessels, causing vascular occlusions and water deficit (Hopkins, 1989; Purcell and Hopkins, 1996). This vascular occlusion hypothesis implies a positive correlation between symptom severity and pathogen concentration. Indeed, some studies showed correlations between high Xf populations and the apparent susceptibilities of grapevine genotypes (Raju and Goheen, 1981; Hopkins and Thompson, 1984; Fry and Milholland, 1990; Krivanek and Walker, 2005). However, the relationship between PD symptoms and bacterial populations is more complex. First, Thorne et al. (2006a) showed that visual symptoms of PD were qualitatively and quantitatively different from those of various water deficits, although water deficits exacerbate the development of PD symptoms and, at times, localized water deficits are possible (Gambetta et al., 2007; Choat et al., 2009). Secondly, multiple studies showed that the overall proportion of vessels occluded by Xf and associated gums was very low (Hopkins, 1989; Newman et al., 2003; Alves et al., 2004; Krell et al., 2006), and was unlikely to induce water deficit. Finally, more recently, Gambetta et al. (2007) demonstrated, via a novel, robust quantitative PCR (qPCR) assay to quantify Xf in planta, that there was very little correlation between Xf concentrations in leaves and symptom severity. That study showed that the Xf populations were patchily distributed across whole leaves, and that leaves could exhibit severe leaf scorch symptoms with low bacterial concentrations. An alternative to the occlusion hypothesis is that disease symptoms result from a systemic plant response to the presence of the gums and tyloses (Stevenson et al., 2004), via a higher rate of ethylene production in the infected leaves (Sun et al., 2006, 2007; Perez-Donoso et al., 2007). Phytotoxins and programmed cell death are also considered in the induction of PD symptoms (Gilchrist and Lincoln, 2006; Reddy et al., 2007).
Pierce's disease is presently controlled in California by reducing vector populations through habitat management (Purcell et al., 1999) and insecticide applications, although development of Vitis vinifera cultivars resistant or tolerant to Xf remains an active area of research. Vitis vinifera cultivars vary in their susceptibilities to PD, while other Vitis species are tolerant of Xf colonization (Purcell, 1981; Raju and Goheen, 1981; Hopkins and Thompson, 1984; Fry and Milholland, 1990; Krivanek and Walker, 2005). In addition to grapevine, Xf multiplies harmlessly within numerous plant species (Freitag, 1951). Most of those alternative hosts of Xf do not show PD symptoms, despite allowing bacterial proliferation and movements beyond the inoculation point, although at much lower levels than in grapevine (Hill and Purcell, 1995; Purcell and Saunders, 1999; Costa et al., 2004; Baumgartner et al., 2005; Wistrom and Purcell, 2005). However, most of these studies recorded plant longevity, the appearance and severity of the symptoms, and the Xf populations in the plant. More detailed investigations of the nature of the plant–pathogen interaction are necessary to identify possible reasons for differences in plant susceptibility to Xf colonization. Since Xf is a xylem-limited bacterium, further investigation of the xylem structure in resistant and susceptible plants is needed. For example, since Xf must digest the pit membrane that separates vessels (Newman et al., 2003; Scarpari et al., 2003), shorter and narrower vessels, and internal separation of xylem tissue by rays of non-conducting tissue, could limit Xf movement. Conversely, the presence of long open conduits would allow fairly quick movement of Xf over long distances (Chatelet et al., 2006; Thorne et al., 2006b).
There are three known xylem-limited bacterial species: Xylella fastidiosa, Clavibacter xyli and Pseudomonas syzygii. Xylella fastidiosa is the most economically important and the most studied; C. xyli subsp. xyli is the agent of ratoon stunting disease of sugar cane (Davis et al., 1980); C. xyli subsp. cynodontis causes stunting disease of Bermuda grass (Davis and Augustin, 1984); and P. syzygii causes Sumatra disease of cloves (Bennett et al., 1985; Roberts et al., 1990). Similar to Xf, the other two xylem-limited bacteria are found in a multitude of plant hosts (Kamiunten and Wakimoto, 1976; Davis et al., 1983; Roberts et al., 1990). However, it is not known how these bacteria spread within the xylem system, and how the plants respond to this invasion. The same applies for exogenous bacteria that can also invade xylem tissues, such as Erwinia stewartii, the agent of corn wilt (Pepper, 1967), Xylophilus ampelinus (ex Xanthomonas ampelina), the agent of grapevine bacterial necrosis (Panagopoulos, 1969; Grall and Manceau, 2003), or Ralstonia solanacearum, responsible for bacterial wilt of solanaceous crop plants (Hayward, 1991). A better understanding of the propagation of these bacteria within the xylem may reveal multiple methods of bacterial movement and of plant defences.
General grapevine anatomy and its vascular tissue have been investigated by various researchers (Pratt, 1974; Mullins et al., 1992; Gerrath et al., 2001). Several anatomical aspects related to PD development have also been documented (Esau, 1948; Hopkins, 1976; Mollenhauer and Hopkins, 1976; Milholland et al., 1981; Stevenson et al., 2004, 2005; Chatelet et al. 2006; Thorne et al., 2006b). However, generally these studies were limited to a few plants and/or a few xylemic characters. The objective of this study was to expand the scope of these studies by examining the xylem structure of grapevines with different susceptibilities to Xf infection, as well as the xylem structure of non-grape plant species that do and do not support the movement of Xf to determine if anatomical differences might explain some of the differences in susceptibility to Xf.
MATERIALS AND METHODS
Plant materials
Grapevines (Vitis vinifera, varieties ‘Sylvaner’, ‘Cabernet Sauvignon’, ‘Pinot Noir’ and ‘Chardonnay’) were propagated from seed or cuttings. These four cultivars are tolerant (‘Sylvaner’), susceptible (‘Cabernet Sauvignon’) and highly susceptible (‘Pinot Noir’ and ‘Chardonnay’) to PD (Purcell, 1977, 1980; Raju and Goheen, 1981; Hopkins and Purcell, 2002; Krivanek et al., 2005). Twelve plant species that varied in their ability to support Xf were chosen for anatomical characterization (Costa et al., 2004; Baumgartner et al., 2005; Wistrom and Purcell, 2005). Species supporting Xf movement included Ipomoea purpurea (Convolvulaceae), Vinca major (Apocynaceae), Citrus sinensis (Rutaceae), Prunus amygdalus (Rosaceae), Helianthus annuus (Asteraceae) and Nicotiana sanderae (Solanaceae). Species that supported limited Xf movement included Umbellularia californica (Lauraceae), Alnus rhombifolia (Betulaceae), Datura meteloides (Solanaceae), Eucalyptus globulus (Myrtaceae), Artemisia douglasiana (Asteraceae) and Chenopodium quinoa (Amaranthaceae). Ipomoea purpurea, H. annuus, D. meteloides, C. quinoa and N. sanderae were grown from seeds (Lake Valley Seed, Boulder, CO, and Botanical Interests, Inc., Broomfield, CO, USA), while cuttings of other plants were collected on the UC Davis campus. All greenhouse-grown plants were grown in 3·78 L pots containing U.C. Davis Mix (equal parts peat moss, coarse sand and nitrolysed redwood sawdust) in the greenhouse (30/20 ± 3 °C; 40/70 ± 10 % relative humidity; natural light), and watered daily with modified Hoagland's nutrient solution (Wada et al., 2008). Grapevines, A. douglasiana, I. purpurea, H. annuus, D. meteloides, C. quinoa and N. sanderae were trained vertically as a single cane or stem, with lateral branches removed. Grape canes and other plants were approx. 2–4 months old when sampled, and had intact terminal tips.
Air movement in petioles and stems
One petiole and attached leaf each from nodes 3, 7, 12, 16 and 20 were carefully removed from stems at the base of the petioles under water. Petioles were attached to a rubber tube under water, and air was infused into the cut ends at a pressure of 35 kPa controlled by a pressure regulator. This pressure is 7 % that of the lowest air-seeding threshold reported for grapevine (Sperry et al., 1987) and about 20 % that of the lowest value reported for trees (Choat et al., 2003; Hacke et al., 2004; Sperry and Hacke, 2004). After a few seconds under pressure, the major veins and their secondary veins were cut with a razor blade every few millimetres starting from the margin, and moving toward the base of the leaf. The incisions were made until a stream of air bubbles appeared at the cut. The distance from where the bubbles first appeared to the air loading point and the distance from the leaf margin to the loading point were measured. Leaf lengths were variable between species, ranging from 20 to 360 mm. In order to compare plant species, the farthest position of bubble appearance was reported as a percentage of the total length of their leaves. Air injection in leaves was replicated five times for each selected node for both grapes and alternative hosts. Data are expressed as calculated means and their standard error (n = 5). Statistical analysis was performed using analyis of variance (ANOVA). P < 0·05 was considered statistically significant.
For stems, plants were brought to the lab and the stems were cut under water, connected to a plastic tube and pressurized air was pushed into the stem base at 35 kPa. Starting from the stem apex, the primary veins of each leaf were cut as previously described until a stream of air bubbles appeared. If air did not appear in the vein, the leaf lamina was separated from the petiole below the lamina/petiole junction. Air exiting at the apical end of the petiole signified that the lamina/petiole junction was blocking the air. If no air exited the apical end of the petiole, it was cut at its base to verify whether air was able to travel within the petiole. The distance from the loading point in the stem to the node where air appeared in leaves was measured, and was calculated as a percentage of the total length of the stem. Finally the stem was cut every few millimetres starting from the apex toward the base until a stream of air bubble appeared at the cut, and the distance from the loading point to the appearance of the bubble was measured. The stem lengths were very variable, ranging from 6 to 240 cm. Therefore, for comparison, the farthest position attained by the air in the stem of every species was calculated as a percentage of the total length of their stem. Air was injected in five different stems for both grapes and alternative hosts. Data are expressed as calculated means and their standard error (n = 5) and the statistical analysis was performed using ANOVA.
Vessel length distribution in stems and petioles
Grape canes and other plants were approx. 2 months old when sampled, and had intact terminal tips. Plants were kept in a cool, dark location overnight prior to measurement, and thoroughly watered to decrease transpiration and xylem tension. The length, diameter and number of nodes were measured for each stem or cane. Vessel length was measured according to the technique of Ewers and Fisher (1989), modified to infuse paint under pressure. Stems were cut underwater, and submerged until attached to the paint infusion apparatus. Latex paint (ACE Royal High Gloss Clean Red Enamel; ACE Hardware, Oak Brook, IL, USA) was diluted 1:300 in deionized water, filtered through Whatman #1 filter paper, and degassed prior to use. A modified stainless steel sprayer (B and G Equipment Co., Jackson, GA, USA) was filled with 3·5 L of diluted paint and pressurized with compressed air to 100 kPa, so the paint solution flowed out of the sprayer and into clear plastic tubing. Air was removed from the system prior to attachment of the stem to the tube. Stems were forced into tubing underwater and secured with wire. All leaves were removed and the stem was placed inside a plastic bag. Paint was infused into the stem for approx. 96 h, until liquid ceased emerging from the distal end. Paint infusion in leaves and stems was replicated five times for both grapes and alternative hosts. Vessel length distribution was calculated using Excel (Microsoft, Redmond, WA, USA), and statistical analysis was performed using ANOVA.
Paint particles filled vessels, but were stopped by pit membranes, indicating one continuous xylem vessel element. Particles in the latex paint solution were larger than 0·22 µm in diameter, since they did not pass through a sterilizing filter (Millipore Corporation, Billerica, MA, USA). Pigment and additive particles in latex paint measured between 0·3 and 7·5 µm in diameter (Croll, 2002), but pores in vessel pit membranes measured between 0·005 and 0·17 µm, depending on the plant species (Siau, 1984).
In a first experiment, vessel lengths were calculated for stems of each plant species or grapevine cultivar. The paint solution was loaded into stems as described above. Thin (∼1 mm) cross-sections were cut by hand with a Platinum Injector razor blade (Longs Corporation, Walnut Creek, CA, USA) every 1 cm and placed on a glass slide in 50 % glycerol. Sections were photographed with an Olympus Vanox-AHBT (Olympus America, Melville, NY, USA) compound light microscope linked to a Pixera 600ES digital camera. Vessels with paint were counted from the digital image of each section.
In a second experiment, vessel length distribution was calculated for leaves from node 3, 7, 12, 16 and 20 from the stem apex with the same technique. The leaves were excised under water and their petiole was connected to silicone tubing linked to the reservoir filled with the paint solution. The leaves were kept under water and were infused with the paint suspension at a pressure of 35 kPa until the paint solution stopped moving. At the end of the infusion time, thin freehand cross-sections were cut every 5 mm with a razor blade. The petiole and major veins were sectioned every 5 mm starting from the paint infusion point, and progressing toward the margin of the leaf. The vessels with paint were counted in each section and vessel length distribution was calculated as described for stems.
Tylose formation
Dental paste and a 0·40 mm hypodermic needle were used to grossly imitate the wounds left by a feeding sharpshooter (Leopold et al., 2003). Five stems of similar age from each species mentioned above were selected to evaluate the tylose formation after wounding. A drop of dental paste (CutterSil Mucosa, Heraeus Kulzer, Inc., Armonk, NY, USA) was placed above the first mature leaf proximal to the shoot apex. The hypodermic needle filled with dental paste was driven four times through the dental paste drop, a few millimetres into the stem xylem. The dental paste sealed the wound upon withdrawing the needle. Stem segments were collected at 0, 1, 3 and 6 d after wounding, and the presence of tyloses in the vessels was observed in cross-sections made within the wounded area, 5, 10 and 100 mm above the wound. For each distance, the proportion of vessels with tyloses was calculated.
Vessel diameter distribution at the base of the stem and petiole
Five stems from each species were cross-sectioned at the base. For each stem, five zones of the xylem were randomly selected and the number and diameter of the vessels within each zone were counted and measured. From the same plants, five mature leaves were collected and cross-sectioned at the base of the petiole. For each section, all the vessels were counted and their diameter was measured. The diameter distributions of the vessels, at the base of the stem, and in the petiole of a mature leaf, were calculated for each species.
Anatomical comparisons among grape cultivars and other plant species
Segments of 1 cm from the base of the stem and petiole were sectioned with a sliding microtome (AO-860, American Optical, Buffalo, NY, USA) in transverse, tangential and radial planes with a section thickness of 25 µm. The sections were dehydrated through an ethanol series (Ruzin, 1999). Each step lasted 1 h, except for the 4 h step in 50 % ethanol with 1 % safranin O, and the 1 min 95 % ethanol step with 0·5 % fast green FCF. Sections were further dehydrated and cleared in an ethanol–xylene series (2:1, 1:1, 1:2) followed by two xylene rinses of 10 min each. Sections were mounted with coverslips in Permount (Fisher Scientific, Fair Lawn, NJ, USA), and photographed with a Olympus Vanox-AHBT (Olympus America, Melville, NY, USA) compound light microscope linked to a Pixera 600ES digital camera. The total numbers of vessels, bundles, rays and paratracheal parenchyma cells were counted. Data are expressed as calculated means and their standard error (n = 5). Statistical analysis was performed using ANOVA (P < 0·05 was considered statistically significant).
RESULTS
Air movement in the leaves
The farthest distance travelled by air in the plant species tested ranged from 20 to 86 % of the total length of the vascular path from the petiole base to individual leaf vein endings (Table 1). This range indicated that the lengths of open, continuous xylem vessels (conduits) are highly variable, and that it is possible for bacteria to move passively from the base of the petiole toward the tip of the leaves.
Table 1.
Farthest position reached by air in leaf primary veins expressed as a percentage of the total distance from the beginning of the petiole to the margin of the leaf
| Node 3 | Node 7 | Node 12 | Node 16 | Node 20 | |
|---|---|---|---|---|---|
| Grapevine | |||||
| V. vinifera ‘Sylvaner’ | 71·9 (2·9)a | 68·6 (2·1)a | 71·1 (2·4)a | 71·5 (2·6)a | 69·5 (1·9)a |
| V. vinifera ‘Cabernet Sauvignon’ | 71·7 (2·8)a | 69·9 (2·3)a | 73·6 (3·1)a | 70·6 (2·6)a | 69·4 (2·9)a |
| V. vinifera ‘Pinot Noir’ | 71·8 (2·9)a | 64·3 (3·8)a | 68·7 (4·0)a | 71·2 (2·9)a | 69·9 (2·9)a |
| V. vinifera ‘Chardonnay’ | 52·9 (4·2)b | 47·0 (2·9)b | 54·9 (4·3)b | 61·2 (2·0)b | 62·5 (2·4)b |
| Movement | |||||
| I. purpurea | 67·1 (2·0)ab | 73·1 (2·1)ab | 77·7 (1·6)b | 86·6 (1·6)a | |
| V. major | 30·6 (1·6)c | 34·9 (2·2)de | |||
| C. sinensis | 69·5 (1·6)a | 68·9 (2·4)ab | |||
| P. amygdalus | 52·7 (2·6)b | 53·4 (3·5)c | 53·9 (1·8)c | 54·5 (2·9)cd | 54·8 (2·7)b |
| H. annuus | 59·0 (3·4)bc | 55·9 (3·6)c | 58·8 (5·9)c | 58·9 (2·9)b | |
| N. sanderae | 42·3 (4·2)d | 50·0 (4·5)d | 56·1 (2·7)b | ||
| Limited movement | |||||
| U. californica | 45·4 (2·9)b | 39·6 (3·2)d | 29·7 (2·7)e | ||
| A. rhombifolia | 30·2 (2·4)c | 27·9 (2·1)e | 70·3 (1·9)b | 84·1 (2·5)f | |
| D. meteloides | 70·9 (2·7)a | 76·6 (2·6)a | 35·3 (2·3)de | 34·8 (1·9)ab | |
| E. globulus | 34·1 (4·2)c | 32·2 (8·6)de | 20·0 (2·2)f | 20·8 (1·1)e | 21·1 (1·4)c |
| A. douglasina | 19·9 (1·1)f | 79·9 (1·8)a | 86·8 (1·4)a | 80·8 (2·4)a | |
| C. quinoa | 66·1 (4·3)a | 72·6 (4·5)a | |||
Measurements were made in leaves of four grapevine varieties as well as in plant species that do and do not support the movement of Xylella fastidiosa beyond the inoculation site.
Data are average means (s.e.) of five leaves for each node. Values in columns with different letters are significantly different at the 95 % confidence level according to ANOVA (Turkey–Kramer test).
In grapevine, air travelled up to 70 % of the leaf length in tolerant ‘Sylvaner’, moderately susceptible ‘Cabernet Sauvignon’ and highly susceptible ‘Pinot Noir’. In highly susceptible ‘Chardonnay’, air only travelled 47–60 % of the leaf length. Overall, air travelled >50 % of the total leaf length in species allowing Xf movement, compared with 30–40 % of the leaf length in plants limiting Xf movement. Although V. major is able to support extensive Xf movement, air only moved into the first third of its leaves. Conversely, in the Xf movement-limiting hosts D. meteloides and C. quinoa, air moved as far as in leaves of non-limiting species. In sum, the lengths of the open xylem conduits did not correspond with the tolerant/susceptible category in grapevine and the observed ability of alternative hosts to limit Xf movement.
Air movement within stems and from stem to leaves
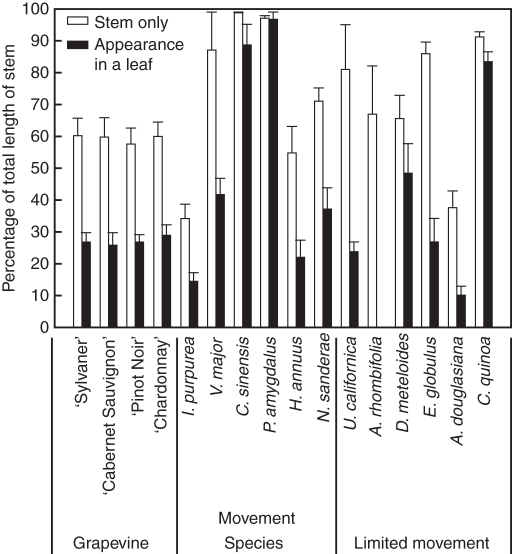
The farthest position travelled by air within the stem of the different species was also highly variable, ranging from 30 % to almost 100 % of the stem length (Fig. 1). The farthest position travelled by air in the stem before it branched off into a leaf was also very variable, ranging from 10 % to almost 100 % of the stem length. In all species, air travelled up the stem beyond the last detection point in the leaves. In the four grapevine varieties tested, air travelled 25–30 % of the length of the stem before going into a leaf, but was found up to about 60 % of the total length of the stems. In alternative hosts, the farthest position that air travelled in the stem before entering a leaf depended upon the plant species tested, ranging from 10–15 % in I. purpurea and C. quinoa to almost 100 % of the total length of the stem in C. sinensis, P. amygdalus and C. quinoa. Likewise, the farthest position attained by air in the stem alone was very variable, ranging from 30 % in I. purpurea and A. douglasiana to almost 100 % of the total length of the stem in V. major, C. sinensis, P. amygdalus, U. californica and C. quinoa. Again, the length of the open xylem conduits and the connections between stem and leaves did not correspond to any significant difference between tolerant/susceptible grapevine and between alternative host groups.
Fig. 1.
Farthest position attained by air infused into stems (white) and into stems with adjacent leaves (black), expressed as a percentage of the total length of the stem. Data are the mean + s.e., n = 5 stems.
Leaf and stem vessel length distribution
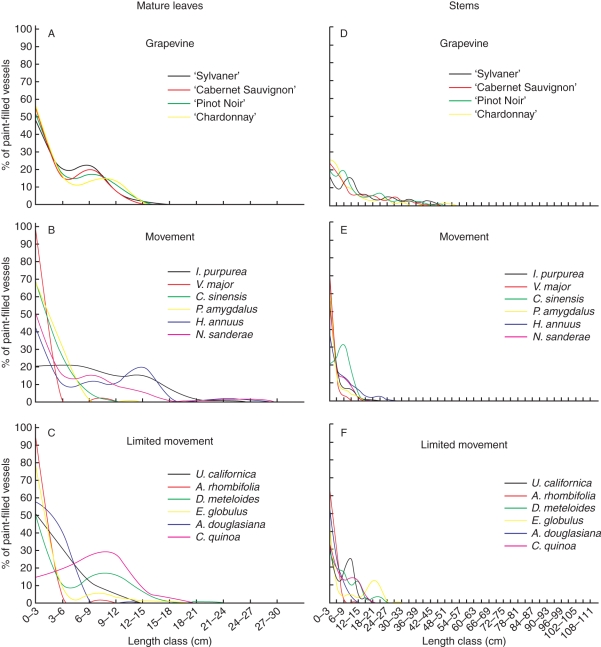
Vessel length distributions were similar in leaves of the four grapevine varieties tested. Most of the vessels were <18 cm and roughly 50 % of them were shorter than 3 cm (Fig. 2A). These are similar to vessel lengths in alternative hosts (Fig. 2B, C), where at least 40 % of the vessels in alternative Xf hosts were <3 cm, except for I. purpurea and C. quinoa. The longest vessels, 24–27 cm, were found in species supporting Xf movement: I. purpurea, H. annuus and N. sanderae.
Fig. 2.
Vessel length distribution in mature leaves (A–C) and stems (D–F) of grapevines and alternative hosts of Xylella fastidiosa. For each length class, the number of paint-infused vessels was calculated as a percentage of the total number of painted vessels at the base of the petiole or stem. n = 5 leaves, 5 stems.
In grapevine stems, most of the vessels were <50 cm, with 55 % of them being <6 cm long and the longest measuring about 1 m (Fig. 2D). In contrast, in all alternative hosts, except C. sinensis, most of the vessels of the stems were shorter than 27 cm, with 30–80 % of them being <3 cm long (Fig. 2E, F). The vessel length distributions in stems of alternative hosts were similar. The longest vessel measured in alternative hosts was about 30 cm (E. globulus).
Petiole and stem vessel diameter distribution
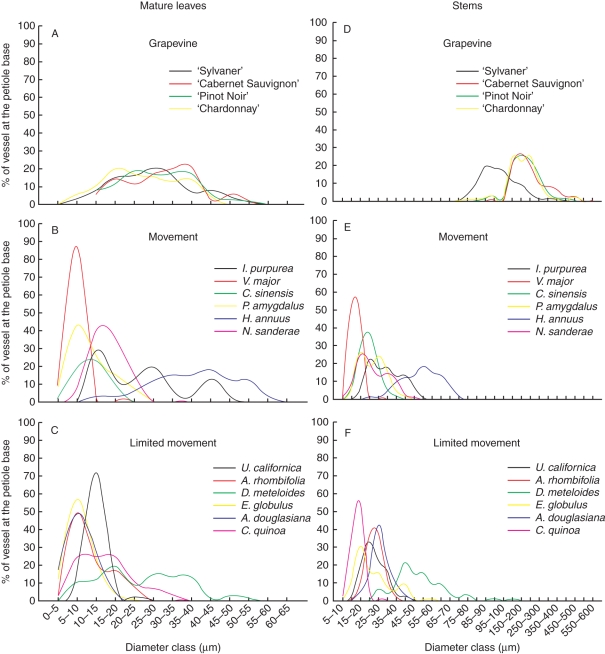
Vessel diameters were similar in petioles of the four grapevine varieties tested, with about 70 % of the vessels ranging from 10 to 45 µm (Fig. 3A). Alternative hosts (Fig. 3B, C) had smaller vessel diameters, mostly <25 µm, with the exception of H. annuus, whose vessels were between 30 and 55 µm, and D. meteloides, whose vessels were between 15 and 45 µm in diameter.
Fig. 3.
Vessel diameter distribution at the base of the petiole of mature leaves (A–C) and stems (A–F) from grapevines and alternative host species of Xylella fastidiosa. n = 5 leaves, 5 stems.
Grapevine stem vessel diameters ranged from 150 to 400 µm, except for ‘Sylvaner’, which had slightly smaller vessels, between 80 and 250 µm (Fig. 3D). In contrast, the vessel diameters at the base of the stem of the alternative hosts were similar to vessel diameters in petioles (Fig. 3E, F), ranging mostly from 10 to 35 µm, except for the vessels from H. annuus and D. meteloides whose diameter was between 40 and 65 µm.
Tylose development
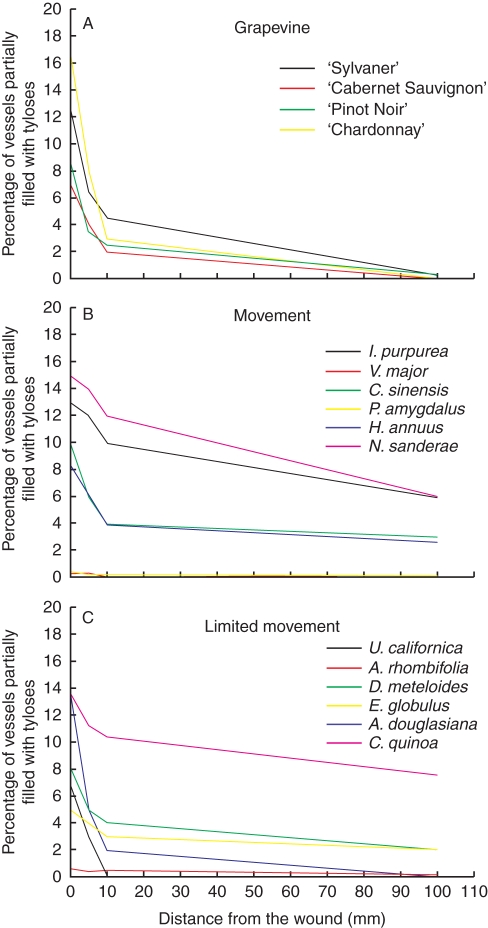
There were no major differences in tylose production between grapevine varieties and between alternative hosts (Fig. 4A–C) except for P. amygdalus, V. major and A. rhombifolia that did not produce any tyloses in response to wounding. In all species, the production of tyloses was greatest near the wound. Six days after wounding, between 5 and 15 % of the vessels had tyloses at the wounding site and the amount of tyloses decreased as the distance from the wound increased, until the vessels became eventually free of them. For example, no tyloses were observed in U. californica past 10 mm from the wound and, in all grapevine varieties and A. douglasiana, the vessels became free of tylose at 100 mm. In all remaining species, some tyloses were still present at 100 mm.
Fig. 4.
Percentage of stem vessel with tyloses distal from a simulated sharpshooter feeding site, 6 d after wounding with a needle. n = 5 stems.
Anatomical comparison of stem and leaf cross-sections
There were no significant differences in the total vessel number, the proportion of short vessels or the longest vessels between resistant and susceptible grape varieties for greenhouse-grown canes of similar length, age and diameter (Table 2). The average grape cane measured 240 cm, with 24–36 nodes. The only significant xylem anatomy difference noted among grape cultivars was the number of rays in ‘Sylvaner’, with about 20 % more rays compared with the other grapevine varieties (Table 2). The average longest vessel measured by paint and air infusion was 72 cm, but most of the vessels were <15 cm long in all cultivars. Stems from the alternative host plants were between 6 and 150 cm long, depending on the species. The longest vessel measured in any alternative host was 28 cm long, in E. globulus, and the percentage of vessels <3 cm long ranged from 21 to 84 %. We observed no discernible differences in vessel density, vessel length or number of rays between alternative hosts limiting and allowing Xf movement. Likewise, when the xylem structure of the petiole was compared, there were no significant differences among the four grapevine varieties or between the alternative hosts (Table 3).
Table 2.
Anatomical comparisons of stems of similar age from four grape cultivars and the alternative host species of Xylella fastidiosa
| No. of vessels at cane/stem base | Vessel density | % vessel ≤3 cm | Longest vessel | No. of rays/stem base | |
|---|---|---|---|---|---|
| Grapevine | |||||
| V. vinifera ‘Sylvaner’ | 513 (38)a | 12 (2)a | 17 (5)a | 69 (9)a | 40 (2)a |
| V. vinifera ‘Cabernet Sauvignon’ | 487 (27)a | 14 (1)a | 24 (2)a | 60 (3)b | 34 (1)b |
| V. vinifera ‘Pinot Noir’ | 474 (27)a | 13 (2)a | 20 (3)a | 64 (9)a | 34 (2)b |
| V. vinifera ‘Chardonnay’ | 433 (19)a | 10 (1)a | 26 (2)a | 72 (9)a | 35 (1)b |
| Movement | |||||
| I. purpurea | 298 (26)g | 15 (2)fg | 66 (5)bc | 13 (2)ef | 84 (3)f |
| V. major | 584 (5)bc | 58 (5)c | 84 (2)a | 17 (1)cde | 82 (2)f |
| C. sinensis | 446 (4)de | 75 (5)b | 21 (1)g | 12 (1)f | 137 (1)d |
| P. amygdalus | 731 (11)a | 28 (2)e | 70 (2)ab | 18 (1)bcd | 146 (2)c |
| H. annuus | 314 (22)fg | 8 (2)g | 42 (7)ef | 21 (2)b | 19 (1)i |
| N. sanderae | 474 (23)de | 6 (1)g | 64 (7)bc | 15 (2)def | 116 (1)e |
| Limited movement | |||||
| U. californica | 434 (19)de | 14 (1)fg | 37 (4)f | 20 (1)bc | 56 (2)g |
| A. rhombifolia | 657 (18)ab | 87 (7)a | 71 (3)ab | 6 (1)g | 170 (3)b |
| D. meteloides | 485 (14)de | 25 (1)ef | 45 (2)def | 27 (1)a | 32 (2)h |
| E. globulus | 507 (5) cd | 65 (3)bc | 53 (2)cde | 28 (1)a | 198 (3)a |
| A. douglasina | 489 (52)de | 40 (4)d | 58 (5)bcd | 12 (1)f | 18 (1)i |
| C. quinoa | 391 (20)ef | 20 (3)efg | 35 (3)f | 18 (1)bcd | 30 (2)h |
Data are average means (s.e.) of five stems. Values in columns with different letters are significantly different at the 95 % confidence level according to ANOVA (Turkey–Kramer test).
Table 3.
Anatomical comparisons of petioles of mature leaves from four grape cultivars and 12 alternative host plant species of Xylella fastidiosa
| No. of vessels at cane/petiole base | Vessel density | % vessel ≤1 cm | Longest vessel | No. of rays/petiole base | |
|---|---|---|---|---|---|
| Grapevine | |||||
| V. vinifera ‘Sylvaner’ | 130 (5)a | 22 (3)a | 40 (3)a | 8 (1)a | 17 (1)b |
| V. vinifera ‘Cabernet Sauvignon’ | 133 (7)a | 22 (2)a | 45 (3)a | 9 (1)a | 18 (1)ab |
| V. vinifera ‘Pinot Noir’ | 129 (3)a | 22 (4)a | 47 (4)a | 8 (1)a | 19 (1)a |
| V. vinifera ‘Chardonnay’ | 103 (9)b | 33 (8)a | 42 (6)a | 9 (1)a | 18 (1)ab |
| Movement | |||||
| I. purpurea | 158 (21)ef | 14 (3)gh | 6 (1)h | 16 (2)a | 10 (1)h |
| V. major | 47 (2)h | 45 (2)e | 68 (3)b | 2 (1)c | 14 (1)g |
| C. sinensis | 134 (6)fg | 50 (1)de | 17 (2)g | 6 (1)b | 44 (1)c |
| P. amygdalus | 151 (8)efg | 85 (4)c | 42 (2)d | 6 (1)b | 34 (1)d |
| H. annuus | 174 (8)de | 2 (1)h | 36 (3)def | 14 (1)a | 11 (1)h |
| N. sanderae | 204 (19)cd | 72 (7)cd | 41 (2)de | 14 (2)a | 23 (1)e |
| Limited movement | |||||
| U. californica | 181 (16)cde | 32 (3)efg | 34 (5)ef | 8 (1)b | 54 (1)b |
| A. rhombifolia | 595 (10)a | 375 (21)a | 49 (2)c | 4 (1)c | 66 (1)a |
| D. meteloides | 217 (9)c | 44 (2)ef | 39 (2)de | 15 (1)a | 21 (1)e |
| E. globulus | 404 (25)b | 259 (21)b | 75 (2)a | 8 (1)b | 53 (1)b |
| A. douglasina | 24 (1)h | 19 (1)fgh | 32 (1)f | 2 (1)c | 17 (1)f |
| C. quinoa | 117 (6)g | 57 (3)de | 7 (1)h | 15 (1)a | 10 (1)h |
Data are average means (s.e.) of five stems. Values in columns with different letters are significantly different at the 9 5 % confidence level according to ANOVA (Turkey–Kramer test).
This was also true for the paratracheal parenchyma cells (Table 4). With the exception of V. major, P. amygdalus and A. rhombifolia, where they were absent, paratracheal parenchyma cells were scanty to vasicentric. In the four grapevine cultivars, vessels had 6–7 paratracheal parenchyma cells, whereas 2–3 cells were present in most of the alternative hosts. Helianthus annuus and E. globulus were the exceptions, with 13·5 and ten cells, respectively. In longitudinal section, strands of paratracheal parenchyma cells had up to ten cells for grapevines, while it was slightly less for the alternative species, mostly 1–4 cells.
Table 4.
Paratracheal parenchyma cells in the stem of the grape cultivars and the alternative host species of Xylella fastidiosa
| Paratracheal parenchyma cell arrangement | Cell number/vessel element CS | Average cell number/vessel element | Cell number/strand | |
|---|---|---|---|---|
| Grapevine | ||||
| V. vinifera ‘Sylvaner’ | Scanty to vasicentric | Up to 14 | 6·5 (0·5) | Up to 10 |
| V. vinifera ‘Cabernet Sauvignon’ | Scanty to vasicentric | Up to 18 | 6·7 (0·6) | Up to 8 |
| V. vinifera ‘Pinot Noir’ | Scanty to vasicentric | Up to 15 | 6·8 (0·4) | Up to 9 |
| V. vinifera ‘Chardonnay’ | Scanty to vasicentric | Up to 13 | 6·4 (0·5) | Up to 8 |
| Movement | ||||
| I. purpurea | Scanty | Up to 5 | 2·9 (0·5) | 2–3 |
| V. major | Absent | |||
| C. sinensis | Scanty | Up to 5 | 3·0 (0·2) | 1–2 |
| P. amygdalus | Absent | |||
| H. annuus | Vasicentric | Up to18 | 13·5 (0·4) | 1–2 |
| N. sanderae | Scanty | Up to 3 | 2·1 (0·1) | 2–4 |
| Limited movement | ||||
| U. californica | Scanty | Up to 5 | 2·9 (0·2) | 2–4 |
| A. rhombifolia | Absent | |||
| D. meteloides | Scanty to vasicentric | Up to 5 | 2·7 (0·2) | 2–4 |
| E. globulus | Scanty to vasicentric | Up to 15 | 10·0 (0·4) | Up to 8 |
| A. douglasina | Scanty to vasicentric | Up to 4 | 2·6 (0·1) | 1–2 |
| C. quinoa | Scanty | Up to 4 | 2·6 (0·2) | 1–2 |
Data are mean (s.e.), n = 5 stems.
CS, cross-section.
DISCUSSION
This study examined four varieties of grapes with different susceptibilities to Xf infection, and 12 alternative host plant species categorized into two groups: those that allow Xf movement, and those that limit Xf movement, to determine whether gross xylem physical characteristics had a role in Xf colonization. The results showed that there were few or minor differences among the grapevine varieties or between the alternative hosts. There was only one grape varietal difference: the stem of the tolerant variety ‘Sylvaner’ had smaller vessel diameters and 20 % more parenchyma rays than the other three varieties. Among the alternative hosts, the xylem in leaves, and the xylem connecting the stem to the leaves was slightly more open in the hosts, allowing more bacterial movement as compared with hosts in which bacterial movement was limited. Therefore, the results show that gross xylem structure and organization are not responsible for genotypic differences in susceptibility to Xf infection and PD.
Earlier studies on different plant species showed that particle movement was limited by the frequency of vessel endings, especially at the stem–leaf junction, where most vessels were thought to end, except for the few vessels crossing the junction (Larson and Isebrands, 1978; Wiebe et al., 1984; André, 1999, 2002; Martre et al., 2000; Tyree and Zimmermann, 2002). Such vascular arrangement was thought to act as a safety mechanism against embolism (Zimmermann, 1983; Aloni and Griffith, 1991; Tyree and Ewers, 1991; Choat et al., 2005) and bacterial movement (Zimmermann, 1983; Tarbah and Goodman, 1987; Bové and Garnier, 2002). Indeed the tracheids and short vessels in these junctions would stop the propagation of air from the leaf to the stem, or vice versa, and are thought to facilitate the shedding of embolized leaves (Tyree et al., 1993; Rood et al., 2000). Although the long open conduits described here would allow free movement of air into the leaf blade if they became embolized, this can be expected to have a limited impact on overall leaf hydraulic conductance because they are so few in number (1–2 % of all vessels) and because water can bypass the obstruction easily through the finely reticulate vein network of a leaf (Wylie, 1938; Roth-Nebelsick et al., 2001; Salleo et al., 2001; Cochard et al., 2004). However, allowing a few bacterial cells to pass unimpeded from stem to leaves or leaves to stems via these open conduits could have a considerably greater impact on pathogenesis by allowing the bacteria to move rapidly throughout the infected plant, particularly because Xf can degrade and traverse pit membranes (Roper et al., 2007; Pérez-Donoso et al., 2010) unlike inert gas embolisms.
The presence of long xylem vessel conduits connecting leaves to the stem several internodes below the leaf in all the species we examined (Chatelet et al. 2006; Thorne et al. 2006b; this study) suggests that most plant species with vessels could have such characteristics. Since bacterial infection affects many different species, an interesting question arises as to how such paths might be exploited by invading bacteria. Thorne et al. (2006b) and Chatelet et al. (2006) speculated on the importance of these conduits for the passive systemic spread of pathogens via the xylem and their role in the development of diseases such as PD in grapevine. Similarly, it was suggested that the inability to restrict bacterial movement by the xylem was a key determinant of the appearance of symptoms (Fry and Milholland, 1990; Krivanek and Walker, 2005). However, in this study, the interconnectedness of the organs and the vessel length distribution profiles were similar between plant varieties and species that have been reported to differ in susceptibility to PD, and to limit and not limit Xf movement. These observations indicate that susceptibility to PD is not controlled by physical limitations in xylem organization to bacterial movement and imply that factors other than maximum open conduit length and vessel length distribution determine the extent of bacterial movement.
The lack of differences in vessel length distributions or open paths among the alternative hosts characterized in the literature as ‘systemic’ and ‘non-systemic’ is interesting. There are at least two possible implications. First, the characteristics measured here are not the pertinent ones to ascertain the potential for Xf movement. However, in our previous studies, experiments tested for open pathways in several ways including measurements of the movement of light-emitting bacteria, green fluorescent protein (GFP)–Xf, air and fluorescent beads, and all produced similar results quantifying long, open pathways in shoots, petioles and leaf lamina (Chatelet et al., 2006; Thorne et al., 2006b). Thus, the potential for passive movement of Xf is probably reflected in the measurements reported here. However, it is clear that the bacterium is motile (Meng et al., 2005; De La Fuente et al., 2007), and it may well be that the environment within the xylem of different genotypes is important to that motility. Secondly, this classification did not account for the difference in Xf strain specificity with the plant host. Strains of Xf differ greatly in their abilities to move and colonize various host plant species systemically. For example, oleander leaf scorch strains of Xf colonized and caused disease in oleander (Neerium oleander) but not in grape, whereas the reverse was true for grape strains (Purcell et al., 1999). Almond strains of Xf were weakly systemic and non-pathogenic in grape, but grape strains were systemic and pathogenic to grape and almond (Almeida et al., 2003). However, all the alternative hosts in this study were chosen from previous work that had used the Xf strain specific to PD, thereby eliminating genetic differences in virulence and intra-plant movements. Thirdly, there may have been false negatives in earlier work using the less sensitive enzyme-linked immunosorbent assay (ELISA) compared with a more sensitive qPCR assay, leading to ‘non-systemic’ interpretation for some species (Gambetta et al., 2007). This possibility raises the further question of the role of bacterial distribution and population in the development of PD.
Although high bacterial populations were previously thought necessary for symptom development (Hopkins, 1985; Fry and Milholland, 1990; Hill and Purcell, 1995; Krivanek and Walker, 2005), that is evidently not the case for Xf in leaves (Alves et al., 2004; Krell et al., 2006; Gambetta et al., 2007). Thus, the movement of small amounts of bacteria through a limited number of open conduits may be important in disease development. The presence of Xf is patchy in infected susceptible plants (Newman et al., 2003; Krell et al., 2006; Gambetta et al., 2007), and not correlated with symptoms (Krell et al., 2006; Gambetta et al., 2007). In the present study, there were no significant differences in open pathways between susceptible and tolerant species. These results point toward a systemic response of susceptible grapevines to the presence of Xf in its xylem sap, possibly involving ethylene (Hopkins 1985; Thorne et al., 2006a; Pérez-Donoso et al., 2007) and programmed cell death (Gilchrist and Lincoln, 2006).
Sun et al. (2007) demonstrated that ethylene is necessary for tylose development in wounded grapevine stems. Tyloses are produced by paratracheal parenchyma cells, outgrowing into the vessel lumen via vessel–parenchyma pits and eventually occluding the vessel (Esau, 1977). They occur naturally in a wide range of species and can be induced by wounding and pathogen infection (Wallis and Truter, 1978; Beckman and Talboys, 1981; Biggs, 1987; Bonsen and Kučera, 1990; Cochard and Tyree, 1990; Pearce, 1991; Saitoh et al., 1993; Schmitt and Liese, 1993; Clerivet et al., 2000; Salleo et al., 2002; Sun et al., 2006). These vascular occlusions that develop in response to infection are often thought to be involved in the isolation of pathogens for disease defence. In grapevines infected with Xf, tyloses are observed in primary and secondary xylem (Esau, 1948; Hopkins and Mollenhauer, 1975; Stevenson et al., 2004). However, their role in PD of grapevine is not clear as some studies reported their frequency in PD-infected grapevines to be greater in resistant genotypes (Mollenhauer and Hopkins, 1976), greater in susceptible genotypes (Krivanek et al., 2005) and unrelated to the susceptibility of the grape genotype (Fry and Milholland, 1990). Our results showed that the amount of tylose produced in response to needle wounding as well as the type and number of paratracheal parenchyma cells was similar among the grapevine varieties and among the alternative hosts, regardless of their ability to limit Xf movement. This is in accordance with the low fraction of vessels being occluded observed in PD studies (Hopkins, 1989; Newman et al., 2003; Alves et al., 2004; Krell et al., 2006) and the wounding study by Sun et al. (2006). These results provide further evidence that tyloses may be unable to limit Xf movement and spread in tolerant plants. The mechanisms for Xf tolerance observed in many species are still not resolved.
The only significant differences between the tolerant and susceptible grapevines in this study were the smaller vessel diameters and the higher number of rays in the stem of the tolerant ‘Sylvaner’ cultivar compared with the more susceptible grapevine varieties. A smaller vessel diameter suggests that Xf would self-aggregate more easily, possibly leading to a more rapid maturation of biofilms. However, the role of biofilm formation in disease development is unclear (Hopkins, 1989; De Souza et al., 2003, 2004; Koide et al., 2004; Newman et al., 2004; Guilhabert and Kirkpatrick, 2005; Feil et al., 2007; Chatterjee et al., 2008a, b). In addition to narrower vessels, the higher number of rays in the tolerant cultivar could also slow down the bacterial infection through active secretion of defence chemical compounds. Several studies suggested that the xylem sap composition influences the expression of genes involved in growth, aggregation, attachment and virulence of Xf (Bi et al., 2007; Reddy et al., 2007; Zaini et al., 2009; Shi et al., 2010). Recently, Basha et al. (2010) showed differences in the xylem sap composition (amino acids, sugars and proteins) of susceptible and tolerant Vitis species. Although more work is needed to clarify those differences, a higher number of parenchyma cells around the xylem could certainly facilitate the secretion of antimicrobial compounds in the stem xylem of the tolerant Vitis cultivar as a response to infection by Xf. In addition, the higher number of rays in the tolerant cultivar suggests that rays could also play a role in limiting the lateral spread of the bacteria. Rays are composed of one to several layers of dense living ray parenchyma cells, without tracheids or vessel elements (Esau, 1977), and they separate the water-conducting xylem into longitudinal zones. These rays would present a barrier to bacteria moving exclusively in the xylem. The lateral spread of bacteria from one zone to another can only occur when vessels bridge the two zones, either by crossing the ray or where a ray ends and two contiguous zones merge. In any case, lateral movement would be limited by the degree of connectivity of the vessels within a zone as bacteria move from vessel to vessel by digesting the pit membrane.
The work by Zimmermann and Brown (1971) and Newbanks et al. (1983) demonstrated that there are many lateral pit field connections between vessels along their length. The connectivity of vessels is presumed to be a key determinant in the vulnerability of plants to the spread of embolisms between vessels (Wheeler et al., 2005). Indeed the more surface area of pit membrane existing between vessels, the greater the chance of having a large pore that will allow the movement of gas to the next vessel. A similar logic could be applied to the movement of Xf in the xylem of grapevine genotypes. The xylem of susceptible grapevines could have greater intervessel pitting, thus allowing Xf access to adjacent vessels via pit membranes, while the xylem of tolerant grapevine would have more isolated vessels, preventing bacterial movement to other vessels. Differences in pit structure could also produce a difference between tolerance and susceptibility by presenting pit membranes that are susceptible to different degrees to digestion and breaching.
Conclusions
Our investigation of the xylem structure of PD-susceptible and tolerant grapevines, and of alternative host species that allow or restrict Xf movement produced no evidence that the gross xylem anatomy (vessel length or number, or organ connectivity) was responsible for tolerance or for limiting bacterial movement. However, the narrower vessels and higher numbers of parenchyma rays found in tolerant compared with susceptible plants imply that bacterial movement might be restricted by more subtle differences in vessel morphology. More subtle differences in the vessel network, such as the degree of intervessel pitting or the thickness of the pit membrane, coupled with the secretion of defensive chemical compounds may play a role in limiting Xf movement and disease development. Another important result of this study is the observation of long, open conduits in all examined species. The role of such conduits in bacterial colonization of plants is largely unstudied. Future analyses of the role of the xylem in bacterial movement should examine vessel overlapping, spatial organization of the pit fields, and pit membrane thickness and porosity.
ACKNOWLEDGEMENTS
We thank U.C. Davis Foundation Plant Materials Service for grape cuttings; and D. Chiniquy, S. Ching, S. Hernandez, R. Zintzun and J. Fei for technical assistance. This project was funded by a grant from the California Department of Food and Agriculture (Contract 01-0712).
LITERATURE CITED
- Almeida RPP, Purcell AH. Biological traits of Xylella fastidiosa strains from grapes and almonds. Applied and Environmental Microbiology. 2003;69:7447–7452. doi: 10.1128/AEM.69.12.7447-7452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R, Griffith M. Functional xylem anatomy in root–shoot junctions of six cereal species. Planta. 1991;184:123–129. doi: 10.1007/BF00208245. [DOI] [PubMed] [Google Scholar]
- Alves E, Marucci CR, Lopes JRS, Leite B. Leaf symptoms on plum, coffee and citrus and the relationship with the extent of xylem vessels colonized by Xylella fastidiosa. Journal of Phytopathology. 2004;152:291–297. [Google Scholar]
- André JP. Organisation vasculaire des angiospermes: une vision nouvelle. Paris: Institut Nationale de la Recherche Agronomique (INRA); 2002. [Google Scholar]
- André JP, Catesson AM, Liberman M. Characters and origin of vessels with heterogenous structure in leaf and flower abscission zones. Canadian Journal of Botany. 1999;77:253–261. [Google Scholar]
- Basha SM, Mazhar H, Vasanthaiah HKN. Proteomic approach to identify unique xylem sap proteins in Pierce's Disease-tolerant Vitis species. Applied Biochemistry and Biotechnology. 2010;160:932–944. doi: 10.1007/s12010-009-8620-1. [DOI] [PubMed] [Google Scholar]
- Baumgartner K, Warren JG. Persistence of Xylella fastidiosa in riparian hosts near northern California vineyards. Plant Disease. 2005;89:1097–1102. doi: 10.1094/PD-89-1097. [DOI] [PubMed] [Google Scholar]
- Beckman CH, Talboys PW. Anatomy of resistance. In: Mace ME, Bell AA, Beckman CH, editors. Fungal wilt diseases of plants. New York: Academic Press; 1981. pp. 487–521. [Google Scholar]
- Bennett CPA, Hunt P, Asman A. Association of a xylem-limited bacterium with Sumatra disease of cloves in Indonesia. Plant Pathology. 1985;34:487–494. [Google Scholar]
- Bi JL, Dumenyo CK, Hernandez-Martinez R, Cooksey DA, Toscano NC. Effect of host plant xylem fluid on growth, aggregation, and attachment of Xylella fastidiosa. Journal of Chemical Ecology. 2007;33:493–500. doi: 10.1007/s10886-006-9248-z. [DOI] [PubMed] [Google Scholar]
- Biggs AR. Occurrence and location of suberin in wound reaction zones in xylem of 17 tree species. Phytopathology. 1987;77:718–725. [Google Scholar]
- Bonsen KJM, Kucera LJ. Vessel occlusions in plants: morphological, functional and evolutionary aspects. International Association of Wood Anatomists Bulletin. 1990;11:393–399. [Google Scholar]
- Bové JM, Garnier M. Phloem- and xylem-restricted plant pathogenic bacteria. Plant Science. 2002;163:1083–1098. [Google Scholar]
- Chatelet D, Matthews MA, Rost TL. Xylem structure and connectivity in grapevine (Vitis vinifera L.) shoots provides a passive mechanism for the spread of bacteria in grape plants. Annals of Botany. 2006;98:483–494. doi: 10.1093/aob/mcl124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Almeida RPP, Lindow SE. Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa. Annual Review of Phytopathology. 2008a;46:243–271. doi: 10.1146/annurev.phyto.45.062806.094342. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Newman KL, Lindow SE. Cell-to-cell signaling in Xylella fastidiosa suppresses movement and xylem vessel colonization in grape. Molecular Plant-Microbe Interactions. 2008b;21:1309–1315. doi: 10.1094/MPMI-21-10-1309. [DOI] [PubMed] [Google Scholar]
- Choat B, Ball M, Luly J, Holtum J. Pit membrane porosity and water stress-induced cavitation in four co-existing dry rainforest tree species. Plant Physiology. 2003;31:41–48. doi: 10.1104/pp.014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Lahr E, Melcher PJ, Zwieniecki MA, Holbrook NM. The spatial pattern of air seeding thresholds in mature sugar maple trees. Plant, Cell and Environment. 2005;28:1082–1089. [Google Scholar]
- Choat B, Gambetta GA, Wada H, Shackel KA, Matthews MA. The effects of Pierce's disease on leaf, petiole hydraulic conductance in Vitis vinifera cv. ‘Chardonnay’. Physiologia Plantarum. 2009;136:384–394. doi: 10.1111/j.1399-3054.2009.01231.x. [DOI] [PubMed] [Google Scholar]
- Clérivet A, Déon V, Alami I, Lopez F, Geiger J-P, Nicole M. Tyloses and gels associated with cellulose accumulation in vessels are responses of plane tree seedlings (Platanus × acerifolia) to the vascular fungus Ceratocystis fimbriata f.sp platani. Trees. 2000;15:25–31. [Google Scholar]
- Cochard H, Tyree MT. Xylem dysfunction in Quercus: vessel sizes, tyloses, cavitation, and seasonal changes in embolism. Tree Physiology. 1990;6:393–408. doi: 10.1093/treephys/6.4.393. [DOI] [PubMed] [Google Scholar]
- Cochard H, Nardini A, Coll L. Hydraulic architecture of leaf blades: where is the main resistance? Plant, Cell and Environment. 2004;27:1257–1267. [Google Scholar]
- Costa HS, Raetz E, Pinckard TR, et al. Plant hosts of Xylella fastidiosa in and near southern California vineyards. Plant Disease. 2004;88:1255–1261. doi: 10.1094/PDIS.2004.88.11.1255. [DOI] [PubMed] [Google Scholar]
- Croll SG. DLVO theory applied to TiO2 pigments and other materials in latex paints. Progress in Organic Coatings. 2002;44:131–146. [Google Scholar]
- Davis MJ, Augustin BJ. Occurrence in Florida of the bacterium that causes bermudagrass stunting disease. Plant Disease. 1984;68:1095–1097. [Google Scholar]
- Davis MJ, Gillaspie AG, Harris RW, Lawson RH. Ratoon stunting disease of sugarcane – isolation of the causal bacterium. Science. 1980;210:1365–1367. doi: 10.1126/science.210.4476.1365. [DOI] [PubMed] [Google Scholar]
- Davis MJ, Raju BC, Brlansky RH, et al. Periwinkle wilt bacterium: axenic culture, pathogenicity, and relationships to other gram-negative, xylem-inhabiting bacteria. Phytopathology. 1983;73:1510–1515. [Google Scholar]
- De La Fuente L, Burr TJ, Hoch HC. Mutations in type I and type IV pilus biosynthetic genes affect twitching motility rates in Xylella fastidiosa. Journal of Bacteriology. 2007;189:7507–7510. doi: 10.1128/JB.00934-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza AA, Takita MA, Coletta-Filho HD, et al. Analysis of gene expression in two growth states of Xylella fastidiosa and its relationship with pathogenicity. Molecular Plant-Microbe Interactions. 2003;16:867–875. doi: 10.1094/MPMI.2003.16.10.867. [DOI] [PubMed] [Google Scholar]
- De Souza AA, Takita MA, Coletta-Filho HD, et al. Gene expression profile of the plant pathogen Xylella fastidiosa during biofilm formation in vitro. FEMS Microbiology Letters. 2004;237:341–353. doi: 10.1016/j.femsle.2004.06.055. [DOI] [PubMed] [Google Scholar]
- Esau K. Anatomic effects of the viruses of Pierce's disease and phony peach. Hilgardia. 1948;18:423–482. [Google Scholar]
- Esau K. Anatomy of seed plants. New York, NY: John Wiley and Sons; 1977. [Google Scholar]
- Ewers FW, Fisher JB. Techniques for measuring vessel lengths and diameters in stems of woody plants. American Journal of Botany. 1989;76:645–656. [Google Scholar]
- Feil H, Feil WS, Lindow SE. Contribution of fimbrial and afimbrial adhesins of Xylella fastidiosa to attachment to surfaces and virulence to grape. Phytopathology. 2007;97:318–324. doi: 10.1094/PHYTO-97-3-0318. [DOI] [PubMed] [Google Scholar]
- Freitag JH. Host range of Pierce's disease virus of grapes as determined by insect transmission. Phytopathology. 1951;41:920–934. [Google Scholar]
- Fry SM, Milholland RD. Multiplication and translocation of Xylella fastidiosa in petioles and stems of grapevine resistant, tolerant and susceptible to Pierce's disease. Phytopathology. 1990;80:61–65. [Google Scholar]
- Gambetta GA, Fei J, Rost TL, Matthews MA. Leaf scorch symptoms are not correlated with bacterial populations during Pierce's disease. Journal of Experimental Botany. 2007;58:4037–4046. doi: 10.1093/jxb/erm260. [DOI] [PubMed] [Google Scholar]
- Gerrath JM, Posluszny U, Dengler NG. Primary vascular patterns in the Vitaceae. International Journal of Plant Sciences. 2001;162:729–745. [Google Scholar]
- Gilchrist D, Lincoln J. Proceedings of the 2006 Pierce's disease research symposium. San Diego, CA: California Department of Food and Agriculture; 2006. Resistance to Pierce's by transgenic expression of plant-derived anti-apoptotic genes. [Google Scholar]
- Grall S, Manceau C. Colonization of Vitis vinifera by a green fluorescence protein-labeled, gfp-marked strain of Xylophilus ampelinus, the causal agent of bacterial necrosis of grapevine. Applied and Environmental Microbiology. 2003;69:1904–1912. doi: 10.1128/AEM.69.4.1904-1912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilhabert MR, Kirkpatrick BC. Identification of Xylella fastidiosa antivirulence genes: hemagglutinin adhesins contribute to X. fastidiosa biofilm maturation and colonization and attenuate virulence. Molecular Plant-Microbe Interactions. 2005;18:856–868. doi: 10.1094/MPMI-18-0856. [DOI] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS, Pittermann J. Analysis of circular bordered pit function. II. Gymnosperm tracheids with torus-margo pit membranes. American Journal of Botany. 2004;91:386–400. doi: 10.3732/ajb.91.3.386. [DOI] [PubMed] [Google Scholar]
- Hayward AC. Biology and epidemiology of bacterial wilt caused by Pseudomonas-solanacearum. Annual Review of Phytopathology. 1991;29:65–87. doi: 10.1146/annurev.py.29.090191.000433. [DOI] [PubMed] [Google Scholar]
- Hill BL, Purcell AH. Multiplication and movement of Xylella fastidiosa within grape and four other plants. Phytopathology. 1995;85:1368–1372. [Google Scholar]
- Hopkins DL. Pierce's disease of grapevines. American Wine Society Journal. 1976;8:26–27. [Google Scholar]
- Hopkins DL. Physiological and pathological characteristics of virulent and avirulent strains of the bacterium that causes Pierces disease of grapevine. Phytopathology. 1985;75:713–717. [Google Scholar]
- Hopkins DL. Xylella-fastidiosa – xylem-limited bacterial pathogen of plants. Annual Review of Phytopathology. 1989;27:271–290. [Google Scholar]
- Hopkins DL, Mollenhauer HH. Tylose and gum formation in the xylem of Pierce's disease infected grapevines. Proceedings of the American Phytopathological Society. 1975;2:65. (abstract) [Google Scholar]
- Hopkins DL, Purcell AH. Xylella fastidiosa: cause of Pierce's disease of grapevine and other emergent diseases. Plant Disease. 2002;86:1056–1066. doi: 10.1094/PDIS.2002.86.10.1056. [DOI] [PubMed] [Google Scholar]
- Hopkins DL, Thompson CM. Seasonal concentration of the Pierce's disease bacterium in ‘Carlos’ and ‘Welder’ muscadine grapes as compared with ‘Schuyler’ bunch grape. Horticultural Sciences. 1984;19:419–420. [Google Scholar]
- Houston BR, Hewitt WE, Esau K. The mode of vector feeding and the tissues involved in the transmission of Pierce's disease virus in grape and alfalfa. Phytopathology. 1947;37:247–253. [Google Scholar]
- Kamiunten H, Wakimoto S. Coryneform bacteria found in xylem of the ratoon stunting diseased sugarcane. Annals of the Phytopathological Society of Japan. 1976;42:500–503. [Google Scholar]
- Koide T, Zaini PA, Moreira LM, et al. DNA microarray-based genome comparison of a pathogenic and a nonpathogenic strain Xylella fastidiosa delineates genes important for bacterial virulence. Journal of Bacteriology. 2004;186:5442–5449. doi: 10.1128/JB.186.16.5442-5449.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell RK, Perring TM, Farrar CA, Park YL, Gispert C. Intraplant sampling of grapevines for Pierce's disease diagnosis. Plant Disease. 2006;90:351–357. doi: 10.1094/PD-90-0351. [DOI] [PubMed] [Google Scholar]
- Krivanek AF, Walker MA. Vitis resistance to Pierce's disease is characterized by differential Xylella fastidiosa populations in stems and leaves. Phytopathology. 2005;95:44–52. doi: 10.1094/PHYTO-95-0044. [DOI] [PubMed] [Google Scholar]
- Larson PR, Isebrands JG. Functional significance of nodal constricted zone in Populus-deltoides. Canadian Journal of Botany. 1978;56:801–804. [Google Scholar]
- Leopold RA, Freeman TP, Buckner JS, Nelson DR. Mouthpart morphology and stylet penetration of host plants by the glassy-winged sharpshooter, Homalodisca coagulata, (Homoptera: Cicadellidae) Arthropod Structure and Development. 2003;32:189–199. doi: 10.1016/S1467-8039(03)00047-1. [DOI] [PubMed] [Google Scholar]
- Martre P, Durand JL, Cochard H. Changes in axial hydraulic conductivity along elongating leaf blades in relation to xylem maturation in tall fescue. New Phytologist. 2000;146:235–247. doi: 10.1046/j.1469-8137.2000.00641.x. [DOI] [PubMed] [Google Scholar]
- Meng Y, Li Y, Galvani CD, et al. Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. Journal of Bacteriology. 2005;187:5560–5567. doi: 10.1128/JB.187.16.5560-5567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milholland RD, Huang PY, Clayton CN, Jones RK. Pierce's disease on muscadine grapes in North Carolina USA. Plant Disease. 1981;65:73–74. [Google Scholar]
- Mollenhauer HA, Hopkins DL. Xylem morphology of Pierce's disease-infected grapevines with different levels of tolerance. Physiological Plant Pathology. 1976;9:95–100. [Google Scholar]
- Mullins MG, Bouquet A, Williams LE. Biology of the grapevine. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- Newbanks D, Bosch A, Zimmermann MH. Evidence for xylem dysfunction by embolization in Dutch elm disease. Phytopathology. 1983;73:1060–1063. [Google Scholar]
- Newman KL, Almeida RPP, Purcell AH, Lindow SE. Use of a green fluorescent strain for analysis of Xylella fastidiosa colonization of Vitis vinifera. Applied and Environmental Microbiology. 2003;69:7319–7327. doi: 10.1128/AEM.69.12.7319-7327.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman KL, Almeida RPP, Purcell AH, Lindow SE. Cell–cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proceedings of the National Academy of Sciences, USA. 2004;101:1737–1742. doi: 10.1073/pnas.0308399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagopoulos CG. The disease of Tsilik Marasi of grapevine – its description and identification of the causal agent Xanthomonas-ampelina new species. Annals of the Institute of Phytopathology Benaki. 1969;9:59–81. [Google Scholar]
- Pearce RB. Reaction zone relics and the dynamics of fungal spread in the xylem of woody angiosperms. Physiology and Molecular Plant Pathology. 1991;39:41–56. [Google Scholar]
- Pepper EH. Stewart's bacterial wilt of corn. Monographs of the American Phytopathological Society No. 4. Paul, MN: American Phytopathological Society; 1967. St. [Google Scholar]
- Pérez-Donoso AG, Greve LC, Walton JH, Shackel KA, Labavitch JM. Xylella fastidiosa infection and ethylene exposure result in xylem and water movement disruption in grapevine shoots. Plant Physiology. 2007;143:1024–1036. doi: 10.1104/pp.106.087023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Donoso AG, Sun Q, Roper CM, Greve CL, Kirkpatrick B, Labavitch JM. Cell wall-degrading enzymes enlarge the pore size of intervessel pit membranes in healthy and Xylella fastidiosa-infected grapevines. Plant Physiology. 2010;152:1748–1759. doi: 10.1104/pp.109.148791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt C. Vegetative anatomy of cultivated grapes – a review. American Journal of Enology and Viticulture. 1974;25:131–150. [Google Scholar]
- Purcell AH. Cold therapy of Pierce's disease of grapevines. Plant Disease Reporter. 1977;61:514–518. [Google Scholar]
- Purcell AH. Vector preference and inoculation efficiency as components of resistance to Pierce's disease in European grape cultivars. Phytopathology. 1981;71:429–435. [Google Scholar]
- Purcell AH, Hopkins DL. Fastidious xylem-limited bacterial plant pathogens. Annual Review of Phytopathology. 1996;34:131–151. doi: 10.1146/annurev.phyto.34.1.131. [DOI] [PubMed] [Google Scholar]
- Purcell AH, Saunders SR. Glassy-winged sharpshooters expected to increase plant disease. California Agriculture. 1999;53:26–27. [Google Scholar]
- Purcell AH, Saunders SR, Norberg E, McBryde JR. Reductions of Pierce's disease vector activity by management of riparian woodlands. Phytopathology. 1999;89:S62. [Google Scholar]
- Raju BC, Goheen AC. Relative sensitivity of selected grapevine cultivars to Pierce's disease bacterial infections. American Journal of Enology and Viticulture. 1981;32:155–158. [Google Scholar]
- Redak RA, Purcell AH, Lopes JRS, Blua MJ, Mizell RF, III, Andersen PC. The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annual Review of Entomology. 2004;49:243–270. doi: 10.1146/annurev.ento.49.061802.123403. [DOI] [PubMed] [Google Scholar]
- Reddy JD, Reddy SL, Hopkins DL, Gabriel DW. ToIC is required for pathogenicity of Xylella fastidiosa in Vitis vinifera grapevines. Molecular Plant-Microbe Interactions. 2007;20:403–410. doi: 10.1094/MPMI-20-4-0403. [DOI] [PubMed] [Google Scholar]
- Roberts SJ, Edengreen SJ, Jones P, Ambler DJ. Pseudomonas-syzygii, sp-nov, the cause of Sumatra disease of cloves. Systematic and Applied Microbiology. 1990;13:34–43. [Google Scholar]
- Rood SB, Patino S, Coombs K, Tyree MT. Branch sacrifice: cavitation-associated drought adaptation of riparian cottonwoods. Trees. 2000;14:248–257. [Google Scholar]
- Roper MC, Greve LC, Warren JG, Labavitch JM, Kirkpatrick BC. Xylella fastidiosa requires polygalacturonase for colonization and pathogenicity in Vitis vinifera grapevines. Molecular Plant-Microbe Interactions. 2007;20:411–419. doi: 10.1094/MPMI-20-4-0411. [DOI] [PubMed] [Google Scholar]
- Roth-Nebelsick A, Uhl D, Mosbrugger V, Kerp H. Evolution and function of leaf venation architecture: a review. Annals of Botany. 2001;87:553–566. [Google Scholar]
- Ruzin SE. Plant microtechnique and microscopy. New York: Oxford University Press; 1999. [Google Scholar]
- Saitoh T, Ohtani J, Fukazawa K. The occurrence and morphology of tyloses and gums in the vessels of Japanese hardwoods. International Association of Wood Anatomists Journal. 1993;14:359–371. [Google Scholar]
- Salleo S, Lo Gullo MA, Raimondo F, Nardini A. Vulnerability to cavitation of leaf minor veins: any impact on leaf gas exchange? Plant, Cell and Environment. 2001;24:851–859. [Google Scholar]
- Salleo S, Nardini A, Lo Gullo MA, Ghirarrdelli LA. Changes in stem and leaf hydraulics preceding leaf shedding in Castanea sativa L. Biologia Plantarum. 2002;45:227–234. [Google Scholar]
- Scarpari LM, Lambais MR, Silva DS, Carraro DM, Carrer H. Expression of putative pathogenicity-related genes on Xylella fastidiosa grown at low and high cell density conditions in vitro. FEMS Microbiology Letters. 2003;222:83–92. doi: 10.1016/S0378-1097(03)00251-9. [DOI] [PubMed] [Google Scholar]
- Schmitt U, Liese W. Response of xylem parenchyma by suberization in some hardwoods after mechanical injury. Trees. 1993;8:23–30. [Google Scholar]
- Shi X, Bi J, Morse JG, Toscano NC, Cooksey DA. Differential expression of genes of Xylella fastidiosa in xylem fluid of citrus and grapevine. FEMS Microbiology Letters. 2010;304:82–88. doi: 10.1111/j.1574-6968.2009.01885.x. [DOI] [PubMed] [Google Scholar]
- Siau JF. Transport processes in wood. Berlin: Springer-Verlag; 1984. [Google Scholar]
- Sperry JS, Hacke UG. Analysis of circular bordered pit function. I. Angiosperm vessels with homogenous pit membranes. American Journal of Botany. 2004;91:369–385. doi: 10.3732/ajb.91.3.369. [DOI] [PubMed] [Google Scholar]
- Sperry JS, Holbrook NM, Zimmermann MH, Tyree MT. Spring filling of xylem vessels in wild grapevine. Plant Physiology. 1987;83:414–417. doi: 10.1104/pp.83.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JF, Matthews MA, Greve LC, Labavitch JM, Rost TL. Grapevine susceptibility to Pierce's disease II: progression of anatomical symptoms. American Journal of Enology and Viticulture. 2004;55:238–245. [Google Scholar]
- Stevenson JF, Matthews MA, Rost TL. The developmental anatomy of Pierce's disease symptoms in grapevines: green islands and matchsticks. Plant Disease. 2005;89:543–548. doi: 10.1094/PD-89-0543. [DOI] [PubMed] [Google Scholar]
- Sun Q, Rost TL, Matthews MA. Pruning-induced tylose development in stems of current-year shoots of Vitis vinifera (Vitaceae) American Journal of Botany. 2006;93:1567–1576. doi: 10.3732/ajb.93.11.1567. [DOI] [PubMed] [Google Scholar]
- Sun Q, Rost TL, Reid MS, Matthews MA. Ethylene and not embolism is required for wound-induced tylose development in stems of grapevines. Plant Physiology. 2007;145:1629–1636. doi: 10.1104/pp.107.100537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbah F, Goodman RN. Systemic spread of Agrobacterium tumefaciens biovar 3 in the vascular system of grapes. Phytopathology. 1987;77:915–920. [Google Scholar]
- Thorne ET, Stevenson JF, Rost TL, Labavitch JM, Matthews MA. Pierce's disease symptoms: comparison with symptoms of water deficit and the impact of water deficits. American Journal of Enology and Viticulture. 2006a;57:1–11. [Google Scholar]
- Thorne ET, Young BM, Young GM, et al. The structure of xylem vessels in grapevine and a possible passive mechanism for the systemic spread of bacterial disease. American Journal of Botany. 2006b;93:497–504. doi: 10.3732/ajb.93.4.497. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Ewers FW. The hydraulic architecture of trees and other woody plants. New Phytologist. 1991;119:345–360. [Google Scholar]
- Tyree MT, Zimmermann MH. Xylem structure and the ascent of sap. 2nd edn. New York: Springer; 2002. [Google Scholar]
- Tyree MT, Cochard H, Cruiziat P, Sinclair B, Ameglio T. Drought-induced leaf shedding in walnut: evidence for vulnerability segmentation. Plant, Cell and Environment. 1993;16:879–882. [Google Scholar]
- Wada H, Shackel K, Matthews M. Fruit ripening in Vitis vinifera: apoplastic solute accumulation accounts for pre-veraison turgor loss in berries. Planta. 2008;227:1351–1361. doi: 10.1007/s00425-008-0707-3. [DOI] [PubMed] [Google Scholar]
- Wallis FM, Truter SJ. Histopathology of tomato plants infected with Pseudomonas solanacearum with emphasis on ultrastructure. Physiological Plant Pathology. 1978;13:307–318. [Google Scholar]
- Wheeler JK, Sperry JS, Hacke UG, Hoang N. Inter-vessel pitting and cavitation in woody Rosaceae and other vesselled plants: a basis for a safety versus efficiency trade-off in xylem transport. Plant, Cell and Environment. 2005;28:800–812. [Google Scholar]
- Wiebe HH, Greer RL, Vanalfen NK. Frequency and grouping of vessel endings in alfalfa (Medicago sativa) shoots. New Phytologist. 1984;97:583–590. [Google Scholar]
- Wistrom C, Purcell AH. The fate of Xylella fastidiosa in vineyard weeds and other alternate hosts in California. Plant Disease. 2005;89:994–999. doi: 10.1094/PD-89-0994. [DOI] [PubMed] [Google Scholar]
- Wylie RB. Concerning the conductive capacity of the minor veins of foliage leaves. American Journal of Botany. 1938;25:567–572. [Google Scholar]
- Zaini PA, De La Fuente L, Hoch HC, Burr TJ. Grapevine xylem sap enhances biofilm development by Xylella fastidiosa. FEMS Microbiology Letters. 2009;295:129–134. doi: 10.1111/j.1574-6968.2009.01597.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann MH, Brown CL. Trees: structure and function. New York: Springer-Verlag; 1971. [Google Scholar]
- Zimmermann MH. Xylem structure and the ascent of sap. Berlin: Springer-Verlag; 1983. [DOI] [PubMed] [Google Scholar]