Abstract
Background and Aims
The amount of data collected previously for Velloziaceae neither clarified relationships within the family nor helped determine an appropriate classification, which has led to huge discordance among treatment by different authors. To achieve an acceptable phylogenetic result and understand the evolution and roles of characters in supporting groups, a total evidence analysis was developed which included approx. 20 % of the species and all recognized genera and sections of Velloziaceae, plus outgroups representatives of related families within Pandanales.
Methods
Analyses were undertaken with 48 species of Velloziaceae, representing all ten genera, with DNA sequences from the atpB-rbcL spacer, trnL-trnF spacer, trnL intron, trnH-psbA spacer, ITS ribosomal DNA spacers and morphology.
Key Results
Four groups consistently emerge from the analyses. Persistent leaves, two phloem strands, stem cortex divided in three regions and violet tepals support Acanthochlamys as sister to Velloziaceae s.s., which are supported mainly by leaves with marginal bundles, transfusion tracheids and inflorescence without axis. Within Velloziaceae s.s., an African Xerophyta + Talbotia clade is uniquely supported by basal loculicidal capsules; an American clade, Barbacenia s.l. + Barbaceniopsis + Nanuza + Vellozia, is supported by only homoplastic characters. Barbacenia s.l. (= Aylthonia + Barbacenia + Burlemarxia + Pleurostima) is supported by a double sheath in leaf vascular bundles and a corona; Barbaceniopsis + Nanuza + Vellozia is not supported by an unambiguous character, but Barbaceniopsis is supported by five characters, including diclinous flowers, Nanuza + Vellozia is supported mainly by horizontal stigma lobes and stem inner cortex cells with secondary walls, and Vellozia alone is supported mainly by pollen in tetrads.
Conclusions
The results imply recognition of five genera (Acanthochlamys (Xerophyta (Barbacenia (Barbaceniopsis, Vellozia)))), solving the long-standing controversies among recent classifications of the family. They also suggest a Gondwanan origin for Velloziaceae, with a vicariant pattern of distribution.
Keywords: Acanthochlamys, Barbacenia, Barbaceniopsis, Gondwanian origin, morphological and molecular characters, phylogenetic analysis, Vellozia, Velloziaceae, vicarious distribution, Xerophyta
INTRODUCTION
Velloziaceae have approx. 250 species (Cronquist, 1981; Dahlgren et al., 1985; Kubitzki, 1998; Mello-Silva, 1991a, 2004; Smith and Ayensu, 1974, 1976) of which one occurs in China, another in Yemen and Saudi Arabia, approx. 30 in Africa and Madagascar, and the rest in South America with the exception of one species that reaches Panama in Central America (Mello-Silva, 1995, 2004). Velloziaceae are one of the best examples of a family of consistently heliophile species (Smith, 1962), and the great majority of species are concentrated in the phytochoria campo rupestre archipelago (Prance, 1994) of central Brazil.
Since the first Velloziaceae monograph in the 20th century (Smith, 1962), new data for systematics of the family have been produced, including anatomy (Ayensu, 1968, 1969, 1974; Menezes, 1970, 1971a, 1973, 1975, 1980, 1984, 1988; Coetzee, 1974; Menezes and Semir, 1990; Mello-Silva, 1990; Sajo et al., 2010), chromosomes (Goldblatt and Poston, 1988; Melo et al., 1997), pollen (Ayensu, 1972; Ayensu and Skvarla, 1974) and phytochemistry (Salatino et al., 1989, 1991; Williams et al., 1991, 1992, 1993, 1994). Nevertheless, species and, more often, generic delimitation within Velloziaceae have often been a source of discordance among authors. One of the reasons for this situation, as in many others in a gradistic context, was the differential emphasis on the various characters used for delimiting groups (Smith and Ayensu, 1974; Menezes, 1980; Mello-Silva, 1991a). Morphological (Menezes et al., 1994; Mello-Silva, 2000, 2005) and molecular (Salatino, 1999; Behnke et al., 2000; Salatino et al., 2001) cladistic analyses have cast light on intra-familial relationships, although the results have been conflicting to some degree, mostly because different sets of taxa and types of characters have been included (Mello-Silva, 2005).
This work is a combined analysis of 67 morphological and five datasets of molecular characters from almost the same species used by Mello-Silva (2005) in his solely morphological analysis. This represents all genera and sections within the genera of Velloziaceae so far established, together with a number of morphological characters long utilized in the systematics of the family. Groups and characters are discussed, as well as implications for the evolution, classification and biogeography of Velloziaceae.
MATERIALS AND METHODS
Total DNA from samples of Velloziaceae was extracted according to the CTAB method of Doyle and Doyle (1987) from fragments of leaves previously dried on silica gel (Chase and Hills, 1991). Primers used for amplification and thermocycler conditions are presented in Table 1.
Table 1.
Primers used for amplification and thermocycler conditions
| DNA markers | Primers | Reference | Thermocycler conditions |
|---|---|---|---|
| trnL intron, trnL-F spacer | tab c (CGA AAT CGG TAG ACG CTA CG) | Taberlet et al. (1991) | Denaturation at 94 °C 2 min, 33 cycles with denaturation at 94 °C 1 min, annealing 54–62 °C 45 s, extension 72 °C 1 min 20 s and last extension 5 min |
| tab f (ATT TGA ACT GGT GAC ACG AG) | |||
| atpB-rbcL spacer | atpB-1 (ACA TCK ART ACK GGA CCA ATA A) | Chiang et al. (1998) | Denaturation at 94 °C 2 min, 28 cycles with denaturation at 94 °C 1 min, annealing at 52 °C 1 min, extension at 72 °C 1 min and last extension 7 min |
| rbcL-1 (AAC ACC AGC TTT RAA TCC AA) | |||
| trnH-psbA spacer | trnHGUG (CGC GCA TGG TGG ATT CAC AAT CC) | Shaw et al. (2005) | Denaturation at 80 °C, 33 cycles with denaturation at 94 °C 30 s, annealing at 50–56 °C 30 s, extension at 72 °C 1 min and last extension 10 min |
| psbA (GTT ATG CAT GAA CGT AAT GCT C) | |||
| ITS rnDNA | 26SE (ACG AAT TCA TGG TCC GGT GAA GTG TTC G) | Sun et al. (1994) | Denaturation at 94 °C 2 min, 28 cycles with denaturation at 94 °C 1 min, annealing at 52 °C 1 min, extension at 72 °C 1 min and last extension 7 min |
| 17SE (TAG AAT TCC CCG GTT CGC TCG CCG TTA C) | |||
| (internal primers) ITS 2F (GCT GCG TTC TTC ATC GAT GC) | Kumar and Shukla (2005) | ||
| ITS 3R (GCA TCG ATG AAG AAC GCA GC) |
The products were purified with GFX PCR purification kit (Amersham Biosciences). The same primers were used for sequencing reactions of the corresponding regions. Sequence analyses were run in automatic sequencers models 3100 and 3700 (Applied Biosystems), using Big Dye 3·0–3·1 and the manufacturer's protocol. Sequences were aligned manually following the guidelines of Kelchner (2000) and the criterion of similarity (Simmons, 2004). Gaps were not treated as characters. Insertions, mainly in ITS, are generally direct repeats of a neighbouring sequence (‘type 1a’ gap; Golenberg et al., 1993; Kelchner, 2000). Other types of insertions are found, mostly as a result of differences from the outgroup but they are too random for appropriate treatment. A total analysis with the majority of indels removed was performed for comparisons.
Morphological characters are mainly from Mello-Silva (2005), but ptyxis, vessels in leaves, septal nectaries, stigmatic surfaces, nucellus and cyanogenic compounds are not utilized here because they were generalizations and not actual observations in the terminals. Accordingly, chromosome number, epicuticular waxes and flavonoid compounds also could not be analysed for a satisfactory number of terminals. Stomata and outer integument of empty cells in seeds are also not considered here due to new evidence for homology (respectively, Amaral and Mello-Silva, 2009; Sousa 2005). On the other hand, there are nine new characters added from root and stem anatomy, taken from Cattai (2007).
Morphological characters are listed in Table 2 and the matrix in Table 3. Molecular voucher materials are listed in Table 4. Additional morphological vouchers can be found in Mello-Silva (2000, 2005) and in Cattai (2007). Herbaria acronyms follow Thiers (2010). Taxonomic decisions about lumping versus splitting were based on the priorities as discussed in Backlund and Bremer (1998).
Table 2.
Character analysis and coding
|
M & al. = Menezes et al. (1994); S & L = Stevenson and Loconte (1995).
Table 3.
Morphological matrix
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Acanthochlamys bracteata | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | - | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | - | ? | ? | ? | - | 0 | 0 | 0 | 1 | A | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 2 | ? | ? | 0 | - | 0 | 2 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 1 | 0 |
| Aylthonia blackii | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 0 | 1 | D | 0 | 1 | 0 | 3 | 1 | 5 | 1 | 0 | 0 | 0 | - | 1 | 1 | 0 | 1 | 3 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 1 | 0 |
| Aylthonia umbrosa | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | D | 0 | 1 | 0 | 3 | 1 | 3 | 1 | 0 | 0 | 0 | - | 1 | 1 | 0 | 1 | 3 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 |
| Barbacenia flava | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | - | 1 | 1 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 |
| Barbacenia ignea | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 1 | 1 | F | 1 | 0 | 0 | 0 | - | 1 | 1 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 |
| Barbacenia markgrafii | 1 | 1 | 0 | 0 | 1 | 1 | A | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 3 | 1 | E | 1 | 0 | 0 | 0 | - | 1 | 1 | 0 | 1 | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 |
| Barbacenia reflexa | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 3 | 1 | 4 | 1 | 0 | 0 | 0 | - | 1 | 1 | 0 | 1 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 |
| Barbaceniopsis boliviensis | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | ? | 0 | ? | 0 | ? | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 3 | 0 | 0 | 1 | 2 | 1 | 0 |
| Barbaceniopsis castillonii | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 3 | 0 | 0 | 1 | 2 | 1 | 0 |
| Barbaceniopsis humahuaquensis | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 1 | 0 | 3 | 0 | 0 | 1 | 2 | 1 | 0 |
| Burlemarxia pungens | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | ? | 1 | 1 | 1 | 0 | 1 | 0 | 2 | 1 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 |
| Burlemarxia spiralis | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | - | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 1 | 0 |
| Cyclanthus bipartitus | ? | 0 | ? | 1 | ? | ? | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | - | 2 | 1 | 3 | 2 | 0 | 0 | 0 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| Encholirium scrutor | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 2 | - | 2 | - | 0 | 2 | 0 | 0 | 0 | 0 | 0 | - | 1 | 1 | 1 | - | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | ? | ? | ? | 4 | 0 | 0 | 1 | 1 | 2 | 4 | - | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nanuza plicata | 0 | 1 | 1 | A | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | N | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 | 1 | A | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 1 |
| Pandanus pygmaeus | 1 | 0 | 0 | 0 | 0 | B | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 2 | - | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | - | ? | ? | ? | - | - | - | 1 | 1 | 1 | 0 | 2 | - | 0 | 1 | 8 | 0 | ? | 0 | ? | 1 | 0 | ? | ? | ? | 3 | ? | 0 | - | ? | 2 | 5 | - | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| Pleurostima longiscapa | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 2 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 | 1 | 0 | 0 | 0 | - | 1 | 0 | 0 | 1 | 3 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 2 | 1 | 0 |
| Pleurostima plantaginea | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | - | 0 | 0 | 0 | 1 | 0 | 3 | 1 | 3 | 1 | 0 | 0 | 0 | - | 1 | 0 | 0 | 1 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 2 | 1 | 0 |
| Pleurostima purpurea | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | - | 2 | 0 | 1 | 1 | 3 | 3 | 0 | 1 | 1 | 0 | 1 | 0 | 2 | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 1 | 0 |
| Pleurostima riparia | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | - | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | - | 2 | 0 | 1 | 1 | 3 | 3 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 1 | 0 |
| Talbotia elegans | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | B | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | - | 0 | 0 | 0 | 0 | 0 | 0 | 1 | A | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 |
| Thoracocarpus bissectus | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | - | 2 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | - | ? | ? | ? | - | - | - | 1 | ? | 1 | 0 | 1 | 4 | 0 | 1 | K | 0 | ? | 0 | 0 | 0 | ? | ? | ? | ? | ? | ? | 0 | - | - | 0 | 5 | - | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| Vellozia abietina | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia alata | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 5 | 1 | - | 0 | 0 | 0 | 0 | 0 | 1 | 1 | A | 0 | 0 | L | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia aloifolia | 0 | 1 | 1 | A | 1 | 2 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia burlemarxii | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | D | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia campanuloides | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia candida | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 3 | 0 | 0 | B | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia canelinha | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | D | 1 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 8 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia caput-ardeae | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | B | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 3 | 1 | A | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia caudata | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 0 | D | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia aff. caudata | 0 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 0 | D | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | D | 1 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia compacta | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | C | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | A | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia dasypus | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 4 | 0 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia epidendroides | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | A | 0 | 0 | J | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia glauca | 0 | 1 | 1 | 0 | 0 | B | 1 | 0 | 2 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 5 | 0 | 1 | B | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | G | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia hatschbachii | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | ? | ? | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia hemisphaerica 1 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 0 | D | 0 | 0 | 0 | 0 | 1 | 0 | A | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | M | 1 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 1 | A | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia hemisphaerica 2 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 1 | 2 | 0 | 0 | D | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | M | 1 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 1 | A | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia hirsuta | 0 | 1 | A | A | A | B | 1 | 0 | 1 | H | 1 | A | A | A | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | A | 0 | O | 0 | 0 | B | 2 | 0 | 0 | 0 | 3 | 1 | A | 0 | 0 | P | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia jolyi | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | B | 0 | 0 | D | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | J | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia minima | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | A | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia prolifera | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia punctulata | 1 | 1 | 0 | A | 1 | 2 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | I | 1 | 0 | 0 | A | 0 | 0 | 0 | 1 | 1 | A | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia religiosa | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | B | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Vellozia sessilis | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | B | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 2 | 1 | 1 |
| Vellozia tubiflora | 0 | 1 | 1 | 0 | A | B | 1 | 0 | 1 | H | 0 | A | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 3 | 1 | A | 0 | 0 | J | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 1 |
| Xerophyta dasylirioides | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | B | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 1 | 0 |
| Xerophyta eglandulosa | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 0 | B | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 1 | 0 |
| Xerophyta equisetoides | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 2 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | ? | ? | ? | ? | ? | ? | ? | ? | ? |
| Xerophyta pinifolia | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ? | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | - | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 2 | 1 | 0 |
| Xerophyta retinervis | ? | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 3 | ? | ? | ? | ? | ? | ? | ? | ? | ? | ? |
‘?’, missing data; ‘–’, inapplicable; A, 0 and 1; B, 0 and 2; C, 0 and 4; D, 1 and 2; E, 1 and 4; F, 2 and 5; G, 6 and 7; H, 0, 1 and 2; I, 1, 2 and 3; J, 2, 3 and 4; K, 5, 6 and 7; L, 6, 7 and 8; M, 0, 1, 2 and 3; N, 0, 2, 3 and 4; O, 2, 3, 4 and 5; P, 1, 2, 3, 4 and 5.
Table 4.
List of plant samples with voucher information and GenBank accession numbers
| Species | atpB-rbcL | trnL-trnF | trnH-psbA | ITS | Voucher and herbaria |
|---|---|---|---|---|---|
| Acanthochlamys bracteata P.C.Kao | JN016989 | JN016885 | JN017041 | JN016937 | Chase 842 (K) |
| Aylthonia blackii (L.B.Sm.) N.L.Menezes | JN016990 | JN016886 | JN017042 | JN016938 | Mello-Silva 1103 (SPF) |
| Aylthonia umbrosa (L.B.Sm. & Ayensu) | JN016991 | JN016887 | JN017043 | JN016939 | Mello-Silva CFCR9658 (F, K, MBM, RB, SPF) |
| Barbacenia flava Mart. ex Schult. & Schult.f. | JN016992 | JN016888 | JN017044 | JN016940 | Mello-Silva 2662 (SPF) |
| Barbacenia ignea Mart. ex Schult. & Schult.f. | JN016993 | JN016889 | JN017045 | JN016941 | Mello-Silva 2554 (B, K, RB, SPF, US) |
| Barbacenia markgrafii Schulze-Menz | JN016994 | JN016890 | JN017046 | JN016942 | Mello-Silva 1504 (BHCB, K, NY, SPF, W) |
| Barbacenia reflexa L.B.Sm. & Ayensu | JN016995 | JN016891 | JN017047 | JN016943 | Mello-Silva CFCR10793 (F, SPF) |
| Barbaceniopsis boliviensis (Baker) L.B.Sm. | JN016996 | JN016892 | JN017048 | JN016944 | Mello-Silva 2107 (B, BHCB, CDBI, CTES, L, LPB, K, MBM, NY, SI, SPF) |
| Barbaceniopsis castillonii (Hauman) Ibisch | JN016997 | JN016893 | JN017049 | JN016945 | Mello-Silva 1857 (B, CESJ, CTES, L, K, MBM, MCNS, NY, SI, SPF, US) |
| Barbaceniopsis humahuaquensis Noher | JN016998 | JN016894 | JN017050 | JN016946 | Mello-Silva 1872 (B, CESJ, CTES, K, MCNS, SI, SPF, US) |
| Burlemarxia pungens N.L.Menezes & Semir | JN016999 | JN016895 | JN017051 | JN016947 | Mello-Silva 319 (SPF) |
| Burlemarxia spiralis (L.B.Sm. & Ayensu) N.L.Menezes & Semir | JN017000 | JN016896 | JN017052 | JN016948 | Mello-Silva 2548 (SPF) |
| Cyclanthus bipartitus Poit. ex A.Rich. | JN017001 | JN016897 | JN017053 | JN016949 | Mello-Silva 3180 (SPF) |
| Encholirium scrutor (L.B.Sm.) Rauh | JN017002 | JN016898 | JN017054 | JN016950 | Forzza 1488 (BHCB, K, SP, SPF, US) |
| Nanuza plicata (Mart.) L.B.Sm. & Ayensu | JN017003 | JN016899 | JN017055 | JN016951 | Mello-Silva 2133 (SPF) |
| Pandanus pygmaeus Thouars | JN017004 | JN016900 | JN017056 | JN016952 | Pirani 4755 (SPF) |
| Pleurostima longiscapa (Goethart & Henrard) N.L.Menezes | JN017005 | JN016901 | JN017057 | JN016953 | Mello-Silva 2553 (K, SPF) |
| Pleurostima plantaginea (L.B.Sm.) | JN017006 | JN016902 | JN017058 | JN016954 | Salatino CFCR11901 (K, SPF) |
| Pleurostima purpurea (Hook.) Raf. | JN017007 | JN016903 | JN017059 | JN016955 | Menezes 511 (SPF) |
| Pleurostima riparia N.L.Menezes & Mello-Silva | JN017008 | JN016904 | JN017060 | JN016956 | Menezes 1167 (SPF) |
| Talbotia elegans Balf. | JN017009 | JN016905 | JN017061 | JN016957 | Chase 253 (K) |
| Thoracocarpus bissectus (Vell.) Harling | JN017010 | JN016906 | JN017062 | JN016958 | Fiaschi 603 (SPF) |
| Vellozia abietina Mart. | JN017011 | JN016907 | JN017063 | JN016959 | Mello-Silva 1733 (B, K, NY, RB, SPF, US) |
| Vellozia alata L.B.Sm. | JN017013 | JN016909 | JN017065 | JN016961 | Mello-Silva 2368 (K, SPF) |
| Vellozia aloifolia Mart. | JN017014 | JN016910 | JN017066 | JN016962 | Salatino 67 (SPF) |
| Vellozia burlemarxii L.B.Sm. & Ayensu | JN017015 | JN016911 | JN017067 | JN016963 | Mello-Silva 2148 (B, CDBI, CTES, HUEFS, K, M, NY, RB, SPF) |
| Vellozia campanuloides Mello-Silva | JN017016 | JN016912 | JN017068 | JN016964 | Mello-Silva 2770 (K, SPF) |
| Vellozia candida J.C.Mikan | JN017017 | JN016913 | JN017069 | JN016965 | Mello-Silva 2877 (SPF) |
| Vellozia canelinha Mello-Silva | JN017018 | JN016914 | JN017070 | JN016966 | Mello-Silva 2131 (K, SPF) |
| Vellozia caput-ardeae L.B.Sm. & Ayensu | JN017019 | JN016915 | JN017071 | JN016967 | Mello-Silva 1520 (G, NY, SPF, UB) |
| Vellozia caudata Mello-Silva | JN017020 | JN016916 | JN017072 | JN016968 | Mello-Silva 2132 (HUEFS, K, SPF) |
| Vellozia aff. caudata Mello-Silva | JN017012 | JN016908 | JN017064 | JN016960 | Mello-Silva 2135 (SPF) |
| Vellozia compacta Mart. ex Schult. & Schult.f. | JN017021 | JN016917 | JN017073 | JN016969 | Mello-Silva 1386 (MBM, MO, SP, SPF) |
| Vellozia dasypus Seub. | JN017022 | JN016918 | JN017074 | JN016970 | Mello-Silva 2578 (SPF) |
| Vellozia epidendroides Mart. ex Schult. & Schult.f. | JN017023 | JN016919 | JN017075 | JN016971 | Mello-Silva 1772 (G, SPF) |
| Vellozia glauca Pohl | JN017024 | JN016920 | JN017076 | JN016972 | Mello-Silva CFCR11585 (BHCB, F, K, MBM, RB, SPF, UEC, US) |
| Vellozia hatschbachii L.B.Sm. & Ayensu | JN017025 | JN016921 | JN017077 | JN016973 | Mello-Silva 2474 (G, SPF) |
| Vellozia hemisphaerica Seub. 1 | JN017026 | JN016922 | JN017078 | JN016974 | Mello-Silva 2576 (SPF) |
| Vellozia hemisphaerica Seub. 2 | JN017027 | JN016923 | JN017079 | JN016975 | Mello-Silva 2800 (B, HUEFS, K, M, NY, RB, SPF, US) |
| Vellozia hirsuta Goethart & Henrard | JN017028 | JN016924 | JN017080 | JN016976 | Mello-Silva 1503 (SPF) |
| Vellozia jolyi L.B.Sm. | JN017029 | JN016925 | JN017081 | JN016977 | Mello-Silva 2146 (B, K, NY, RB, SPF, US) |
| Vellozia minima Pohl | JN017030 | JN016926 | JN017082 | JN016978 | Mello-Silva 1735 (CTES, K, M, NY, RB, SPF) |
| Vellozia prolifera Mello-Silva | JN017031 | JN016927 | JN017083 | JN016979 | Mello-Silva CFCR10000 (BHCB, CEPEC, NY, SPF) |
| Vellozia punctulata Seub. | JN017032 | JN016928 | JN017084 | JN016980 | Mello-Silva 2587 (HUEFS, K, SPF) |
| Vellozia religiosa Mello-Silva & D.Sasaki | JN017033 | JN016929 | JN017085 | JN016981 | Mello-Silva 2577 (SPF) |
| Vellozia sessilis L.B.Sm. ex Mello-Silva | JN017034 | JN016930 | JN017086 | JN016982 | Mello-Silva 2263 (CTES, K, SPF) |
| Vellozia tubiflora (A.Rich.) Kunth | JN017035 | JN016931 | JN017087 | JN016983 | Mello-Silva 2158 (BHCB, HRCB, HUFU, K, MBM, NY, SP, SPF, SPFR, UEC) |
| Xerophyta dasylirioides Baker | JN017036 | JN016932 | JN017088 | JN016984 | Treutlein 412 (TEX) |
| Xerophyta eglandulosa H.Perr. | JN017037 | JN016933 | JN017089 | JN016985 | Treutlein 410 (TEX) |
| Xerophyta equisetoides Baker | JN017038 | JN016934 | JN017090 | JN016986 | Rodrigues s.n. (SPF 181828) |
| Xerophyta pinifolia Lam. | JN017039 | JN016935 | JN017091 | JN016987 | Treutlein 406 (TEX) |
| Xerophyta retinervis Baker | JN017040 | JN016936 | JN017092 | JN016988 | Cultivated (UConn 199700041) |
Six datasets, plastid atpB-rbcL spacer, trnH-psbA spacer, trnL-trnF spacer, trnL intron, ITS nrDNA and morphology, from 48 terminals of Velloziaceae, including Acanthochlamys bracteata (APG, 2003, 2009) were analysed. Six sets of analyses were performed. Data from plastid DNA, nuclear ribosomal spacers (ITS nrDNA) and morphology were analysed separately and, then, together in a combined molecular and total evidence analysis. External outgroups are Encholirium scrutor, Bromeliaceae (Dahlgren and Rasmussen, 1983; Dahlgren et al., 1985; Gilmartin and Brown, 1987), Cyclanthus bipartitus and Thoracocarpus bissectus, Cyclanthaceae, and Pandanus pygmaeus, Pandanaceae (Chase et al., 1993, 1995, 2006; Clark et al., 1993; Duvall et al., l993; APG, 2003, 2009). There are in total 52 terminals. Criteria for choosing the Velloziaceae terminals are provided in Mello-Silva (2000, 2005). Trees were arranged with Encholirium scrutor as the ultimate outgroup (Farris, 1972, 1982; Nixon and Carpenter, 1993; Ferrarezzi and Marques, 1997) based on results of broader analyses conducted for the monocots (e.g. Chase et al., 2006). The combined dataset was analysed using heuristic searches in PAUP 4·0b10 for Macintosh (Swofford, 2002) with 10 000 replicates of randomized taxa entries with tree–bissection–reconnection (TBR) swapping and a tree limit of 100 trees per replicate. Other datasets were analysed using 1000 replicates with ten trees per replicate because of limits on computational memory for performing them. The bootstrap was used to estimate internal support; 1000 replicates of simple-taxon addition, with a limit of ten trees per replicate and TBR swapping were used for the combined data. ‘Fast’ stepwise addition with 100 000 replicates was used for the other datasets, for the same reason as above. One single tree, the first found, is shown for all analyses; groups not present in the strict consensus tree are marked with an asterisk. Bayesian inference of the combined datasets was conducted in Mr Bayes v.3·1·2 (Huelsenbeck and Ronquist, 2001). The best model of nucleotide substitution for each dataset was determined under AIC criteria using MrModeltest 2·2 (Nylander, 2004). A general time reversible (GTR) model with gamma distribution was selected for atpB-rbcL and trnL-trnF for total analysis and the Hasegawa, Kishino and Yano (HKY) model of nucleotide distribution with gamma distribution for atpB-rbcL in total analysis with indels removed. For ITS nrDNA and trnH-psbA, the GTR model followed a gamma distribution combined with a proportion of invariable sites. For morphological data, the standard discrete model was used. Four simultaneous chains were run starting from random trees for 5 million generations, sampling every 100 generations. Examination of the average standard deviation of split frequencies suggested that stationarity was reached. To ensure sampling of topologies after chain convergence, 10 % of generations were discarded as burn-in; posterior probabilities were calculated from the remaining trees and shown as a majority-rule consensus tree. Numbers above branches illustrate branch lengths (DELTRAN optimization), and those below branches are bootstrap percentages (bp) and posterior probability (pp) either equal or greater than 50. MacClade version 4·07 for OS X (Maddison and Maddison, 2005) was used for optimizing morphological characters on trees and presenting results.
RESULTS
The combined matrix has 3614 aligned characters, of which 67 are non-molecular, 2607 are from the plastid genome and 952 from ITS nrDNA. Of these, 542 are variable but uninformative, and 733 are potentially parsimony-informative. As regards the clades, results from different analyses, i.e. morphological, plastid, ITS, combined DNA, total evidence with indels removed and total evidence analyses, do not show strong conflicts. Therefore only detailed information about (a) a morphology-based tree, (b) a total DNA-based tree (including indels) and (c) a total evidence tree (including indels) is presented here. Removing the indels served to diminish resolution and to depress bootstrap values across a subset of the branches.
Morphological analysis
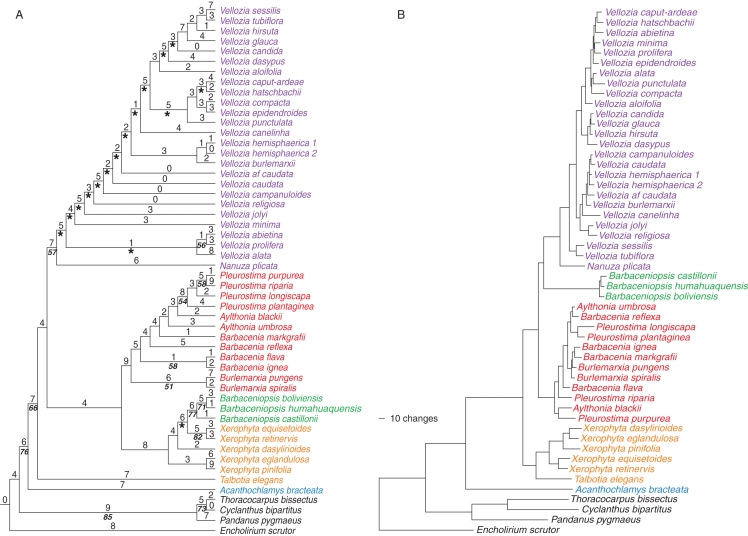
Morphological analysis found 697 most-parsimonious trees with 464 steps, a consistency index (CI) of 0·44 and a retention index (RI) of 0·70 (Fig. 1A). Velloziaceae emerge as monophyletic (76 bp), with Acanthochlamys bracteata as sister of all remaining species. In the ‘core’ Velloziaceae (66 bp), i.e. Velloziaceae s.s. (not including Acanthochlamys), Talbotia is sister to all other species (bp < 50), which are divided in two major clades. One includes Barbacenia s.l. (Aylthonia + Barbacenia s.s. + Burlemarxia + Pleurostima; bp < 50) and Xerophyta + Barbaceniopsis (77 bp). The other (57 bp) includes Nanuza sister to Vellozia (bp < 50). The morphology consensus tree resulting from the improved matrix with stem characters is almost identical to the one from Mello-Silva (2005). The main differences are, apart from some different terminals, the status of Aylthonia and Barbacenia, now paraphyletic, and of Pleurostima, now monophyletic (54 bp). Nevertheless, conclusions regarding genera and other groups are the same.
Fig. 1.
(A) Single tree selected from the 697 equally most-parsimonious trees produced from analysis of the morphological data matrix. Numbers above branches are estimated substitutions and numbers below branches are bootstrap percentages. Clades not present in all trees are marked with an asterisk. (B) Single tree selected from the 72 equally most-parsimonious trees produced from a combined matrix of all DNA regions. The length of branches is proportional to substitutions.
Combined DNA analysis
Analysis of the combined DNA matrix found 72 most-parsimonious trees of 2376 steps, CI of 0·70 and RI of 0·79 (Fig. 1B). Velloziaceae are monophyletic (100 bp) with Acanthochlamys bracteata as sister of the rest. In Velloziaceae s.s. (100 bp), Talbotia + Xerophyta (91 bp) is sister to American clade (bp < 50). In the latter, Barbacenia s.l. (98 bp) is sister to Barbaceniopsis + Nanuza + Vellozia (bp < 50). Barbaceniopsis (100 bp) is sister to Nanuza + Vellozia (99 bp). Nanuza is sister to Vellozia (96 bp). Xerophyta is monophyletic (68 bp).
Total evidence analysis
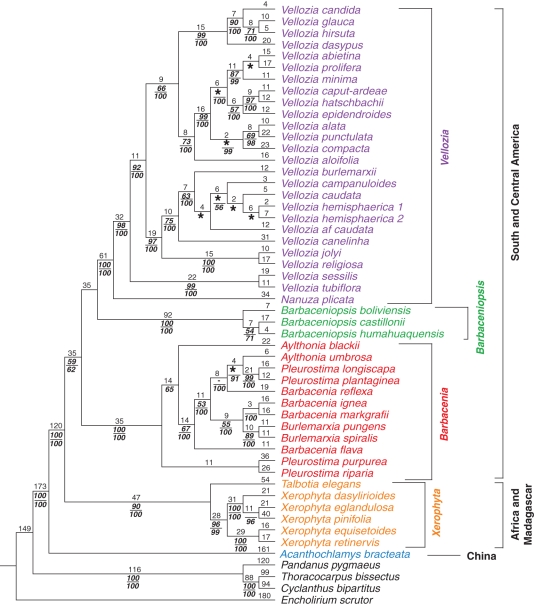
The total evidence analysis found 48 most-parsimonious trees of 2807 steps, CI of 0·63 and RI of 0·76 (Figs 2 and 3). Velloziaceae are again monophyletic (100 bp/100 pp), with Acanthochlamys bracteata as sister of all remaining species. Three non-homoplastic characters, persistent leaves (or at least the sheath; character 2:state 1), two phloem strands (20:0 and 1), and stem cortex divided in three regions (66:1), and one homoplastic character, violet tepals (38:0), support Velloziaceae including Acanthochlamys. There are, possibly, four more characters supporting this clade, stem vascular bundles with fibres next to xylem (65:2), Barbacenia type of leaf sclerenchyma (19:2), a loculicidal capsule (57:0) and a continuous belt of fibres in stem cortex (61:2). The first and the second are invariable, and the last three present transformations within Velloziaceae. Nevertheless, their optimization is uncertain. Acanthochlamys is supported by five homoplastic characters: abaxial strands on leaf blades (14:1), aquiferous hypodermis extending to bundle sheaths only (17:0), hypanthial tube longer than ovary (36:3), cylindrical filaments (43:0) and fibres uniting the stem vascular bundles in maturity (63:1). Perhaps three other homoplastic characters also support it, spiral phyllotaxis (1:2) and dorsifixed, bisporangiate anthers (45:1 and 50:2), all with equivocal optimization.
Fig. 2.
Single tree selected from the 48 equally most-parsimonious trees produced from the combined matrix of all data. Numbers above branches are estimated substitutions and numbers below branches are bootstrap percentages/posterior probability. Clades not present in all trees are marked with an asterisk.
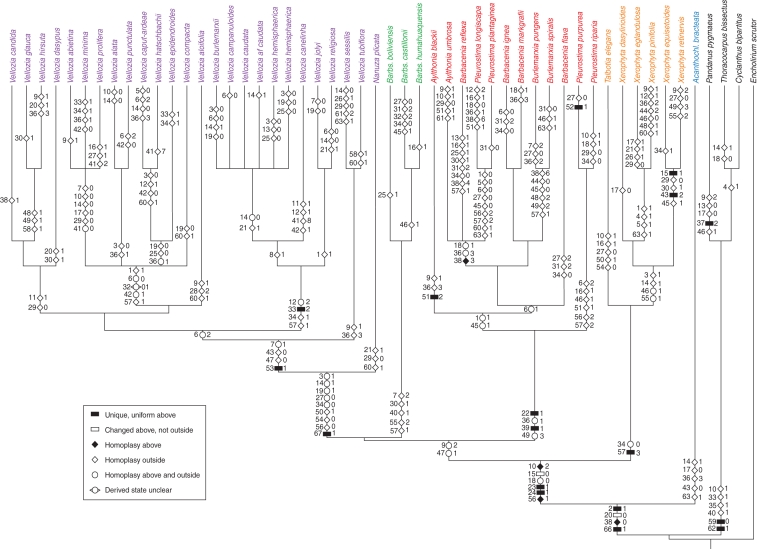
Fig. 3.
Strict consensus tree generated from the 48 equally most-parsimonious trees produced from the combined matrix of all data. Character bars are shown in the key (unambiguous changes only). Numbers at the left side of character bars refer to character numbers, and those at the right indicate the character state.
Velloziaceae s.s. (100 bp/100 pp) are supported by non-homoplastic synapomorphies once attributed to the family: leaves with marginal bundles (15:0 and 1; Mello-Silva, 2005), transfusion tracheids (23:1) and inflorescence without major axis (24:1; Menezes et al., 1994). Three more characters, amphistomatic leaves (10:2), aquiferous parenchyma absent between bundles (18:0) and stigma lobes vertical and fused at apex (56:1), are homoplastic. This clade is perhaps also supported by a non-homoplastic, Barbacenia-type of sclerenchyma pattern (19:2) and a homoplastic character, tristichous phyllotaxis (character 1:0), both with equivocal optimization.
Within Velloziaceae s.s., there are two major clades. One, Talbotia + Xerophyta (90 bp/100 pp), is African and supported by trigonous ovary in transverse section (34:0, homoplastic) and basal loculicidal capsules (57:3, non-homoplastic). Xerophyta (96 bp/99 pp) is monophyletic and supported by four homoplastic characters, among them style and stigma of the same length (55:1). The African clade is sister to the American clade (59 bp/62pp), which is supported by two homoplastic characters, leaf trichomes or emergences with multicellular base on leaf margins and midrib (9:2) and auriculate anthers (47:1), and includes Barbacenia s.l. + Barbaceniopsis + Nanuza + Vellozia. Barbacenia s.l. (100 bp/100 pp) is supported by non-homoplastic double sheath in leaf vascular bundles (22:1), and corona (39:1), and by hypanthial tube shorter than ovary (36:1) and introrse antipetalous anthers (49:3), which are homoplastic. Absence of filaments (44:1 and 2) is also characteristic of this clade, although its transformation series could not be determined. There are also two more homoplastic characters, 12 vascular bundles in pedicel (28:2) and introrse antisepalous anthers (48:3), of uncertain status due to equivocal optimization. Barbacenia s.l. is sister to Barbaceniopsis + Nanuza + Vellozia (bp/pp < 50), which is supported only by bundles of fibres in stem cortex not forming a continuous belt (61:1 and 3, state 1 homoplastic, transformation series not determined) and, perhaps, by a central fibrous bundle in stem (64:1, ambiguous). Barbaceniopsis (100 bp/100 pp) is supported by a non-homoplastic character within Velloziaceae, diclinous flowers (40:1) and, potentially, by U-shaped bundles of fibres in stem cortex (61:3, ambiguous). There are also four homoplastic ones, leaf blade with furrows in both surfaces (7:2), flowers with subulate emergences (30:1), style much shorter than stigma (55:2) and poricidal capsules (57:1). It is sister to Nanuza + Vellozia (100 bp/100 pp), which, in its turn, is supported by two non-homoplastic characters within Velloziaceae, stigma lobes horizontal and fused at the centre (56:0) and stem inner cortex cells with secondary walls (67:1), and nine homoplastic characters, leaves with abscission line between sheath and lamina (3:1), with abaxial strands (14:1), with aquiferous hypodermis (17:0 and 1, transformation series not determined), and with Vellozia type of sclerenchyma pattern (19:1), plus trigonous pedicel (27:0) and ovary (34:0), each pair of microsporangia in anther dehiscing by a separated slit (50:1), and stigma positioned above stamens (54:0). This clade could also be supported by two ambiguous and homoplastic characters, adaxial strands in leaves (13:1) and bundles of round fibres in stem cortex (61:1). Nanuza, supported by three homoplastic autapomorphies, minor fibro-vascular bundles in leaves (21:1), absence of a belt of sclerified cells in pedicel (29:0) and trigonous stem (60:1), is sister to Vellozia (98 bp/100 pp), which is supported by a non-homoplastic character, pollen in tetrads (53:1), and three homoplastic ones, leaf blade with furrows only on abaxial surface (7:1), filaments cylindrical (43:0) and anthers non-auriculate (47:0). Within Vellozia, the V. sessilis + V. tubiflora clade (99 bp/100 pp) is sister to all other species. It is supported by two homoplastic characters, leaf trichomes or emergences with multicellular bases disposed on lamina (9:1) and hypanthial tube longerthan ovary (36:3). In that main clade, (((V. burlemarxii, V. campanuloides, V. caudata, V. aff. caudata, V. hemisphaerica 1, V. hemisphaerica 2), V. canelinha) (V. jolyi, V. religiosa; 97 bp/100 pp))) is supported by a non-homoplastic character, hemispheric ovary (33:2), and by four homoplastic characters, specialized cells on both surfaces of lamina (12:2), minor vascular bundles in leaf blade (21:1), circular-trilobate ovary (34:1) and poricidal capsules (57:1). A second group, composed of nine species, ((V. abietina, V. minima, V. prolifera) (V. alata, V. punctulata) ((V. caput-ardeae, V. hatschbachii) V. epidendroides) V. compacta; 99 bp/100 pp), has almost no internal resolution. Five homoplastic characters, spirotristichous phyllotaxis (1:1), leaf blade involute when dry (6:0), hypanthial emergences absent to laxly disposed (32:0 and 1), staminal appendages (42:1) and poricidal capsules (57:1), support it. A third and final group (99 bp/100 pp) is composed of four species, (((V. hirsuta, V. glauca) V. candida) V. dasypus), and supported by two homoplastic characters, stomata with ridged subsidiary cells (11:1) and pedicel without a belt of sclerified cells (29:0), and perhaps, also by aquiferous hypodermis extending to bundle sheaths only (17:0, ambiguous).
DISCUSSION
Characters and classification in total evidence analysis
In both total analyses, monophyly of Velloziaceae and the position of Acanthochlamys bracteata as sister to all other members of the family is in accordance with Chase et al. (1995, 2006), Behnke et al. (2000), Salatino et al. (2001) and Mello-Silva (2005). Thus, it supports sinking of Acanthochlamydaceae into Velloziaceae (Wu, 1988; APG, 1998, 2003, 2009; contra Kao and Kubitzki, 1998), maximizing phylogenetic information of classification. In Mello-Silva (2005), synapomorphies of the family including Acanthochlamys were only perianth violet and tenuinucellate ovules, the latter from Stevenson and Loconte (1995) and not analysed here. Velloziaceae are now supported by at least four character states, three of them non-homoplastic. Persistent leaves, or at least the sheath (2:1), are easily observable and a good diagnostic characteristic for the family (Martius and Zuccarini, 1823; Seubert, 1847; Smith, 1962; Menezes, 1976; Kubitzki, 1998). This character has been misinterpreted in Mello-Silva (2000, 2005), who associated it with presence of an abscission zone (character 3). Two phloem strands (20:0) characterize all Velloziaceae (contra McPherson et al., 1997), although in some species strands are united at the bottom (20:1; see Mello-Silva, 2000, fig. 1M, N). This character was also misinterpreted in Mello-Silva (2000, 2005), who assigned state 2 (two strands united at bottom) as homologous to state 3 (one strand), which they are not. Stem cortex divided in three regions (66:1) is a new synapomorphy of Velloziaceae. Finally, violet tepals (38:0) still support Velloziaceae as in Mello-Silva (2005), although the transformation series for this character within the family is complex.
In the African clade of Velloziaceae s.s., Talbotia + Xerophyta, recognition of monospecific Talbotia, although possible because of its position, would imply disregarding an easily observable fruit character, the basal loculicidal dehiscence (57:3), for delimiting the group. Besides, Talbotia elegans and all studied species of Xerophyta are hexaploid (Goldblatt and Poston, 1988; Melo et al., 1997). For maximizing phylogenetic information and ease of identification, Talbotia should be transferred to Xerophyta. The American clade, including Barbacenia s.l., Barbaceniopsis, Nanuza and Vellozia, is a new result among Velloziaceae phylogenetic studies, although it has low support. Its two supporting homoplastic characters, leaf trichomes or emergences with multicellular base on leaf margins and midrib (9:2) and auriculate anthers (47:1), have been used by Menezes (1980, 1981) to characterize Pleurostima.
Within American Velloziaceae, the same characters used by Menezes (1971b, 1980) to define subfamily Barbacenioideae, double sheath in leaf vascular bundles (22:1) and corona (39:1), support the Barbacenia s.l. clade. The relationships among genera involved, in both total analyses, show them all polyphyletic except Burlemarxia, but even that, if maintained, would leave Barbacenia paraphyletic. Similar conclusions could be obtained from Behnke et al. (2000) and Salatino et al. (2001). Thus, with respect to the criterion of monophyly, all genera in that clade should be considered synonymous with Barbacenia, with the same circumscription as subfamily Barbacenioideae sensu Menezes (1971b, 1980). This result also renders Menezes' subfamily Vellozioideae paraphyletic.
The clade of Barbaceniopsis + Nanuza + Vellozia, present in the total analyses, is also a new result for Velloziaceae phylogenetic studies, but because of its weak support, both by clearly optimized morphological characters and DNA, it is better to recognize at least the Andean Barbaceniopsis as a separate genus. This would be also a reasonable conclusion from the total analysis with indels removed, in which Barbaceniopsis is in a basal trichotomy within the American clade. Within Barbaceniopsis, the analyses support recognition of Barbaceniopsis castillonii, as did Ibisch et al. (2001), and it appears more closely related to B. humahuaquensis than to B. boliviensis, in which synonymy it had been placed since Smith (1962).
The Nanuza + Vellozia clade corresponds to subfamily Vellozioideae sensu Smith and Ayensu (1976), thus rendering their subfamily Barbacenioideae paraphyletic. Sinking Nanuza in Vellozia, as proposed by Mello-Silva (2005), would leave Vellozia characterized by stigma lobes (Seubert, 1847; Smith, 1962), an easily observed character. On the other hand, Vellozia s.s. is characterized mainly by pollen in tetrads, and Nanuza alone is only supported by homoplastic, mostly anatomical characters. Nanuza is monospecific (contra Alves, 2002), and merging it with Vellozia would maximize phylogenetic information and ease of identification.
Within Vellozia, the analysis without indels fails to establish relationships among the main groups, which are almost the same as in the total analysis with indels. In this analysis, V. sessilis + V. tubiflora are sister to all other species. Vellozia tubiflora is the most widespread species of Velloziaceae. It is highly variable (Mello-Silva, 2011), and several of its morphological characters are coded as polymorphic. An analysis of its populations could reveal a paraphyletic taxon in relation to V. sessilis, a narrow endemic species with several autapomorphies (Mello-Silva, 1997, 2000). Nine terminals, V. burlemarxii, V. campanuloides, V. caudata, V. aff. caudata, V. canelinha, V. hemisphaerica 1 and 2, V. jolyi and V. religiosa belong to the V. hemisphaerica group, representing its five species (R. Mello-Silva and D. Sasaki, unpubl. res.). Smith and Ayensu (1976) informally defined the group by placing V. burlemarxii near V. hemisphaerica in a separate group in their identification key, characterized by a hemispherical ovary (33:2) and minor vascular bundles in leaf blade (21:1). The first character proved to be a non-homoplastic synapomorphy of the group, but the second appears to have originated twice within the group and again in Nanuza. Another group is composed of nine species, V. abietina, V. alata, V. caput-ardeae, V. compacta, V. epidendroides, V. hatschbachii, V. minima, V. prolifera and V. punctulata. This group also occurs in the analyses of Mello-Silva (2000), but in no other Velloziaceae phylogenetic analyses or classifications. Despite that, Smith and Ayensu (1976) used the hypanthial emergences absent to laxly disposed (32:0 and 1) to link together a large group. Nonetheless, that group did not include species of sect. Xerophytoides (Smith and Ayensu, 1976; Mello-Silva, 1991b), represented here by V. abietina, V. minima and V. prolifera. A final group (((V. hirsuta, V. glauca) V. candida) V. dasypus) is also present in Behnke et al. (2000) as ((V. crassicaulis, V. glochidea) V. hirsuta) and in Salatino et al. (2001) as (V. candida, V. hirsuta). The placement of V. caput-ardeae, V. hirsuta and V. tubiflora completely apart from one another reinforces the polyphyletic condition of Vellozia section Radia (Smith and Ayensu, 1976), which had previously been merged with Vellozia (Mello-Silva, 2000).
Historical taxonomic characters and their evolution
The taxonomic history of Velloziaceae, as of many other families under gradistic concepts, is linked mainly to floral characters. When describing the family, Vandelli (1788) also set up two genera distinguished by stamen number and stigma form. Jussieu (1789) followed him, describing a third genus also based on a combination of those characters. Such combinations are still in evidence in most recent systems (Mello-Silva, 1991a; Kubitzki, 1998).
Six stamens (41:0) is the ancestral condition in Velloziaceae (contra Menezes, 1980), and it is constant in all genera, except for Vellozia s.s. (i.e. excluding Nanuza). It is also an odd characteristic within Pandanales, in which stamen number is variable. Within Vellozia s.l. (i.e. including Nanuza) the ancestral condition is also six stamens. However, the most common situation, flowers with 18 stamens, cannot be established as primitive in Vellozia s.s. The sister clade of all other Vellozia s.s., V. sessilis + V. tubiflora, brings together a species with six stamens and another with 12, 15 or 18 stamens, rendering equivocal this character optimization. Analysis of representative populations of V. tubiflora will cast light on polarization of transformations within that species, as well as their relationship with V. sessilis, thus defining the basal situation within Vellozia s.s. Similar situations mask evolution of stamen number in less inclusive clades. Within the V. hemisphaerica group, 12 stamens could be a synapomorphy of clade, V. jolyi + V. religiosa, that reverted to 18 in some populations of V. jolyi, or a derived situation independently acquired by V. religiosa and some populations of V. jolyi. Within clade V. abietina–V. compacta, a reduction to 12 and 6 stamens has occurred in V. prolifera and in V. abietina plus V. minima, respectively, the primitive condition of which is undetermined. Six stamens have lead Sprengel (1827), Schultes and Schultes (1829), Baker (1875) and Menezes (1980) to classify as Xerophyta those species and their relatives later placed together in Vellozia sect. Xerophytoides (Smith and Ayensu, 1976; Mello-Silva, 1991b). It has also helped to make those species sister to the rest of the Vellozia clade in Mello-Silva (2005), a situation that also occurs in the present morphological analysis (Fig. 1A). Other reductions took place within species, such as in V. epidendroides, with 18–12 stamens. However, not only reductions but also multiplications of stamens have occurred within Vellozia, sometimes dramatically, as in V. canelinha, which has 48–66 (Mello-Silva, 1993), and V. alata with 30–72 stamens (Sazima, 1978).
Vertical, fused at apex stigma lobes (56:1) appears as a synapomorphic condition of Velloziaceae s.s. (i.e. excluding Acanthochlamys), with one later transformation into horizontal, centrally fused stigmas in Vellozia s.l. (i.e. including Nanuza), and two independent transformations into free, lateral stigmas in species of Pleurostima. Those species (P. longiscapa, P. plantaginea) and (P. purpurea, P. riparia) have been classified as P. sects. Graziela and Pleurostima, respectively (Menezes, 1981). These transformations are congruent with basal attachment of anthers, another attribute used by Menezes (1981) to define Pleurostima. Although it is a synapomorphy of P. sect. Graziela, it is a symplesiomorphy in P. sect. Pleurostima. Some other attributes, such as a hypanthial tube, auricles in anthers and anther position, that have been used to justify the splitting of Barbacenia s.l. into four genera (Menezes, 1971b, 1980, 1981; Menezes and Semir, 1991) are homoplastic and could perhaps be explained by shifts in pollination strategies.
Characters and outgroups
As in most analyses focusing on Velloziaceae, some questions (mostly minor) regarding evolution of some morphological characters remain open due to the absence of representatives from Stemonaceae, Triuridaceae and Dioscoreales in the present study. These problems do not affect the topology of the trees, just interpretation of morphological change. Stemonaceae are closely related to Velloziaceae as they are the sister family of the sister clade of Velloziaceae, which is, in its turn, the sister of the rest of Pandanales, the sister-order of Dioscoreales (Chase et al., 2006). Triuridaceae have been absent from most analyses due to their modified (reduced) plastid genomes, but now they are positioned within Pandanales (Chase et al., 2000; Stevens, 2001). For these families, assessing morphological data similar to those here analysed is not an easy task, although necessary for better evaluation of homologies and, thus, character evolution. Tenuinucellate ovules are not found in Stemonaceae or Dioscoreales (Cronquist, 1981; Stevenson and Loconte, 1995; Kubitzki, 1998), but they do occur in Triuridaceae (Maas-van de Kamer and Weustenfeld, 1998). Leaves with a sheath seem to be deciduous in all taxa of Dioscoreales and Pandanales except Velloziaceae. A violet perianth, together with other colours, occurs in Burmanniaceae (Maas-van de Kamer, 1998), Aletris (Nartheciaceae) (Tamura, 1998; Tamura et al., 2004), Stemonaceae (Kubitzki, 1998) and Triuridaceae (Maas-van de Kamer and Weustenfeld, 1998). Six stamens are here a symplesiomorphy shared by Velloziaceae and the outgroup, but the situation could be different as stamen number is variable and something other than six in many members of Dioscoreales and Pandanales. Loculicidal capsules are present in some Burmanniaceae (Maas-van de Kamer, 1998), Nartheciaceae subfamily Narthecioideae (Tamura, 1998) and some Dioscoreaceae (e.g. the former Taccaceae; Kubitzki, 1998). Anatomical characters, phloem strands, fibres in stem vascular bundles, divisions of and bundles of fibres in the stem cortex have never been investigated in the other groups. The same situation applies to phyllotaxis (1), sclerenchyma pattern (19), and anther insertion and dehiscence (45 and 50), which could be synapomorphies of Acanthochlamys or Velloziaceae s.s., depending on the optimization provided by the inclusion of other outgroups. Two floral structures of Stemonaceae (Kubitzki, 1998), not analysed here, could be parallel with those in Velloziaceae and deserve investigation. The anthers are apiculate, as in species of ‘Pleurostima’ (Menezes, 1981; Kubitzki, 1998), and the connectives are also long and resemble the corona of Barbacenia or the enlarged filaments of several species of Xerophyta. A search for more outgroup data is one of the investigative efforts to be accomplished by future students of Velloziaceae.
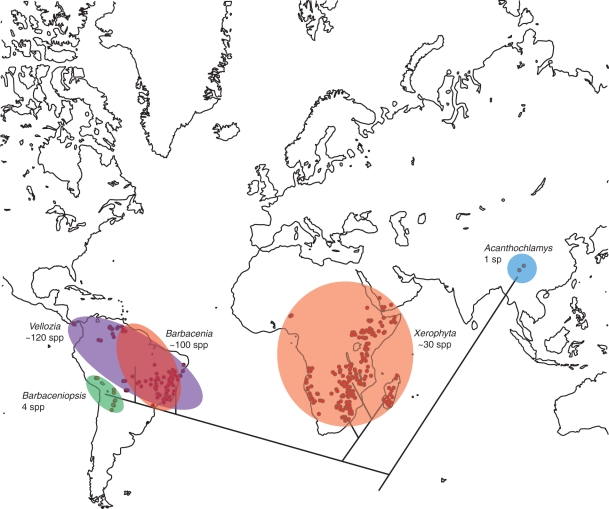
Biogeography
The distribution of Velloziaceae (Fig. 4) suggests a Gondwanan origin of the family and a possible vicarious splitting of main clades. In this scenario, long-distance dispersal as claimed by Ayensu (1973) and Menezes (1980) is not necessary to explain the geographical distribution of Velloziaceae, although it is the main explanation for other amphi-Atlantic taxa (e.g. Givnish et al., 2004; Renner, 2004). The first cladogenetic event in the family, the splitting of Acanthochlamys from remaining genera, should have been consequence of the separation of Indian Plate from Gondwanaland, as first suggested by Wu (1988), which began about 115 Mya. The next split, between African and South American species, could correspond to the splitting of those two continents, which took place around 100 Mya (Scotese et al., 1988). These ages are compatible with the age of the stem group of Velloziaceae stated by Janssen and Bremer (2004), although they did not include Acanthochlamys in their analysis. Nevertheless, the results are more compatible with Velloziaceae sister to the remaining Pandanales (Chase et al., 2006) than to Velloziaceae sister to Stemonaceae (Bremer and Janssen, 2006). The absence of Velloziaceae fossils impedes a concrete evaluation of these estimated dates but fossils from Triuridaceae (Gandolfo et al., 2002) and Pandanaceae (Kvacek and Herman, 2004) do suggest that Pandanales were well diversified by the late Cretaceous. Subsequent cladogenesis within both African and American clades could be explained also by events other than vicariance. In Africa, results point to dispersal of Xerophyta into Madagascar, as continental species form a clade in which the Madagascan taxa are embedded. In South America, Barbacenia and Vellozia are largely sympatric, and the history of their distribution must be related to several minor factors. However, the split of Barbaceniopsis could be related to the Andean orogeny, as it is endemic to that mountain range, with allopatric distribution. This uplift occurred in the last 20–15 Mya (Burnham and Graham, 1993), during the time of establishment of the crown group of Velloziaceae (Janssen and Bremer, 2004).
Fig. 4.
Correlations between phylogenetic relationship of the five accepted genera of Velloziaceae and their geographical distribution.
Conclusions
Results from all analyses reinforce inclusion of Acanthochlamys bracteata in Velloziaceae and its position as sister to the rest of the family. Total analysis adds also at least three non-homoplastic characters, viz., persistent leaves, two phloem strands, and stem cortex divided in three regions, to the tenuinucellate nucellus (Stevenson and Loconte, 1995) and violet perianth (Mello-Silva, 2005) as synapomorphies of the family.
The American clade and its subclade Barbaceniopsis + Nanuza + Vellozia are not well supported, neither by non-homoplastic, conspicuous characters nor bootstrap percentages. On the other hand, these are groups that more or less correspond to accepted genera and are well established.
Xerophyta, including Talbotia or not, and Vellozia, including Nanuza or not, are well supported. However, for reasons of maximizing phylogenetic information and ease of identification (Backlund and Bremer, 1998), Talbotia should be transferred to Xerophyta and Nanuza to Vellozia. Barbacenia s.l., encompassing Aylthonia, Burlemarxia and Pleurostima, is also well circumscribed, and it is impossible to recognize the smaller genera without violating the principle of monophyly.
ACKNOWLEDGEMENTS
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo and Kew Latin America Research Fellowships. Myndel Botánica Foundation funded field trips to Argentina and Bolivia. Lucimar Motta, Denise Sasaki and Cristiane Rodrigues were funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico, and Cíntia Rocini by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Maria Luiza Salatino and Renato Mello-Silva are CNPq research fellows. We thank Amauri Marcato and Richard Winkworth for help with PAUP and MacClade; to Mariane Sousa for help at University of São Paulo; to Pao-Chung Kao, Clinton Morse, Miguel Rodrigues, and curator of living collections of B for kindly providing material of A. bracteata, X. retinervis, X. equisetoides and X. elegans, respectively; to James Clarkson, Edith Kapinos, Laslo Cziba, Laura Kelly, Daniela Zappi, Eimear NicLughadha, Amelia Baracat and Odile Weber for assistance at the Jodrell Laboratory and Kew Herbarium; and to the reviewers that helped to improve the text.
LITERATURE CITED
- Alves RJV. Two new species of Nanuza (Velloziaceae) from Brazil. Novon. 2002;12:12–17. [Google Scholar]
- Amaral MM, Mello-Silva R. Ontogenesis of stomata in Velloziaceae: paracytic versus tetracytic? Revista Brasileira de Botânica. 2009;31:529–536. (‘2008’) [Google Scholar]
- APG. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden. 1998;85:531–553. [Google Scholar]
- APG. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Botanical Journal of the Linnean Society. 2003;141:399–436. [Google Scholar]
- APG. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121. [Google Scholar]
- Ayensu ES. The anatomy of Barbaceniopsis, a new genus recently described in Velloziaceae. American Journal of Botany. 1968;55:399–405. [Google Scholar]
- Ayensu ES. Leaf-anatomy and systematics of Old World Velloziaceae. Kew Bulletin. 1969;23:315–335. [Google Scholar]
- Ayensu ES. Studies on pollen morphology in the Velloziaceae. Proceedings of the Biological Society of Washington. 1972;85:469–480. [Google Scholar]
- Ayensu ES. Phytogeography and evolution of the Velloziaceae. In: Meggers BJ, Ayensu ES, Duckworth WD, editors. Tropical forest ecosystems in Africa and South America: a comparative review. Washington, WA: Smithsonian Institution Press; 1973. pp. 105–119. [Google Scholar]
- Ayensu ES. Leaf anatomy and systematics of New World Velloziaceae. Smithsonian Contributions to Botany. 1974;15:1–125. i–vi + [Google Scholar]
- Ayensu ES, Skvarla JJ. Fine structure of Velloziaceae pollen. Bulletin of the Torrey Botanical Club. 1974;101:250–266. [Google Scholar]
- Backlund A, Bremer K. To be or not to be – principles of classification and monotypic plant families. Taxon. 1998;47:391–400. [Google Scholar]
- Baker JG. Synopsis of the African species of Xerophyta. Journal of Botany. 1875;13:231–236. [Google Scholar]
- Behnke H-D, Treutlein J, Wink M, Kramer K, Schneider C, Kao PC. Systematics and evolution of Velloziaceae, with special reference to sieve-element plastids and rbcL sequence data. Botanical Journal of the Linnean Society. 2000;134:93–129. [Google Scholar]
- Bremer K, Janssen T. Gondwanan origin of major monocot groups inferred from dispersal-vicariance analysis. Aliso. 2006;22:22–27. [Google Scholar]
- Burnham RJ, Graham A. The history of Neotropical vegetation: new developments and status. Annals of the Missouri Botanical Garden. 1993;86:546–589. [Google Scholar]
- Cattai MB. Anatomia em Velloziaceae: caracteres, evolução e filogenia. 2007 M.Sc. Thesis, University of São Paulo, Brazil. [Google Scholar]
- Chase MW, Hills HH. Silica gel: an ideal material for field preservation of leaf samples for DNA studies. Taxon. 1991;40:215–220. [Google Scholar]
- Chase MW, Soltis DE, Olmstead RG, et al. Phylogenetics of seed plants, an analysis of nucleotide sequences from the plastid gene rbcL. Annals of the Missouri Botanical Garden. 1993;80:528–580. [Google Scholar]
- Chase MW, Stevenson DW, Wilkin P, Rudall PJ. Monocot systematics: a combined analysis. In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ, editors. Monocotyledons: systematics and evolution. Kew: Royal Botanic Gardens; 1995. pp. 685–730. [Google Scholar]
- Chase MW, Soltis DE, Soltis PS, et al. Higher-level systematics of the monocotyledons: an assessment of current knowledge and a new classification. In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution. Melbourne: CSIRO; 2000. pp. 3–16. [Google Scholar]
- Chase MW, Fay MF, Devey DS, et al. Multigene analyses of monocot relationships: a summary. Aliso. 2006;22:63–75. [Google Scholar]
- Chiang T, Schaal BA, Peng C. Universal primers for sequencing a noncoding spacer between the atpB and rbcL genes of chloroplast DNA. Botanical Bulletin of Academia Sinica. 1998;39:245–250. [Google Scholar]
- Clark WD, Gaut BS, Duvall MR, Clegg MT. Phylogenetic relationships of the Bromeliiflorae-Commeliniflorae-Zingiberiflorae complex of monocots based on rbcL sequence comparisons. Annals of the Missouri Botanical Garden. 1993;80:987–998. [Google Scholar]
- Coetzee H. Anatomy of the leaves of the Velloziaceae in South Africa and South West Africa and a key based on leaf anatomy. Dinteria. 1974;9:3–8. [Google Scholar]
- Cronquist A. An integrated system of classification of flowering plants. New York, NY: Columbia University Press; 1981. [Google Scholar]
- Dahlgren RMT, Rasmussen FN. Monocotyledon evolution: characters and phylogenetic estimation. Evolutionary Biology. 1983;16:255–395. [Google Scholar]
- Dahlgren RMT, Clifford HT, Yeo PF. The families of the monocotyledons. Berlin: Springer-Verlag; 1985. [Google Scholar]
- Doyle JJ, Doyle JS. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemistry Bulletin. 1987;19:11–15. [Google Scholar]
- Duvall MR, Clegg MT, Chase MW, et al. Phylogenetic hypothesis for the monocotyledons constructed from rbcL sequence data. Annals of the Missouri Botanical Garden. 1993;80:607–619. [Google Scholar]
- Farris JS. Estimating phylogenetic trees from distance matrices. American Naturalist. 1972;106:645–668. [Google Scholar]
- Farris JS. Outgroups and parsimony. Systematic Zoology. 1982;31:328–334. [Google Scholar]
- Ferrarezzi H, Marques AC. Análise cladística numérica e recursos computacionais. In: Amorim DS, editor. Elementos básicos de sistemática filogenética. 2nd edn. Ribeirão Preto: Holos Editora e Sociedade Brasileira de Entomologia; 1997. pp. 163–186. [Google Scholar]
- Gandolfo MA, Nixon KC, Crepet WL. Triuridaceae fossil flowers from the Upper Cretaceous of New Jersey. American Journal of Botany. 2002;89:1940–1957. doi: 10.3732/ajb.89.12.1940. [DOI] [PubMed] [Google Scholar]
- Gilmartin AJ, Brown GK. Bromeliales, related monocots, and resolution of relationships among Bromeliaceae subfamilies. Systematic Botany. 1987;12:493–500. [Google Scholar]
- Givnish TJ, Millam KC, Evans TM, et al. Ancient vicariance or recent long-distance dispersal? Inferences about phylogeny and South American–African disjunctions in Rapateaceae and Bromeliaceae based on ndhF sequence data. International Journal of Plant Sciences. 2004;165(Suppl. 4):35–54. [Google Scholar]
- Goldblatt P, Poston ME. Observations on the chromosome cytology of Velloziaceae. Annals of the Missouri Botanical Garden. 1988;75:192–195. [Google Scholar]
- Golenberg EM, Clegg MT, Burbin ML, Doebley J, Ma DP. Evolution of a non-coding region of the chloroplast genome. Molecular Phylogenetics and Evolution. 1993;2:52–64. doi: 10.1006/mpev.1993.1006. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist FP. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Ibisch PL, Nowicki C, Vásquez R, Koch K. Taxonomy and biology of Andean Velloziaceae: Vellozia andina sp. nov. and notes on Barbaceniopsis (including Barbaceniopsis castillonii comb. nov.) Systematic Botany. 2001;26:5–16. [Google Scholar]
- Janssen T, Bremer K. The age of major monocot groups inferred from 800+ rbcL sequences. Botanical Journal of the Linnean Society. 2004;146:385–398. [Google Scholar]
- Jussieu AL. Genera plantarum. Paris: Hérissant and Th. Barrois; 1789. [Google Scholar]
- Kao PC, Kubitzki K. Acanthochlamydaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Vol. 3. Berlin: Springer Verlag; 1998. pp. 55–58. Flowering plants, monocotyledons, Lilianae (except Orchidaceae) [Google Scholar]
- Kelchner SA. The evolution of non-coding chloroplast DNA and its application in plant systematics. Annals of the Missouri Botanical Garden. 2000;87:482–498. [Google Scholar]
- Kubitzki K. Stemonaceae. Velloziaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Vol. 3. Berlin: Springer Verlag; 1998. pp. 422–425. Flowering plants, monocotyledons, Lilianae (except Orchidaceae) 459–467. [Google Scholar]
- Kumar M, Shukla PK. Use of PCR targeting of internal transcribed spacer regions and single-stranded conformation polymorphism analysis of sequence variation in different regions of rRNA genes in fungi for rapid diagnosis of mycotic keratitis. Journal of Clinical Microbiology. 2005;43:662–668. doi: 10.1128/JCM.43.2.662-668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvacek J, Herman AB. Monocotyledons from the Early Campanian (Cretaceous) of Grünbach, Lower Austria. Review of Palaeobotany and Palynology. 2004;128:323–353. [Google Scholar]
- McPherson G, van der Werff H, Keating RC. A new species of Xerophyta (Velloziaceae) from Madagascar. Novon. 1997;7:387–394. [Google Scholar]
- Maas-van de Kamer H. Burmanniaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Vol. 3. Berlin: Springer Verlag; 1998. pp. 154–164. Flowering plants, monocotyledons, Lilianae (except Orchidaceae) [Google Scholar]
- Maas-van de Kamer H, Weustenfeld T. Triuridaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Vol. 3. Berlin: Springer Verlag; 1998. pp. 452–458. Flowering plants, monocotyledons, Lilianae (except Orchidaceae) [Google Scholar]
- Maddison DR, Maddison WP. MacClade version 4·07 for Mac OS X. Sunderland, MA: Sinauer Associates; 2005. [Google Scholar]
- Martius CFP, Zuccarini JG. Nova genera et species plantarum. Vol. 1. München: Typis Lindaueri; 1823. [Google Scholar]
- Mello-Silva R. Morphological and anatomical differentiation of Vellozia hirsuta populations (Velloziaceae) Plant Systematics and Evolution. 1990;173:197–208. [Google Scholar]
- Mello-Silva R. The infra-familial taxonomic circumscription of the Velloziaceae: a historical and critical analysis. Taxon. 1991a;40:45–51. [Google Scholar]
- Mello-Silva R. A new species of Vellozia from the Espinhaço Range, Brazil, with some considerations on the section Xerophytoides. Kew Bulletin. 1991b;46:321–326. [Google Scholar]
- Mello-Silva R. Three new species of Vellozia from Pico das Almas, Bahia, Brazil, with an account of their leaf anatomy. Kew Bulletin. 1993;48:1–8. [Google Scholar]
- Mello-Silva R. Aspectos taxonômicos, biogeográficos, morfológicos e biológicos das Velloziaceae de Grão-Mogol, Minas Gerais, Brasil. Boletim de Botânica da Universidade de São Paulo. 1995;14:49–79. [Google Scholar]
- Mello-Silva R. Vellozia sessilis L.B.Sm. ex Mello-Silva (Velloziaceae), espécie nova de Goiás, Brasil. Boletim de Botânica da Universidade de São Paulo. 1997;16:65–69. [Google Scholar]
- Mello-Silva R. Partial cladistic analysis of Vellozia and characters for the phylogeny of Velloziaceae. In: Wilson KL, Morrison DA, editors. Monocots: systematics and evolution. Melbourne: CSIRO; 2000. pp. 505–522. [Google Scholar]
- Mello-Silva R. Velloziaceae. In: Smith N, Mori SA, Henderson A, Stevenson DW, Heald SV, editors. Flowering plants of the Neotropics. Princeton, NJ: Princeton University Press; 2004. pp. 490–491. [Google Scholar]
- Mello-Silva R. Morphological analysis, phylogenies and classification in Velloziaceae. Botanical Journal of the Linnean Society. 2005;148:157–173. [Google Scholar]
- Mello-Silva R. Circumscribing Vellozia hirsuta and V. tubiflora (Velloziaceae) Hoehnea. 2011 in press. [Google Scholar]
- Melo NF, Guerra M, Bemko-Iseppon AM, Menezes NL. Cytogenetics and cytotaxonomy of Velloziaceae. Plant Systematics and Evolution. 1997;204:257–273. [Google Scholar]
- Menezes NL. Estudos anatômicos e a taxonomia da família Velloziaceae. 1970 PhD Thesis, University of São Paulo, Brazil. [Google Scholar]
- Menezes NL. Traqueídes de transfusão no gênero Vellozia Vand. (Velloziaceae) Ciência e Cultura. 1971a;23:389–409. [Google Scholar]
- Menezes NL. New taxa and new combination in Velloziaceae. Ciência e Cultura. 1971b;23:421–422. [Google Scholar]
- Menezes NL. Natureza dos apêndices petalóides em Barbacenioideae (Velloziaceae) Boletim de Zoologia e Biologia Marinha. 1973;30:713–755. N.S. [Google Scholar]
- Menezes NL. Presença de traqueídes de transfusão e bainha mestomática em Barbacenioideae (Velloziaceae) Boletim de Botânica da Universidade de São Paulo. 1975;3:29–60. [Google Scholar]
- Menezes NL. Megasporogênese, megagametogênese e embriogênese em Velloziaceae. Boletim de Botânica da Universidade de São Paulo. 1976;4:41–60. [Google Scholar]
- Menezes NL. Evolution in Velloziaceae with special reference to androecial characters. In: Brickell CD, Cutler DF, Gregory M, editors. Petaloid monocotyledons: horticultural and botanical research. London: Academic Press; 1980. pp. 117–139. [Google Scholar]
- Menezes NL. Re-establishment of genus Pleurostima Rafinesque (Velloziaceae) Revista Brasileira de Botânica. 1981;3:37–47. (‘1980’) [Google Scholar]
- Menezes NL. Características anatômicas e a filogenia na família Velloziaceae. 1984 Associate Professor Thesis, University of São Paulo, Brazil. [Google Scholar]
- Menezes NL. Evolution of the anther in the family Velloziaceae. Boletim de Botânica da Universidade de São Paulo. 1988;10:33–41. [Google Scholar]
- Menezes NL, Semir J. New considerations regarding the corona in the Velloziaceae. Annals of the Missouri Botanical Garden. 1990;77:539–544. [Google Scholar]
- Menezes NL, Semir J. Burlemarxia, a new genus of Velloziaceae. Taxon. 1991;40:413–426. [Google Scholar]
- Menezes NL, Mello-Silva R, Mayo SJ. A cladistic analysis of the Velloziaceae. Kew Bulletin. 1994;49:71–92. [Google Scholar]
- Nylander JA. MrModelTest ed. 2·2. Uppsala: Uppsala University; 2004. [Google Scholar]
- Nixon KC, Carpenter JM. On outgroups. Cladistics. 1993;5:275–289. doi: 10.1111/j.1096-0031.1993.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Prance GT. The use of phytogeographic data for conservation planning. In: Forey PL, Humphries CJ, Vane-Wright RI, editors. Systematics and conservation evaluation. Oxford: Clarendon Press; 1994. pp. 145–163. Systematics Association Special Volume No. 50. [Google Scholar]
- Renner S. Plant dispersal across the tropical Atlantic by wind and sea currents. International Journal of Plant Sciences. 2004;165(Suppl. 4):23–33. [Google Scholar]
- Sajo MG, Mello-Silva R, Rudall PJ. Homologies of floral structures in Velloziaceae with particular reference to the corona. International Journal of Plant Sciences. 2010;171:595–606. [Google Scholar]
- Salatino A. Main results from trnL-F sequencing of Velloziaceae and allied taxa. Anais da Academia Brasileira de Ciências. 1999;71:203–206. [Google Scholar]
- Salatino MLF, Salatino A, Menezes NL, Mello-Silva R. Alkanes of foliar epicuticular waxes of Velloziaceae. Phytochemistry. 1989;28:1105–1114. [Google Scholar]
- Salatino A, Salatino MLF, Mello-Silva R, Duerholt-Oliveira I. An appraisal of the plasticity of alkane profiles of some species of Velloziaceae. Biochemical Systematics and Ecology. 1991;19:241–248. [Google Scholar]
- Salatino A, Salatino MLF, Mello-Silva R, Sluys M-A, Giannasi DE, Price RA. Phylogenetic inference in Velloziaceae using chloroplast trnL-F sequences. Systematic Botany. 2001;26:92–103. [Google Scholar]
- Sazima M. Biologia floral de espécies de Velloziaceae na Serra do Cipó, Minas Gerais. 1978 PhD Thesis, University of São Paulo, Brazil. [Google Scholar]
- Schultes JA, Schultes JH. In: Barbacenia. Xerophyta. Vellozia. 1. Roemer JJ, Schultes JA, editors. Vol. 7. Stuttgart: J. G. Cottae; 1829. pp. 284–293. Systema vegetabilium (‘1826’) [Google Scholar]
- Scotese CR, Gahagan LM, Larson RL. Plate tectonics reconstructions of the Cretaceous and Cenozoic ocean basins. Tectonophysics. 1988;155:27–48. [Google Scholar]
- Seubert MA. Vellosieae. In: Martius CFP, editor. Flora brasiliensis. 1. Vol. 3. Leipzig: Fridrich Fleischer; 1847. pp. 65–84. t. 8–10. [Google Scholar]
- Shaw J, Lickey EB, Beck JT, et al. The tortoise and the hare. II. Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. American Journal of Botany. 2005;92:142–166. doi: 10.3732/ajb.92.1.142. [DOI] [PubMed] [Google Scholar]
- Simmons MP. Independence of alignment and tree search. Molecular Phylogenetics and Evolution. 2004;31:874–879. doi: 10.1016/j.ympev.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Smith LB. A synopsis of the American Velloziaceae. Contributions from the United States National Herbarium. 1962;35:251–292. pl. 1–12. [Google Scholar]
- Smith LB, Ayensu ES. Classification of Old World Velloziaceae. Kew Bulletin. 1974;29:181–205. [Google Scholar]
- Smith LB, Ayensu ES. A revision of American Velloziaceae. Smithsonian Contributions to Botany. 1976;30:1–172. i–viii + [Google Scholar]
- Sousa MS. Morfogênese de frutos e sementes em Velloziaceae. 2005 M.Sc. Thesis, University of São Paulo, Brazil. [Google Scholar]
- Sprengel KPJ. Systema vegetabilium. 2. Vol. 4. Göttingen: Dieterich; 1827. [Google Scholar]
- Stevens PF. Angiosperm phylogeny website, version 9, June 2008 [more or less continuously updated] 2001 http://www.mobot.org/MOBOT/research/APweb/ (accessed 25 June 2010) [Google Scholar]
- Stevenson DW, Loconte H. Cladistic analysis of monocot families. In: Rudall PJ, Cribb PJ, Cutler DF, Humphries CJ, editors. Monocotyledons: systematics and evolution. Kew: Royal Botanic Gardens; 1995. pp. 543–578. [Google Scholar]
- Sun Y, Skinner DZ, Liang GH, Hulbert SH. Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theoretical and Applied Genetics. 1994;89:26–32. doi: 10.1007/BF00226978. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4·0b10 for Macintosh. Sunderland, MA: Sinauer Associates; 2002. [Google Scholar]
- Taberlet P, Gielly L, Pautou G, Bouvet J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology. 1991;17:1105–1109. doi: 10.1007/BF00037152. [DOI] [PubMed] [Google Scholar]
- Tamura MN. Nartheciaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Vol. 3. Berlin: Springer Verlag; 1998. pp. 381–392. Flowering plants, monocotyledons, Lilianae (except Orchidaceae) [Google Scholar]
- Tamura MN, Yamashita J, Fuse S, Haraguchi M. Molecular phylogeny of monocotyledons inferred from combined analysis of plastid matK and rbcL gene sequences. Journal of Plant Research. 2004;117:109–120. doi: 10.1007/s10265-003-0133-3. [DOI] [PubMed] [Google Scholar]
- Thiers B. Index herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. 2010 [continuously updated] http://sweetgum.nybg.org/ih/ (accessed 25 June 2010) [Google Scholar]
- Vandelli DA. Florae lusitanicae et brasiliensis specimen. Coimbra: Typographia Academico-Regia; 1788. [Google Scholar]
- Williams CA, Harborne JB, Menezes NL. The utility of leaf flavonoids as taxonomic markers in the subfamily and generic classification of the Velloziaceae. Biochemical Systematics and Ecology. 1991;19:483–495. [Google Scholar]
- Williams CA, Greenham J, Harborne JB, Eagles J, Markham KR. Occurrence of c-methylflavonols in leaves of Vellozia. Phytochemistry. 1992;31:555–557. [Google Scholar]
- Williams CA, Harborne JB, Greenham J, Eagles J, Markham KR. Six further lipophilic flavonols from the leaves of Vellozia stipitata. Phytochemistry. 1993;32:731–735. [Google Scholar]
- Williams CA, Harborne JB, Greenham J, Eagles J. Differences in flavonoid patterns between genera within the Velloziaceae. Phytochemistry. 1994;36:931–940. [Google Scholar]
- Wu CY. Hengduan mountain flora and its significance. Journal of Japanese Botany. 1988;63:297–310. [Google Scholar]