Abstract
Background
Plasmodium vivax shows a small prevalence in West and Central Africa due to the high prevalence of Duffy negative people. However, Duffy negative individuals infected with P. vivax have been reported in areas of high prevalence of Duffy positive people who may serve as supply of P. vivax strains able to invade Duffy negative erythrocytes. We investigated the presence of P. vivax in two West African countries, using blood samples and mosquitoes collected during two on-going studies.
Methodology/Findings
Blood samples from a total of 995 individuals were collected in seven villages in Angola and Equatorial Guinea, and 820 Anopheles mosquitoes were collected in Equatorial Guinea. Identification of the Plasmodium species was achieved by nested PCR amplification of the small-subunit rRNA genes; P. vivax was further characterized by csp gene analysis. Positive P. vivax-human isolates were genotyped for the Duffy blood group through the analysis of the DARC gene. Fifteen Duffy-negative individuals, 8 from Equatorial Guinea (out of 97) and 7 from Angola (out of 898), were infected with two different strains of P. vivax (VK210 and VK247).
Conclusions
In this study we demonstrated that P. vivax infections were found both in humans and mosquitoes, which means that active transmission is occurring. Given the high prevalence of infection in mosquitoes, we may speculate that this hypnozoite-forming species at liver may not be detected by the peripheral blood samples analysis. Also, this is the first report of Duffy negative individuals infected with two different strains of P. vivax (VK247 and classic strains) in Angola and Equatorial Guinea. This finding reinforces the idea that this parasite is able to use receptors other than Duffy to invade erythrocytes, which may have an enormous impact in P. vivax current distribution.
Author Summary
Recent reports of Plasmodium vivax infections, the most widely distributed species of human malaria, show that this parasite is evolving and adapting, becoming not only more aggressive but also more frequent in countries where it was not present in the past, becoming, therefore, a major source of concern. Thus, it is extremely important to perform new studies of its distribution in West and Central Africa, where there are few reports of its presence, due to the high prevalence of Duffy-negative individuals. The aim of this study was to investigate the presence of P. vivax in Angola and in Equatorial Guinea, using blood samples and mosquitoes. The results showed that P. vivax seems to be able to invade erythrocytes using receptors other than Duffy, and this new capacity is not exclusive to one strain of P. vivax, since we have found samples infected with two different strains: VK247 and classic. Additionally we demonstrated that the parasite has a greater distribution than previously thought, calling for a reevaluation of its worldwide distribution.
Introduction
Plasmodium vivax has been neglected by the scientific community since it has been seen as a “benign” parasite. Nowadays this scenario has changed and the infection caused by P. vivax gained higher importance, firstly because it has a very wide distribution, being found both in tropical and subtropical areas [1], [2], [3]; and secondly because of the high number of clinical cases reported, ranging from 70 million to 300 million [2], [4], [5]. Although clinical symptoms are usually considered as not severe, some reports documented cases of severe disease and even death [6], [7], [8], [9].
This parasite has traditionally shown a small prevalence in West and Central Africa, attributed to the high prevalence of Duffy negative people [Fy(a−b−)] who are described as being resistant to P. vivax infection [10], [11]. Culleton et al. [12] performed a study including nine endemic countries of West and Central Africa using a high sensitive PCR-based protocol for the detection and identification of Plasmodium species reporting only one case out of 2588 individuals infected with P. vivax - one Duffy-positive individual from São Tomé. Although the exact prevalence of P. vivax in Africa is unknown, this parasite tends to be endemic in countries of East Africa, like Sudan, Somalia and Ethiopia, where the majority of the population is Duffy-positive.
The Duffy antigen, also called Duffy antigen receptor for chemokines (DARC), is a multimeric red cell membrane protein organized into seven transmembrane domains, and it is the unique known erythrocyte receptor for P. vivax invasion. DARC-coding gene is polymorphic with multiple alleles as the codominant FY*A and FY*B, which encode for the two antigens – Fya and Fyb. Four genotypes are possible as a result of the combination of the major alleles, Fy(a+b+), Fy(a+b−), Fy(a−b+) and Fy(a−b−) [13], [14], [15]. The first three correspond to a Duffy-positive phenotype, mostly prevalent in Asian and in Caucasian populations and the last one correspond to the Duffy-negative phenotype, mainly prevalent in African people, who are consequently resistant to P. vivax infection. The Fy(a−b−) genotype results from a point mutation, -33T>C, in the promoter region of allele FY*B, in the GATA box region [13].
Recent data showed that Duffy binding protein, the main vaccine candidate for P. vivax [16], [17], seems no longer to protect against P. vivax infection. Rosenberg [18] hypothesized that P. vivax could infect Duffy negative erythrocytes, since there were reports of European travellers and immigrants from West and Central Africa who were infected with P. vivax [19], [20], [21]. In fact, there are now other reports that seem to support this hypothesis [18].
In a case-control study conducted in Kenya, an East African country, with children with severe malaria caused by Plasmodium falciparum, it was found that there were children infected with P. vivax VK247 despite being Duffy-negative [22]. Similar results were found in the Amazon region in Brazil [23], [24] and more recently in Madagascar [25]. These new data suggest that P. vivax may be evolving by using alternative receptors to bind and invade erythrocytes or it may be a “vivax-like” that do not require Duffy antigen for the invasion [26].
Currently, three different strains of P. vivax have been described – classic P. vivax (also called P. vivax VK 210), P. vivax VK 247 and P. vivax-like. These strains, although morphologically similar, differ in the central portion of circunsporozoite surface protein (csp), an abundant polypeptide present at the sporozoite surface [27]. The variant VK247 was first described by Rosenberg et al. [28] in isolates from Thailand and differs from the P. vivax classic in the nonapeptide repeat units of the central portion of CSP gene: ANGA(G/D)(N/D)QPG in P. vivax VK247 and GDRA(A/D)GQPA in P. vivax classic (described in [29]). Qari et al. [26] identified the strain P. vivax-like, characterised by having a 11-mer repeat sequence, APGNQ(E/G)GGAA in the central portion of the CSP gene.
With new cases of P. vivax infections appearing every day, especially in countries where this parasite has not been reported before, it becomes essential not to underestimate it, since P. vivax may be swiftly evolving and infecting people that were thought to be protected.
The aims of this study were to investigate the presence of P. vivax in Angola and in Equatorial Guinea, using blood samples and mosquitoes, and analyze the presence of P. vivax infection in Duffy-negative individuals.
Methods
Ethics statement
Each person (or parent) was informed of the nature and aims of the study and told that participation was voluntary and that they could withdraw from the study at any time. Blood samples were collected after informed consent from all donors (parents or guardians respond on behalf of children). In Equatorial Guinea, written consent was not obtainable because of the community-wide mistrust of signing any official forms and the low level of literacy in the population. Viewing this, written consent was only assented by population in case of the legal guardians of the recruited children and only non-documented oral consent was requested on adults. The study was approved by the Ethical Committee of the Equatorial Guinea's Ministry of Health and Social Welfare, the National Malaria Control Programme and the local health authorities from these villages, which accepted this constraint and did not find bio-ethical impediments to disallow the study. In Angola, written informed consent was obtained from each person (or parent/guardian) and the study was approved by the Ethical Committee of the Angola's Ministry of Health. Ethical clearance was also given by the Ethical Committees of IHMT and the ISCIII, according to EU norms.
Sampling
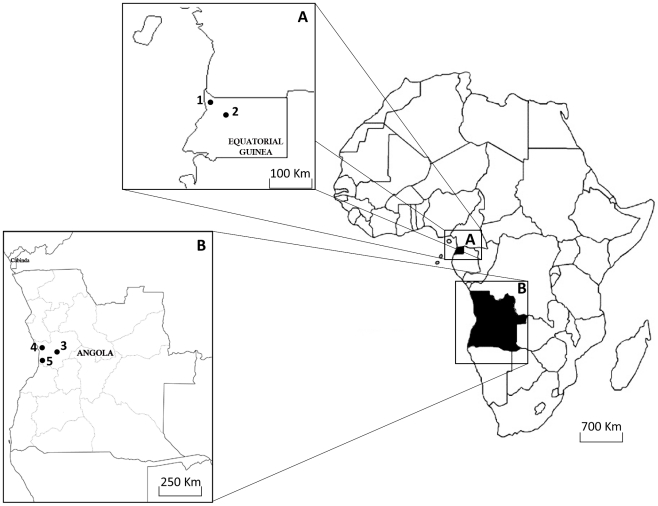
Blood samples were collected as part of two on-going studies in Angola and Equatorial Guinea (see figure 1).
Figure 1. Map of the five collection places in Equatorial Guinea and Angola.
A- Equatorial Guinea; B- Angola; 1- Ngonamanga; 2- Miyobo; 3- Gabela; 4 – Porto Amboim and 5- Sumbe.
Angola samples were collected in Gabela (10°S51′/14°E22′), Porto Amboim (10°S43′/13°E46′), Kissala-Sumbe (11°S12′/13°E50′), Praia-Sumbe (11°S12′/13°E50′) [Kuanza Sul province] and Funda (8°S50′/13°E33′) [Bengo province] between June 2006 and May 2007. In these two provinces malaria is mesoendemic stable and the climate is tropical, characterised by a wet and warm season, from September to April, and a dry and cold season, from May to August. In each village, blood samples were collected by fingerprick on filter paper, from asymptomatic children older than 2 months.
In Equatorial Guinea, blood samples and mosquito specimens were collected from 20 households in two different villages - Miyobo (1°N45′/10°E10′) in May and August of 2005 and Ngonamanga (2°N9′/9°E48′) in February and May of 2005. The two villages present different ecological characteristics: Miyobo is located in the interior of the country in a forested area, while Ngonamanga is a coastal area. In both, malaria is classified as hyperendemic, and it is possible to distinguish four seasons, two dry seasons from December to March and from July to September; and two wet seasons, one more intense from September to November and the other from March to the end of June. In each household, blood fed resting mosquitoes were collected early in the morning (5.00–7.00am), followed by blood sample collection by fingerprick from all inhabitants, during four consecutive days. Mosquitoes were kept in paper-cups corresponding to each house/room for 8 days to enable the development of oocysts from infections acquired the night prior to collection. Head/thorax and abdomen from each mosquito were kept separately for subsequent molecular processing.
Sample collection and DNA extraction
Blood samples from a total of 995 individuals (898 from Angola and 97 from Equatorial Guinea) were collected by fingerprick on filter paper and stored at room temperature until DNA extraction, which was carried out using the chelex protocol as described by Plowe et al. [30].
DNA from the 819 mosquitoes captured in Equatorial Guinea was extracted using the chelex protocol described by Arez et al. [31]. DNA from portions head/thorax and abdomen of each mosquito was extracted separately.
Detection and identification of Plasmodium species
For all samples, detection of malaria infection and identification of Plasmodium species was made using nested-PCR amplification of the small subunit ribosomal RNA genes as described by Snounou et al. [32].
Genotyping of Pvcsp genes
In samples infected with P. vivax, parasite characterisation was carried out by analysis of the central region of the Pvcsp gene, following a slightly modified version of the protocol described by Alves et al. [33]. This was amplified in a MyCyclerTM Thermal cycler (Biorad), using the primers VivF 5′- TCCATCCTGTTGGTGGACTT – 3′ and VivR 5′ – TCACAACGTTAAATATGCCAG – 3′ with final reagent concentrations of 1× reaction buffer, 1 mM of MgCl2, 100 µM of each dNTPs, 0.5 µM of each primer and 1 U/µl of Taq DNA Polymerase (Promega), in a total volume of 50 µl for each reaction. The PCR cycle conditions were: initial denaturation at 94°C for 5 minutes, followed by 35 cycles of 93°C for 1 minute, 60°C for 90 seconds and 72°C for 1 minute, with a final extension at 72°C for 10 minutes.
In order to distinguish the three P. vivax strains (VK210, VK247 and P. vivax-like), restriction fragment length polymorphism (RFLP) analysis was performed using the restriction endonucleases (AluI and DpnI), following the recommended protocol (New England Biolabs, Ipswich, MA). PCR-RFLP products were run in a 2% agarose gel.
Genotyping of Duffy blood group
Duffy genotypes were also determined in P. vivax human isolates. To detect the point mutation -33T>C, which correspond to a Duffy-negative phenotype, the DARC gene promoter regions were amplified by PCR, followed by enzymatic restriction with StyI (New England Biolabs, Ipswich, MA) (adapted from [13]). Briefly, the PCR was performed using the primer P38 5′- AGGCTTGTGCAGGCAGTG - 3′ and P39 5′- GGCATAGGGATAAGGGACT - 3′, 0.5 pmol/µl of each, 1 mM of MgCl2, 200 µM of dNTP's and 1 U/µl of Taq DNA Polymerase (Promega), in a total volume of 30 µl. Cycling parameters were as follows: 94°C for 5 minutes, pursued by 30 cycles of 94°C for 1 minute, 59°C for 1 minute and 72°C for 30 seconds, with a final extension at 72°C for 10 minutes.
Endonuclease StyI was used for RFLP analysis of PCR products, according to the supplier's specifications (New England Biolabs, Ipswich, MA). Restriction fragments were separated on an 18% acrylamide/bis-acrylamide (39.5∶1) gel and silver stained.
For confirmation, some samples were purified with the SureClean Kit (Bioline) according to manufacturer's recommendations and were sequenced in both directions by Macrogen, Korea.
Results
Detection and identification of Plasmodium species
The four species of Plasmodium were identified in both countries. Plasmodium vivax had not been previously described in the mainland of Equatorial Guinea.
Prevalence of infection in both blood samples and mosquitoes is presented in Table 1. Regarding the human host, overall prevalence of infection was much higher in Equatorial Guinea than in Angola (86.6% versus 28.9%, respectively), with P. falciparum showing the highest infection rate in both countries (95.2% in Equatorial Guinea and 97.9% in Angola). Plasmodium vivax was detected in 15 individuals, 8 from Equatorial Guinea (9.5% of infected individuals) and 7 from Angola (2.8% of infected individuals). From these 15 cases, 5 exhibited a single P. vixax infection, 8 a mixed infection with P. falciparum and 2 a triple infection with P. falciparum and Plasmodium malariae. In Equatorial Guinea, the overall prevalence of infected mosquitoes was 26.7% (219/819). From these, P. vivax infections were found in 10.9% (24/219), both in head/thorax (salivary glands) and abdomen (midgut): 22 were a single P. vixax infection and 2 a mixed infection with P. falciparum.
Table 1. Prevalence of infection in both humans and mosquitoes, in Angola and Equatorial Guinea.
| Prevalence of infection | Individuals | Mosquitoes | |
| Angola | Equatorial Guinea | ||
| n | 898 | 97 | 819 |
| Overall infection | 28.9% (245/848) | 86.6% (84/97) | 26.7% (219/819) |
| Overall infection F | 97.9% (240/245) | 95.2% (80/84) | 89.0% (195/219) |
| Overall infection V | 2.8% (7*/245) | 9.5% (8*/84) | 10.9% (24/219) |
| V | 3 ind. | 2 ind. | 22 mosq. |
| F+V | 3 ind. | 5 ind. | 2 mosq. |
| F+V+M | 1 ind. | 1 ind. | 0 |
*All Duffy-negatives.
n - Sample size; F: P. falciparum; V: P. vivax; F+V: mixed infection by P. falciparum and P. vivax; F+V+M: mixed infection by P. falciparum, P. vivax and P. malariae.
Genotyping of Pvcsp genes
Using the endonuclease AluI the fragments obtained for the P. vivax classic were: 243, 135, 133, 108, 90, 78, 57, 54, 30, 27 bp and for P. vivax VK247 were: 673, 243, 90, 78 bp. Using the endonuclease DpnI it was possible to identify fragments of 969, 71 and 50 bp in the case of P. vivax classic, and fragments of 360, 225, 108, 81, 71, 54, 50, 27 bp for P. vivax VK247. Fragments below 50 bp were not considered for variant determination due to the low molecular weight.
According to this, it was possible to identify 6 blood samples infected with P. vivax classic, 6 blood samples with P. vivax VK247 and 3 blood samples infected with two strains of P. vivax: classic and VK247.
No samples were identified as being infected with P. vivax-like. In this case, it was expected to obtain fragments of 786, 101, 83, 70 and 62 bp when using AluI, and fragments of 883, 169 and 50 bp when using DpnI.
For the 24 mosquitoes infected with P. vivax, the same procedure was used for the parasite characterisation but unfortunately no successful amplification of specific sequences was achieved.
Genotyping of Duffy blood group
All the human isolates P. vivax infected were genotyped for the Duffy gene by PCR-RFLP (82, 77 and 64 bp for Duffy positive genotypes and 82, 65, 64 and 12 bp for Duffy negative genotypes; the fragment of 12 bp was not considered due to the low molecular weight, not visible in gel). Results showed that all samples analysed were genotyped as FY*B-33/FY*B-33 (Duffy-negative homozygous) being therefore classified as Fy(a−b−).
Given that differentiation of bands in acrylamide gel is sometimes dubious, some samples were sequenced to confirm results. Sequencing (figure 2) confirmed the Duffy-negative genotype, since all of them contained the point mutation -33T→C.
Figure 2. Multiple sequences alignment of promoter region from the DARC gene, allele FY*B, in the GATA box region.
Discussion
Despite all the efforts that have been made to control malaria, many of them having a real effect, the prevalence of infection is still very high, even in countries with active control campaigns, like Equatorial Guinea (86.6%) and Angola (28.9%).
Particularly, P. vivax seems not only to be evolving and adapting, causing more severe forms of the disease [6], [8], [34], [35], [36] but also appears to be more frequent in countries where either it was not present or it was not detected by the available techniques in the past, as is the case of some countries of West and Central Africa like, Congo [37], São Tomé and Principe [37], [38], Gabon [37], [39] and Cameroon [37], becoming a major source of concern. Our results corroborate these assumptions, since for the first time we were able to detect P. vivax on mainland Equatorial Guinea in humans and mosquitoes, which imply well-established whole life-cycles and active transmission.
Further, a relevant aspect needs to be stressed - the proportion of P. vivax infected mosquitoes is higher than the proportion of P. vivax infected individuals. This may be associated with the fact that in the human host this parasite may be “hidden” since it forms dormant forms in the liver – hypnozoites - and go unnoticed, being much more “visible” in mosquitoes. If this is the case, these results suggest that the prevalence of this species may be underestimated, not only in this country but in other parts of Africa.
Other factors associated with parasite-human interaction and immune response could be conditioning this variable prevalence in P. vivax infection in mosquitoes and human host.
In this study we were able to detect Duffy negative people carrying P. vivax infections, both in Angola and Equatorial Guinea, two countries located in West Africa, where the prevalence of Duffy negative individuals is near 95% [11], confirming thereby the suspicion of some authors [18], [22]–[25]. Similar results were found in other studies, but always in areas where the prevalence of Duffy positive is significantly higher: in Kenya - East Africa [22], Amazon region in Brazil [23], [24] and more recently, in Madagascar [25].
Ménard et al. [25] suggested that Duffy positive individuals may serve as a reservoir of P. vivax providing an opportunity for this parasite to infect hepatocytes of Duffy negative people and the selection of new P. vivax strains with capacity to invade Duffy negative erythrocytes. In the present case, it is likely that the evolutionary process has been the same, although these two countries showed low prevalence of Duffy positive autochthonous individuals. From the beginning of the 90 s, these countries have experienced a marked increase in economic development with the finding of important oil reserves. Related to this development, intensive migration processes are occurring from outside and inside of the African continent. Therefore, workers from countries with higher Duffy positive and P. vivax prevalence could be circulating in Angola and Equatorial Guinea, thus increasing the reservoir of P. vivax.
Although we do not know which main force was contributing for the evolution of P. vivax and why it is able to infect Duffy negative erythrocytes, one thing seems to be clear - P. vivax may have an extraordinary ability to adapt. In addition, the African continent has both the ideal temperature and highly competent vectors for its transmission [3], [40]. Altogether, these factors show that this parasite can become a serious public health problem in West and Central Africa, both for locals and travelers.
The results obtained in this work are highly relevant. First, it demonstrates that P. vivax is able to invade erythrocytes using other receptors than Duffy, and this new capacity is not exclusive of one strain of P. vivax, since we found samples infected with two different strains: VK247 and VK210/classic. Other species of Plasmodium, as Plasmodium knowlesi (phylogenetically close to P. vivax) and P. falciparum have more than one receptor for the invasion of erythrocytes [41]. Considering that these two phylogenetically distant species have evolved in order to recognize more than one receptor for erythrocyte invasion, it is expected that P. vivax is also evolving, becoming capable of using more than one path of invasion.
Second, this parasite seems to be expanding, and now it can be found in areas where it was not present in the past. Some approaches to determine the distribution limits of P. vivax have been carried out, although areas with high prevalence of Duffy negative were virtually considered free of this parasite [3]. So it is expectable that the real distribution of this parasite is greater than that found by these authors.
In conclusion, this study present the first cases of Duffy negative individuals infected with different strains of P. vivax (VK247 and classic) in two West African countries. This finding reinforces the idea that this parasite is rapidly evolving, being able to use other receptors than Duffy to invade the erythrocytes.
The presence of P. vivax infection both in blood samples and mosquitoes indicates that this parasite is well adapted. Further, the higher number of infected mosquitoes shows that this species is more “visible” in mosquitoes and may go unnoticed if blood samples are only analyzed.
It is therefore important to establish the real distribution of P. vivax, since new and more aggressive cases of infection by this parasite are reported every day, in countries where this parasite has not been noticed before having significant implications in the design of control measures and implementation of prophylactic and therapeutic regimens.
Supporting Information
STROBE checklist.
(DOC)
Acknowledgments
We thank all families who accepted to participate in this study.
We also thank researchers and technicians from the National Malaria Control Program from the Republic of Equatorial Guinea's Ministry of Health and Social Welfare and “Centro de Referencia para el Control de Endemias” (Instituto de Salud Carlos III, Equatorial Guinea), and the Health Delegates of Health Care Units of Hospital, Programa Nacional de Controlo de Malária and Ministério da Saúde, Angola.
Footnotes
The authors have declared that no competing interests exist.
This study was supported by “Faculdade de Medicina Américo Boavida”, Angola; by the “Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación”, Madrid, Spain; by “Financiamento Programático do Laboratório Associado CMDT.LA/IHMT” (http://www.ihmt.unl.pt/) and PTDC/SAU-EPI/113326/2009, “Fundacão para a Ciência e Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior”, FCT/MCTES (http://alfa.fct.mctes.pt/index.phtml.pt), Portugal. Cristina Mendes holds a FCT grant (SRFH/BD/41473/2007). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Guerra CA, Snow RW, Hay SI. Mapping the global extent of malaria in 2005. Trends Parasitol. 2006;22:353–358. doi: 10.1016/j.pt.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galinski MR, Barnwell JW. Plasmodium vivax: who cares? Malar J. 2008;7:S9. doi: 10.1186/1475-2875-7-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, et al. The International Limits and Population at Risk of Plasmodium vivax Transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baird JK. Neglect of Plasmodium vivax malaria. Trends Parasit. 2007;23:533–539. doi: 10.1016/j.pt.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis. 2009;9:555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 6.Genton B, D'Acremont V, Rare L, Baea K, Reeder JC, et al. Plasmodium vivax and Mixed Infections Are Associated with Severe Malaria in Children: A Prospective Cohort Study from Papua New Guinea. PLoS Med. 2008;5:881–889. doi: 10.1371/journal.pmed.0050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogerson SJ, Carter R. Severe Vivax Malaria: Newly Recognised or Rediscovered? PLoS Med. 2008;5:e136. doi: 10.1371/journal.pmed.0050136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tjitra E, Anstey NM, Sugiarto P, Warikar N, Kenangalem E, et al. Multidrug-Resistant Plasmodium vivax Associated with Severe and Fatal Malaria: A Prospective Study in Papua, Indonesia. PLoS Med. 2008;5:e128. doi: 10.1371/journal.pmed.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexandre MA, Ferreira CO, Siqueira AM, Magalhães BL, Mourão MPG, et al. Severe Plasmodium vivax Malaria, Brazilian Amazon. Emerg Infect Dis. 2010;16:1611–1614. doi: 10.3201/eid1610.100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller LH, Mason SJ, Dvorak JA, McGinniss MH, Rothman IK. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science. 1975;189:561–563. doi: 10.1126/science.1145213. [DOI] [PubMed] [Google Scholar]
- 11.Langhi DM, Jr, Bordin JO. Duffy blood group and malaria. Hematology. 2006;11:389–398. doi: 10.1080/10245330500469841. [DOI] [PubMed] [Google Scholar]
- 12.Culleton RL, Mita T, Ndounga M, Unger H, Cravo PVL, et al. Failure to detect Plasmodium vivax in West and Central Africa by PCR species typing. Malar J. 2008;7:174. doi: 10.1186/1475-2875-7-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Van Kim C. Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy-negative individuals. Nat Genet. 1995;10:224–228. doi: 10.1038/ng0695-224. [DOI] [PubMed] [Google Scholar]
- 14.Castilho L, Rios M, Pellegrinno J, Jr, Saad STO, Costa FF, et al. A novel FY allele in Brazilians. Vox Sang. 2004;87:190–195. doi: 10.1111/j.1423-0410.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho TL, Ribolla PEM, Curi RA, Mota LS. Characterization and transcriptional analysis of the promoter region of the Duffy blood group, chemokine receptor (DARC) in cattle. Vet Immunol Immunopathol. 2009;132:153–159. doi: 10.1016/j.vetimm.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Cerávolo IP, Bruña-Romero O, Braga EM, Fontes CJ, Brito CF, et al. Anti-Plasmodium vivax duffy binding protein antibodies measure exposure to malaria in the Brazilian Amazon. Am J Trop Med Hyg. 2005;72:675–681. [PubMed] [Google Scholar]
- 17.Beeson JG, Crabb BS. Towards a Vaccine against Plasmodium vivax Malaria. PLoS Med. 2007;4:1862–1864. doi: 10.1371/journal.pmed.0040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg R. Plasmodium vivax in Africa: hidden in plain sight? Trends Parasitol. 2007;23:193–196. doi: 10.1016/j.pt.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Rubio JM, Benito A, Berzosa PJ, Roche J, Puente S, et al. Usefulness of Seminested Multiplex PCR in Surveillance of Imported Malaria in Spain. J Clin Microbiol. 1999;37:3260–3264. doi: 10.1128/jcm.37.10.3260-3264.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gautret P, Legros F, Koulmann P, Rodier MH, Jacquemin JL. Imported Plasmodium vivax malaria in France: geographical origin and report of an atypical case acquired in Central or Western Africa. Acta Trop. 2001;78:177–181. doi: 10.1016/s0001-706x(00)00181-9. [DOI] [PubMed] [Google Scholar]
- 21.Rubio JM, Benito A, Roche J, Berzosa PJ, García ML, et al. Semi-nested, multiplex polymerase chain reaction for detection of human malaria parasites and evidence of Plasmodium vivax infection in Equatorial Guinea. Am J Trop Med Hyg. 1999;1999 Feb;60(2):183–7. doi: 10.4269/ajtmh.1999.60.183. [DOI] [PubMed] [Google Scholar]
- 22.Ryan JR, Stoute JA, Amon J, Dunton RF, Mtalib R, et al. Evidence for transmission of Plasmodium vivax among a duffy antigen negative population in Western Kenya. Am J Trop Med Hyg. 2006;75:575–581. [PubMed] [Google Scholar]
- 23.Cavasini CE, de Mattos LC, Couto AA, Couto VS, Gollino Y, et al. Duffy blood group gene polymorphisms among malaria vivax patients in four areas of the Brazilian Amazon region. Malar J. 2007b;6:167–175. doi: 10.1186/1475-2875-6-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavasini CE, Mattos LC, Couto AA, Bonini-Domingos CR, Valencia SH, et al. Plasmodium vivax infection among Duffy antigen-negative individuals from the Brazilian Amazon region: an exception? Trans R Soc Trop Med Hyg. 2007a;101:1042–1044. doi: 10.1016/j.trstmh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Ménard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, et al. Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci U S A. 2010;107:5967–5971. doi: 10.1073/pnas.0912496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qari SH, Shi YP, Goldman IF, Udhayakumar V, Alpers MP, et al. Identification of Plasmodium vivax-like human malaria parasite. Lancet. 1993;341:780–783. doi: 10.1016/0140-6736(93)90559-y. [DOI] [PubMed] [Google Scholar]
- 27.Kappe SH, Buscaglia CA, Nussenzweig V. Plasmodium sporozoite molecular cell biology. Annu Rev Cell Dev Biol. 2004;20:29–59. doi: 10.1146/annurev.cellbio.20.011603.150935. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg R, Wirtz RA, Lanar DE, Sattabongkot J, Hall T, et al. Circumsporozoite protein heterogeneity in the human malaria parasite Plasmodium vivax. Science. 1989;245:973–976. doi: 10.1126/science.2672336. [DOI] [PubMed] [Google Scholar]
- 29.Souza-Neiras WC, Storti-Melo LM, Cassiano GC, Couto VS, Couto AA, et al. Plasmodium vivax circumsporozoite genotypes: a limited variation or new subspecies with major biological consequences? Malar J. 2010;9:178. doi: 10.1186/1475-2875-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plowe CV, Djimde A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–568. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 31.Arez AP, Lopes D, Pinto J, Franco AS, Snounou G, et al. – Plasmodium sp.: Optimal protocols for PCR amplification from mosquito (Anopheles sp.) samples. Exp Parasitol. 2000;94:269–272. doi: 10.1006/expr.2000.4496. [DOI] [PubMed] [Google Scholar]
- 32.Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58:283–292. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- 33.Alves RT, Póvoa MM, Goldman IF, Cvasini CE, Rossit ARB, et al. A new polymerase chain/restriction fragment length polymorphism protocol for Plasmodium vivax circumsporozoite protein genotype (VK210, VK247 and P. vivax–like) determination. 2007;59:415–419. doi: 10.1016/j.diagmicrobio.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Barcus MJ, Basri H, Picarima H, Manyakori C, Sekartuti, et al. Demographic risk factors for severe and fatal vivax and falciparum malaria among hospital admissions in northeastern Indonesian Papua. Am J Trop Med Hyg. 2007;77:984–991. [PubMed] [Google Scholar]
- 35.Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, et al. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77:79–87. [PMC free article] [PubMed] [Google Scholar]
- 36.Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, et al. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. 2009;80:194–198. [PubMed] [Google Scholar]
- 37.Gautret P, Legros F, Koulmann P, Rodier MH, Jacquemin JL. Imported Plasmodium vivax malaria in France: geographical origin and report of an atypical case acquired in Central or Western Africa. Acta Trop. 2001;78:177–181. doi: 10.1016/s0001-706x(00)00181-9. [DOI] [PubMed] [Google Scholar]
- 38.Snounou G, Pinheiro L, Antunes AM, Ferreira C, do Rosario VE. Nonimmune patients in the Democratic Republic of Sao Tome e Principe reveal a high level of transmission of P. ovale and P. vivax despite low frequency in immune patients. Acta Trop. 1998;70:197–203. doi: 10.1016/s0001-706x(98)00021-7. [DOI] [PubMed] [Google Scholar]
- 39.Poirriez J, Landau I, Verhaeghe A, Savage A, Dei-Cas E. [Atypical forms of Plasmodium vivax. Apropos of a case]. Ann Parasitol Hum Comp. 1991;66:149–154. doi: 10.1051/parasite/1991664149. [DOI] [PubMed] [Google Scholar]
- 40.Collins WE, Roberts JM. Anopheles gambiae as a host for geographic isolates of Plasmodium vivax. 1991;7:569–573. [PubMed] [Google Scholar]
- 41.Michon P, Stevens JR, Kaneko O, Adams JH. Evolutionary relationships of conserved cysteine-rich motifs in adhesive molecules of malaria parasites. Mol Biol Evol. 2002;19:1128–42. doi: 10.1093/oxfordjournals.molbev.a004171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
STROBE checklist.
(DOC)