Abstract
Background
Albuminuria has been associated with cardiovascular risk, but the relationship of high-normal albuminuria to subsequent heart failure has not been well established.
Study Design
Prospective observational study, the Atherosclerosis Risk in Communities (ARIC) Study.
Setting & Participants
10,975 individuals free from heart failure were followed up from the fourth ARIC Study visit (1996–1998) through January 2006.
Predictor
Urinary albumin-creatinine ratio (UACR), analyzed continuously and categorically as optimal (<5 mg/g), intermediate-normal (5–9 mg/g), high-normal (10–29 mg/g), microalbuminuria (30–299 mg/g), and macroalbuminuria (≥300 mg/g).
Outcomes & Measurements
Incident heart failure was defined as a heart failure–related hospitalization or death. Cox proportional hazard models were used to calculate the HR of heart failure after adjustment for age, race, sex, estimated glomerular filtration rate (eGFR), and other cardiovascular risk factors.
Results
Individuals were followed up for a median of 8.3 years and experienced 344 heart failure events. Compared with normal UACR, albuminuria was associated with a progressively increased risk of heart failure from intermediate-normal (adjusted HR, 1.54; 95% CI, 1.12–2.11) and high-normal UACR (adjusted HR, 1.91; 95% CI, 1.38–2.66) to microalbuminuria (adjusted HR, 2.49; 95% CI, 1.77–3.50) and macroalbuminuria (adjusted HR, 3.47; 95% CI, 2.10–5.72). Results were similar in secondary analyses of participants censored at the time of coronary heart disease event and along a range of eGFRs.
Limitations
UACR was measured as a single random sample.
Conclusions
Albuminuria is associated with subsequent heart failure, even in individuals with few cardiovascular risk factors and UACR within the normal range. Our results suggest that the association between albuminuria and heart failure may not be mediated fully by ischemic heart disease or kidney disease, measured using eGFR.
INDEX WORDS: Albuminuria, urinary albumin-creatinine ratio, heart failure, epidemiology
Albuminuria is a marker of kidney damage1 and has been associated with heart failure, possibly due to its correlation with vascular damage2 and activation of the renin-angiotensin-aldosterone system.3 Although albuminuria generally has been defined at albumin levels ≥30 mg/d (or urinary albumin-creatinine ratio [UACR] ≥30 mg/g), previous research suggests that more modest increases in albuminuria may be associated with poor clinical outcomes.3–5 Albuminuria, even at levels considered to be high-normal, has been associated with increased rates of cardiovascular disease.6–9 Although albuminuria has been associated with heart failure in high-risk individuals in clinical trials,7,10–12 the association of lower levels of albuminuria and incident heart failure has been less well established in the community, particularly in a racially diverse sample.13
Our primary hypothesis was that albuminuria, even at levels considered normal, would be associated with incident heart failure events in a community-based sample, the Atherosclerosis Risk in Communities (ARIC) Study. Additionally, we explored whether the relationship between albuminuria and heart failure was mediated by known risk factors, including coronary heart disease (CHD) and kidney disease, measured using estimated glomerular filtration rate (eGFR).14
METHODS
Study Design and Population
The ARIC Study is a prospective cohort study of the cause and outcomes of cardiovascular disease in 15,792 individuals from 4 communities (Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and Washington County, MD). Individuals aged 45–64 years were recruited using probability sampling for the initial evaluation in 1987–1989.15 Three additional study examinations were performed approximately every 3 years. For this study, we included all participants who had a urinary albumin and creatinine sample, which was collected during the fourth study visit (1996–1998). Exclusion criteria included individuals with a history of heart failure, eGFR <30 mL/min/1.73 m2, or self-reported history of dialysis or transplant. We also excluded individuals who self-reported race as neither white nor black given their small numbers in the ARIC cohort (n = 48 at visit 1). The ARIC Study was approved by the institutional review boards at each site. Informed consent was provided by all study participants.
Assessment of Albuminuria and Covariates
Level of albuminuria was calculated using the UACR from a random urine sample.16 Urine albumin was measured using a nephelometric method on either the Dade Behring BN100 (Dade Behring Inc, www.dadebehring.com) or Beckman Image Nephelometer (Beckman Coulter Inc, www.beckmancoulter.com), and urine creatinine was measured using the Jaffé method. Quality control was assessed on 516 blinded samples, with a correlation coefficient of r = 0.95 for log-transformed UACR.16 We analyzed UACR as both a continuous and categorical variable, with categories of optimal (<5 mg/g), intermediate-normal (5–9 mg/g), high-normal (10–29 mg/g), microalbuminuria (30–299 mg/g), and macroalbuminuria (≥300 mg/g). The cutoff for high-normal was chosen because it was near the population mean17 and has been examined previously as a cutoff for cardiovascular prediction.18
All laboratory measurements and covariates were obtained from visit 4 through the standard ARIC protocol.19 eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation20 and categorized as 30–44, 45–59, 60–74, 75–89, 90–104, and ≥105 mL/min/1.73 m2. Education level was classified as basic (≤11 years), intermediate (12–16 years), and advanced (≥17 years). Smoking was categorized as current smoker, previous smoker, and never smoker. Alcohol use was dichotomized as current drinker and previous/never drinker. Body mass index was determined by dividing weight in kilograms by height in meters squared. Participant medications were reviewed to determine current use. Blood pressure was measured by a certified technician using a random-zero sphygmomanometer. Individuals with blood pressure ≥140/90 mm Hg or who were using antihypertensive medication were considered to have prevalent hypertension. Pulse pressure was calculated by taking the difference of systolic and diastolic blood pressures. History of diabetes was defined as fasting blood glucose level ≥126 mg/dL, nonfasting glucose level ≥200 mg/dL, report of a physician diagnosis of diabetes, or current use of diabetes medication. CHD was defined as history of myocardial infarction, silent myocardial infarction, coronary artery bypass surgery, or angioplasty and was adjudicated by the end points committee.
Assessment of Heart Failure
Individuals were considered to have prevalent heart failure if they met any of the following criteria: heart failure hospitalization before visit 4, symptomatic heart failure using Gothenberg (stage 3) criteria21,22 assessed at ARIC visit 1 or 2, or medication use for heart failure at any ARIC Study visit. Participants were not asked about clinical symptoms at visits 3 and 4; thus, symptomatic heart failure could not be assessed at these visits. However, intervening heart failure hospitalizations were available.
In individuals free of heart failure at baseline (visit 4), incident heart failure was defined as the first heart failure hospitalization or death from heart failure for those without a prior heart failure hospitalization. Heart failure diagnosis was established using International Classification of Disease, Ninth Revision (ICD-9) code 428 or Tenth Revision (ICD-10) code I50 in the first position.23 Additionally, to improve sensitivity of our outcome, we performed a secondary analysis using a definition of heart failure based on ICD-9 code 428 in any position,24 as in previous ARIC studies.25,26 Individuals were followed up for events through January 1, 2006.
Statistical Analysis
Baseline characteristics were compared using χ2 tests for categorical variables and analysis of variance for continuous variables. Incidence graphs were created using the Kaplan-Meier method. Differences between groups were compared using log-rank test. Hazard ratios (HRs) with multivariate adjustment were estimated using a Cox proportional hazard model. The proportionality assumption was evaluated graphically using the −log(−log-[survival function]) plot and statistically using the Schoenfeld goodness-of-fit test. Follow-up time was defined from study visit 4 until time of heart failure hospitalization, death, or January 1, 2006.
UACR was evaluated as both a categorical and continuous variable. For the continuous model, UACR was log-transformed given a non-normal distribution and examined graphically using a linear spline model with knots at 10, 30, and 300 mg/g. To eliminate the influence of very high levels of albuminuria on its association with heart failure, we performed a sensitivity analysis excluding participants with UACR ≥30 mg/g.
We assessed potential interactions between UACR categories and sex, race, hypertension status, diabetes status, prevalent CHD, and eGFR category using likelihood ratio tests. Because albuminuria has been a marker of disease severity in diabetes and hypertension, both of which are major clinical risk factors for heart failure,14 we also examined whether albuminuria was independently associated with incident heart failure in subgroups of individuals with and without these 2 conditions. To minimize the influence of CHD as a mediator of the association of low levels of albuminuria on heart failure, we analyzed the association between individuals free from CHD at baseline and who were censored at CHD event during study follow-up. Finally, we examined the independent relationship of albuminuria on heart failure after stratification by eGFR because both now are recognized to be important in chronic kidney disease staging.27
Given potential sex and racial differences in albuminuria, we performed sensitivity analyses in which we calculated an adjusted UACR based on these characteristics. Using methods described by Jacobs et al,28 the adjusted UACR was calculated by dividing measured UACR by a factors for both sex (0.68 if male, 1 if female) and race (0.88 if black, 1 if white). This calculated adjusted UACR was categorized as optimal, intermediate-normal, high-normal, microalbuminuria, and macroalbuminuria using the same cutoff values as in the primary analysis. Additionally, we analyzed adjusted UACR as a continuous variable.
Statistical significance was prespecified with α = 0.05 (2 tailed). Statistical analyses were performed using Stata 10 (Stata Statistical Software, release 10; StataCorp, www.stata.com).
RESULTS
Of 11,656 participants who presented for the fourth study visit, 10,975 were included in the analysis because of exclusions for prevalent heart failure (n = 455), missing UACR information (n = 160), history of dialysis or transplant (n = 6), eGFR <30 mL/min/1.73 m2 (n = 30), and race other than black or white (n = 30). Compared with individuals included in the study, individuals with missing UACR values had similar characteristics of age, sex, education level, and smoking status, but were more likely to be black, have hypertension, or have diabetes. In study participants, mean ± standard deviation and median UACRs were 22.9 ± 184.9 and 3.7 mg/g, respectively. Most individuals were found to have optimal UACRs (n = 6,733; 61.4% of cohort), with 19.4% (n = 2,128) and 11.8% (n =1,294) of individuals having intermediate-normal and high-normal UACRs, respectively. Prevalences of microalbuminuria (n = 678) and macroalbuminuria (n = 142) were 6.2% and 1.3%, respectively.
Table 1 lists baseline characteristics of participants by UACR category. Higher UACR was associated with older age, lower educational attainment, and higher prevalences of CHD, hypertension, and diabetes. Participants with micro- or macroalbuminuria had on average lower eGFRs and high-density lipoprotein cholesterol levels and higher systolic blood pressures and BMI than participants with normal UACR.
Table 1.
Baseline Characteristics of Participants by UACR Category
| Optimal (<5 mg/g) (n = 6,733) |
Intermediate-Normal (5–9 mg/g) (n = 2,128) |
High-Normal (10–29 mg/g) (n = 1,294) |
Microalbuminuria (30–299 mg/g) (n = 678) |
Macroalbuminuria (≥300 mg/g) (n = 142) |
P Trend | |
|---|---|---|---|---|---|---|
| UACR (mg/g) | 2.1 (1.1, 3.3) | 6.6 (5.6, 7.7) | 14.6 (11.8, 19.5) | 60.0 (41.7, 116.7) | 594 (405, 949) | |
| Age (y) | 62.2 ± 5.5 | 63.0 ± 5.7 | 63.7 ± 5.8 | 64.1 ± 5.8 | 65.4 ± 6.2 | <0.001 |
| Women (%) | 52.5 | 66 | 61.0 | 53.7 | 54.9 | <0.001 |
| Blacks (%) | 23.7 | 13.2 | 18.6 | 36.1 | 40.9 | <0.001 |
| Education level | <0.001 | |||||
| <12th grade | 18.1 | 15.8 | 19.5 | 29.6 | 31.0 | |
| High school degree or college | 42.1 | 44.8 | 40.8 | 37.1 | 43.0 | |
| Graduate school | 40.0 | 39.4 | 39.7 | 33.4 | 26.1 | |
| Smoking status | <0.001 | |||||
| Current smoker | 13.5 | 15.5 | 17.0 | 21.5 | 15.7 | |
| Former smoker | 44.5 | 40.6 | 42.7 | 40.7 | 49.3 | |
| Never smoker | 42.0 | 43.9 | 40.3 | 37.9 | 35.0 | |
| Current drinker (%) | 51.0 | 51.8 | 48.5 | 42.2 | 29.3 | <0.001 |
| Coronary heart disease (%) | 6.3 | 6.7 | 7.7 | 12.8 | 16.6 | <0.001 |
| eGFR | <0.001 | |||||
| 30–44 mL/min/1.73 m2 | 0.7 | 0.9 | 1.5 | 4.4 | 14.8 | |
| 45–59 mL/min/1.73 m2 | 7.4 | 6.7 | 7.0 | 15.3 | 18.3 | |
| 60–74 mL/min/1.73 m2 | 26.9 | 24.1 | 23.2 | 22.1 | 23.2 | |
| 75–89 mL/min/1.73 m2 | 37.0 | 37.8 | 36.8 | 28.6 | 21.1 | |
| 90–104 mL/min/1.73 m2 | 23.7 | 26.8 | 26.8 | 21.2 | 16.9 | |
| >105 mL/min/1.73 m2 | 4.4 | 3.6 | 4.7 | 8.3 | 5.6 | |
| SBP (mm Hg) | 124.4 ± 17.0 | 128.2 ± 19.1 | 133.3 ± 19.9 | 140.2 ± 22.3 | 145.6 ± 24.1 | <0.001 |
| Pulse pressure (mm Hg) | 54.0 ± 14.4 | 57.3 ± 16.0 | 60.5 ± 16.8 | 65.7 ± 18.7 | 71.6 ± 18.4 | <0.001 |
| Hypertension (%) | 39.9 | 47.7 | 59.4 | 73.1 | 90.8 | <0.001 |
| Diabetes (%) | 12.3 | 13.5 | 21.3 | 37.9 | 59.4 | <0.001 |
| Diuretic use (%) | 15.6 | 17.4 | 19.6 | 26.6 | 39.4 | <0.001 |
| ACEi or ARB use (%) | 11.2 | 11.8 | 16.2 | 20.8 | 44.4 | <0.001 |
| Statin use (%) | 10.0 | 10.4 | 12.0 | 13.9 | 21.8 | <0.001 |
| BMI (kg/m2) | 28.8 ± 5.3 | 28.1 ± 5.6 | 28.6 ± 5.8 | 29.6 ± 6.3 | 30.4 ± 6.2 | <0.001 |
| LDL cholesterol (mg/dL) | 123.3 ± 33.3 | 122.8 ± 32.8 | 121.9 ± 33.0 | 120.6 ± 34.7 | 124.5 ± 36.8 | 0.2 |
| HDL cholesterol (mg/dL) | 49.8 ± 16.1 | 51.9 ± 16.9 | 50.6 ± 17.3 | 48.7 ± 17.5 | 47.9 ± 16.2 | <0.001 |
Note: Baseline corresponds to Atherosclerosis Risk in Communities (ARIC) Study visit 4. Continuous variables expressed as median (25th, 75th percentile) or mean ± standard deviation; categorical variables, as percentage. Conversion factors for units: eGFR in mL/min/1.73 m2 to mL/s/1.37 m2, ×0.01667; LDL and HDL cholesterol in mg/dl to mmol/L, ×0.02856.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; UACR, urinary albumin-creatinine ratio.
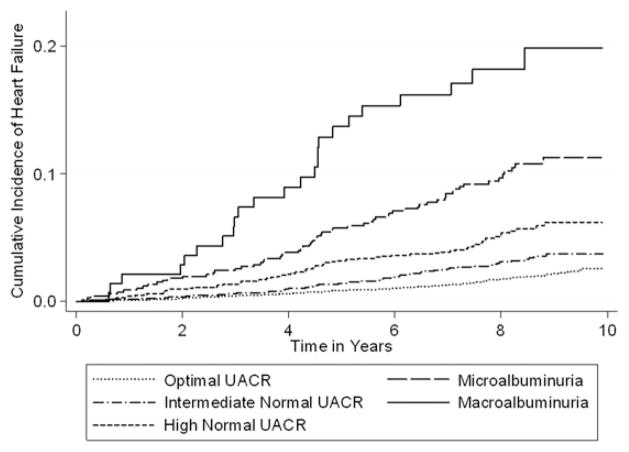
Participants were followed up for a median of 8.3 years and experienced 344 incident heart failure events for an incident rate of 3.9 events/1,000 person-years. Incidence rates progressively increased with higher levels of UACR (P <0.001 for trend). After adjustment for covariates, intermediate-normal UACR was associated with a 54% increase in the relative hazard of heart failure compared with normal UACR, whereas high-normal UACR was associated with a 91% increase in the relative hazard of heart failure (Table 2). Figure 1 shows the incidence of heart failure; survival curves were statistically different both globally (P < 0.001) and among all individual curves (P < 0.01 for all comparisons).
Table 2.
Incident Heart Failure Events by UACR Category
| UACR Category | No. | Median Follow-up (y) | Incident Heart Failure (%) | Incidence Rate (/1,000 person-years) | Relative Hazard (95% CI)
|
|||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model 2a | Model 3b | Fully Adjustedc | |||||
| Optimal (<5 mg/g) | 6,733 | 8.4 | 126 (1.9) | 2.3 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Intermediate-normal (5–9 mg/g) | 2,128 | 8.1 | 65 (3.1) | 3.8 | 1.70 (1.26–2.30) | 1.74 (1.29–2.36) | 1.55 (1.13–2.11) | 1.54 (1.12–2.11) |
| High-normal (10–29 mg/g) | 1,294 | 8.0 | 65 (5.0) | 6.5 | 2.89 (2.14–3.90) | 2.69 (1.99–3.64) | 2.11 (1.54–2.90) | 1.91 (1.38–2.66) |
| Microalbuminuria (30–299 mg/g) | 678 | 8.0 | 64 (9.4) | 12.7 | 5.69 (4.21–7.68) | 4.70 (3.47–6.36) | 3.00 (2.17–4.14) | 2.49 (1.77–3.50) |
| Macroalbuminuria (≥300 mg/g) | 142 | 7.7 | 24 (16.9) | 24.8 | 11.29 (7.30–17.48) | 8.16 (5.24–12.71) | 4.49 (2.83–7.13) | 3.47 (2.10–5.72) |
Abbreviations: CI, confidence interval; UACR, urinary albumin-creatinine ratio.
Adjusted for age, sex, and race.
Adjusted for age, sex, race, coronary heart disease, hypertension, and diabetes.
Adjusted for age, sex, race, education attainment, smoking status, drinking status, coronary heart disease, diuretic use, use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, hypertension, diabetes, statin use, systolic blood pressure, pulse pressure, body mass index, low- and high-density lipoprotein cholesterol levels, and estimated glomerular filtration rate.
Figure 1.
Incidence of heart failure by albuminuria category in 10,975 Atherosclerosis Risk in Communities (ARIC) Study participants. Albuminuria categories were based on urinary albumin-creatinine ratios (UACRs) as macroalbuminuria (UACR ≥300 mg/g), microalbuminuria (UACR <300 to ≥30 mg/g), high-normal (<30 to ≥10 mg/g), intermediate-normal (<10 to ≥5 mg/g), and optimal (<5 mg/g). P < 0.01 for differences among curves using the log-rank test.
In the analysis of UACR as a continuous log-transformed variable, the relative hazard of heart failure was 1.15 (95% confidence interval [CI], 1.10–1.21) for each doubling of UACR (eg, 20 vs 10 or 10 vs 5 mg/g). The risk associated with doubling of UACR was similar after exclusion of individuals with micro- or macroalbuminuria (HR, 1.20; 95% CI, 1.10–1.31). Overall, we found a linear relationship between level of albuminuria and relative hazard of the development of heart failure. Figure 2 shows the linear spline model of continuous UACR with knots at 10, 30, and 300 mg/g. The relative hazard begins to increase linearly from the baseline value of 1 mg/g.
Figure 2.
Adjusted relative hazard of heart failure by continuous level of urinary albumin-creatinine ratio (UACR) in 10,975 Atherosclerosis Risk in Communities (ARIC) Study participants. Reference point is UACR of 1 mg/g. Graph represents a linear spline model, with spline terms at UACRs of 10, 300, and 300 mg/g. Shading represents the 95% confidence interval. Hazard ratio (HR) adjusted for age, sex, race, education attainment, smoking status, drinking status, coronary heart disease, hypertension, diabetes, systolic blood pressure, pulse pressure, use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, use of diuretic, use of statin therapy, body mass index, low- and high-density lipoprotein cholesterol level, and estimated glomerular filtration rate.
In the secondary analysis using the more sensitive definition of heart failure, there were 752 incident cases of heart failure over a median of 8.2 years, for an incident rate of 8.7 cases/1,000 person-years. The incidence of heart failure appeared to increase with each category of UACR (P < 0.001 for trend; Fig S1, available as online supplementary material). After adjustment for covariates, the HR of heart failure was significantly increased in individuals with intermediate-normal UACR (1.25; 95% CI, 1.01–1.55), high-normal UACR (1.68; 95% CI, 1.34–2.11), microalbuminuria (2.35; 95% CI, 1.86–2.96), and macroalbuminuria (4.56; 95% CI, 3.29–6.31; Table S1).
No interaction was found between UACR category and race, sex, diabetes, hypertension, or CHD (P > 0.1 for all comparisons). In subgroup analysis, continuous UACR was associated with increased risk of incident heart failure in individuals with and without hypertension, with and without diabetes, with and without baseline CHD, and free from CHD during follow up (Table 3). This relationship persisted even after excluding individuals with micro- or macroalbuminuria. In individuals with UACR <30 mg/g who were free from diabetes and hypertension and censored at the time of the CHD event, doubling of UACR was associated with a 49% increase in the relative hazard of heart failure.
Table 3.
Adjusted HR of Heart Failure for Doubling of UACR
| No. of Participants | HR (95% CI)
|
||
|---|---|---|---|
| All Participants | Participants With UACR <30 mg/ga | ||
| Full sample | 10,975 | 1.15 (1.10–1.21) | 1.20 (1.10–1.31) |
| Prevalent diabetes | 1,716 | 1.15 (1.08–1.22) | 1.15 (0.99–1.32) |
| No diabetes | 9,177 | 1.17 (1.10–1.24) | 1.24 (1.11–1.40) |
| Prevalent hypertension | 5,074 | 1.14 (1.09–1.21) | 1.15 (1.03–1.29) |
| No hypertension | 5,860 | 1.19 (1.09–1.32) | 1.27 (1.09–1.47) |
| No diabetes or hypertension | 5,227 | 1.12 (0.97–1.29) | 1.35 (1.11–1.63) |
| Prevalent CHD | 763 | 1.15 (1.05–1.26) | 1.31 (1.06–1.63) |
| No CHD | 10,001 | 1.15 (1.10–1.21) | 1.18 (1.07–1.30) |
| Censored if CHD | 10,001 | 1.18 (1.11–1.25) | 1.20 (1.07–1.34) |
| No hypertension or diabetes, censored at CHD event | 4,894 | 1.22 (1.04–1.45) | 1.49 (1.19–1.87) |
Note: Adjusted for age, sex, race, education attainment, smoking status, drinking status, coronary heart disease, diuretic use, use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, hypertension, diabetes, statin use, systolic blood pressure, pulse pressure, body mass index, low- and high-density lipoprotein cholesterol levels, and estimated glomerular filtration rate. Values estimated from a model with UACR as a continuous variable.
Abbreviations: CHD, coronary heart disease; CI, confidence interval; HR, hazard ratio; UACR, urinary albumin-creatinine ratio.
Excludes participants with microalbuminuria (UACR, 30 to 299 mg/g) and macroalbuminuria (UACR ≥300 mg/g).
No interaction was found between UACR and eGFR category (P = 0.4). Subgroup analysis by eGFR category showed that increased levels of albuminuria were associated with increased risk of incident heart failure along categories of eGFR (Table 4). This association was particularly evident in the analysis that used the more sensitive definition of heart failure, resulting in increased power (Table S2). In this analysis, the trend for association of albuminuria and incident heart failure was highly significant along all categories of eGFR, with the exception of eGFR >120 mL/min/1.73 m2 (P trend =0.2; P trend <0.01 for all other categories).
Table 4.
Adjusted Relative Hazard of Heart Failure by Category of UACR and eGFR
| eGFR (mL/min/1.73 m2) | No. | UACR (mg/g)
|
|||||
|---|---|---|---|---|---|---|---|
| <5 | 5–9 | 10–29 | 30–299 | >300 | Total | ||
| ≥105 | 499 | 1.2 (0.5, 2.9) | 2.0 (0.6, 6.7) | 0.6 (0.1, 4.4) | 1.2 (0.2, 5.1) | 3.3 (0.4, 25.0) | 0.8 (0.4, 1.5) |
| 90–104 | 2,705 | Ref (for joint analysis) | 2.3 (1.2, 4.2) | 1.6 (0.7, 3.4) | 3.2 (1.5,6.7) | 3.1 (0.9,10.5) | Ref (for this column) |
| 75–89 | 4,030 | 0.8 (0.5, 1.3) | 1.4 (0.7, 2.6) | 1.9 (1.0, 3.5) | 1.5 (0.7, 3.2) | 2.6 (0.8, 8.9) | 0.7 (0.5, 1.0) |
| 60–74 | 2,824 | 1.4 (0.8, 2.3) | 1.3 (0.6, 2.6) | 2.5 (1.3, 4.9) | 1.5 (0.6, 3.7) | 4.9 (1.8, 13.4) | 1.0 (0.7, 1.4) |
| 45–59 | 890 | 1.1 (0.5, 2.2) | 1.7 (0.7, 4.1) | 2.5 (1.1, 5.7) | 5.5 (3.0, 10.4) | 5.9 (2.2, 16.1) | 1.4 (1.2.0, 2.1) |
| 30–44 | 144 | 2.5 (0.7, 8.5) | 2.8 (0.6, 12.1) | 6.0 (2.0, 17.8) | 8.7 (3.8, 19.9) | 7.4 (2.7, 20.4) | 3.2 (1.9, 5.3) |
| All | 10,975 | Ref (for this row) | 1.6 (1.1, 2.1) | 2.0 (1.4, 2.7) | 2.7 (1.9, 3.8) | 4.2 (2.6, 6.9) | |
Note: Hazard ratio adjusted for age, sex, race, education attainment, smoking status, drinking status, coronary heart disease, hypertension, diabetes, systolic blood pressure, pulse pressure, use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, use of diuretic, use of statin therapy, low- and high-density lipoprotein cholesterol level, and body mass index. Column totals represent the adjusted hazard ratio for the UACR category compared with UACR <5 mg/g. Row totals represent the adjusted hazard ratio for the eGFR category compared with eGFR of 90–104 mL/min/1.73 m2. Conversion factor eGFR in mL/min/1.73 m2 to mL/s/1.37 m2, ×0.01667.
Abbreviations: eGFR, estimated glomerular filtration rate; ref, reference; UACR, urinary albumin-creatinine ratio.
In the sensitivity analysis using calculated adjusted UACR based on sex and race, we obtained results similar to results in our primary analyses. Adjusted relative hazards of heart failure for intermediate-normal adjusted UACR (1.57; 95% CI, 1.14–2.15), high-normal adjusted UACR (1.95; 95% CI, 1.38–2.67), microalbuminuria (2.46; 95% CI, 1.75–3.48), and macroalbuminuria (3.53; 95% CI, 2.21–5.66) were all increased compared with optimal adjusted UACR. In the analysis of adjusted UACR as a continuous log-transformed variable, the relative hazard of heart failure was 1.15 (95% CI, 1.10–1.21) for each doubling in adjusted UACR.
DISCUSSION
Our findings suggest that albuminuria is an independent risk factor for heart failure in the community. The relationship between UACR and incident heart failure hospitalization or death was linear across the continuum of UACR, with risk present even at levels less than current definitions of albuminuria. This risk was similar in individuals with and without diabetes, hypertension, and incident CHD and across the range of eGFRs.
Of 3 previous community-based studies, 2 showed an association between albuminuria and incident heart failure,13,29 whereas 1 study30 did not. We found albuminuria, even at low levels, to be associated with incident heart failure. Similar to our results, 1 previous study had found a linear relationship between albuminuria and heart failure in a study of European men older than 70 years.13 However, in that study, the linear association was not significant after excluding individuals with macro- or microalbuminuria; thus, the association between albuminuria and heart failure may have been driven by individuals with current definitions of albuminuria. The investigators note that their study may have been underpowered to differentiate risk between high-normal and normal urinary albumin excretion.13 In our study, both intermediate- and high-normal UACR were associated with incident heart failure in a community setting. Furthermore, the relative hazard of heart failure was similar across sex and race categories, which, to our knowledge, has not been examined previously.
We found albuminuria to be associated with heart failure in individuals with diabetes, hypertension, or CHD. Previous clinical trials have found an association between albuminuria and heart failure in individuals at high risk of cardiovascular disease.7,31 Ingelsson et al13 found albuminuria was associated with heart failure in subgroups of individuals free from antihypertensive treatment, diabetes, and myocardial infarction. However, their results were not significant after excluding individuals with currently defined levels of albuminuria.13 In our study, intermediate- and high-normal albuminuria were associated with heart failure in individuals without diabetes and CHD. The association of low-grade albuminuria in individuals without hypertension, diabetes, and CHD was evident in the model of continuous UACR. The finding that low levels of albuminuria are a risk factor for heart failure in low-risk individuals is novel.
There are a number of possible mechanisms for the association between albuminuria and heart failure. Albuminuria has been associated with traditional risk factors for heart failure, including hypertension,32 diabetes,9 hyperlipidemia,33 and CHD.5,34 However, our results were significant even after adjustment and exclusion of many of these risk factors. Albuminuria may be associated with activation of the renin-angiotensin-aldosterone system and subsequent cardiovascular effects.3 Alternatively, the increased permeability of renal vessels in albuminuria may be a manifestation of general vascular endothelial dysfunction,2,35 which has been associated with atherosclerosis.36 Microalbuminuria has been associated with small-artery remodeling,37 suggesting that impaired vascular function may represent a common link between albuminuria and cardiovascular disease. Finally, albuminuria has been associated with left ventricular hypertrophy,38,39 which suggests there may be a mechanism for ventricular dysfunction that is not mediated by ischemic disease.13 In agreement, we found albuminuria to be associated with subsequent heart failure events even after censoring individuals at the time of CHD events.
We found a strong relationship between UACR and heart failure that was independent of eGFR. As a marker of decreased kidney function, eGFR is a known risk factor for the development of heart failure.25 However, at normal levels of albuminuria, eGFR was not associated significantly with incident heart failure. Conversely, we found a strong association with UACR and incident heart failure regardless of eGFR. These findings suggest that the mechanism by which kidney function and albuminuria are associated with heart failure may be different.
Although our results indicate that high-normal levels of albuminuria are associated with increased risk of heart failure, they do not provide insight regarding whether interventions to reduce albuminuria will improve outcomes. Previous trials suggest that inhibition of the renin-angiotensin-aldosterone system may decrease the risk of cardiovascular disease in individuals with albuminuria.40–42 The benefit of treatment for individuals with high-normal albuminuria is uncertain.5 Additional trials could determine whether a decrease in these levels of albuminuria will decrease the risk of heart failure in the general population.
In this study, the primary outcome of incident heart failure was based on hospitalization or death and relied on hospital diagnostic coding. This outcome may have excluded individuals with clinical heart failure who were never hospitalized. In a recent clinical trial of individuals with heart failure, albuminuria was associated with increased heart failure hospitalizations.43 Our findings therefore may reflect that albuminuria is associated with increased disease severity for those with an outpatient diagnosis of heart failure. Nonetheless, such results would still have clinical importance from a risk prediction standpoint.
Other limitations of our study include measurement of UACR as a single random sample. Although this method has been recommended,1 it also is subject to considerable variability.44 Nonetheless, any nondifferential misclassification of UACR would more likely bias our results toward the null.38 We were unable to determine the effect of certain markers of risk of heart failure, including echocardiographic parameters, natriuretic peptide levels, and novel markers of inflammation, because these measurements currently are not available in the ARIC Study. Furthermore, we do not have follow-up clinical measurements, including albuminuria, creatinine, and blood pressure, in our data set and thus are unable to observe how changes in these factors affect the relationship between albuminuria and heart failure. Finally, although we found a strong relative risk of association between categories of UACR and incident heart failure, the absolute risk difference was relatively small given our baseline incidence rate in the study population. However, using a more sensitive definition for the outcome, we found the incidence of heart failure to be more than double the rate in our baseline analysis. In the secondary analysis, the relative risk across UACR categories was similar to our baseline analysis, implying a more substantial difference in absolute risk between categories.
Our study has several strengths. The ARIC Study represents a prospective community-based cohort of individuals who are of an age at risk of cardiovascular events. The biracial sample further improves the generalizability of our results. ARIC participants had detailed and standardized characterization of their cardiovascular risk. The large sample size and significant follow-up allowed for various subgroup analyses to assess the risk in important subpopulations.
In conclusion, increased levels of normal albuminuria, defined as UACR of 5–30 mg/g, was associated with incident heart failure events. The risk of heart failure appeared to be linearly related to increases in urinary albumin, with a relative hazard increase of 15% for each doubling of UACR at levels currently considered to be normal. Increased risk of heart failure was present even when censoring individuals at the time of CHD events during follow-up, which suggests that the relationship between UACR and heart failure may not be explained fully by ischemic heart disease. Further research is needed to elucidate the mechanism of the albuminuria–heart failure relationship and address whether interventions to reduce low levels of albuminuria will improve clinical outcomes.
Supplementary Material
Table S1: Number of heart failure events by albuminuria category, using more sensitive definition of heart failure outcome.
Table S2: Adjusted relative hazard of heart failure by category of UACR and eGFR, using more sensitive definition of heart failure outcome.
Figure S1: Incidence of heart failure by albuminuria category among 10,975 ARIC participants, using more sensitive definition of heart failure outcome.
Acknowledgments
We thank the staff and participants of the ARIC Study for important contributions.
Support: The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. Dr Blecker was supported by NHLBI grant 5T32HL007024.
Footnotes
Because the Editor-in-Chief recused himself from consideration of this manuscript, the Deputy Editor (Daniel E. Weiner, MD, MS) served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1–266. [PubMed] [Google Scholar]
- 2.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32(4):219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 3.Dobre D, Nimade S, de Zeeuw D. Albuminuria in heart failure: what do we really know? Curr Opin Cardiol. 2009;24(2):148–154. doi: 10.1097/HCO.0b013e328323aa9a. [DOI] [PubMed] [Google Scholar]
- 4.Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem. 2009;46(pt 3):205–217. doi: 10.1258/acb.2009.009007. [DOI] [PubMed] [Google Scholar]
- 5.Danziger J. Importance of low-grade albuminuria. Mayo Clin Proc. 2008;83(7):806–812. doi: 10.4065/83.7.806. [DOI] [PubMed] [Google Scholar]
- 6.Hallan S, Astor B, Romundstad S, Aasarod K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: the HUNT II Study. Arch Intern Med. 2007;167(22):2490–2496. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 7.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 8.Klausen K, Borch-Johnsen K, Feldt-Rasmussen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension, and diabetes. Circulation. 2004;110(1):32–35. doi: 10.1161/01.CIR.0000133312.96477.48. [DOI] [PubMed] [Google Scholar]
- 9.Arnlov J, Evans JC, Meigs JB, et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112(7):969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 10.Okin PM, Wachtell K, Devereux RB, et al. Combination of the electrocardiographic strain pattern and albuminuria for the prediction of new-onset heart failure in hypertensive patients: the LIFE Study. Am J Hypertens. 2008;21(3):273–279. doi: 10.1038/ajh.2007.66. [DOI] [PubMed] [Google Scholar]
- 11.Carr AA, Kowey PR, Devereux RB, et al. Hospitalizations for new heart failure among subjects with diabetes mellitus in the RENAAL and LIFE studies. Am J Cardiol. 2005;96(11):1530–1536. doi: 10.1016/j.amjcard.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 12.Vaur L, Gueret P, Lievre M, Chabaud S, Passa P DIABHY-CAR Study Group (type 2 DIABetes, Hypertension and CARdiovascular Events and Ramipril) Study. Development of congestive heart failure in type 2 diabetic patients with microalbuminuria or proteinuria: observations from the DIABHYCAR (type 2 DIABetes, Hypertension, CArdiovascular Events and Ramipril) study. Diabetes Care. 2003;26(3):855–860. doi: 10.2337/diacare.26.3.855. [DOI] [PubMed] [Google Scholar]
- 13.Ingelsson E, Sundstrom J, Lind L, et al. Low-grade albuminuria and the incidence of heart failure in a community-based cohort of elderly men. Eur Heart J. 2007;28(14):1739–1745. doi: 10.1093/eurheartj/ehm130. [DOI] [PubMed] [Google Scholar]
- 14.Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117(19):2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 15.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 16.Hsu CC, Brancati FL, Astor BC, et al. Blood pressure, atherosclerosis, and albuminuria in 10,113 participants in the Atherosclerosis Risk in Communities Study. J Hypertens. 2009;27(2):397–409. doi: 10.1097/hjh.0b013e32831aede6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 18.Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collaborative Studies Coordinating Center, Department of Biostatistics, Gillings School of Public Health, University of North Carolina at Chapel Hill. [Accessed September 14, 2010];Atherosclerosis Risk In Communities (ARIC) manuals. http://www.cscc.unc.edu/aric/visit/index.php.
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eriksson H, Caidahl K, Larsson B, et al. Cardiac and pulmonary causes of dyspnoea—validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8(9):1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelmsen L, Eriksson H, Svardsudd K, Caidahl K. Improving the detection and diagnosis of congestive heart failure. Eur Heart J. 1989;10(suppl C):13–18. doi: 10.1093/eurheartj/10.suppl_c.13. [DOI] [PubMed] [Google Scholar]
- 23.Lee DS, Donovan L, Austin PC, et al. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43(2):182–188. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Goff DC, Jr, Pandey DK, Chan FA, Ortiz C, Nichaman MZ. Congestive heart failure in the United States: is there more than meets the I(CD code)? The Corpus Christi Heart Project. Arch Intern Med. 2000;160(2):197–202. doi: 10.1001/archinte.160.2.197. [DOI] [PubMed] [Google Scholar]
- 25.Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2007;18(4):1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 26.Matsushita K, Blecker S, Pazin-Filho A, et al. The association of hemoglobin A1c with incident heart failure among people without diabetes: the Atherosclerosis Risk in Communities Study. Diabetes. 2010;59(8):2020–2026. doi: 10.2337/db10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs DR, Jr, Murtaugh MA, Steffes M, Yu X, Roseman J, Goetz FC. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2002;155(12):1114–1119. doi: 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]
- 29.Bahrami H, Bluemke DA, Kronmal R, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) Study. J Am Coll Cardiol. 2008;51(18):1775–1783. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 30.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-Terminal probrain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293(13):1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 31.Okin PM, Wachtell K, Devereux RB, et al. Combination of the electrocardiographic strain pattern and albuminuria for the prediction of new-onset heart failure in hypertensive patients: the LIFE Study. Am J Hypertens. 2008;21(3):273–279. doi: 10.1038/ajh.2007.66. [DOI] [PubMed] [Google Scholar]
- 32.Winocour PH, Harland JO, Millar JP, Laker MF, Alberti KG. Microalbuminuria and associated cardiovascular risk factors in the community. Atherosclerosis. 1992;93(1–2):71–81. doi: 10.1016/0021-9150(92)90201-q. [DOI] [PubMed] [Google Scholar]
- 33.Campese VM, Bianchi S, Bigazzi R. Association between hyperlipidemia and microalbuminuria in essential hypertension. Kidney Int Suppl. 1999;71:S10–13. [PubMed] [Google Scholar]
- 34.Castells I, Salinas I, Rius F, et al. Inducible myocardial ischaemia in asymptomatic type 2 diabetic patients. Diabetes Res Clin Pract. 2000;49(2–3):127–133. doi: 10.1016/s0168-8227(00)00154-6. [DOI] [PubMed] [Google Scholar]
- 35.de Zeeuw D, Parving HH, Henning RH. Microalbuminuria as an early marker for cardiovascular disease. J Am Soc Nephrol. 2006;17(8):2100–2105. doi: 10.1681/ASN.2006050517. [DOI] [PubMed] [Google Scholar]
- 36.Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol. 2006;17(8):2106–2111. doi: 10.1681/ASN.2005121288. [DOI] [PubMed] [Google Scholar]
- 37.Peralta CA, Katz R, Madero M, et al. The differential association of kidney dysfunction with small and large arterial elasticity: the Multiethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169(6):740–748. doi: 10.1093/aje/kwn392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kramer H, Jacobs DR, Jr, Bild D, et al. Urine albumin excretion and subclinical cardiovascular disease. The Multi-Ethnic Study of Atherosclerosis. Hypertension. 2005;46(1):38–43. doi: 10.1161/01.HYP.0000171189.48911.18. [DOI] [PubMed] [Google Scholar]
- 39.Lieb W, Mayer B, Stritzke J, et al. Association of low-grade urinary albumin excretion with left ventricular hypertrophy in the general population: the MONICA/KORA Augsburg Echocardiographic Substudy. Nephrol Dial Transplant. 2006;21(10):2780–2787. doi: 10.1093/ndt/gfl364. [DOI] [PubMed] [Google Scholar]
- 40.de Zeeuw D, Remuzzi G, Parving HH, et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110(8):921–927. doi: 10.1161/01.CIR.0000139860.33974.28. [DOI] [PubMed] [Google Scholar]
- 41.Asselbergs FW, Diercks GF, Hillege HL, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110(18):2809–2816. doi: 10.1161/01.CIR.0000146378.65439.7A. [DOI] [PubMed] [Google Scholar]
- 42.Ibsen H, Olsen MH, Wachtell K, et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2005;45(2):198–202. doi: 10.1161/01.HYP.0000154082.72286.2a. [DOI] [PubMed] [Google Scholar]
- 43.Jackson CE, Solomon SD, Gerstein HC, et al. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009;374(9689):543–550. doi: 10.1016/S0140-6736(09)61378-7. [DOI] [PubMed] [Google Scholar]
- 44.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Number of heart failure events by albuminuria category, using more sensitive definition of heart failure outcome.
Table S2: Adjusted relative hazard of heart failure by category of UACR and eGFR, using more sensitive definition of heart failure outcome.
Figure S1: Incidence of heart failure by albuminuria category among 10,975 ARIC participants, using more sensitive definition of heart failure outcome.