Abstract
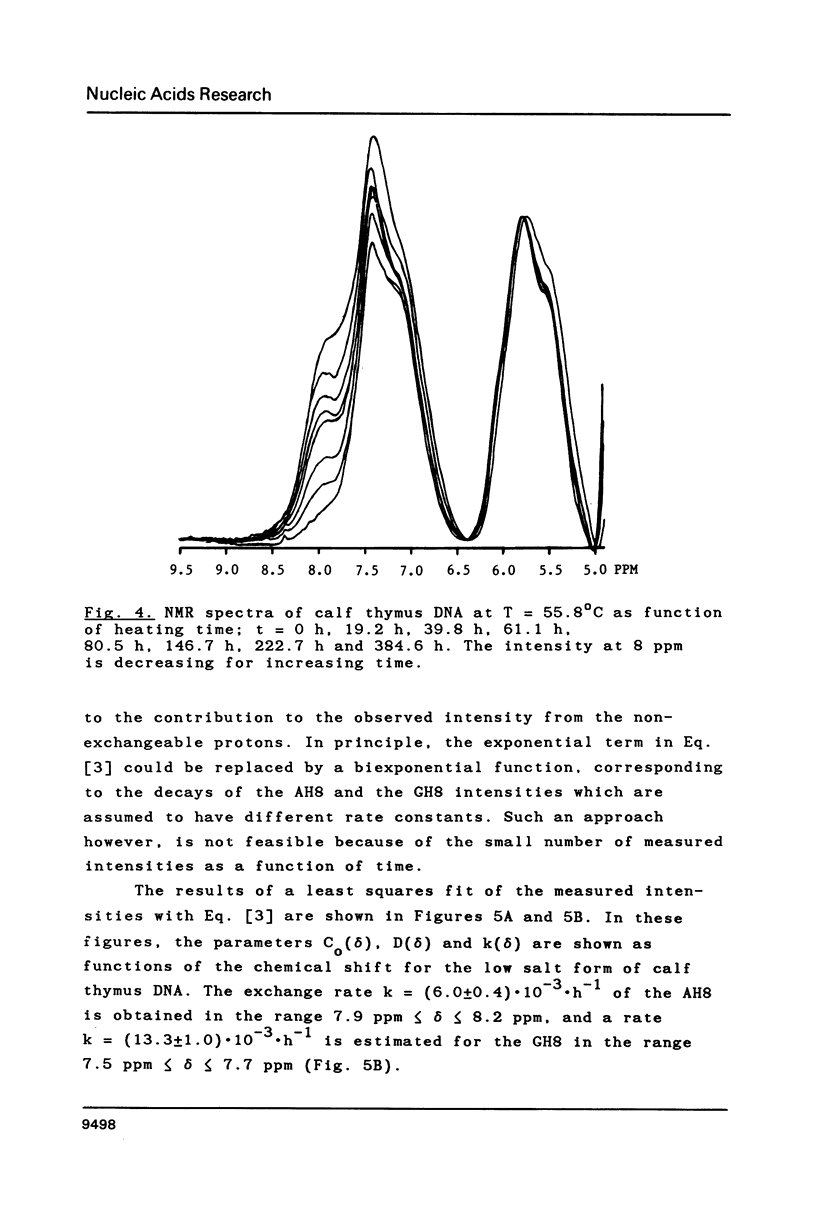
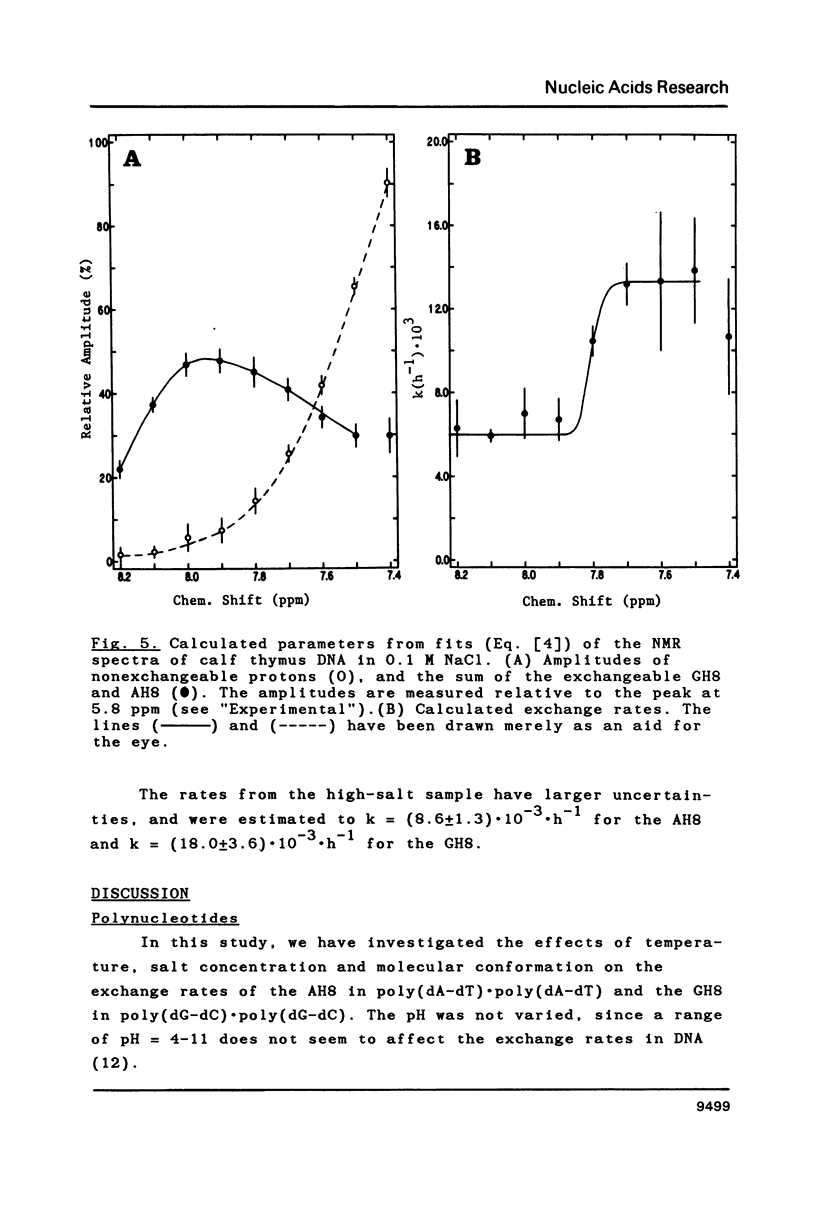
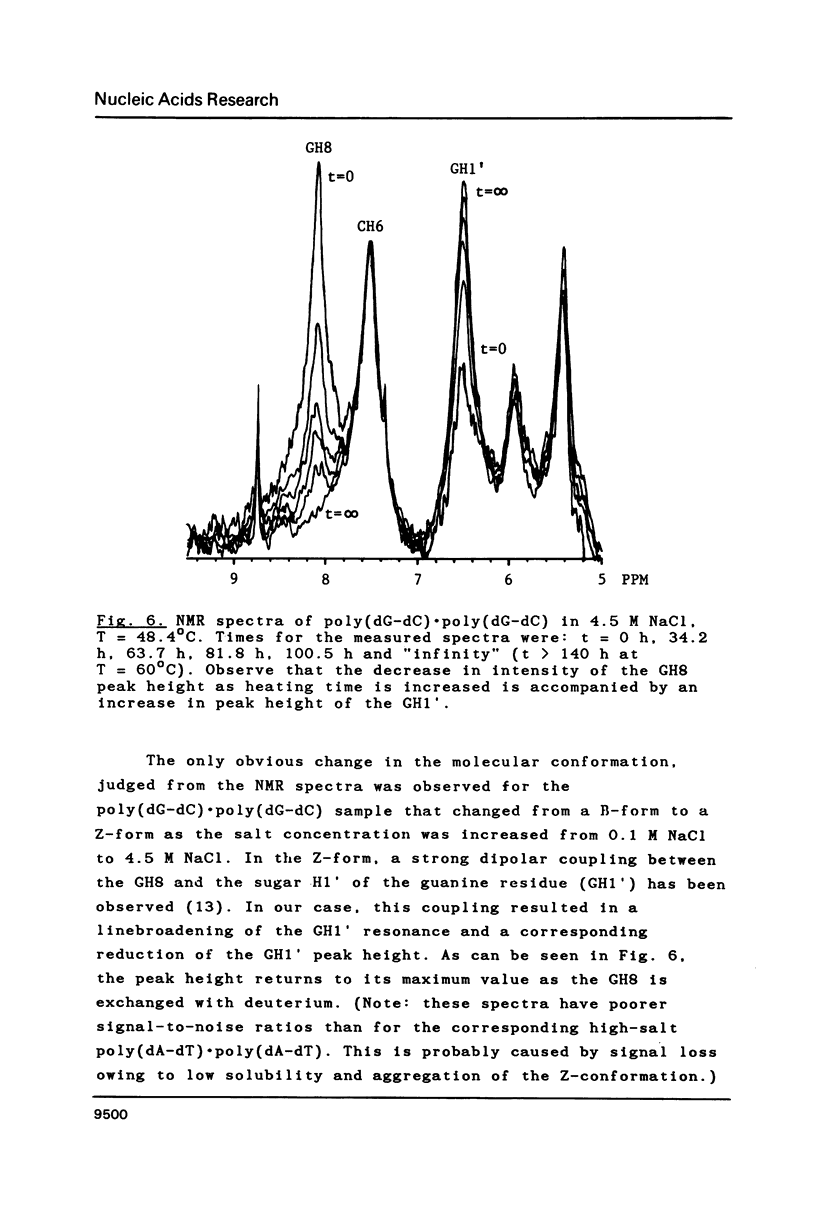
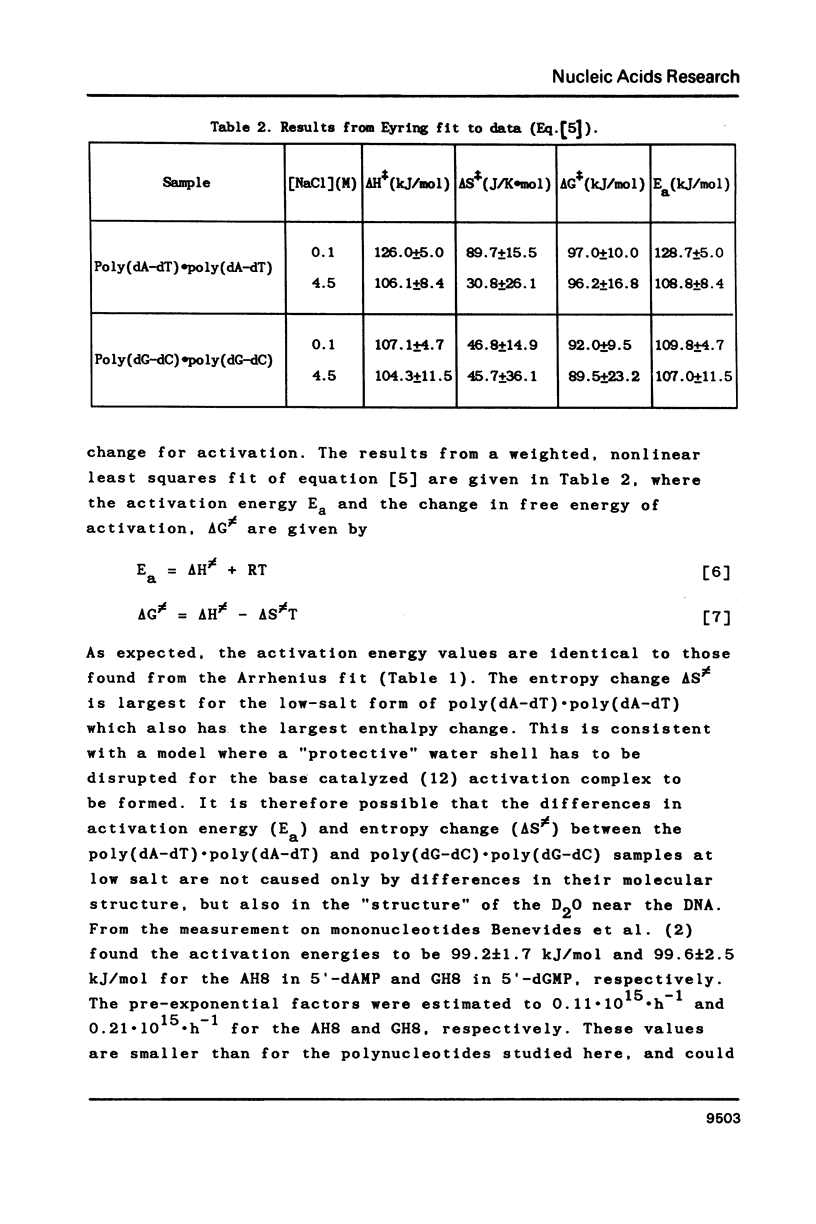
Proton-NMR has been used to determine the activation energies and pre-exponential factors for the deuterium exchange of AH8 in poly(dA-dT).poly(dA-dT), and for GH8 in poly(dG-dC).poly(dG-dC). No simple relationship between the kinetic parameters and molecular conformation was found. By addition of 4.5 M NaCl a transition from the B to the Z conformation was induced for poly(dG-dC).poly(dG-dC), and an increased exchange rate was observed. The exchange rate for poly(dA-dT).poly(dA-dT) also increased below 64 degrees C, and a significant decrease in activation energy on addition of 4.5 M NaCl was observed. The exchange rates at T = 55.8 degrees C were also measured for the AH8 and GH8 in random sequence calf thymus DNA. From the difference in exchange rates, a method of preferential labeling of either the AH8 or the GH8 in high molecular weight DNA is evaluated.
Full text
PDF


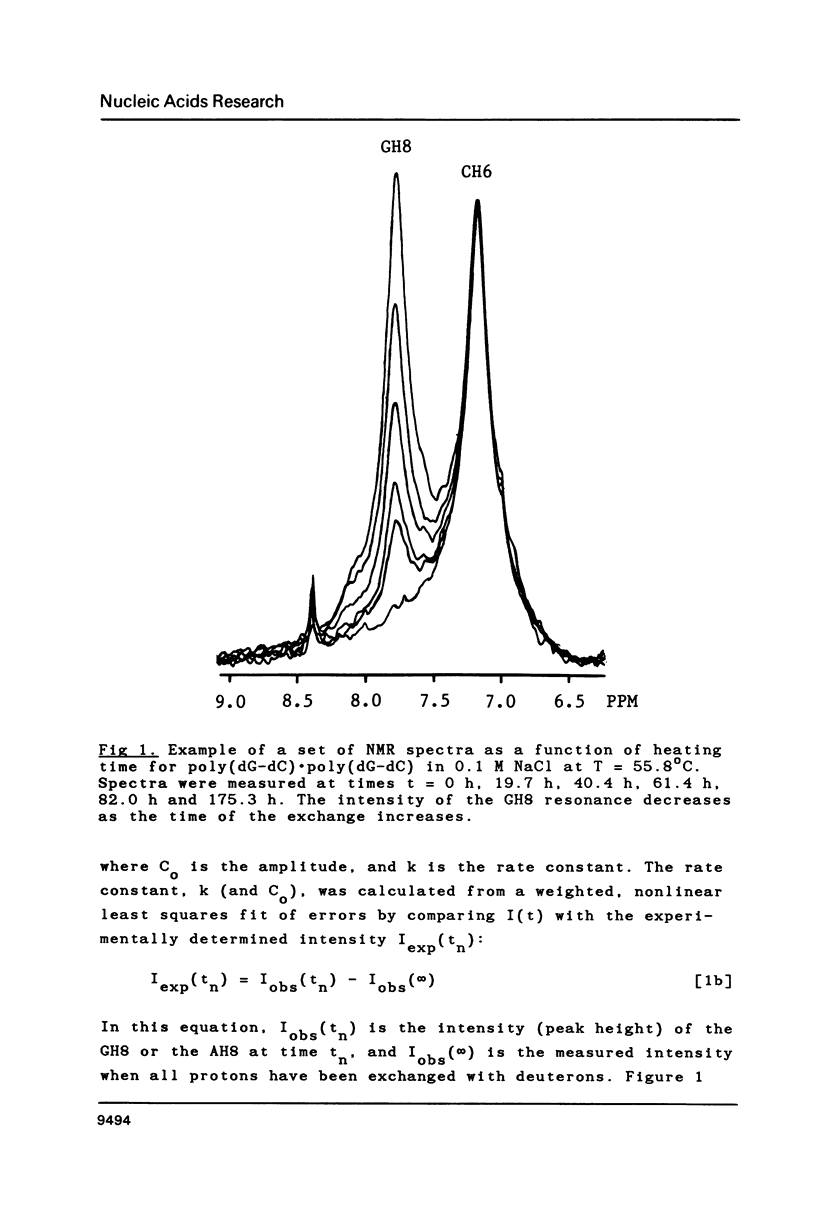
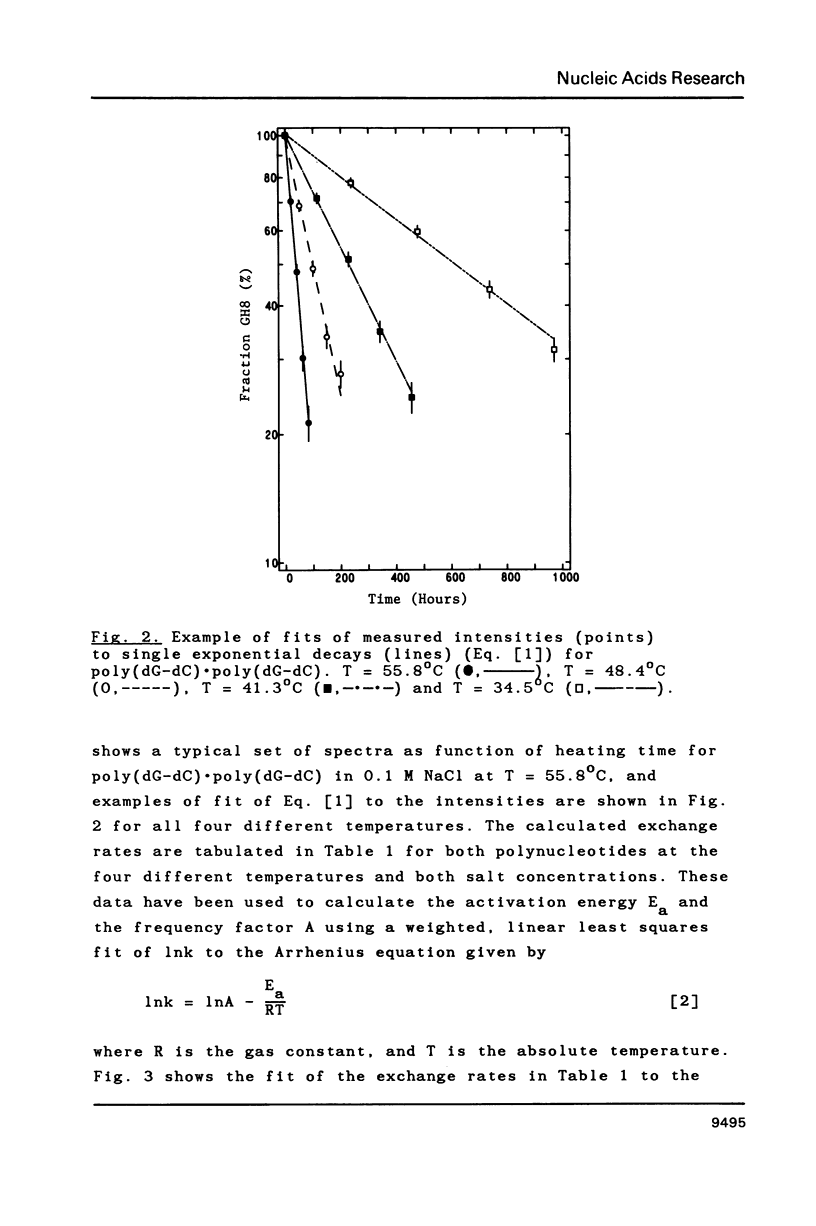
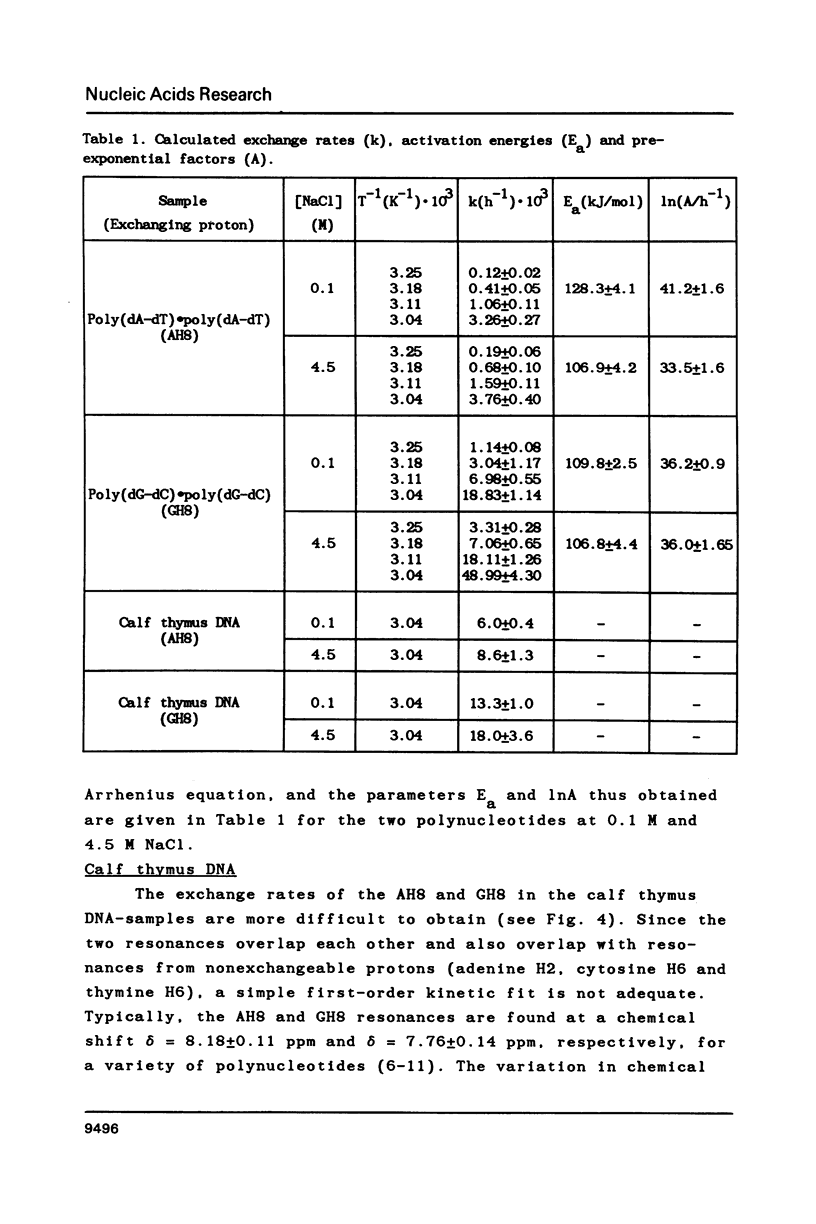
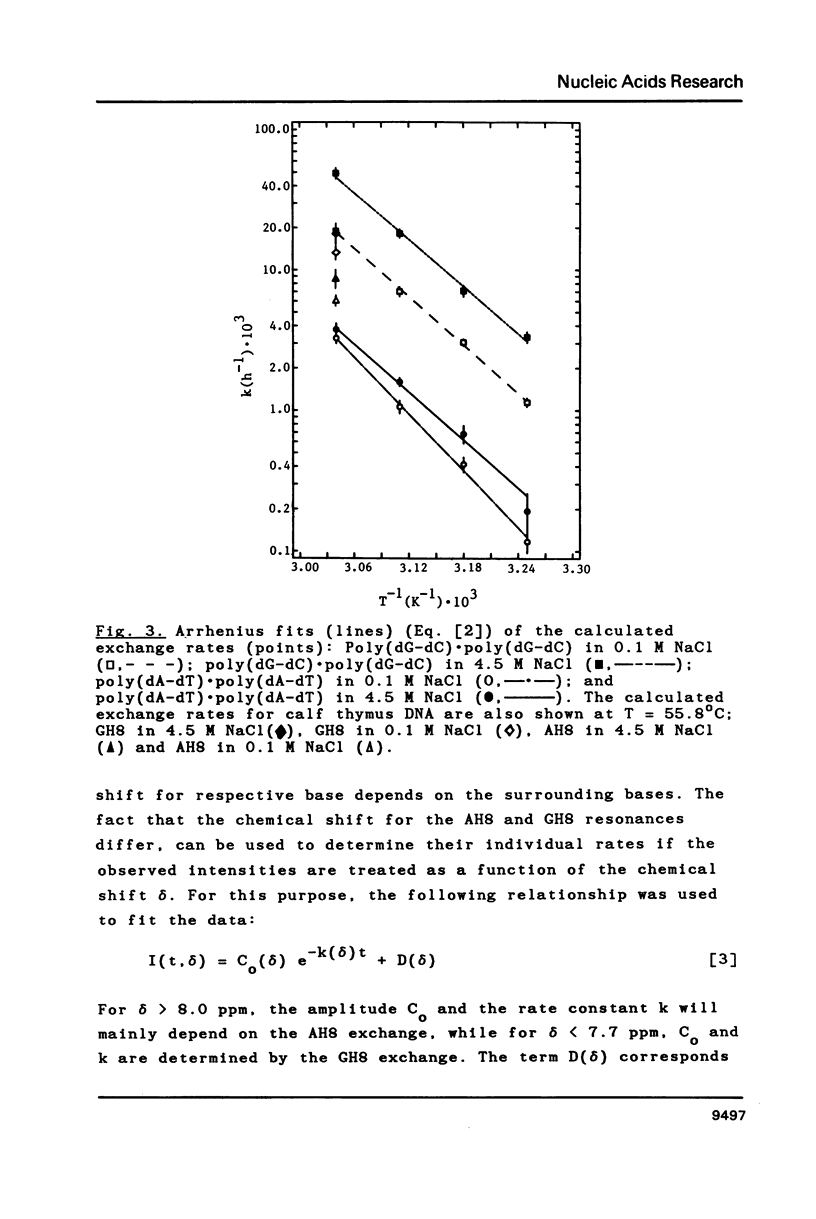






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benevides J. M., Lemeur D., Thomas G. J., Jr Molecular conformations and 8-CH exchange rates of purine ribo- and deoxyribonucleotides: investigation by Raman spectroscopy. Biopolymers. 1984 Jun;23(6):1011–1024. doi: 10.1002/bip.360230604. [DOI] [PubMed] [Google Scholar]
- Benevides J. M., Thomas G. J., Jr Dependence of purine 8C-H exchange on nucleic acid conformation and base-pairing geometry: a dynamic probe of DNA and RNA secondary structures. Biopolymers. 1985 Apr;24(4):667–682. doi: 10.1002/bip.360240407. [DOI] [PubMed] [Google Scholar]
- Borah B., Cohen J. S., Bax A. Conformation of double-stranded polydeoxynucleotides in solution by proton two-dimensional nuclear Overhauser enhancement spectroscopy. Biopolymers. 1985 May;24(5):747–765. doi: 10.1002/bip.360240503. [DOI] [PubMed] [Google Scholar]
- Clementi E., Corongiu G. Interaction of water with DNA single-helix in the a conformation. Biopolymers. 1979 Oct;18(10):2431–2450. doi: 10.1002/bip.1979.360181005. [DOI] [PubMed] [Google Scholar]
- Clore G. M., Lauble H., Frenkiel T. A., Gronenborn A. M. A two-dimensional NMR study of the solution structure of a DNA dodecamer comprising the concensus sequence for the specific DNA-binding sites of the glucocorticoid receptor protein. Eur J Biochem. 1984 Dec 17;145(3):629–636. doi: 10.1111/j.1432-1033.1984.tb08603.x. [DOI] [PubMed] [Google Scholar]
- Feigon J., Leupin W., Denny W. A., Kearns D. R. Two-dimensional proton nuclear magnetic resonance investigation of the synthetic deoxyribonucleic acid decamer d(ATATCGATAT)2. Biochemistry. 1983 Dec 6;22(25):5943–5951. doi: 10.1021/bi00294a038. [DOI] [PubMed] [Google Scholar]
- Goldblum A., Perahia D., Pullman A. Hydration scheme of the complementary base-pairs of DNA. FEBS Lett. 1978 Jul 15;91(2):213–215. doi: 10.1016/0014-5793(78)81175-2. [DOI] [PubMed] [Google Scholar]
- Hare D. R., Wemmer D. E., Chou S. H., Drobny G., Reid B. R. Assignment of the non-exchangeable proton resonances of d(C-G-C-G-A-A-T-T-C-G-C-G) using two-dimensional nuclear magnetic resonance methods. J Mol Biol. 1983 Dec 15;171(3):319–336. doi: 10.1016/0022-2836(83)90096-7. [DOI] [PubMed] [Google Scholar]
- Mirau P. A., Behling R. W., Kearns D. R. Internal motions in B- and Z-form poly(dG-dC).poly(dG-dC): 1H NMR relaxation studies. Biochemistry. 1985 Oct 22;24(22):6200–6211. doi: 10.1021/bi00343a026. [DOI] [PubMed] [Google Scholar]
- Mirau P. A., Kearns D. R. Comparison of the conformation of poly(dI-dC) with poly(dI-dbr5C) and the B and Z forms of poly(dG-dC). One- and two-dimensional NMR studies. Biochemistry. 1984 Nov 6;23(23):5439–5446. doi: 10.1021/bi00318a010. [DOI] [PubMed] [Google Scholar]
- Perahia D., Jhon M. S., Pullman B. Theoretical study of the hydration of B-DNA. Biochim Biophys Acta. 1977 Feb 3;474(3):349–362. doi: 10.1016/0005-2787(77)90265-9. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Rupprecht A. Preparation of oriented DNA by wet spinning. Acta Chem Scand. 1966;20(2):494–504. doi: 10.3891/acta.chem.scand.20-0494. [DOI] [PubMed] [Google Scholar]
- Scheek R. M., Boelens R., Russo N., van Boom J. H., Kaptein R. Sequential resonance assignments in 1H NMR spectra of oligonucleotides by two-dimensional NMR spectroscopy. Biochemistry. 1984 Mar 27;23(7):1371–1376. doi: 10.1021/bi00302a006. [DOI] [PubMed] [Google Scholar]
- Tomasz M., Olson J., Mercado C. M. Mechanism of the isotopic exchange of the C-8 hydrogen of purines in nucleosides and in deoxyribonucleic acid. Biochemistry. 1972 Mar 28;11(7):1235–1241. doi: 10.1021/bi00757a019. [DOI] [PubMed] [Google Scholar]
- Unis M. J., Hearst J. E. On the hydration of DNA. II. Base composition dependence of the net hydration of DNA. Biopolymers. 1968;6(9):1345–1353. doi: 10.1002/bip.1968.360060909. [DOI] [PubMed] [Google Scholar]
- Vorlícková M., Kypr J., Kleinwächter V., Palecek E. Salt-induced conformational changes of poly(dA-dT). Nucleic Acids Res. 1980 Sep 11;8(17):3965–3973. doi: 10.1093/nar/8.17.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. A., Patel D. J., Sauer R. T., Karplus M. Two-dimensional 1H NMR study of the lambda operator site OL1: a sequential assignment strategy and its application. Proc Natl Acad Sci U S A. 1984 Jan;81(1):130–134. doi: 10.1073/pnas.81.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmer D. E., Chou S. H., Hare D. R., Reid B. R. Sequence-specific recognition of DNA: assignment of nonexchangeable proton resonances in the consensus Pribnow promoter DNA sequence by two-dimensional NMR. Biochemistry. 1984 May 8;23(10):2262–2268. doi: 10.1021/bi00305a027. [DOI] [PubMed] [Google Scholar]



