SUMMARY
This study examined a two-dimensional approach to assessing affective states among good and poor sleepers using the Self-Assessment Manikin (SAM), a brief non-verbal self-report measure of affective states with separate ratings of valence and arousal. A sample of 286 undergraduate students completed the Pittsburgh Sleep Quality Index (PSQI) and the SAM. Participants were classified post-hoc as either good (PSQI ≤ 5) or poor sleepers (PSQI > 5) using the PSQI and used the SAM to rate their current affective states (day) and their affective state at bedtime (night) the previous night. Compared to good sleepers, poor sleepers reported more negative affect and arousal at night and more negative affect during the day. Among poor sleepers, lower sleep quality and shorter sleep duration on the components of the PSQI were associated with more negative daytime valence. Among good sleepers, higher scores on the sleep medication and daytime dysfunction components of the PSQI were associated with more negative daytime valence. These findings indicate that the SAM appears to detect differences between good and poor sleepers on both valence and arousal of current daytime and retrospective nighttime emotional states. This approach could be useful for the assessment of affective states related to sleep disturbance.
Keywords: Sleep Disturbance, Affect, Assessment, Daytime Functioning, Arousal, Insomnia
INTRODUCTION
Sleep disturbance is a prevalent problem and a major health issue (National Sleep Foundation, 2005). Complaints about poor sleep include difficulty initiating or maintaining sleep, as well as non-restorative sleep. An insomnia disorder is present when these nocturnal symptoms are associated with sleep-related complaints of mood dysregulation, cognitive or social impairment, fatigue or sleepiness, or concerns about sleep (American Academy of Sleep Medicine, 2005). Although several methods are available to assess nocturnal symptoms (e.g., sleep diaries, actigraphy, polysomnography), novel approaches to measuring sleep-related complaints are needed to improve the assessment of insomnia disorders.
Using the Pre-Sleep Arousal Scale (PSAS), previous research has found that people with insomnia report higher levels of pre-sleep cognitive activity (Nicassio et al., 1985) and more negative tone of sleep-related cognitions compared to good sleepers (Kuisk et al., 1989). Also, higher scores on the PSAS and on the Glasgow Sleep Effort Scale (Broomfield and Espie, 2005) at the end of treatment were found to be related to the future occurrence of insomnia episodes during follow-up (Ong et al., 2009). Negative affect is recognized to be associated with insomnia, but instruments such as the Positive and Negative Affect Schedule (PANAS) and the Profile of Mood States (POMS) are used less frequently than the Beck Depression Inventory (BDI), which assesses symptoms of depression and not the affective state. Since the relationship between affective tone and arousal has not been adequately evaluated, an instrument that measures both these dimensions across the day and night could be useful.
Studies of human emotion (Barrett, 1996, Yik et al., 1999) have utilized a two-dimensional approach to assessing affective states that could improve the assessment of sleep-related complaints. The first dimension is valence, or hedonic tone, with pleasantness and unpleasantness serving as anchors on each end. The second dimension is arousal, or alertness, with deactivation and activation serving as anchors on each end. These two dimensions are consistently produced across studies on human emotion using factor analysis and correlational techniques (Posner et al., 2005).
The aim of the present study was to obtain evidence for the utility of this two-dimensional approach using a brief self-report measure of affective states. The goal was to examine differences in affective states between good and poor sleepers.
METHODS
Participants and Procedures
Data were collected from undergraduate students at Stanford University during the Fall of 2007 and Spring of 2008. A total of 296 participants completed a questionnaire packet as part of course credit that included the two measures described below in addition to other questionnaires used as part of other studies at the university. Ten participants did not provide complete data on the PSQI and were excluded from the analyses because group membership (good vs. poor sleeper) could not be determined. Demographic data were unavailable for 80 participants (28%) who completed the questionnaire. There were no significant differences (p < .05) between these 80 participants and those who provided complete data on any of the measures below so these participants were included in the main analyses. The study was approved by the Institutional Review Board at Stanford University and all participants provided written informed consent.
Measures
Pittsburgh Sleep Quality Index (PSQI)
The PSQI is a 19-item self-report measure of sleep quality and sleep disturbances over the past month (Buysse et al., 1989). Each item is scored on a 0 to 3 scale. Items are combined to yield seven component scores addressing different sleep domains and the component scores are added to yield a global sleep quality score. A total score > 5 represents clinically meaningful insomnia and was used in this study as a cutoff to identify good (total score ≤ 5) and poor sleepers (total score > 5). The seven component scores assess: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, use of sleep medication, and daytime dysfunction. Wake time after sleep onset is not directly assessed by the PSQI.
Self-Assessment Manikin (SAM)
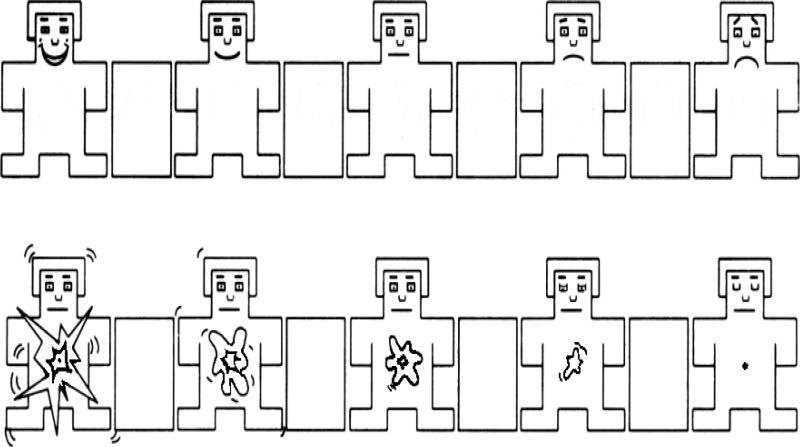
The SAM is a 9-point pictorial scale that is a non-verbal self-report measure of affective state using cartoon-like manikins (see Figure 1) (Bradley and Lang, 1994). In the present study, two sets of manikins were used. In one set, ratings for valence were scored from one (happy) to nine (sad). In the second set, ratings for arousal were scored from one (calm) to nine (excited). Participants were asked to use the two sets of SAM (valence and arousal) rating scales to assess current affective states (daytime) and recall of affective state at bedtime the previous night (nighttime).
Figure 1.
The Self-Assessment Manikin (SAM) adapted with permission from (Bradley and Lang, 1994). The first set of manikins (top row) consists of ratings for the valence dimension (happy to sad). The second set of manikins (bottom row) consists of ratings for the arousal dimension (excited to calm).
RESULTS
Participant Characteristics
No significant differences were found between good and poor sleepers on gender, ethnicity, or age (see Table 1). As expected, the poor sleepers reported more sleep disturbance on the sleep parameters derived from the PSQI as well as the subscales of the PSQI. A significantly greater number of poor sleepers (40.7%) relative to good sleepers (4.3%) also met quantitative criteria for insomnia (sleep latency > 30 minutes or sleep efficiency < 85%), proposed by Lichstein and colleagues (2003) and Edinger and colleagues (2004). Moreover, 23.4% of poor sleepers reported these nocturnal symptoms (sleep latency > 30 minutes or sleep efficiency < 85%) with daytime dysfunction (PSQI daytime dysfunction subscale score ≥ 2), consistent with criteria for an insomnia disorder (Edinger et al., 2004). In contrast, none of the good sleepers met criteria for an insomnia disorder.
Table 1.
Participant Characteristics
| Good Sleepers (n=143) | Poor Sleepers (n=143) | Total (n=286) | p value | |
|---|---|---|---|---|
| Gender (n, %) | .095 | |||
| Male | 41 (40.6%) | 30 (28.6%) | 71 (34.5%) | |
| Female | 60 (59.4%) | 75 (71.4%) | 135 (65.5%) | |
| Ethnicity (n, %) | .206 | |||
| Caucasian | 50 (49.5%) | 46 (43.8%) | 96 (46.6%) | |
| African American | 5 (5.0%) | 7 (6.7%) | 12 (5.8%) | |
| Asian/Pacific Islander | 12 (11.9%) | 22 (21.0%) | 34 (16.52%) | |
| Hispanic/Latino | 17 (16.8%) | 11 (10.5%) | 28 (13.6%) | |
| Other | 1 (1.0%) | 5 (4.8%) | 6 (2.9%) | |
| More than one race | 16 (15.8%) | 14 (13.3%) | 30 (14.6%) | |
| Age (Mean, SD) | .238 | |||
| Years | 19.49 (1.18) | 19.30 (1.10) | 19.39 (1.14) | |
| PSQI Component scores | ||||
| Sleep Quality | 0.76 (0.52) | 1.55 (0.66) | 1.16 (0.71) | <.001 |
| Sleep Latency | 0.56 (0.66) | 1.42 (0.90) | 0.99 (0.90) | <.001 |
| Sleep Duration | 0.39 (0.61) | 1.02 (0.77) | 0.70 (0.76) | <.001 |
| Sleep Efficiency | 0.04 (0.19) | 0.37 (0.67) | 0.20 (0.52) | <.001 |
| Sleep Disturbances | 1.00 (0.39) | 1.29 (0.48) | 1.14 (0.46) | <.001 |
| Sleep Medication | 0.06 (0.26) | 0.35 (0.77) | 0.20 (0.60) | <.001 |
| Daytime Dysfunction | 1.11 (0.69) | 1.77 (0.81) | 1.44 (0.82) | <.001 |
| Total Global Score | 3.87 (1.13) | 7.75 (2.08) | 5.81 (2.56) | <.001 |
| PSQI Sleep Parameters | ||||
| Sleep Onset Latency (minutes) | 11.83 (7.76) | 26.07 (33.29) | 18.95 (25.16) | .001 |
| Total Sleep Time (minutes) | 451.27 (62.18) | 390.70 (62.99) | 421.20 (69.45) | <.001 |
| Sleep Efficiency (%) | 95.92 (5.83) | 87.89 (10.06) | 91.90 (9.14) | <.001 |
| Time in Bed (minutes) | 472.23 (69.40) | 445.38 (64.51) | 458.76 (68.21) | <.001 |
| Evening Bedtime | 00:55 | 1:20 | 1:08 | .001 |
| Morning Risetime | 8:48 | 8:46 | 8:47 | .765 |
| Insomnia Symptoms (n, %) | ||||
| Sleep Latency > 30 min | 1 (0.7%) | 31 (21.7%) | 32 (11.2%) | <.001 |
| Sleep Efficiency < 85% | 5 (3.5%) | 40 (28.0%) | 45 (15.9%) | <.001 |
| Sleep Latency > 30 min or Sleep Efficiency < 85% | 6 (4.3%) | 57 (40.7%) | 63 (22.5%) | <.001 |
| Insomnia Disorder | 0 (0.0%) | 33 (23.4%) | 33 (11.7%) | <.001 |
| Group Comparisons on SAM (Mean, SD) | ||||
| Daytime Valence | 3.68 (1.51) | 4.19 (1.46) | 3.93 (1.51) | .004 |
| Daytime Arousal | 4.00 (1.91) | 3.90 (1.80) | 3.95 (1.85) | .649 |
| Nighttime Valence | 3.93 (1.94) | 4.62 (2.24) | 4.27 (2.12) | .006 |
| Nighttime Arousal | 3.96 (2.26) | 4.56 (2.29) | 4.26 (2.29) | .025 |
Note. Data for gender, ethnicity, and age were unavailable for 80 participants (42 good sleepers, 38 poor sleepers) and percentages are based on available data. Chi-square tests were conducted for gender, ethnicity, and sleep measures derived form the PSQI. One-way ANOVAs were conducted for all other variables. Data for Insomnia Symptoms were derived from the PSQI. Insomnia Disorder = (Sleep latency > 30 min or Sleep Efficiency < 85%) + daytime dysfunction (score of 2 or greater on the daytime dysfunction subscale of the PSQI). Daytime and nighttime arousal recoded such that high numbers reflect greater arousal.
SAM Valence and Arousal
Differences between good and poor sleepers were tested using a series of one-way Analysis of Variance (ANOVA) on daytime and nighttime SAM ratings. Significant differences were found on daytime valence, F(1, 272) = 8.23, p < .005, nighttime valence, F(1, 283) = 7.70, p < .01, and nighttime arousal, F(1, 283) = 5.05, p < .05. In each analysis, poor sleepers reported higher scores (more negative valence, more arousal) relative to good sleepers (see Table 1). No significant difference was found on daytime arousal.
SAM Ratings and PSQI Components
To examine the relationship between components of sleep quality and affective states, a linear regression analysis was conducted for each of the four SAM rating as the dependent variable. In each model, the seven PSQI component scores were entered as independent variables. To examine evidence for discriminant validity, regression analyses were conducted separately for each group.
Good sleepers
The regression model for daytime valence was significant (F = 2.32, df = 7, 126, p < .05) accounting for 11% of the variance. The PSQI components for sleep medication use and daytime dysfunction were significant predictors (p < .05) of daytime valence. The regression model for nighttime arousal was also significant (F = 2.38, df = 7, 129, p < .05) accounting for 11% of the variance. The PSQI component for sleep duration was the only significant predictor (p < .05) of nighttime arousal. The models for daytime arousal and nighttime valence were not statistically significant.
Poor Sleepers
The regression model for daytime valence was significant (F = 3.60, df = 7, 124, p < .005), accounting for 17% of the variance. The PSQI components for sleep quality and sleep duration were significant predictors of daytime valence (p < .05). The regression model for nighttime arousal was significant (F = 2.47, df = 7, 132, p < .05), accounting for 12% of the variance. The PSQI components for sleep quality and sleep disturbances were significant predictors (p < .05). The models for daytime arousal and nighttime valence were not statistically significant.
DISCUSSION
The present study examined a two-dimensional approach to assessing affective states using the SAM. Compared to good sleepers, poor sleepers reported more negative affect and arousal at night and more negative affect during the day. We found different predictors of daytime valence ratings for good and poor sleepers. Among poor sleepers, lower sleep quality and shorter sleep duration as derived from the PSQI were associated with more negative daytime valence. Among good sleepers, more frequent use of sleep medication (albeit a small percentage of users) and more frequent daytime dysfunction as derived from the PSQI were associated with more negative daytime valence. Not surprisingly, components related to nocturnal symptoms were associated with nighttime arousal for both groups. These findings suggest that the SAM can be used to assess emotional states of individuals with poor sleep quality. Good and poor sleepers differed on both valence and arousal dimensions. Therefore, the SAM might clarify ambiguous sleep-related complaints from insomnia patients such as feeling “tired but wired” that would be difficult to explain using a single dimension. In the two-dimensional model, this affective state would be characterized by elevations in both negative valence and arousal, similar to that found for poor sleepers in this study. A recent study using visual analog scales taken four times per day also found differences between good sleepers and people with insomnia across multiple domains, including ratings of alert cognition, negative mood, positive mood, and sleepiness/fatigue (Buysse et al., 2007). The simple non-verbal scales employed by the SAM could be an alternative for capturing affective states across multiple time points, especially for non-English speakers or those with cognitive impairments. It might also be used with other standard instruments such as the PSAS, PANAS, POMS, or BDI to clarify the relationship between affect state and depressive symptoms.
The different predictors of daytime valence ratings found for good and poor sleepers provide some evidence of discriminant validity for the SAM. For poor sleepers, negative daytime mood was predicted by nocturnal symptoms, which is consistent with the tendency of people with insomnia to attribute daytime dysfunction to poor sleep (Harvey, 2002). In contrast, for good sleepers, negative daytime mood was predicted by daytime symptoms (i.e., sleepiness, anhedonia), and possibly carry over effects from sleep medications use. This is consistent with a reduced focus on nighttime symptoms among good sleepers and suggests that good sleepers might use different attributions and salient cues to rate their daytime valence.
These findings should be considered within the limitations of this study. An important limitation is that the nighttime assessment was retrospective while the daytime assessment was current. Therefore, causality cannot be determined and it is possible that other factors such as underlying mood disorders, which were not assessed, were present. Also, no formal screening of sleep disorders was administered, thus precluding characterization of specific sleep disorders in this sample. Because sleep-related breathing disorders have a low prevalence among adolescent and young adult students it is not likely that sleep apnea contributed to the elevation in PSQI scores. However, we cannot rule out the possibility that delayed sleep phase syndrome may have been present, as the poor sleepers reported a later bedtime along with a longer sleep latency. Finally, this sample consisted of late adolescent and young adult students which might limit the generalizability of the findings to populations that are more heterogeneous with regards to age and other demographic characteristics.
Despite these limitations, the two-dimensional approach could be a useful tool for the assessment of affect states related to sleep disturbance. Given recent findings of changes in brain metabolism within wake-promoting and emotion-regulating regions of the brain (Nofzinger et al., 2004), future studies might consider using the SAM along with physiological measures. Such an approach might be useful in connecting the self-reported affective state with physiological measures and improve the understanding of the relationship between sleep-related complaints and nocturnal symptoms.
ACKNOWLEDGEMENTS
Portions of the data from this study were presented at the annual meeting of the Associated Professional Sleep Societies, Baltimore, MD, June 2008.
REFERENCES
- American Academy of Sleep Medicine The International Classification of Sleep Disorders-2. Rochester, MN: 2005. [Google Scholar]
- Barrett LF. Hedonic tone, perceived arousal, and item desirability: Three components of self-reported mood. Cognition & Emotion. 1996;10:47–68. [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Broomfield NM, Espie CA. Towards a valid, reliable measure of sleep effort. J Sleep Res. 2005;14:401–7. doi: 10.1111/j.1365-2869.2005.00481.x. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Thompson W, Scott J, Franzen PL, Germain A, Hall M, Moul DE, Nofzinger EA, Kupfer DJ. Daytime symptoms in primary insomnia: a prospective analysis using ecological momentary assessment. Sleep Med. 2007;8:198–208. doi: 10.1016/j.sleep.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, Jamieson AO, Mccall WV, Morin CM, Stepanski EJ. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- Kuisk LA, Bertelson AD, Walsh JK. Presleep cognitive hyperarousal and affect as factors in objective and subjective insomnia. Percept Mot Skills. 1989;69:1219–25. doi: 10.1177/00315125890693-228. [DOI] [PubMed] [Google Scholar]
- Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation Sleep in America Poll. Washington, D.C.: 2005. [Google Scholar]
- Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther. 1985;23:263–71. doi: 10.1016/0005-7967(85)90004-x. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- Ong JC, Shapiro SL, Manber R. Mindfulness meditation and cognitive behavioral therapy for insomnia: a naturalistic 12-month follow-up. Explore (NY) 2009;5:30–6. doi: 10.1016/j.explore.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Russell JA, Peterson BS. The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev. Psychopathol. 2005;17:715–34. doi: 10.1017/S0954579405050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yik MSM, Russell JA, Barrett LF. Structure of self-reported current affect: Integration and beyond. J. Pers. Soc. Psychol. 1999;77:600–19. [Google Scholar]