Abstract
Human metapneumovirus (hMPV) is a major cause of upper and lower respiratory infections in children and adults. Recent work from our group demonstrated that hMPV G glycoprotein is an important virulence factor, responsible for inhibiting innate immune responses in airway epithelial cells. Myeloid dendritic cells are potent antigen presenting cells and play a major role in initiating and modulating the innate and adaptive immune responses. In this study, we found that TLR4 plays a major role in hMPV-induced activation of monocyte-derived dendritic cells (moDCs), as downregulation of its expression by siRNA significantly blocked hMPV-induced chemokine and type I interferon expression. Similar results were found in bone marrow derived-dendritic cells (BM-DCs) from TLR4 deficient mice. MoDCs infected with a virus lacking G protein expression (rhMPV-ΔG) produced higher levels of cytokines and chemokines, compared to cells infected with wild-type virus (rhMPV-WT), suggesting that G protein plays an inhibitory role in viral-induced cellular responses. Specifically, G protein affects TLR4-dependent signaling, as rhMPV-ΔG infection of moDCs inhibited LPS-induced production of cytokine and chemokines significantly less than rhMPV-WT, and treatment of moDCs with purified G protein resulted in a similar inhibition of LPS-dependent signaling. Our results demonstrate that hMPV G protein plays an important role in inhibiting host innate immune responses, likely affecting adaptive responses too.
INTRODUCTION
Human metapneumovirus (hMPV) is a recently identified RNA virus belonging to the Paramyxoviridae family, which includes several major human and animal pathogens (1). Epidemiological studies indicate that hMPV is a significant human respiratory pathogen with worldwide distribution (2,3). It is associated with respiratory illnesses in children, adults, and immunocompromised patients, ranging from upper respiratory tract infections to severe bronchiolitis and pneumonia (4–6). Evidence suggests that the virus may also cause repeated infection throughout life (7,8).
Toll like receptors (TLRs) have been shown to be involved in the activation of innate immune responses by recognizing different pathogen-associated molecular patterns (PAMPs) (9–13). Their role in virus-triggered cellular signaling is stimulus- and cell-type-dependent [Reviewed in (14)]. After recognition of their own PAMP(s), following viral infection, TLRs trigger intracellular signaling pathways that are necessary to the induction of inflammatory cytokines, chemokines, as well as type I IFNs. Respiratory syncytial virus (RSV) fusion (F) protein, and the envelop proteins of mammary tumor virus, and murine leukemia virus, have been shown to activate TLR4 (15,16), however, its role in other viral infections, such as hMPV, is not known.
Dendritic cells (DCs) play a pivotal role in shaping antiviral immune responses in the respiratory tract. They can efficiently sense invading pathogens by TLRs and, because of their strategic localization at mucosal sites, are involved in the response to viral infections (17,18). We have previously shown that hMPV is able to infect human monocytes-derived dendritic cells (moDCs) and plasmacytoid dendritic cells (pDCs), and that infection of these two cell-types can effectively block the production of type I IFN in response to TLR agonists (19). Similarly, following infection with hMPV, mice showed a significant inhibition of IFN-α production in the lung in response to intranasal inoculation with TLR3, or TLR9 agonists (20). However, the mechanism(s) of inhibition of innate immune responses by hMPV is not known.
Since the discovery of hMPV in 2001, several research groups have developed vaccine candidates that may be used to protect different risk groups against hMPV-induced respiratory disease. Recombinant hMPV lacking G protein expression (rhMPV-ΔG) exhibited reduced replication in the upper and lower respiratory tract of Syrian hamsters and African green monkeys (21,22). We have previously shown that rhMPV-ΔG induces higher levels of cytokines, chemokines, and type I IFN, in cultured human alveolar epithelial cells, compared to rhMPV-WT (23). The mechanism(s) underlying rhMPV-ΔG attenuation, as well as, the role of the hMPV G protein in modulating host cell responses, are largely unknown. Although reduced attachment ability might contribute to the observed attenuation of rhMPV-ΔG in vivo, it is possible that hMPV G protein has an inhibitory role in immune cell activation, similar to what we have recently reported in airway epithelial cells, leading to reduced secretion of pro-inflammatory and/or antiviral molecules upon infection, therefore, affecting innate and adaptive immune responses.
The results of our study indicate that TLR4 plays an important role in hMPV-induced secretion of pro-inflammatory cytokines and chemokines, as well as type I IFN in moDCs, and that G protein inhibits moDC cellular responses by affecting TLR4-dependent signaling.
MATERIALS AND METHODS
Recombinant hMPV preparation
The hMPV strain CAN97-83, referred as naïve hMPV, was obtained from the Centers for Disease Control (CDC), Atlanta, GA, with permission from Dr. Guy Boivin at the Research Center in Infectious Diseases, Regional Virology Laboratory, Laval University, Quebec City, Canada. The virus was propagated in LLC-MK2 cells (ATCC, Manassas, VA) in serum-free MEM (Invitrogen GIBCO, Carlsbad, CA) containing 1 μg trypsin/ml, followed by purification on a 60% sucrose-cushion. Wild-type hMPV (rhMPV-WT), as well as hMPV lacking G protein (rhMPV-ΔG) was generated by a reverse genetic technology, using the CAN97-83 as backbone, as previously described (23). The virus titer was determined by a cell-based immunoassay, as previously described (20).
Preparation of purified hMPV G protein
The construct for G protein purification was created by PCR using hMPV antigenome as a template with EcoRI and XhoI restriction sites added at the 5′ and 3′ ends. The 3′ primer contained a histidine tag for subsequent protein purification. The primer sequences used for PCR are available upon request. The PCR fragment was first cloned into TOPO® cloning vector (Invitrogen, Carlsbad, CA), and then digested with the appropriate enzymes, followed by ligation into the pCAGGS vector. Plasmid was purified by ion exchange (Endo-Free Qiagen Kit, Qiagen, Chatsworth, CA) and sequenced, prior to transfection, into MAX free-style 293 cells grown in suspension, as recommend by the manufacturer (Invitrogen, Carlsbad, CA). After three days, cell pellets were collected and resuspended in buffer NT1-10-G containing 51 mM n-Octyl-β-D-glucopyranoside (OG). After overnight incubation on a shaker, the reaction mixture was centrifuged and the supernatant was pipetted into a 1 ml disposable column containing Ni2+ resin, which was pre-equilibrated with 0.5 ml buffer NT1-10-G with 51 mM OG. The flow-through fraction was reapplied to the column twice. The column was then washed with NTI-25-G containing 37 mM OG, and the protein was eluted in 0.5 ml NTI-500-G with 37 mM OG. Imidazole, a chemical used for disrupting Ni2+ -histidine interaction, was eliminated by passing the purified protein through PD-10 column (Amersham Biosciences, Piscataway, NJ). Eluted protein in NTI-10-G buffer with 37 mM OG was then quantified using a BCA Protein Assay Kit from Thermo Fisher Scientific (Thermo Fisher Scientific, Waltham, MA). Purified G was run on 10% SDS-PAGE to verify preservation of highly glycosylated forms characteristic of the naïve viral protein, as previously shown (23).
Purification of moDCs cells and viral infection
MoDCs were generated from human peripheral blood mononuclear cells (PBMC), as previously described (19). Briefly, whole blood from healthy adult donors, after dextran sedimentation, was layered on top of Ficoll-hypaque, and after centrifugation the layer of mononuclear cells was collected. CD14+ cells were isolated by immunomagnetic selection following manufacturer’s instruction (purity > 93%) (Miltenyi, Auburn, CA). These CD14+ cells were cultured for 7 d in RPMI 1640 supplemented with 2 mMol/liter L-glutamine, 10% FBS, 50 μM 2-mercaptoethanol ME, and 1,000 U/I penicillin-streptomycin medium containing GM-CSF (100 ng/ml) and IL-4 (20 ng/ml). IL-4 was purchased from R&D Systems (Minneapolis, MN) and recombinant human GM-CSF from PeproTech (Rocky Hill, NJ). One-third of the medium and 100% of each cytokine were replaced every other day. MoDCs were used on the seventh day of culture in all experiments. Cells were infected with naïve hMPV, rhMPV-WT or -ΔG at an MOI of 2 (unless otherwise specified) in the presence of 1 μg/ml trypsin (19). Uninfected cells, treated with cell culture media containing same amounts of sucrose and trypsin of infected cells, were defined as mock-infected cells. In some experiments moDCs were also exposed to ultraviolet-inactivated(non-replicating) preparations of rhMPV-WT or -ΔG (24). In experiments investigating hMPV interference with TLR4 signaling, 2×105 cells were infected with rhMPV-WT or -ΔG, at MOI of 2, for 24 h. Cell supernatants were removed and cells were then stimulated with LPS (E. coli K12 LPS, InvivoGen, San Diego, CA) at a final concentration of 100 ng/ml in fresh media. Cell supernatants were harvested 24 h later to measure cytokine, chemokine, and type I IFN secretion.
To assess the effect of isolated G protein on TLR4-dependent signaling, 2×105 moDCs were pretreated with purified G protein at different concentrations (2, 1, 0.5, 0, 1 and 0.05μg/ml) for 30 min, followed by the addition of LPS at a final concentration of 100 ng/ml. Cell supernatants were harvested at 24 h to measure cytokine, chemokine, and type I IFN production.
RNA interference and real time PCR
TLR2, 3 and 4 expression was downregulated using 100 nM of specific human TLR siRNA sequences (ON-TARGETplus SMARTpool, Dharmacon) and the electroporation system from Amaxa Biosystem, Gaithersburg, MD, which uses cell-type specific buffers and reagents to allow high transfection efficiency in primary immune cells. Non-targeting sequences (siCONTROL pools, Dharmacon) were used as negative control. At 48 h post-transfection, moDCs were infected with hMPV and harvested at 24 h post infection (p.i) to collect cell supernatants and extract total RNA, using RNeasy kit (Qiagen, Chatsworth, CA), according to manufacturer’s instructions. TLR2, 3, and 4, as well as cytokine, chemokine, and type I IFN gene expression levels were determined by quantitative real-time PCR (Q-RT-PCR). Specific mRNAs were amplified by Q-RT-PCR using Applied Biosystems Assays-On-Demand (AB, Foster City, CA) 20x mix of primers and TaqMan MGB probes (FAM-dye-labeled) for target genes, and 18S rRNA (VIC-dye-labeled probe) TaqMan assay reagent (P/N4319413E) for control. Separate tubes (singleplex) one-stepRT-PCR was performed with 80 ng RNA for both target genes and endogenous control. The cycling parameters for one-step RT-PCR were: reverse transcription, 48°C for 30 min; AmpliTaq activation, 95 °C for 10 min; denaturation 95 °C for 15 s and annealing/extension 60°C for 1 min (repeated 30 times) on an ABI 7000. Duplicate threshold cycle (Ct) values were analyzed in Microsoft Excel using the comparative Ct (ΔΔCT) method as described by the manufacturer(Applied Biosystems). The amount of target (2−ΔΔCT) was obtained by normalizing to an endogenous reference (18S) sample.
Establishment of bone marrow-derived dendritic cells
TLR 4−/− mice, [C.C3-Tlr4Lps-d/J (BALB/c background] and [C57BL/10ScSnJ and C57BL/10ScNJ mice (BL/10) background] were purchased from Jackson Laboratories. TLR7−/− mice [C57BL6 (B6)×129 F2 background] were obtained from Regeneron Inc. (Tarrytown, NY) and Dr. Richard Flavell (Howard Hughes Medical Institute, Yale University School of Medicine, New Haven, CT, USA) and were further bred to the B6 background by back-crossing for seven successive generations (25). Bone marrow dendritic cells were generated as previously described (26,27). Briefly, bone marrow cells isolated from the femur and tibia were cultured in complete DC medium (RPMI-1640 supplemented with 10% FCS, 2 mM L-glutamine, penicillin, streptomycin, 1 mM sodium pyruvate, 0.02 M Hepes and 50 50 μM 2-ME) supplemented with murine recombinant GM-CSF (Peprotech, Rocky Hill, NJ) at a final concentration of 20 ng/mL and seeded at 2×106 cells/mL in 6 well plates. Cells were gently washed every other day to replace the medium and to remove granulocytes and non-adherent T and B cells. After 8–9 days, cells loosely adhered and suspension cells were collected and seeded in 24 well plates at 5×105 per well. Their DC phenotype was confirmed based on the expression of several surface markers, including CD11b and CD11c, MHC II, CD86, as well as CD80. The following day, cells were infected with hMPV (MOI 2) and supernatants were collected at different time p.i. to measure cytokine, chemokine, and type I IFN production.
FACS Analysis of DCs for infectivity
Simultaneous analysis of cell surface CD11c and MHC class II and intracellular hMPV antigens by moDCs was performed as previously described (19). Briefly, cells in suspension were washed once with an ice-cold wash solution (PBS with 1% heat-inactivated FCS) and stained with a PE-conjugated anti-human CD11c (Pharmingen, San Diego, CA) and PerCP-conjugated anti-HLA DR (Miltenyi, Auburn, CA). To detect expression of intracellular viral antigens, cells were fixed with Cytofix/cytoperm (Pharmingen, San Diego, CA)and permeabilized with washing buffer Perm/wash buffer (Pharmingen, Fallbrook, CA). Cells were then incubated with guinea pig anti-hMPV antibody (19), followed by a FITC-goat anti-guinea pig antibody (Zymed, Carlsbad, CA). After the final step, cells were washed and then fixed in 200 μl of 2% para formaldehyde in PBS. Cells were analyzed with a FACScan flow cytometer equipped with CellQuest software (both from Becton Dickinson Immunocytometry Systems, San Jose, CA). Analysis was performed in FlowJo software (Tree Star, Inc. La Jolla, CA).
Bio-Plex and ELISA
Human and mouse IFN-α/-β concentrations were determined by commercial enzyme-linked immunosorbent assays (ELISA), according to the manufacturer’s instructions (PBL, Piscataway, NJ). Selected human and mouse cytokines and chemokines [IL-1α, IL-1β, IL-6, IL-8 (KC for mouse samples), IL-10, IL-12 p40, IL-12 p70, G-CSF, IFN-γ, IP-10, MIP-1α, MIP-1β, MCP-1, RANTES, and TNF-α] were quantified by Luminex-based Bio-Plex system (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. The lower limit of detection for all cytokines measured by this assay is 3 pg/ml.
Statistical Analysis
Statistical analyses were performed by the Mann-Whitney U test using the InStat 3.05 biostatistics package (GraphPad, San Diego, CA). Unless otherwise indicated, mean ± SEM is shown.
RESULTS
TLR4 complex is required for hMPV-induced activation of human moDCs
Among the 10 members of TLRs identified in humans, TLR2, 3, 4, 7, 8 and 9 have been shown to be involved in the innate response to viral stimulation (13,28). TLR2 and 3 recognize components of cytomegalovirus, herpes virus and measles viruses (29,30) and they have also been shown to play an important role in RSV-induced cytokine production in monocytes (31) and in airway epithelial cells (32,33). RSV F protein and envelop proteins of mammary tumor virus and murine leukemia virus have been shown to activate TLR4 (15,16), and the role of TLR4 in the immune pathogenesis of RSV infection has been widely documented (15,34). Overall, the role of TLRs in hMPV-induced signaling in immune cells is not known, with the exception of TLR7 in pDCs, which require its expression to be able to respond to hMPV infection (35).
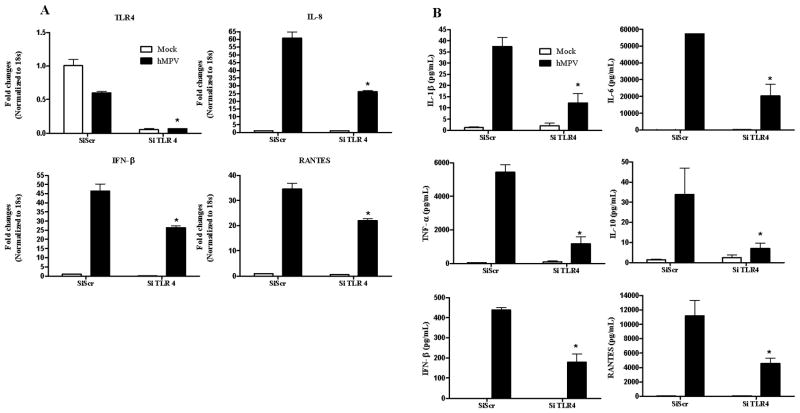
To determine the role of TLR2, 3 and 4 in hMPV infection, we downregulated their expression in human moDCs and we assessed hMPV-induced secretion of type I IFN and proinflammatory mediators. Cells were transfected with either a scrambled siRNA, as control, or one targeting the specific TLR at a final concentration of 100 nM. This concentration was chosen as the one having the greatest effect on reduction of the target gene expression, without greatly affecting cell survival (data not shown) or viral replication (supplementary Fig. 1). At 48 h post-transfection, moDCs were infected with naïve hMPV and harvested at 24 h p.i. to collect cell supernatants and extract total RNA. Our results showed that hMPV infection of moDCs significantly downregulated expression of TLR4 (Fig. 1A) and that treatment with specific siRNA effectively blocked both basal and viral-regulated gene expression (80–90%). There was a significant reduction of IL-8, RANTES and IFN-β gene expression in response to hMPV infection in TLR 4-silenced cells, compared to scramble siRNA-treated cells (Fig. 1A), indicating the involvement of TLR4 in the expression of these viral-induced genes. To determine the effect of TLR4 gene silencing on the production of other immune mediators, supernatants of moDCs transfected with either scramble or TLR4 siRNA and infected with hMPV were assayed for cytokine and chemokine production. In agreement with our gene expression results, TLR4 silencing significantly reduced hMPV-induced secretion of cytokines and chemokines, such as IL-1β, IL-6, IL-10, TNF-α, and RANTES, as well as IFN-β (Fig. 1B).
Fig. 1. Effect of TLR4 gene silencing on hMPV-induced gene expression.
MoDCs were transfected with 100 nM siRNA targeting TLR4 (siTLR4) or a scramble control (SiScr) for 48 h and infected with hMPV. Cells were harvested at 24 h p.i. to prepare total RNA for analysis of TLR4, IL-8, RANTES and IFN-β gene expression by Q-RT-PCR (panel A) and to collect cell supernatants for measuring cytokines, chemokines, and IFN-β secretion by Bio-Plex or ELISA (panel B). Results are representative of two separate experiments. *, P < 0.05 relative to scramble control.
On the other hand, there was no significant difference in IL-8, RANTES and IFN-β gene expression in response to hMPV infection in TLR2 and 3-silenced cells, compared to scramble siRNA-treated cells, although hMPV significantly upregulated mRNA levels of both TLRs (supplementary Fig. 2A and B).
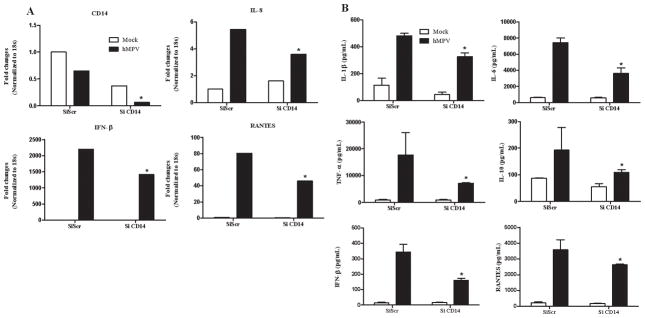
Similar to LPS, the primary ligand of TLR4, RSV engagement of TLR4 requires the presence of CD14 and MD-2 for signaling (34,36). To confirm the involvement of TLR4 complex in hMPV-induced signaling, we downregulated CD14 and investigated hMPV-induced moDCs responses, as described above. Similar to cells treated with siRNA for TLR4, CD14-silenced cells showed a significant reduction in cytokine, chemokine and type I IFN gene expression (Fig. 2A) and protein secretion (Fig. 2B).
Fig. 2. Effect of CD14 gene silencing on hMPV-induced gene expression.
MoDCs were transfected with 100 nM siRNA targeting CD14 (siCD14) or a scramble control (SiScr) for 48 h and infected with hMPV. Cells were harvested at 24 h p.i. to prepare total RNA for analysis of CD14, IL-8, RANTES and IFN-β gene expression by Q-RT-PCR (panel A) and to collect cell supernatants for measuring cytokines, chemokines, and IFN-β secretion by Bio-Plex or ELISA (panel B). Results are representative of two separate experiments. *, P < 0.05 relative to scramble control.
We further investigated the role of TLR4 in hMPV-induced cytokine and chemokine expression using BMDCs from mice with two different types of TLR4 deficiency. C. C3-Tlr4Lps-d/J mice have a spontaneous point mutation in the intracellular domain of TLR4 that blocks LPS signaling (37), whereas C57BL/10ScNJ mice carry a deletion of the TLR4 gene that results in the absence of both mRNA and protein and, thus, is defective in the response to LPS stimulation (34,37). Bone marrow cells isolated from these mice, along with their respective control mice, were differentiated into DCs using mouse rGM-CSF. Their DC phenotype was confirmed based on the expression of several surface markers, including CD11b and CD11c, MHC II, CD86, as well as F4/80 (19).
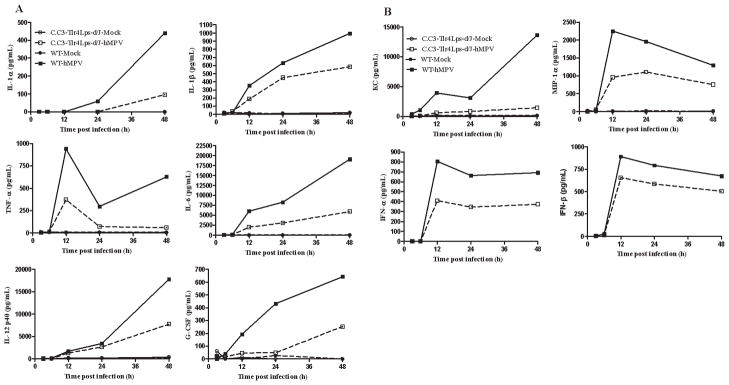
Once differentiated into DCs, cells were infected with naïve hMPV and cell supernatants were collected at various times p.i. for the detection of cytokines, chemokines, and type I IFNs. BMDCs from C.C3-Tlr4Lps-d/J mice responded poorly to hMPV infection, compared to control cells, producing significantly less cytokines, including IL-1α, IL-1β, IL-6, IL-12, TNF-α and G-CSF (Fig. 3A), and chemokines, such as KC, MIP-1α and MIP-β, as well as IFN-α and -β (Fig. 3B). Similar results were obtained in C57BL/10ScNJ mice (data not shown).
Fig. 3. Role of TLR4 in hMPV-induced cytokine, chemokine, and type I IFN secretion.
BMDCs prepared from either wild-type or C.C3-TLR4 Lps-d/J mice were infected with hMPV. Supernatants were harvested at different times p.i. to measure cytokines (panel A), chemokines, and type I IFN (Panel B) by Bio-Plex or ELISA. Results are representative of two separate experiments.
Since TLR7 has been shown to play an important role in hMPV-induced signaling in pDCs (35), we investigated whether it had a similar role in conventional DCs as well. Cells isolated from TLR7−/− mice were prepared as above and, after purity (> 95%) and DC phenotype were confirmed by flow cytometry, cells were either infected with naïve hMPV or treated with a known TLR7 agonist (CL097, 1μg/mL, Invivogen, San Diego, CA). Cell supernatant was harvested at 24 p.i. to measure representative cytokines and chemokines. No change in hMPV-induced proinflammatory mediator secretion was observed in the absence of TLR7, while TLR7 was required for CL097-mediated production of cytokine and chemokines (Supplementary Fig. 3).
HMPV G protein inhibits viral-induced cytokine production in moDCs
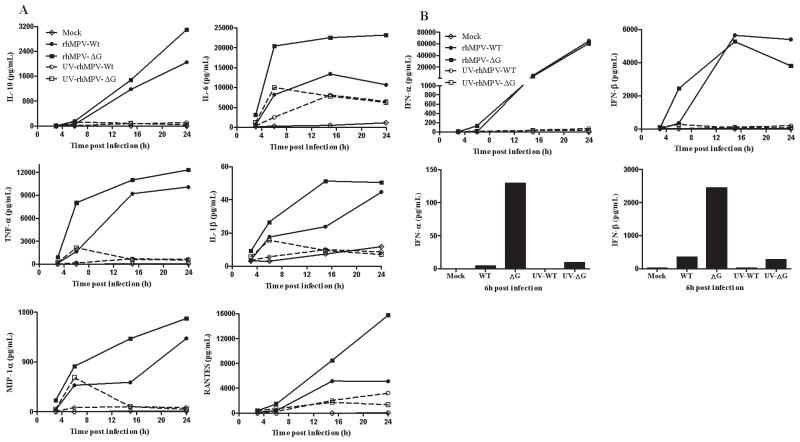
We have recently shown that hMPV G protein is an important virulence factor, as its expression affects viral-induced cytokines, chemokines, as well as IFN production in airway epithelial cells (23). To determine whether this protein plays a role also in hMPV-induced signaling in primary immune cells, we infected human moDCs with recombinant hMPV and investigated the effect of G protein deletion on immune mediator production. Cells were infected with either rhMPV-WT or rhMPV-ΔG, and cell supernatants were collected at various times p.i. to measure levels of cytokines, chemokines, and type I IFN. MoDCs infected with rhMPV-ΔG secreted significantly higher levels of cytokines, such as IL-6, IL-10, IFN-γ, TNF-α, IL-1β, and IL-12p70, and chemokines, such as RANTES and MIP-1α, when compared with cells infected with rhMPV-WT, at all time points tetsed (Fig. 4A). On the other hand, rhMPV-ΔG infection induced significantly higher IFN-α and-β production only at 6 h p.i, while there was no significant difference at later time points of infection (Fig. 4B). When cells were infected with UV-inactivated virus, there was very little or no type I IFN production, indicating the replication-dependent nature of these mediators. Other cytokine and chemokine secretion was at least in part independent of viral replication, with levels of mediators induced by UV- treated rhMPV-ΔG somewhat higher compared to rhMPV-WT.
Fig. 4. Effect of G protein deletion on cytokine, chemokine, and type I IFN secretion.
MoDCs were infected with hMPV, either WT or ΔG, and harvested at different time points p.i. to measure secretion of cytokines, chemokines, (panel A), as well as type I IFN (panel B), by Bio-Plex or ELISA. Results shown are representative of three separate experiments.
As the observed difference in the production of cytokines and chemokines could be due to higher infectivity of the virus lacking G protein expression, we determined rhMPV-WT and rhMPV-ΔG replication in moDCs using intracellular staining for hMPV proteins. MoDCs were infected with hMPV and stained at different times p.i. with a polyclonal anti-hMPV antibody to detect intracellular antigen expression, as previously described (19). A similar percentage of cells were infected with both viruses at an early time point of infection (6 h), while there was a significant increase in antigen staining in moDCs infected with rhMPV-WT at 24 h p.i. compared to rhMPV-ΔG infected cells (Fig. 5).
Fig. 5. Recombinant viral infection of moDCs.
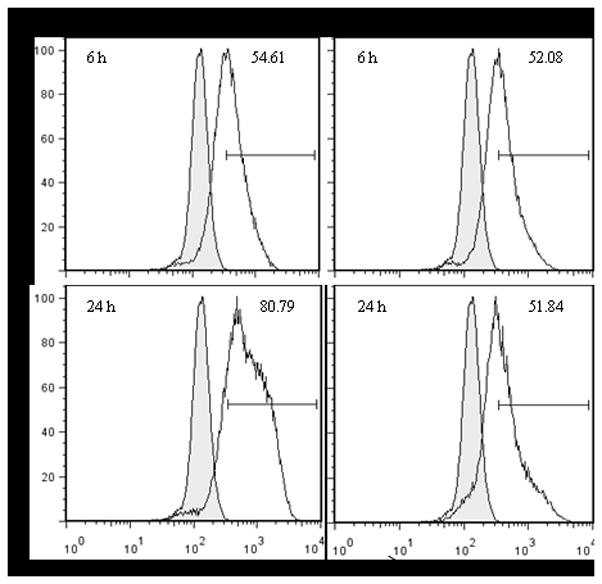
MoDCs were infected with hMPV, either WT or ΔG, and stained for specific cell surface antigens (anti-CD11c and MHC II), and for intracellular viral antigens. Top panels are results at 6 h p.i. and bottom panels represent 24 h p.i. Results are presented as histogram overlays of anti-hMPV staining versus isotype control. Results are representative of three separate experiments.
As naïve hMPV infection induced downregulation of TLR4 expression (Fig. 1A), similar to what has been described for LPS (38), we investigated whether rhMPV-ΔG affected TLR4 expression differently from rhMPV-WT infection. MoDCs were treated with LPS or infected with rhMPV-WT or -ΔG for various length of time and harvested to extract total RNA. TLR4 expression was measured by Q-RT-PCR. There was a time-dependent decrease in TLR4 mRNA levels after stimulation with both LPS and recombinant viruses, with significant TLR4 downregulation starting at 15 h p.i., although we observed some difference between rhMPV-WT and -ΔG infection in their ability to affect TLR4 expression at 6 h p.i. (Supplementary Fig. 4).
HMPV G protein blocks TLR4-mediated expression of immune mediators
We have recently shown that hMPV inhibits TLR-induced IFN-α production in moDCs and pDCs in a replication-dependent manner, suggesting that specific viral proteins affect TLR-mediated signaling pathways (19). To determine whether G protein expression could interfere with TLR4 signaling in hMPV-infected moDCs, leading to a reduction in pro-inflammatory mediator secretion, cells were infected with rhMPV-WT or -ΔG. At 24 h p.i., cells were washed and stimulated with LPS, and cell supernatants were harvested to measure cytokine and chemokine production. We found that LPS-induced secretion of cytokines, such as IL-6, IL-8, IL-10, IFN-γ and TNF-α, as well as chemokines, such as RANTES and MIP-1β, were significantly inhibited by rhMPV-WT infection, but not by rhMPV-ΔG (Fig. 6A), suggesting an inhibitory role of G protein on TLR4-dependent signaling in the context of hMPV infection. There was a minor induction of type I IFN following LPS stimulation, which was not significantly affected by hMPV infection, either WT or ΔG (data not shown).
Fig. 6. Effect of hMPV G protein on TLR4 agonist-induced gene expression.
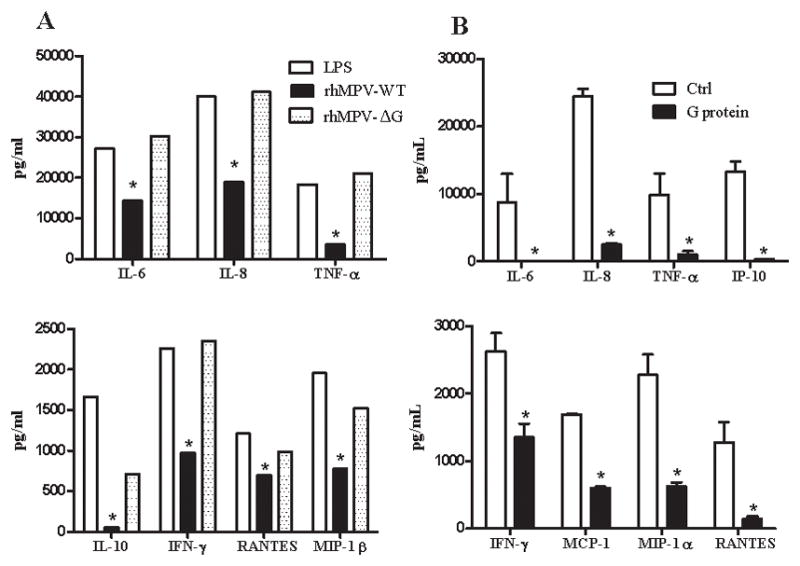
MoDCs were infected with hMPV, either WT or ΔG. After washing, cells were treated with 100 ng/ml of LPS for 18 h and supernatants were harvested to measure cytokines and chemokines by Bio-Plex (panel A) *, P < 0.05 compared to LPS treatment alone. MoDCs were pretreated with either control buffer (Ctrl) or purified G protein at 1μg (final concentration) for 30 min. Cells were then stimulated with 100 ng/ml of LPS for 18 h and supernatants were harvested to measure cytokine and chemokine secretion by Bio-Plex (panel B). Results are representative two separate experiments. *, P < 0.05 compared to control.
To confirm the role of hMPV G protein in blocking TLR4 activation, moDCs were pretreated with either control buffer or purified G protein at different concentrations for 30 min and then stimulated with LPS. Cell supernatants were harvested to measure cytokine and chemokine secretion. Cells treated with LPS alone produced significant amounts of proinflammatory mediators which were significantly reduced by pretreatment of cells with purified G protein in a dose-dependent manner (results are shown for a dose of 1 μg/ml) (Fig. 6B). An 80–90 fold reduction in secretion was observed for IL-6, IL-8, TNF-α, IP-10 and RANTES, while a 50–70 fold reduction was seen with other mediators such as IFN-γ, MCP-1 and MIP-1α. These results indicate that hMPV G protein indeed inhibits TLR4-dependent cellular signaling.
DISCUSSION
The innate immune response represents a critical component of the host defense against viruses and is coordinated at the cellular level by activation of transcription factors that regulate the expression of inducible gene products with antiviral and/or inflammatory activity. As the immune system evolved to combat viral infections, viruses have developed strategies to evade the host immune responses, mainly by targeting the type I IFN system. HMPV is the second most common cause of epidemic respiratory infections in infants and young children, and a significant cause of respiratory tract infections in the elderly and immunocompromised patients (4–6). The availability of the reverse genetic system for negative sense RNA viruses has allowed the dissection of viral protein functions in viral replication, as well as, in cellular signaling. In a recent investigation, we have shown that hMPV G protein affects cellular signaling in airway epithelial cells (23). In this study, we found that TLR4 plays an important role in hMPV-induced secretion of proinflammatory cytokines and chemokines, as well as, type I IFN in moDCs, and that hMPV G protein modulates cytokine and chemokine secretion in moDCs by targeting TLR4-dependent signaling pathway.
Several viral envelop proteins, including RSV F protein and proteins of mammary tumor virus, murine leukemia virus, vescicular stomatitis virus (15,16,39), and more recently Ebola virus (40), have been shown to activate TLR4 in primary immune cells. Similar to LPS, the primary ligand of TLR4, RSV F protein requires the presence of CD14 and MD-2 for signaling (34,36). TLR4 signaling has been shown to play an important role in controlling paramyxovirus infection. TLR4-deficient mice challenged with RSV exhibited impaired NK cell and CD14+ cell pulmonary trafficking, diminished NK cell function, and impaired IL-12 induction, in addition to impaired RSV clearance (15). In a model of alveolar macrophage depletion of TLR4-defective C3H/HeJ mice, we have shown that the early NF-κB response that occurs in the lung after RSV infection, is dependent upon alveolar macrophages and TLR4 (41). Furthermore, both TLR4 and the adaptor molecule MyD88 have been shown to be required for optimal protection against viral challenge in a mouse model of RSV infection (42). Our results show that down regulation of TLR4 expression in human moDCs, or lack of functional TLR4 in mouse BMDCs, result in significantly reduced expression of hMPV-induced cytokine, chemokine, and type I IFN secretion, indicating an important role of this TLR in the activation of cellular signaling following hMPV infection. The presence of residual mediator production in both cell types suggest the presence of additional pathways, either TLR-dependent or -independent, involved in hMPV-induced proinflammatory gene expression in moDCs. We are currently investigating the role of TLR4 in the pathophysiology of hMPV infection using C.C3-Tlr4Lps-d/J or C57BL/10ScNJ mice, which are defective in TLR4 signaling.
RSV G protein has been shown to modulate cytokine and chemokine production in monocytes, as infection with a mutant RSV lacking the full-length G protein, or the soluble part of G protein (sG) enhanced production of IL-6 and IL-8 (43). Addition of a specific peptide derived from RSV sG, the GC-rich region, inhibited proinflammatory responses elicited by LPS stimulation, indicating that RSV sG affects TLR4-dependent signaling (43). In our experiments, deletion of hMPV G protein resulted in enhanced production of cytokines, and chemokines, with minor changes in type I IFN secretion, indicating an important role of this surface glycoprotein in modulating cellular signaling in moDCs. The enhanced response of moDCs to rhMPV-ΔG infection was not due to an increased ability of rhMPV-ΔG to replicate, as initial staining of viral proteins was similar in cells infected with rhMPV-ΔG, compared to rhMPV-WT. Both naïve and recombinant viruses downregulated TLR4 expression, similar to what described for LPS (38), with rhMPV-ΔG inducing a later inhibition of TLR4 expression compared to WT. However, this finding is unlikely to explain the highly significant difference we observed in cytokine and chemokine secretion in response to rhMPV-ΔG at all time points of infection.
Three structural domains, i.e., a Leucine Rich Region (LRR) in the N-terminal ectodomain, a transmembrane region, and a Toll/IL-1R resistance (TIR) domain in the intracellular region, are structural hallmarks of all known Toll/TLRs. Differential utilization of four TIR-containing adapter molecules (i.e., MyD88, TIRAP, TRIF, and TRAM) by distinct TLRs leads to activation of downstream signaling pathways, findings based largely on studies in adapter knockout mice. Two major TLR signaling pathways have been identified, i.e., one that is MyD88-dependent, and gives rise to strong and early activation of NF-κB, and a TRIF-dependent, MyD88-independent pathway that primarily drives strong activation of IRF-3, with later activation of NF-κB. The MyD88-dependent pathway results in induction of highly NF-κB-dependent, proinflammatory genes (TNF-α, IL-1β, IL-6), while the MyD88-independent pathway leads to gene induction that is highly IRF-3-dependent (IFN-β, RANTES). TLR4 activates both pathways for gene expression, as it is the only TLR that uses both adapter proteins. In a recent study, Shingai et al. (44) have shown that addition of RSV soluble G (sG) protein suppresses the production of IFN-β in moDCs stimulated with LPS. RSV sG protein expression in HEK293 cells could inhibit the TLR adaptor TRIF/TICAM-1-dependent activation of an interferon-stimulated responsive element (ISRE) in transient transfection assays, suggesting that RSV G targets this adaptor to inhibit TLR3/4-dependent signaling. Our initial investigations have shown that infection of moDCs with rhMPV-ΔG affects primarily cytokine and chemokine production and much less type I IFN (only at early time points). Similarly, inhibition of TLR4-dependent signaling by hMPV G protein significantly modulates hMPV-induced cytokine and chemokine secretion, but not type I IFN. This could be due to a preferential inhibition of MyD88 versus TRIF in response to hMPV infection, or to the concomitant activation of TLR-independent pathways, such as the one dependent on protein kinase R (PKR), which has been shown to play a significant role in IFN production in response to certain viral infections (45,46).
In conclusion, we show that hMPV G protein is an important virulence factor, as it inhibits production of important immune mediators not only in airway epithelial cells (23), but also in DCs, by targeting TLR4 signaling. This is an important finding, as inadequate TLR stimulation, with subsequent lack of antibody affinity maturation has been recently identified as an important cause of vaccine failure and enhanced disease following administration of the formaline-inactivated RSV vaccine (47).
Supplementary Material
Acknowledgments
The authors would like to thank Deborah Prusak for assistance with Q-RT-PCR work and Cynthia Tribble for assistance in the manuscript submission.
Abbreviations
- hMPV
human metapneumovirus
- RSV
Respiratory syncytial virus
- mDC
myeloid dendritic cells
- moDCs
monocyte derived dendritic cells
- TLR
toll like receptors
- rhMPV-ΔG
recombinant hMPV lacking G protein
- rhMPV-WT
wild type virus
- BMDC
bone marrow derived dendritic cells
- PAMPs
different pathogen-associated molecular patterns
- pDCs
plasmacytoid dendritic cells
- PBMC
peripheral blood mononuclear cells
- ELISA
enzyme-linked immunosorbent assays
- LRR
Leucine Rich Region
- TIR
Toll/IL-1R resistance
- ISRE
interferon-stimulated responsive element
Footnotes
This work was supported by grants NIAID P01 062885 and R01 AI079246. X. B. was supported by the NIAID training grant T32 AI07536 and Parker B. Francis Award.
References
- 1.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jartti T, van den Hoogen BG, Garofalo RP, Osterhaus AD, Ruuskanen O. Metapneumovirus and acute wheezing in children. Lancet. 2002;360:1393–1394. doi: 10.1016/S0140-6736(02)11391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullins JA, Erdman DD, Weinberg GA, Edwards K, Hall CB, Walker FJ, Iwane M, Anderson LJ. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis. 2004;10:700–705. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boivin G, Abed Y, Pelletier G, Ruel L, Moisan D, Cote S, Peret TC, Erdman DD, Anderson LJ. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–1334. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 5.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 6.Esper F, Boucher D, Weibel C, Martinello RA, Kahn JS. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics. 2003;111:1407–1410. doi: 10.1542/peds.111.6.1407. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier G, Dery P, Abed Y, Boivin G. Respiratory tract reinfections by the new human Metapneumovirus in an immunocompromised child. Emerg Infect Dis. 2002;8:976–978. doi: 10.3201/eid0809.020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelletier G, Abed Y, Ruel L, Moisan D, Cote’ S, Peret TCT, Erdman DD, Anderson LJ, Boivin G, Chuq-Chul . Virological features and clinical manifestations associated with the human metapneumovirus, a newly discovered paramyxovirus. 2002. [DOI] [PubMed] [Google Scholar]
- 9.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 10.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Akira S, Takeda K. Functions of toll-like receptors: lessons from KO mice. C R Biol. 2004;327:581–589. doi: 10.1016/j.crvi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 13.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 14.Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16:141–147. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- 15.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc Natl Acad Sci U S A. 2002;99:2281–2286. doi: 10.1073/pnas.042355399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253–256. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 18.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 19.Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo RP. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol. 2006;34:320–329. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerrero-Plata A, Baron S, Poast JS, Adegboyega PA, Casola A, Garofalo RP. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J Virol. 2005;79:10190–10199. doi: 10.1128/JVI.79.16.10190-10199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biacchesi S, Skiadopoulos MH, Yang L, Lamirande EW, Tran KC, Murphy BR, Collins PL, Buchholz UJ. Recombinant human Metapneumovirus lacking the small hydrophobic SH and/or attachment G glycoprotein: deletion of G yields a promising vaccine candidate. J Virol. 2004;78:12877–12887. doi: 10.1128/JVI.78.23.12877-12887.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biacchesi S, Pham QN, Skiadopoulos MH, Murphy BR, Collins PL, Buchholz UJ. Infection of nonhuman primates with recombinant human metapneumovirus lacking the SH, G, or M2-2 protein categorizes each as a nonessential accessory protein and identifies vaccine candidates. J Virol. 2005;79:12608–12613. doi: 10.1128/JVI.79.19.12608-12613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao X, Liu T, Shan Y, Li K, Garofalo RP, Casola A. Human metapneumovirus glycoprotein G inhibits innate immune responses. PLoS Pathog. 2008;4:e1000077. doi: 10.1371/journal.ppat.1000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garofalo RP, Mei F, Espejo R, Ye G, Haeberle H, Baron S, Ogra PL, Reyes VE. Respiratory syncytial virus infection of human respiratory epithelial cells up-regulates class I MHC expression through the induction of IFN-β and IL-1α. J Immunol. 1997;157:2506–2513. [PubMed] [Google Scholar]
- 25.Welte T, Reagan K, Fang H, Machain-Williams C, Zheng X, Mendell N, Chang GJ, Wu P, Blair CD, Wang T. Toll-like receptor 7-induced immune response to cutaneous West Nile virus infection. J Gen Virol. 2009;90:2660–2668. doi: 10.1099/vir.0.011783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matheu MP, Sen D, Cahalan MD, Parker I. Generation of bone marrow derived murine dendritic cells for use in 2-photon imaging. J Vis Exp. 2008 doi: 10.3791/773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006 doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 29.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci U S A. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murawski MR, Bowen GN, Cerny AM, Anderson LJ, Haynes LM, Tripp RA, Kurt-Jones EA, Finberg RW. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol. 2009;83:1492–1500. doi: 10.1128/JVI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groskreutz DJ, Monick MM, Powers LS, Yarovinsky TO, Look DC, Hunninghake GW. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol. 2006;176:1733–1740. doi: 10.4049/jimmunol.176.3.1733. [DOI] [PubMed] [Google Scholar]
- 33.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic Acid-inducible gene I mediates early antiviral response and toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, Walsh EE, Freeman MW, Golenbock DT, Anderson LJ, Finberg RW. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 35.Goutagny N, Jiang Z, Tian J, Parroche P, Schickli J, Monks BG, Ulbrandt N, Ji H, Kiener PA, Coyle AJ, Fitzgerald KA. Cell type-specific recognition of human metapneumoviruses (HMPVs) by retinoic acid-inducible gene I (RIG-I) and TLR7 and viral interference of RIG-I ligand recognition by HMPV-B1 phosphoprotein. J Immunol. 2010;184:1168–1179. doi: 10.4049/jimmunol.0902750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lizundia R, Sauter KS, Taylor G, Werling D. Host species-specific usage of the TLR4-LPS receptor complex. Innate Immun. 2008;14:223–231. doi: 10.1177/1753425908095957. [DOI] [PubMed] [Google Scholar]
- 37.Ehl S, Bischoff R, Ostler T, Vallbracht S, Schulte-Monting J, Poltorak A, Freudenberg M. The role of Toll-like receptor 4 versus interleukin-12 in immunity to respiratory syncytial virus. Eur J Immunol. 2004;34:1146–1153. doi: 10.1002/eji.200324449. [DOI] [PubMed] [Google Scholar]
- 38.Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 39.Beutler B, Hoebe K, Georgel P, Tabeta K, Du X. Genetic analysis of innate immunity: identification and function of the TIR adapter proteins. Adv Exp Med Biol. 2005;560:29–39. doi: 10.1007/0-387-24180-9_4. [DOI] [PubMed] [Google Scholar]
- 40.Okumura A, Pitha PM, Yoshimura A, Harty RN. Interaction between Ebola virus glycoprotein and host toll-like receptor 4 leads to induction of proinflammatory cytokines and SOCS1. J Virol. 2010;84:27–33. doi: 10.1128/JVI.01462-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haeberle H, Takizawa R, Casola A, Brasier AR, Dieterich H-J, van Rooijen N, Gatalica Z, Garofalo RP. Respiratory syncytial virus-induced activation of NF-kB in the lung involves alveolar macrophages and Toll-like receptor 4-dependent pathways. J Infect Dis. 2002;186:1199–1206. doi: 10.1086/344644. [DOI] [PubMed] [Google Scholar]
- 42.Cyr SL, Angers I, Guillot L, Stoica-Popescu I, Lussier M, Qureshi S, Burt DS, Ward BJ. TLR4 and MyD88 control protection and pulmonary granulocytic recruitment in a murine intranasal RSV immunization and challenge model. Vaccine. 2009;27:421–430. doi: 10.1016/j.vaccine.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 43.Polack FP, Irusta PM, Hoffman SJ, Schiatti MP, Melendi GA, Delgado MF, Laham FR, Thumar B, Hendry RM, Melero JA, Karron RA, Collins PL, Kleeberger SR. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc Natl Acad Sci U S A. 2005;102:8996–9001. doi: 10.1073/pnas.0409478102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shingai M, Azuma M, Ebihara T, Sasai M, Funami K, Ayata M, Ogura H, Tsutsumi H, Matsumoto M, Seya T. Soluble G protein of respiratory syncytial virus inhibits Toll-like receptor 3/4-mediated IFN-beta induction. Int Immunol. 2008;20:1169–1180. doi: 10.1093/intimm/dxn074. [DOI] [PubMed] [Google Scholar]
- 45.Stojdl DF, Abraham N, Knowles S, Marius R, Brasey A, Lichty BD, Brown EG, Sonenberg N, Bell JC. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. J Virol. 2000;74:9580–9585. doi: 10.1128/jvi.74.20.9580-9585.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilfoy FD, Mason PW. West Nile Virus-Induced Interferon Production Is Mediated by the Double-Stranded RNA-Dependent Protein Kinase PKR. J Virol. 2007;81:11148–11158. doi: 10.1128/JVI.00446-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.