Abstract
Background
Sleep difficulty is a common symptom of cannabis withdrawal, but little research has objectively measured sleep or explored the effects of hypnotic medication on sleep during cannabis withdrawal.
Methods
Twenty daily cannabis users completed a within-subject crossover study. Participants alternated between periods of ad-libitum cannabis use and short-term cannabis abstinence (3 days). Placebo was administered at bedtime during one abstinence period (withdrawal test) and extended-release zolpidem, a non-benzodiazepine GABAA receptor agonist, was administered during the other. Polysomnographic (PSG) sleep architecture measures, subjective ratings, and cognitive performance effects were assessed each day.
Results
During the placebo-abstinence period, participants had decreased sleep efficiency, total sleep time, percent time spent in Stage 1 and Stage 2 sleep, REM latency and subjective sleep quality, as well as increased sleep latency and time spent in REM sleep compared with when they were using cannabis. Zolpidem attenuated the effects of abstinence on sleep architecture and normalized sleep efficiency scores, but had no effect on sleep latency. Zolpidem was not associated with any significant side effects or next-day cognitive performance impairments.
Conclusions
These data extend prior research that indicates abrupt abstinence from cannabis can lead to clinically significant sleep disruption in daily users. The findings also indicate that sleep disruption associated with cannabis withdrawal can be attenuated by zolpidem, suggesting that hypnotic medications might be useful adjunct pharmacotherapies in the treatment of cannabis use disorders.
Keywords: Cannabis, Marijuana, Sleep, Withdrawal, Zolpidem, Pharmacotherapy
1. Introduction
Cannabis (marijuana, hashish) is the most widely used illicit drug in the world (UNODC, 2007). Cannabis dependence develops in a subset of users, typically daily or near daily users. Prevalence rates of cannabis dependence vary by region, but generally exceed the rate for dependence on any other illicit drug (AIHW, 2008; EMCDDA, 2008; SAMHSA, 2008; UNODC, 2007). Clinical trials have demonstrated clinical efficacy for several psychosocial interventions, but, to date, there are no medications known to improve the clinical outcomes of those seeking treatment for cannabis use disorders (Benyamina et al., 2008; Nordstrom and Levin, 2007; Vandrey and Haney, 2009).
Cannabis dependence is associated with a drug withdrawal syndrome that appears comparable to that observed with tobacco withdrawal (Vandrey et al., 2008; Vandrey et al., 2005b), and dependent users often fail at attempts to quit or reduce use (Budney and Moore, 2002). The cannabis withdrawal syndrome has been characterized in a number of controlled laboratory and clinical survey studies (Budney et al., 2004; Haney, 2005). One of the hallmark symptoms of cannabis withdrawal is self-reported sleep difficulty. In clinical survey studies, sleep difficulty during attempts to quit cannabis use has been endorsed by 67–73% of adults (Budney et al., 1999; Budney et al., 2008) and 33–43% of adolescents (Crowley et al., 1998; Vandrey et al., 2005a). In prospective laboratory studies, sleep difficulty has been consistently rated as one of the most severe symptoms of cannabis withdrawal (Budney et al., 2001; Budney et al., 2003; Vandrey et al., 2008).
There is also growing evidence that cannabis withdrawal, and sleep difficulty in particular, may negatively impact attempts to stop cannabis use. In a recent survey of adult cannabis users in four U.S. cities, 65% of respondents indicated that sleep difficulty during abstinence contributed to relapse on at least one previous quit attempt (Budney et al., 2008). In two survey studies of non-treatment seekers, sleep difficulty was reported by 32–47% of cannabis users during prior periods of cannabis abstinence, of which 48–77% reported having resumed cannabis use or used sedative/hypnotic drugs, alcohol, or other substances in attempts to alleviate their sleep problems (Copersino et al., 2006; Levin et al., 2010). These findings indicate that sleep disturbance associated with cannabis withdrawal is clinically important, and suggest that treatment of this key withdrawal symptom may facilitate efforts to discontinue cannabis use. However, it is unknown whether use of sedative/hypnotic medications would actually be effective in ameliorating sleep disturbance induced by cannabis withdrawal.
Another limitation to understanding sleep problems associated with cannabis withdrawal is that polysomnographic (PSG) characterization of sleep during cannabis withdrawal has not been sufficiently studied. Studies conducted in the 1970’s indicated that chronically administered oral THC decreased REM sleep and increased Stage 3/4 (slow-wave) sleep when the drug was introduced, as well as increased sleep latency and REM sleep, and decreased Stage 3/4 sleep during THC withdrawal (for review, see Schierenbeck et al., 2008). However, the generality of these findings is limited because the results were inconsistent across studies. During withdrawal periods, sleep continuity (sleep latency or wake after sleep onset) was significantly affected in 4 of 7 studies, REM sleep was affected in 3 of 7 studies, and Stage 3/4 sleep was affected in 3 of 7 studies. Other limitations were that sample sizes were small (2–7 participants) and oral THC rather than smoked cannabis was administered in 5 of 7 studies.
More recently, a cross-sectional study was conducted in which PSG sleep data were obtained from daily cannabis users during a period of abrupt abstinence as well as a control group of participants without a history of substance abuse (Bolla et al., 2008; Bolla et al., 2010). The abstinent cannabis users had less total sleep time, decreased sleep efficiency, increased sleep latency, decreased REM latency, less Stage 3/4 sleep and more nighttime awakenings compared with the control group (Bolla et al., 2008). The cannabis users also had reduced total sleep time, sleep efficiency and REM sleep, and an increase in time awake after sleep onset and periodic limb movements across 13 days of abstinence (Bolla et al., 2010). These findings clearly indicate that, relative to healthy controls, cannabis users experience disturbed sleep during cannabis withdrawal. However, because baseline sleep data (during active cannabis use) were not obtained in the abstinent cannabis users and sleep was only assessed in the control group during one night (compared with 5 in cannabis users) it is not possible to discern whether the disordered sleep patterns observed in the cannabis users were indeed caused by the imposed period of cannabis abstinence.
The present study was conducted to extend this prior research. A within-subject crossover study design with baseline administration of smoked cannabis was used to address the methodological limitations of the aforementioned studies, and hypnotic medication was administered in an attempt to normalize sleep during withdrawal. Specific aims of the study were to 1) examine the effects of cannabis withdrawal on sleep continuity and architecture, and 2) determine whether administration of extended-release zolpidem (Ambien CR®) could attenuate withdrawal-induced sleep disturbance. Zolpidem was selected because it is widely used for the treatment of sleep disorders (e.g. insomnia), it has a good safety, side effect, and abuse liability profile among hypnotic medications, and it does not appear to have deleterious effects on sleep architecture or produce rebound insomnia when use is discontinued. Demonstration that zolpidem can suppress cannabis withdrawal-induced sleep disturbance would suggest that administration of sedative-hypnotic medications could be a useful symptom-specific intervention strategy in the treatment of cannabis use disorders.
2. Methods
2.1 Participants
Cannabis users were recruited through newspaper advertisements. Volunteers were included in the study if they: 1) were 18–55 years old, 2) used cannabis at least 25 days/month and provided a urine specimen positive for cannabinoids (qualitative rapid test, Medical Disposables Corp., Orlando, FL, USA), 3) had at least an 8th grade level of education and demonstrated literacy, 4) were not taking psychoactive medication, 5) did not meet criteria for primary sleep or Axis-I psychiatric disorders (DSM-IV) other than nicotine or cannabis dependence; 6) were not seeking treatment for cannabis-related problems or using cannabis for a medical disorder, 7) had a negative urine toxicology test for drugs other than cannabis (cocaine, opioids, amphetamine, methamphetamine, PCP, benzodiazepines, barbiturates, methadone, and MDMA) and a negative breath alcohol test on the day of admission, 8) had a negative urine pregnancy test, 9) had no history of seizures, severe hepatic impairment, or conditions associated with clinically significant cognitive impairment, 10) were not taking drugs that affect drug metabolism via cytochrome P450, 11) had not used hypnotic medication in the past month or reported a history of repeated use of hypnotics to resolve sleep difficulties in the past year. Also, due to large increases in systolic and diastolic blood pressure observed during cannabis abstinence in an initial subset of study participants (Vandrey et al., In Press), we excluded study volunteers with high (>140/90 mmHg) or unstable blood pressure midway through the study.
A telephone interview was conducted followed by a laboratory screening to determine study eligibility. The Time-Line Follow-Back (TLFB) method (Sobell and Sobell, 1992) was used to obtain the amount and frequency of substance use during the previous 90 days. The DSM Checklist (Hudziak et al., 1993) was used to diagnose current Axis I psychiatric disorders. The Marijuana Quit Questionnaire (Boyd et al., 2005) was conducted to obtain a detailed history of cannabis use and prior quit attempts. The Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989) was used to detect clinically relevant sleep problems. The Marijuana Withdrawal Checklist (MWC) (Budney et al., 1999) was used to assess the presence of cannabis withdrawal symptoms during prior periods of abstinence.
Written informed consent was obtained prior to study participation. The study was approved by the John Hopkins Medicine IRB and conducted in accordance with the ethical standards of the Helsinki Declaration. Twenty of 25 participants successfully completed the study. Of the 5 who did not complete the study: 3 left to pursue employment, 1 did not tolerate the PSG monitoring procedures, and 1 left due to legal obligations. Study completers had an average (SD) age of 29 (8) years, and were 85% male, 65% African American, 25% Caucasian, and 10% Asian or of mixed ethnicity. Total score on the Pittsburgh Sleep Quality Index (PSQI) at intake was 5 (3). Participants on average first used cannabis at 14 (2) years of age, had been using cannabis regularly for 14 (8) years, and currently used cannabis 4 (3) times per day. All participants met DSM-IV criteria for either cannabis abuse (N=10) or dependence (N=10). Use of drugs other than cannabis was infrequent (Table 1). On the MWC, participant-rated sleep difficulty during the most recent 24-hour period of cannabis abstinence was “none” for 20% of participants, “mild” for 40% of participants, “moderate” for 20% of participants, and “severe” for 20% of participants. The most common route of cannabis administration was via “blunt”, cannabis rolled in a hollowed out cigar.
Table 1.
Drug use characteristics of study participants.
| Ever Used? | Used Past 30 Days? |
Frequency Past 30 Days‡ |
|
|---|---|---|---|
| Alcohol | 19/20 | 14/20 | 2 (2) drinks/week |
| Cannabis | 20/20 | 20/20 | 4 (3) times/day |
| Cocaine | 8/20 | 3/20 | 1 time |
| Hallucinogens | 7/20 | 1/20 | 1 time |
| MDMA | 3/20 | 1/20 | 1 time |
| Nicotine/Tobacco* | 20/20 | 16/20 | 9 (6) times/day |
| Opioids | 6/20 | 0/20 | -- |
| Hypnotics/Tranquilizers | 6/20 | 0/20 | -- |
2 participants smoked cigars rather than cigarettes. Tobacco use was not restricted during the study.
Mean (SD) among those who used in the prior month
2.2 Procedures
A within-subject crossover design was used. The study lasted for 20 days, of which 11 days required overnight inpatient housing at the Johns Hopkins Bayview Medical Center (JHBMC). On Day 1, participants received training on study procedures, smoked cannabis ad libitum after training until 21:00, and completed an overnight polysomnography (PSG) sleep assessment to acclimate participants to sleeping while connected to PSG equipment and screen for sleep apnea and periodic limb movement disorder. A cannabis-use period was conducted on Days 2 and 3, during which participants used cannabis ad libitum between 12:00 and 21:00, and were administered an oral capsule containing placebo at bedtime each night. A cannabis-abstinence period was conducted on Days 4–6. On these days participants were not permitted to use cannabis and capsules administered at bedtime contained either placebo or 12.5 mg extended-release zolpidem (Ambien CR, Sanofi-Aventis LLC). Total cannabis abstinence, rather than administration of placebo cannabis, was used in this study order to better model cannabis quit attempts as they occur naturalistically. Subjective, physiological, cognitive performance, and sleep PSG assessments were conducted each day.
After completing cognitive tasks and self-report assessments on the morning of Day 7, participants were discharged from the residential facility for 8 days. The purpose of this outpatient study period was to allow participants to re-establish baseline levels of cannabis use. On the morning of Day 15, participants were re-admitted to the residential facility. Participants self-reported continued daily cannabis use on the TLFB during the outpatient phase and provided a urine specimen that was qualitatively positive for THC and negative for other drugs of abuse at the time of the second inpatient admission. For the following 5 days they repeated the 2-day cannabis-use and 3-day cannabis-abstinence periods exactly as conducted on Days 2–7. If participants received placebo medication at bedtime during the first cannabis-abstinence period, they received 12.5 mg extended-release zolpidem during the second period or vice-versa. The order in which participants received zolpidem or placebo was counterbalanced.
Use of tobacco products (cigarettes, cigars) was allowed ad-libitum during daytime hours. Participants were allowed to drink coffee or other caffeinated beverages with breakfast, but were restricted from caffeine containing food and beverages after 10:00 each day of the inpatient phases. The allowance of tobacco and limited caffeine use was implemented in order to limit any effects of tobacco or caffeine withdrawal on study outcome measures.
2.3 Drug Administration
The cannabis provided during the acclimation day and two cannabis-use periods was obtained from the National Institute on Drug Abuse (NIDA). Cannabis was prepared in cigarettes that contained approximately 0.8 grams of cannabis each and the cannabis contained approximately 3% THC by volume. Cannabis smoking occurred in a specially ventilated room. During the outpatient study phase, participants obtained cannabis by means normally used when not participating in the study.
During inpatient study periods, except on the acclimation day, participants were administered an oral (size 00) capsule each night at bedtime. During cannabis-use periods the capsules always contained placebo (capsule packed with lactose). During one cannabis-abstinence period, the capsules administered contained placebo each night, and during the other cannabis-abstinence period the capsules contained one commercially purchased 12.5 mg extended-release zolpidem pill with lactose packed around it. All bedtime medication was administered double blind by a registered nurse.
2.4 Objective Sleep Assessment
Participants’ sleep was monitored nightly using an Embla N-7000 digital polysomnography (PSG) data recorder (Broomfield, CO). A standard PSG montage was used: 1) five EEGs (F3-A2, F4-A1, C4-A1, C3-A2, O1-A2, O2-A1); 2) right and left electro-oculograms; 3) three EMG’s (submental and anterior tibialis muscles). Respiratory function was measured via oronasal thermister, nasal air pressure transducer, pulse oximetry, and abdominal and thoracic strain gages. Respiratory sensors and tibialis EMGs were used during the acclimation night to screen for undiagnosed sleep apnea, and periodic limb movement disorder, respectively, and were removed on subsequent nights for participant comfort. A study technician monitored biometric signals throughout the night in a separate control room. A certified sleep technician followed standardized procedures of the American Academy of Sleep Medicine (Iber et al., 2007) to score the PSGs, and a physician certified in sleep medicine reviewed each scored record prior to data analysis. Technicians responsible for recording and scoring PSGs were blind to the study conditions of participants.
Outcome variables included measures of sleep continuity (initial sleep latency, time awake after sleep onset) and sleep architecture (% time spent in sleep stages 1, 2, 3/4 (slow-wave sleep) and REM). Sleep efficiency, an index of sleep continuity that incorporates initial sleep latency and time awake after sleep onset (total sleep time/time in bed) was also calculated. Sleep efficiency ratios below .85 are generally considered indicative of disordered sleep.
Participants were allowed to retire between the hours of 23:00 and 24:00 and slept ad libitum until they decided to wake for the day or 9:00, whichever came first. Oral medication was administered at lights out. PSG data collection began when the participants turned out the lights to go to sleep and stopped when they woke for the day. Participants were not permitted to sleep during daytime hours. The timing of lights out, morning awakening, and medication administration was conducted with limited structure in an attempt to approximate naturalistic sleep patterns of the study volunteers without impacting the daytime protocol requirements.
2.5 Self-Report Measures
At 10:00 each day, participants completed a sleep diary using items from the PSQI to rate how they slept the previous night, the MWC to indicate experience of cannabis withdrawal symptoms the previous day, and the Marijuana Craving Questionnaire (MCQ) (Heishman et al., 2009) to indicate current craving for cannabis. Participants also completed a medication side effect questionnaire derived from the medication package insert for extended-release zolpidem. Side effects experienced in the prior 24 hours were rated from 0–3 (not at all, mild, moderate, severe).
2.6 Physiological Measures
Vital signs were assessed at 10:00, 16:00 and 21:30 via automated monitor.
2.7 Cognitive Performance Measures
A cognitive performance battery was completed daily at 10:30 to assess daytime cognitive functioning. Performance tasks took approximately 75 minutes to complete and all were completed prior to cannabis use to avoid any effects of acute cannabis intoxication. Tasks included: Flicker-Fusion, a sensory perception task (Simonson and Brozek, 1952); Digit Symbol Substitution Test (DSST), a measure of psychomotor ability (McLeod et al., 1982); a divided attention task (Kleykamp et al., 2010); N-Back, a measure of working memory (Gevins and Cutillo, 1993); Word Memory, a measure of episodic memory and metamemory (Mintzer et al., 1997); and Tower of London (TOL), a measure of executive function and planning (Ramaekers et al., 2006; Shallice, 1982). On Day 1 participants completed each task until competency was demonstrated and performance was stable on primary outcome variables (within 20% for at least three consecutive trials).
2.8 Data Analysis
Data collected on Day 1 were excluded from analysis because this was a training/acclimation day. Study outcome measures were analyzed using a repeated measures regression with an AR(1) covariance structure. Factors in the analyses included cannabis use condition (ad-libitum use, abstinence), bedtime medication condition (zolpidem, placebo), and time (study day). When significant main effects or interactions were observed, Tukey’s post-hoc tests were used to detect differences within factors. Data analysis was completed using SAS statistical software (Version 9.1), and α was set at 0.05 for all tests of significance.
3. Results
3.1 Objective Sleep Assessment
Analysis of PSG data indicated significant main effects for cannabis use condition on total sleep time (F = 23.3, p < .01), sleep efficiency (F = 14.4, p < .01), sleep latency (F = 36.7, p < .01), REM latency (F = 11.1, p < .01), %Stage 1 sleep (F = 16.6, p < .01), and %REM sleep (F = 5.5, p < .05) (see Table 2 for details). Significant cannabis use by medication interactions were observed for %Stage 2 sleep (F = 7.1, p < .05) and %REM sleep (F = 4.5, p < .05). Post-hoc tests indicated that participants had reduced total sleep time, sleep efficiency, latency to REM onset, %Stage 1 sleep, and %Stage 2 sleep, and increased sleep latency and %REM sleep during the placebo-abstinence period compared with cannabis use periods. Participants had a reduction in total sleep time and sleep efficiency, and an increase in sleep latency during the zolpidem-abstinence period compared with cannabis use periods. Participants had reduced %Stage 2 and increased %REM sleep during the placebo-abstinence period compared with the zolpidem-abstinence period. No significant effects were observed for Stage 3/4 sleep or time awake after sleep onset.
Table 2.
Mean PSG Outcomes By Study Condition.
| Placebo | Extended-Release Zolpidem | |||
|---|---|---|---|---|
| Cannabis Use | Cannabis Abstinence | Cannabis Use | Cannabis Abstinence | |
| Total Sleep Time (min) | 451 (10) | 419 (9.6) * | 464 (10) | 419 (10) * |
| Sleep Efficiency | .89 (.01) | .85 (.01) * | .91 (.01) | .88 (.01) * |
| Sleep Latency (min) | 8 (1) | 29 (5) * | 8 (1) | 22 (3) * |
| REM Latency (min) | 103 (8) | 71 (5) * | 90 (6) | 83 (5) |
| Wake After Sleep Onset (min) | 45 (6) | 44 (6) | 40 (6) | 36 (5) |
| % Stage 1 Sleep | 10 (1) | 8 (1) * | 9 (1) | 8 (1) |
| % Stage 2 Sleep | 50 (1) | 46 (1) *‡ | 49 (1) | 51 (1)‡ |
| % Stage 3/4 Sleep | 15 (1) | 16 (1) | 16 (1) | 15 (1) |
| % REM Sleep | 25 (1) | 30 (1) *‡ | 26 (1) | 26 (1)‡ |
Denotes a significant abstinence effect
Denotes a significant difference between abstinence conditions
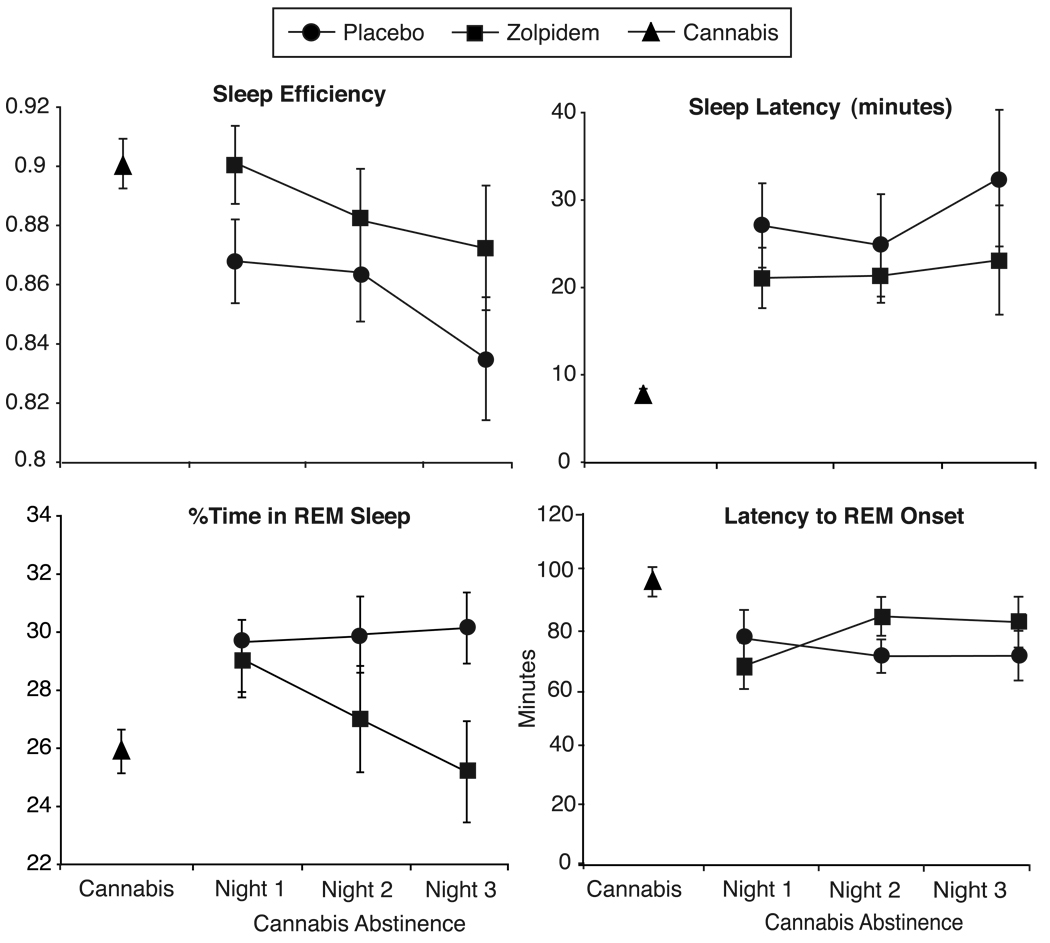
Figure 1 illustrates mean scores for sleep continuity and REM measures on each day of abstinence. Sleep continuity appears increasingly degrade over the three days of abstinence, while abstinence effects on REM sleep remain stable. The exception to this is that the effect of zolpidem on REM sleep appears to be delayed until the second day of abstinence.
Figure 1.
Mean sleep continuity and REM measures by day during abstinence. For parsimony, the 4 days of ad-libitum cannabis use have been averaged and are represented by triangles. Circles indicate cannabis abstinence nights in which placebo medication was administered at bedtime and squares indicate cannabis abstinence nights in which extended-release zolpidem was administered at bedtime.
3.2 Self-Report Measures
A main effect of cannabis use was observed for PSQI sleep latency (F = 4.5, p < .05), with participants reporting increased time to get to sleep during both abstinence periods compared with cannabis use periods. Significant interactions (cannabis use × medication) were observed for PSQI nocturnal awakenings (F = 7.5, p < .05) and PSQI subjective sleep quality (F = 4.2, p < .05). Participants reported more nocturnal awakenings and worse sleep quality during the placebo-abstinence phase compared with other study conditions.
Significant main effects of cannabis use were observed for Marijuana Withdrawal Checklist ratings of decreased appetite (F = 33.9, p < .05), dizziness (F = 4.6, p < .05), restlessness (F = 7.7, p < .05), strange/wild dreams (F = 9.6, p < .05), increased sexual arousal (F = 6.1, p < .05), and the composite Withdrawal Discomfort Score (WDS) (F = 13.6, p < .05). All of these items increased in severity during abstinence relative to cannabis use periods, indicating cannabis withdrawal effects. No differences in withdrawal severity were observed between the placebo-abstinence and zolpidem-abstinence periods.
No significant differences were observed for ratings of cannabis craving (MCQ) or medication side effects during the study.
Main effects of cannabis use and time were observed for reaction time assessments on the N-back (1, 2, and 3-back) and Tower of London, and for the number of attempted and correct DSST trials completed. Post-hoc analyses for all of these measures showed that, despite the competency training conducted on Day 1, performance improved (reduced reaction time, increased # of DSST trials) over time. This indicates that differences were due to practice effects, and the main effects of cannabis use observed simply reflect the fact that cannabis use periods always preceded cannabis abstinence periods. No significant differences were observed on other cognitive performance measure outcomes.
3.3 Physiological Measures
Analysis of blood pressure and heart rate assessments were limited to those obtained at the 10:00 am time point for consistency with other performance measures and to eliminate the effects caused by acute cannabis intoxication. A main effect of cannabis was observed for diastolic blood pressure (F = 13.2, p < .05), with increased blood pressure observed during cannabis abstinence periods compared with cannabis use periods.
4. Discussion
Clinical surveys and laboratory models of withdrawal indicate that sleep disturbance is common among frequent cannabis users and may directly contribute to either relapse to cannabis use or compensatory use of other drugs (Budney et al., 2004; Budney et al., 2008; Copersino et al., 2006; Haney, 2009; Peters and Hughes, 2010). However, most studies of cannabis withdrawal have relied on self-reported sleep assessments, which do not always accurately reflect actual sleep quality and do not provide information about sleep architecture (Morgan et al., 2006). The present study provides objective evidence that abrupt cessation of daily cannabis use can indeed result in disrupted sleep continuity and altered sleep architecture, with latency to sleep onset and REM sleep most notably affected. These findings confirm and extend previous studies indicating that abrupt marijuana cessation leads to sleep disruption, and provide the first data demonstrating that these effects can be attenuated with administration of hypnotic medication.
The decreased sleep efficiency and increased sleep latency observed during cannabis withdrawal in the present study is consistent with prior studies (Bolla et al., 2008; Schierenbeck et al., 2008). Mean sleep efficiency scores during the cannabis withdrawal period exceeded the cutoff (0.85) generally used to indicate clinically important sleep disturbance by the third day of abstinence. Chronic decreases in sleep continuity have been associated with a variety of negative sequelae including fatigue, cognitive decline, decreased pain thresholds and spontaneous pain, increased anxiety and negative mood (Stores, 2007). Also, %REM sleep increased and REM latency decreased during withdrawal in this study. REM sleep is increasingly believed to play a role in affect regulation and memory related processes (Stickgold and Walker, 2007; Wagner et al., 2001; Walher, 2010). Reduced REM latency and increased %REM, due to heritable or situational factors, have been linked to depression (Cartwright and Wood, 1991) and heightened pain sensitivity (Smith et al., 2005). Thus, it is possible that secondary effects of reduced sleep continuity (e.g. cognitive decline, anxiety, negative mood) and increased REM (depression, pain sensitivity) during cannabis withdrawal could put users at an increased risk for relapse. However, research is needed to determine whether or not these associations extend to changes in sleep induced by cannabis withdrawal.
Some differences between the findings of the present study and those reported by Bolla and colleagues (2008, 2010) warrant discussion. First, in contrast to the present study, Bolla et al. found no abstinence effects on %REM sleep, reported that %REM sleep declined over time, and that cannabis abstinence was associated with less Stage 3/4 (slow-wave) sleep. These differences could be accounted for by the possibility that the cannabis users in the study by Bolla and colleagues had lower REM and Stage 3/4 sleep compared with controls at baseline. For REM sleep, an increase during abstinence, consistent with what was observed in our study, would have resulted in more similarity to the control group (no difference observed), and the gradual reduction in REM sleep observed over time may reflect a return to baseline. Stage 3/4 sleep was stable within-subjects across both studies, but relatively large inter-subject variability was observed (mean %Stage 3/4 sleep across nights ranged from 4.4% to 26.5%) in the present study. Thus, it is possible that a larger percentage of cannabis users with low Stage 3/4 sleep was enrolled in the study by Bolla et al. That said, we cannot eliminate the possibility that factors other than cannabis use impacted the data in the present study because a control group of non-cannabis users was not included.
At this point it remains unclear whether the reduced Stage 3/4 sleep observed among a subset of daily cannabis users across studies is related to chronic use of cannabis, cannabis withdrawal, or an unrelated cause. That said, Stage 3/4 sleep is integral to the restorative effects of sleep and has been shown to predict relapse among those in treatment for alcohol use disorders (Brower et al., 1998). Thus, it will be important for future studies to further delineate the effects of cannabis use and withdrawal on Stage 3/4 sleep, and, to identify heritable or substance use risk factors that predict the effects of cannabis withdrawal on Stage 3/4 sleep.
In this study, administration of extended-release zolpidem attenuated the effects of cannabis withdrawal on sleep. No differences were observed between periods of cannabis use and the zolpidem-abstinence condition for measures of sleep architecture (% time spent in different sleep stages). Also, zolpidem administration significantly reversed abstinence induced changes in Stage 2 and REM sleep relative to the placebo-abstinence condition. While sleep efficiency was worse during the zolpidem-abstinence condition compared with cannabis use, mean sleep efficiency scores were maintained in the range of normal sleep (>.85) on all three nights. Zolpidem did not reduce sleep latency as expected. This may be because it was administered when participants turned out the lights for the night, which likely did not allow enough time for the onset of clinical effects with the medication. Thus, it is possible that zolpidem administered 20–30 minutes prior to bedtime would improve sleep continuity (reduced sleep latency and increased sleep efficiency).
Study participants self-reported significantly better sleep quality when they received active medication compared with placebo. This suggests that zolpidem may be useful for the treatment of sleep disturbance associated with cannabis withdrawal. Additional research is warranted to determine whether the level of improvement in sleep quality obtained with zolpidem during cannabis withdrawal can translate to improved clinical outcomes for those trying to quit use of cannabis. Other medications that can improve sleep continuity and/or reverse the effects of cannabis withdrawal on REM sleep should also be explored.
With the exception of the speed with which responses were made on some tasks, cognitive performance assessed each morning remained stable throughout the study. On one hand, this indicates that there was no “hangover” effect of zolpidem on cognitive performance. This is consistent with prior research (cf. Blin et al., 2006). On the other hand, clinically significant sleep deficits have been associated with impaired cognitive abilities (cf. Altena et al., 2008; Edinger et al., 2008). Because sleep continuity and REM measures were significantly degraded during the cannabis withdrawal period it was somewhat surprising that a corresponding decrease in cognitive performance was not observed. It is possible that the duration of abstinence was not long enough for the development of cognitive impairment associated with cumulative sleep loss, or that the tasks used were not sensitive to the type (e.g. sustained or shifting attention) or magnitude of change in cognitive ability associated with sleep loss. A recent literature review suggests that cognitive deficits among people with primary insomnia are subtle and inconsistently observed in controlled research studies not involving experimenter induced sleep deprivation (Shekleton et al., 2010). Thus, the failure to find impairment on cognitive performance in the present study is not entirely unexpected or inconsistent with research on the cognitive consequences of poor sleep.
There are limitations of the present study that warrant discussion. The duration of abstinence studied was relatively short and there was a trend for sleep to become progressively worse over the three days of abstinence. Previous findings suggest that changes in sleep architecture persist for at least 2 weeks (Bolla et al., 2010), and subjective ratings of sleep difficulty and strange dreams have been observed for up to 7 weeks in an outpatient study of continuous cannabis abstinence (Budney et al., 2003). A study of longer duration is needed to determine the peak severity and time course of abstinence-induced alterations of sleep architecture in daily cannabis users. While extended-release zolpidem appears to have improved objective and subjective measures of sleep quality, this did not translate to a reduction in overall cannabis withdrawal severity or craving. That said, participant-rated withdrawal and craving scores were relatively low in this study, possibly because the duration of abstinence was short and the study was conducted in a residential setting devoid of the environmental cues associated with cannabis use that can exacerbate craving and withdrawal. Replication of these effects using ambulatory sleep collection methods will be important to validate the present findings and the inpatient model. Also, participants in the present study were not seeking treatment. Additional research is needed to prospectively assess the correlation between sleep and relapse in a clinical population, and to determine whether an improvement in sleep quality can positively impact treatment success among those with cannabis use disorders.
In summary, this study provides objective evidence of sleep disturbance during cannabis withdrawal and suggests that pharmacological, and, possibly, behavioral interventions known to reduce sleep latency and normalize REM could be useful in the treatment of cannabis use disorders. Significant sleep disruption appears to be a common feature of withdrawal across drugs of abuse, and it has been suggested that sleep disturbance is a universal risk factor for relapse among those with drug use disorders (Brower and Perron, 2010). Additional research is recommended to 1) examine the time course of cannabis withdrawal effects on sleep continuity and architecture, 2) replicate these effects in outpatient clinical samples, 3) prospectively establish the association between sleep and relapse during a quit attempt, 4) determine whether alternative medications or doses of zolpidem can further attenuate sleep disturbance, and 5) examine the commonality and treatment implications of abstinence-induced sleep disturbance in drug use disorders more broadly.
Acknowledgements
The authors wish to thank Maxine Stitzer, Miriam Mintzer, Karen Bolla, Linda Felch, and the staff of the Behavioral Pharmacology Research Unit (BPRU) and Clinical Research Unit (CRU) at Johns Hopkins University for their valuable contributions in the design and execution of this research.
Role of Funding Source. This research was supported by grants DA025794 (Vandrey) and DA12471 (Budney) from the National Institute on Drug Abuse, grant NS4716 (Smith) from the National Institute of Neurological Disorders and Stroke, and grant UL1-RR025005 from the National Center for Research Resources, a component of the National Institutes of Health, and NIH Roadmap for Medical Research. The study design; collection, analysis and interpretation of data; writing of the report; and decision to submit the paper for publication were all completed at the sole discretion of the authors with no role of any funding agencies. This study was registered at clinical trials.gov (NCT00893269).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors. Authors Vandrey, Smith, and Budney designed the study and wrote the protocol. Authors Vandrey, Smith, McCann, and Curran participated in data collection/interpretation. Authors Vandrey and Curran undertook data analysis. Author Vandrey wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest. The authors have no conflicts of interest to declare.
References
- AIHW. Alcohol and Other Drug Treatment Services in Australian Capital Territory 2006–07: Findings from the National Minimum Data Set. Canberra: Australian Institute of Health and Welfare; 2008
- Altena E, Van Der Werf YD, Strijers RL, Van Someren EJ. Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. J. Sleep Res. 2008;17:335–343. doi: 10.1111/j.1365-2869.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- Benyamina A, Lecacheux M, Blecha L, Reynaud M, Lukasiewcz M. Current state of phamacotherapy and psychotherapy in cannabis withdrawal and dependence. Expert Rev. Neurother. 2008;8:479–491. doi: 10.1586/14737175.8.3.479. [DOI] [PubMed] [Google Scholar]
- Blin O, Micallef J, Audebert C, Legangneux E. A double-blind, placebo- and flurazepam-controlled investigation of the residual psychomotor and cognitive effects of modified release zolpidem in young healthy volunteers. J. Clin. Psychopharmacol. 2006;26:284–289. doi: 10.1097/01.jcp.0000218985.07425.d9. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Funderburk FR, Lud-Cadet J, David PM, Verdejo-Garcia A, Benbrook AR. Sleep disturbance in heavy marijuana users. Sleep. 2008;31:901–908. doi: 10.1093/sleep/31.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Wang NY, Funderburk FR, Allen RP, David PM, Cadet JL. Polysomnogram changes in marijuana users who report sleep disturbances during prior abstinence. Sleep Med. 2010;11:882–889. doi: 10.1016/j.sleep.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Strategies for quitting among non-treatment-seeking marijuana users. Am. J. Addict. 2005;14:35–42. doi: 10.1080/10550490590899835. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcohol relapse. Alcohol. Clin. Exp. Res. 1998;22:1864–1871. [PubMed] [Google Scholar]
- Brower KJ, Perron BE. Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Med. Hypotheses. 2010;74:928–933. doi: 10.1016/j.mehy.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch. Gen. Psychiatry. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey RG. A review of the validity and significance of the cannabis withdrawal syndrome. Am. J. Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA. Development and consequences of cannabis dependence. J. Clin. Pharmacol. 2002;42:28S–33S. doi: 10.1002/j.1552-4604.2002.tb06000.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J. Abnorm. Psychol. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy P, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J. Subst. Abuse Treat. 2008;35:362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cartwright RD, Wood E. Adjustment disorders of sleep: the sleep effects of a major stressful event and its resolution. Psychiatry Res. 1991;39:199–209. doi: 10.1016/0165-1781(91)90088-7. [DOI] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am. J. Addict. 2006;15:8–14. doi: 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, MacDonald MJ, Whitmore EA, Mikulich SK. Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct disorder symptoms and substance use disorders. Drug Alcohol Depend. 1998;50:27–37. doi: 10.1016/s0376-8716(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep. 2008;31:599–607. doi: 10.1093/sleep/31.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMCDDA. Annual report on the state of the drugs problem. Lisbon: European Monitoring Centre for Drugs and Drug Addiction; 2008
- Gevins A, Cutillo B. Spatiotemporal dynamics of component processes in human working memory. Electroencephalogr. Clin. Neurophysiol. 1993;87:128–143. doi: 10.1016/0013-4694(93)90119-g. [DOI] [PubMed] [Google Scholar]
- Haney M. The marijuana withdrawal syndrome: diagnosis and treatment. Curr. Psychiatry Rep. 2005;7:360–366. doi: 10.1007/s11920-005-0036-1. [DOI] [PubMed] [Google Scholar]
- Haney M. Role of withdrawal in relapse to marijuana use. Paper presented at the College on Problems of Drug Dependence; Reno, NV. 2009. [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend. 2009;102:35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudziak J, Helzer JE, Wetzel MW, Kessel KB, McBee B, Janca A, Przybeck P. The use of the DSM-III-R Checklist for initial diagnostic assessments. Compr. Psychiatry. 1993;34:375–383. doi: 10.1016/0010-440x(93)90061-8. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF, editors. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Kleykamp BA, Griffiths RR, Mintzer MZ. Dose effects of triazolam and alcohol on cognitive performance in healthy volunteers. Exp. Clin. Psychopharm. 2010;18:1–16. doi: 10.1037/a0018407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin KH, Copersino ML, Heishman SJ, Liu F, Kelly DL, Boggs DL, Gorelick DA. Cannabis withdrawal symptoms in non-treatment-seeking adult cannabis smokers. Drug Alcohol Depend. 2010;111:120–127. doi: 10.1016/j.drugalcdep.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav. Res. Methods. 1982;14:463–466. [Google Scholar]
- Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: a comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behav. Pharmacol. 1997;8:561–574. doi: 10.1097/00008877-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: evidence for occult insomnia. Drug Alcohol Depend. 2006;82:238–249. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Nordstrom BR, Levin FR. Treatment of cannabis use disorders: a review of the literature. Am. J. Addict. 2007;16:331–342. doi: 10.1080/10550490701525665. [DOI] [PubMed] [Google Scholar]
- Peters EN, Hughes JR. Daily marijuana users with past alcohol problems increase alcohol consumption during marijuana abstinence. Drug Alcohol Depend. 2010;106:111–118. doi: 10.1016/j.drugalcdep.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacol. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Treatment episode data set (TEDS) 1995–2007: National admissions to substance abuse treatment services. Rockville, MD: US Department of Health and Human Services; 2008
- Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: cocaine, ecstasy and marijuana. Sleep Med. Rev. 2008;12:381–389. doi: 10.1016/j.smrv.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shekleton JA, Rogers NL, Rajaratnam SM. Searching for the daytime impairments of primary insomnia. Sleep Med. Rev. 2010;14:47–60. doi: 10.1016/j.smrv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Simonson E, Brozek J. Flicker fusion frequency background and applications. Physiol. Rev. 1952;32:349–378. doi: 10.1152/physrev.1952.32.3.349. [DOI] [PubMed] [Google Scholar]
- Smith MT, Edwards RR, Stonerock GL, McCann UD. Individual variation in rapid eye movement sleep is associated with pain perception in healthy women: preliminary data. Sleep. 2005;28:809–812. doi: 10.1093/sleep/28.7.809. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A Technique for Assessing Self-Reported Alcohol Consumption. In: Allen JP, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Human Press; 1992. pp. 41–72. [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8:331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stores G. Clinical diagnosis and misdiagnosis of sleep disorders. J. Neurol. Neurosurg. Psychiatry. 2007;78:1293–1297. doi: 10.1136/jnnp.2006.111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC. World Drug Report - 2007. New York, NY: United Nations Office on Drugs and Crime; 2007
- Vandrey R, Budney AJ, Kamon JL, Stanger C. Cannabis withdrawal in adolescent treatment seekers. Drug Alcohol Depend. 2005a;78:205–210. doi: 10.1016/j.drugalcdep.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs. 2009;23:543–553. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Umbricht A, Strain EC. Increased blood pressure following abrupt cessation of daily cannabis use. J. Addict. Med. doi: 10.1097/ADM.0b013e3181d2b309. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subjects comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 2008;92:48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey RG, Budney AJ, Moore BA, Hughes JR. A cross-study comparison of cannabis and tobacco withdrawal. Am J Addict. 2005b;14:54–63. doi: 10.1080/10550490590899853. [DOI] [PubMed] [Google Scholar]
- Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walher MP. Sleep memory and emotion. Prog. Brain Res. 2010;185:49–68. doi: 10.1016/B978-0-444-53702-7.00004-X. [DOI] [PubMed] [Google Scholar]