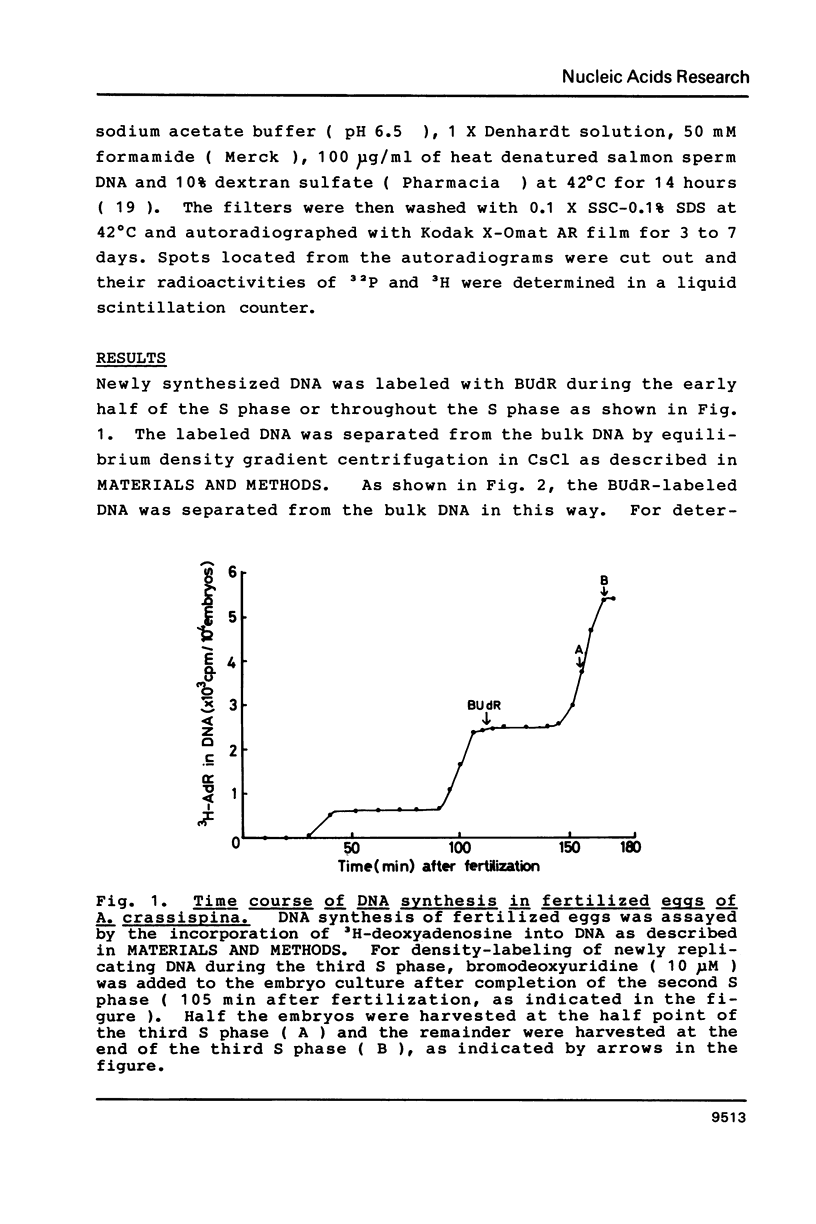
Abstract
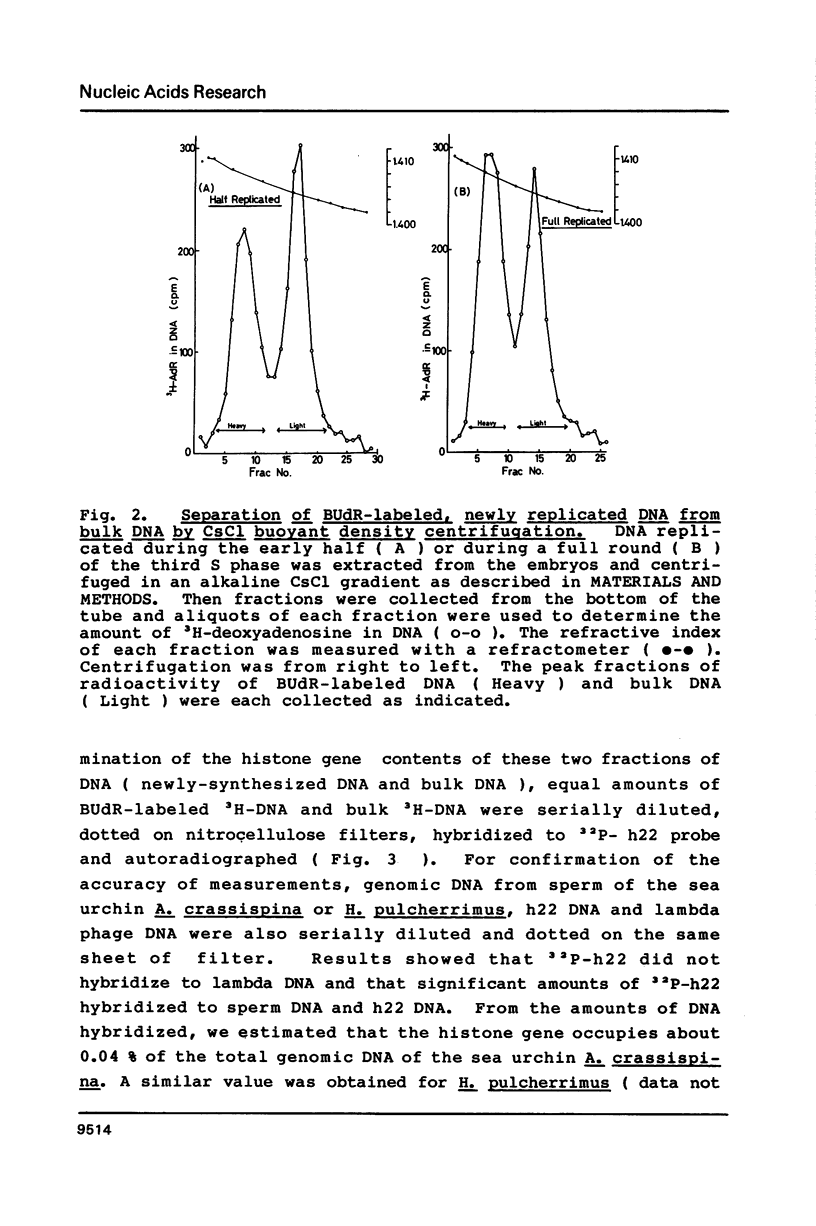
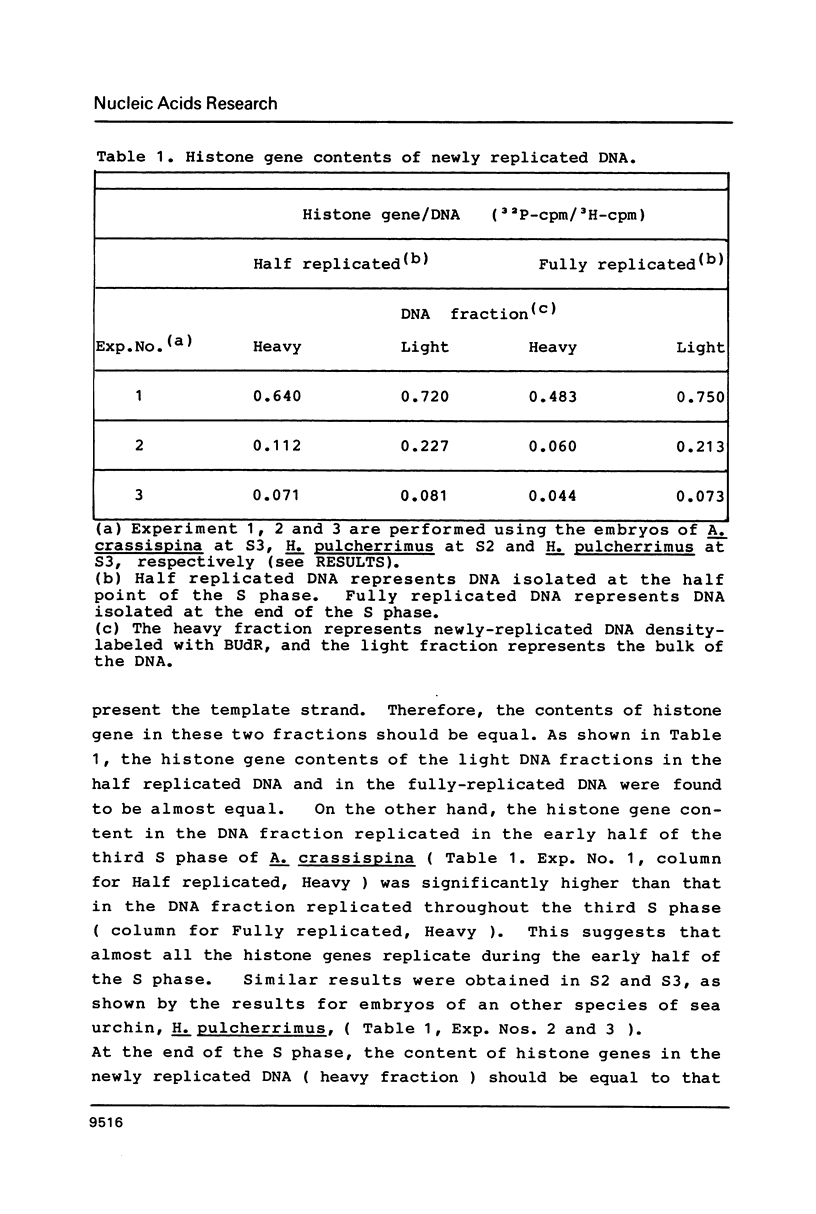
Newly synthesized DNA was separated from the bulk of the DNA by pulse-labeling with BUdR and centrifugation in an alkaline CsCl buoyant density gradient. The content of histone gene in the newly synthesized DNA was determined by DNA dot hybridization. The gene contents in DNA replicated during the early half of S phase and during the whole S phase were compared. Results showed that histone genes were replicated during the first half of the S phase in embryos in the early cleavage stage.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldari C. T., Amaldi F., Buongiorno-Nardelli M. Electron microscopic analysis of replicating DNA of sea urchin embryos. Cell. 1978 Nov;15(3):1095–1107. doi: 10.1016/0092-8674(78)90293-3. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Schaffner W., Smith H. O. DNA sequences coding for the H2B histone of Psammechinus miliaris. Nature. 1977 Apr 14;266(5603):603–607. doi: 10.1038/266603a0. [DOI] [PubMed] [Google Scholar]
- Braunstein J. D., Schulze D., DelGiudice T., Furst A., Schildkraut C. L. The temporal order of replication of murine immunoglobulin heavy chain constant region sequences corresponds to their linear order in the genome. Nucleic Acids Res. 1982 Nov 11;10(21):6887–6902. doi: 10.1093/nar/10.21.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. W. Heterochromatin. Science. 1966 Jan 28;151(3709):417–425. doi: 10.1126/science.151.3709.417. [DOI] [PubMed] [Google Scholar]
- Burke W., Fangman W. L. Temporal order in yeast chromosome replication. Cell. 1975 Jul;5(3):263–269. doi: 10.1016/0092-8674(75)90101-4. [DOI] [PubMed] [Google Scholar]
- Chambers S. A., Vaughn J. P., Shaw B. R. Shortest nucleosomal repeat lengths during sea urchin development are found in two-cell embryos. Biochemistry. 1983 Nov 22;22(24):5626–5631. doi: 10.1021/bi00293a026. [DOI] [PubMed] [Google Scholar]
- Clarkson S. G., Smith H. O., Schaffner W., Gross K. W., Birnstiel M. L. Integration of eukaryotic genes for 5S RNA and histone proteins into a phage lambda receptor. Nucleic Acids Res. 1976 Oct;3(10):2617–2632. doi: 10.1093/nar/3.10.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M. A., Holmquist G. P., Gray M. C., Caston L. A., Nag A. Replication timing of genes and middle repetitive sequences. Science. 1984 May 18;224(4650):686–692. doi: 10.1126/science.6719109. [DOI] [PubMed] [Google Scholar]
- Gourlie B. B., Infante A. A. Pool sizes of the deoxynucleoside triphosphates in the sea urchin egg and developing embryo. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1206–1214. doi: 10.1016/0006-291x(75)90821-9. [DOI] [PubMed] [Google Scholar]
- Harrison M. F., Wilt F. H. The program of Hl histone synthesis in S. purpuratus embryos and the control of its timing. J Exp Zool. 1982 Nov 1;223(3):245–256. doi: 10.1002/jez.1402230306. [DOI] [PubMed] [Google Scholar]
- Hentschel C. C., Birnstiel M. L. The organization and expression of histone gene families. Cell. 1981 Aug;25(2):301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Iqbal M. A., Plumb M., Stein J., Stein G., Schildkraut C. L. Coordinate replication of members of the multigene family of core and H1 human histone genes. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7723–7727. doi: 10.1073/pnas.81.24.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedes L. H., Chang A. C., Houseman D., Cohen S. N. Isolation of histone genes from unfractionated sea urchin DNA by subculture cloning in E. coli. Nature. 1975 Jun 12;255(5509):533–538. doi: 10.1038/255533a0. [DOI] [PubMed] [Google Scholar]
- Kee S. G., Haber J. E. Cell cycle-dependent induction of mutations along a yeast chromosome. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1179–1183. doi: 10.1073/pnas.72.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews C. K. Giant pools of DNA precursors in sea urchin eggs. Exp Cell Res. 1975 Apr;92(1):47–56. doi: 10.1016/0014-4827(75)90635-7. [DOI] [PubMed] [Google Scholar]
- Pierron G., Sauer H. W., Toublan B., Jalouzot R. Physical relationship between replicons and transcription units in Physarum polycephalum. Eur J Cell Biol. 1982 Nov;29(1):104–113. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Kunz G., Daetwyler H., Telford J., Smith H. O., Birnstiel M. L. Genes and spacers of cloned sea urchin histone DNA analyzed by sequencing. Cell. 1978 Jul;14(3):655–671. doi: 10.1016/0092-8674(78)90249-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N., Shimada H. Effects of emetine on initiation of DNA synthesis in embryonic cells of sea urchin. Cell Differ. 1983 Nov;13(3):239–245. doi: 10.1016/0045-6039(83)90095-7. [DOI] [PubMed] [Google Scholar]




