Abstract
The N1 component of the auditory evoked potential (AEP) is a robust and easily recorded metric of auditory sensory-perceptual processing. In patients with schizophrenia, a diminution in the amplitude of this component is a near-ubiquitous finding. A pair of recent studies has also shown this N1 deficit in first-degree relatives of schizophrenia probands, suggesting that the deficit may be linked to the underlying genetic risk of the disease rather than to the disease state itself. However, in both these studies, a significant proportion of the relatives had other psychiatric conditions. As such, although the N1 deficit represents an intriguing candidate endophenotype for schizophrenia, it remains to be shown whether it is present in a group of clinically unaffected first-degree relatives. In addition to testing first-degree relatives, we also sought to replicate the N1 deficit in a group of first-episode patients and in a group of chronic schizophrenia probands. Subject groups consisted of 35 patients with schizophrenia, 30 unaffected first-degree relatives, 13 first-episode patients, and 22 healthy controls. Subjects sat in a dimly lit room and listened to a series of simple 1,000-Hz tones, indicating with a button press whenever they heard a deviant tone (1,500 Hz; 17% probability), while the AEP was recorded from 72 scalp electrodes. Both chronic and first-episode patients showed clear N1 amplitude decrements relative to healthy control subjects. Crucially, unaffected first-degree relatives also showed a clear N1 deficit. This study provides further support for the proposal that the auditory N1 deficit in schizophrenia is linked to the underlying genetic risk of developing this disorder. In light of recent studies, these results point to the N1 deficit as an endophenotypic marker for schizophrenia. The potential future utility of this metric as one element of a multivariate endophenotype is discussed.
Keywords: Schizophrenia, N1 deficit, Electrophysiology, Endophenotype, Event-related potential, ERP, Genetic liability
Introduction
Deficits in generation of the N1 component of the auditory evoked potential (AEP) are now well established in patients with schizophrenia [2, 8–11, 13, 14, 21, 26, 27, 37, 38, 41, 47, 51, 60]. With a peak latency of approximately 100 ms, the N1 component receives its main contributions from generators in and around primary auditory cortex [13, 27, 38], although both frontal [27] and parietal sources [43] also contribute. This component, marking a crucial auditory sensory-perceptual processing period, is best observed at electrode sites over the fronto-central scalp. As well as its nearly ubiquitous dysfunction in chronic patients, a growing number of studies have found a similar deficit in new-onset [48, 57] and medication-free patients [57, 62, 66]. A key question is whether this deficit is a consequence of the expression of the disease state itself, or alternatively, whether it is associated with the underlying genetic liability for developing the disorder. Given the ease with which the auditory N1 can be recorded and its general robustness across individuals, it could ultimately have great utility as an endophenotypic marker if it proves to be related to the underlying genetic risk of schizophrenia.
In 1984, Saitoh et al. [56] provided the first evidence that we are aware of for an N1 deficit in unaffected siblings of patients with schizophrenia, thereby suggesting that it might well be an endophenotype. This aspect of auditory sensory processing was not the primary concern of the Saitoh study, however, which was focused instead on the effects of selective attention manipulations on later cognitive components of the AEP. As such, the N1 finding appears to have been largely overlooked at the time. Fortunately, there has been a recent resurgence of interest in the N1 deficit as a potential endophenotypic marker. Crucially, this abnormality has now been extended to studies of twins non-concordant for schizophrenia where the deficit is also seen in the unaffected twin [1, 4], strongly suggesting that the deficit is linked to the underlying genetic risk of developing schizophrenia rather than to the progress of the disease state itself. A recent study by Turetsky et al. lends some additional support to the notion that this N1 deficit has a genetic component [65]. In a large-scale study of first-degree non-psychotic relatives of schizophrenia probands, these authors found a similar N1 deficit to that seen in patients. However, in this study, when the first-degree sample was “narrowed” to only those relatives without non-psychotic psychiatric comorbidities, the N1 deficit was no longer observed. The implication of their results was that the N1 deficit was limited to those relatives with the highest genetic liability for psychiatric symptoms. Force et al. [20] have also shown an N1 deficit in first-degree relatives, but as in the Turetsky study, their sample of relatives (N = 37) also had a high incidence of psychiatric comorbidities. Further, a number of additional studies have failed to find N1 abnormalities in first-degree relatives [33, 67]. Thus, while there is mounting evidence that amplitude attenuation of the N1 component of the AEP may be endophenotypic for schizophrenia, the picture is far from clear at this stage.
In the current study, we used high-density electrical mapping to measure the auditory N1 in patients with schizophrenia, their clinically unaffected first-degree relatives, first-episode schizophrenia patients, and healthy controls. An important feature of the current study was that any individuals with previous psychiatric history in the sample of first-degree relatives were carefully excluded. A second key aspect of this study was the use of high sweep counts for each subject's AEP recordings (600 trials) as we suspected that some of the previous negative findings may have resulted from rather noisy estimations of the N1 amplitude. As such, we hypothesized that these improved methods and better classification of participants would likely reveal an N1 deficit in the first-degree relative group. Our results provide further support for the proposal that the N1 deficit is an endophenotype for schizophrenia in that we find a clear N1 deficit in both first-episode and first-degree relative groups.
Methods and materials
Subjects
Informed consent was obtained from the three subject groups: 35 schizophrenia patients (12 women, mean age ± SD = 41.5 ± 12.4), 30 first-degree relatives (16 women, mean age ± SD = 34.1 ± 15.5), and 13 first-episode schizophrenia patients (1 woman, mean age ± SD = 25.7 ± 7.83). All subjects were between 18 and 65 years of age. All patients met DSM-IV criteria for schizophrenia [5] using the SCID, and axis II comorbidities were ruled out. Patients were recruited from the St. Vincent's Hospital Catchment Area in Fairview, Dublin, Ireland. The ethics committee at St. Vincent's Hospital approved all procedures, and all participants signed a written informed consent after the details of the study were fully explained to them and before participating in the study. Healthy control subjects and first-degree relatives received a modest remuneration of €40 for their time. All procedures were conducted in accordance with the ethical principles developed by the World Medical Association, as put forth in the Declaration of Helsinki.
The Brief Psychiatric Rating Scale (BPRS) [50] and the Scale for the Assessment of Negative Symptoms (SANS) [3] were used to assess the severity of the current illness. All the patients with chronic schizophrenia were medicated (average chlorpromazine equivalent dose = 420 mg/day). Medications comprised of typical and atypical antipsychotics. First-episode schizophrenia patients were on an average dose of 262 mg/day of chlorpromazine equivalents of antipsychotics. Of these, three subjects were unmedicated and were neuroleptic-naïve. The average number of days on medication was 20 days. Control subjects were recruited from the local and hospital staff community and comprised of 22 (11 women) volunteers (mean age ± SD = 40.6 ± 12.7). Controls and first-degree relatives were medication-free and free of any psychiatric illness or symptoms by self-report using criteria from the SCID-NP [61] and reported no history of alcohol or substance abuse. All subjects were also assessed for handedness using the Edinburgh Handedness Inventory [49] and reported normal or corrected-to-normal vision. Participant demographics and clinical characteristics are summarized in Table 1.
Table 1. Demographic and clinical characteristics of all the participant groups.
| Characteristics | Chronics | Relatives | 1st Episodes | Controls |
|---|---|---|---|---|
| Number of subjects | 35 | 30 | 13 | 22 |
| Sex | 23 M, 12F | 14 M, 16F | 12 M, 1F | 11 M, 11F |
| Mean age (and SD) | 41.5 (12.4) | 34.1 (15.5) | 25.7 (7.8) | 40.6 (12.7) |
| Left-handedness | 5 | 6 | 1 | 4 |
| Mean duration of illness (and SD), year | 14.9 (9.8) | 0 | 20.2 (12.5) days | 0 |
| Mean chlorpromazine dose (and SD), mg | 427.2 (341.6) | 0 | 262.3 (280.2) | 0 |
| Mean BPRS (and SD) | 38.2 (10.0) | 0 | 43.3 (12.1) | 0 |
| Mean SANS (and SD) | 38.9 (25.2) | 0 | 33.0 (20.8) | 0 |
SD standard deviation, BPRS Brief Psychiatric Rating Scale, SANS Scale for the Assessment of Negative Symptoms
Stimuli and experimental design
In a single experimental block, subjects were presented with 750 standard tone stimuli (1,000 Hz; 60-ms duration; 70 dB SPL) and 150 deviant tone stimuli (1,500-Hz; 60 ms duration; 70 dB SPL). Stimuli were presented through headphones (Sennheiser-HD600), and subjects were required to press a button with their right thumb whenever they detected the occurrence of a deviant tone. Only standard stimuli were analyzed here. In addition, any standard that immediately followed a deviant stimulus was excluded from this analysis since it has been shown that such stimuli can contain mismatch negativities that might contaminate the analysis of the N1 [46]. Thus, responses to 600 standard stimuli were entered into the present analysis. Total block run-length lasted for 23.4 min if uninterrupted, but subjects could pause to rest at will during the block if they felt fatigued. Subjects sat in a dimly lit room and looked at a fixation point located 160 cm directly in front of them. Each tone was presented with a fixed interstimulus interval (ISI) of 1,500 ms.
Electrophysiological recording and analysis
Continuous EEG was acquired through the ActiveTwo Biosemi™ electrode system from 72 scalp electrodes, digitized at 512 Hz with an open pass-band from DC to 150 Hz. For analysis and display purposes, data were subsequently filtered with a 0-phase-shift 45-Hz low-pass filter (24 dB/octave) after acquisition. No high-pass filter was applied. With the Biosemi system, every electrode or combination of electrodes can be assigned as the “reference”, and this is done purely in software after acquisition. Biosemi replaces the “ground” electrodes used in conventional systems with two separate electrodes: common mode sense (CMS) active electrode and driven right leg (DRL) passive electrode. These 2 electrodes form a feedback loop, which drives the average potential of the subject (the common mode voltage) as close as possible to the ADC reference voltage in the AD box (the ADC reference can be considered as the amplifier “zero”). For a detailed description of the referencing and grounding conventions used by the Biosemi active electrode system, the interested reader is referred to the following Web site: http://www.biosemi.com/faq/cms&drl.htm. All data were re-referenced to the nasion after acquisition, for analysis.
Data were analyzed using BESA version 5.08 (Brain Electric Source Analysis) software (http://www.besa.de). All electrode channels were subjected to an artifact criterion of ± 120 μV from −200 to 1,200 ms to reject trials with excessive EMG (electromyography) or other noise transients. The vertical and horizontal electro-oculograms were also visually inspected for blinks and large eye movements. Epochs were calculated for a time window from 200 ms pre-stimulus to 1,200 ms post-stimulus and baseline-corrected relative to the interval −80 to 20 ms. Accepted trials were then averaged for the standard tone only.
A measure of N1 amplitude was defined as the greatest negativity over the interval 96–116 ms, spanning the N1 component, chosen based on grand average waveforms. These peak measures were then submitted to a repeated-measures multivariate analysis of variance (MANOVA; using SPSS software, Chicago, IL) with between-subjects factor of group (patients, relatives, first episodes, and controls) and within-subjects factors of electrode (FC1, FCZ, FC2), covering the left, midline, and right fronto-central scalp regions, respectively. All tests were two-tailed with a preset α-level of p < 0.05. In all statistical tests, Greenhouse–Geiser Correction was used.
Results
The mean ± SD target detection rate was 91.03 ± 14.53 for patients, 96.56 ± 5.76 for first-episode patients, 98.43 ± 2.73 for relatives, and 99.03 ± 1.64 for controls. There was no significant difference between groups (p ≥ 0.2). These high accuracy rates across groups confirmed that all groups were attending the stimuli adequately.
Visual inspection of the grand average waveforms across groups revealed a peak latency of approximately 106 ms for the N1 that was consistent across groups. Therefore, we defined a window from 96 to 116 ms during which the largest negative deflection was chosen as the dependent measure. The scalp waveforms (Fig. 1a) clearly illustrate the group differences within the time range of the N1. This negativity is again distinctly observed using topographic voltage maps of the distribution of the amplitude on the scalp (Fig. 1b).
Fig. 1.
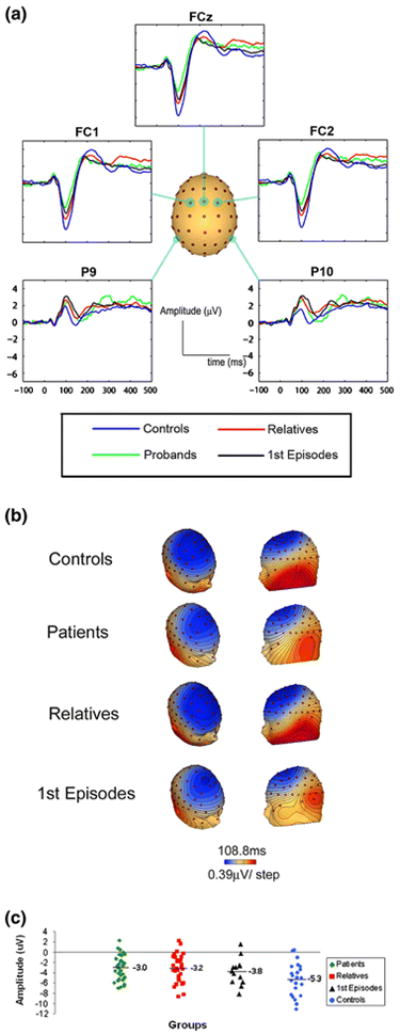
a Waveforms illustrating the AEP in all four groups from 5 selected scalp electrodes (FC1, FCz, FC2, P9, and P10). b Scalp topographies at 108 ms showing the distribution of the N1 and the marked differences between first-episode patients, chronic patients, and controls. c A scatter plot of the distribution of the N1 peak amplitude for each of the 4 groups taken at the scalp site FCz
An analysis of variance (ANOVA) (4 groups × 3 electrodes) showed a significant main effect of group [F(3,96) = 6.506, p ≤ 0.01] with no group × electrode interaction (p = 0.23). The overall effect size was f = 0.41, which constitutes a large effect size according to the criterion of Cohen [15]. Unadjusted pairwise comparisons showed all three groups significantly different in comparison with the healthy controls (chronic patients: p ≤ 0.001; first-episode patients: p = 0.028; and first-degree relatives: p = 0.034).
Discussion
Here, we found that the amplitude of the N1 component of the AEP was significantly reduced in patients with schizophrenia, and this was also the case in a group of newly psychotic patients, when compared to a healthy comparison group. Crucially, this N1 amplitude reduction was also evident in clinically unaffected first-degree relatives of the schizophrenia probands, providing further evidence that this deficit is likely to be linked to the underlying genetic liability for schizophrenia and that the N1 deficit may have utility as an endophenotypic marker for risk assessment. These results add to a growing body of literature where similar amplitude reductions in the auditory N1 in first-degree biological relatives have also been reported [20, 56, 65]. Unfortunately, the emerging picture is not as straightforward as one might hope. In the most recent pair of these studies, the samples of first-degree relatives also included individuals with axis I disorders, and this somewhat complicates the picture. In the case of the largest and most comprehensive of these studies [65], removal of the relatives with psychiatric comorbidities from the sample resulted in loss of the N1 deficit. A somewhat similar picture emerges from the study of Force et al. [20]. They used a dichotic listening task to assess neural abnormalities during auditory processing in four cohorts: patients with schizophrenia, patients with bipolar disorder, and the respective first-degree biological relatives for each of the two clinical groups. They found that only schizophrenia patients and their relatives had a significant decrease in N1 amplitude compared to a non-psychotic healthy comparison group, whereas neither the bipolar patients nor their relatives showed the deficit. However, the first-degree schizophrenia group in the Force study also included relatives with other psychiatric comorbidities, again somewhat clouding interpretability of these results. The present study had the advantage that the sample of first-degree relatives used was completely free from other DSM-IV axis I diagnoses, and yet the N1 deficit was seen to persist in this group.
Twin studies
Another very powerful method of determining whether genetic contributions are a major source of a given ERP difference is to look at concordant and discordant twin pairs. One such study interrogated the N1 in mono- and di-zygotic twin pairs discordant for schizophrenia and found a significant decrease in N1 amplitude in unaffected co-twins when compared to control subjects [1]. More recently, Anokhin et al. [4] also looked at the auditory N1 in twin pairs and found substantial heritability of N1 peak amplitudes, strongly implicating genetic influences at early stages of auditory processing in schizophrenia. Thus, in conjunction with the relatives studies discussed above where the N1 deficit was seen in first-degree relatives, these twin studies add to a significant bolus of work pointing to a genetic link.
Evidence against an N1 deficit in first-degree relatives
Unfortunately, the picture is simply not as clear as one would like. Evidence against an N1 deficit in siblings of schizophrenia patients comes from five studies that we are aware of [7, 25, 33, 58, 67]. However, we believe that certain aspects of these prior studies suggest that such negative findings could well be due to so-called type II error. For example, Winterer et al. [67] did not find differences in auditory N1 amplitude between unaffected relatives and control subjects, but neither did they find differences in N1 amplitude between their patient group with schizophrenia and controls. However, the basic N1 deficit in schizophrenia has been demonstrated by dozens of independent studies with varying task parameters over the course of three decades and seems beyond doubt. In light of this, it is likely that the observations of Winterer et al. may have been falsely negative for the N1 period.
Another illustrative example is that of Karoumi et al. [33]. These researchers reported significant differences in N1 amplitude between patients and controls but found that relatives did not differ from either of these two groups. Clearly, this must reflect type II error, since if the population of people with schizophrenia differs from the healthy population, then it cannot be the case that the population of relatives of schizophrenia patients is identical to both of these groups. In fact, an independent-samples t-test of the data provided by Karoumi et al. for the comparison of relatives to controls shows a p-value of 0.043 (one-tailed), which misses significance after Bonferroni correction. However, the achieved power (1 – β) for the observed effect size (d = 0.541) is only 0.53 (α = 0.05). Simply stated, there was a 46% chance of type II error in this study. In our case, the observed effect size was larger (d = 0.69), due to less variance in the amplitude measures across subjects (see below), and our achieved power for the same test was 0.78. Even after Bonferroni correction for three comparisons to α = 0.017, our achieved power is a tolerable 0.61.
Frangou et al. [25] showed no difference in N1 amplitude between 33 probands and 57 of their first-degree relatives. The latter group included parents and siblings from 16 multiplex families, with presumably high genetic liability for schizophrenia. Although recordings were made from only 3 midline scalp sites, which is clearly not ideal, and the number of epochs included in each subject's averaged response could be relatively low (circa 60 sweeps), likely leading to less reliable estimates of the AEP, it is nonetheless unclear why their results are not in accordance with the current results. This study did show differences in later AEP components (N200 and P300) since subjects were required to respond to the auditory stimuli as part of an active discrimination task. Thus, another possibility is that estimates of N1 amplitude were affected by overlap from these later cognitive components, which could have obscured potential differences. Another potential cause of falsely negative findings, alluded to above, relates to signal-to-noise issues and the possibility of poor estimation of the evoked component of interest. Frangou et al. reported using only 60 trials for at least some of the averages, which, in our experience, is not enough to reliably estimate the evoked response. Additional within-group variability due to residual noise from estimation procedures will of course adversely affect statistical power. Our procedure yielded smaller standard deviations for N100 amplitudes (±2.7 to 3.2 uV for relatives and controls, respectively, compared to ±3.2 to 4.7 uV reported by Frangou et al. [25]). This led to a greater observed effect size and statistical power as described above.
Thus, while there exists studies where an N1 deficit was not seen in first-degree relatives, many of these studies are open to criticism on methodological and statistical grounds. Nonetheless, these studies do speak to the relative robustness of the N1 effect or lack thereof, and clearly, one would prefer a much more stable measure if it is ultimately to prove useful as an endophenotypic marker.
First-episode patients
With respect to the N1 in first-episode schizophrenia patients, once again the experimental results are rather unfortunately mixed. In one case, Valkonen-Korhonen et al. [66] found the N1 unchanged in 25 never-medicated acutely psychotic patients compared to healthy controls. In contrast, Sumich et al. [62] found a significant reduction in the anterior N1 in a group of twenty men with recent-onset psychosis. A third study examined forty young first-episode schizophrenia patients and showed the same pattern of N1 amplitude disturbances as seen in chronic patients [10]. Most recently, a report from Salisbury et al. [57] adds further support for an auditory N1 deficit in first-episode schizophrenia. It is important though to note that the first-episode patients from these latter three studies, where an N1 deficit was seen, were all on antipsychotic medications at the time of testing. Our finding supports the results of the latter three studies showing significant N1 amplitude reduction in newly diagnosed first-episode patients with schizophrenia. The first-episode patients in our study had been exposed to a total of less than 20 days of antipsychotic medication. They had also never previously been on antipsychotic medication. The short amount of time of antipsychotic exposure rules out the long-term effects of antipsychotic medication, which is often a potentially confounding factor in studies of schizophrenia patients. Thus, the weight of evidence in first-episode patients also points to an N1 deficit, with the proviso that the patients were generally medicated at test time.
Toward a multivariate endophenotype
The concept of a composite multivariate endophenotype consisting of a battery of easily obtainable complementary measures is an intuitively appealing one [53]. Not only would such an entity provide greater experimental stability but also test–retest reliability compared to just a single endophenotypic measure [64]. There are now a handful of very promising candidate endophenotypes emerging from ERP studies that could play pivotal roles in such a multivariate metric. For example, studies from our own laboratory have shown that there is a prominent deficit in generation of the so-called P1 component of the visual evoked potential (VEP) [22, 23, 34, 68, 69], a deficit that is of even greater amplitude to the one reported here in the auditory N1. Yeap et al. [70] showed that this deficit was robustly present in unaffected first-degree biological relatives, and in turn, Donohoe et al. [19] showed a relationship between this measure of visual sensory dysfunction and a known genetic risk variant for schizophrenia (Dysbindin—DTNBP1). Thus, the VEP P1 deficit has all the hallmarks of a measure related to the underlying genetic liability for schizophrenia. Other groups have established similar endophenotypy for measures of both sensory (P50) and cognitive (P300) components of the AEP [e.g. 6, 29, 31, 44, 45, 65], although in much the same way as the auditory N1, findings are not always consistent [17, 18]. Very recently, Hall et al. [30] showed that the early auditory-evoked gamma-band response was another potential endophenotype in that unaffected identical twins of patients with schizophrenia showed a robust reduction in the amplitude of this very easily measured oscillatory response. Another well-characterized measure of auditory sensory function is the mismatch negativity (MMN) response, and there is some evidence that MMN might also be endophenotypic [e.g., 42], although we and others have found essentially normal MMNs in first-degree relatives, suggesting that it may not be a terribly robust measure, if it is endophenotypic at all [e.g., 39]. Finally, the so-called N400 component has also emerged as a potential endophenotype. Guerra et al. [28] used simple pairings of line drawings of common objects to elicit N400 responses in a cohort of first-degree relatives, their schizophrenic probands, and a group of matched healthy controls. When the picture pairings, which are presented in sequence, are semantically congruent (e.g., both pictures are animals), the N400 is small or absent; in contrast, when the pairings are incongruent (e.g., a picture of a book followed by a picture of a car), a robust N400 is typically recorded to the second picture of the pair. This N400 response is well known to be impaired in schizophrenia patients [40], and Guerra et al. [28] found a clear deficit in their patient group as well as in the unaffected first-degree relatives. Taken together, the emerging picture is one of a sizable and growing battery of candidate endophenotypes from ERP measures across both the visual and auditory sensory modalities, not all of which are as robust as one might hope.
It would also be very interesting to see whether similar sensory deficits in somatosensory processing can be found to be endophenotypic where early suggestions of abnormalities in the evoked responses to median nerve stimulation [59] have recently been confirmed using magneto-encephalographic (MEG) measures [32]. In turn, given the likelihood that deficits exist across all three major sensory modalities (audition, vision, and somatosensation), another fruitful area for investigation will be indices of multisensory integration in schizophrenia [16, 24, 54]. In the cases of many of the simple ERP measures of sensory processing described above, they are typically obtainable to a high signal-to-noise ratio within a matter of minutes, so measures from 4 to 6 of these methods are certainly feasible in a short clinical testing session. Recent innovations in the acquisition of sensory evoked responses may even speed these measures further [35, 36]. In addition, both structural and functional neuroimaging measures could easily be combined with electrophysiological measures, as there is also strong emerging evidence for endophenotypic measures using magnetic resonance imaging (MRI). For example, Camchong et al. [12] used diffusion tensor imaging to make two key observations. First, in a cohort of monozygotic twins, they showed stronger correlations between fractional anisotropy (FA) measures across twin pairs within medial wall frontal cortex white matter structures such as the anterior cingulum. FA is commonly used as a proxy measure of structural organization within white matter tracts. They then found that non-affected first-degree relatives of schizophrenia patients showed reduced FA in frontal white matter structures, pointing to this measure as another potential endophenotype.
One obvious issue arises when one considers the multivariate endophenotypic strategy. Will it simply be the case that if a given high-risk individual shows a deficit in one measure of auditory function (e.g., the N1), they will show similar deficits across all measures of auditory function (MMN, P50, P300, etc.)? In other words, are these all essentially measures of the same underlying dysfunction, and as such, will combination of the measures add little to our diagnostic abilities? The answer is as yet unknown but clearly needs to be investigated. There certainly seems a reasonable likelihood that measures of dysfunction within a sensory system could be strongly correlated within subject. But, on a more promising note, it also seems a reasonable proposal that measures of dysfunction across sensory systems should not be as tightly coupled. As such, the use of multiple endophenotypes across multiple sensory systems and across physiological methodologies (ERP and fMRI) may yet lead to considerably increased diagnostic sensitivity. An obvious next step will be to assess whether deficits across sensory systems are correlated within subject.
In turn, the field has yet to adequately leverage advanced multivariate methods for classification analysis, methods that continue to be developed and improved in the applied mathematical and statistics fields. To date, the only study that we are aware of to have used the multivariate endophenotype approach in schizophrenia employed basic logistic regression methods [53]. While this was a perfectly appropriate approach, there are other potentially powerful methods such as discriminant function analysis (DA) that could prove more effective [52]. Another method that might be applicable is the minimum covariance determinant (MCD) estimation. MCD is a robust estimator of multivariate location and scatter, finding the half of a given data set for which the determinant of the covariance matrix of the data is smallest, allowing all data points to be evaluated against this half [55]. One could certainly imagine a scenario where healthy subjects might be likely to cluster more tightly in an n-dimensional multivariate space than their patient counterparts. Fuzzy clustering is another potentially compelling method that has clear applicability [63]. A comparative application of these and other classification techniques to ERP and MRI data has not yet been conducted to our knowledge and is clearly warranted. Another important distinction needs to be made here. It is one matter to use a method such as DA to optimize separation of two groups where group membership is already known (i.e., controls vs. patients), but rather a different matter to then use the derived predictive functions to classify individuals in a new data set where group membership is unknown. In this regard, despite the huge upsurge in efforts to identify potential endophenotypes for schizophrenia, there is much ground yet to be covered. In conclusion, we and others strongly believe that substantial gains in sensitivity can be achieved using this multivariate endophenotypic approach. The current study suggests that simple AEP measures of the auditory N1 have excellent potential as one element of just such an approach.
Acknowledgments
This work was supported in part by an RO1 grant from the US National Institute of Mental Health (NIMH) to Professor Foxe (MH65350). Dr. Yeap was supported by a fellowship from the Irish Health Research Board (HRB). The authors would like to thank the Chief Executive Officer at St. Vincent's Hospital, Mr. Edward Byrne, and the Director of Nursing, Mrs. Phil Burke, for their crucial support of the Cognitive Neurophysiology Laboratory (CNL). The authors are hugely indebted to the patients at St. Vincent's Hospital and their dedicated relatives who so graciously gave of their time for this project.
References
- 1.Ahveninen J, Jaaskelainen IP, Osipova D, Huttunen MO, Ilmoniemi RJ, Kaprio J, Lonnqvist J, Manninen M, Pakarinen S, Therman S, Naatanen R, Cannon TD. Inherited auditory-cortical dysfunction in twin pairs discordant for schizophrenia. Biol Psychiatry. 2006;60:612–620. doi: 10.1016/j.biopsych.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Alain C, Cortese F, Bernstein LJ, He Y, Zipursky RB. Auditory feature conjunction in patients with schizophrenia. Schizophr Res. 2001;49:179–191. doi: 10.1016/s0920-9964(00)00138-9. [DOI] [PubMed] [Google Scholar]
- 3.Andreasen N. Scale for the Assessment of Negative Symptoms (SANS) Department of Psychiatry, University of Iowa; Iowa City: 1984. [Google Scholar]
- 4.Anokhin AP, Vedeniapin AB, Heath AC, Korzyukov O, Boutros NN. Genetic and environmental influences on sensory gating of mid-latency auditory evoked responses: a twin study. Schizophr Res. 2007;89:312–319. doi: 10.1016/j.schres.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 5.PA A. Diagnostic and statistical manual of mental disorders. 4th. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 6.Bestelmeyer PE, Phillips LH, Crombie C, Benson P, St Clair D. The P300 as a possible endophenotype for schizophrenia and bipolar disorder: evidence from twin and patient studies. Psychiatry Res. 2009;169(3):212–219. doi: 10.1016/j.psychres.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 7.Blackwood DH, St Clair DM, Muir WJ, Duffy JC. Auditory P300 and eye tracking dysfunction in schizophrenic pedigrees. Arch Gen Psychiatry. 1991;48:899–909. doi: 10.1001/archpsyc.1991.01810340031004. [DOI] [PubMed] [Google Scholar]
- 8.Boutros NN, Korzyukov O, Jansen B, Feingold A, Bell M. Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Res. 2004;126:203–215. doi: 10.1016/j.psychres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Boutros NN, Korzyuko O, Oliwa G, Feingold A, Campbell D, McClain-Furmanski D, Struve F, Jansen BH. Morphological and latency abnormalities of the mid-latency auditory evoked responses in schizophrenia: a preliminary report. Schizophr Res. 2004;70:303–313. doi: 10.1016/j.schres.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Brown KJ, Gonsalvez CJ, Harris AW, Williams LM, Gordon E. Target and non-target ERP disturbances in first episode vs. chronic schizophrenia. Clin Neurophysiol. 2002;113:1754–1763. doi: 10.1016/s1388-2457(02)00290-0. [DOI] [PubMed] [Google Scholar]
- 11.Bruder G, Kayser J, Tenke C, Amador X, Friedman M, Sharif Z, Gorman J. Left temporal lobe dysfunction in schizophrenia: event-related potential and behavioural evidence from phonetic and tonal dichotic listening tasks. Arch Gen Psychiatry. 1999;56:267–276. doi: 10.1001/archpsyc.56.3.267. [DOI] [PubMed] [Google Scholar]
- 12.Camchong J, Lim KO, Sponheim SR, Macdonald AW. Frontal white matter integrity as an endophenotype for schizophrenia: diffusion tensor imaging in monozygotic twins and patients' nonpsychotic relatives. Front Hum Neurosci. 2009;3:35. doi: 10.3389/neuro.09.035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celesia GG. Organization of auditory cortical areas in man. Brain. 1976;99:403–414. doi: 10.1093/brain/99.3.403. [DOI] [PubMed] [Google Scholar]
- 14.Clunas NJ, Ward PB. Auditory recovery cycle dysfunction in schizophrenia: a study using event-related potentials. Psychiatry Res. 2005;136:17–25. doi: 10.1016/j.psychres.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Lawrence Erlbaum Associates Publishers; Hillsdale: 1988. [Google Scholar]
- 16.de Jong JJ, Hodiamont PP, Van den Stock J, de Gelder B. Audiovisual emotion recognition in schizophrenia: reduced integration of facial and vocal affect. Schizophr Res. 2009;107(2–3):286–293. doi: 10.1016/j.schres.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 17.de Wilde OM, Bour LJ, Dingemans PM, Koelman JH, Linszen DH. Failure to find P50 suppression deficits in young first-episode patients with schizophrenia and clinically unaffected siblings. Schizophr Bull. 2007;33(6):1319–1323. doi: 10.1093/schbul/sbm001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Wilde OM, Bour LJ, Dingemans PM, Koelman JH, Boerée T, Linszen DH. P300 deficits are present in young first-episode patients with schizophrenia and not in their healthy young siblings. Clin Neurophysiol. 2008;119(12):2721–2726. doi: 10.1016/j.clinph.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Donohoe G, Morris DW, De Sanctis P, Magno E, Montesi JL, Garavan HP, Robertson IH, Javitt DC, Gill M, Corvin AP, Foxe JJ. Early visual processing deficits in dysbindin-associated schizophrenia. Biol Psychiatry. 2008;63(5):484–489. doi: 10.1016/j.biopsych.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Force RB, Venables NC, Sponheim SR. An auditory processing abnormality specific to liability for schizophrenia. Schizophr Res. 2008;103:298–310. doi: 10.1016/j.schres.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ford JM, White PM, Csernansky JG, Faustman WO, Roth WT, Pfefferbaum A. ERPs in schizophrenia: effects of antipsychotic medication. Biol Psychiatry. 1994;36:153–170. doi: 10.1016/0006-3223(94)91221-1. [DOI] [PubMed] [Google Scholar]
- 22.Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. Neuroreport. 2001;12(17):3815–3820. doi: 10.1097/00001756-200112040-00043. [DOI] [PubMed] [Google Scholar]
- 23.Foxe JJ, Murray MM, Javitt DC. Filling-in in schizophrenia: a high-density electrical mapping and source-analysis investigation of illusory contour processing. Cereb Cortex. 2005;15(12):1914–1927. doi: 10.1093/cercor/bhi069. [DOI] [PubMed] [Google Scholar]
- 24.Foxe JJ, Molholm S. Ten years at the Multisensory Forum: musings on the evolution of a field. Brain Topogr. 2009;21(3–4):149–154. doi: 10.1007/s10548-009-0102-9. [DOI] [PubMed] [Google Scholar]
- 25.Frangou S, Sharma T, Alarcon G, Sigmudsson T, Takei N, Binnie C, Murray RM. The Maudsley family study, II: endogenous event-related potentials in familial schizophrenia. Schizophr Res. 1997;23:45–53. doi: 10.1016/S0920-9964(96)00089-8. [DOI] [PubMed] [Google Scholar]
- 26.Gallinat J, Mulert C, Bajbouj M, Herrmann WM, Schunter J, Senkowski D, Moukhtieva R, Kronfeldt D, Winterer G. Frontal and temporal dysfunction of auditory stimulus processing in schizophrenia. Neuroimage. 2002;17:110–127. doi: 10.1006/nimg.2002.1213. [DOI] [PubMed] [Google Scholar]
- 27.Giard MH, Perrin F, Echallier JF, Thevenet M, Froment JC, Pernier J. Dissociation of temporal and frontal components in the human auditory N1 wave: a scalp current density and dipole model analysis. Electroencephalogr Clin Neurophysiol. 1994;92:238–252. doi: 10.1016/0168-5597(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 28.Guerra S, Ibáñez A, Martín M, Bobes MA, Reyes A, Mendoza R, Bravo T, Domínguez M, Sosa MV. N400 deficits from semantic matching of pictures in probands and first-degree relatives from multiplex schizophrenia families. Brain Cogn. 2009;70(2):221–230. doi: 10.1016/j.bandc.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Hall MH, Rijsdijk F, Picchioni M, Schulze K, Ettinger U, Toulopoulou T, Bramon E, Murray RM, Sham P. Substantial shared genetic influences on schizophrenia and event-related potentials. Am J Psychiatry. 2007;164(5):804–812. doi: 10.1176/ajp.2007.164.5.804. [DOI] [PubMed] [Google Scholar]
- 30.Hall MH, Taylor G, Sham P, Schulze K, Rijsdijk F, Picchioni M, Toulopoulou T, Ettinger U, Bramon E, Murray RM, Salisbury DF. The early auditory gamma-band response is heritable and a putative endophenotype of schizophrenia. Schizophr Bull. doi: 10.1093/schbul/sbp134. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong LE, Summerfelt A, Mitchell BD, McMahon RP, Wonodi I, Buchanan RW, Thaker GK. Sensory gating endophenotype based on its neural oscillatory pattern and heritability estimate. Arch Gen Psychiatry. 2008;65(9):1008–1016. doi: 10.1001/archpsyc.65.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang MX, Lee RR, Gaa KM, Song T, Harrington DL, Loh C, Theilmann RJ, Edgar JC, Miller GA, Canive JM, Granholm E. Somatosensory system deficits in schizophrenia revealed by MEG during a median-nerve oddball task. Brain Topogr. 2010;23(1):82–104. doi: 10.1007/s10548-009-0122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karoumi B, Laurent A, Rosenfeld F, Rochet T, Brunon AM, Dalery J, d'Amato T, Saoud M. Alteration of event related potentials in siblings discordant for schizophrenia. Schizophr Res. 2000;41:325–334. doi: 10.1016/s0920-9964(99)00062-6. [DOI] [PubMed] [Google Scholar]
- 34.Lalor EC, Yeap S, Reilly RB, Pearlmutter BA, Foxe JJ. Dissecting the cellular contributions to early visual sensory processing deficits in schizophrenia using the VESPA evoked response. Schizophr Res. 2008;98(1–3):256–264. doi: 10.1016/j.schres.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 35.Lalor EC, Power AJ, Reilly RB, Foxe JJ. Resolving precise temporal processing properties of the auditory system using continuous stimuli. J Neurophysiol. 2009;102(1):349–359. doi: 10.1152/jn.90896.2008. [DOI] [PubMed] [Google Scholar]
- 36.Lalor EC, Foxe JJ. Neural responses to uninterrupted natural speech can be extracted with precise temporal resolution. Eur J Neurosci. 2010;31(1):189–193. doi: 10.1111/j.1460-9568.2009.07055.x. [DOI] [PubMed] [Google Scholar]
- 37.Laurent A, Garcia-Larrea L, d'Amato T, Bosson JL, Saoud M, Marie-Cardine M, Maugiere F, Dalery J. Auditory event-related potentials and clinical scores in unmedicated schizophrenic patients. Psychiatry Res. 1999;86:229–238. doi: 10.1016/s0165-1781(99)00038-4. [DOI] [PubMed] [Google Scholar]
- 38.Leavitt VM, Molholm S, Ritter W, Shpaner M, Foxe JJ. Auditory processing in schizophrenia during the middle latency period (10–50 ms): high-density electrical mapping and source analysis reveal subcortical antecedents to early cortical deficits. J Psychiatry Neurosci. 2007;32:339–353. [PMC free article] [PubMed] [Google Scholar]
- 39.Magno E, Yeap S, Thakore JH, Garavan H, De Sanctis P, Foxe JJ. Are auditory-evoked frequency and duration mismatch negativity deficits endophenotypic for schizophrenia? High-density electrical mapping in clinically unaffected first-degree relatives and first-episode and chronic schizophrenia. Biol Psychiatry. 2008;64(5):385–391. doi: 10.1016/j.biopsych.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathalon DH, Roach BJ, Ford JM. Automatic semantic priming abnormalities in schizophrenia. Int J Psychophysiol. 2010;75(2):157–166. doi: 10.1016/j.ijpsycho.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michie PT, Fox AM, Ward PB, Catts SV, McConaghy N. Event-related potential indices of selective attention and cortical lateralization in schizophrenia. Psychophysiology. 1990;27:209–227. doi: 10.1111/j.1469-8986.1990.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 42.Michie PT, Innes-Brown H, Todd J, Jablensky AV. Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biol Psychiatry. 2002;52(7):749–758. doi: 10.1016/s0006-3223(02)01379-3. [DOI] [PubMed] [Google Scholar]
- 43.Molholm S, Sehatpour P, Mehta AD, Shpaner M, Gomez-Ramirez M, Ortigue S, Dyke JP, Schwartz TH, Foxe JJ. Audio-visual multisensory integration in superior parietal lobule revealed by human intracranial recordings. J Neurophysiol. 2006;96:721–729. doi: 10.1152/jn.00285.2006. [DOI] [PubMed] [Google Scholar]
- 44.Myles-Worsley M. P50 sensory gating in multiplex schizophrenia families from a Pacific island isolate. Am J Psychiatry. 2002;159(12):2007–2012. doi: 10.1176/appi.ajp.159.12.2007. [DOI] [PubMed] [Google Scholar]
- 45.Myles-Worsley M, Ord L, Blailes F, Ngiralmau H, Freedman R. P50 sensory gating in adolescents from a pacific island isolate with elevated risk for schizophrenia. Biol Psychiatry. 2004;55(7):663–667. doi: 10.1016/j.biopsych.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Nousak JM, Deacon D, Ritter W, Vaughan HG., Jr Storage of information in transient auditory memory. Brain Res Cogn Brain Res. 1996;4:305–317. doi: 10.1016/s0926-6410(96)00068-7. [DOI] [PubMed] [Google Scholar]
- 47.O'Donnell BF, Hokama H, McCarley RW, Smith RS, Salisbury DF, Mondrow E, Nestor PG, Shenton ME. Auditory ERPs to non-target stimuli in schizophrenia: relationship to probability, task-demands, and target ERPs. Int J Psychophysiol. 1994;17:219–231. doi: 10.1016/0167-8760(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 48.Ogura C, Nageishi Y, Matsubayashi M, Omura F, Kishimoto A, Shimokochi M. Abnormalities in event-related potentials, N100, P200, P300 and slow wave in schizophrenia. Jpn J Psychiatry Neurol. 1991;45:57–65. doi: 10.1111/j.1440-1819.1991.tb00506.x. [DOI] [PubMed] [Google Scholar]
- 49.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 50.Overall J. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:13. [Google Scholar]
- 51.Potts GF, Hirayasu Y, O'Donnell BF, Shenton ME, McCarley RW. High-density recording and topographic analysis of the auditory oddball event-related potential in patients with schizophrenia. Biol Psychiatry. 1998;44:982–989. doi: 10.1016/s0006-3223(98)00223-6. [DOI] [PubMed] [Google Scholar]
- 52.Press SJ, Wilson S. Choosing between logistic regression and discriminant analysis. J Am Stat Assoc. 1978;73:699–705. [Google Scholar]
- 53.Price GW, Michie PT, Johnston J, Innes-Brown H, Kent A, Clissa P, Jablensky AV. A multivariate electrophysiological endophenotype, from a unitary cohort, shows greater research utility than any single feature in the Western Australian family study of schizophrenia. Biol Psychiatry. 2006;60:1–10. doi: 10.1016/j.biopsych.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 54.Ross LA, Saint-Amour D, Leavitt VM, Molholm S, Javitt DC, Foxe JJ. Impaired multisensory processing in schizophrenia: deficits in the visual enhancement of speech comprehension under noisy environmental conditions. Schizophr Res. 2007;97(1–3):173–183. doi: 10.1016/j.schres.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Rousseeuw PJ, Van Driessen K. A fast algorithm for the minimum covariance determinant estimator. Technometrics. 1999;41:212–223. [Google Scholar]
- 56.Saitoh O, Niwa S, Hiramatsu K, Kameyama T, Rymar K, Itoh K. Abnormalities in late positive components of event-related potentials may reflect a genetic predisposition to schizophrenia. Biol Psychiatry. 1984;19:293–303. [PubMed] [Google Scholar]
- 57.Salisbury DF, Collins KC, McCarley W. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Biol Psychiatry. 2010;36:991–1000. doi: 10.1093/schbul/sbp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schreiber H, Stolz-Born G, Rothmeier J, Kornhuber A, Kornhuber HH, Born J. Endogenous event-related brain potentials and psychometric performance in children at risk for schizophrenia. Biol Psychiatry. 1991;30:177–189. doi: 10.1016/0006-3223(91)90172-i. [DOI] [PubMed] [Google Scholar]
- 59.Shagass C, Roemer RA, Straumanis JJ, Amadeo M. Temporal variability of somatosensory, visual, and auditory evoked potentials in schizophrenia. Arch Gen Psychiatry. 1979;36(12):1341–1351. doi: 10.1001/archpsyc.1979.01780120071009. [DOI] [PubMed] [Google Scholar]
- 60.Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophr Res. 1999;37:65–79. doi: 10.1016/s0920-9964(98)00138-8. [DOI] [PubMed] [Google Scholar]
- 61.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 62.Sumich A, Harris A, Flynn G, Whitford T, Tunstall N, Kumari V, Brammer M, Gordon E, Williams LM. Event-related potential correlates of depression, insight and negative symptoms in males with recent-onset psychosis. Clin Neurophysiol. 2006;117:1715–1727. doi: 10.1016/j.clinph.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 63.Szendi I, Racsmány M, Cimmer C, Csifcsák G, Kovács ZA, Szekeres G, Galsi G, Tóth F, Nagy A, Garab EA, Boda K, Gulyás G, Kiss JG, Dombi J, Pléh C, Janka Z. Two subgroups of schizophrenia identified by systematic cognitive neuropsychiatric mapping. Eur Arch Psychiatry Clin Neurosci. 2010;260(3):257–266. doi: 10.1007/s00406-009-0073-6. [DOI] [PubMed] [Google Scholar]
- 64.Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turetsky BI, Greenwood TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Light GA, Mintz J, Nuechterlein KH, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Calkins ME. Abnormal auditory N100 amplitude: a heritable endophenotype in first-degree relatives of schizophrenia probands. Biol Psychiatry. 2008;64(10):1051–1059. doi: 10.1016/j.biopsych.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valkonen-Korhonen M, Purhonen M, Tarkka IM, Sipila P, Partanen J, Karhu J, Lehtonen J. Altered auditory processing in acutely psychotic never-medicated first-episode patients. Brain Res Cogn Brain Res. 2003;17:747–758. doi: 10.1016/s0926-6410(03)00199-x. [DOI] [PubMed] [Google Scholar]
- 67.Winterer G, Egan MF, Radler T, Coppola R, Weinberger DR. Event-related potentials and genetic risk for Schizophrenia. Biol Psychiatry. 2001;50:407–417. doi: 10.1016/s0006-3223(01)01072-1. [DOI] [PubMed] [Google Scholar]
- 68.Yeap S, Kelly SP, Sehatpour P, Magno E, Garavan H, Thakore JH, Foxe JJ. Visual sensory processing deficits in Schizophrenia and their relationship to disease state. Eur Arch Psychiatry Clin Neurosci. 2008;258:305–316. doi: 10.1007/s00406-008-0802-2. [DOI] [PubMed] [Google Scholar]
- 69.Yeap S, Kelly SP, Thakore JH, Foxe JJ. Visual sensory processing deficits in first-episode patients with Schizophrenia. Schizophr Res. 2008;102(1–3):340–343. doi: 10.1016/j.schres.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 70.Yeap S, Kelly SP, Sehatpour P, Magno E, Javitt DC, Garavan H, Thakore JH, Foxe JJ. Early visual sensory deficits as endophenotypes for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives. Arch Gen Psychiatry. 2006;63:1180–1188. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]


