Abstract
The steroid hormone estradiol plays an important role in reproductive development and behavior and modulates a wide array of physiological and cognitive processes. Recently, reports from several research groups have converged to show that estradiol also powerfully modulates sensory processing, specifically, the physiology of central auditory circuits in songbirds. These investigators have discovered that (1) behaviorally-relevant auditory experience rapidly increases estradiol levels in the auditory forebrain; (2) estradiol instantaneously enhances the responsiveness and coding efficiency of auditory neurons; (3) these changes are mediated by a non-genomic effect of brain-generated estradiol on the strength of inhibitory neurotransmission; and (4) estradiol regulates biochemical cascades that induce the expression of genes involved in synaptic plasticity. Together, these findings have established estradiol as a central regulator of auditory function and intensified the need to consider brain-based mechanisms, in addition to peripheral organ dysfunction, in hearing pathologies associated with estrogen deficiency.
Keywords: 17β-Estradiol, Audition, Selectivity, Fadrozole, Estrogen, Aromatase, Behavior, Vocal learning, Selectivity, Auditory coding
1. Introduction: changing signals, changing senses
The neural systems that we use to perceive the world are, by necessity, extraordinarily plastic. The processes by which external stimuli are encoded, represented and interpreted change dynamically in order to emphasize stimuli that are the most behaviorally relevant and minimize responses to those that are not. Which stimuli are behaviorally relevant is in many cases learned, and sensory systems revised accordingly – for example by increasing the neural space devoted to the representation of a stimulus (for reviews, see [29,52,91,160,216]) or the magnitude of the neural response to that stimulus [75,87,181].
Many signals contain information for which the behavioral relevance changes through time. A mating call, for example, contains information that is relevant to a receiver in reproductive condition. Outside a breeding context, the same call may have a different meaning. For example, in the winter a female sparrow listening to male song is almost certainly the receiver of an aggressive display, not courtship [13]. Sensory systems are thus presented with a quandary: similar signals can have different meanings in different contexts. In the case of a mating call, the meaning can be predicted by the receiver’s reproductive condition and is therefore connected to the hormonal milieu. The processing of external signals may therefore be modulated according to internal cues, independently of learning, via hormones.
Beach [17] noted that social signals, which can take the form of courtship displays, aggressive threats, or hungry offspring, act as triggers for behavioral responses, and that hormones raise and lower the threshold for performing those responses. The same social signal, in other words, may elicit very different kinds of behavior depending on the receiver’s hormone level. In midshipman fish (Porichthys notatus) and many species of frogs, for example, females exhibit phonotaxis in response to males’ courtship vocalizations only when gravid [27,67]. Females that have already released their eggs do not respond to the sounds, suggesting that phonotaxis in these species may depend on reproductive condition and therefore have a hormonal basis.
Even in species with presumably more complex responses to social signals, reproductive state can alter those responses. Female rhesus monkeys looked longer at photos of conspecific males during the periovulatory period, whereas at other times they preferred to look at photos of females [103]. A large literature in humans also indicates that women’s preferences for male attributes change over the course of the menstrual cycle. Women looking at photos of male faces preferred more masculine and symmetric features during the time of peak fertility than outside it [90,107,143,144,170]. This phenomenon is not limited to the visual modality – in fact, during peak fertility, women also prefer lower voices [59,159] and the body odors of more dominant males [74]. There is little doubt that hormonal modulation of responses to social signals is widespread among vertebrates, and that it occurs in a variety of sensory systems.
The neural substrates involved in steroid-dependent social responses are well-studied and usually include one or more brain regions collectively referred to as a “social behavior network” (for reviews, see [70,138]). These regions, which include the medial extended amygdala and several hypothalamic areas such as the medial preoptic area and ventromedial nucleus, are interconnected, contain sex steroid receptors, and are implicated in a variety of social behaviors including sexual behavior, aggression, and parenting. Sociosexual signals, such as conspecific vocalizations or pheromones, regulate neural activity and/or induce gene transcription in neuronal populations of many of these regions. The modulation of those responses by steroid hormones, however, is not well-studied. In songbirds, there good evidence that the transcription of immediate early genes (IEGs) is modulated across the entire network by hormones: both the magnitude and selectivity of genomic responses to song are increased by treatment with estradiol [118]. In male ferrets, however, the response to pheromones is not altered in any node of this network by castration or testosterone replacement [95]. Rather, in this model, responses to such signals appear to be modulated by steroids more peripherally, in the olfactory bulb itself. Indeed, a growing literature supports the hypothesis that in many species, from fish to birds to humans, steroids may alter sensory processing, thus affecting the way sensory signals are perceived.
Beach himself argued that hormones affect the periphery and thus the sensations that are experienced [17]. Over the last 50 years, it has become clear that hormones have profound effects on sensory systems, to the extent that detection thresholds and discrimination can be altered. In both rats and humans, for example, the ability to detect odors is greater during periods of high estradiol than during other phases of the reproductive cycle, and estradiol treatment improves olfactory sensitivity in ovariectomized rats and postmenopausal women [35,36,149]. Estrogen receptors can be found in the olfactory periphery [16], cochlea [122,139,182,191], dorsal root ganglia [190], and retina [19,98,218], and are likely to impact sensory processing at every level in the brain. In other words, sex steroids affect the way we perceive the world.
2. The songbird as a model for estrogen-dependent behaviors
2.1. Why songbirds?
Since the pioneering work of Marler (e.g., [121]) songbirds are among the most popular models for studying the development and neural control of communication. Their songs, which function primarily in contexts of courtship and aggression (for review, see [38]), serve as an ideal social stimulus for playback studies in a laboratory setting. One can test, for example, how and when song is learned, which features are important for song learning or mate choice, and how song interacts with other cues, such as photoperiod or social context, to affect physiology. Playback studies have also allowed us to delineate the brain circuits involved in auditory processing, which are highly similar to that of mammals in their general anatomical organization (see below). Because the behavioral and neural responses to song are well-studied, songbirds represent excellent models with which to study the effects of hormones on those responses. Much of Marler’s early work was conducted on white-crowned sparrows (Zonotrichia leucophrys), and common seasonally breeding models now include the east coast congener of the white-crowned sparrow, the white-throated sparrow (Zonotrichia albicollis) and its cousins the dark-eyed junco (Junco hyemalis). Some researchers focus on European starlings (Sturnus vulgaris), which, because of their status as an invasive pest species in the US, are more convenient to collect in the field than sparrows. None of these species breed in captivity, however, which necessitated including the domesticated canary (Serinus canaria), a finch similar to wild sparrows with regard to its behavior and reproductive biology.
All of the above species, including canaries, synchronize their reproductive development each year to changing day length. Although other cues, such as temperature and food availability, can affect the timing of reproduction in these species, the length of the day is a primary cue that cannot normally be overridden. When birds of these species are held in captivity on short days, their ovaries and testes regress. Plasma levels of gonadal steroids decrease dramatically – in some species to non-detectable levels [219]. As long as the days remain short, the birds can be maintained in this state for many months. Because the secretion of endogenous gonadal steroids can be suppressed in this way without the need for surgery or drugs, manipulation via administration of exogenous hormone is relatively straightforward. Breeding levels of gonadal steroids can be achieved using subcutaneous implants of testosterone or estradiol [119,135,184], which allow the normal expression of many steroid-dependent reproductive behaviors including song, other courtship displays, and nest-building, with the gonads in a regressed state. Alternatively, endogenous levels of gonadal steroids can be manipulated by increasing the day length; many researchers have looked at seasonal effects simply by comparing long-day versus short-day birds. The neural effects of such manipulations can be profound; one of the earliest demonstrations of hormone-induced neuroplasticity in adult vertebrates was a comparison of the size of song control nuclei in spring versus fall canaries [141].
Seasonal breeders have proven useful for understanding how relatively slow, large-scale changes in steroid levels can affect brain structure and function. In some cases we are more interested in rapid effects, and for these it is more convenient to work with songbirds that can breed at any time of year. Zebra finches (Taeniopygia guttata) are inexpensive and highly social, making them easy to maintain in a laboratory setting. Unlike female sparrows and canaries, female zebra finches do not sing, making this species ideal for studies of sexually dimorphic behavior and its neural control. For these and many other reasons, the zebra finch has emerged as by far the most popular model for the study of songbird neurobiology and behavior. Their genome was recently sequenced, making them also a model for behavioral genetics and genomics [152,215]. Although they can breed continuously in the lab, it is possible to manipulate the reproductive state of females by water deprivation [146] or isolation from males [211]. It is important to note however that even in single-sex groups, reproductive state can vary unpredictably [145] and administration of exogenous estradiol can result in supraphysiological levels. Ovariectomy in songbirds is technically challenging and does not lower plasma levels of estradiol [1]; some investigators have made use of aromatase inhibitors [213]. In general, when interpreting the results of hormonal manipulation in female zebra finches it is important to consider whether the levels that are achieved, be they high or low, are within a physiological range or relevant to the animal.
2.2. Estradiol-dependent courtship displays
The concept of a hormone-dependent behavioral response is perhaps no better exemplified than by the copulation solicitation display (CSD), which is performed by female sparrows, canaries, and blackbirds in response to hearing male song. During the display, the bird raises her tail and head, quivers her wings, and gives a trill-like vocalization [177]. Like phonotaxis in midshipman fish and frogs, CSD is strongly dependent on reproductive state and is performed only by females with sufficiently elevated levels of estradiol [178]. Also like phonotaxis, CSD can be elicited in laboratory-housed females in response to an audio recording of male courtship vocalizations and has been used as an index to quantify both the attractiveness of the signal and the responsiveness of the receiver. In wild-caught sparrows and blackbirds, CSD behavior was directly related to the dose of estradiol [135,178], cf. [105]. In canaries, the latency to perform the display was increased by treatment with fadrozole, a selective inhibitor of aromatase (estrogen-synthase) [105]. No female of any species has been observed to perform CSD when not in breeding condition or otherwise treated with estradiol, even when presented with song that would, in the breeding season, be highly stimulatory [96,118,119,135]. In other words, the behavioral response to exactly the same auditory stimulus changes dramatically according to plasma estradiol level. This change in behavior suggests that estradiol affects the perception of either the song itself or of the behavioral context in which the song is heard. Our research on the effects of estradiol on auditory responses, outlined in the following sections, has addressed this phenomenon at the genomic, electrophysiological and pharmacological levels.
2.3. Effects of hormones on auditory preferences and discrimination
When studying behavioral responses to sensory stimuli, it is crucial to understand whether the experimental paradigm tests for effects on detection, discrimination, or preferences. Imagine a study, for example, in which female rats at different points in the estrous cycle are presented with the urine of a castrated and an intact male. The females in estrus choose to spend more time investigating the urine from the intact male, but those in other phases of the cycle spend equal time with the two samples. What do these results indicate? Can we conclude that the females that are not in estrus cannot discriminate between the two samples? Or perhaps they can discriminate between them but simply do not have a preference? Perhaps they cannot detect the odor of urine at all? The design of this study does not allow us to decide among these conclusions. More importantly for our purposes here, if the non-estrus females can discriminate between the odors but simply have no preference for one or the other, then we cannot necessarily infer an effect of reproductive phase on olfactory processing per se, only on the relative valence of the stimuli.
In female songbirds, behavioral song preferences are typically quantified using CSD to assay the attractiveness of song stimuli [177]. Because CSD requires breeding levels of plasma estradiol, testing behavioral preferences in birds with low estradiol levels is not possible with this type of assay. Four recent studies in zebra finches were done using assays of behaviors that are less likely to be affected by estradiol independently of stimulus: orienting to a sound or spending time in proximity to its source. Svec and Wade [196] reported that females preferred high quality to low quality song regardless of estradiol treatment, but in that study the untreated females had intact ovaries and likely normal plasma estradiol levels. Vyas and colleagues [212] showed that whereas untreated females showed a preference for high quality over low quality song, aromatase inhibitors blocked that preference. When Remage-Healey et al. [163] administered aromatase inhibitors to males, there was a trend toward the suppression of preferences for the bird’s own song. Tremere and Pinaud [206] found that local antagonism of estrogen receptors in a central auditory area (the caudomedial nidopallium; NCM, see below), and consequently interference with the physiological action of estradiol, largely abolished behavioral preferences to tutor songs. The latter finding demonstrates compelling evidence that estradiol may affect preferences by acting directly in the auditory system. Interestingly, estradiol appeared to shape the behavioral responses to learned songs selectively. Phonotaxis of male zebra finches towards female calls, which are similar acoustically in both complexity and frequency range to songs syllables, was not affected by blockade of estrogen receptors or inhibition of aromatase in NCM [206].
In frogs, phonotaxis depends strongly on estradiol [39] and it is possible that even the orienting responses and phonotaxis measured in the zebra finch studies detailed above depended on estradiol such that a lack of preference may be more related to a lack of motivation to respond at all than to an equal attraction to both stimuli. In frogs, the selectivity of the behavioral response goes down, not up, with estradiol treatment; as the estradiol dose increases, females are more likely to respond to any male call, even a low-quality one [114]. This phenomenon has not been demonstrated in songbirds, however. Searcy and Capp [178] reported that in female redwing blackbirds (Agelaius phoeniceus), CSD responses remained selective for high quality song even with increasing doses of estradiol.
Surprisingly few investigations of the effects of gonadal steroids on auditory detection or discrimination have been conducted from a purely behavioral perspective in songbirds. In only two studies have gonadal steroids been manipulated, and only one of those studies was conducted in a seasonal breeder. Reeves and colleagues [162] showed that administration of testosterone to non-breeding male song sparrows (Melospiza melodia) did not improve discrimination between two conspecific songs. Males with regressed testes and low endogenous testosterone reached criterion on a song discrimination task just as quickly as did their testosterone-treated, photostimulated counterparts. A previous study in zebra finches [46] showed similarly that testosterone treatment did not facilitate discrimination between conspecific songs, but it did facilitate discrimination between the bird’s own song and that of a familiar conspecific. The latter study was a follow-up to a finding that, in two other studies from the same authors that year, the acquisition of a song discrimination task took longer in winter than in summer in both male and female zebra finches [45,47]. Although zebra finches are not strictly seasonal breeders, a moderate amount of testicular growth can be induced by photostimulation [20] and it is therefore possible that seasonal effects may be related to gonadal steroid levels even in this species. Because of the small number of studies, however, it remains unclear whether testosterone affects auditory discrimination in songbirds. The effects of estradiol, either on discrimination or detection, have never been tested behaviorally in males or females of any species.
In behavioral studies that test specifically for discrimination, animals are trained to attend to the test stimuli. This training, however, itself increases the salience of the signals in a way that may mask or interfere with effects of hormones. In order to investigate the effects of sex steroids on auditory processing without affecting the way in which the signals are processed, it is for the moment best to look inside the auditory system in untrained animals.
3. Seasonality, estradiol, and the auditory system
3.1. The peripheral auditory system
As is the case in several other vertebrates [122,182,191], the effects of sex steroids on the auditory system in birds begin at the periphery. Noirot and colleagues [139] demonstrated that the hair cells of the inner ear of zebra finches contain estrogen receptor-α and aromatase and that these two molecules colocalize. Estrogen and androgen receptors have been detected in the inner ear of a seasonal breeder, the white-crowned sparrow (Y. Wang, unpublished, as cited in [31]). Cochlear sensitivity to sex steroids may to lead to seasonal plasticity in peripheral auditory function. Auditory brainstem responses, which represent activity generated by the auditory nerve and brainstem, change seasonally in a variety of songbirds including Carolina chickadees (Poecile carolinensis), tufted titmice (Baeolophus bicolor), white-breasted nuthatches (Sitta carolinensis), and white-crowned sparrows [31,111,112]. Any consideration of steroid effects on central auditory processing, therefore, must take into account the possibility that input arriving from the periphery is already affected by hormones.
3.2. The central auditory pathway
The anatomical and, to a large extent, functional organization of the central auditory pathways in songbirds largely resembles those found in other vertebrates, including mammals (Fig. 1). Auditory input in songbirds is transduced in the cochlea and ascends through an array of pontine, mesencephalic and thalamic nuclei before it reaches auditory forebrain areas. In brief, auditory input is conveyed to the songbird analogue of the mammalian cochlear nuclei (nucleus angularis [NA] and nucleus magnocellularis [NM]). Although NA and NM have not been studied in detail in songbirds, significant research in non-oscine avian species (i.e., chick and the barn owl) has shown that these structures encode information regarding sound intensity and the temporal properties of acoustic stimuli, respectively [61,133,195,197,225]. NM provides bilateral input to nucleus laminaris (NL), an auditory brainstem area thought to be the avian analogue of the mammalian medial superior olive [26], and responsible for computing interaural time differences required for sound spatial localization [33,142,225]. We emphasize that spatial tuning, and its neural bases, have not yet been studied in songbirds and may differ from other avian species. In fact, recent anatomical evidence suggests that whereas brainstem projections carrying time and level information, which are segregated in the barn-owl midbrain, appear to be integrated in the zebra finch midbrain [101,102]. NL and NA provide bilateral input to the superior olivary nuclei and to nucleus MLd of the intercollicular complex, the avian homologue of the central nucleus of the inferior colliculus (IC) in mammals. In birds and mammals, processing at the level of the IC is not only associated with the neural representation of sound intensity and perhaps location, but also with the shaping of frequency tuning required for auditory discrimination [97,157,183,221–224,226]. MLd neurons send direct projections to the diencephalic nucleus Ovoidalis (Ov), the avian homologue of the ventral medial geniculate nucleus in mammals [28,93], and a structure thought to play a central role in both frequency and temporal tuning of acoustic signals (Fig. 1).
Fig. 1.
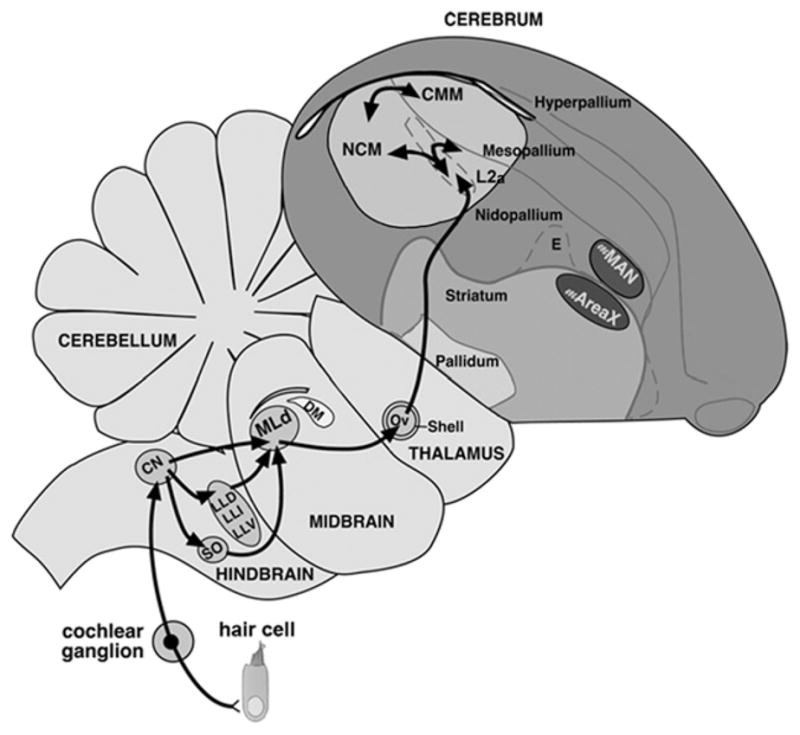
Schematic of a parasagittal view of the zebra finch brain depicting the principal structures of the main auditory pathway. Abbreviations: CN, cochlear nuclei; DM, dorsal medial mesencephalic nucleus; E, entopallium; H, hyperpalium; Hp, hippocampus; L2, field L2a; LaM, lamina mesopallialis; LL, lateral lemniscal nuclei; M, mesopallium; mAreaX, medial area X of the striatum; mMAN, medial magnocellular nucleus of the anterior nidopallium; MLd, dorsal lateral mesencephalic nucleus; N, nidopallium; NCM, caudomedial nidopallium; Ov, ovoidalis; st, striatum; v, ventricle; sO, superior olive. Modified from [154].
The distribution of estrogen-producing (aromatase-positive) and estrogen-sensitive (estrogen-receptor positive) cells has not yet been systematically mapped in sub-telencephalic auditory structures in oscine songbirds. It is clear, however, that the co-chlear nuclei, MLd and Ov in the zebra finch are devoid of aromatase- and estrogen-receptor-expressing cells [62,63]. Despite the lack of estrogen receptors in MLd, systemic estrogen manipulations appear to alter auditory-driven physiological responses of MLd neurons (Vyas and Woolley, unpublished observations), suggesting that estradiol may either modulate the encoding of auditory signals in the cochlea, or in areas that providing descending modulatory inputs to MLd.
Projections from thalamic nucleus Ov target the forebrain, more specifically a pronounced caudomedial lobe where most auditory areas are located (Fig. 1). At the core of this lobe lies the thalamo-recipient field L of the nidopallium, which receives input from Ov and has been proposed to be the analogue of cortical layer IV of the mammalian primary auditory cortex [130,207]. Processing in field L appears to be related to both encoding and decoding of spectro-temporal information, as revealed by electrophysiological studies carried out in both anesthetized and awake songbirds [30,136,222,224]. Field L provides input to caudomedial nidopallium (NCM) which, in turn, exchanges significant reciprocal connectivity with the caudomedial mesopallium (CMM) [28,93,94, 130,207]. Based on the general anatomical organization of these projection systems, it has been proposed that NCM may be analogous to the supragranular layers of the mammalian auditory cortex [127,131,207]. Studies involving activity-dependent gene expression methods as well as electrophysiological measurements indicate, however, that NCM and CMM exhibit properties more congruent with those observed in the mammalian auditory association cortex [155,198,199,205]. Field L, CMM and NCM send efferent projections to other telencephalic areas including those that originate descending auditory connectivity, as is also observed in the mammalian central auditory pathways, which include the shelf region underlying HVC (HVC shelf) and the area surrounding song nucleus RA (RA cup) [130]. Although a significant literature exists on the anatomical and functional organization of field L and NCM, less is known about CMM. It is generally accepted, however, that each of these auditory forebrain areas plays distinctive and central roles in the perceptual processing and discrimination of behaviorally-relevant communication signals and in associative learning involving acoustic cues [23,65,127,131].
The distribution of estrogen-sensitive and -synthetic cells has been studied in more detail in the auditory forebrain than in subcortical auditory regions. Aromatase-positive cells are exclusively located in NCM and are concentrated primarily in a crescent-shaped domain in the caudal aspect of this area [153,173,205]. NCM neurons also express both types of estrogen receptors: ERα is expressed at moderate levels in the dorsal-caudomedial extent of this auditory area ([63] and Maney, unpublished observations), whereas ERβ is expressed at relatively high levels and in a seemingly homogenous distribution [173,205]. Although other auditory forebrain areas, including CMM and Field L, appear to be devoid of cells that are positive for aromatase- or estrogen-receptor, a small cluster of neurons in the RA cup, a well defined auditory area, do appear to express estrogen receptors [63]. Overall, the distribution of aromatase and estrogen receptors suggests that within the central auditory system of songbirds, only NCM is clearly capable of both synthesizing and responding to estradiol. Estrogen-dependent modulation of neuronal responses in other auditory areas may be driven primarily by the sensitivity of the cochlea [139] or by descending input from estrogen-sensitive areas.
3.3. Induction of plasticity-associated genes in auditory areas
The identification and study of brain regions activated by auditory experience in the vertebrate brain has been facilitated by the quantification of IEG expression, which is commonly studied in intact, freely-behaving animals (for reviews, see [80,92,127,156]). In songbirds, several of the telencephalic areas involved in auditory processing were confirmed as auditory structures in part by their expression of the IEG zenk, also called zif-268, egr-1, krox-24 and NGFI-A. Males listening to conspecific songs show robust induction of zenk mRNA and/or its protein product (ZENK) in CMM, the caudolateral mesopallium (CLM), Field L (subfields L1 and L3), NCM, and the cup and shelf regions surrounding the robust nucleus of the arcopallium (RA) and HVC (proper name) song nuclei, respectively, which constitute auditory areas [125,127,129,130]. Both CMM and NCM show greater ZENK responses to conspecific song than to other sounds, suggesting that these areas exhibit selectivity towards conspecific communication signals (see below).
Biochemical analysis and cellular assays of gene expression have revealed a number of molecules, including ZENK, that are regulated in NCM by song [40,41,127,130–132,137,209]. These studies and, more recently, unbiased high-throughput proteomics methods indicate that the song-induced molecular changes in NCM are primarily driven by the mitogen-activated protein kinase (MAPK) pathway [40,108,154,209], a biochemical cascade repeatedly implicated in phenomena related to synaptic plasticity including those supporting learning and memory formation [194,202]. Induction of these genes likely represents the initiation of processes related to experience-dependent synaptic remodeling (for reviews, see [41,127,150,151]). When input from a salient sensory signal stimulates a neuron at the same time as modulatory input relaying contextual information, IEGs initiate possibly hundreds of downstream effects, via multiple intracellular pathways, that may collectively facilitate the formation of an association between stimulus and context [41,193]. Thus, IEGs are thought to play an important role in memory consolidation, particularly in cases of novel or otherwise behaviorally relevant stimuli.
The concept of behaviorally relevant stimuli is key to understanding the selectivity of the IEG response in the auditory forebrain. Social factors known to affect the magnitude of the behavioral response, such as song complexity, dialect, or familiarity to the listener, also affect the ZENK response ([66,120,186,200], cf. [51,220]). The ZENK response to a song can be enhanced by experimentally increasing its salience, for example by pairing it with foot shocks or a novel visual stimulus [87,100]. The response is also related to the attractiveness of the signal; in female canaries, song syllables known to be highly attractive elicit greater ZENK responses in CMM than less “sexy” syllables [106]. Female European starlings show greater ZENK induction in NCM when listening to long-bout song [66], which is thought to be more attractive to them than short-bout song [55]. Finally, the magnitude of ZENK induction in NCM is proportional to the number of CSDs given in response to a song stimulus [120], suggesting again that ZENK induction is related to behavioral relevance, not simply novelty. Because of the close association between the behavioral relevance of a stimulus and the ZENK response to that stimulus, factors that affect the former can be expected to affect the latter.
3.4. Estradiol-induced selectivity of the ZENK response in NCM
Of the factors that can affect the behavioral relevance of conspecific song, estradiol is one of the most important. In seasonal breeders, estradiol clearly modulates the behavioral response to song and appears to alter the song’s context or meaning; estradiol could therefore affect the ZENK response in NCM. Remarkably, as discussed above, a large fraction of NCM neurons express the estrogen-synthetic enzyme aromatase and estrogen receptors (ERα and ERβ) indicating that this auditory area is the site of an intriguing and robust overlap between sensory and neuroendocrine systems. The first study in which the ZENK response was compared across seasons was conducted by Duffy et al. [53] in female European starlings. They found that that the ZENK response to conspecific song was identical in reproductively active and non-active females. In that study, therefore, the magnitude of the ZENK response to song was not modulated by reproductive state. Because the control sound was silence, it was still unknown whether reproductive state could affect the magnitude of the response to song relative to the response to other sounds – in other words, whether estradiol could affect the selectivity of the ZENK response.
Working with white-throated sparrows, Maney et al. [117] showed that in this seasonal breeder, the selectivity of the ZENK response depends on estradiol. Non-breeding females were held on short days and treated with estradiol or placebo for 7 days. Half of the birds in each treatment group were then exposed to conspecific song, and the others heard synthetic tone sequences that were matched to the song stimuli with regard to the frequencies, amplitude, duration, number of onsets and offsets, and total sound energy. In estradiol-treated females, the ZENK response was selective for song over tones not only in NCM but also in CMM and the auditory midbrain (MLd). In the birds not treated with estradiol, however, the ZENK response to song did not differ from that to tones in any of these regions. These results suggest that in females, ZENK induction in the auditory system is selective for song only when plasma estradiol exceeds non-breeding levels and that estradiol-dependent plasticity of auditory centers may be related to learning or attention processes during the breeding season.
Maney et al. [117] noted that the effect of estradiol on the selectivity of ZENK induction in the auditory forebrain was not attributable simply to song-induced increases in the number of labeled cells. Hearing song induced similar ZENK responses in both estradiol-treated and blank-implanted birds (see also [53]). Rather, estradiol affected selectivity by reducing the ZENK response to frequency-matched tones, which elicited lower numbers of ZENK-positive cells in estradiol-treated birds than in the other groups. This pattern suggests estradiol-dependent modulatory input to the auditory forebrain that sharpens the response to behaviorally relevant signals by inhibiting the response to non-relevant stimuli.
A non-selective, high IEG response in non-breeding individuals has also been described in male zebra finches during song learning. In that species, whereas the ZENK response is selective for conspecific song in adults, at 30 days after hatching the responses to heterospecific and conspecific song do not differ and are as high as the adult response to conspecific song [192]. As is the case in female white-throated sparrows [117], selectivity of the ZENK response in male zebra finches arises not from an increase in the response to conspecific song but from a decrease in the response to sounds other than conspecific song. The development of this selectivity coincides with a rise in testosterone levels during song learning (e.g., [85]); in white-throated sparrows, it is induced by hormonal treatment that mimics adult breeding levels. Together, these results suggest that steroid hormones could induce selectivity in the auditory forebrain during both gonadal maturation and seasonal recrudescense. This selectivity could therefore wax and wane every year in the white-throated sparrow as well as other seasonally breeding songbirds such as the canary and the European starling.
3.5. NCM is a big place
NCM, the largest and best-studied region in the auditory fore-brain, is bounded dorsally and medially by the ventricular zone and overlying hippocampus, and rostrally by the mesopallial lamina and Field L (Figs. 2 and 3). Its lateral boundary is more difficult to define, but the region is thought to extend ~1.2 mm from the midline in zebra finches and a correspondingly larger area in larger songbirds. The distribution of song-induced zenk expression extends laterally to the level of the song nuclei HVC and caudally to the level of RA [125]. Most studies of ZENK induction in NCM have been conducted using sections cut in the parasagittal plane and focus on an area roughly half a millimeter from the midline (reviewed in [201]).
Fig. 2.
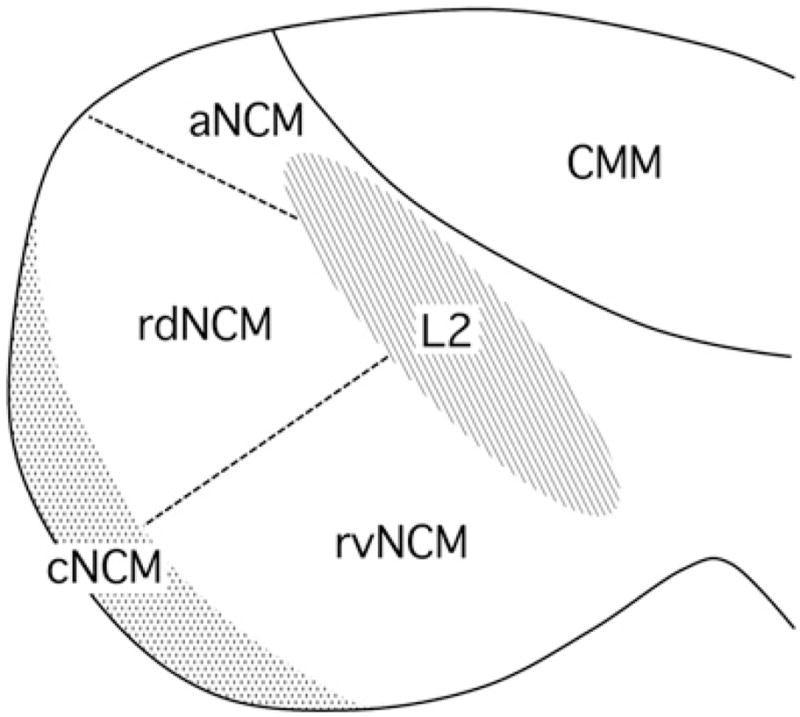
Domains of the auditory forebrain as defined by Sanford et al. [174] and Matragrano et al. [123]. Areas delineated by hatch marks are defined by cytoarchitecture and neurochemistry ([123,174]; see text), whereas the borders depicted by dashed lines are more arbitrary [66,174]. The border between aNCM and Field L2b, which appears more laterally [207], has not been characterized in sparrows. Abbreviations: CMM, caudomedial mesopallium; NCM, caudomedial nidopallium; aNCM, apical NCM; rdNCM, rostrodorsal NCM; rvNCM, rostroventral NCM; cNCM, caudal NCM. Rostral is to the right.
Fig. 3.
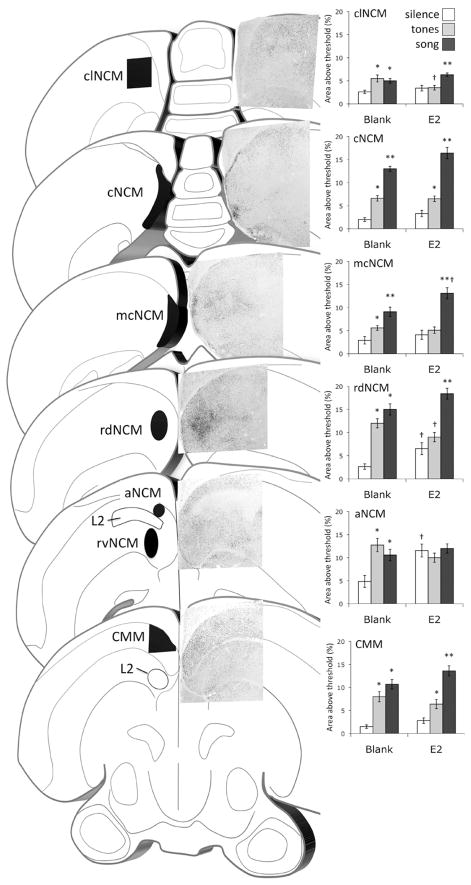
The ZENK response in the auditory forebrain of a white-throated sparrow as seen in coronal sections spanning approximately 4 mm. Each section is 450 μm thick with the exception of the one containing cNCM, which is 1650 μm thick. Photomicrographs depict ZENK immunolabelled material [117]. Graphs illustrate the effects of estradiol treatment on ZENK immunoreactivity in females listening to silence, tones, or conspecific song. In some cases (clNCM, mcNCM, rdNCM, and CMM) data collected from the material used in other published studies [117,174] have been combined. Data for rvNCM are not shown but are summarized in Table 1. *Significantly different from silence; **significantly different from both silence and tones; †significantly different from the same stimulus group in the blank condition. Alpha = 0.05. Abbreviations, see Table 1.
Throughout most of NCM, responses to sound are organized tonotopically [166,198], and the ZENK response to song is generally more robust in the dorsomedial aspect than the ventro-lateral aspect ([9,66,113,120,148,185,187,201,210], cf. [54]). Some researchers studying NCM have treated it as a single entity, sampling from a portion or randomly chosen portions within it (e.g., [24,25,53,126,189,217]). Gentner and colleagues [66] introduced the practice of dividing NCM into dorsal and ventral domains by bisecting it orthogonally to its dorso-ventral axis. Since that time, the majority of published studies have followed this convention (e.g., [9,10,54,81,113,117,124,148,187,188,204,208]). Despite numerous reports that dorsal and ventral NCM differ in their sensitivity to and selectivity for a variety of experimental stimuli, these domains do not appear to have a cytoarchitectonic, neurochemical, or hodological basis.
Pinaud et al. [153] argued that based on connectivity, electrophysiological responses and neurochemical markers, NCM could be divided into rostral and caudal domains. They demonstrated that in zebra finches, immunoreactivity for calbindin is concentrated along the caudal boundary of NCM, as is the expression of aromatase. Work by Maney and colleagues has shown that the caudomedial aspect of NCM is particularly rich in tyrosine hydroxylase-immunopositive fibers [123,174], receptors that bind the avian homologues of vasopressin and oxytocin (C.H. Leung, unpublished data), and estrogen receptors [21,62,63]. This region is located adjacent to the ventricular zone [2], and thus may contain or regulate the migration of newly generated neurons. Overall, the caudomedial border of NCM appears to be distinct, hodologically and neurochemically, from the more rostral and lateral regions.
Sanford and colleagues [174] sought to map the effects of estradiol on ZENK induction in order to determine whether some domains are more sensitive to estradiol treatment than others. They quantified sound-induced ZENK induction in distinct subregions, or domains, of NCM (Fig. 2)in non-breeding female white-throated sparrows treated systemically with estradiol or placebo. Surprisingly, estradiol-dependent selectivity of the ZENK response was limited primarily to the rostral domains of NCM. In the caudomedial domains, song induced more ZENK expression than did tones regardless of hormone treatment. They concluded that that estradiol-dependent plasticity in NCM is localized primarily to rostral NCM, the activity and selectivity of which may be seasonally regulated.
Maney and colleagues [117] quantified ZENK expression in a relatively caudal and lateral area, dorsal to the rostral-most aspect of RA (see clNCM, Fig. 3). We present here an analysis of the ZENK responses that occurred more rostrally and medially, in areas of NCM more commonly studied, in the animals from that experiment. Fig. 3 shows the ZENK immunostaining in the auditory fore-brain in a female white-throated sparrow that was listening to male song. Note in Fig. 3 that the ZENK induction is heterogeneously distributed within the auditory lobule, forming clusters of labeled cells particularly along the medial edge. These clusters were first described by Sanford et al. [174] and appear as a band on the caudal edge of sections cut in the parasagittal plane. Analysis of the labeling in this area revealed that song induced a greater ZENK response than tones in both the estradiol- and placebo-treated animals. This result is consistent with the findings of Sanford et al. [174], who reported estradiol-independent selectivity in cNCM (Fig. 2). In the most medial portions of this area (see mcNCM, Fig. 3, and [174]), estradiol significantly increased the response to song in this area. Also consistent with their findings, the present analysis in coronal sections shows that laterally, selective responses disappear in animals with low plasma levels of estradiol (see rdNCM, clNCM, Fig. 3 and Table 1). Thus, we show here that the findings of Sanford et al., namely the phenomenon of estradiol-dependent selectivity in the inner core of NCM, but not along the caudomedial edge, can be observed in coronal sections. Since the rostral portion of this interesting edge is easily lost when brains are cut in the parasagittal plane [125,174], it is perhaps preferable to study it in coronal sections where it remains fully connected to the rest of the telencephalon. Table 1 summarizes the effects of estradiol treatment in the domains of NCM that have been studied to date, and incorporates data from material generated by Maney et al. [117] and Sanford et al. [174].
Table 1.
The effects of 7–9 days of estradiol treatment on (A) ZENK expression in the absence of sound playback; (B) selectivity of the ZENK response (“increase” indicates a higher response to song than to tones in estradiol-treated birds only); (C) density of fibers immunoreactive for tyrosine hydroxylase (TH) or (D) dopamine beta hydroxylase (DBH). In some regions, the ZENK expression (A and B) has been reanalyzed using combined material from previously published studies [117,174]; see also Fig. 3. Data on catecholaminergic fiber density (C and D) are summarized from previously published data [104,123]. In some regions there is a good match regarding the effects of estradiol on the four variables measured. In rvNCM, for example, estradiol increases ZENK, selectivity, TH- and DBH-immunoreactivity, whereas in cNCM, estradiol has no effect on any of these variables. Interesting exceptions include aNCM, where estradiol has a profound effect on ZENK induction but does not increase selectivity, and Ov, where estradiol affects selectivity but not TH- or DBH-IR.
| Region | (A) ZENK expression | (B) ZENK selectivity for song over tones | (C) TH-IR fiber density | (D) DBH-IR fiber density |
|---|---|---|---|---|
| CMM | No change | Increase | Increase | Increase |
| aNCM | Increase | No change | Increase | Increase |
| rvNCM | Increase | Increase | Increase | Increase |
| rdNCM | Increase | Increase | No change | Increase |
| mcNCM | No change | No change | n.d. | n.d. |
| cNCM | No change | No change | No change | No change |
| clNCM | No change | Increase | Increase | n.d. |
| MLd | No change | Increase | Increase | Increase |
| Ov | No change | Increase | No change | No change |
Abbreviations: CMM, caudomedial mesopallium; NCM, caudomedial nidopallium; aNCM, apical NCM; rvNCM, rostroventral NCM; rdNCM, rostrodorsal NCM; mcNCM, medial caudal NCM; cNCM, caudal NCM; cl NCM, caudolateral NCM; MLd, auditory midbrain; Ov, nucleus ovoidalis.
n.d. = Not determined.
3.6. Estradiol is necessary and sufficient to induce ZENK and other MAPK-dependent genes
The second major finding of Sanford and colleagues [174] was that estradiol treatment increased ZENK expression in NCM in birds that were not exposed to sound at all. This result was consistent with previous observations by Tremere and colleagues [205], who showed that infusions of estradiol into the NCM of awake, sound-isolated zebra finches markedly induced the expression of MAPK-dependent genes, including zenk, in NCM neurons. In other words, estradiol upregulates plasticity-associated gene expression, even in the absence of auditory stimulation, to levels that were quantitatively indistinguishable from levels in the NCM of song-stimulated control animals. Sanford and colleagues [174] demonstrated this phenomenon in the rostral regions of NCM. In aNCM, an under-studied region immediately dorsal to Field L2, the ZENK response to estradiol was so great that it appeared to mask sound-driven responses. The region responds to both song and tones in placebo-treated birds but not in estradiol-treated birds ([174]; Fig. 3). Overall, Sanford and colleagues reported that estradiol treatment induced ZENK expression in the rostral areas of NCM, which were the same areas in which the treatment enhanced selectivity for conspecific song (Table 1).
Some authors have argued that gonadal hormones increase constitutive activity in brain regions associated with social behavior and may thus promote context-appropriate social responses by biasing the system toward certain behaviors over others [50,72,86,168]. Estradiol may therefore prime the auditory system to respond differently to sound during the breeding season by biasing responses to acoustic cues that become more behaviorally relevant at that time of year. High constitutive levels of IEG expression in NCM may be related to learning; young male zebra finches show high levels of ZENK expression, even in the absence of sound stimulation, during the period of song memorization [192]. Plasma testosterone is high during this period, suggesting that this hormone, or perhaps its conversion to estradiol, may play a role in the sound-independent ZENK expression in juvenile zebra finches.
In addition to their finding that local infusion of estradiol induces MAPK-dependent genes in NCM, Tremere and colleagues [205] reported two other important findings. First, they found that unilateral blockade of estrogen receptors markedly decreased the song-induced expression of these genes, including zenk. This finding demonstrated that endogenous estradiol acts locally to induce the zenk response and that it is not only sufficient, but also necessary for that response. Second, they found that local inhibition of aromatase suppressed the expression of these plasticity-associated genes to negligible levels while leaving contralateral song-induced expression intact. The latter finding provides additional compelling evidence not only that estradiol acts locally, but that it is synthesized locally. In the following sections we consider the growing literature on brain-derived estradiol and its role in the processing of acoustic communication signals.
4. The brain as a source of estradiol
4.1. The brain makes its own sex steroids
The effects of estradiol on auditory processing and selectivity need not be limited to the breeding season or to breeding individuals. Evidence accumulated over the last two decades shows clearly that the brain itself, in addition to the gonads, serves as a primary source of sex steroids. Conclusive evidence supporting these findings derived from key contributions from multiple research groups [11,147,172,175,179]. Thorough reviews on the topic of brain-generated steroids have been published in the past few years and, therefore, this topic will not be extensively covered here (for example, see [110]).
The brain can synthesize both testosterone and estradiol either from steroid precursors that arrive from the periphery or de novo from cholesterol [109]. The brain is not reliant, therefore, on the gonads for a supply of steroids that can alter central auditory function. NCM in particular, especially the caudal domain, is rich in cells that express the estrogen-synthetic enzyme aromatase [153]. This population of neurons is neurochemically heterogeneous, comprised of both inhibitory and excitatory cells, and is directly engaged by auditory experience [88]. Remage-Healey and colleagues [164] demonstrated via in vivo micro dialysis that estradiol concentrations increased rapidly in NCM in male zebra finches as they listened to song or interacted with females. An aromatase inhibitor administered into NCM decreased estradiol levels [164]. Singing itself, or possibly hearing oneself sing, also alters estradiol synthesis; in male zebra finches that sang for 30 min, aromatase activity increased in synaptic terminals of the caudal telencephalon [165]. The levels of aromatase mRNA did not change, suggesting that estradiol levels are regulated rapidly by post-transcriptional activation of aromatase.
4.2. Brain-derived estradiol alters behavior
As reviewed above in Section 2.3, systemic aromatase treatment of male zebra finches tended to reduce behavioral preferences for the bird’s own song [163]. When the aromatase inhibitors were administered directly into the auditory forebrain, the suppression of those preferences was robust and significant [163]. These results suggested that estradiol generated in NCM as a function of auditory experience rapidly regulates auditory-based behaviors. Further support for this hypothesis was provided by Tremere and Pinaud [206], who showed that local blockade of estrogen receptors in NCM interferes with behavioral preferences to tutor songs in adult zebra finches. This effect was mediated by estradiol generated in NCM, given that local inhibition of aromatase with fadrozole or ATD suppressed behavioral preferences to the same degree as blockade of estrogen receptors. Remarkably, male preference towards female calls was affected neither by estrogen receptor blockade nor by aromatase inhibition in NCM, suggesting that local estradiol appears to shape behavioral responses to learned song selectively.
4.3. Interactions between brain-derived and gonadal steroids
The aromatase-positive neurons in NCM are concentrated in the caudal domain (cNCM) in zebra finches [153] and in white-throated sparrows (D.L. Maney, unpublished observations). Because estradiol concentrations increase in NCM after song but not other sounds [164] and because estradiol alone can induce zenk expression [205] (see below), it is possible that the selective ZENK response observed by Sanford et al. [174] in cNCM is regulated by the aromatase-positive neurons in that area. Such a scenario could explain why the ZENK responses in cNCM remain selective for song over tones even when plasma estradiol levels are low. In cNCM, high plasma estradiol may be unnecessary for selective ZENK responses because estradiol can be synthesized locally. In contrast, the more rostral domains of NCM, which have lower levels of aromatase, may rely on estradiol from the plasma for selective responses (Table 1). In zebra finches, aromatase inhibitors readily suppress both mIPSCs and ZENK responses in rostral NCM [205], suggesting that although estradiol is primarily synthesized in caudal NCM, in this species it significantly impacts rostral NCM presumably by readily and robustly diffusing to this region. More research is needed to determine definitively whether the distribution of aromatase differs between species as well as whether and how it is transported. It is possible that seasonal breeders have evolved mechanisms by which plasma steroids can act in concert with brain-derived steroids. In such animals, steroids synthesized in the brain are thought to help maintain steroid-dependent behaviors during the non-breeding season when plasma levels are low [158,176]. Selective ZENK responses in cNCM may be an example of this phenomenon.
5. Brain-generated estradiol affects auditory neuronal physiology
5.1. Estradiol generated in NCM regulates hearing-driven neurophysiological responses
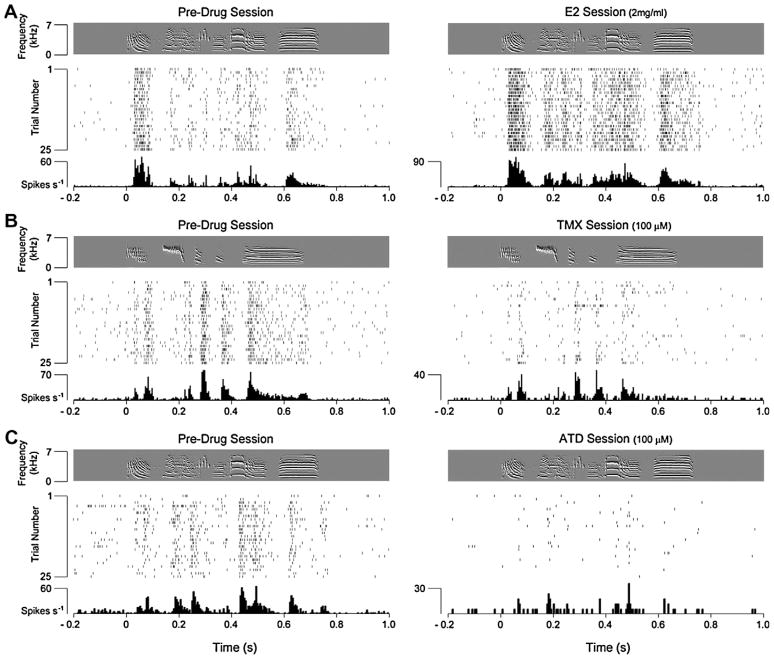
The fact that estradiol levels rapidly, robustly and selectively increase in NCM as a result of auditory experience suggests that local production of this steroid hormone may directly modulate auditory-driven responses in the NCM of awake songbirds. Tremere and colleagues [205] directly tested this possibility by recording auditory-driven neurophysiological responses in awake, restrained zebra finches during bilateral, intracerebral pharmacological manipulations directed at estrogen-associated circuits in NCM. They found that local infusions of estradiol markedly increased hearing-evoked discharge rates of NCM neurons in a dose-dependent manner (Fig. 4A). In contrast, firing rates of NCM neurons decreased, also in a dose-dependent way, when estrogen receptors were locally blocked or when aromatase was inhibited in NCM (Fig. 4B, C). These findings have been recently independently reproduced via the combination of in vivo microdialysis and multi-unit recordings in the anesthetized zebra finch [163]. Remarkably, the disparate effects of estradiol, tamoxifen (TMX) and ATD on NCM neuronal discharge rates reported by Tremere and colleagues were virtually instantaneous, suggesting that non-genomic mechanisms are at play.
Fig. 4.
Brain-generated estradiol rapidly increases discharge rates of NCM neurons. (A) Effects of exogenous infusions of estradiol to a single-unit in NCM. Shown is the spectrogram of a stimulus (a conspecific song segment; top panel), time-aligned with a spike raster plot (middle panel) and the peri-stimulus time histogram (bottom panel) for an NCM neuron, both before (left) and during (right) estradiol infusions. Note that estradiol dramatically increases the firing rate of this representative neuron, and that this effect occurs virtually instantaneously. (B) Blockade of estrogen receptors with tamoxifen (TMX) significantly decreases song-induced discharge rates of NCM neurons. The raster plots shown on (B) derive from the responses of a single NCM neuron before (left) and during (right) TMX infusions. (C) Estradiol locally produced in NCM markedly increases song-driven discharge rates. Shown is the response profile of a single NCM neuron before (left) and during (right) blockade of local estradiol production with the aromatase inhibitor ATD. Note that song-driven responses are largely abolished in the absence of local estradiol. From [205].
5.2. Estradiol acts through a non-genomic mechanism involving GABAergic neurotransmission
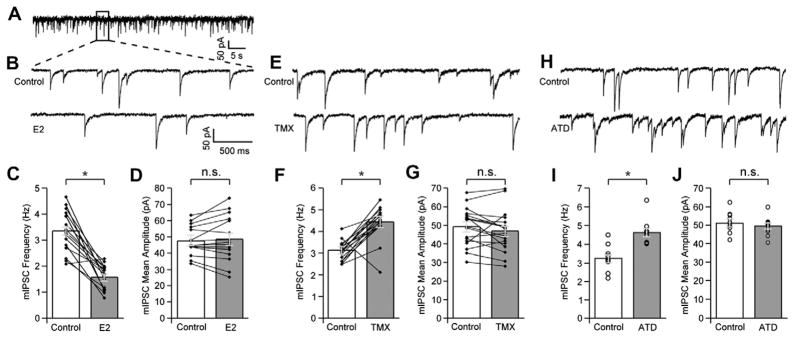
In addition to studying the instantaneous effects of estradiol on the physiology of NCM neurons, Tremere and colleagues explored their biophysical basis. Given that estradiol affected NCM’s neuronal responses on a rapid timescale (seconds), the authors hypothesized that this neurohormone exerted its effects through a non-genomic mechanism, likely through the modulation of local neurotransmission. To address this possibility directly, whole-cell patch-clamp recordings were obtained from NCM slices and the effects of estradiol, antagonism of the estrogen receptors, or inhibition of aromatase on excitatory and inhibitory synaptic transmission were determined [205]. By pharmacologically isolating miniature spontaneous inhibitory post-synaptic currents (mIPSCs), the authors found that estradiol had a profound effect on local GABAergic transmission (Fig. 5). Specifically, estradiol significantly suppressed the frequency of mIPSCs but had no effect on the amplitude of such events. In contrast, local aromatase inhibition or blockade of estrogen receptors increased the frequency but failed to affect the amplitude of mIPSCs. The fact that estradiol affected the frequency but not the amplitude of mIPSCs indicated that this neurohormone shaped NCM’s neuronal physiology through a pre-synaptic mechanism. This mechanism putatively involves an estradiol-mediated change in the probability of GABA release onto NCM neurons. Importantly, as indicated above, Tremere and colleagues also assessed whether estradiol affected the synaptic physiology of excitatory NCM circuitry. Surprisingly, it was found that bath application of estradiol, antagonism of estrogen receptors, or aromatase inhibition affected neither the frequency nor the amplitude of miniature excitatory post-synaptic currents (mEPSCs), suggesting that estradiol selectively modulates inhibitory (GAB-Aergic) synaptic neurotransmission in NCM. More generally, the findings of Tremere and colleagues provided the first demonstration that brain-generated estradiol modulates auditory-driven responses, in real-time, in the awake vertebrate brain by suppressing the strength of local inhibitory transmission via a pre-synaptic, non-genomic mechanism.
Fig. 5.
Estradiol suppresses the strength of local inhibition via a pre-synaptic, non-genomic mechanism. (A) Shown is a 1-min trace obtained from an NCM neuron via whole-cell patch-clamp recordings, depicting miniature inhibitory post-synaptic currents (mIPSCs) that were pharmacologically isolated. (B) Changes in mIPSC properties are shown in a representative neuron before (top trace) and during (bottom trace) application of estradiol to the recording bath. (C and D) Estradiol significantly decreases the frequency (C), but not the amplitude (D) of mIPSCs, suggesting that this steroid hormone influences inhibitory transmission through a pre-synaptic mechanism. Diamonds depict values obtained for each recorded cell that contributed to the mean (bars). (E) Blockade of estrogen receptors with tamoxifen significantly impact mIPSC properties. Shown are traces obtained from a representative neuron before (top trace) and during (bottom trace) application of TMX to the recording bath. (F and G) TMX increases mIPSC frequency (F) but does not alter the amplitude of these events (G). (H) Representative traces obtained from a control neuron (top trace) and from a neuron treated with ATD (bottom trace). Inhibition of aromatase activity with ATD significantly increases the frequency of mIPSCs (I), but not their amplitude (J). *p < 0.0001. From [205].
5.3. Estradiol enhances the information that NCM neurons carry about the stimulus to facilitate the neural discrimination of songs
Although Tremere et al. [205] directly demonstrated that estradiol generated in NCM regulates the gain of auditory-driven responses in the awake songbird, the functional relevance of this estrogenic modulation was not yet known. In other words, what does estradiol offer NCM neurons in terms of auditory processing capabilities and/or computational power? To address this problem, Tremere and Pinaud [206] combined multi-electrode neurophysiological recordings in the NCM of awake zebra finches with bilateral local pharmacological manipulations, and used information theoretical methods and machine learning algorithms to analyze their neural data. More specifically, Tremere and Pinaud used these approaches to determine how much information NCM neurons carry about stimulus structure and to determine how well NCM cells can discriminate among different songs. The authors found that local blockade of estrogen receptors, and consequently the effects of estradiol, markedly decreased the information transmitted about song stimuli. As a result, this pharmacological manipulation significantly impaired a neuron’s ability to discriminate among different songs. Tremere and Pinaud [206] also determined that it is the local production of estradiol that shapes the efficiency with which songs are encoded and discriminated by NCM neurons. Local inhibition of aromatase suppressed these phenomena to levels indistinguishable from those observed after blockade of estrogen receptors in NCM. Interestingly, local infusions of exogenous estradiol in NCM significantly enhanced auditory coding efficiency and, consequently, the neural discrimination of songs by NCM neurons. Remarkably, the local effects of estradiol, blockade of estrogen receptors and inhibition of aromatase were long-lasting, persisting for several after local pharmacological manipulations were interrupted [206]. Finally, it was shown that the optimization of auditory coding driven by estradiol is not merely explained by increased discharge rates elicited by this neurohormone on auditory-driven responses, but rather it was found that estradiol modulates the neural coding of songs by shaping both rate and the temporal coding [206]. These data provided the first demonstration that one of the main functional consequences of estradiol’s modulation of NCM’s neuronal physiology is to enhance the information that these central auditory neurons carry about stimulus structure, such that the neural discrimination of song stimuli is optimized [206].
5.4. Brain-derived estradiol does not affect selectivity
Tremere and co-workers [205] tested whether estradiol selectively modulates the auditory processing of songs or whether this neurosteroid affects the neural responses to song and non-song stimuli equally. To this end, an array of artificial stimuli, consisting of songs that were degraded in both spectral and temporal domains, were presented and electrophysiological responses recorded. They found that local infusions of estradiol, blockade of estrogen receptor, or suppression of the local production of estrogens in NCM affected the auditory processing of song and non-song stimuli equally. These findings suggested that although estradiol may not acutely regulate the selectivity of song-driven responses in NCM in real-time, it perhaps serves a more fundamental purpose: to regulate the gain of auditory-evoked responses in the NCM of awake animals irrespective of the behavioral relevance of the acoustic cues.
The finding that estradiol does not alter the selectivity of electrophysiological responses in NCM at first glance may appear to contradict the findings of Maney and colleagues that estradiol treatment is required for selective ZENK responses in non-breeding females [117,174] The apparent discrepancy is not actually in the findings but rather in the use of the term “selectivity”. For the electrophysiologist, selectivity refers to a single neuron (or ensemble of neurons) firing more robustly to stimulus A over stimulus B. Such a result cannot be achieved with ZENK mapping, especially because the ZENK response is engaged by estradiol in the absence of a sound stimulus [174,205]. Whereas estradiol does not appear to modulate the selectivity of auditory-driven neurophysiological responses in NCM neurons [205], it does appear to interact with the biochemical/molecular cascades that derive from that activity [174,205]. For example, estradiol may prime co-regulators of the ZENK response that, when engaged by song but not non-song stimuli, lead to differential induction of ZENK. In this case, estradiol may not necessarily impact the selectivity of the neural activity, but may directly influence the outcome of that process. Moreover, at least some of the processes responsible for estradiol-dependent selectivity of the ZENK response in NCM may actually occur outside this area, in other brain regions. In other words, selective ZENK responses in NCM may represent an end result of estradiol- and stimulus-dependent input arriving from outside NCM. Below we outline evidence that estradiol-dependent selective responses in the auditory forebrain may be mediated by catecholaminergic innervation.
6. Catecholamine systems as mediators of estrogenic effects
The caudal domain of NCM is rich in catecholaminergic (CA) fibers that form basket-like structures around the aromatase cell bodies, completely surrounding them [5]. Clearly, CA innervation may be important for the regulation of estradiol synthesis and for auditory processing in general. Dopamine (DA) and norepinephrine (NE) are widely understood to shape the response properties of sensory networks involved in selective attention (for reviews, see [8,84]) and context-dependent modulation of forebrain plasticity [12,32,37,42,43,48]. In songbirds, CA projections to the forebrain have been hypothesized to affect the auditory processing of song (e.g., [4,6,7,22,32,104,115,169]). Noradrenergic denervation of the forebrain reduces behavioral and neural responses to song as well as behavioral and neural selectivity for sexually stimulating song [6,113,212]. Modulation of catecholaminergic systems is therefore likely to affect the auditory processing of conspecific song as well as the resulting behavioral responses.
The anatomical relationship between CA fibers in NCM and the aromatase neurons suggests that CAs could modulate estradiol synthesis in the brain [5]. The converse, that CA systems are themselves sensitive to sex steroids, is also likely true. In male zebra finches, catecholamine levels and turnover in the auditory fore-brain are regulated by both androgens and estrogens [14]. LeBlanc and colleagues [104] reported that estradiol treatment of female white-throated sparrows increased the number of tyrosine hydroxylase (TH)-immunoreactive cells in the ventral tegmental area (VTA), a major source of DA in the forebrain, and in the locus coeruleus (LoC), the primary source of NE. Both of these brainstem CA cell groups, which likely project to the auditory forebrain [3,5,128,167], contain estrogen receptor [116] and therefore could be directly affected by estradiol treatment. Recently, Matragrano et al. [123] tested the hypothesis that the heterogeneous effects of estradiol treatment on ZENK expression and selectivity in the auditory system are mediated by CA innervation. Of the eight regions of the auditory system they looked at, estradiol significantly increased immunoreactivity for CA synthetic enzymes only in the regions previously demonstrated by Sanford et al. [174] to be sensitive to estradiol (Table 1). The caudal domain of NCM, in which ZENK expression was unaffected by estradiol treatment, showed no estrogen-dependent modulation of either TH or dopamine β-hydroxylase (DBH). The strong anatomical match between the estradiol’s effects on the ZENK response and on CA innervation suggests that the two might be related.
How might CA input affect auditory selectivity? If the CA cells that project to auditory areas themselves fire in response to song, the ensuing catecholamine release may cause or modulate subsequent activity. Gale and Perkel [64] recently demonstrated that in male zebra finches, TH-immunoreactive cells in the VTA fire in response to sound and, in fact, are highly selective for conspecific song. LeBlanc et al. [104] reported that in estradiol-treated female white-throated sparrows, hearing song induces ZENK expression not only in the VTA but also in the LoC, substantia nigra, and periaqueductal gray, and that these responses are selective for song only in birds with physiological breeding levels of plasma estradiol. This effect was particularly striking in the LoC; in estradiol-treated birds, the ZENK response to song was threefold higher than the response to tones, but in blank-implanted birds the responses to song and tones were identical. Although LeBlanc and colleagues [104] reported that the ZENK response in CA cell groups of the brainstem was not localized in TH-positive cells, other authors have reported co-localization of IEG expression and TH in these cell groups after presentation of song or other social stimuli [22,71,140]. Experiments are currently underway to determine whether levels of catecholamines or their metabolites change rapidly in NCM in response to hearing song, and whether those changes depend on plasma estradiol.
The CA cells in the brainstem need not be actively firing during song in order to play a neuromodulatory role in estradiol-dependent auditory selectivity. Some CA terminals may release transmitter in a non-synaptic, or paracrine fashion, which may alter the responsivity or spontaneous firing activity in forebrain neurons (for review, see [18]). Such changes, which may be sustained over prolonged periods [161], may enable forebrain areas to respond differently to the same input depending on the behavioral state of the animal (e.g., season or social context), without changes in the selectivity of CA neurons themselves. Seasonal changes in catecholamine-based neuromodulation may enable auditory systems of female songbirds to respond selectively to male song during the breeding season and to respond less selectively outside it.
7. Evolution of estrogen-dependent sensory processes
In this review we have shown evidence that in songbirds, the steroid hormone estradiol modulates the processing of a behaviorally relevant signal, conspecific song. We have argued that in seasonally breeding sparrows estradiol affects auditory selectivity for this signal, which presumably increases in behavioral relevance during the breeding season. We have also shown, however, that estradiol modulates the processing of conspecific song in zebra finches, which show neither sex differences nor seasonal changes in plasma estradiol levels. In fact estradiol affects many sensory processes, such as nociception in quail [58] and speech understanding in humans [79], for which the connections to reproduction are not obvious. Why might a hormone associated with reproductive behavior alter sensory processing so broadly?
We believe that sensitivity to estradiol may be an evolutionarily ancient property common to all sensory systems. Estradiol and proteins that bind it can be found in a wide range of species, including unicellular organisms [60,171], and application of estradiol can alter their behavior [99]. The earliest steroid receptors, which evolved more than 600 million years ago prior to the split between deuterostomes and protostomes, functioned as estrogen receptors [203]. Estrogen-mediated signaling mechanisms are therefore among the most ancient forms of intercellular communication. The most primitive of sensory systems, for example the external chemoreceptors in unicellular organisms, make use of the same compounds and transduction mechanisms used for internal cell signaling in multicellular organisms [34]. The molecules important for communication in unicellular systems evolved into the neuromodulators and receptors of vertebrate sensory systems; for example, the vertebrate β-adrenergic receptor shares ancestry with an external chemoreceptor for a mating factor in yeast [68]. Given the age of estrogens and their receptors, it is possible that the role of these molecules in sensory processing predates their role in reproduction! Thus, although it is possible that sensitivity to reproductive hormones is a relatively new adaptation in some sensory systems, it is unlikely that every instance of steroid modulation of sensory systems is related to the tuning of that system to reproductive signals.
8. Broader implications for estradiol’s regulation of auditory processing
The link between sensory processing and hormonal regulation was clearly demonstrated over 30 years ago in the olfactory system, in which the perception of species-specific olfactory cues were shown to be markedly affected by sex hormones (for review, see [134]). However, it has also been widely accepted that primary, “cortically-represented” sensory systems, including vision and audition, are not modulated by steroid hormones. In the auditory system in particular, this dogma has permeated scientific wisdom despite over three decades of sporadic reports in the literature suggesting a link between steroidal hormones, especially estradiol, and hearing function. For instance, auditory processing was shown to be marginally but significantly more efficient in women than in men, a phenomenon that has now been reproduced by an array of research groups employing varied methodological approaches. Likewise, auditory-event related potentials (ERPs) and hearing thresholds have long been known to oscillate across the menstrual cycle in women: whereas higher plasma estrogen levels are associated with lower thresholds and more robust ERPs, lower plasma estrogen levels are associated with higher thresholds and more variable ERPs [49,89,214]. Such findings were amongst the earlier work that suggested a sensory–endocrine interaction in the auditory system. This literature was further supported by clinical studies that demonstrated significant hearing pathologies in women with Turner’s syndrome, who are deficient in estrogens [73,83]. Although these findings collectively suggest that a steroid hormone may modulate hearing function, it remained unclear where such interaction occurred in the auditory system – peripherally, centrally or both – and whether gonadal or brain-derived hormones were at play (but see below).
As in humans, periodic findings in experimental models have suggested that steroid hormones may influence hearing function. For example, progressive hearing loss leading to early deafness has been recently described for mice that are deficient for ERη [180]. Ovariectomized non-human primates and rodents displayed significant distortions in the normal profile of auditory brainstem responses; interestingly, such distortions could be rescued by estrogen replacement therapy [44,69]. As with the human findings discussed above, it is unclear at this point whether peripheral or centrally-generated hormone is related to these changes in sensory processing, whether the locus of action is in the peripheral organ or within the central nervous system, or whether these phenomena are causally linked at all in these experimental models. Over the past 3 years, as discussed in the sections above, research carried out in songbirds has suggested that brain-generated estradiol has a profound and local effect on the cellular biology and physiology of central auditory neurons. If sex hormone-dependent regulation of auditory processing is shown to be a general phenomenon across vertebrates, including humans, a number of translational issues will be raised, some of which we consider here. First, hearing impairments are overwhelmingly studied in the context of peripheral organ (cochlear) dysfunction. The data discussed above suggest that deficiency in estradiol levels largely impair central auditory function. It is, therefore, highly conceivable that hearing impairments associated with estrogen deficiency may be a process that occurs entirely, or at least partially, in the brain. This novel way of considering hearing dysfunction goes against the generally accepted view that sex hormones may influence hearing function by affecting the functionality and/or integrity of the cochlea [15,44,57,69,76–78,82]. We do not reject the view that estrogens may influence peripheral organ function, but rather emphasize the need to seriously consider phenomena in the central nervous system as a mechanistic source of hearing impairments. Second, conditions associated with significant hormonal shifts in humans, including menopause, contraceptive regimens, hormonal supplements, chemotherapeutic strategies involving anti-estrogenic compounds (e.g., tamoxifen), as well as genetic conditions such as Turner’s syndrome, have been reported to alter hearing function. Studying the modulation of auditory processing by steroid hormones may therefore impact a variety of human health issues [56,57,78,82]. Third, for decades research in both humans and experimental models has established in detail how age-associated processes and an array of stressors profoundly affect both peripheral and central estrogen levels, all of which could modulate hearing function.
We strongly underscore that these suggestions are highly fragile and will rely on future research aimed at establishing causal interactions between neurosteroids and central auditory circuits in mammals, including humans. However, the sensory–neuroendocrine interactions so clearly demonstrated in the songbird brain to date, as discussed above, have further influenced the study of how steroid hormones regulate cellular biological processes, contextualized how sensory systems operate and, more generally, have initiated discussions on the potential use of hormonal replacement therapy to recover attributes of hearing function degraded by estrogen insufficiency.
Acknowledgments
The authors thank Anjani Chitrapu, Ellen Cho, Chris Goode, David Gutman, Jin Kwon Jeong, Henry Lange, Cary Leung, Lisa Matragrano, Katrina Salvante, Sara Sanford, Keith Sockman, Tony Tran, and Liisa Tremere for their contributions to the work described in this review. The research discussed here from the authors’ laboratories was funded by the Center for Behavioral Neuroscience and NSF IBN 0346984 (to DLM), and NIH/NIDCD R01-DC-010181-01, NSF IOS-1064684, the Schmitt Foundation and the Reynolds Foundation (to RP).
References
- 1.Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA. Sex steroid levels in developing and adult male and female zebra finches (Poephila guttata) Gen Comp Endocrinol. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–109. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- 3.Appeltants D, Ball GF, Balthazart J. The distribution of tyrosine hydroxylase in the canary brain: demonstration of a specific and sexually dimorphic catecholaminergic innervation of the telencephalic song control nuclei. Cell Tissue Res. 2001;304:237–259. doi: 10.1007/s004410100360. [DOI] [PubMed] [Google Scholar]
- 4.Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002;13:649–653. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- 5.Appeltants D, Ball GF, Balthazart J. Catecholaminergic inputs to aromatase cells in the canary auditory forebrain. Neuroreport. 2004;15:1727–1730. doi: 10.1097/01.wnr.0000135920.75925.1e. [DOI] [PubMed] [Google Scholar]
- 6.Appeltants D, Del Negro C, Balthazart J. Noradrenergic control of auditory information processing in female canaries. Behav Brain Res. 2002;133:221–235. doi: 10.1016/s0166-4328(02)00005-0. [DOI] [PubMed] [Google Scholar]
- 7.Appeltants D, Gentner TQ, Hulse SH, Balthazart J, Ball GF. The effect of auditory distractors on song discrimination in male canaries (Serinus canaria) Behav Process. 2005;69:331–341. doi: 10.1016/j.beproc.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus–norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 9.Avey MT, Kanyo RA, Irwin EL, Sturdy CB. Differential effects of vocalization type, singer and listener on ZENK immediate early gene response in black-capped chickadees (Poecile atricapillus) Behav Brain Res. 2008;188:201–208. doi: 10.1016/j.bbr.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Avey MT, Phillmore LS, MacDougall-Shackleton SA. Immediate early gene expression following exposure to acoustic and visual components of courtship in zebra finches. Behav Brain Res. 2005;165:247–253. doi: 10.1016/j.bbr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Balthazart J, Schumacher M, Evrard L. Sex differences and steroid control of testosterone-metabolizing enzyme activity in the quail brain. J Neuroendocrinol. 1990;2:675–683. doi: 10.1111/j.1365-2826.1990.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 12.Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 13.Baptista LF, Trail PW, DeWolfe BB, Morton ML. Singing and its functions in female white-crowned sparrows. Anim Behav. 1993;46:511–524. [Google Scholar]
- 14.Barclay SR, Harding CF. Differential modulation of monoamine levels and turnover rates by estrogen and or androgen in hypothalamic and vocal control nuclei of male zebra finches. Brain Res. 1990;523:251–262. doi: 10.1016/0006-8993(90)91494-2. [DOI] [PubMed] [Google Scholar]
- 15.Barnes P, Staal V, Muir J, Good MA. 17-Beta estradiol administration attenuates deficits in sustained and divided attention in young ovariectomized rats and aged acyclic female rats. Behav Neurosci. 2006;120:1225–1234. doi: 10.1037/0735-7044.120.6.1225. [DOI] [PubMed] [Google Scholar]
- 16.Barni T, Maggi M, Fantoni G, Granchi S, Mancina R, Gulisano M, Marra F, Macorsini E, Luconi M, Rotella C, Serio M, Balboni GC, Vannelli GB. Sex steroids and odorants modulate gonadotropin-releasing hormone secretion in primary cultures of human olfactory cells. J Clin Endocrinol Metab. 1999;84:4266–4273. doi: 10.1210/jcem.84.11.6150. [DOI] [PubMed] [Google Scholar]
- 17.Beach FA. Hormones and Behavior: A Survey of Interrelationships between Endocrine Secretions and Patterns of Overt Response. Paul B. Hoeber Inc; New York: 1948. [Google Scholar]
- 18.Beaudet A, Descarries L. The monoamine innervation of rat cerebral cortex: synaptic and nonsynaptic axon terminals. Neuroscience. 1978;3:851–860. doi: 10.1016/0306-4522(78)90115-x. [DOI] [PubMed] [Google Scholar]
- 19.Begay V, Valotaire Y, Ravault JP, Collin JP, Falcon J. Detection of estrogen receptor mRNA in trout pineal and retina: estradiol-17 beta modulates melatonin production by cultured pineal photoreceptor cells. Gen Comp Endocrinol. 1994;93:61–69. doi: 10.1006/gcen.1994.1008. [DOI] [PubMed] [Google Scholar]
- 20.Bentley GE, Wingfield JC, Morton ML, Ball GF. Stimulatory effects on the reproductive axis in female songbirds by conspecific and heterospecific male song. Horm Behav. 2000;37:179–189. doi: 10.1006/hbeh.2000.1573. [DOI] [PubMed] [Google Scholar]
- 21.Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF. Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology. 1999;140:4633–4643. doi: 10.1210/endo.140.10.7024. [DOI] [PubMed] [Google Scholar]
- 22.Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- 24.Bolhuis JJ, Hetebrij E, Den Boer-Visser AM, De Groot JH, Zijlstra GGGG. Localized immediate early gene expression related to the strength of song learning in socially reared zebra finches. Eur J Neurosci. 2001;13:2165–2170. doi: 10.1046/j.0953-816x.2001.01588.x. [DOI] [PubMed] [Google Scholar]
- 25.Bolhuis JJ, Zijlstra GG, Den Boer-Visser AM, Van der Zee EA. Localized neuronal activation in the zebra finch brain is related to the strength of song learning. Proc Natl Acad Sci USA. 2000;97:2282–2285. doi: 10.1073/pnas.030539097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boord RL. Ascending projections of the primary cochlear nuclei and nucleus laminaris in the pigeon. J Comp Neurol. 1968;133:523–541. doi: 10.1002/cne.901330410. [DOI] [PubMed] [Google Scholar]
- 27.Brantley RK, Bass AH. Alternative male spawning tactics and acoustic signals in the plainfin midshipman fish Porichthys notatus Girard. Ethology. 1994;96:213–232. [Google Scholar]
- 28.Brauth SE, McHale CM, Brasher CA, Dooling RJ. Auditory pathways in the budgerigar. I. Thalamo-telencephalic projections. Brain Behav Evol. 1987;30:174–199. doi: 10.1159/000118645. [DOI] [PubMed] [Google Scholar]
- 29.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 30.Capsius B, Leppelsack H. Response patterns and their relationship to frequency analysis in auditory forebrain centers of a songbird. Hear Res. 1999;136:91–99. doi: 10.1016/s0378-5955(99)00112-4. [DOI] [PubMed] [Google Scholar]
- 31.Caras ML, Brenowitz EA, Rubel EW. Peripheral auditory processing changes seasonally in Gambel’s white-crowned sparrow. J Comp Physiol A. 2010;196:581–599. doi: 10.1007/s00359-010-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardin JA, Schmidt MF. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. J Neurosci. 2004;24:7745–7753. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci. 1990;10:3227–3246. doi: 10.1523/JNEUROSCI.10-10-03227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carr WES, Gleeson RA, Trapido-Rosenthal HG. Chemosensory systems in lower organisms: correlations with internal receptor systems for neurotransmitters and hormones. Adv Comp Environ Physiol. 1989;5:25–52. [Google Scholar]
- 35.Caruso S, Grillo C, Agnello C, Di Mari L, Farina M, Serra A. Olfactometric and rhinomanometric outcomes in post-menopausal women treated with hormone therapy: a prospective study. Hum Reprod. 2004;19:2959–2964. doi: 10.1093/humrep/deh465. [DOI] [PubMed] [Google Scholar]
- 36.Caruso S, Grillo C, Agnello C, Maiolino L, Intelisano G, Serra A. A prospective study evidencing rhinomanometric and olfactometric outcomes in women taking oral contraceptives. Hum Reprod. 2001;16:2288–2294. doi: 10.1093/humrep/16.11.2288. [DOI] [PubMed] [Google Scholar]
- 37.Castelino CB, Ball GF. A role for norepinephrine in the regulation of context-dependent ZENK expression in male zebra finches (Taeniopygia guttata) Eur J Neurosci. 2005;21:1962–1972. doi: 10.1111/j.1460-9568.2005.04028.x. [DOI] [PubMed] [Google Scholar]
- 38.Catchpole CK, Slater PJB. Bird Song: Biological Themes and Variations. Cambridge University Press; Cambridge: 1995. [Google Scholar]
- 39.Chakraborty M, Burmeister SS. Estradiol induces sexual behavior in female Túngara frogs. Horm Behav. 2009;55:106–112. doi: 10.1016/j.yhbeh.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Cheng HY, Clayton DF. Activation and habituation of extracellular signal-regulated kinase phosphorylation in zebra finch auditory forebrain during song presentation. J Neurosci. 2004;24:7503–7513. doi: 10.1523/JNEUROSCI.1405-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clayton DF. The genomic action potential. Neurobiol Learn Memory. 2000;74:85–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- 42.Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: a role for the locus coeruleus. Science. 1996;274:1211–1215. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- 43.Cirelli C, Tononi G. Locus ceruleus control of state-dependent gene expression. J Neurosci. 2004;24:5410–5419. doi: 10.1523/JNEUROSCI.0949-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coleman JR, Campbell D, Cooper WA, Welsh MG, Moyer J. Auditory brainstem responses after ovariectomy and estrogen replacement in rat. Hear Res. 1994;80:209–215. doi: 10.1016/0378-5955(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 45.Cynx J, Nottebohm F. Role of gender, season, and familiarity in discrimination of conspecific song by zebra finches (Taeniopygia guttata) Proc Natl Acad Sci USA. 1992;89:1368–1371. doi: 10.1073/pnas.89.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cynx J, Nottebohm F. Testosterone facilitates some conspecific song discriminations in castrated zebra finches (Taeniopygia guttata) Proc Natl Acad Sci USA. 1992;89:1376–1378. doi: 10.1073/pnas.89.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cynx J, Williams H, Nottebohm F. Hemispheric differences in avian song discrimination. Proc Natl Acad Sci USA. 1992;89:1372–1375. doi: 10.1073/pnas.89.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dave AS, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- 49.Davis MJ, Ahroon WA. Fluctuations in susceptibility to noise-induced temporary threshold shift as influenced by the menstrual cycle. J Audit Res. 1982;22:173–187. [PubMed] [Google Scholar]
- 50.Dimeo AN, Wood RI. ICV testosterone induces Fos in male Syrian hamster brain. Psychoneuroendocrinology. 2006;31:237–249. doi: 10.1016/j.psyneuen.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Dong S, Clayton DF. Partial dissociation of molecular and behavioral measures of song habituation in adult zebra finches. Genes Brain Behav. 2008;7:802–809. doi: 10.1111/j.1601-183X.2008.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dreher B, Burke W, Calford MB. Cortical plasticity revealed by circumscribed retinal lesions or artificial scotomas. Prog Brain Res. 2001;134:217–246. doi: 10.1016/s0079-6123(01)34016-5. [DOI] [PubMed] [Google Scholar]
- 53.Duffy DL, Bentley GE, Ball GF. Does sex or photoperiodic condition influence ZENK induction in response to song in European starlings? Brain Res. 1999;844:78–82. doi: 10.1016/s0006-8993(99)01915-0. [DOI] [PubMed] [Google Scholar]
- 54.Eda-Fujiwara H, Satoh R, Bolhuis JJ, Kimura T. Neuronal activation in female budgerigars is localized and related to male song complexity. Eur J Neurosci. 2003;17:149–154. doi: 10.1046/j.1460-9568.2003.02414.x. [DOI] [PubMed] [Google Scholar]
- 55.Eens M, Pinxten R, Verheyen RF. Male song as a cue for mate choice in the European starling. Behaviour. 1991;116:210–238. [Google Scholar]
- 56.Elkind-Hirsch KE, Wallace E, Malinak LR, Jerger JJ. Sex hormones regulate ABR latency. Otolaryngol Head Neck Surg. 1994;110:46–52. doi: 10.1177/019459989411000105. [DOI] [PubMed] [Google Scholar]
- 57.Elkind-Hirsch KE, Wallace E, Stach BA, Jerger JF. Cyclic steroid replacement alters auditory brainstem responses in young women with premature ovarian failure. Hear Res. 1992;64:93–98. doi: 10.1016/0378-5955(92)90171-i. [DOI] [PubMed] [Google Scholar]
- 58.Evrard HC, Balthazart J. Aromatase (estrogen synthase) activity in the dorsal horn of the spinal cord: functional implications. Ann N Y Acad Sci. 2003;1007:263–271. doi: 10.1196/annals.1286.025. [DOI] [PubMed] [Google Scholar]
- 59.Feinberg DR, Jones BC, Law-Smith MJ, Moore FR, DeBruine LM, Cornwell RE, Hillier SG, Perrett DI. Menstrual cycle, trait estrogen level, and masculinity preferences in the human voice. Horm Behav. 2006;49:215–222. doi: 10.1016/j.yhbeh.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Feldman D, Do Y, Burshell A, Stathis P, Loose DS. An estrogen-binding protein and endogenous ligand in Saccharomyces cerevisiae: possible hormone receptor system. Science. 1982;218:297–298. doi: 10.1126/science.6289434. [DOI] [PubMed] [Google Scholar]
- 61.Fukui I, Ohmori H. Tonotopic gradients of membrane and synaptic properties for neurons of the chicken nucleus magnocellularis. J Neurosci. 2004;24:7514–7523. doi: 10.1523/JNEUROSCI.0566-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gahr M. Distribution of sex steroid hormone receptors in the avian brain: functional implications for neural sex differences and sexual behaviors. Microsc Res Technol. 2001;55:1–11. doi: 10.1002/jemt.1151. [DOI] [PubMed] [Google Scholar]
- 63.Gahr M, Guttinger HR, Kroodsma DE. Estrogen receptors in the avian brain: survey reveals general distribution and forebrain areas unique to songbirds. J Comp Neurol. 1993;327:112–122. doi: 10.1002/cne.903270109. [DOI] [PubMed] [Google Scholar]
- 64.Gale SDSD, Perkel DJ. A basal ganglia pathway drives selective auditory responses in songbird dopaminergic neurons via disinhibition. J Neurosci. 2010;30:1027–1037. doi: 10.1523/JNEUROSCI.3585-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gentner TQ. Neural systems for individual song recognition in adult birds. Ann NY Acad Sci USA. 2004:1016. doi: 10.1196/annals.1298.008. [DOI] [PubMed] [Google Scholar]
- 66.Gentner TQ, Hulse SH, Duffy D, Ball GF. Response biases in auditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. J Neurobiol. 2001;46:48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 67.Gerhardt HC. The significance of some spectral features in mating call recognition in the green tree frog. J Exp Biol. 1974;61:229–241. doi: 10.1242/jeb.61.1.229. [DOI] [PubMed] [Google Scholar]
- 68.Gocayne J, Robinson DA, FitzGerald MG, Chung FZ, Kerlavage AR, Lentes KU, Lai J, Wang CD, Fraser CM, Venter JC. Primary structure of rat cardiac beta-adrenergic and muscarinic cholinergic receptors obtained by automated DNA sequence analysis: further evidence for a multigene family. Proc Natl Acad Sci USA. 1987;23:8296–8300. doi: 10.1073/pnas.84.23.8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Golub MS, Germann SL, Hogrefe CE. Endocrine disruption and cognitive function in adolescent female rhesus monkeys. Neurotoxicol Teratol. 2004;26:799–809. doi: 10.1016/j.ntt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 70.Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc Natl Acad Sci USA. 2009;106:8737–8742. doi: 10.1073/pnas.0811821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci USA. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gungor N, Boke B, Belgin E, Tuncbilek E. High frequency hearing loss in Ullrich–Turner syndrome. Eur J Pediatr. 2000;159:740–744. doi: 10.1007/pl00008338. [DOI] [PubMed] [Google Scholar]
- 74.Havlicek J, Roberts SC, Flegr J. Women’s preference for dominant male odour: effects of menstrual cycle and relationship status. Biol Lett. 2005;22:256–259. doi: 10.1098/rsbl.2005.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayden BY, Nair AC, McCoy AN, Platt ML. Posterior cingulate cortex mediates outcome-contingent allocation of behavior. Neuron. 2008;60:19–25. doi: 10.1016/j.neuron.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hederstierna C, Hultcrantz M, Collins A, Rosenhall U. The menopause triggers hearing decline in healthy women. Hear Res. 2009;259:31–35. doi: 10.1016/j.heares.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 77.Hederstierna C, Hultcrantz M, Rosenhall U. Estrogen and hearing from a clinical point of view; characteristics of auditory function in women with Turner syndrome. Hear Res. 2009;252:3–8. doi: 10.1016/j.heares.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 78.Hederstierna C, Hultcrantz M, Rosenhall U. A longitudinal study of hearing decline in women with Turner syndrome. Acta Otolaryngol. 2009;129:1434–1441. doi: 10.3109/00016480902741962. [DOI] [PubMed] [Google Scholar]
- 79.Helfer KS. Cross-sectional study of differences in speech understanding between users and nonusers of estrogen replacement therapy. Exp Aging Res. 2004;30:195–204. doi: 10.1080/03610730490274257. [DOI] [PubMed] [Google Scholar]
- 80.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 81.Hernandez AM, MacDougall-Shackleton SA. Effects of early song experience on song preferences and song control and auditory brain regions in female house finches (Carpodacus mexicanus) J Neurobiol. 2004;59:247–258. doi: 10.1002/neu.10312. [DOI] [PubMed] [Google Scholar]
- 82.Hultcrantz M, Simonoska R, Stenberg AE. Estrogen and hearing: a summary of recent investigations. Acta Otolaryngol. 2006;126:10–14. doi: 10.1080/00016480510038617. [DOI] [PubMed] [Google Scholar]
- 83.Hultcrantz M, Sylven L, Borg E. Ear and hearing problems in 44 middle-aged women with Turner’s syndrome. Hear Res. 1994;76:127–132. doi: 10.1016/0378-5955(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 84.Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 85.Hutchison JB, Wingfield JC, Hutchison RE. Sex differences in plasma concentrations of steroids during the sensitive period for brain differentiation in the zebra finch. J Endocrinol. 1984;103:363–369. doi: 10.1677/joe.0.1030363. [DOI] [PubMed] [Google Scholar]
- 86.Insel TR. Regional induction of c-Fos-like protein in rat brain after estradiol administration. Endocrinology. 1990;126:1849–1853. doi: 10.1210/endo-126-4-1849. [DOI] [PubMed] [Google Scholar]
- 87.Jarvis ED, Mello CV, Nottebohm F. Associative learning and stimulus novelty influence the song-induced expression of an immediate early gene in the canary forebrain. Learn Memory. 1995;2:62–80. doi: 10.1101/lm.2.2.62. [DOI] [PubMed] [Google Scholar]
- 88.Jeong JK, Guo K, Tremere LA, Pinaud R. Neurochemical organization and experience-dependent activation of estrogen-associated circuits in the songbird auditory forebrain. doi: 10.1111/j.1460-9568.2011.07743.x. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jerger J, Hall J. Effects of age and sex on auditory brainstem response. Arch Otolaryngol. 1980;106:387–391. doi: 10.1001/archotol.1980.00790310011003. [DOI] [PubMed] [Google Scholar]
- 90.Jones BC, DeBruine LM, Perrett DI, Little AC, Feinberg DR, Smith MJL. Effects of menstrual cycle phase on face preference. Arch Sex Behav. 2008;37:8–84. doi: 10.1007/s10508-007-9268-y. [DOI] [PubMed] [Google Scholar]
- 91.Kaas JH. Sensory loss and cortical reorganization in mature primates. Prog Brain Res. 2002;138:167–176. doi: 10.1016/S0079-6123(02)38077-4. [DOI] [PubMed] [Google Scholar]
- 92.Kaczmarek L, Robertson HA. Immediate Early Genes and Inducible Transcription Factors in Mapping of the Central Nervous System Function and Dysfunction. Elsevier Science B.V; Amsterdam: 2002. [Google Scholar]
- 93.Karten HJ. The organization of the ascending auditory pathway in the pigeon (Columba livia). I. Diencephalic projections of the inferior colliculus (nucleus mesencephali lateralis, pars dorsalis) Brain Res. 1967;6:409–427. doi: 10.1016/0006-8993(67)90055-8. [DOI] [PubMed] [Google Scholar]
- 94.Kelley DB, Nottebohm F. Projections of a telencephalic auditory nucleus-field L-in the canary. J Comp Neurol. 1979;183:455–469. doi: 10.1002/cne.901830302. [DOI] [PubMed] [Google Scholar]
- 95.Kelliher KR, Chang YM, Wersinger SR, Baum MJ. Sex difference and testosterone modulation of pheromone-induced neuronal Fos in the ferret’s main olfactory bulb and hypothalamus. Biol Reprod. 1998;59:1454–1463. doi: 10.1095/biolreprod59.6.1454. [DOI] [PubMed] [Google Scholar]
- 96.Kern MD, King JR. Testosterone-induced singing in female white-crowned sparrows. Condor. 1972;74:204–209. [Google Scholar]
- 97.Knudsen EI, Zheng W, DeBello WM. Traces of learning in the auditory localization pathway. Proc Natl Acad Sci USA. 2000;97:11815–11820. doi: 10.1073/pnas.97.22.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kobayashi K, Kobayashi H, Ueda M, Honda Y. Estrogen receptor expression in bovine and rat retinas. Invest Ophthalmol Vis Sci. 1998;39:2105–2110. [PubMed] [Google Scholar]
- 99.Kohidai L, Katona J, Csaba G. Effects of steroid hormones on five functional parameters of Tetrahymena: evolutionary conclusions. Cell Biochem Funct. 2003;21:19–26. doi: 10.1002/cbf.996. [DOI] [PubMed] [Google Scholar]
- 100.Kruse AA, Stripling R, Clayton DF. Context-specific habituation of the zenk gene response to song in adult zebra finches. Neurobiol Learn Memory. 2004;82:99–108. doi: 10.1016/j.nlm.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 101.Krutzfeldt NO, Logerot P, Kubke MF, Wild JM. Connections of the auditory brainstem in a songbirdTaeniopygia guttata. I. Projections of nucleus angularis and nucleus laminaris to the superior olive and lateral lemniscal nuclei. J Comp Neurol. 2010;518:2109–2134. doi: 10.1002/cne.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krutzfeldt NO, Logerot P, Kubke MF, Wild JM. Connections of the auditory brainstem in a songbirdTaeniopygia guttata. II. Projections of nucleus angularis and nucleus laminaris to the superior olive and lateral lemniscal nuclei. J Comp Neurol. 2010;518:2135–2148. doi: 10.1002/cne.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lacreuse A, Martin-Malivel J, Lange HS, Herndon JG. Effects of the menstrual cycle on looking preferences for faces in female rhesus monkeys. Anim Cogn. 2007;10:105–115. doi: 10.1007/s10071-006-0041-8. [DOI] [PubMed] [Google Scholar]
- 104.LeBlanc MM, Goode CT, MacDougall-Shackleton EA, Maney DL. Estradiol modulates brainstem catecholaminergic cell groups and projections to the auditory forebrain in a female songbird. Brain Res. 2007;1171:93–103. doi: 10.1016/j.brainres.2007.06.086. [DOI] [PubMed] [Google Scholar]
- 105.Leboucher G, Beguin N, Mauget R, Kreutzer M. Effects of fadrozole on sexual displays and reproductive activity in the female canary. Physiol Behav. 1998;65:233–240. doi: 10.1016/s0031-9384(98)00079-1. [DOI] [PubMed] [Google Scholar]
- 106.Leitner S, Voigt C, Metzdorf R, Catchpole CK. Immediate early gene (ZENK, Arc) expression in the auditory forebrain of female canaries varies in response to male song quality. J Neurobiol. 2005;64:275–284. doi: 10.1002/neu.20135. [DOI] [PubMed] [Google Scholar]
- 107.Little AC, Jones BC, Burris RP. Preferences for masculinity in male bodies change across the menstrual cycle. Horm Behav. 2007;51:633–639. doi: 10.1016/j.yhbeh.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 108.London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci. 2008 doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.London SE, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinology. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.London SE, Remage-Healey L, Schlinger BA. Neurosteroid production in the songbird brain: a re-evaluation of core principles. Front Neuroendocrinol. 2009;30:302–314. doi: 10.1016/j.yfrne.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lucas JR, Freeberg TM, Krishnan A, Long GR. A comparative study of avian auditory brainstem responses: correlations with phylogeny and vocal complexity, and seasonal effects. J Comp Physiol A. 2002;188:981–992. doi: 10.1007/s00359-002-0359-x. [DOI] [PubMed] [Google Scholar]
- 112.Lucas JR, Freeberg TM, Long GR, Krishnan A. Seasonal variation in avian auditory evoked responses to tones: a comparative analysis of Carolina chickadees, tufted titmice, and white-breasted nuthatches. J Comp Physiol A. 2007;193:201–215. doi: 10.1007/s00359-006-0180-z. [DOI] [PubMed] [Google Scholar]
- 113.Lynch KS, Ball GF. Noradrenergic deficits alter processing of communication signals in female songbirds. Brain Behav Evol. 2008;72:207–214. doi: 10.1159/000157357. [DOI] [PubMed] [Google Scholar]
- 114.Lynch KS, Crews D, Ryan MJ, Wilczynski W. Hormonal state influences aspects of female mate choice in the Túngara frog (Physalaemus pustulosus) Horm Behav. 2006;49:450–457. doi: 10.1016/j.yhbeh.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J Neurobiol. 2003;56:163–170. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- 116.Maney DL, Bernard DJ, Ball GF. Gonadal steroid receptor mRNA in catecholaminergic nuclei of the canary brainstem. Neurosci Lett. 2001;311:189–192. doi: 10.1016/s0304-3940(01)02157-7. [DOI] [PubMed] [Google Scholar]
- 117.Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23:1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- 118.Maney DL, Goode CT, Lange HS, Sanford SE, Solomon BL. Estradiol modulates neural responses to song in a seasonal songbird. J Comp Neurol. 2008;511:173–186. doi: 10.1002/cne.21830. [DOI] [PubMed] [Google Scholar]
- 119.Maney DL, Lange HS, Raees MQ, Reid AE, Sanford SE. Behavioral phenotypes persist after gonadal steroid manipulation in white-throated sparrows. Horm Behav. 2009;55:113–120. doi: 10.1016/j.yhbeh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 120.Maney DL, MacDougall-Shackleton EA, MacDougall-Shackleton SA, Ball GF, Hahn TP. Immediate early gene response to hearing song correlates with receptive behavior and depends on dialect in a female songbird. J Comp Physiol A. 2003;189:667–674. doi: 10.1007/s00359-003-0441-z. [DOI] [PubMed] [Google Scholar]
- 121.Marler P, Tamura M. Culturally transmitted patterns of vocal behavior in sparrows. Science. 1964;146:1483–1486. doi: 10.1126/science.146.3650.1483. [DOI] [PubMed] [Google Scholar]
- 122.Maruska KP, Fernald RD. Steroid receptor expression in the fish inner ear varies with sex, social status, and reproductive state. BMC Neurosci. 2010;11:58. doi: 10.1186/1471-2202-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matragrano LL, Sanford SE, Salvante KG, Sockman KW, Maney DL. Estradiol-dependent catecholaminergic innervation of auditory areas in a seasonally breeding songbird. doi: 10.1111/j.1460-9568.2011.07751.x. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McKenzie TL, Hernandez AM, MacDougall-Shackleton SA. Experience with songs in adulthood reduces song-induced gene expression in songbird auditory forebrain. Neurobiol Learn Memory. 2006;86:330–335. doi: 10.1016/j.nlm.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 125.Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mello CV, Nottebohm F, Clayton D. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene’s response to that song in zebra finch telencephalon. J Neurosci. 1995;15:6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mello CV, Pinaud R. Immediate early gene regulation in the auditory system. In: Pinaud R, Tremere LA, editors. Immediate Early Genes in Sensory Processing Cognitive Performance and Neurological Disorders. Springer Verlag; New York: 2006. pp. 35–56. [Google Scholar]
- 128.Mello CV, Pinaud R, Ribeiro S. Noradrenergic system of the zebra finch brain: immunocytochemical study of dopamine-β-hydroxylase. J Comp Neurol. 1998;400:207–228. [PubMed] [Google Scholar]
- 129.Mello CV, Ribeiro S. ZENK protein regulation by song in the brain of songbirds. J Comp Neurol. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 130.Mello CV, Vates GE, Okuhata S, Nottebohm F. Descending auditory pathways in the adult male zebra finch (Taeniopygia guttata) J Comp Neurol. 1998;395:137–160. [PubMed] [Google Scholar]
- 131.Mello CV, Velho TA, Pinaud R. Song-induced gene expression: a window on song auditory processing and perception. Ann NY Acad Sci. 2004;1016:263–281. doi: 10.1196/annals.1298.021. [DOI] [PubMed] [Google Scholar]
- 132.Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Monsivais P, Rubel EW. Accommodation enhances depolarizing inhibition in central neurons. J Neurosci. 2001;21:7823–7830. doi: 10.1523/JNEUROSCI.21-19-07823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moffatt CA. Steroid hormone modulation of olfactory processing in the context of socio-sexual behaviors in rodents and humans. Brain Res Rev. 2003;43:192–206. doi: 10.1016/s0165-0173(03)00208-x. [DOI] [PubMed] [Google Scholar]
- 135.Moore MC. Effect of female sexual displays on the endocrine physiology and behavior of male white-crowned sparrowsZonotrichia leucophrys. J Zool. 1983;199:137–148. [Google Scholar]
- 136.Muller CM, Leppelsack HJ. Feature extraction and tonotopic organization in the avian auditory forebrain. Exp Brain Res. 1985;59:587–599. doi: 10.1007/BF00261351. [DOI] [PubMed] [Google Scholar]
- 137.Nastiuk KL, Mello CV, George JM, Clayton DF. Immediate-early gene responses in the avian song control system: cloning and expression analysis of the canary c-jun cDNA. Brain Res Mol Brain Res. 1994;27:299–309. doi: 10.1016/0169-328x(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 138.Newman SW. The medial extended amygdala in male reproductive behavior: a node in the mammalian social behavior network. Ann NY Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 139.Noirot IC, Adler HJ, Cornil CA, Harada N, Dooling RJ, Balthazart J, Ball GF. Presence of aromatase and estrogen receptor alpha in the inner ear of zebra finches. Hear Res. 2009;252:49–55. doi: 10.1016/j.heares.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 140.Nordeen EJ, Holtzman DA, Nordeen KW. Increased Fos expression among midbrain dopaminergic cell groups during birdsong tutoring. Eur J Neurosci. 2009;30:662–670. doi: 10.1111/j.1460-9568.2009.06849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- 142.Overholt EM, Rubel EW, Hyson RL. A circuit for coding interaural time differences in the chick brainstem. J Neurosci. 1992;12:1698–1708. doi: 10.1523/JNEUROSCI.12-05-01698.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Penton-Voak IS, Jones BC, Little AC, Baker S, Tiddeman B, Burt DM, Perrett DI. Symmetry, sexual dimorphism in facial proportions and male facial attractiveness. Proc Biol Sci. 2001;268:1617–1623. doi: 10.1098/rspb.2001.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Penton-Voak IS, Perrett DI, Castles DL, Kobayashi T, Burt DM, Murray LK, Minamisawa R. Menstrual cycle alters face preference. Nature. 1999;399:741–742. doi: 10.1038/21557. [DOI] [PubMed] [Google Scholar]
- 145.Perfito N. The reproductive and stress physiology of zebra finches in context: integrating field and laboratory studies. Emu. 2010:199–208. [Google Scholar]
- 146.Perfito N, Bentley G, Hau M. Tonic activation of brain GnRH immunoreactivity despite reduction of peripheral reproductive parameters in opportunistically breeding zebra finches. Brain Behav Evol. 2006;67:123–134. doi: 10.1159/000090977. [DOI] [PubMed] [Google Scholar]
- 147.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Phillmore LS, Bloomfield LL, Weisman RG. Effects of songs and calls on ZENK expression in the auditory telencephalon of field- and isolate-reared black capped chickadees. Behav Brain Res. 2003;147:125–134. doi: 10.1016/s0166-4328(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 149.Pietras RJ, Moulton DG. Hormonal influences on odor detection in rats: changes associated with the estrous cycle, pseudopregnancy, ovariectomy, and administration of testosterone propionate. Physiol Behav. 1974;12:475–491. doi: 10.1016/0031-9384(74)90125-5. [DOI] [PubMed] [Google Scholar]
- 150.Pinaud R. Experience-dependent immediate early gene expression in the adult central nervous system: evidence from enriched-environment studies. Int J Neurosci. 2004;114:321–333. doi: 10.1080/00207450490264142. [DOI] [PubMed] [Google Scholar]
- 151.Pinaud R. Critical calcium-regulated biochemical and gene expression programs involved in experience-dependent plasticity. In: Pinaud R, Tremere LA, DeWeerd P, editors. Plasticity in the Visual System: From Genes to Circuits. Springer Verlag; New York: 2005. pp. 153–180. [Google Scholar]
- 152.Pinaud R. Genome of a songbird unveiled. J Biol. 2010;9:19. doi: 10.1186/jbiol222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pinaud R, Fortes AF, Lovell P, Mello CV. Calbindin-positive neurons reveal a sexual dimorphism within the songbird analogue of the mammalian auditory cortex. J Neurobiol. 2006;66:182–195. doi: 10.1002/neu.20211. [DOI] [PubMed] [Google Scholar]
- 154.Pinaud R, Osorio C, Alzate O, Jarvis ED. Profiling of experience-regulated proteins in the songbird auditory forebrain using quantitative proteomics. Eur J Neurosci. 2008;27:1409–1422. doi: 10.1111/j.1460-9568.2008.06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pinaud R, Terleph TA. A songbird forebrain area potentially involved in auditory discrimination and memory formation. J Biosci. 2008;33:145–155. doi: 10.1007/s12038-008-0030-y. [DOI] [PubMed] [Google Scholar]
- 156.Pinaud R, Tremere LA. Cognitive Performance and Neurological Disorders. Springer Verlag; New York: 2006. Immediate Early Genes in Sensory Processing. [Google Scholar]
- 157.Pollak GD, Burger RM, Klug A. Dissecting the circuitry of the auditory system. Trends Neurosci. 2003;26:33–39. doi: 10.1016/s0166-2236(02)00009-7. [DOI] [PubMed] [Google Scholar]
- 158.Pradhan DS, Newman AE, Wacker DW, Wingfield JC, Schlinger BA, Soma KK. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm Behav. 2010;57:381–389. doi: 10.1016/j.yhbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Puts DA. Mating context and menstrual phase affect women’s preference for male voice pitch. Evol Human Behav. 2005;26:388–397. [Google Scholar]
- 160.Rauschecker JP. Auditory cortical plasticity: a comparison with other sensory systems. Trends Neurosci. 1999;22:74–80. doi: 10.1016/s0166-2236(98)01303-4. [DOI] [PubMed] [Google Scholar]
- 161.Reader TA, Ferron A, Descarries L, Jasper HH. Modulatory role for biogenic amines in the cerebral cortex: microiontophoretic studies. Brain Res. 1979;160:217–229. doi: 10.1016/0006-8993(79)90420-7. [DOI] [PubMed] [Google Scholar]
- 162.Reeves BJ, Beecher MD, Brenowitz EA. Seasonal changes in avian song control circuits do not cause seasonal changes in song discrimination in song sparrows. J Neurobiol. 2003;57:119–129. doi: 10.1002/neu.10257. [DOI] [PubMed] [Google Scholar]
- 163.Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci USA. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Remage-Healey L, Oyama RK, Schlinger BA. Elevated aromatase activity in forebrain synaptic terminals during song. J Neuroendocrinol. 2009;21:191–199. doi: 10.1111/j.1365-2826.2009.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ribeiro S, Cecchi GA, Magnasco MO, Mello CV. Toward a song code: evidence for a syllabic representation in the canary brain. Neuron. 1998;21:359–371. doi: 10.1016/s0896-6273(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 167.Ribeiro S, Mello CV. Gene expression and synaptic plasticity in the auditory forebrain of songbirds. Learn Memory. 2000;7:235–243. doi: 10.1101/lm.34400. [DOI] [PubMed] [Google Scholar]
- 168.Ricci LA, Grimes JM, Melloni RH. Lasting changes in neuronal activation patterns in select forebrain regions of aggressive, adolescent anabolic/androgenic steroid treated hamsters. Behav Brain Res. 2007;176:344–352. doi: 10.1016/j.bbr.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Riters LV, Pawlisch BA. Evidence that norepinephrine influences responses to male courtship song and activity within song control regions and the ventromedial nucleus of the hypothalamus in female European starlings. Brain Res. 2007;1149:127–140. doi: 10.1016/j.brainres.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 170.Roney JR, Simmons ZL. Women’s estradiol predicts preference for facial cues of men’s testosterone. Horm Behav. 2008;53:14–19. doi: 10.1016/j.yhbeh.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 171.Rowland SS, Falkler WA, Jr, Bashirelahi N. Identification of an estrogen-binding protein in Pseudomonas aeruginosa. J Steroid Biochem. 1992;42:721–727. doi: 10.1016/0960-0760(92)90113-w. [DOI] [PubMed] [Google Scholar]
- 172.Saldanha CJ, Popper P, Micevych PE, Schlinger BA. The passerine hippocampus is a site of high aromatase: inter- and intraspecies comparisons. Horm Behav. 1998;34:85–97. doi: 10.1006/hbeh.1998.1447. [DOI] [PubMed] [Google Scholar]
- 173.Saldanha CJ, Coomaralingam L. Overlap and co-expression of estrogen synthetic and responsive neurons in the songbird brain – a double-label immunocytochemical study. Gen Comp Endocrinol. 2005;141:66–75. doi: 10.1016/j.ygcen.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 174.Sanford SE, Lange HS, Maney DL. Topography of estradiol-modulated genomic responses in the songbird auditory forebrain. Dev Neurobiol. 2010;70:73–86. doi: 10.1002/dneu.20757. [DOI] [PubMed] [Google Scholar]
- 175.Schlinger BA, Arnold AP. Brain is the major site of estrogen synthesis in a male songbird. Proc Natl Acad Sci USA. 1991;88:4191–4194. doi: 10.1073/pnas.88.10.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schmidt KL, Pradhan DS, Ahah AH, Charlier TD, Chin EH, Soma KK. Neurosteroids, immunosteroids, and the balkanization of endocrinology. Gen Comp Endocrinol. 2008;157:266–274. doi: 10.1016/j.ygcen.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 177.Searcy WA. Measuring responses of female birds to male song. In: McGregor PK, editor. Playback and Studies of Animal Communication. Plenum; New York: 1992. pp. 175–189. [Google Scholar]
- 178.Searcy WA, Capp MS. Estradiol dosage and the solicitation display assay in red-winged blackbirds. Condor. 1997;99:826–828. [Google Scholar]
- 179.Shen P, Campagnoni CW, Kampf K, Schlinger BA, Arnold AP, Campagnoni AT. Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Mol Brain Res. 1994;24:227–237. doi: 10.1016/0169-328x(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 180.Simonoska R, Stenberg AE, Duan M, Yakimchuk K, Fridberger A, Sahlin L, Gustafsson JA, Hultcrantz M. Inner ear pathology and loss of hearing in estrogen receptor-beta deficient mice. J Endocrinol. 2009;201:397–406. doi: 10.1677/JOE-09-0060. [DOI] [PubMed] [Google Scholar]
- 181.Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- 182.Sisneros JA, Forlano PM, Deitcher DL, Bass AH. Steroid-dependent auditory plasticity leads to adaptive coupling of sender and receiver. Science. 2004;305:404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- 183.Sivaramakrishnan S, Sterbing-D’Angelo SJ, Filipovic B, D’Angelo WR, Oliver DL, Kuwada S. GABA(A) synapses shape neuronal responses to sound intensity in the inferior colliculus. J Neurosci. 2004;24:5031–5043. doi: 10.1523/JNEUROSCI.0357-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Smith GT, Brenowitz EA, Wingfield JC. Roles of photoperiod and testosterone in seasonal plasticity of the avian song control system. J Neurobiol. 1997;32:426–442. doi: 10.1002/(sici)1097-4695(199704)32:4<426::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 185.Sockman KW, Ball GF. Independent effects of song quality and experience with photostimulation on expression of the immediate early gene ZENK (EGR-1) in the auditory telencephalon of female European starlings. Dev Neurobiol. 2009;69:339–349. doi: 10.1002/dneu.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sockman KW, Gentner TQ, Ball GF. Recent experience modulates forebrain gene-expression in response to mate-choice cues in European starlings. Proc Roy Soc Lond B. 2002;269:2479–2485. doi: 10.1098/rspb.2002.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sockman KW, Gentner TQ, Ball GF. Complementary neural systems for the experience-dependent integration of mate-choice cues in European starlings. J Neurobiol. 2005;62:72–81. doi: 10.1002/neu.20068. [DOI] [PubMed] [Google Scholar]
- 188.Sockman KW, Salvante KG. The integration of song environment by catecholaminergic systems innervating the auditory telencephalon of adult female European starlings. Dev Neurobiol. 2008;68:656–668. doi: 10.1002/dneu.20611. [DOI] [PubMed] [Google Scholar]
- 189.Soderstrom K, Tian Q, Valenti M, Di Marzo V. Endocannabinoids link feeding state and auditory perception-related gene expression. J Neurosci. 2004;24:10013–10021. doi: 10.1523/JNEUROSCI.3298-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sohrabji F, Miranda RC, Toran-Allerand CD. Estrogen differentially regulates estrogen and nerve growth factor receptor mRNAs in adult sensory neurons. J Neurosci. 1994;14:459–471. doi: 10.1523/JNEUROSCI.14-02-00459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Stenberg AE, Wang H, Sahlin L, Hultcrantz M. Mapping of estrogen receptors alpha and beta in the inner ear of mouse and rat. Hear Res. 1999;136:29–34. doi: 10.1016/s0378-5955(99)00098-2. [DOI] [PubMed] [Google Scholar]
- 192.Stripling R, Kruse AA, Clayton DF. Development of song responses in the zebra finch caudomedial neostriatum: role of genomic and electrophysiological activities. J Neurobiol. 2001;48:163–180. doi: 10.1002/neu.1049. [DOI] [PubMed] [Google Scholar]
- 193.Stripling R, Volman SF, Clayton DF. Response modulation in the zebra finch neostriatum: relationship to nuclear gene regulation. J Neurosci. 1997;17:3883–3893. doi: 10.1523/JNEUROSCI.17-10-03883.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 195.Sullivan WE, Konishi M. Segregation of stimulus phase and intensity coding in the cochlear nucleus of the barn owl. J Neurosci. 1984;4:1787–1799. doi: 10.1523/JNEUROSCI.04-07-01787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Svec LA, Wade J. Estradiol induces region-specific inhibition of ZENK but does not affect the behavioral preference for tutored song in adult female zebra finches. Behav Brain Res. 2009;199:298–306. doi: 10.1016/j.bbr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Takahashi T, Moiseff A, Konishi M. Time and intensity cues are processed independently in the auditory system of the owl. J Neurosci. 1984;4:1781–1786. doi: 10.1523/JNEUROSCI.04-07-01781.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Terleph TA, Mello CV, Vicario DS. Auditory topography and temporal response dynamics of canary caudal telencephalon. J Neurobiol. 2006;66:281–292. doi: 10.1002/neu.20219. [DOI] [PubMed] [Google Scholar]
- 199.Terleph TA, Mello CV, Vicario DS. Species differences in auditory processing dynamics in songbird auditory telencephalon. Dev Neurobiol. 2007;67:1498–1510. doi: 10.1002/dneu.20524. [DOI] [PubMed] [Google Scholar]
- 200.Terpstra NJ, Bolhuis JJ, Riebel K, van der Burg JM, den Boer-Visser AM. Localized brain activation specific to auditory memory in a female songbird. J Comp Neurol. 2006;494:784–791. doi: 10.1002/cne.20831. [DOI] [PubMed] [Google Scholar]
- 201.Terpstra NJ, Bolhuis JJ, Den Boer-Visser AM. An analysis of the neural representation of birdsong memory. J Neurosci. 2004;24:4971–4977. doi: 10.1523/JNEUROSCI.0570-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 203.Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301:1714–1717. doi: 10.1126/science.1086185. [DOI] [PubMed] [Google Scholar]
- 204.Tomaszycki ML, Sluzas EM, Sundberg KA, Newman SW, DeVoogd TJ. Immediate early gene (ZENK) responses to song in juvenile female and male zebra finches: effects of rearing environment. J Neurobiol. 2006;66:1175–1182. doi: 10.1002/neu.20275. [DOI] [PubMed] [Google Scholar]
- 205.Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 208.Velho TAF, Mello CV. Synapsins are late activity-induced genes regulated by birdsong. J Neurosci. 2008;28:11871–11882. doi: 10.1523/JNEUROSCI.2307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Velho TA, Pinaud R, Rodrigues PV, Mello CV. Co-induction of activity-dependent genes in songbirds. Eur J Neurosci. 2005;22:1667–1678. doi: 10.1111/j.1460-9568.2005.04369.x. [DOI] [PubMed] [Google Scholar]
- 210.Vignal C, Bouchut C, Mathevon N. Sound-induced brain activity depends on stimulus subjective salience in female zebra finches. CR Biol. 2008;331:347–356. doi: 10.1016/j.crvi.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 211.Vyas AA, Harding C, Borg L, Bogdan D. Acoustic characteristics, early experience, and endocrine status interact to modulate female zebra finches’ behavioral responses to songs. Horm Behav. 2009;55:50–59. doi: 10.1016/j.yhbeh.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 212.Vyas A, Harding C, McGowan J, Snare R, Bogdan D. Noradrenergic neurotoxin, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4), treatment eliminates estrogenic effects on song responsiveness in female zebra finches (Taeniopygia guttata) Behav Neurosci. 2008;122:1148–1157. doi: 10.1037/0735-7044.122.5.1148. [DOI] [PubMed] [Google Scholar]
- 213.Wade J, Schlinger BA, Hodges L, Arnold AP. Fadrozole: a potent and specific inhibitor of aromatase in the zebra finch brain. Gen Comp Endocrinol. 1994;94:53–61. doi: 10.1006/gcen.1994.1059. [DOI] [PubMed] [Google Scholar]
- 214.Walpurger V, Pietrowsky R, Kirschbaum C, Wolf OT. Effects of the menstrual cycle on auditory event-related potentials. Horm Behav. 2004;46:600–606. doi: 10.1016/j.yhbeh.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 215.Warren WC, Clayton DF, Ellegren H, Arnold AP, Hillier LW, Künstner A, et al. The genome of a songbird. Nature. 2010;464:757–762. doi: 10.1038/nature08819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annu Rev Neurosci. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Whitney O, Soderstrom K, Johnson F. CB1 cannabinoid receptor activation inhibits a neural correlate of song recognition in an auditory/perceptual region of the zebra finch telencephalon. J Neurobiol. 2003;56:266–274. doi: 10.1002/neu.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wickham LA, Gao J, Toda I, Rocha EM, Ono M, Sullivan DA. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol Scand. 2000;78:146–153. doi: 10.1034/j.1600-0420.2000.078002146.x. [DOI] [PubMed] [Google Scholar]
- 219.Wingfield JC, Farner DS. The determination of five steroids in avian plasma by radioimmunoassay and competitive protein-binding. Steroids. 1975;26:311–321. doi: 10.1016/0039-128x(75)90077-x. [DOI] [PubMed] [Google Scholar]
- 220.Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biol. 2008;6:e62. doi: 10.1371/journal.pbio.0060062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Woolley SM, Casseday JH. Response properties of single neurons in the zebra finch auditory midbrain: response patterns, frequency coding, intensity coding, and spike latencies. J Neurophysiol. 2004;91:136–151. doi: 10.1152/jn.00633.2003. [DOI] [PubMed] [Google Scholar]
- 222.Woolley SM, Fremouw TE, Hsu A, Theunissen FE. Tuning for spectro-temporal modulations as a mechanism for auditory discrimination of natural sounds. Nat Neurosci. 2005;8:1371–1379. doi: 10.1038/nn1536. [DOI] [PubMed] [Google Scholar]
- 223.Woolley SM, Gill PR, Theunissen FE. Stimulus-dependent auditory tuning results in synchronous population coding of vocalizations in the songbird midbrain. J Neurosci. 2006;26:2499–2512. doi: 10.1523/JNEUROSCI.3731-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Woolley SM, Gill PR, Fremouw T, Theunissen FE. Functional groups in the avian auditory system. J Neurosci. 2009;29:2780–2793. doi: 10.1523/JNEUROSCI.2042-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Young SR, Rubel EW. Frequency-specific projections of individual neurons in chick brainstem auditory nuclei. J Neurosci. 1983;3:1373–1378. doi: 10.1523/JNEUROSCI.03-07-01373.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zheng W, Knudsen EI. Functional selection of adaptive auditory space map by GABAA-mediated inhibition. Science. 1999;284:962–965. doi: 10.1126/science.284.5416.962. [DOI] [PubMed] [Google Scholar]