Abstract
Sleep deprivation can acutely reverse depressive symptoms in some patients with major depression. Because abnormalities in slow wave sleep are one of the most consistent biological markers of depression, it is plausible that the antidepressant effects of sleep deprivation are due to the effects on slow wave homeostasis. This study tested the prediction that selectively reducing slow waves during sleep (slow wave deprivation; SWD), without disrupting total sleep time, will lead to an acute reduction in depressive symptomatology. As part of a multi-night, cross-over design study, participants with major depression (non-medicated; n = 17) underwent baseline, SWD, and recovery sleep sessions, and were recorded with high-density EEG (hdEEG). During SWD, acoustic stimuli were played to suppress subsequent slow waves, without waking up the participant. The effects of SWD on depressive symptoms were assessed with both self-rated and researcher-administered scales. Participants experienced a significant decrease in depressive symptoms according to both self-rated (p = .007) and researcher-administered (p = .010) scales, while vigilance was unaffected. The reduction in depressive symptoms correlated with the overnight dissipation of fronto-central slow wave activity (SWA) on baseline sleep, the rebound in right frontal all-night SWA on recovery sleep, and the amount of REM sleep on the SWD night. In addition to highlighting the benefits of hdEEG in detecting regional changes in brain activity, these findings suggest that SWD may help to better understand the pathophysiology of depression and may be a useful tool for the neuromodulatory reversal of depressive symptomatology.
Keywords: Depression, Slow Wave Deprivation, Sleep Deprivation, Slow Wave Sleep, Sleep Homeostasis, high-density EEG
1. Introduction
Sleep disturbances are an integral part of the diagnostic criteria for major depression (American Psychiatric Association, 2004; Peterson & Benca, 2006), and sleep may provide biomarkers for treatment response to antidepressant medication (Steiger & Kimura, 2010). Since the initial investigation of a therapeutic benefit of sleep deprivation (SD) in depression (Pflug & Tölle, 1971), many studies have attempted to understand the link between sleep and depression. SD interventions, including total sleep deprivation (TSD), partial sleep deprivation and selective REM sleep deprivation (REMD) can acutely reverse depressive symptoms in approximately 50–60% of patients with major depression (Gillin, 1983; Wu & Bunney, 1990; Kuhs & Tölle, 1991; Wirz-Justice & Van den Hoofdakker, 1999; Hemmeter et al., 2010). Decreased REM sleep latency and increased REM density are hallmark features of depression (Kupfer & Foster, 1972; Benca et al., 1992). Given these features, and the observation of suppressed REM sleep with antidepressant medication (Riemann et al., 1990; Jobert et al., 1999), it has been postulated that selectively suppressing REM sleep may yield an antidepressant response (Vogel et al., 1975).
Slow wave sleep abnormalities, however, are also prominent in depression (Borbély & Wirz-Justice, 1982; Benca et al., 1992) and likely play a role in the modulation of depressive symptomatology. For instance, Nissen and colleagues (2001) found that a high delta sleep ratio (quotient of slow wave activity [SWA] in the first to the second NREM sleep cycle) on the night prior to SD predicted the antidepressant response. In addition, Duncan et al. (1980) showed that the participants who responded to SD treatment exhibited a greater rebound of slow wave sleep and total sleep time upon recovery sleep compared to their baseline. Finally, Borbély (1987) proposed that sleep in depression is characterized by abnormal slow wave homeostasis, which may be renormalized by SD therapy.
The current study directly investigated the role of slow wave homeostasis in the antidepressant action of SD by using a selective slow wave deprivation (SWD) technique. As the first study to assess the antidepressant effects of SWD in depression, this exploratory investigation had two primary aims. First, we sought to determine the efficacy of the SWD technique in suppressing SWA in depressed participants, to measure the overnight change in depressive symptomatology, and to examine the extent to which these two variables are correlated. Second, we aimed with this initial investigation, using a simple, randomized crossover design, to provide the foundation for larger, more controlled, comparison-based, double-blinded studies to rigorously assess the usefulness of SWD in treating major depression.
2. Methods
2.1. Participants
17 right-handed individuals (9 female; mean age 23.94 ± 2.31 years, M ± S.E.M.) participated in the study (approved by the Institutional Review Board of the University of Wisconsin-Madison). Participants were medication-free for ≥ 6 months prior to enrollment, diagnosed with Major Depressive Disorder via the Structural Clinical Interview for DSM-IV Axis 1 disorders (SCID) (First et al., 1995) and initially evaluated with the researcher-administered 17-item Hamilton Rating Scale for Depression (score: 14.25 ± 1.31, range 7–25) (Hamilton, 1960). During the informed consent, participants were informed that the study aimed to understand brain waves during sleep in depressed individuals compared to healthy controls, to examine motor learning in depressed individuals, and to track changes in mood over the course of the study, during which tones may or may not be played during sleep. Participants were instructed to maintain regular sleep-wake schedules, avoid napping, and refrain from alcohol, caffeine, and nicotine for the duration of the study. Compliance was verified by sleep diaries and wrist motor actigraphy (Actiwatch, Mini-Mitter, Bend, OR).
2.2. Study Design
The current study consisted of 3 overnight sessions in the following order: baseline (BSL), slow wave deprivation (SWD) intervention, and recovery (RCV) sleep nights. Each sleep session was recorded in a sound-attenuated room. These recordings were part of a larger, randomized cross-over design study that involved 2 additional visits to the laboratory, which minimized possible order effects (9 participants underwent SWD on the 2nd visit overall and 8 underwent SWD on the 4th visit). The BSL night included full clinical polysomnography, which was reviewed by an American Board of Sleep Medicine-certified physician. The clinical polysomnography entailed electrooculogram (EOG), submental electromyogram (EMG), electrocardiogram (ECG), bilateral tibial EMG, and respiratory recordings, as well a position sensor and pulse oximetry. Participants with sleep disorders (including apnea-hypopnea index >10 or periodic limb movement-arousal index >10) or a sleep efficiency < 80% were excluded from the study.
2.3. Data Collection and Processing
EEG recordings of sleep were acquired using high-density EEG (256 electrode nets; Electrical Geodesics, Eugene, OR). Specifications for sampling and filtering of the hd-EEG signal, as well as for artifact rejection and spectral analysis, have been previously described (Landsness et al., 2009). To increase the signal-to-noise ratio, hdEEG processing and analysis were restricted to the 185 channels overlaying the scalp, removing channels around the cheeks, ears, and neck that are susceptible to artifacts (Goncharova et al., 2003). For sleep scoring, 6 bipolar mastoid-referenced channels (F3, F4, C3, C4, O1, O2) were extracted from the high-density montage. Sleep stages and arousals were visually scored by a registered polysomnographic technologist in 30-second epochs according to standard criteria (Iber et al., 2007). Evaluations of mood were obtained in the evening before and after SWD treatment via a modified version of the self-rated Inventory of Depressive Symptomatology (see Section 2.4 “Modified IDS Scale”) (Rush et al., 1996). We evaluated a subset (n = 6) of participants with a modified version of the researcher-administered 17-item Hamilton Rating Scale for Depression (HRSD-13; 4 questions about appetite/weight and insomnia removed) (Hamilton, 1960; Hernandez et al., 2000; Smith et al., 2009). The researcher administering the HRSD-13 was blind to the treatment condition and thus unaware of the expected amelioration of depressive symptoms following SWD. To measure changes in vigilance for all participants, data from the Psychomotor Vigilance Task (PVT) (Dinges et al., 1997), VAS for alertness and sleepiness (Folstein & Luria, 1973), and Stanford Sleepiness Scale (SSS) (Herscovitch & Broughton, 1981) were collected in the evening and following morning of each overnight visit.
2.4. Modified IDS Scale
Rush and colleagues found that the self-rated 16-item version of the IDS (QIDS-SR16) yielded strong psychometric properties and correlated well with longer self-rated scales in assessing change in depressive symptomatology (Rush et al., 2003, 2005). Because the current study evaluated the overnight change in depressive symptomatology, it was important to implement a scale that could assess such an acute change in scale ratings. In a similar fashion as previous SD studies with the HRSD-13 (Hernandez et al., 2000; Smith et al., 1999, 2009), we modified the QIDS-SR16 by removing questions about sleep and weight (Smith et al., 1999) and adding items from the full-length version of the IDS (IDS-SR30) about irritability, anxiety, response of mood to good events, view of future, and general capacity for enjoyment. Participants were instructed to rate each item according to how they felt since the last rating. The modified IDS self-rated scale (mIDS-SR) showed sufficient internal reliability as measured by Cronbach’s alpha for the two time points (Pre-SWD: α = .731; Post-SWD: α = .774) and was therefore used as the primary outcome measure.
2.5. SWD Intervention
SWD was performed with a technique similar to previous studies (Dijk & Beersma, 1989; Ferrara et al., 1999; Aeschbach et al., 2008; Tasali et al., 2008; Landsness et al, 2009). While the participant was asleep in a sound-attenuated room, the hdEEG recording was remotely monitored in real-time. Each time a slow wave (0.5–4.5 Hz; > 75 µV) was visually detected, an auditory tone (500–2000 Hz; 40–110 DB; 1–2 sec.) was delivered through a loudspeaker placed next to the participant’s bed using LabView (National Instruments, Austin, TX). The type and incidence of tones played were tailored to each participant to suppress slow waves without arousing the subject.
For 2 participants, a greater all-night average SWA value was observed in the SWD night relative to the baseline night (1 due to a technical failure and 1 due to difficulty in inhibiting slow waves with the tones). These participants, for whom the SWD intervention was not successful, were excluded from the analysis, and the results from the remaining 15 participants are reported.
2.6. Statistical Analysis
To examine changes in mood and vigilance, we directly compared the evening pre-treatment (Pre-SWD) and post-treatment (Post-SWD) scores using 2-tailed, paired t-tests, controlling for possible diurnal variation. For each sleep variable, we conducted a repeated-measures analysis of variance (ANOVA) with post-hoc paired t-tests (Bonferroni corrected). To assess the relationship between sleep variables and the decrease in depressive symptoms (via the mIDS-SR), correlational analyses were performed. In order to minimize the possibility of spurious findings for the hdEEG data from 185 channels, topographical correlations were investigated only if 3 contiguous channels showed a significant correlation. All comparisons and correlations were conducted in Statistica 6.0 software package (StatSoft, Tulsa, OK) and MATLAB (Mathworks, Nantucket, MA).
3. Results
3.1. Impact of SWD on Sleep Variables
Measures of sleep quality and quantity for the BSL, SWD, and RCV conditions are summarized in Table 1A. A repeated-measures ANOVA indicated a treatment effect for sleep stage N3 (% of total sleep time [TST]) and SWA. Post-hoc t-tests revealed a 54% and 37% reduction from BSL to SWD for N3% and SWA, respectively. Other sleep measures, including N1%, N2%, REM%, REM latency (REML), and spindle power (12–15Hz range) were not significantly affected. However, there was also a treatment effect for wake after sleep onset (WASO), arousal index, and sleep efficiency. Post-hoc comparisons showed significantly greater WASO on BSL compared to both SWD and RCV, suggesting a first-night effect. The arousal index was significantly higher on SWD compared to both BSL and RCV, although participants did not report waking up more than usual when questioned about their sleep the following morning. Sleep efficiency was significantly higher on RCV compared to BSL, possibly reflecting a first-night effect on BSL and a sleep rebound on RCV.
Table 1.
Impact of SWD on Sleep, Depressive Symptoms, and Measures of Sleepiness and Alertness (n = 15)
| A | BSL (1) | SWD (2) | RCV (3) | Overall ANOVA | p Value for Comparisona | ||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | M (± SEM) | M (± SEM) | M (± SEM) | df | F | p | 1 vs. 2 | 2 vs. 3 | 1 vs. 3 |
| TST, min | 372.33 (11.85) | 381.03 (11.13) | 387.73 (5.70) | 2,28 | 0.92 | .412 | 1.000 | 1.000 | 0.564 |
| WASO, min | 53.03 (9.02) | 29.67 (5.29) | 23.47 (4.54) | 2,28 | 7.12 | .003 | .026 | 1.000 | .004 |
| AI | 10.83 (1.86) | 16.85 (1.83) | 12.27 (1.12) | 2,28 | 6.37 | .005 | .006 | .044 | 1.000 |
| SE | 84.10 (2.09) | 88.95 (1.95) | 90.73 (1.39) | 2,28 | 4.86 | .015 | .107 | 1.000 | .016 |
| N1, % | 9.53 (1.64) | 11.39 (1.27) | 7.68 (0.67) | 2,28 | 2.73 | .083 | .759 | .081 | .759 |
| N2, % | 58.38 (1.95) | 60.43 (1.69) | 55.03 (2.02) | 2,28 | 2.69 | .086 | 1.000 | .089 | .496 |
| N3, % | 13.58 (2.22) | 6.21 (1.48) | 17.19 (2.57) | 2,28 | 16.84 | <.001 | .002 | <.001 | .214 |
| REM, % | 18.51 (1.39) | 21.99 (1.82) | 20.13 (1.76) | 2,28 | 1.55 | .230 | .269 | 1.000 | 1.000 |
| REML, min | 92.73 (14.13) | 92.70 (16.05) | 92.3 (13.36) | 2,28 | <.001 | 1.000 | 1.000 | 1.000 | 1.000 |
| SWA | 20.27 (2.54) | 12.71 (1.27) | 21.56 (2.83) | 2,28 | 12.05 | <.001 | .002 | <.001 | 1.000 |
| Spindle Power | 1.07 (0.13) | 1.21 (0.14) | 1.03 (0.12) | 2,28 | 2.98 | .067 | .572 | .065 | .850 |
| B | Pre-SWD | Post-SWD | Pre-SWD vs. Post-SWDb | |
|---|---|---|---|---|
| Variables | M (± SEM) | M (± SEM) | t | p |
| mIDS-SR | 16.53 (1.20) | 15.00 (1.13) | 3.15 | 0.007 |
| HRSD-13 (N=6) | 10.67 (1.33) | 7.83 (1.25) | 4.03 | 0.010 |
| VAS-Sleepiness | 6.37 (0.23) | 5.92 (0.45) | 0.88 | 0.393 |
| SSS | 3.47 (0.31) | 3.40 (0.32) | 0.16 | 0.872 |
| VAS-Alertness | 5.27 (0.43) | 5.22 (0.47) | 0.09 | 0.927 |
| PVT Reaction Time†, msec | 296.84 (12.55) | 288.22 (13.26) | 1.00 | 0.334 |
ANOVA, analysis of variance; SEM, standard error of the mean. (A) BSL, baseline; SWD, slow wave deprivation; RCV, recovery; TST, total sleep time; WASO, wake (time) after sleep onset; AI, arousal index; SE, sleep efficiency; N1/2/3, NREM stage 1/2/3 (% of TST); REM, stage REM (% of TST); REML, REM latency; SWA, slow wave activity ([µV2/0.25] in 1–4.5Hz range); Spindle Power ([µV2/0.25] in 12–15Hz range). (B) mIDS-SR, modified Inventory of Depressive Symptomatology (self-rated); HRSD, Hamilton Rating Scale for Depression; VAS, Visual Analogue Scale; SSS, Stanford Sleepiness Scale; PVT, Psychomotor Vigilance Task. Data for Pre-SWD and Post-SWD were recorded at approximately 9:00pm the evening prior to SWD treatment and 9:00pm the following evening, respectively.
n = 14 due to technical error during collection of one data point (Pre-SWD)
Post-hoc t-tests. p values are expressed with Bonferroni adjustment, where p < .05 is considered significant.
2-tailed, paired t-tests.
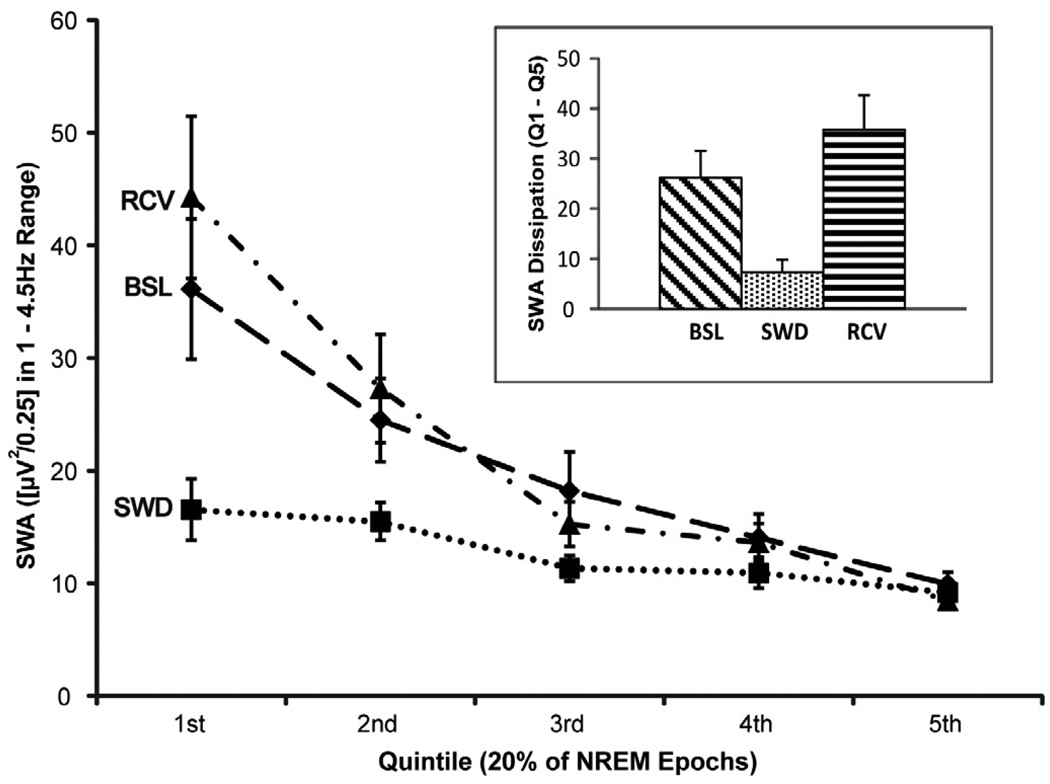
To illustrate the impact of SWD on global SWA across the night compared to the BSL and RCV conditions, all artifact-free NREM sleep epochs for each condition were divided into quintiles, and SWA for each quintile is displayed in Figure 1. A 3 (condition) × 5 (quintile) repeated-measures ANOVA indicated a main effect for condition (F2,28 = 12.45, p = .0001) and quintile (F4,56 = 22.43, p < .0001), as well as a condition × quintile interaction (F8,112 = 7.19, p < .0001). Post-hoc tests demonstrated that global SWA was lower in the first quintile on SWD compared to both BSL (p < .0001) and RCV (p < .0001), although global SWA in the last quintile did not differ among conditions (p = n.s.).
Figure 1.
Global SWA time course across the night for baseline (BSL), slow wave deprivation (SWD), and recovery (RCV) nights. Artifact-free NREM epochs for each night were divided into quintiles. Inset: comparison of SWA dissipation across the entire night for BSL, SWD, and RCV, expressed as the first quintile (Q1) minus the last quintile (Q5).
Another measure to evaluate the success of SWD in suppressing SWA involves the dissipation of global SWA across the night. To capture the robust change in SWA that occurs from high sleep pressure to low sleep pressure, which has been linked to distinct changes in neuronal firing patterns that parallel sleep homeostasis (Vyazovskiy et al., 2009), this study defined SWA dissipation as the SWA of the first quintile minus the SWA of the last quintile. A 1 × 3 ANOVA indicated a significant difference in SWA dissipation among the three study conditions (F2,28 = 15.21, p < .0001), whereby SWA dissipation was greater on both BSL (p = .004) and RCV (p < .0001) compared to SWD (see Figure 1 inset).
3.2. Vigilance Measures
Ruling out confounding effects of alertness or sleepiness that often accompany total sleep deprivation, we found no significant differences on vigilance measures between Pre- and Post-SWD, including the PVT, VAS-Alertness, VAS-Sleepiness, and the SSS. See Table 1B.
3.3. Decrease in Depressive Symptoms
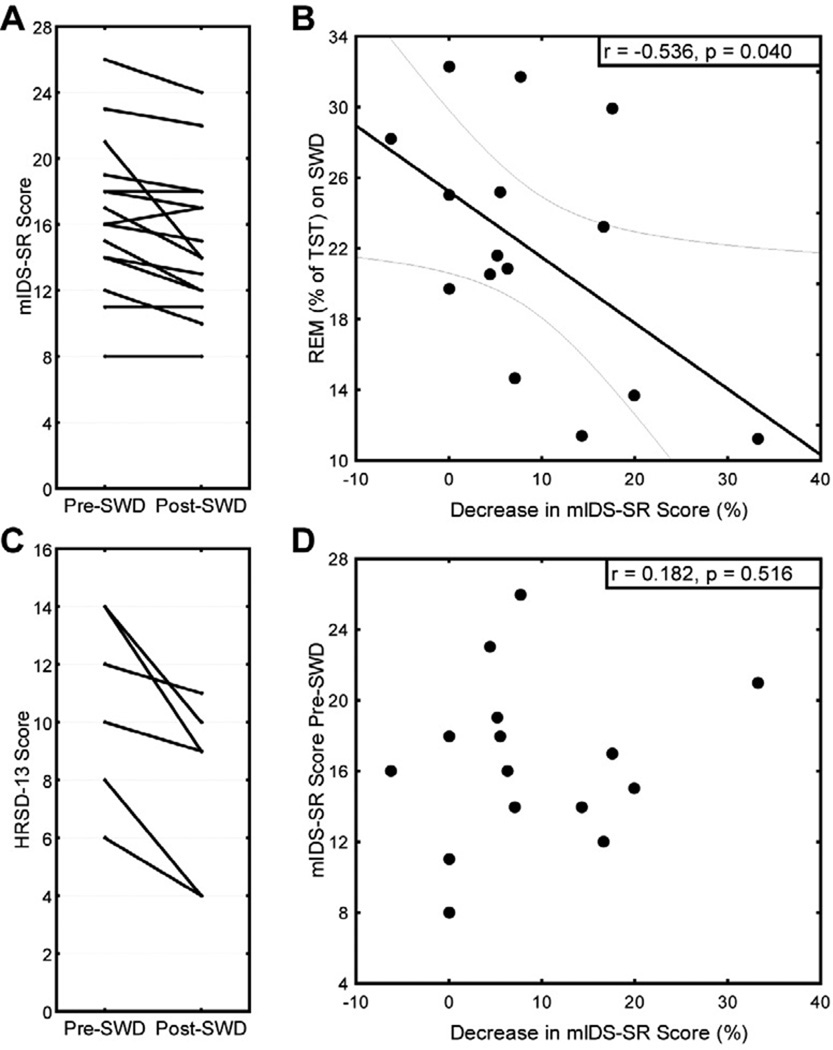
After one night of SWD, depressed participants showed a significant reduction in depressive symptoms on both the self-rated mIDS-SR (10%) and the HRSD-13 (27%). Individual scores are displayed in Figure 2A and 2C. Ensuring that the subset of participants with HRSD-13 scores was representative of the total group of participants, we found similar results for the mIDS-SR in this subset as we found with the entire sample, constituting a 12% reduction in symptoms. When comparing subgroups based on the order of experimental session (whether participants underwent SWD on their 2nd or 4th night during the study), we found no significant differences in the evening pre-sleep scores on the mIDS-SR (16.86 ± 0.46 and 16.25 ± 0.63, respectively). Likewise, there was no significant difference in the overnight decrease of mIDS-SR score (11.03% ± 4.65 and 6.84% ± 2.78, respectively).
Figure 2.
Impact of slow wave deprivation (SWD) on the (A) modified Inventory of Depressive Symptomatology, self-rated (mIDS-SR) and (C) Hamilton Rating Scale for Depression (HRSD-13) scores. (B) The amount of REM sleep obtained during the treatment correlates with the antidepressant response (displayed with 95% confidence regression bands), but (D) initial severity of depression does not predict level of response.
3.4. Predictors of Change in Mood
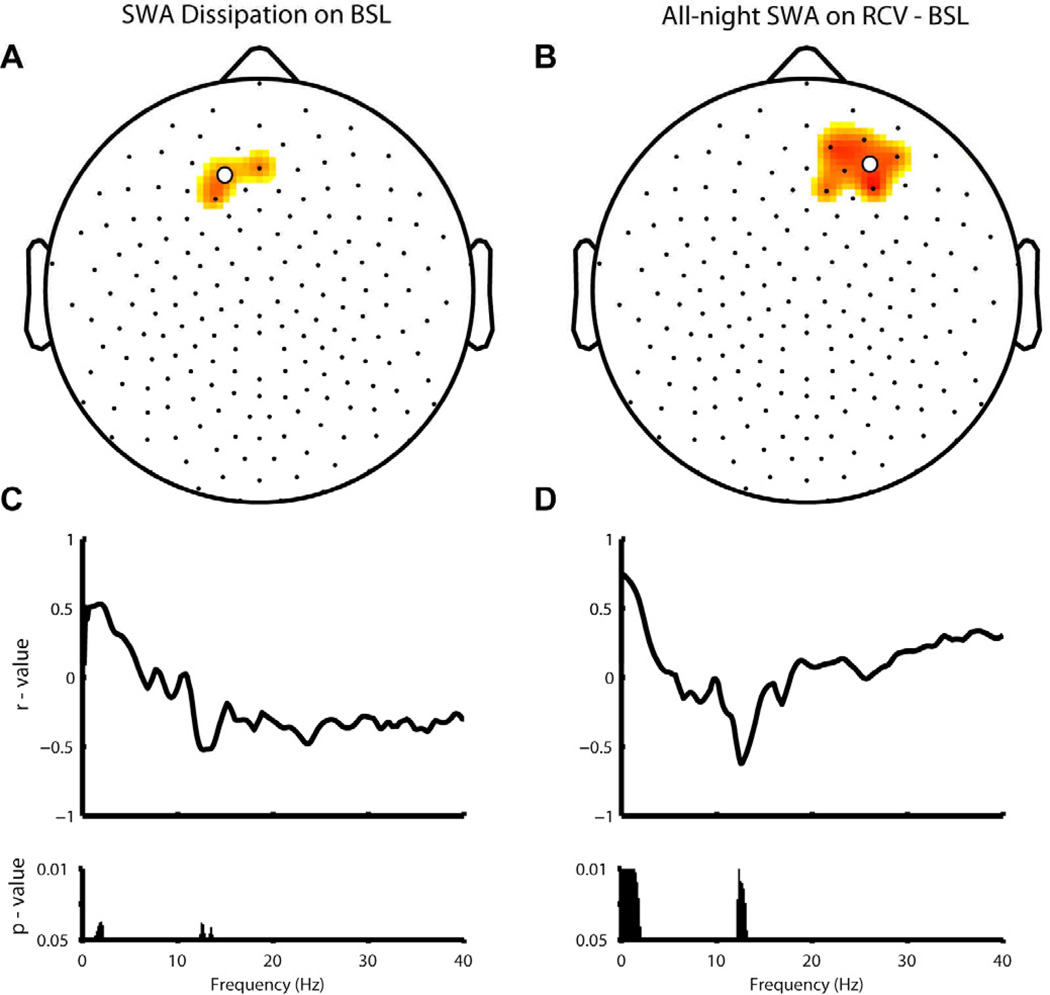
Because hdEEG was recorded and a treatment effect was observed for global SWA, topographical analyses were conducted to investigate correlations of regional changes in SWA with the overnight change in depressive symptoms. When examining the dissipation of SWA across the night (first – last 20% of total artifact-free NREM sleep epochs) on BSL, the decrease of SWA in a fronto-central cluster of channels (Figure 3A) correlated with the decrease in mIDS-SR score. The more SWA was dissipated across the night on baseline, the greater the reduction in depressive symptoms after SWD. As shown in Figure 3C, this correlation was specific for the low SWA frequency range (1–2 Hz) and was inversely related to low spindle power (12–13 Hz).
Figure 3.
Topographic correlation of antidepressant response (via mIDS-SR) with (A) dissipation of SWA across the baseline (BSL) night (high sleep pressure – low sleep pressure, expressed as a percentage of SWA averaged across the entire night) and (B) all-night SWA rebound on RCV (relative to BSL). (C–D) Frequency-specific correlations are shown for the central channel in the cluster (white dot) of each SWA measure.
Given that the homeostatic dissipation of SWA on BSL predicted subsequent response to SWD, we sought to establish whether the homeostatic rebound of SWA on RCV also correlated with response. As seen in Figure 3B, greater all-night average SWA on RCV (relative to BSL) in a right frontal cluster of channels was related to greater decreases in symptom severity. Again, this correlation was specific to the low SWA frequency range (< 2Hz) and was inversely related to low spindle power (12–13Hz).
Previous literature (Vogel et al., 1975) has suggested a role of REM sleep in the antidepressant response to sleep deprivation. Therefore, REM sleep variables were also examined. Despite no significant differences in REM% of total sleep time (Table 1A), participants with less REM% on SWD showed a greater decrease in mIDS-SR score. See Figure 2B.
To examine the relative impact of baseline SWA dissipation, SWA rebound, and REM%, a multiple regression was conducted with the overnight change in mIDS-SR score as the dependent variable. The results demonstrated that SWA dissipation on BSL [β = 0.30], all-night SWA rebound (BSL – RCV) [β = −0.23], and REM% on SWD [β = −0.32] had similar weights in predicting response.
3.5. Pertinent Negative Findings
Ensuring that the treatment effect for arousal index was not related to the decrease in depressive symptoms, no correlation between the two variables was found (r = 0.262; p > .05). In addition, baseline severity rating on the mIDS-SR did not predict response to SWD (Figure 2D), suggesting that the SWD intervention does not preferentially benefit moderate depression severity (baseline HRSD ≥ 17) compared to more mild levels of depression. Upon questioning during an exit interview at the end of the study, all but one participant reported hearing no tones being played during any of the nights. The one participant who reported hearing tones did not show a greater reduction in depressive symptoms than the group mean, nor did this participant associate a change in mood specific to the SWD night.
4. Discussion
Extending previous literature on the benefits of SD in major depression, the current investigation found that the SWD intervention selectively suppressed SWA, while leading to a small, but significant reduction in depressive symptomatology.
4.1. SWA and Depressive Symptoms
When correlating hdEEG measures of SWA with the overnight change in depressive symptoms, two specific relationships were revealed that support a direct link between SWA and change in depressive symptomatology. Extending previous literature (Nissen et al., 2001), a greater overnight dissipation of SWA on the baseline night in a fronto-central cluster of channels was predictive of a decrease in depressive symptom ratings. In addition, the observed relationship between a greater rebound in all-night average SWA upon recovery sleep (relative to baseline) and the antidepressant response corroborates and extends previous studies (Duncan et al., 1980; Reynolds et al., 1987; Gillin et al., 1989), suggesting that the SWA rebound in the right frontal region may be particularly important for an antidepressant response to SWD. Given that both correlations of SWA with the antidepressant response were specific to a frequency of < 2 Hz, it is possible that low frequencies of the SWA range may be of particular importance for the modulation of depressive symptomatology. Moreover, these findings support the notion that the depressed individuals with a more normal regulation of slow wave homeostasis are more responsive to the SWD treatment, which may be an important consideration for future studies that wish to further investigate the mechanism of SD’s antidepressant action (Borbély, 1987; Wirz-Justice & Van den Hoofdakker, 1999; Hemmeter et al., 2010).
Functional brain imaging studies in depression have provided evidence for abnormalities in frontal brain regions related to depressive symptomatology (Mayberg, 1997). Response to SD has been positively correlated with baseline, waking hyperactivity in the anterior cingulate cortex (ACC) (Benedetti & Smeraldi, 2009; Clark et al., 2006) and medial prefrontal cortex (Wu et al., 1999, 2001). Nofzinger and colleagues (2005) demonstrated that in addition to ACC hypermetabolism at waking baseline, depressed individuals experienced higher levels of ACC metabolism during NREM sleep compared to healthy controls, as well as relatively greater decreases in ACC metabolism from waking to NREM sleep. Given evidence that the ACC may be involved in slow waves (Murphy et al., 2009), our findings of a correlation between overnight dissipation in SWA and the response to SWD may reflect frontal cortex hyperactivity during baseline sleep in these participants. Other studies have shown that prefrontal abnormalities normalized by SD therapy may be predictive of response (Wu et al., 2001, 2008; Clark et al., 2006). In this regard, our finding of a homeostatic rebound in SWA upon recovery sleep that correlated with the SWD response may also reflect a renormalization of prefrontal abnormalities. Together, the results of the current investigation emphasize the importance of considering both frontal brain activity and the nature of slow wave homeostasis in the reduction of depressive symptomatology.
4.2. Role of REM Sleep
Although REM sleep was not directly manipulated in this experiment, a relationship between REM (% of total sleep time) and the overnight change in depressive symptoms was found. Supporting the REMD literature and the REM sleep-suppressing action of many antidepressant medications (Vogel, 1983), lower amounts of REM sleep (relative to TST) were correlated with a greater reduction in depressive symptom ratings. Given this unexpected finding, it is possible that a combined SWD and REMD intervention may be particularly beneficial for alleviating depressive symptomatology. In agreement with both the REM sleep-suppressing effects of antidepressant medications and proven benefits of adjunct pharmacotherapy in TSD studies (Wu & Bunney, 1990; Wirz-Justice & Van den Hoofdakker, 1999; Giedke & Schwärzler, 2002), concomitant use of REM sleep-suppressing antidepressant medications, as well as chronotherapy (Wu et al., 2009), could also be beneficial in potentiating and maintaining the response to SWD.
4.3. Implications for Future Research
In this exploratory investigation, the SWD intervention yielded a 37% suppression in all-night SWA and led to a 10% overnight reduction in depressive symptoms. It is possible that the response to SWD may be dose-dependent, whereby either refining the SWD technique to suppress a greater amount of SWA or implementing multiple nights of SWD could yield a greater response.
When further examining the SWD technique in depression, however, it will also be important to evaluate other effects associated with the intervention, such as decrements memory consolidation (Landsness et al., 2009) or immune regulation (Tasali et al., 2008) that have been demonstrated when applying SWD in healthy controls. These considerations will be critical when fully evaluating the usefulness of SWD therapy in depression.
Because the focus of this study was to evaluate the acute effect of SWD on depressive symptoms, changes in mood following recovery sleep were not assessed. Given previous findings on depression relapse following recovery sleep after SD (Hemmeter et al., 2010), it would be important for future studies to evaluate this phenomenon in relation to SWD to determine both the duration of its antidepressant effect and the usefulness of combined therapies in prolonging the effect.
It is possible that the observed antidepressant effects in this study may be in part due to a placebo or order effect of being in the sleep laboratory. However, we found that the subgroup of participants who underwent SWD on their 4th overall night of the study did not have evening pre-sleep depression ratings that were significantly different than the subgroup who experienced SWD on their 2nd night, nor did they respond more favorably. In addition, all but one participant did not report hearing tones specific to the SWD night when questioned during an exit interview. The one subject that did report hearing tones did not associate the tones to a change in mood, and the change in the subject’s mood was not significantly different than the mean change in mood for all the subjects. Given these observations, it is unlikely that the acute reduction in depressive symptoms following SWD were due to an order or placebo effect.
Due to the difficulty in recruiting non-medicated depressed participants, the average intake depression ratings tended to be lower than other TSD studies. Despite this, baseline depression severity did not correlate with subsequent response to SWD. However, it is possible that low intake levels of depression may have caused a floor effect that contributed to a relatively small reduction in symptoms (i.e. compared to TSD studies). Interestingly, these results suggest that the SWD intervention may be beneficial for even mild to moderate levels of depression.
This study used the self-rated mIDS-SR as the primary outcome measure, while collecting data from the researcher-administered HRSD in a subset of participants. When comparing the scales directly, this subset of individuals showed a greater reduction in depressive symptoms with the HRSD (27% in HRSD vs. 12% in mIDS-SR). However, these results are from a small number of participants (n = 6) and need to be replicated with a larger sample. This discrepancy could also be explained by a self-rater bias that is commonly observed in depression, especially when assessing improvement in symptoms (Koenig et al., 1995), suggesting that the researcher-administered scale could be more accurate in estimating antidepressant response. To avoid this subjective bias, an alternative could be to use more objective measures that correlate to mood when assessing response to SWD, such as spontaneous waking EEG (Steiger & Kimura, 2010), or auditory evoked potentials (Hegerl & Juckel, 2001).
Results from this study demonstrate the potential application of hdEEG as a functional brain imaging technique in psychiatric disorders. Source modeling of hdEEG data has already linked the ACC, a critical brain structure in major depression, to sleep slow waves (Murphy et al., 2009). Our findings also suggest localized effects of SWD on SWA in frontal regions. In conjunction with MRI co-localization of electrode placement, hdEEG offers a versatile, yet less expensive approach to studying specific brain regions involved in the persistence and treatment of depressive symptomatology when compared with other brain imaging techniques, such as fMRI or PET.
4.4. Conclusion
This initial investigation provides further evidence for the known role of slow waves in depression and suggests that SWD may help acutely reverse depressive symptomatology, although its effect may be less robust compared to the response demonstrated with total sleep deprivation treatments. Future studies will benefit from SWD and/or hdEEG as useful tools in understanding the underlying pathophysiology of depression and the response to sleep deprivation therapy.
Acknowledgments
Role of the funding source
This research was funded by the National Institute of Mental Health (5P20MH077967 to GT and RB, and F30MH082601 to EL) and the National Alliance for Research on Schizophrenia and Depression Young Investigator Award to MP. The NIMH and NARSAD had no further role in the study design, data collection, analysis and interpretation of the data, and the decision to submit the paper for publication. Statistical consultation during the data analysis was supported by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Eric Landsness contributed to designing the study, performing the experiments and data analysis, and writing the article.
Michael Goldstein contributed by conducting the experiments and data analysis, and writing the article.
Michael Peterson contributed to the study design, psychiatric evaluations of the participants, and writing the article.
Giulio Tononi contributed to the study design and writing the article.
Ruth Benca contributed by being the principal investigator, designing the study, participating in the data analysis and interpretation, and writing the article.
Conflict of interest statement
Dr. Peterson has received unrelated research support from Sanofi-Aventis. Dr. Tononi has consulted for Sanofi-Aventis and Takeda, and he is currently the David P. White Chair in Sleep Medicine at the University of Wisconsin – Madison, endowed by Phillips Respironics. Dr. Tononi has also received unrelated research support from Phillips Respironics. Dr. Benca has consulted for Merck and Sanofi-Aventis. The other authors have indicated no financial conflicts of interest.
References
- Aeschbach D, Cutler AJ, Ronda JM. A role for non-rapid-eye-movement sleep homeostasis in perceptual learning. J Neurosci. 2008;28:2766–2772. doi: 10.1523/JNEUROSCI.5548-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association., & American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Benca R, Obermeyer W, Thisted R, Gillin J. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. discussion 669-70. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Smeraldi E. Neuroimaging and genetics of antidepressant response to sleep deprivation: implications for drug development. Curr Pharm Des. 2009;15:2637–2649. doi: 10.2174/138161209788957447. [DOI] [PubMed] [Google Scholar]
- Borbély A. The S-deficiency hypothesis of depression and the two-process model of sleep regulation. Pharmacopsychiatry. 1987;20:23–29. doi: 10.1055/s-2007-1017069. [DOI] [PubMed] [Google Scholar]
- Borbély A, Wirz-Justice A. Sleep, sleep deprivation and depression. A hypothesis derived from a model of sleep regulation. Hum Neurobiol. 1982;1:205–210. [PubMed] [Google Scholar]
- Clark C, Brown G, Archibald S, Fennema-Notestine C, Braun D, Thomas L, Sutherland A, Gillin J. Does amygdalar perfusion correlate with antidepressant response to partial sleep deprivation in major depression? Psychiatry Res. 2006;146:43–51. doi: 10.1016/j.pscychresns.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG. Effects of SWS deprivation on subsequent EEG power density and spontaneous sleep duration. Electroencephalogr Clin Neurophysiol. 1989;72:312–320. doi: 10.1016/0013-4694(89)90067-9. [DOI] [PubMed] [Google Scholar]
- Dinges D, Pack F, Williams K, Gillen K, Powell J, Ott G, Aptowicz C, Pack A. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–277. [PubMed] [Google Scholar]
- Duncan WJ, Gillin J, Post R, Gerner R, Wehr T. Relationship between EEG sleep patterns and clinical improvement in depressed patients treated with sleep deprivation. Biol Psychiatry. 1980;15:879–889. [PubMed] [Google Scholar]
- Ferrara M, De Gennaro L, Bertini M. Selective slow-wave sleep (SWS) deprivation and SWS rebound: do we need a fixed SWS amount per night? Sleep Res Online. 1999;2:15–19. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis1 Disorders - Patient Edition (SCID-I/P) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Folstein M, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Giedke H, Schwärzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6:361–377. [PubMed] [Google Scholar]
- Gillin J. The sleep therapies of depression. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:351–364. doi: 10.1016/0278-5846(83)90123-9. [DOI] [PubMed] [Google Scholar]
- Gillin J, Kripke D, Janowsky D, Risch S. Effects of brief naps on mood and sleep in sleep-deprived depressed patients. Psychiatry Res. 1989;27:253–265. doi: 10.1016/0165-1781(89)90141-8. [DOI] [PubMed] [Google Scholar]
- Goncharova II, McFarland DJ, Vaughan TM, Wolpaw JR. EMG contamination of EEG: spectral and topographical characteristics. Clin Neurophysiol. 2003;114:1580–1593. doi: 10.1016/s1388-2457(03)00093-2. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegerl U, Gallinat J, Juckel G. Event-related potentials. Do they reflect central serotonergic neurotransmission and do they predict clinical response to serotonin agonists? J Affect Disord. 2001;62:93–100. doi: 10.1016/s0165-0327(00)00353-0. [DOI] [PubMed] [Google Scholar]
- Hemmeter U, Hemmeter-Spernal J, Krieg J. Sleep deprivation in depression. Expert Rev Neurother. 2010;10:1101–1115. doi: 10.1586/ern.10.83. [DOI] [PubMed] [Google Scholar]
- Hernandez CR, Smith GS, Houck PR, Pollock BG, Mulsant B, Dew MA, Reynolds CF. The clinical response to total sleep deprivation and recovery sleep in geriatric depression: potential indicators of antidepressant treatment outcome. Psychiatry Res. 2000;97:41–49. doi: 10.1016/s0165-1781(00)00225-0. [DOI] [PubMed] [Google Scholar]
- Herscovitch J, Broughton R. Sensitivity of the stanford sleepiness scale to the effects of cumulative partial sleep deprivation and recovery oversleeping. Sleep. 1981;4:83–91. doi: 10.1093/sleep/4.1.83. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chessonn A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specificiations. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Jobert M, Jähnig P, Schulz H. Effect of two antidepressant drugs on REM sleep and EMG activity during sleep. Neuropsychobiology. 1999;39:101–109. doi: 10.1159/000026568. [DOI] [PubMed] [Google Scholar]
- Koenig L, Ragin A, Harrow M. Accuracy and Bias in Depressives' Judgments for Self and Other. Cognitive Therapy and Research. 1995;19:505–517. [Google Scholar]
- Kuhs H, Tölle R. Sleep deprivation therapy. Biol Psychiatry. 1991;29:1129–1148. doi: 10.1016/0006-3223(91)90255-k. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Foster FG. Interval between onset of sleep and rapid-eye-movement sleep as an indicator of depression. Lancet. 1972;2:684–686. doi: 10.1016/s0140-6736(72)92090-9. [DOI] [PubMed] [Google Scholar]
- Landsness E, Crupi D, Hulse B, Peterson M, Huber R, Ansari H, Coen M, Cirelli C, Benca R, Ghilardi M, Tononi G. Sleep-dependent improvement in visuomotor learning: a causal role for slow waves. Sleep. 2009;32:1273–1284. doi: 10.1093/sleep/32.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen C, Feige B, König A, Voderholzer U, Berger M, Riemann D. Delta sleep ratio as a predictor of sleep deprivation response in major depression. J Psychiatr Res. 2001;35:155–163. doi: 10.1016/s0022-3956(01)00021-8. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Price JC, Meltzer CC, Miewald JM, Kupfer DJ. Alterations in regional cerebral glucose metabolism across waking and non-rapid eye movement sleep in depression. Arch Gen Psychiatry. 2005;62:387–396. doi: 10.1001/archpsyc.62.4.387. [DOI] [PubMed] [Google Scholar]
- Peterson M, Benca R. Sleep in mood disorders. Psychiatr Clin North Am. 2006;29:1009–1032. doi: 10.1016/j.psc.2006.09.003. abstract ix. [DOI] [PubMed] [Google Scholar]
- Pflug B, Tölle R. Therapy of endogenous depressions using sleep deprivation. Practical and theoretical consequences. Nervenarzt. 1971;42:117–124. [PubMed] [Google Scholar]
- Reynolds Cr, Kupfer D, Hoch C, Stack J, Houck P, Berman S. Sleep deprivation effects in older endogenous depressed patients. Psychiatry Res. 1987;21:95–109. doi: 10.1016/0165-1781(87)90068-0. [DOI] [PubMed] [Google Scholar]
- Riemann D, Velthaus S, Laubenthal S, Müller WE, Berger M. REM-suppressing effects of amitriptyline and amitriptyline-N-oxide after acute medication in healthy volunteers: results of two uncontrolled pilot trials. Pharmacopsychiatry. 1990;23:253–258. doi: 10.1055/s-2007-1014515. [DOI] [PubMed] [Google Scholar]
- Rush A, Trivedi M, Carmody T, Ibrahim H, Markowitz J, Keitner G, Kornstein S, Arnow B, Klein D, Manber R, Dunner D, Gelenberg A, Kocsis J, Nemeroff C, Fawcett J, Thase M, Russell J, Jody D, Borian F, Keller M. Self-reported depressive symptom measures: sensitivity to detecting change in a randomized, controlled trial of chronically depressed, nonpsychotic outpatients. Neuropsychopharmacology. 2005;30:405–416. doi: 10.1038/sj.npp.1300614. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Smith GS, Reynolds CF, Houck PR, Dew MA, Ginsberg J, Ma Y, Mulsant BH, Pollock BG. Cerebral glucose metabolic response to combined total sleep deprivation and antidepressant treatment in geriatric depression: a randomized, placebo-controlled study. Psychiatry Res. 2009;171:1–9. doi: 10.1016/j.pscychresns.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GS, Reynolds CF, Pollock B, Derbyshire S, Nofzinger E, Dew MA, Houck PR, Milko D, Meltzer CC, Kupfer DJ. Cerebral glucose metabolic response to combined total sleep deprivation and antidepressant treatment in geriatric depression. Am J Psychiatry. 1999;156:683–689. doi: 10.1176/ajp.156.5.683. [DOI] [PubMed] [Google Scholar]
- Steiger A, Kimura M. Wake and sleep EEG provide biomarkers in depression. J Psychiatr Res. 2010;44:242–252. doi: 10.1016/j.jpsychires.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G. Evidence for REM sleep deprivation as the mechanism of action of antidepressant drugs. Prog Neuropsychopharmacol Biol Psychiatry. 1983;7:343–349. doi: 10.1016/0278-5846(83)90122-7. [DOI] [PubMed] [Google Scholar]
- Vogel G, Thurmond A, Gibbons P, Sloan K, Walker M. REM sleep reduction effects on depression syndromes. Arch Gen Psychiatry. 1975;32:765–777. doi: 10.1001/archpsyc.1975.01760240093007. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy V, Olcese U, Lazimy Y, Faraguna U, Esser S, Williams J, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A, Van den Hoofdakker R. Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry. 1999;46:445–453. doi: 10.1016/s0006-3223(99)00125-0. [DOI] [PubMed] [Google Scholar]
- Wu J, Buchsbaum MS, Gillin JC, Tang C, Cadwell S, Wiegand M, Najafi A, Klein E, Hazen K, Bunney WE, Fallon JH, Keator D. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry. 1999;156:1149–1158. doi: 10.1176/ajp.156.8.1149. [DOI] [PubMed] [Google Scholar]
- Wu J, Bunney W. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147:14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- Wu JC, Buchsbaum M, Bunney WE. Clinical neurochemical implications of sleep deprivation's effects on the anterior cingulate of depressed responders. Neuropsychopharmacology. 2001;25:S74–S78. doi: 10.1016/S0893-133X(01)00336-0. [DOI] [PubMed] [Google Scholar]
- Wu JC, Gillin JC, Buchsbaum MS, Schachat C, Darnall LA, Keator DB, Fallon JH, Bunney WE. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. 2008;107:181–186. doi: 10.1016/j.jad.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Wu JC, Kelsoe JR, Schachat C, Bunney BG, DeModena A, Golshan S, Gillin JC, Potkin SG, Bunney WE. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66:298–301. doi: 10.1016/j.biopsych.2009.02.018. [DOI] [PubMed] [Google Scholar]