Abstract
Objectives
This study assessed the antibacterial activity of short-, medium-, and long-chain fatty acids against various oral microorganisms.
Methods
The short-chain fatty acids [formic acid (C1), acetic acid (C2), propionic acid (C3), butyric acid (C4), isobutyric acid (C4), isovaleric acid (C5), hexanoic acid (C6)], medium-chain fatty acids [octanoic acid (C8), capric acid (C10), lauric acid (12)], and long-chain fatty acids [myristic acid (C14), palmitic acid (C16)], were investigated for antimicrobial activity against Streptococcus mutans, S. gordonii, S. sanguis, Candida albicans, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, and Porphyromonas gingivalis.
Results
The data demonstrated that the fatty acids exhibited patterns of inhibition against oral bacteria with some specificity that appeared related more to the bacterial species that the general structural characteristics of the microorganism. As a group the fatty acids were much less effective against C. albicans than the oral bacteria, with effectiveness limited to hexanoic, octanoic, and lauric acids. Formic acid, capric, and lauric acids were broadly inhibitory for the bacteria. Interestingly, fatty acids that are produced at metabolic end-products by a number of these bacteria, were specifically inactive against the producing species, while substantially inhibiting the growth of other oral microorganisms.
Conclusions
The results indicate that the antimicrobial activity of short-chain fatty acids (SCFAs), medium-chain fatty acids (MCFAs), long-chain fatty acids (LCFAs) could influence the microbial ecology in the oral cavity via at least 2 potential pathways. First, the agents delivered exogenously as therapeutic adjuncts could be packaged to enhance a microbial-regulatory environment in the subgingival sulcus. Second, it would be the intrinsic nature of these fatty acid inhibitors in contributing to the characteristics of the microbial biofilms, their evolution, and emergence of species within the biofilms. Further studies on these functions are required to better understand the nature of these potential microbial interactions in the biofilms.
Keywords: Short-chain, Medium-chain, Long-chain fatty acids, Antimicrobial activity, Bacterial ecology
1. Introduction
Long chain fatty acids (LCFAs) including n-3, n-6, n-7, and n-9 fatty acids have been reported to have anti-inflammatory and antibacterial activities. 1-6 For example, the n-3 fatty acids, eicosapentanoic acid (EPA) and docosahexanoic acid (DHA), can serve as precursors to eicosanoids, which can reduce inflammation. 1,3 A recent study demonstrated three major n-3 PUFA, EPA, DHA, α-linolenic acid (ALA), and their ester derivatives exhibited strong antimicrobial activity against various oral pathogens. 5 Similar findings were also reported for n-6, n-7, and n-9 fatty acids related to antimicrobial activities against oral microorganisms. 6
Oral bacteria are known to produce many short chain fatty acids (SCFAs, isobutyric, butyric, isovaleric, propionic, lactic acids), 4,7-8 and even LCFAs (myristic acid, palmitic acid), 9 as end-products of their metabolism. Oral bacterial strains produce and secrete a range of products that appear to function in providing individual species a competitive advantage within a complex microbial environment, including bacteriocins, 10 quorum sensing molecules, 11 and metabolic end-products. 12. Thus, there is a potential for these fatty acids to contribute to ecological and biological interactions among the oral bacteria. Fatty acid elaboration could contribute to a natural “food web” in oral biofilms resulting in competitive or mutualistic relationships with other oral bacterial co-inhabitants. 13 The fatty acid-secreting bacteria are present in lesions of both dental caries and periodontitis and likely contribute to the biology of oral biofilms. It has been posited that cell-to-cell metabolic communication within these bacterial communities can occur via short chain organic acids. 11, 13, 14 These studies concluded that “cross-feeding” takes place between individual bacterial species and that short-chain fatty acids produced by oral bacteria could serve as an essential carbon source for certain other oral bacteria and that these SCFA may be “competitive factors in oral biofilms”. 15
We hypothesized that an array of fatty acids could function in chronic oral infections as extrinsic or intrinsic agents. Extrinsically, SCFA and MCFA may also function as a dietary supplement or in an oral delivery medicament that could alter the microbial ecology. Alternatively, the fatty acids produced by various oral bacterial strains may be contribute to maintaining the commensal ecology through inhibiting additional bacterial colonization and/or emergence, or in some cases inhibit existing species and enable pathogens to emerge in the microbial biofilms. Thus, determining the profiles of fatty acids are most effective and specifically inhibit selected oral bacteria would provide some insights into potential therapeutic approaches, as well as naturally occurring activities in established and transitional biofilms.
2. Materials and Methods
2.1 Reagents
The short-chain fatty acids, formic acid (C1), acetic acid (C2), propionic acid (C3), butyric acid (C4), isobutyric acid (C4), isovaleric acid (C5), hexanoic acid (C6), and medium-chain fatty acids, octanoic acid (C8), capric acid (C10), lauric acid (C12), myristic acid (C14), palmitic acid (C16) were purchased from Cayman Chemicals (Ann Arbor, Michigan) or from Sigma (St. Louis, MO). Most of the fatty acids used in this study were obtained as fatty acid stock solutions (in H2O or ethyl alcohol) at mg/ml concentrations. Oral microbial species Streptococcus mutans (ATCC 25175), Porphyromonas gingivalis (ATCC 33277), Candida albicans (ATCC 2091), Aggregatibacter actinomycetemcomitans JP2, S. sanguis (ATCC 10556), S. gordonii (ATCC 10558), and Fusobacterium nucleatum (ATCC 25586) were purchased from the American Type Culture Collection (Manassas, VA). TSBYE media and Anaerobe Broth were purchased from Oxoid Ltd. (Cambridge, UK). Growth conditions for most of the bacteria were at 37°C in an anaerobic chamber (Plas-Labs, Lansing, MI) in an atmosphere of 85% N2, 10% H2, and 5% CO2. S. sanguis and S. gordonii were grown at 37°C in 5% CO2 and air, while C. albicans (ATCC 2091) was grown aerobically at 37°C.
2.2 Antimicrobial screening of fatty acids
Initially a dosage of 25μg/ml was tested for the fatty acids based upon or previous observations regarding the effectiveness of this concentration with long-chain fatty acids and their derivatives. 5,6 Additionally, various concentrations (125μM, 500μM, 1250μM and 2500μM) were used in dose-response experiments to determine an inhibitory concentration at 80% killing (IC80). SCFAs and MCFAs were prepared in ethanol stock solutions, and antimicrobial activity was tested against the oral microorganisms by adding 5 μl of the fatty acid solutions to each well of a 96-well plate containing 200 μl of TSBYE medium and a 10% bacterial inoculum from an overnight culture. The plates were then incubated under appropriate growth conditions for the specific microorganism for approximately 16 hours. After overnight incubation, 3 μl of the culture solution was diluted 105 times and plated onto blood agar plates (Remel®). The plates were again incubated under specific environmental conditions for each microorganism for 24~48 hours, at which time colony forming units (CFU) were determined.
3. Results and Discussion
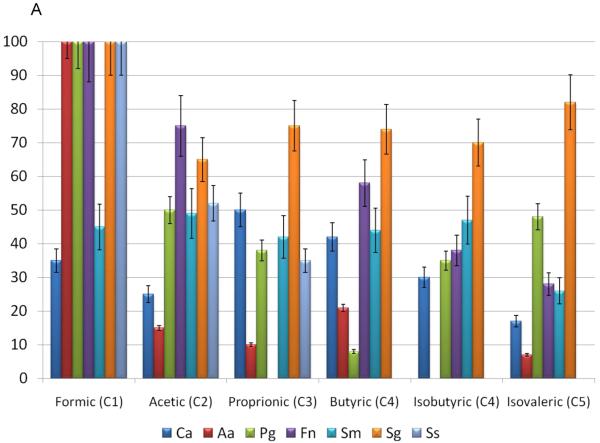
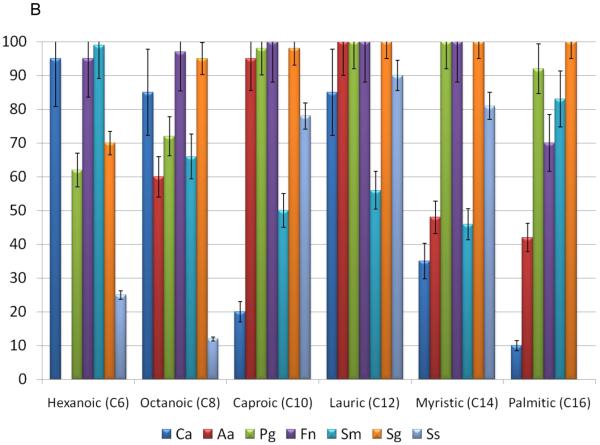
The effect of the various sized fatty acids at 25μg/ml, on the survival of various oral bacteria and C. albicans is depicted in Figure 1A & B. The SCFAs and LCFAs had limited bioactivity against the oral fungal species. .However, the MCFAs, hexanoic and octanoic acids, had significant anti-Candida activity, as did the LCFAs, lauric acid. The effect of the fatty acids on the viability of Gram-negative oral bacteria is depicted in Figure 1A & B. Generally, P. gingivalis and A. actinomycetemcomitans were resistant to most of the SCFA and MCFA. This might be expected for P. gingivalis, due to its robust production of butyric acid as a major end-product and noted resistance to the effects of the SCFA. 16-18 Interestingly, in a similar fashion A. actinomycetemcomitans produces both LCFAs that were tested and is quite resistant to each of these. In contrast, other Gram-negative bacteria were effectively killed by myristic and palmitic acid. Of the Gram-negative species examined F. nucleatum appeared to be the most generally susceptible to killing by a range of fatty acids. Interestingly, F. nucleatum produces butyric, isovaleric, and propionic acids, which demonstrated the least effective killing among the fatty acids tested. These findings are consistent with these types of fatty acids providing a competitive advantage for the range of bacteria in complex oral biofilms, and support the consideration of a strategy to use combinations of fatty acids as a potential therapeutic adjunct.
Figure 1.
Effects of small-, medium-, and long-chain fatty acids on the various oral microorganisms. A concentration 25 μg/mL of small-chain fatty acids (formic acid, acetic acid, butyric acid) and medium-chain fatty acids (capric acid, myristic acid, (long-chain fatty acids (lauric acid and octanoic acid) were used. The percent inhibition is based upon the ratio of CFU at 24 hr between triplicate plates treated with small-, medium-, and long- chain fatty acids and the negative control. (A) Effects of small-chain fatty acids on the growth of gram-positive and gram-negative oral microorganisms, which include Ca (Candida albicans), Aa (A. actinomycetemcomitans), Pg (P. gingivalis), Fn (F. nucleatum), Sm (S. mutans), Sg (S. gordonii), Ss (S sanguis). (B) Effects of medium-, and long-chain fatty acids on the growth of gram-positive and gram-negative oral microorganisms
We examined 3 different oral streptococcal species to better understand the specificity or promiscuity of fatty acid effects within a genus, as well as the importance of these species to both supra- and subgingival biofilms related to health and disease. These fatty acids were minimally effective against S. mutans, except hexanoic and palmitic acids. Interestingly, formic acid was very inhibitory for each of the other streptococcal species, with capric, aluric and myristic acids also toxic for S. gordonii and S. sanguinis. S. gordonii was generally the most susceptible to killing by the fatty acids (Figure 1A & B) with C8 to C16 fatty acids uniformly able to effectively kill this species. As S. mutans is a major acidogenic and aciduric microorganism in the oral cavity, 19 it is consistent that this species would be relatively resistant to the effects of these acids.
To further confirm the bioactivity, different doses of the short-chain, mid-chain and long-chain fatty acids were used and compared for antibacterial activity against gram-positive oral bacteria. Short-chain fatty acids C2-C5 exhibited minimal antimicrobial activity for this range of bacteria as estimated by the inhibitory concentration at 80% (IC80) (Table 1). Among the MCFA, the IC80 decreased with increasing size of the fatty acid. However, the variation in susceptibility among the oral streptococci would suggest the potential for a range of molecular mechanisms by which these fatty acids express their antibacterial activity. As an example, there appears to be a corresponding bioactivity increase as the carbon chain increases for MCFAs and LCFAs. However, the single carbon fatty acid, formic acid, had the strongest antibacterial activity against the range of oral bacteria. Interestingly, formic acid has been used extensively in animal feeds and the food industry for its strong antibacterial functions, 20, 21, suggesting a potential use in managing oral infections.
Table 1.
Inhibitory concentration at 80% (IC80) effectiveness for fatty acids
| Fatty Acid | Microorganism (IC80) | |||||
|---|---|---|---|---|---|---|
| Aa | Pg | Fn | Sm | Sg | Ss | |
| Formic | 1462* | <125 | 1220 | 991 | <125 | 1123 |
| Acetic | >2500 | >2500 | 2377 | 1365 | 1661 | >2500 |
| Proprionic | >2500 | >2500 | >2500 | 1634 | 2463 | >2500 |
| Butyric | >2500 | >2500 | >2500 | 1684 | >2500 | >2500 |
| Isobutyric | >2500 | >2500 | >2500 | 1374 | >2500 | >2500 |
| Isovaleric | >2500 | >2500 | >2500 | >2500 | 2130 | >2500 |
| Hexanoic | >2500 | 1161 | >2500 | 631 | 1151 | >2500 |
| Octanoic | >2500 | 2294 | 1403 | <125 | <125 | >2500 |
| Caproic | 1881 | 766 | <125 | <125 | <125 | <125 |
Inhibitory concentration in μM
In this study, the myristic and palmitic acid needed to be dissolved in ethanol, thus, the short-chain and medium-chain fatty acids were also dissolved in this solvent. While the solubility of the fatty acids could potentially be a major concern if very high concentrations were needed to achieve the anti-bacterial activity, the fatty acids functionally inhibited the bacteria at μg/ml or μM levels. The studies required dilution of the fatty acids from stock solutions; so the ethanol solvent was also diluted over 40-fold in the TSBYE media. Thus, in our experiment, the ethanol in the stock solution was diluted to an approximately 2.5% final concentration, and a similarly diluted ethanol control absent the fatty acids was used as negative control in all experiments with no inhibitory activity. Even in an extended experiment, where a final 25% ethanol solution was used as the negative control, no significant antibacterial activity was observed. However, there is a possibility that the killing/bacteriostatic activity of the fatty acids could be enhanced in the presence of ethanol, which could not be unequivocally eliminated in these studies, but does not undermine the importance of the observations of the effect of these fatty acid solutions.
SCFA have been documented to express a number of other important biological functions. For example, acetic and butyric SCFAs are found in the colon, as well as other parts of the digestive tract. The levels of these SCFA found in the fecal material can inhibit the growth of Salmonella, 22 contributing to the innate protective capacity of the commensal microbiota in the gut. Furthermore, acetic acid was found to inhibit Shigella flexneri multiplication by interfering with glucose metabolism. 23
While SCFAs have numerous reported biological activities, including negative impacts on various host cells, 17, 24, and the ability of molecules such as butyric acid to induced HIV reactivation, 8 there is minimal literature describing antimicrobial activity for the SCFAs and MCFAs against oral bacteria. These data also appear to support that these types of fatty acids secreted by oral bacteria could play an important role in the oral bacteria ecology and biofilm formation by influencing the growth of competitor microorganisms. The bacterial ecology that creates biofilms at sites of periodontital lesions includes increases in the total numbers and complexity of species, particularly related to increases in Gram-negative bacteria, in anaerobic species, and in nonfermentative/asaccharolytic species. 17 Thus, within the “foodweb” at these sites, it is possible that individual oral bacteria secrete certain profiles of fatty acids that inhibit the growth of other species and provide the individual a competitive edge to emerge in the pathogenic biofilms. Future studies could be specifically designed to examine the potential biological role of fatty acids in the characteristics of biofilm formation and biofilm dynamics.
Acknowledgements
This work was supported by grant R41DE17265-01 from the NIH/NIDCR. We thank Molini Patel and Susan Wofford for their technical contribution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
No conflict of interest.
REFERENCES
- 1.Raffaelli L, Serini S, Piccioni E, Manicone PF, Berardi D, Perfetti G, Calviello G. N-3 polyunsaturated fatty acid effect in periodontal disease: state of art and possible mechanisms involved. Int J Immunopathol Pharmacol. 2008;21:261–6. doi: 10.1177/039463200802100202. [DOI] [PubMed] [Google Scholar]
- 2.Campan P, Planchand PO, Duran D. Pilot study on n-3 polyunsaturated fatty acids in the treatment of human experimental gingivitis. J Clin Periodontol. 1997;24:907–3. doi: 10.1111/j.1600-051x.1997.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 3.Kesavalu L, Bakthavatchalu V, Rahman MM, Su J, Raghu B, Dawson D, Fernandes G, Ebersole JL. Omega-3 fatty acid regulates inflammatory cytokine/mediator messenger RNA expression in Porphyromonas gingivalis-induced experimental periodontal disease. Oral Microbiol Immunol. 2007;22:232–9. doi: 10.1111/j.1399-302X.2007.00346.x. [DOI] [PubMed] [Google Scholar]
- 4.Bendyk A, Marino V, Zilm PS, Howe P, Bartold PM. Effect of dietary omega-3 polyunsaturated fatty acids on experimental periodontitis in the mouse. J Periodontal Res. 2009;44:211–6. doi: 10.1111/j.1600-0765.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- 5.Huang CB, George B, Ebersole JL. Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Arch Oral Biol. 2010;55:555–0. doi: 10.1016/j.archoralbio.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang CB, Ebersole JL. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol Oral Microbiol. 2010;25(1):75–0. doi: 10.1111/j.2041-1014.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 7.Imai K, Ochiai K, Okamoto T. Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J Immunol. 2009;182:3688–95. doi: 10.4049/jimmunol.0802906. [DOI] [PubMed] [Google Scholar]
- 8.Kurita-Ochiai T, Seto S, Suzuki N, Yamamoto M, Otsuka K, Abe K, Ochiai K. Butyric acid induces apoptosis in inflamed fibroblasts. J Dent Res. 2008;87:51–5. doi: 10.1177/154405910808700108. [DOI] [PubMed] [Google Scholar]
- 9.Braunthal Sabina, Holt SC, Tanner ACR, Socransky SS. Cellular fatty acid composition of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. Journal of Clinical Microbiology. 2005;187:625–630. doi: 10.1128/jcm.11.6.625-630.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreth J, Merritt J, Shi W, Qi F. Competition and Coexistence between Streptococcus mutans and Streptococcus sanguinis in the Dental Biofilm. Journal of Bacteriology. 2005;187:7193–03. doi: 10.1128/JB.187.21.7193-7203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frias J, Olle E, Alsina M. Periodontal pathogens produce quorum sensing signal molecules. Infect Immun. 2001;69:3431–3434. doi: 10.1128/IAI.69.5.3431-3434.2001. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillman JD, Shivers M. Interaction between wild-type,mutant and revertant forms of the bacterium Streptococcus sanguis and the bacterium Actinobacillus actinomycetemcomitans in vitro and in the gnotobiotic rat. Arch Oral Biol. 1988;33:395–401. doi: 10.1016/0003-9969(88)90196-3. [DOI] [PubMed] [Google Scholar]
- 13.Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ., Jr. Communication among oral bacteria. Microbiol Mol Biol Rev. 2002;66:486–505. doi: 10.1128/MMBR.66.3.486-505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whittaker CJ, Klier CM, Kolenbrander PE. Mechanisms of adhesion by oral bacteria. Ann Rev Microbiol. 1996;50:513–552. doi: 10.1146/annurev.micro.50.1.513. 1996. [DOI] [PubMed] [Google Scholar]
- 15.Hojo KS, Nagaoka T, Ohshima, Maeda N. Bacterial Interactions in Dental Biofilm Development. J Dent Res. 2009;88:982–990. doi: 10.1177/0022034509346811. [DOI] [PubMed] [Google Scholar]
- 16.Kurita-Ochiai T, Seto S, Suzuki N, Yamamoto M, Otsuka K, Abe K, Ochiai K. Butyric acid induces apoptosis in inflamed fibroblasts. J Dent Res. 2008;87:51–55. doi: 10.1177/154405910808700108. [DOI] [PubMed] [Google Scholar]
- 17.Morris TL, Arnold RR, Webster-Cryiaque J. Signaling cascades triggered by bacterial metabolic end products. Journal of Virology. 2007;81:6032–6042. doi: 10.1128/JVI.02504-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwardsson S. Characteristics of caries-inducing human streptococci resembling Streptococcus mutans. Arch Oral Biol. 1968;13:637–646. doi: 10.1016/0003-9969(68)90142-8. [DOI] [PubMed] [Google Scholar]
- 19.Selwet M. Effect of organic acids on numbers of yeasts and mould fungi and aerobic stability in the silage of corn. Pol J Vet Sci. 2008;11:119–123. [PubMed] [Google Scholar]
- 20.Eisemann JH, van Heugten E. Response of pigs to dietary inclusion of formic acid and ammonium formate. J Anim Sci. 2007;85:1530–1539. doi: 10.2527/jas.2006-464. [DOI] [PubMed] [Google Scholar]
- 21.Bohnhoff M, Miller CP, Martin WR. Resistance of the mouse’s intestinal tract to experimental Salmonella infection. I. Factors which interfere with the initiation of infection by oral inoculation. J Exp Med. 1964;120:805–16. doi: 10.1084/jem.120.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baskett RC, Hentges DJ. Shigella flexneri inhibition by acetic acid. Infect Immun. 1973;8:91–97. doi: 10.1128/iai.8.1.91-97.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niederman R, Zhang J, Kashket S. Short-chain carboxylic-acid-stimulated, PMN-mediated gingival inflammation. Crit Rev Oral Biol Med. 1997;8:269–290. doi: 10.1177/10454411970080030301. [DOI] [PubMed] [Google Scholar]
- 24.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]