Abstract
Gut-associated dendritic cells (DC) synthesize all-trans retinoic acid (RA), which is required for inducing gut-tropic lymphocytes. Gut-associated DC from MyD88-/- mice, which lack most TLR signals, expressed low levels of retinal dehydrogenases (Raldh, critical enzymes for RA biosynthesis) and were significantly impaired in their capacity to induce gut-homing T cells. Pre-treatment of extra-intestinal DC with a TLR1/2 agonist was sufficient to induce Raldh and to confer these DC with the capacity to induce gut-homing lymphocytes via a mechanism dependent on MyD88 and JNK/MAPK. Moreover, gut-associated DC from TLR2-/- mice or from mice in which JNK was pharmacologically blocked were impaired in their education to imprint gut-homing T cells, which correlated with a decreased induction of gut-tropic T cells in TLR2-/- mice upon immunization. Thus, MyD88-dependent TLR2 signals are necessary and sufficient to educate DC with gut-specific imprinting properties and contribute in vivo to the generation of gut-tropic T cells.
Introduction
In addition to their well-known role as antigen-presenting cells, DC can modulate immune responses in a tissue-specific manner (1). DC from Peyer's patches (PP-DC) promote the induction of Th2 T cell responses and IgA immunoglobulin class-switching (1). In addition, DC from Peyer's patches, mesenteric lymph nodes, and small intestine lamina propria (gut-associated DC), but not DC from extra-intestinal sites, induce the expression of the gut-homing receptors integrin α4β7 and chemokine receptor CCR9 on mouse and human T and B cells upon activation (2-5). The selective capacity of gut-associated DC to induce gut-tropic lymphocytes is explained by their ability to metabolize vitamin A (retinol) into RA (3, 5, 6). RA is both necessary and sufficient to imprint gut-homing lymphocytes and it also potentiates the induction of gut-homing Foxp3+ regulatory T cells (TREG) while reciprocally inhibiting the development of Th17 cells (7-9). Gut-associated DC, but not peripheral extra-intestinal DC, express high levels of Raldh, which are key enzymes for RA biosynthesis. However, it is unknown how these enzymes are specifically induced in gut-associated DC. We reasoned that bone marrow-derived uncommitted DC (or their precursors) enter the intestine from the blood and might then acquire their imprinting properties in response to local differentiation signal(s) that are present in the gut mucosa. Among those environmental cues, the gut is highly exposed to pathogen-associated molecular patterns derived from the intestinal microbiota, which are recognized by pathogen recognition receptors, the best characterized of which are Toll-like receptors (TLR) (10). Since DC can express several combinations of TLR (11) and stimulation via TLR can differentially modulate some functional properties of DC (12), we hypothesized that TLR-mediated signals are critical for the acquisition of tissue-specific functional properties by gut-associated DC, including their capacity to produce RA, to imprint gut-homing lymphocytes.
Materials and Methods
Mice
OT-IxRAG2-/-, P14xTCRα-/-, C57BL/6, and C57BL/6/Thy1.1+ were obtained from Taconic (Germantown, NY). TLR2-/-, JNK1-/- and JNK2-/- mice were obtained from Jackson Laboratories (Bar Harbor, ME). TLR6-/- mice were provided by Dr. Adam Lacy-Hulbert (Massachusetts General Hospital, Boston, MA). MyD88-/- TRIF-/- and TLR4-/- mice (13) were provided by Drs. Cathryn Nagler, Nir Hacohen and Hans-Christian Reinecker (Massachusetts General Hospital, Boston, MA). Nalp3-/- mice were provided by Dr. Jurg Tschopp (University of Lausanne, Switzerland). GM-CSF-/- mice (14) were provided by Drs. Glenn Dranoff (Harvard Medical School, Boston, MA) and Bruce C. Trapnell (University of Cincinnati, Cincinnati, OH). DR5-luciferase mice (15) were provided by Dr. Rune Blomhoff (Cgene AS, Oslo, Norway). Mice were maintained in specific pathogen free conditions and used Massachusetts in accordance with the guidelines from the Subcommittee on Research Animal Care at Massachusetts General Hospital and Harvard Medical School.
DC/T cell isolation and co-culture
Mice (8-16 weeks old) were injected subcutaneously with 5-10×106 B16 melanoma cells secreting Flt3-L as described (2). After 12 to 14 days the mice were sacrificed and PLN, MLN and PP were digested using 250 μg/ml Liberase CI (Roche Diagnostics, Indianapolis, In) plus 50 μg/ml DNAse-I (Roche) in IMDM (no additives) for 40 min at 37°C with mild agitation. For lamina propria (LP) DC isolation (16) the small bowels were cut into 0.5 cm pieces, placed in a 50 ml tube and shaken at 250 rpm for 20 min at 37°C in HBSS + 5% low-endotoxin FBS (Gibco, Invitrogen) + 2 mM EDTA. Cell suspensions were passed through a strainer and the remaining intestinal tissue was washed, placed in a 50 ml tube and enzymatically digested as described above. Cell separations were performed at 4°C in PBS + 2 mM EDTA + 2% low-endotoxin FBS. DC were immunomagnetically isolated by negative selection as described (5). Negatively selected DC (85-90% CD11c+) were resuspended at 107/ml and pulsed for 2 hours at 37°C with 100 nM LCMVgp33-41 or SIINFEKL peptides in IMDM + 10% low-endotoxin FBS + 50 μM β-mercaptoethanol plus standard supplements (complete IMDM). For some experiments, negatively selected DC were sorted into CD11c+CD103+ and CD11c+CD103- fractions (FACS Vantage, BD Biosciences, >95% purity for each DC subset). Naïve P14xTCRα-/- or OT-IxRAG2-/- CD8 T cells were purified from splenocytes after RBC lysis in ACK buffer by negative selection as described (2). For CFSE labeling T cells were resuspended at 107 cells/ml in DMEM + 1% FBS + 20 mM Hepes, incubated with 2.5 μM CFSE for 20 min at 37°C and then washed using an FBS gradient. 1×105 CFSE-labeled naïve CD8 T cells were co-cultured with peptide-pulsed DC in a 1:1 ratio in flat bottom 96-well plates (Falcon, BD Biosciences).
Bone marrow chimeras
C57BL/6 wild type or MyD88-/- mice were irradiated with 550 cGy plus 500 cGy, with 3 h between doses, and 5 × 106 total bone marrow (BM) cells from C57BL/6 wild type or MyD88-/- mice were injected intravenously 3 h later, as described (17). Mice were fed with antibiotic-supplemented food (TestDiet) and were given free access to water. Chimeric mice were used for experiments 8 wk after BM transplantation. For mixed BM chimeras, irradiated wild type mice were reconstituted with a 1:1 ratio of BM from wild type or MyD88-/- mice plus BM from CD11c-DTR mice, in which DC can be specifically depleted by diphtheria toxin (DT) treatment (18). By using this strategy we could selectively deplete wild type DC, without affecting other wild type BM-derived cells or MyD88-/- DC. For DC depletion, mixed BM chimeras were inoculated i.p. with DT (Sigma-Aldrich) 8 ng/g every other day for 4 times.
Immunization experiments
5 × 106 CFSE-labeled OT-IxRAG2-/- T-cells were adoptively transferred into wild type or MyD88-/- mice. 24 hours later recipients were injected i.p. with 10 mg ovalbumin (OVA, grade VI; Sigma-Aldrich) (19) plus 1 mg Alum (resuspended in 500 μl PBS) (20). In some experiments mice were immunized with 30 mg OVA via oral gavage. 3-4 days later the mice were sacrificed and single cell suspensions from spleen, PLN, MLN, IEL and LP were collected and stained for CCR9 or α4β7. The samples were analyzed in a FACScalibur flow cytometer by gating on viable CD8+ and CFSEint cells.
Spleen-DC conditioning
Spleen DC were isolated from Flt3-L-treated C57BL/6 mice as described above. After negative selection, DC were positively selected with anti-CD11c magnetic beads (Miltenyi Biotec). DC (>98% CD11c+) were pre-treated for 24 h with or without different TLR ligands (InvivoGene TLR ligands kits: tlrl-kit1m2 or tlrl-kit2), washed, pulsed with antigenic peptide and used for co-culture with naïve T and B cells, mRNA extraction, aldefluor assay or luciferase assay (Spleen-DC from DR5-luciferase mice). In some experiments Spleen-DC were treated with 1 μg/ml Pam3CSK4 (TLR1/2 agonist) plus/minus pharmacological inhibitors for P38/MAPK (10 μM SB203580), ERK1/2/MAPK (10 μM FR180204), JNK/MAPK (50 μM SP600125), NF-κB (50 μM SN50) (21) or 1 μM LE540 RAR antagonist. In some experiments spleen DC were pre-incubated for 24 hr with live E. Coli (K12 strain) in a 1:10 DC:bacteria ratio, washed, pulsed with antigenic peptide and co-cultured with T cells. In other experiments spleen DC were pre-treated for 24 h with 300 μg/ml alum (77161, Thermo) and/or 500 μM ATP (FLAAS, Sigma), as described (22). For some experiments DC were pre-treated for 24 h with Pam3CSK4 in FBS-free media (X-vivo15 ™, Lonza) plus/minus 50 nM retinol (R7632, Sigma-Aldrich, St. Louis, MO), as described (23).
DC immunization
Spleen-DC were treated with or without 1 μg/ml Pam3CSK4 for 24 hours, washed 3 times with PBS and pulsed with LCMVgp33-41 peptide. 1×106 DC were injected i.v. into Thy1.1+ mice that were adoptively transferred with 4×106 naïve CFSE-labeled P14xTCRα-/- CD8 T cells (Thy1.2+). 4 days post-DC injection the mice were euthanized and cell suspensions from the spleen, MLN, and PLN were analyzed by flow cytometry by gating on viable Thy1.2+CD8+ T cells.
In vivo JNK inhibition
Mice were treated with 50 μg/g SP600125 (Calbiochem) i.p. once a day for 5 days, as described (24). After that, the mice were euthanized and analyzed for their Raldh activity (aldefluor staining) in MLN DC.
Statistical analysis
Unless specified otherwise, data are presented as mean ± SEM and were analyzed using GraphPad Prism Software 5.0c. Significance was set at p < 0.05
Results
MyD88 is required for educating gut-associated DC with gut-homing imprinting capacity and for in vivo generation of gut-tropic T cells
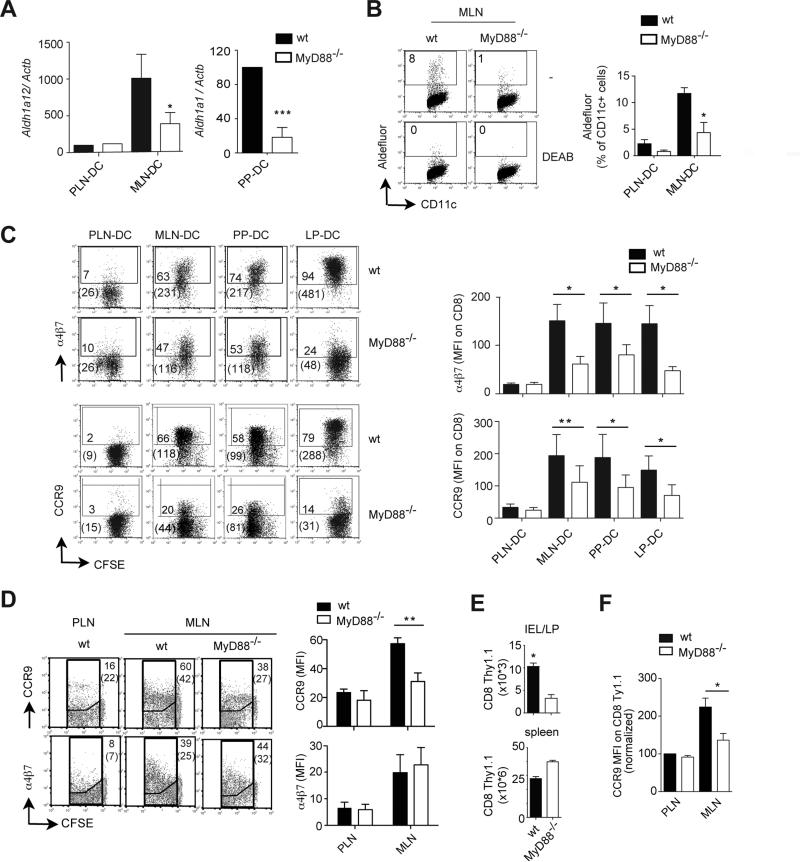
In agreement with a major role of MyD88-dependent signals in gut-associated DC education, gut-associated DC isolated from mice lacking MyD88, which is an essential intracellular signaling adaptor for most TLR signals(10), showed an impaired RA-synthesizing capacity, as determined by their decreased levels of Aldh1a2 mRNA (encoding Raldh2) and Aldh1a1 mRNA (encoding Raldh1) in DC from mesenteric lymph nodes (MLN-DC) and Peyer's patches (PP-DC), respectively (Figure 1A). As a functional readout for Raldh enzymatic activity we used the Aldefluor® assay, which is based on a fluorescent substrate (bodipy-aminoacetaldehyde) that upon being metabolized by Raldh enzymes accumulates in the cytoplasm of viable cells, making them fluorescent (25). In agreement with a decreased expression of Aldh1a2 mRNA, MyD88-/- MLN-DC exhibited lower Raldh activity as compared to wild type MLN-DC (Figure 1B). Importantly, consistent with a reduced ability to synthesize RA, MyD88-/- gut-associated DC were significantly impaired in inducing the gut-homing receptors α4β7 and CCR9 on activated TCR transgenic CD8 T cells (Figure 1C). This was not due to a defective T cell activation, since MyD88-/- DC induced a similar degree of 1B11/CD43 expression (effector CD8 T cell marker (26)) and T cell proliferation (CFSE dilution) as compared to wild type DC (Figure S1A).
Figure 1. MyD88-dependent signals are necessary to confer gut-associated DC with gut-homing imprinting capacity and for in vivo generation of gut-homing T cells.
(A) TaqMan qPCR for Aldh1a1 and Aldh1a2 mRNA expression in DC (encoding for Raldh1 and Raldh2, respectively) (n=4). Results were normalized respect to Actb mRNA (encoding β-actin) (B) Raldh activity (aldefluor assay) in DC either in the absence or the presence of the Raldh inhibitor DEAB (n=4). (C) Naïve CFSE-labeled P14xTCRα-/- or OT-IxRAG2-/- CD8 T cells were activated with peptide-pulsed DC from peripheral lymph nodes (PLN-DC), mesenteric lymph nodes (MLN-DC), Peyer's patches (PP-DC) or small intestine lamina propria (LP-DC) from wild type or MyD88-/- mice. After 4-5 days T cells were analyzed for α4β7 and CCR9 expression by flow cytometry (n=6). Numbers indicate percentage of positive cells and in parenthesis the mean fluorescence intensity (MFI) of total cells. (D-E) Wild type CFSE-labeled OT-1xRAG2-/- CD8 T cells (Thy1.1+) were adoptively transferred into wild type or MyD88-/- mice (Thy1.2+), which were immunized i.p. with ovalbumin plus Alum. 4 days post-immunization the mice were analyzed for (D) the expression of α4β7 and CCR9 on transferred activated CFSELow CD8 T cells in PLN, MLN (n=4) and (E) the numbers of Thy1.1+ CD8 T cells recovered from the small bowel lamina propria (LP) and intraepithelial lymphocyte compartment (IEL) or the spleen (n=6-8). (F) Same as in (D), but the mice were immunized orally with ovalbumin (n=4). Mean ± SEM. * p<0.05, **p<0.01, ***p<0.001
It has been shown that the gut-homing imprinting capacity is restricted to the CD103+ DC subset (27), and it remained possible that CD103+ gut-associated DC were specifically reduced in MyD88-/- mice, hence accounting for their reduced gut-homing inducing capacity. However, in agreement with a previous report showing that MyD88-/- mice do not show significant changes in DC subsets or in the degree of DC maturation in MLN (28), CD103+ gut-associated DC were only slightly decreased in MyD88-/- mice (Figure S1B). Moreover, sorted CD103+ MLN-DC from MyD88-/- mice expressed lower levels of Aldh1a2 mRNA (Figure S1C), exhibited decreased Raldh activity (aldefluor staining) (Figure S1D) and induced lower levels of CCR9 on T cells (Figure S1E) as compared to wild type CD103+ MLN-DC. Therefore, the decreased gut-homing imprinting capacity observed in MyD88-/- gut-associated DC is likely explained by an intrinsic functional impairment in RA production in DC rather than by a reduction in the proportion of CD103+ DC.
In order to address the possibility that some compensatory mechanisms might offset the requirement of MyD88 signaling for the generation of gut-homing T cells in vivo, we adoptively transferred OT-1/RAG2-/- TCR-transgenic CD8 T cells (specific for an ovalbumin epitope) into wild type or MyD88-/-mice and immunized them intraperitoneally with ovalbumin plus an aluminum-based adjuvant (Alum), which does not require MyD88-dependent signals for its adjuvant effect (29). Although α4β7 was similarly induced on T cells activated in the MLN of wild type or MyD88-/- mice, the proportion of CCR9+ T cells was significantly reduced in MyD88-deficient mice (Figure 1D), without a decrease in T cell proliferation (Figure S1F). Moreover, MyD88-/- exhibited a reduced number of recently activated T cells in the small bowel LP, whereas their number in the spleen was slightly increased in these mice (Figure 1E). Although lower numbers of activated T cells were recruited to the small bowel of MyD88-/-as compared to wild type mice, T cells that homed to the small bowel LP/IEL in MyD88-/- mice were selected for their high expression of CCR9 (Figure S1G). Of note, the proportion of CCR9+ T cells was also reduced in MLN from MyD88-/- mice immunized via oral gavage (Figure 1F). Thus, MyD88-dependent signals are required in vivo for educating gut-associated DC with RA-synthesizing and gut-homing imprinting capacity and are also necessary for the efficient generation of gut-tropic T cells upon immunization.
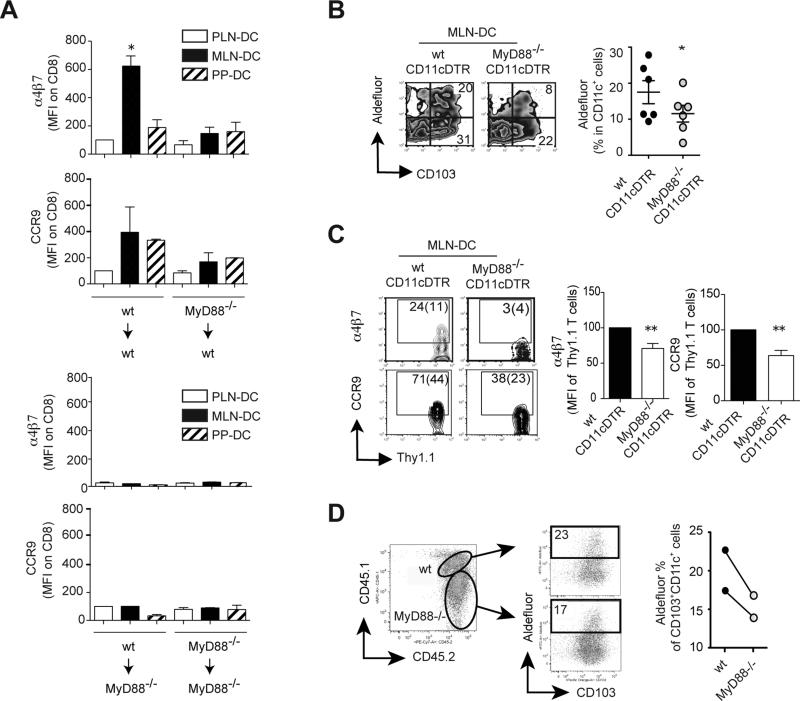
Next, we asked whether gut-specific DC education requires MyD88-dependent signals acting on bone marrow (BM)-derived cells. Our experiments using BM chimeras showed that gut-homing receptors were induced on T cells only when MLN-DC were isolated from mice in which both donor BM and recipient irradiated hosts were wild type (Figure 2A and Figure S2A). Moreover, consistent with an essential role of RA synthesis in gut-homing induction by MLN-DC, their imprinting capacity correlated with Aldh1a2 mRNA expression (Figure S2B) and Raldh activity (Figure S2C). These experiments suggest that gut-specific DC education depends on MyD88-dependent signals acting on BM-derived cells as well as in the radioresistant compartment.
Figure 2. Gut-associated DC education requires MyD88 expression in bone marrow (BM)-derived cells and radioresistant compartment.
(A) α4β7 and CCR9 staining on CFSE-labeled P14xTCRα-/-CD8 T cells activated by CD11c+ DC from BM chimeras in which BM from either wild type or MyD88-/- donors was transplanted into wild type or MyD88-/- recipient mice (n=3). (B-D) Mixed BM chimeras were generated by transplanting irradiated wild type mice with a 1:1 ratio of BM from MyD88-/- mice plus BM from CD11c-DTR mice. (B, C) 10 weeks later the mice were treated with diphtheria toxin (DT), which depleted CD11c+ DC derived from CD11c-DTR BM. (B) MLN-DC were analyzed for their Raldh activity (n=7). (C) Alternatively, the chimeric mice were adoptively transferred with OT1 CD8 T cells and then immunized orally with ovalbumin. Four days later the activated CD8 T cells were analyzed for their expression of α4β7 and CCR9 in MLN (n=5). (D) MyD88-/- and MyD88-sufficient CD103+ MLN-DC from chimeric mice (without DT treatment) were analyzed for their Raldh activity (n=2). Mean ± SEM. * p<0.05
In order to dissect whether DC need to express MyD88 to be educated in vivo or if other BM-derived cells can compensate for the lack of MyD88 in DC, we made mixed bone marrow (BM) chimeras by reconstituting irradiated wild type (CD45.1+) mice with a 1:1 ratio of BM from MyD88-/- mice (CD45.2+) plus BM from CD11c-DTR mice (CD45.1+CD45.2+), in which DC can be specifically depleted by diphtheria toxin (DT) treatment (18). By using this strategy we could selectively deplete wild type DC without affecting other wild type BM-derived cells or MyD88-/- DC. Even though the remaining BM-derived cells from CD11c-DTR BM express MyD88, our results indicate that they were not sufficient to rescue the education of MyD88-/- MLN-DC, as the latter exhibited lower Raldh activity than MLN-DC from control mixed BM chimeras in which wild type BM plus CD11c-DTR BM was transplanted into irradiated recipients (and also treated with DT) (Figure 2B). In addition, the MyD88-/-/CD11c-DTR mixed BM chimeras exhibited a decreased in vivo induction of α4β7+ and CCR9+ T cells upon oral immunization as compared to control mixed BM chimeras (Figure 2C). Finally, we took advantage of the congenic markers to distinguish between wild type and MyD88-/- DC in the same mouse. We observed that MyD88-/- CD103+ MLN-DC have lower Raldh activity when compared side by side with their MLN counterpart in the same mouse (Figure 2D). Thus, our data suggest that DC need to express MyD88 in order to acquire optimal RA-synthesizing capacity and gut-homing imprinting ability and that the MyD88 deficiency cannot be recued “in trans” by other MyD88-sufficient BM-derived cells (including DC).
TLR1/2 stimulation is sufficient to educate extra-intestinal DC with gut-homing imprinting capacity
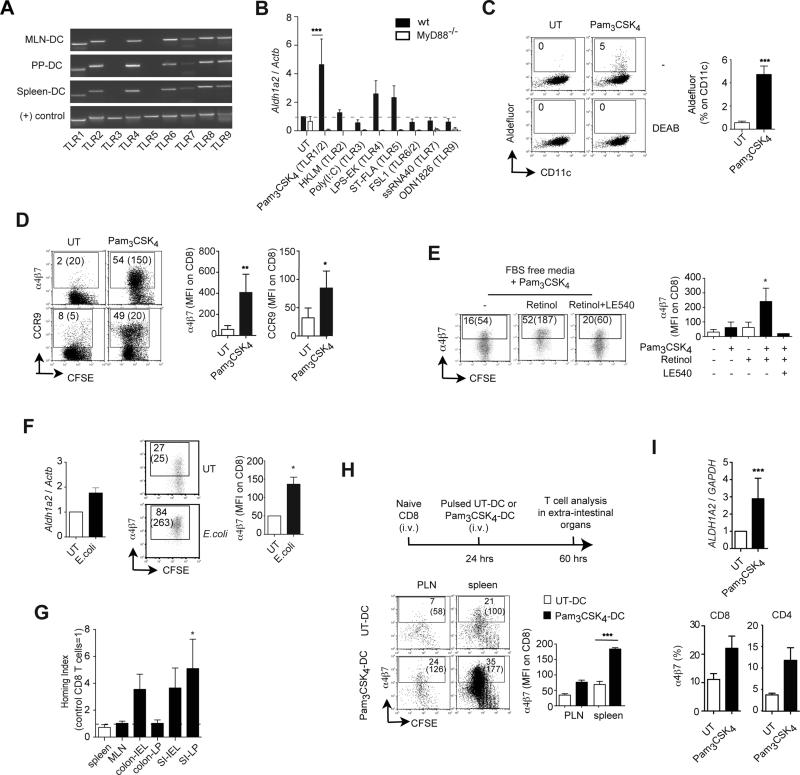
Having established that MyD88-dependent signals are critical for gut-associated DC education in vivo we asked whether stimulation via MyD88-dependent TLR might be sufficient to educate extra-intestinal DC with gut-specific imprinting properties. Similar to MLN-DC and PP-DC, Spleen-DC expressed mRNA for most TLR, except TLR3 and TLR5 (Figure 3A). To define the role of different TLR in educated extra-intestinal DC, Spleen-DC were pre-incubated with different TLR agonists and then used to activate naïve CD8 T cells. Only Spleen-DC pre-incubated with the TLR1/2 agonist Pam3CSK4 (triacylated lipopeptide), but not with other TLR agonists, consistently upregulated Aldh1a2 mRNA (Figure 3B and Figure S3A). Accordingly, Pam3CSK4-stimulated Spleen-DC also exhibited Raldh activity (Figure 3C) and acquired the capacity to induce α4β7 and CCR9 on CD8 T cells (Figure 3D and Figure S3B) and on CD4 T cells (Figure S3C). Since Spleen-DC expressed mRNA for most TLR, absence of specific TLR could only partially explain their lack of education by other TLR agonists. In fact, although Spleen-DC express TLR4 (Figure S3A and refs. (30-32)), the TLR4 agonist lipopolysaccharide (LPS) was not sufficient to induce Aldh1a2 mRNA in these DC (Figure 4A), which is in agreement with previous reports (25, 32). Moreover, MLN-DC from TLR4-/- mice were not impaired in inducing gut-homing receptors (Figure S3E). Thus, TLR4 stimulation is neither sufficient in vitro nor necessary in vivo for DC education.
Figure 3. TLR1/2 stimulation is sufficient to confer extra-intestinal DC with gut-homing imprinting capacity.
(A) TLR mRNA expression in freshly isolated DC. Representative of two experiments with similar results. (B) Aldh1a2 mRNA expression in wild type or MyD88-/- Spleen-DC untreated (UT) or pre-treated with the indicated TLR ligands (n=3-6). (C) Raldh activity in DC. Numbers indicate percentage of aldefluor positive cells in the indicated gate (n=5). (D) α4β7 and CCR9 expression on CD8 T cells activated with Spleen-DC untreated (UT) or pre-treated with Pam3CSK4 (1 μg/ml) (n=6). Flow cytometry plots show percentage of positive cells and in parenthesis MFI of total cells. (E) α4β7 expression on T cells activated with Spleen-DC UT or pre-treated with Pam3CSK4 in FBS-free media with or without 50 nM retinol or 1 μM LE540 (RAR antagonist) (n=3). (F) Spleen-DC UT or pre-incubated for 24h with E. coli (K12 strain) and then analyzed for their expression of Aldh1a2 mRNA (n=2) or used to activate naïve CD8 T cells. T cells were analyzed after 4 days for their expression of α4β7 (n=3). (G) P14xTCRα-/- CD8 T cells activated with Spleen-DC UT or pre-treated with Pam3CSK4 were labeled with TRITC or CFSE, respectively, mixed in a 1:1 ratio and injected i.v. into congenic Thy-1.1+ recipient mice. After 18h the ratio of CFSE+ to TRITC+ cells was analyzed in the indicated tissues. The homing indices (HI) were calculated as the ratio of CFSE+/TRITC+ in each tissue divided by the input ratio (n= 4). Statistics were calculated using a one sample t-test versus a HI=1 (equal migration). (H) Thy-1.1+ mice were adoptively transferred with naïve CFSE-labeled P14xTCRα-/-CD8 T cells and then immunized i.v. with LCMVgp33-41-pulsed Spleen-DC either UT or pre-treated with Pam3CSK4. Three days later α4β7 expression was analyzed on Thy1.2+ CD8 T cells in PLN, MLN, and spleen (n=3). (I) Human monocyte-derived DC (moDC) were UT or pre-treated with Pam3CSK4 and then analyzed for their expression of ALDH1A2 mRNA (n=4) or co-cultured with human T cells activated with plate-bound anti-CD3 plus anti-CD28 antibodies. T cells were analyzed after 5 days for their expression of α4β7 and CCR9 (n=3). Mean ± SEM. * p<0.05, ** p<0.01, ***p<0.001
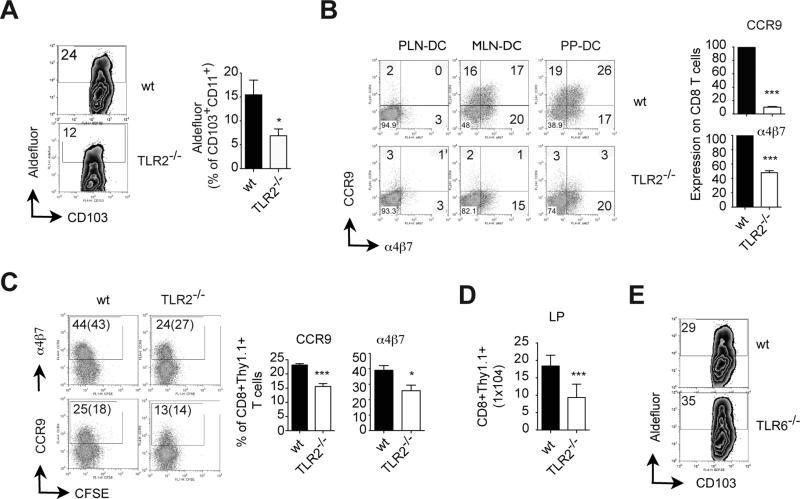
Figure 4. TLR2 is necessary for gut-associated DC education and for in vivo generation of gut-homing T cells.
(A) Raldh activity (aldefluor assay) in CD103+ MLN-DC from wild type or TLR2-/-untreated (non-Flt3L DC expanded) mice (n=8 mice/group). (B) Naïve OT-IxRAG2-/- CD8 T cells were activated with peptide-pulsed PLN-DC, MLN-DC or PP-DC from wild type or TLR2-/- mice. After 4 days T cells were analyzed for α4β7 and CCR9 expression (n=4). Numbers indicate percentage of positive cells. (C-D) Wild type CFSE-labeled OT-1xRAG2-/- CD8 T cells (Thy1.1+) were adoptively transferred into wild type or TLR2-/- mice (Thy1.2+), which were immunized i.p. with ovalbumin plus LPS. (C) Four days post-immunization the mice were analyzed for the expression of α4β7 and CCR9 on activated Thy1.1+ CD8 T cells in MLN (n=5). (D) Numbers of Thy1.1+ CD8 T cells recovered from the small bowel lamina propria (LP) and intraepithelial lymphocyte compartment (IEL) 4 days post-immunization. (E) Raldh activity in CD103+ MLN-DC from wild type or TLR6-/- untreated (non-Flt3L DC expanded) mice (n=2 mice/group). Mean ± SEM. * p<0.05, ***p<0.001
Of note, the preferential induction of Aldh1a2 mRNA by Pam3CSK4 correlated with an enhanced capacity to induce IL-1β mRNA in DC (Figure S3E, F), suggesting that Pam3CSK4 is more potent than other TLR agonists and/or that Spleen-DC exhibit decreased functionality for other TLR. Nonetheless, TLR-stimulated and control Spleen-DC induced a similar degree of T cell activation (Figure S3G), suggesting that Spleen-DC stimulated via different TLR do not differ significantly in their basic antigen-presenting functions as compared to control Spleen-DC.
Consistent with a requirement of MyD88 for TLR1/2 signaling, Pam3CSK4 did not induce Aldh1a2 mRNA in MyD88-/- Spleen-DC (Figure 3A). A similar requirement for DC education was observed in BM-derived DC (BM-DC) when MyD88 was knocked down using shRNA (Figure S3I). In addition, gut-homing imprinting by TLR1/2-educated DC only happened when DC and T cells were co-cultured in FBS-free media (devoid of vitamin A) supplemented with retinol and it was abolished in the presence of LE540, an antagonist of RA receptors of the RAR family (Figure 3E). Moreover, Raldh2 knockdown abrogated gut-homing imprinting capacity by Pam3CSK4-educated DC (Figure S3H). These results indicate that TLR1/2-stimulated DC need to actively metabolize retinol into RA in a Raldh2-dependent manner in order to imprint gut-homing T cells. We also explored the possibility that TLR agonists might act directly on T cells to induce gut-homing receptors. However, none of the TLR agonists tested (including Pam3CSK4) significantly induced or enhanced α4β7 or CCR9 when T cells were activated in the absence of DC (Figure S3I and data not shown).
DC in the intestinal lamina propria can extend projections towards the intestinal lumen (33, 34) and therefore might be directly exposed to TLR agonists from the intestinal microbiota. Therefore, in order to test whether bacteria-associated TLR agonists could also educate DC, we pre-incubated Spleen-DC with viable E. coli (K12 strain), which displays multiple TLR ligands including TLR1/2 agonists (35, 36). In agreement with this possibility, Spleen-DC pre-incubated with E. coli expressed Aldh1a2 mRNA and induced higher levels of α4β7 on T cells as compared to control DC (Figure 3F).
As predicted by their expression of α4β7 and CCR9, T cells activated with Pam3CSK4-educated DC were bona fide gut-homing T cells and migrated significantly more to the small bowel lamina propria as compared to T cells activated with control Spleen-DC (Figure 3G). Moreover, immunization with Pam3CSK4-stimulated DC induced higher levels of α4β7-expressing T cells in vivo as compared to immunization with control Spleen-DC (Figure 3H), suggesting that ex vivo-educated DC could be used to improve gut-associated T cell responses. In fact, despite that RA has been shown to potentiate the differentiation of TREG in vitro (7, 8), Pam3CSK4-educated Spleen-DC did not induce higher levels of Foxp3+ T cells than control Spleen-DC (Figure S3J). The lack of higher Foxp3 induction by Pam3CSK4-treated Spleen-DC might be explained by a parallel increase in the production of IL-6 (data not shown), which has been shown to antagonize TREG induction (37) and therefore might offset the effect of RA on TREG differentiation. Thus, our data indicate that TLR1/2 stimulation might promote the induction of immunogenic rather than tolerogenic DC.
Of note, Pam3CSK4 also induced ALDH1A2 mRNA expression in human monocyte-derived DC and conferred them with gut-homing imprinting potential (Figure 3I), suggesting that our findings in the murine system could be extrapolated, at least in part, to humans.
TLR2 is required for gut-associated DC education and for in vivo generation of gut-tropic T cells
Given that TLR1/2 stimulation was sufficient to confer extra-intestinal DC with gut-homing imprinting capacity ex vivo, we tested whether TLR2 plays a physiological role in gut-associated DC education. In agreement with this possibility, CD103+ MLN-DC from TLR2-/- mice exhibited a lower Raldh activity than their wild type counterparts (Figure 4A), which correlated with a decreased induction of gut-homing T cells by MLN-DC (Figure 4B). Of note, TLR2-/- DC did not show differences in their proportion of classical DC subsets or in their expression of maturation markers as compared to their wild type counterparts (Figure S4A).
In order to determine whether TLR2 is also required for the in vivo generation of gut-tropic T cells, we adoptively transferred OT-1/RAG2-/- CD8 T cells into wild type or TLR2-/- mice and immunized them with ovalbumin plus LPS, as described (19). Consistent with a role of TLR2 in the generation of gut-tropic T cells in vivo, the proportions of α4β7+ and CCR9+ CD8 T cells was significantly decreased in T cells activated in MLN from TLR2-/- mice (Figure 4C and Figure S4B, C). Moreover, although TLR2-/-mice did not show a decrease in total CD8 T cells in the small bowel LP/IEL in the steady state (Figure S4D), they exhibited lower number of recently activated CD8 T cells in the small intestine LP as compared with wild type mice (Figure 4D).
TLR2 needs to heterodimerize with either TLR1 or TLR6 in order to mediate intracellular signaling (35). Since Raldh activity was not impaired in MLN-DC from TLR6-/- mice (Figure 4E), our data suggest that in vivo gut-associated DC education might rely on TLR1/2 rather than on TLR2/6.
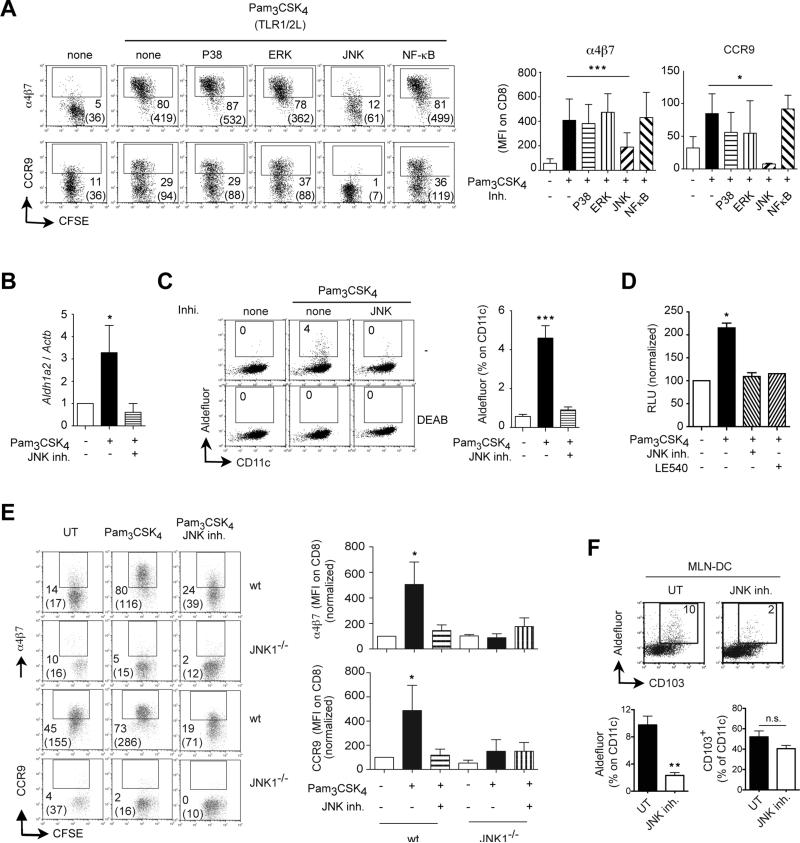
TLR1/2-mediated DC education requires JNK/MAPK signaling
With the aim of obtaining further mechanistic insights on TLR1/2-mediated DC education we explored the role of some canonical signaling pathways in this process. MAP kinases (MAPK) have been involved in TLR-mediated effects on DC (38-40) and we assessed whether these signaling pathways are involved in TLR1/2-mediated DC education. Pharmacological inhibitors of p38/MAPK or ERK/MAPK did not impair TLR1/2-mediated DC education to induce gut-tropic T cells (Figure 5A). Similarly, blocking NF-κB, a well-known downstream signaling complex in TLR signaling (40), did not affect TLR1/2-mediated DC education by Pam3CSK4. However, blocking JNK/MAPK significantly decreased the capacity of TLR1/2-stimulated DC to imprint α4β7 and CCR9 on T cells (Figure 5A). Moreover, JNK inhibition abrogated the induction of Aldh1a2 mRNA (Figure 5B) and Raldh activity (Figure 5C) in Pam3CSK4-treated Spleen-DC, an effect that was mirrored by a blockade of RA activity in Pam3CSK4-stimulated DC, as assessed in Spleen-DC from DR5-luciferase mice in which luciferase is controlled by a promoter with RA response elements (15) (Figure 5D).
Figure 5. Pam3CSK4-mediated DC education requires JNK/MAPK signaling.
(A) Spleen-DC were untreated (UT) or pre-treated for 24 h with 1 μg/ml Pam3CSK4 plus/minus pharmacological inhibitors of p38/MAPK (10 μM SB203580), ERK/MAPK (10 μM FR180204), JNK/MAPK (50 μM SP600125) or NF-kB (50 μM SN50). After that DC were washed, pulsed with peptide and used to activate naïve CFSE-labeled OT-IxRAG2-/- or P14xTCRα-/- CD8 T cells. Four days later T cells were analyzed for their expression of α4β7 and CCR9 (n=6). (B-D) Spleen-DC were UT or pre-treated with Pam3CSK4 plus/minus 50 μM SP600125 (JNK inhibitor) or 1 μM LE540 (RAR antagonist). (B) Aldh1a2 mRNA expression in DC (n=3). (C) Raldh activity (aldefluor staining) in DC (n=3). (D) Luciferase activity in Spleen-DC from DR5-luciferase mice (n=3). Blockade of RA activity using 1 μM LE540 (RAR antagonist) was used as control. (E) Spleen-DC from wild type or JNK1-/- mice were untreated (UT) or pre-treated for 24 h with 1 μg/ml Pam3CSK4 plus/minus 50 μM SP600125. After that DC were washed, pulsed with peptide and used to activate naïve CFSE-labeled OT-IxRAG2-/- CD8 T cells. Four days later T cells were analyzed for their expression of α4β7 and CCR9 (n=3). (F) Wild type mice were orally treated with 50 μg/g SP600125 i.p. once a day for 5 days. After that, CD11c+ MLN-DC were analyzed for CD103 expression and Raldh activity (n=4 mice/group). Mean ± SEM. * p<0.05, ***p<0.001
Consistent with our data using a pharmacological JNK inhibitor, Spleen-DC from JNK1-/- mice were also prevented from being educated by Pam3CSK4 (Figure 5E). By contrast, Pam3CSK4 could efficiently educate JNK2-/- Spleen-DC (Figure S5A), suggesting that JNK1 but not JNK2 is required for Spleen-DC education. However, despite the role of JNK1 in ex vivo Spleen-DC education, MLN-DC from JNK1-/- were not impaired in their Raldh activity or in their gut-homing imprinting capacity (data not shown), suggesting that JNK1 and JNK2 might play redundant roles in vivo. Since JNK1-/-JNK2-/-double deficient mice are embryonic lethal (41), we treated wild type mice with the pan-JNK inhibitor SP600125, which exhibit high specificity for JNK and has been used in vivo (42). In agreement with a physiological role of JNK signaling in gut-associated DC education, mice treated with SP600125 showed a significantly lower Raldh activity (aldefluor staining) in MLN-DC as compared to control mice, without a decrease in the proportion of CD103+ MLN-DC (Figure 5F).
MyD88 is also required for IL-1/IL-18 signaling (13, 43) and the production of mature IL-1or IL-18 requires inflammasome activation (44). Thus, we explored whether treatment with ATP and/or Alum, which activate the inflammasome (45), was sufficient to educate Spleen-DC. However, these inflammasome stimuli were neither sufficient nor enhanced TLR1/2-mediated Spleen-DC education to imprint gut-homing T cells or to produce RA (Figure S5B, C). Moreover, gut-associated DC from mice lacking the Nalp3 inflammasome, which cannot produce mature IL-1 or IL-18 (44), were not impaired in their gut-homing imprinting capacity (Figure S5D), indicating that IL-1/IL-18 signals are not required for gut-associated DC education in vivo. We also explored the role of MyD88-independent/TRIF-dependent TLR signals. However, DC from TRIF-/- mice were not impaired in their in vivo or in vitro education to imprint gut-homing T cells (Figure S5E).
Of interest, a recent study reported that gut-associated DC from mice deficient in the common β subunit of GM-CSF/IL-3/IL-5 receptors were impaired in their gut-homing imprinting capacity, concluding that GM-CSF signals are necessary in vivo to educate gut-associated DC (25). However, our data show that gut-associated DC from GM-CSF-/- mice are not impaired in their capacity to synthesize RA or to induce gut-homing T cells (Figure S5F, G), indicating that this cytokine is not essential for in vivo gut-associated DC education.
Discussion
Gut-associated DC, but not extra-intestinal DC, exhibit gut-specific imprinting properties, including the capacity to generate gut-tropic lymphocytes (1). Our data support a model in which MyD88-dependent TLR1/2 signals are necessary in vivo and sufficient in vitro to educate DC with gut-specific imprinting properties. TLR1/2-agonists (e.g., originated from the gut microbiota or endogenous TLR agonists) act in DC and/or DC precursors in the gut lamina propria, which then acquire the expression of Raldh2 (RA synthesis via a mechanism dependent on MyD88 and JNK signaling.
A recent report showed that GM-CSF is sufficient to induce Raldh enzymes in extra-intestinal DC and also required in vivo for gut-associated DC education (25). In agreement with this study, we observed induction of Aldh1a2 mRNA and Raldh activity in Spleen-DC pretreated with GM-CSF (data not shown). However, gut-associated DC from GM-CSF-/- mice were not impaired in their RA-synthesizing or gut-homing imprinting capacities, suggesting that GM-CSF is not required for gut-associated DC education in vivo. The discrepancies between our results and the aforementioned study could be explained, at least in part, by the fact that we used DC from mice deficient only in GM-CSF, whereas Yokota et al. isolated DC from mice deficient in the common β subunit (Beta-c), which is shared by GM-CSF, IL-3 and IL-5 receptor (25). Therefore, a combined lack of signaling via any of these receptors might potentially contribute to explain their observed phenotype.
With the purpose of obtaining a sufficient number of DC we treated mice with Flt3L, strategy that we as well as other groups have previously used to expand DC in all organs, including gut-associated DC (2, 5, 27, 46). Although DC obtained from Flt3L-treated mice might not be fully representative of their non-expanded counterparts isolated from untreated mice, Flt3L expands all the classically described DC subsets in the gut, including CD103+ DC (27). Importantly, we and others have shown that gut-associated DC from Flt3L-treated mice induce gut-homing receptors on T and B cells (2, 5, 27, 46), which was also observed when using gut-associated DC isolated from non Flt3L-treated mice (4, 6, 25) or from human MLN (3, 5). Thus, DC from Flt3L treated mice seem to maintain most of their gut-specific imprinting capacity, similar to non-expanded murine and freshly isolated human gut-associated DC. These considerations notwithstanding, we also showed that CD103+ MLN-DC from untreated MyD88-/- or TLR2-/- mice exhibited significantly lower Raldh activity as compared to their counterparts from untreated wild type mice, indicating that the requirement of MyD88 and TLR signals applies to DC education in non Flt3L-treated mice.
Depending on the experimental system, it has been shown that MyD88-dependent signals may be required in BM-derived DC (47), the radioresistant compartment (48) or both (49). In this regard, it has been suggested that intestinal epithelial cells (IEC) might contribute to DC education in the gut mucosa. IEC promoted gut-homing imprinting in vitro when co-cultured with T cells activated with extra-intestinal DC (50). Moreover, IEC conditioned extra-intestinal DC ex vivo to induce TREG in a RA- and TGF-β-dependent manner (51). Although the in vivo role of IEC in DC differentiation remains to be demonstrated, our data using BM chimeras suggest that gut-associated DC education depends on MyD88-dependent signals acting in BM-derived cells and also in the radioresistant compartment, such as IEC and/or stromal cells (23).
Lamina propria DC and IEC express TLR1-TLR6, (32, 52, 53) and signals via MyD88-dependent TLR, including TLR2, are necessary for maintaining intestinal epithelial homeostasis (53, 54) and for recovering the IEC compartment post-irradiation (54). Therefore, lack of MyD88 in IEC might affect gut-associated DC education indirectly by disrupting normal epithelial homeostasis. Although assessing the role of MyD88 specifically in IEC would need conditional cell-specific MyD88 deletion (47), our experiments using mixed BM chimeras suggest that MyD88 needs to be expressed in DC for their in vivo education and that MyD88-sufficient BM-derived cells (including DC) cannot compensate “in trans” for the lack of MyD88 in DC. In addition, since Nalp3-/- mice are not impaired in gut-associated DC education (hence excluding a role for IL-1/IL-18), it suggest that MyD88-dependent TLR (at least in part TLR2) expressed on DC and/or DC precursors might sense TLR-agonists coming from the intestinal microbiota and/or from endogenous TLR-agonists, which have been proposed for TLR2 (55, 56).
Interestingly, among MyD88-dependent TLR, only stimulation via TLR1/2 consistently educated Spleen-DC with gut-homing imprinting capacity. Moreover, TLR1/2 was necessary for gut-associated DC education in vivo, whereas other TLR, including TLR4, TLR6 or TRIF-dependent TLR, were not required for DC education. Although the mechanistic basis of these TLR1/2 specific effects remains to be determined, it has been described that TLR2 stimulation triggers different intracellular signals as compared to other MyD88-dependent TLR (38, 39, 57). In addition, it has been reported that stimulation via TLR2, but not via other MyD88-dependent TLR, confers DC with some specific functional properties, including differential cytokine secretion and a more efficient induction of effector/memory T cells (38, 39, 58).
Even though MyD88-/- and TLR2-/- gut-associated DC exhibited a significant reduction in their capacity to induce α4β7 and CCR9, their impairment in gut-homing imprinting was not complete. It is possible that there are alternative pathways compensating for the lack of MyD88 or TLR2 in vivo. Stromal cells in MLN can also produce RA and induce gut-homing receptors on T cells (23, 59). While we demonstrated that CD103+ MLN-DC require MyD88 and TLR1/2 signals for their education, stromal cells from MLN might not need MyD88-dependent signals for acquiring RA-synthesizing potential, hence contributing to partially compensate for the induction of gut-homing T cells in vivo. Moreover, despite that CCR9+ T cells were significantly reduced in MLN from MyD88-/- mice in vivo upon immunization,α4β7 was similarly induced in wild type or MyD88-/- mice. This dissociation in the induction of α4β7 and CCR9 could be due, at least in part, to a greater requirement of RA for CCR9 than for α4β7 induction on lymphocytes (3, 5, 60). A similar explanation has been proposed to explain why T cells upregulate only α4β7 but not CCR9 when the MLN is transplanted in a subcutaneous location (55). Thus, whereas the overall production of RA in MLN of MyD88-/- is apparently not sufficient to promote an optimal induction of CCR9 in vivo, it might suffice for α4β7 induction.
In addition, although our results show that the recruitment of recently activated CD8 T cells was significantly impaired in MyD88-/- and TLR2-/- mice, these mice do not exhibit an obvious defect in the numbers/proportions of T cells in the intestinal mucosa in the steady-state condition (54, 61). This is analogous to the phenotype observed in CCR9-/- or CCL25-/- mice (62, 63), which show virtually normal numbers of T cells in the intestine, but nevertheless exhibit a marked defect in the recruitment of recently activated T cells to the small bowel (4, 19, 63). Therefore, since MyD88-/- and TLR2-/- mice are mostly impaired in acute CCR9 induction on T cells in vivo, it is not unexpected that these mice recapitulate the virtually normal gut T cell phenotype of CCR9-/- or CCL25-/- in the steady-state situation, but nonetheless they show a significantly impaired migration of recently activated T cells to the small bowel.
MAPK signaling has been involved in TLR-mediated effects on DC (38-40). Moreover, it was recently reported that zymosan (TLR2 and Dectin-1 ligand) induces Raldh expression in Spleen-DC via an ERK-dependent mechanism, conferring these DC with the capacity to induce TREG (64). By contrast, TLR1/2-mediated DC education by Pam3CSK4 did not require on ERK, but needed JNK signaling and JNK also played a physiological role in gut-associated DC education. Our results are consistent with previous data showing that TLR1/2 signaling triggered by Pam3 (a triacylated lipopeptide analogue to Pam3CSK4) requires JNK, but not ERK or p38 signaling (40). In addition, Pam3CSK4-stimulated Spleen-DC did not promote an increase in TREG differentiation. All together, these results underscore the complexity of TLR signaling in which even a single TLR stimulated by different agonists might exert varied effects on DC and trigger distinct intracellular pathways.
Interestingly, activation of the inflammasome was neither necessary nor sufficient for DC education to imprint gut-homing T cells or to produce RA. Therefore, in spite of promoting protective systemic immune responses, aluminum-based adjuvants, which are used in many human vaccines (29), might not be suitable for inducing effective intestinal immune responses. Thus, novel strategies for educating DC with gut-specific imprinting properties might help to improve vaccination strategies aimed at enhancing immunity in the gut mucosa.
Supplementary Material
Acknowledgements
We thank Drs. Ulrich von Andrian and Michael Briskin for critical reading of this manuscript and Susan Davis for editorial assistance. J.R.M. is indebted to Ingrid Ramos for constant support.
Footnote: D.D.N. was supported by grants from Crohn's & Colitis Foundation of America and NIH. J.K. was supported by a grant from NIH. Y.C. is a Special Fellow in Clinical Research of the Leukemia & Lymphoma Society. S.B.S. was supported by grants from NIH. J.R.M. was supported by grants from Crohn's & Colitis Foundation of America, Cancer Research Institute, MGH Howard Goodman Fellowship, Massachusetts Life Science Center Award & NIH Director's New Innovator 1DP2OD006512-01.
Footnotes
S.W. and E.J.V. contributed equally to this work.
References
- 1.Mora JR. Homing imprinting and immunomodulation in the gut: role of dendritic cells and retinoids. Inflamm Bowel Dis. 2008;14:275–289. doi: 10.1002/ibd.20280. [DOI] [PubMed] [Google Scholar]
- 2.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 3.Eksteen B, Mora JR, Haughton EL, Henderson NC, Turner LL, Villablanca EJ, Curbishley SM, Aspinall AI, von Andrian UH, Adams DH. Gut Homing Receptors on CD8 T Cells Are Retinoic Acid Dependent and Not Maintained by Liver Dendritic or Stellate Cells. Gastroenterology. 2009;137:320–329. doi: 10.1053/j.gastro.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 2003;198:963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 6.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 8.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-{beta}- and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 12.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 14.Enzler T, Gillessen S, Manis JP, Ferguson D, Fleming J, Alt FW, Mihm M, Dranoff G. Deficiencies of GM-CSF and interferon gamma link inflammation and cancer. J Exp Med. 2003;197:1213–1219. doi: 10.1084/jem.20021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svensson M, Johansson-Lindbom B, Z. F., Jaenssona E, Austenaa LM, Blomhoff R, Agace WW. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunology. 2008;1:38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 17.Klein C, Nguyen D, Liu CH, Mizoguchi A, Bhan AK, Miki H, Takenawa T, Rosen FS, Alt FW, Mulligan RC, Snapper SB. Gene therapy for Wiskott-Aldrich syndrome: rescue of T-cell signaling and amelioration of colitis upon transplantation of retrovirally transduced hematopoietic stem cells in mice. Blood. 2003;101:2159–2166. doi: 10.1182/blood-2002-05-1423. [DOI] [PubMed] [Google Scholar]
- 18.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 19.Svensson M, Marsal J, Ericsson A, Carramolino L, Broden T, Marquez G, Agace WW. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J Clin Invest. 2002;110:1113–1121. doi: 10.1172/JCI15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Q, Egelston C, Vivekanandhan A, Uematsu S, Akira S, Klinman DM, Belyakov IM, Berzofsky JA. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: implications for vaccines. Proc Natl Acad Sci U S A. 2008;105:16260–16265. doi: 10.1073/pnas.0805325105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guarda G, Dostert C, Staehli F, Cabalzar K, Castillo R, Tardivel A, Schneider P, Tschopp J. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- 23.Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, Pabst O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–2490. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokota A, Takeuchi H, Maeda N, Ohoka Y, Kato C, Song SY, Iwata M. GMCSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int Immunol. 2009;21:361–377. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington LE, Galvan M, Baum LG, Altman JD, Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J Exp Med. 2000;191:1241–1246. doi: 10.1084/jem.191.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg PL, Davidsson T, Powrie F, Johansson-Lindbom B, Agace WW. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson NS, Young LJ, Kupresanin F, Naik SH, Vremec D, Heath WR, Akira S, Shortman K, Boyle J, Maraskovsky E, Belz GT, Villadangos JA. Normal proportion and expression of maturation markers in migratory dendritic cells in the absence of germs or Toll-like receptor signaling. Immunol Cell Biol. 2008;86:200–205. doi: 10.1038/sj.icb.7100125. [DOI] [PubMed] [Google Scholar]
- 29.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. Eur J Immunol. 2003;33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 31.Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, Hemmi H, Coban C, Kawai T, Ishii KJ, Takeuchi O, Miyasaka M, Takeda K, Akira S. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006;7:868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 32.Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, Sato S, Tsujimura T, Yamamoto M, Yokota Y, Kiyono H, Miyasaka M, Ishii KJ, Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 33.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 34.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, Littman DR, Reinecker HC. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 35.Farhat K, Riekenberg S, Heine H, Debarry J, Lang R, Mages J, Buwitt-Beckmann U, Roschmann K, Jung G, Wiesmuller KH, Ulmer AJ. Heterodimerization of TLR2 with TLR1 or TLR6 expands the ligand spectrum but does not lead to differential signaling. J Leukoc Biol. 2008;83:692–701. doi: 10.1189/jlb.0807586. [DOI] [PubMed] [Google Scholar]
- 36.Robichon C, Vidal-Ingigliardi D, Pugsley AP. Depletion of apolipoprotein N-acyltransferase causes mislocalization of outer membrane lipoproteins in Escherichia coli. J Biol Chem. 2005;280:974–983. doi: 10.1074/jbc.M411059200. [DOI] [PubMed] [Google Scholar]
- 37.Dove WF, Clipson L, Gould KA, Luongo C, Marshall DJ, Moser AR, Newton MA, Jacoby RF. Intestinal neoplasia in the ApcMin mouse: independence from the microbial and natural killer (beige locus) status. Cancer Res. 1997;57:812–814. [PubMed] [Google Scholar]
- 38.Dillon S, Agrawal A, Van Dyke T, Landreth G, McCauley L, Koh A, Maliszewski C, Akira S, Pulendran B. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol. 2004;172:4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 39.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Cutting Edge: different toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 2003;171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 40.Adhikary G, Sun Y, Pearlman E. C-Jun NH2 terminal kinase (JNK) is an essential mediator of Toll-like receptor 2-induced corneal inflammation. J Leukoc Biol. 2008;83:991–997. doi: 10.1189/jlb.1107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berndt BE, Zhang M, Chen GH, Huffnagle GB, Kao JY. The role of dendritic cells in the development of acute dextran sulfate sodium colitis. J Immunol. 2007;179:6255–6262. doi: 10.4049/jimmunol.179.9.6255. [DOI] [PubMed] [Google Scholar]
- 42.Nagamine CM, Sohn JJ, Rickman BH, Rogers AB, Fox JG, Schauer DB. Helicobacter hepaticus infection promotes colon tumorigenesis in the BALB/c-Rag2(-/-) Apc(Min/+) mouse. Infect Immun. 2008;76:2758–2766. doi: 10.1128/IAI.01604-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 44.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 46.Dudda JC, Lembo A, Bachtanian E, Huehn J, Siewert C, Hamann A, Kremmer E, Forster R, Martin SF. Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur J Immunol. 2005;35:1056–1065. doi: 10.1002/eji.200425817. [DOI] [PubMed] [Google Scholar]
- 47.Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29:272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF, Lagente V, Ryffel B, Couillin I. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007;117:3786–3799. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanders CJ, Moore DA, 3rd, Williams IR, Gewirtz AT. Both radioresistant and hemopoietic cells promote innate and adaptive immune responses to flagellin. J Immunol. 2008;180:7184–7192. doi: 10.4049/jimmunol.180.11.7184. [DOI] [PubMed] [Google Scholar]
- 50.Edele F, Molenaar R, Gutle D, Dudda JC, Jakob T, Homey B, Mebius R, Hornef M, Martin SF. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J Immunol. 2008;181:3745–3749. doi: 10.4049/jimmunol.181.6.3745. [DOI] [PubMed] [Google Scholar]
- 51.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol. 2009 doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 52.Monteleone I, Platt AM, Jaensson E, Agace WW, Mowat AM. IL-10-dependent partial refractoriness to Toll-like receptor stimulation modulates gut mucosal dendritic cell function. Eur J Immunol. 2008;38:1533–1547. doi: 10.1002/eji.200737909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lavelle EC, Murphy C, O'Neill LA, Creagh EM. The role of TLRs, NLRs, and RLRs in mucosal innate immunity and homeostasis. Mucosal Immunol. 2009 doi: 10.1038/mi.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Schoonjans K, Dubuquoy L, Mebis J, Fayard E, Wendling O, Haby C, Geboes K, Auwerx J. Liver receptor homolog 1 contributes to intestinal tumor formation through effects on cell cycle and inflammation. Proc Natl Acad Sci U S A. 2005;102:2058–2062. doi: 10.1073/pnas.0409756102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell AL. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:5540–5544. doi: 10.1073/pnas.0912675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chandran SS, Verhoeven D, Teijaro JR, Fenton MJ, Farber DL. TLR2 Engagement on Dendritic Cells Promotes High Frequency Effector and Memory CD4 T Cell Responses. J Immunol. 2009 doi: 10.4049/jimmunol.0901683. [DOI] [PubMed] [Google Scholar]
- 59.Ahrendt M, Hammerschmidt SI, Pabst O, Pabst R, Bode U. Stromal cells confer lymph node-specific properties by shaping a unique microenvironment influencing local immune responses. J Immunol. 2008;181:1898–1907. doi: 10.4049/jimmunol.181.3.1898. [DOI] [PubMed] [Google Scholar]
- 60.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iiyama R, Kanai T, Uraushihara K, Ishikura T, Makita S, Totsuka T, Yamazaki M, Nakamura T, Miyata T, Yoshida H, Takeuchi O, Hoshino K, Takeda K, Ishikawa H, Akira S, Watanabe M. Normal development of the gut-associated lymphoid tissue except Peyer's patch in MyD88-deficient mice. Scand J Immunol. 2003;58:620–627. doi: 10.1111/j.1365-3083.2003.01346.x. [DOI] [PubMed] [Google Scholar]
- 62.Wurbel MA, Malissen M, Guy-Grand D, Meffre E, Nussenzweig MC, Richelme M, Carrier A, Malissen B. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 2001;98:2626–2632. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- 63.Wurbel MA, Malissen M, Guy-Grand D, Malissen B, Campbell JJ. Impaired accumulation of antigen-specific CD8 lymphocytes in chemokine CCL25-deficient intestinal epithelium and lamina propria. J Immunol. 2007;178:7598–7606. doi: 10.4049/jimmunol.178.12.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.