Abstract
Animals vary considerably in the degree to which they attribute incentive salience to cues predictive of reward. When a discrete cue (conditional stimulus) is repeatedly paired with delivery of a food reward (unconditional stimulus) only some rats (“sign-trackers”; STs) come to find the cue itself an attractive and desirable incentive stimulus. For other rats (“goal-trackers”; GTs) the cue is an effective conditional stimulus – it evokes a conditional response – but it is less attractive and less desirable. Given that STs have particular difficulty resisting reward cues, and are thought to have poor inhibitory control over their behavior, we hypothesized that they may also be more impulsive. There are, however, multiple forms of impulsivity; therefore, we compared STs and GTs on two tests of so-called impulsive action – a 2-choice serial reaction time task and a differential reinforcement of low rates of responding task, and one test of impulsive choice - a delay discounting choice procedure. We found that relative to GTs, STs were more impulsive on the two tests of impulsive action, but not on the test of impulsive choice. We speculate that when these two traits combine, that is, when an individual is not only prone to attribute incentive salience to reward cues but also prone to impulsive action, they may be especially susceptible to impulse control disorders, including addiction.
Keywords: impulsive action, impulsive choice, sign tracking, goal tracking, incentive salience
1. Introduction
Cues associated with rewards (conditional stimuli, CSs) not only evoke conditional responses (CRs), but they can also become attractive and desirable in their own right, acting as incentive stimuli [4, 5, 9, 31]. There is, however, considerable individual variation in the extent to which CSs are attributed with incentive salience, and thus come to motivate behavior. For example, when a spatially discrete cue (a lever) is paired with delivery of a food reward (the unconditional stimulus, US) only in some rats does the lever-CS itself become attractive, eliciting approach towards it [20, 41], and desirable, in that rats will work to get it [37]. These rats are called sign-trackers (STs) because they tend to approach the cue or “sign” that predicts reward [25]. In the same situation other rats, called “goal-trackers” (GTs), do not approach the lever-CS, but CS presentation instigates approach towards the location of food delivery [6], and in GTs, a lever-CS is not a potent conditional reinforcer [37]. Furthermore, both food and cocaine cues are more effective in reinstating seeking for their respective rewards, following extinction, in STs than GTs [39, 53]. Therefore, although reward cues can act as an effective CS for both STs and GTs, they seem to acquire potent incentive properties only in STs [37]. Given their difficulty resisting drug cues, we, and others, have argued that STs may be especially vulnerable to impulse control disorders, including addiction [21, 39, 43].
Another trait thought to confer vulnerability to addiction is impulsivity [13–15, 28, 35, 48, 52], a hypothesis supported by recent preclinical studies. For example, high-impulsive rats are more likely to compulsively self-administer drugs than low-impulsive rats [3] and are more likely to show reinstatement of drug-seeking following punishment-induced extinction [18]. Interestingly, it has been argued that STs may also have poor inhibitory control over their behavior, and are especially impulsive [22, 43], but there are very few studies exploring this topic. Therefore, the purpose of the experiments reported here was to better characterize the relationship between the propensity to attribute incentive salience to a reward cue and the propensity to be impulsive.
Impulsivity is characterized by premature actions, reduced tolerance of delay of gratification and rapid decision-making, and therefore, is not a unitary construct [19]. It has been suggested that forms of impulsivity can be grouped into at least two broad dissociable classes: impulsive action and impulsive choice [15, 16, 19, 33, 34, 50]. Impulsive action refers to phenomena where an individual fails to inhibit premature or poorly timed responses, whereas impulsive choice refers to situations where an organism is faced with action choices, each leading to distinct outcomes [1, 10, 14, 19, 27, 33]. Therefore, we tested STs and GTs on two tests thought to be sensitive to impulsive action, a differential reinforcement of low rats of responding (DRL) task and a 2-choice serial reaction time task (2-CSRTT; a simplified version of 5-CSRTT), as well as a test of so-called impulsive choice, a delay discounting (DD) choice task. We report that, relative to GTs, STs are prone to make impulsive actions, but not impulsive choices.
2. Material and Methods
2.1. Subjects
Male Sprague-Dawley rats (Harlan, IN) were used, and different rats were used in three independent experiments. Upon arrival, rats (~60 days old; ~280g) were housed individually in Plexiglas cages (W 26 × L 38 × H 21 cm), lined with bedding (Bed O Cobs®), in a temperature- and humidity-controlled room (22±1 °C and 40–50%, respectively) with lights off from 2000-0800h. Rats had ad lib access to Purina rat chow (unless otherwise specified, see below) and water. All testing was done between 0900-1800h. Rats were allowed to acclimate for one week before testing commenced. All the procedures were approved by the University of Michigan Committee on the Use and Care of Animals.
2.2. Apparatus
Testing was conducted in 32 modular test chambers (28 × 21 × 21 cm; Med Associates Inc., St. Albans, VT, USA) housed inside sound attenuating cubicles outfitted with ventilation fans, which provided masking noise. The chamber configuration, such as the presence of specific manipulanda (i.e., levers or nose poke ports) and their location, depended on the specific experiment. For Pavlovian training (see below) the chambers were outfitted with a red house light, a food cup (magazine), which contained an infrared photobeam detector, and an illuminated retractable lever (2.5 cm long; 6 cm above the grid floor) located either to the left or right of the food magazine. For the 2-CSRTT task the chambers were outfitted with a food magazine centered on one wall and 2 nose-poke ports on the left and right sides of the opposite wall (2 cm above the floor). For the DRL task the chambers were outfitted with a food magazine, centrally located on one wall, flanked by either a nose-poke port (half of the rats) or a lever. For the delay-discounting task the chambers were outfitted with a food magazine, centrally located along one wall, flanked by two nose-poke ports (2cm above the floor). For all impulsivity tests, a white house light was located on the wall opposite from the food magazine (16 cm above the floor). The chambers were controlled by Med Associates software and a Dell computer.
2.3. Procedures
2.3.1. Pavlovian Conditioned Approach (PCA) Training
All rats were first trained using a Pavlovian ( autoshaping ) procedure similar to that described previously [20]. A day prior to the start of training rats were given approximately twenty 45 mg banana-flavored pellets (BioServe, #F0059, Frenchtown, NJ, USA) in their home cages. The following day rats were trained to retrieve 25 banana-flavored pellets, delivered on variable time (VT) 30-s schedule, from the magazine. The day following magazine training, Pavlovian training commenced. Each training trial consisted of insertion (and simultaneous back illumination) of the lever (conditional stimulus, CS) for 8s, after which the lever was retracted and a single food pellet (unconditional stimulus, US) was delivered into the magazine. Each of five daily training sessions consisted of 25 response-independent trials in which CS-US pairings occurred on a VT 90-s schedule. Lever deflections, magazine entries, latency to the first lever deflection, and latency to food cup entry during CS presentation were recorded. Note that the rats were not food deprived for this test.
PCA Index
Following Pavlovian training rats were assigned to one of three groups based on whether they preferentially interacted with the lever-CS (“sign-trackers”, STs), preferentially interacted with the food magazine during lever-CS presentation (“goal-trackers”, GTs), or had no preference for the lever-CS or magazine (“intermediate group”, IG). We quantified this using a composite Pavlovian conditioned approach (PCA) Index which was the average of three measures of conditioned approach: (1) the probability of contacting either the lever-CS or food cup during the CS period [P(lever) – P(magazine)]; (2) the response bias for contacting the lever-CS or the magazine during the CS period [(#lever-CS contacts – #magazine contacts)/(#lever-CS contacts + #magazine contacts)]; and (3) the latency to contact the lever-CS or the food cup during the CS period [(magazine contact latency – lever-CS contact latency)/8]. This produces values on a scale ranging from −1.0 to +1.0. For the purposes of classification, rats with a score ranging from −1.0 to −0.5 were operationally defined as GTs (i.e., rats that were at least twice as likely to direct behavior towards the magazine than towards the lever), and rats with a score between +0.5 and +1.0 were designated as STs (i.e., rats that were at least twice as likely to direct behavior towards the CS lever than the magazine) [30, 40].
2.3.2. Experiment 1: 2-Choice Serial Reaction Time Task (2-CSRTT)
Following Pavlovian training a subset of rats (N=30) were used in a 2-CSRTT experiment, which is a two choice adaptation of the widely used 5-choice serial reaction time task [2]. One week before 2-CSRTT testing commenced rats were restricted to approximately 15 g of rat chow per day, which maintained their weight at ~90% of their free feeding weight. Two days prior to testing rats were given 45mg chocolate-flavored pellets (BioServe, #F0299) in their home cage. Training sessions began with the illumination of the house light and delivery of a food pellet into the food cup. A nose-poke into the food cup (i.e., collection of unconditioned delivery of the first pellet) initiated the first trial, which consisted of a 5s (or 9s; see below) inter-trial interval (ITI) followed by the illumination of one of the two nose-poke ports (order randomly determined). The length of time the nose-poke port was illuminated (stimulus duration; SD) was fixed during each session (but varied during training; see below). Responses into the illuminated nose-poke port, or within 5s of light termination (Limited Hold), resulted in the delivery of a food pellet (and a Correct response was recorded). The next trial was initiated by collection of the pellet from the food cup. Responses into the non-illuminated nose-poke port were recorded as an Incorrect response (resulting in no pellet delivery), and a failure to respond was recorded as an Omission. An Omission resulted in a 5s Time Out period (TO), during which the house light was extinguished. Responding during the ITI (i.e., before either one of the nose poke ports was illuminated) was recorded as a Premature Response, and also resulted in a TO period.
During training sessions SD was gradually decreased, across 9 sessions, from 60s to 1.5s. During the next 10 (testing) sessions SD was fixed at 1s. On Days 6 and 10 (Probe Sessions) of testing the ITI was suddenly increased from 5 to 9s. Training and test sessions lasted 30 min or until rats completed 80 trials (whichever came first). The Probe Session lasted 40 min or until rats completed 80 trials.
2.3.3. Experiment 2: Differential Reinforcement of Low Rates (DRL) Task
A second independent group of rats (N=35) that completed Pavlovian training were used in a DRL experiment. One week before testing commenced rats were food restricted as in Exp. 1. Rats were first trained on an FR 1 schedule to make an instrumental response (nose-poke or lever press) in order to earn a food pellet (45 mg food pellets; Bio-Serv). Sessions were 30 min long or until rats made 100 responses. All rats had 3 or 4 FR 1 sessions. Following this, rats were tested on DRL-10s schedule for 5 days and DRL-20s schedule for 15 days. On the DRL-10s and DRL-20s schedules, rats were reinforced only if at least 10s or 20s, respectively, elapsed between responses. Responses made before the waiting period had elapsed were not rewarded and resulted in resetting of the waiting period. For each session the following measures were collected: number of responses, number of reinforcers earned, and percent efficiency [(number of reinforcers earned/number of responses made) × 100].
2.3.4. Experiment 3: Delay Discounting
A third independent group of rats (N=24) that completed Pavlovian training were subsequently tested on a delay discounting schedule of reinforcement. One week before testing commenced rats were food restricted as described above. Experimental testing occurred in three distinct phases.
Fixed Ratio Training
Rats were trained to make responses into the left and right (alternate days) nose-poke ports for food reward (45 mg food pellets; Bio-Serv; FR 1 schedule for 7 consecutive days). Each session lasted 30 min or until rats made 100 responses. Rats were considered trained if they made 100 nose-pokes on 2 consecutive days (1 day each on left and right ports).
Choice Training – No Delays to Large Reward Across Blocks
Testing was similar to the procedures used by others [8]. Over 7 test sessions rats were tested for their preference for a large reward (4 pellets) or small reward (1 pellet). The magnitude of reward (i.e., 1 vs. 4 pellets) was based on responses made into the left or the right nose-poke port (randomized across groups and subjects). Each session consisted of 5 blocks of 12 trials. The first two trials within a block were forced trials. Forced trials consisted of illumination of only one of the nose-poke ports and responding in this port resulted in the delivery of reward. Responses made into the non-illuminated port had no consequence. The remaining 10 trials were choice trials. Choice trials consisted of the illumination of both nose-poke ports. Responding into one of the ports resulted in delivery of 1 pellet while responding into the other port resulted in the delivery of 4 pellets. Each trial was 60s long and the entire session lasted 60 min. Rats were deemed to prefer the large reward if they chose it on at least 85% of trials for 2 consecutive days. Rats were tested in this manner for 7 days.
Delay Discounting
Testing was identical as described in the previous section except that a delay to the large reward (i.e., 4 pellets) was introduced and increased across 5 blocks from 0 to 3, 6, 12 and then 24 s. Rats were tested for 30 days in this manner and the criterion was stable performance across 3 consecutive days (i.e., no significant change in preference for large and small reward; repeated measures ANOVA).
2.4. Statistical Analyses
Repeated measures ANOVA were used to analyze data in all 3 experiments. Significant interactions were followed by planned group comparisons. Statistical significance was set at p<0.05.
3. Results
3.1. Pavlovian Conditioned Approach
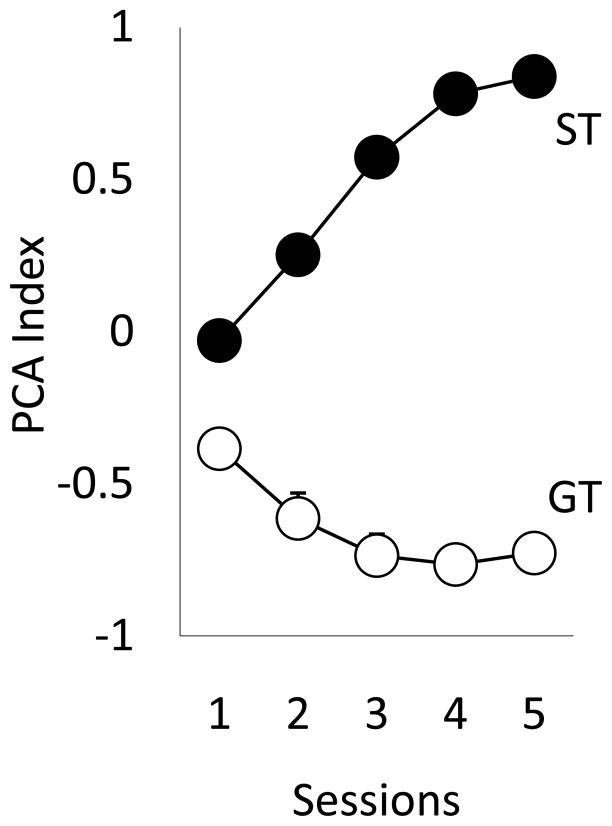
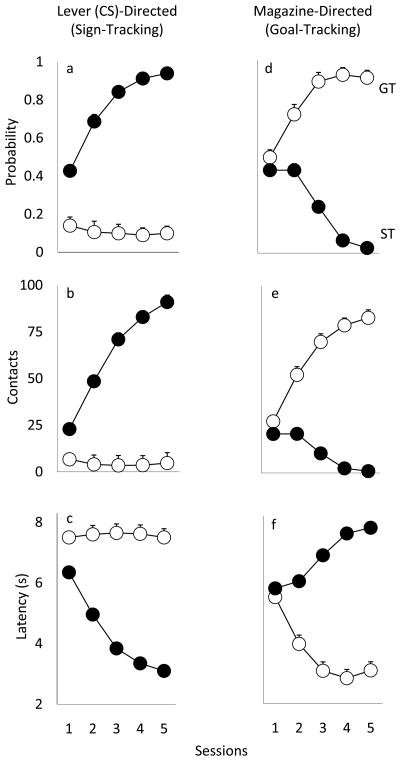
Fig. 1 shows the change in PCA index scores over the 5 days of Pavlovian training in rats classed as STs or GTs based on their performance on days 4 and 5 of training. As would be expected, there was a significant main effect of group [F(1, 85)=636.1, p<0.0001], and session [F(4, 332)=17.79, p<0.0001], and a significant group x session interaction [F(4, 332)=91.13, p<0.0001]. Fig. 2 shows the performance of STs and GTs across days of training on three measures of Pavlovian approach. With training STs (but not GTs) came to reliably (Panel 2a) and rapidly (Panel 2c) approach the lever-CS, and vigorously engage it (Panel 2b; all ps<0.0001). GTs on the other hand learned to reliably and rapidly approach the food magazine during the CS period, and vigorously engaged it (Fig. 2d-f; all ps<0.0001).
Figure 1.
PCA Index scores for ST and GT rats across 5 sessions (mean + SEM; the SEM is smaller than the symbol in most cases). There were significant group differences (ST vs. GT) and significant group x session interactions (ps<0.001).
Figure 2.
Lever- (panels a-c) and magazine-directed (panels d-f) behavior in STs and GTs in each of the 5 training sessions (mean +SEM; the SEM is smaller than the symbol in most cases): (a) probability of contacting the lever-CS (# of trials during which at least one contact has been made with the lever-CS/25), (b) number of lever-CS contacts, (c) latency to first lever-CS contact, (d) probability of magazine entries (# of trials during which at least one contact has been made with the magazine/25), (e) number of magazine contacts (during lever-CS presentation), (f) latency to first magazine contact (during lever-CS presentation). There were significant group (ST vs. GT) differences and significant group x session interactions (ps<0.001).
3.2. Experiment 1: STs Made More Premature Responses than GTs on a 2-CSRTT
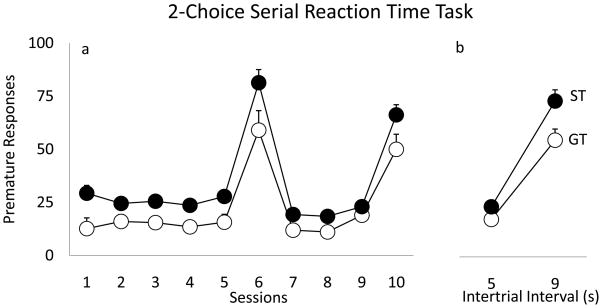
Following PCA training and 9 days of 2-CSRTT training (with gradually decreasing stimulus durations), rats were tested on the 2-CSRTT using a stable (1s) stimulus duration for 10 consecutive sessions. On sessions 1–5 and 7–9 the ITI period was 5s, but on sessions 6 and 10 it was increased to 9s. Rats that omitted more than 25% of the trials (i.e., had impaired attention) or were not correct at least 75% of trials (i.e., showed poor learning) were excluded (the groups did not differ on these two measures). Fig. 3 shows the number of premature responses over these 10 sessions in STs and GTs. When analyzed over all 10 sessions STs made significantly more premature responses than GTs [F(1, 28)=8.08, p<0.01]. These group differences were evident both during baseline test days [5s ITI; F(1, 29)=10.22, p<0.01] as well as during probe test days [9s ITI; F(1, 29)=4.18, p<0.05]. We also looked at the change in premature responses as ITIs increased from 5s (data for sessions 5 and 9 averaged) to 9s (data for sessions 6 and 10 averaged; Fig. 3b). There was a significant effect of group but the interaction (group x ITI) was not statistically significant [F(1, 28)=3.06, p=0.09]. Lastly, variance in the PCA score was positively correlated with the number of premature responses, but variance in one measure only accounted for about 15 percent of the variance in the other (r2 = 0.153, p = 0.016).
Figure 3.
Premature responses (mean +SEM) across 10 test sessions in the 2-CSRRT (a). STs made significantly more premature responses than GTs during baseline trials (Sessions 1–5, 7–9; ITIs 5s) and during probe trials (Sessions 6 and 10; ITIs 9s) (ps<0.05). Comparison of premature responses during baseline (averaged sessions 5 and 9) and probe trials (averaged session 6 and 10) (b).
3.3. Experiment 2: STs Were Less Efficient (More Impulsive) than GTs on a DRL Task
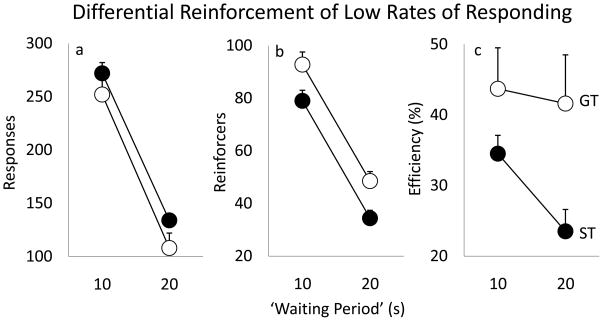
Data from the last 2 days of DRL-10s and the last 2 days of DRL-20s testing, when performance was stable, were averaged for all 3 measures of interest (i.e., responses, reinforcers and percent efficiency). Fig. 4 shows that an increase in the waiting period, from 10s to 20s resulted in a decrease in both the number of responses [Panel 4a, F(1, 33)=100.13, p<0.0001] and the number of reinforcers earned [Panel 4b, F(1, 33)=107.17, p<0.0001], but there were no significant group differences on these measures (p=0.13 and p=0.14, respectively). However, STs were more impulsive than GTs on this task as they were significantly less efficient in obtaining rewards because of their tendency to make premature responses [Panel 4c, effect of group, F(1, 33)=5.7, p<0.05; interaction, F(1, 33)=1.26, p=0.27].
Figure 4.
Performance on the DRL task (Mean +SEM) at a delay of 10 and 20 sec averaged over the last 2 days of testing: (a) total responses, (b) number of reinforcers earned and (c) percent efficiency (reinforcers/response). The groups did not differ in the number of responses made or reinforcers earned but STs had lower percent efficiency than GTs (p<0.05).
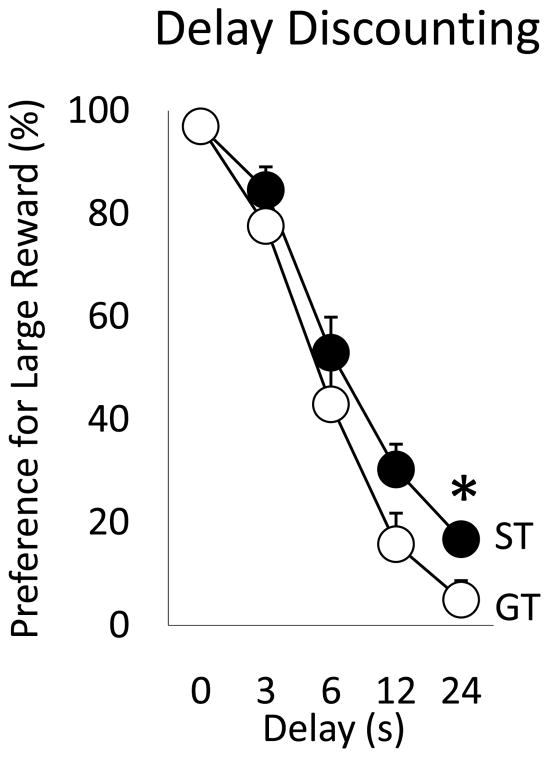
3.4. Experiment 3: STs Showed Lower Discounting Rates (Are Less Impulsive) than GTs on a Delay Discounting Task
No Delays Across Test Blocks
Rats were initially tested on the version of the “delay discounting” schedule without any delays to receive the large or small reward. Within several test sessions, all rats showed high preference for the large reward (>90% across blocks; see the delay of zero in Fig. 5).
Figure 5.
Performance on the delay discounting task averaged over test sessions 28–30, expressed as percent preference for large reward (mean + SEM) across 5 delay periods. Preference for large reward significantly decreased as the delay to its delivery increased. There was significant group x delay interactions and STs showed greater preference for the large reward (24s delay) than GTs (*p<0.05).
Delay Discounting
Rats were tested until their responses were stable across 3 days. Stability was assessed using repeated measures ANOVA (data for individual delay intervals across 3 days). If there were no significant Day effects, we considered the rats performance stable. Stability was achieved by day 30 of testing. Next, data were averaged across the last 3 days of testing and analyzed using repeated measures ANOVA (Group x Delay). Fig. 5 shows that preference for the large reward decreased as the delay to receive the large reward increased in both STs and GTs [effect of delay period, F(1, 22)=1044.5 p<0.0001]. There was no significant effect of group (F(1, 22)=2.9, p=0.1), but there was a significant Group x Delay interaction (F(1, 22)=4.8, p<0.05; see Fig. 5). This was because STs preferred the large reward more than GTs, but only at the longest delay period [24s delay to large reward; t (22)=2.4, p<0.05]. Groups were also marginally different for the 12s delay to large reward [t(22)=1.98, p=0.06].
4. Discussion
We asked whether rats that are prone to attribute incentive salience to reward cues (STs) are also impulsive, as indicated by tests of so-called impulsive action and impulsive choice [14, 19, 33]. We found that, relative to GTs, STs had difficulty withholding a response when required to do so to receive an impending reward, as indicated by more premature responses on both the DRL task and 2-CSRTT. In contrast, at the longest delay interval, STs were more likely than GTs to choose a larger but delayed reward. This pattern of results is consistent with studies using rats selectively bred for reactivity to a novel environment [22]. Selectively bred high responder rats (bHRs) are almost exclusively STs and selectively bred low responder rats (bLRs) are almost exclusively GTs. Flagel et al. [22] reported that bHR/ST rats were more impulsive than bLR/GT rats on a DRL task, whereas bHR/ST rats were less impulsive than bLR/GT rats on a DD choice task. We have not assessed the ability of STs and GTs to stop an action that has already been initiated, for example, using a stop signal reaction time task [17, 29], so we do not know if they differ on this dimension of impulsivity. Thus, more work is required to tease apart the many forms of impulsivity, but the available evidence suggests that rats prone to attribute incentive salience to reward cues are prone to impulsive action.
Impulsivity is a complex and multidimensional construct consisting of a variety of psychologically and neurobiologically dissociable forms [see 14, 34], and there are a number of biobehavioral manipulations that dissociate impulsive action from impulsive choice, as found here. For example, a lesion of the subthalamic nucleus, acute amphetamine administration, or early life social deprivation produce an increase in impulsive action but a decrease in impulsive choice [14, see 34, 44, 46, 49]. However, the question that requires discussion here concerns what psychological and/or neurobiological mechanisms might account for the differences between STs and GTs on tests of these two dimensions of impulsivity. There are at least two possibilities, which are not mutually exclusive. STs may be more action impulsive, relative to GTs, because of (1) exaggerated, “bottom-up” motivation to approach and interact with reward-associated cues and/or (2) poor “top-down” inhibitory control over behavior. We discuss each of these possibilities.
STs and GTs differ in their propensity to attribute incentive salience to spatially discrete cues. This is manifest not only in their propensity to approach a food cue (from which the terms ST and GT are derived), but importantly, a food cue serves as a more effective conditioned reinforcer [37] and is more effective in reinstating food-seeking behavior after extinction of an instrumental response [53], in STs than GTs. Furthermore, a cocaine cue exerts greater control over behavior in STs than GTs, as indicated by approach behavior to a cocaine cue, the influence of cue removal on self-administration behavior and by cue-induced reinstatement of cocaine-seeking behavior [22, 39]. That is, the difference between STs and GTs is in the extent to which reward cues come to act as incentive stimuli capable of instigating motivated behavior [see Fig. 4 in 32], and this difference is evident in both Pavlovian and instrumental settings. Thus, in the present experiment, STs may have not only attributed greater incentive salience to the lever-CS, as indicated by approach behavior in the Pavlovian task, but also differed in their attribution of incentive salience to the manipulandum in the instrumental tasks used to measure impulsivity; i.e., the nose-poke port [e.g., 41].
If STs attributed greater incentive salience to the nose-poke port used in the DRL and 2-CSRTT tasks, relative to GTs, they may have had greater difficulty inhibiting approach and engagement with the port. Tomie [41, 42] has argued that poor inhibitory control over behavior is a prominent feature associated with a propensity for sign-tracking behavior, that this effect is most pronounced when the cue is located at the manipulandum, and that this can promote behavior that is maladaptive in the sense that it can result in loss of the reward [also see 21]. Thus, STs may have made a greater number of responses (often premature) in the DRL and 2-CSRTT tasks because the incentive stimulus properties of the nose port drew them to it. In contrast, in the DD choice procedure STs might approach the nose-poke port associated with the larger reward more often than GTs because STs attribute greater incentive salience to this port. They may do this simply because it is associated with greater reward. However, we hasten to add that, in addition to the differences between STs and GTs, there was also considerable within-group variation. That is, some STs were not especially impulsive on tests of impulsive action and some GTs were impulsive. Indeed, premature responses on the 2-CSRTT only accounted for about 15% of the variance in Pavlovian approach behavior. Thus, the tendency to attribute incentive salience to a reward cue is related some aspects of impulsive behavior, these traits appear to be dissociable.
If STs do attribute greater incentive salience to instrumental manipulanda one might expect STs and GTs would differ in their rate of instrumental responding, but there are a number of situations in which this is not the case. For example, there are no differences in operant responding for food reward on either a FR-1 or VI-5 schedule of reinforcement [37], and STs and GTs also do not differ in rate of responding for cocaine, at least under some circumstances [39]. However, in these studies all rats show relatively high rates of responding and there is no requirement to inhibit actions. Thus, differences between STs and GTs might only become evident when they have to inhibit a prepotent response, or, when motivational requirements are high. For example, although STs and GTs did not differ in their rate of responding for cocaine on a FR-1 schedule of reinforcement, STs maintained responding to a greater extent than GTs on a progressive ratio schedule, achieving higher breakpoints [40, see also 53].
A second, but not mutually exclusive, explanation of ST s greater impulsive action is that STs have relatively poor “top-down” inhibitory control over their behavior [12, 14]. In general, individuals with good top-down control are capable of sustaining attention toward salient stimuli until the appropriate time for action, while simultaneously resisting distracting stimuli and suppressing inappropriate responses [24, 38]. Input from prefrontal cortical (PFC) areas onto subcortical regions including amygdala, hippocampus, and ventral striatum is important for this inhibitory regulation of actions. Damage or reduced activity in PFC and other cortical regions is associated with weaker response inhibition, and thus a propensity to impulsive action [11, 14]. Importantly, Hester and Garavan [26] recently reported that individuals with a history of cocaine use are less able to inhibit prepotent responses, and this was associated with hypoactivity in PFC and anterior cingulate cortex (ACC), suggesting a link between impulsivity, drug addiction, and impaired top-down behavioral control [see also 14]. It is currently unknown if STs have attenuated cortical activity, but our results are compatible with that possibility. Thus, it is possible that two processes: (1) increased, subcortically-driven motivation to seek out reward-associated cues and (2) decreased cortical inhibitory control over responding, might work in concert (i.e., observed in same rats) or independently from each other (i.e., only one of the processes producing premature responses in particular rats) to drive the differences in impulsive behavior we have found in STs and GTs.
Our results appear discrepant with two previous studies that examined the relationship between impulsivity and sign-tracking behavior, but the discrepancies may be due a number of procedural differences. First, Robinson et al. [36] found no difference in the acquisition of a sign-tracking CR in high- vs. low-impulsive rats, as measured by premature responses on a 5-CSRTT. However, in this study Pavlovian conditioned approach behavior was assessed using a discriminative approach procedure that is quite different than that used here, and it may produce a bias toward sign-tracking behavior - no goal tracking during CS+ presentation was reported by Robinson et al. [36]. In a second study, Tomie et al. [42] reported that rats that more readily acquired a sign-tracking CR were also more impulsive on a delay discounting task of impulsive choice, whereas we found the opposite. However, in the Tomie et al. [42] study the same lever that predicted food delivery during Pavlovian training was consistently associated with the smaller, but immediate reward in the DD task. Thus, rats that more avidly approached the lever-CS in the Pavlovian task may have established a strong bias for that lever that carried over to the DD task, which would have been manifest as a preference for the lever that delivered the smaller reward.
The neurobiological bases for individual differences in impulsive action versus impulsive choice are not known, but these different forms of impulsivity are thought to depend on distinct, although overlapping, corticostriatal circuits and variation in dopamine (DA) and serotonin neurotransmission [10, 14, 34]. Furthermore, there are at least two key areas in which lesions dissociate them: the infralimbic cortex and the subthalamic nucleus. Lesion of either one of these structures produces an increase in impulsive action but a decrease in impulsive choice [12, 44], and importantly, a subthalamic nucleus lesion also facilitates approach (sign-tracking) to both food and cocaine-associated cues [45, but see also 51]. These effects may be mediated in part by alterations in DA neurotransmission. Increasing DA neurotransmission with drugs such as amphetamine, cocaine, or nicotine, or with specific DA receptor agonists, produces an increase in impulsive action [46, 47], and some of these agents, such as amphetamine, produce a concomitant decrease in impulsive choice [49]. DA has been implicated in human impulsivity as well given that highly impulsive individuals have fewer midbrain DA autoreceptors and enhanced DA release [7]. STs and GTs also differ on several indices of DA function. For example, relative to GTs, STs show greater expression of dopamine D1 receptor mRNA and lower levels of DA transporter and tyrosine hydroxylase [20]. Furthermore, learning a ST CR is DA-dependent but learning a GT CR is not, and learning a ST CR (but not a GT CR) is associated with a transfer of a phasic DA signal from the US to CS in the accumbens core [23]. It is possible, therefore, that under some circumstances DA neurotransmission is facilitated in STs, which results in them attributing greater incentive salience to reward cues and also makes them prone to impulsive action, though the exact relation between these traits remains unclear.
In conclusion, rats prone to attribute incentive salience to rewards cues are also prone to impulsive action. However, our results suggest that while these traits are related, they remain largely dissociable psychological constructs, each of which may independently contribute to vulnerability to impulse control disorders, such as addiction. Which trait contributes a greater proportion of the variance for which symptoms of addiction remains an open question. One hypothesis is that those individuals in which these traits combine, that is, those who are both prone to attribute inordinate incentive salience to reward cues and are also impulsive, may be especially vulnerable.
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse (R37 DA04294) to TER. We thank Alex Belakovskiy and Tony McClafferty for technical assistance.
Footnotes
Disclosure
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–96. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- 2.Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3:759–67. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- 3.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge KC. Psychology of Learning and Motivation: Advances in Research and Theory. Vol. 40. San Diego: Academic Press Inc; 2001. Reward learning: Reinforcement, incentives, and expectations; pp. 223–78. [Google Scholar]
- 5.Bindra D. How adaptive behavior is produced: A perceptual-motivation alternative to response reinforcement. Behav Brain Sci. 1978;1:41–91. [Google Scholar]
- 6.Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian Interactions. Hillsdale, NJ: Earlbaum; 1977. pp. 67–97. [Google Scholar]
- 7.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 9.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neuroscience and Biobehavioral Reviews. 2002;26:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 10.Cardinal RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw. 2006;19:1277–301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–46. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–19. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Dalley JW, Fryer TD, Brichard L, Robinson ESJ, Theobald DEH, Laane K, et al. Nucleus Accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–94. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 15.de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dellu-Hagedorn F. Relationship between impulsivity, hyperactivity and working memory: a differential analysis in the rat. Behav Brain Funct. 2006;2:10. doi: 10.1186/1744-9081-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eagle DM, Robbins TW. Inhibitory Control in Rats Performing a Stop-Signal Reaction-Time Task: Effects of Lesions of the Medial Striatum and d-Amphetamine* 1. Behavioral neuroscience. 2003;117:1302. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- 18.Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High Impulsivity Predicts Relapse to Cocaine-Seeking After Punishment-Induced Abstinence. Biological Psychiatry. 2009;65:851–6. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 20.Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology. 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- 21.Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56:139–48. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, et al. An Animal Model of Genetic Vulnerability to Behavioral Disinhibition and Responsiveness to Reward-Related Cues: Implications for Addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasselmo ME, Sarter M. Modes and Models of Forebrain Cholinergic Neuromodulation of Cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hearst E, Jenkins H. Sign-tracking: The stimulus-reinforcer relation and directed action. Austin: Monograph of the Psychonomic Society; 1974. [Google Scholar]
- 26.Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–22. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho MY, Mobini S, Chiang TJ, Bradshaw CM, Szabadi E. Theory and method in the quantitative analysis of “impulsive choice” behaviour: implications for psychopharmacology. Psychopharmacology (Berl) 1999;146:362–72. doi: 10.1007/pl00005482. [DOI] [PubMed] [Google Scholar]
- 28.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 29.Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological review. 1984;91:295. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- 30.Lomanowska AM, Lovic V, Rankine MJ, Mooney SJ, Robinson TE, Kraemer GW. Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behav Brain Res. 2011;220:91–9. doi: 10.1016/j.bbr.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 31.Lovibond PF. Facilitation of instrumental behavior by a Pavlovian appetitive conditioned stimulus. J Exp Psychol Anim Behav Process. 1983;9:225–47. [PubMed] [Google Scholar]
- 32.Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci. 2010;31:2308–19. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- 33.Olmstead MC. Animal models of drug addiction: Where do we go from here? Q J Exp Psychol (Colchester) 2006;59:625–53. doi: 10.1080/17470210500356308. [DOI] [PubMed] [Google Scholar]
- 34.Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–9. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- 36.Robinson ES, Eagle DM, Economidou D, Theobald DE, Mar AC, Murphy ER, et al. Behavioural characterisation of high impulsivity on the 5-choice serial reaction time task: specific deficits in ‘waiting’ versus ‘stopping’. Behav Brain Res. 2009;196:310–6. doi: 10.1016/j.bbr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 37.Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biological Psychiatry. 2009;65:869–73. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–60. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: Implications for addiction. Biological Psychiatry. 2010;67:730–6. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. doi: 10.1038/npp.2011.48. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomie A. Locating reward cue at response manipulandum (CAM) induces symptoms of drug abuse. Neuroscience and Biobehavioral Reviews. 1996;20:505–35. doi: 10.1016/0149-7634(95)00023-2. [DOI] [PubMed] [Google Scholar]
- 42.Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Ethanol induces impulsive-like responding in a delay-of-reward operant choice procedure: impulsivity predicts autoshaping. Psychopharmacology (Berl) 1998;139:376–82. doi: 10.1007/s002130050728. [DOI] [PubMed] [Google Scholar]
- 43.Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Research Reviews. 2008;58:121–35. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uslaner JM, Robinson TE. Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice - mediation by enhanced incentive motivation? European Journal of Neuroscience. 2006;24:2345–54. doi: 10.1111/j.1460-9568.2006.05117.x. [DOI] [PubMed] [Google Scholar]
- 45.Uslaner JM, Dell’Orco JM, Pevzner A, Robinson TE. The influence of subthalamic nucleus lesions on sign-tracking to stimuli paired with food and drug rewards: Facilitation of incentive salience attribution? Neuropsychopharmacology. 2008;33:2352–61. doi: 10.1038/sj.npp.1301653. [DOI] [PubMed] [Google Scholar]
- 46.van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) 2006;187:73–85. doi: 10.1007/s00213-006-0396-1. [DOI] [PubMed] [Google Scholar]
- 47.van Gaalen MM, Unger L, Jongen-Relo AL, Schoemaker H, Gross G. Amphetamine decreases behavioral inhibition by stimulation of dopamine D2, but not D3, receptors. Behav Pharmacol. 2009;20:484–91. doi: 10.1097/FBP.0b013e3283305e3b. [DOI] [PubMed] [Google Scholar]
- 48.Verdejo-Garcia A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 2003;170:320–31. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- 50.Winstanley CA, Dalley JW, Theobald DEH, Robbins TW. Fractionating impulsivity: Contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004;29:1331–43. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- 51.Winstanley CA, Baunez C, Theobald DE, Robbins TW. Lesions to the subthalamic nucleus decrease impulsive choice but impair autoshaping in rats: the importance of the basal ganglia in Pavlovian conditioning and impulse control. Eur J Neurosci. 2005;21:3107–16. doi: 10.1111/j.1460-9568.2005.04143.x. [DOI] [PubMed] [Google Scholar]
- 52.Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–18. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yager LM, Robinson TE. Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav Brain Res. 2010;214:30–4. doi: 10.1016/j.bbr.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]