Abstract
Interleukin (IL)-1β is a cytokine critical to several inflammatory diseases in which pathogenic TH17 responses are implicated. Activation of the NLRP3 inflammasome by microbial and environmental stimuli can enable the caspase-1 dependent processing and secretion of IL-1β. The acute phase protein serum amyloid A (SAA) is highly induced during inflammatory responses, wherein it participates in systemic modulation of innate and adaptive immune responses. Elevated levels of IL-1β, SAA, and IL-17 are present in subjects with severe allergic asthma, yet the mechanistic relationship between these mediators has yet to be identified. Herein, we demonstrate that Saa3 is expressed in the lung of mice exposed to several mixed Th2/Th17-polarizing allergic sensitization regimens. SAA instillation into the lungs elicits robust TLR2-, MyD88-, and IL-1-dependent pulmonary neutrophilic inflammation. Furthermore, SAA drives production of IL-1α, IL-1β, IL-6, IL-23, and PGE2, causes dendritic cell maturation, and requires TLR2, MyD88, and the NLRP3 inflammasome for secretion of IL-1β by dendritic cells and macrophages. CD4+ T cells polyclonally stimulated in the presence of conditioned media from SAA-exposed dendritic cells produced IL-17 and the capacity of polyclonally-stimulated splenocytes to secrete IL-17 is dependent upon IL-1, TLR2, and the NLRP3 inflammasome. Additionally, in a model of allergic airway inflammation, administration of SAA to the lungs functions as an adjuvant to sensitize mice to inhaled ovalbumin, resulting in leukocyte influx after antigen challenge and a predominance of IL-17 production from restimulated splenocytes that is dependent upon IL-1 receptor signaling.
Introduction
The Interleukin 1 (IL-1) family of cytokines is critical to the host response to infection, playing a variety of functions not only in the acute phase response from the liver, but also in alterations in metabolism, induction of fever, and lymphocyte activation (1). Overproduction of IL-1β, in particular, is thought to be responsible for a variety of autoinflammatory syndromes such as Familial Mediterranean Fever and Muckle-Wells Syndrome, and is also a contributing factor in osteoarthritis, rheumatoid arthritis, gout, Multiple Sclerosis (EAE), colitis, diabetes, and Alzheimer’s disease (2–9). Setting IL-1β apart from other acute phase cytokines such as IL-6 and TNF-α is the requirement for processing from an inactive pro-form to an active secreted form by caspase-1 cleavage, which itself is activated by the assembly of a cytoplasmic inflammasome complex. The NLRP3 inflammasome is not only critical for IL-1β release in response to a variety of stimuli, but has also been implicated in several of the same autoimmune and autoinflammatory disorders in which IL-1β plays a causative role. Key to these models of inappropriate adaptive immune responsiveness is the development of a TH17-biased phenotype, and recent research has highlighted the importance of IL-1β in TH17 development (10, 11). IL-1β, in conjunction with IL-6 and TGF-β, is critical in humans for the development of the TH17 lineage. Not only do TH17 cells upregulate mRNA expression of the IL-1 Receptor (IL-1R) compared to TH1 and TH2 cells, but lack of this receptor on polyclonally stimulated T cells results in significant reduction of IL-17A, IL-17F, IL-21, and IL-22 production (10, 12).
Long known to be a biomarker of inflammation in a multitude of diseases (13), serum amyloid A (SAA) is also a critical mediator of disease pathogenesis. SAA can stimulate cells via TLR2 to elicit a robust signaling cascade in human monocytes (14) and mouse macrophages (15), while it can also signal through the formyl-peptide receptor like 1 (FPRL1/FPR2) to promote neutrophil chemotaxis and activation (16–18). In addition, SAA induces expression of matrix metalloproteinases and collagenases that are pivotal in tissue remodeling after injury (19). SAA can also promote the development of TH17 responses, as has been demonstrated to be an important mechanism by which segmented filamentous bacteria induce intestinal disease (20). However, the importance of SAA on modulating adaptive immune responses in other tissues and beyond the scope of infection has not yet been demonstrated. Most well known is the role of SAA fibril deposition in severe conditions such as amyloidosis (21). It has recently been demonstrated that β-amyloid fibrils in Alzheimer’s disease (22) and islet amyloid polypeptide in type 2 diabetes (2) signal through the NLRP3 inflammasome and drive caspase-1 dependent cleavage of IL-1β.
Asthma is conventionally considered to be a TH2-driven disease associated with wheezing, airway hyperresponsiveness, IgE, eosinophilia, and mucus metaplasia. However, in a substantial percentage of patients, asthma presents as non-atopic, instead manifesting as a neutrophilic and steroid-resistant phenotype that results in increased severity and morbidity of disease (23, 24). Severe allergic asthma is associated with elevated levels of several mediators, including SAA (25–27), IL-1β (28), and IL-17 (29–36), although a mechanistic link between these molecules has not yet been established. IL-1β and IL-17A have been demonstrated to upregulate expression of the mucin gene Muc5ac (37), and IL-1β also acts via cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) production to desensitize airway smooth muscle cells to β-adrenergic agonists (38).
Mouse models of allergic asthma have classically exploited the TH2-promoting adjuvant, aluminum hydroxide (Alum), delivered as an emulsion with antigen via intraperitonel injection (39). However, allergic asthma models are evolving to encompass inhalational methods of sensitization to aeroantigens, which promote a mixed TH2/TH17 allergic airway disease phenotype (40). While the TH17 response in mouse models of allergic airway disease is associated with neutrophilia, tissue destruction, and steroid unresponsiveness, little is known about the endogenous mediators that are critical to this response. Recent reports have implicated IL-1β, IL-6, and IL-23 in the initiation and expansion of IL-17-producing T cells, three cytokines that are highly induced by SAA (14, 41).
Herein, we report that multiple models of respiratory system exposure that promote mixed TH2/TH17 responses also induce pulmonary Saa3 expression. SAA signals through TLR2 to induce inflammatory mediators and through the Nlrp3 inflammasome to induce IL-1β secretion. In addition, SAA induces dendritic cells to undergo maturation and produce soluble mediators, including IL-1α, IL-1β, IL-6, PGE2, and IL-23, that function in an IL-1-dependent manner to promote CD4+ T cells to secrete IL-17A upon stimulation. Finally, SAA sensitizes mice to a mixed TH2/TH17 allergic airway disease via an IL-1 receptor-dependent mechanism. Together, these data implicate pulmonary SAA as a pro-inflammatory mediator capable of promoting antigen-specific pulmonary TH17 responses through the activities of the cytokine mediator, IL-1.
Materials and Methods
Mice
C57BL/6 and IL-1Rα−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME). TLR2−/− (42), TLR4−/− (43), MyD88−/− (44), NLRP3−/− (45), ASC−/− (45), Caspase1−/− (46), and CC10-rtTA x TetOP-CAIKKβ bitransgenic mice on the C57BL/6 background (47), which express a constitutively-active IKKβ (CAIKKβ) in bronchiolar epithelium following administration of 6 g/kg doxycycline (Dox) in chow (TestDiet, Richmond, IN), and age- and sex-matched transgene-negative littermates, were also bred at the University of Vermont. Mice were housed in an AAALAC approved facility, maintained on a 12-hour light/dark cycle, and were provided food and water ad libitum. All animal studies were approved by the University of Vermont IACUC.
Exposures
For acute studies, mice were anesthetized with inhaled isoflurane and received either 50 μl of sterile saline, 100 ng ultra-pure LPS (InvivoGen, San Diego, CA), or 10 μg apo-SAA (Peprotech, Rocky Hill, NJ) in 50 μl sterile saline by oropharyngeal aspiration, and analyzed 24 hours later. Mice were exposed to 15ppm NO2 or HEPA-filtered room air for 1 hour and analyzed 24 hours later as previously-described (48). CAIKKβ mice and transgene-negative littermates were provided Dox-containing chow for 60 hours prior to analysis. For treatment with Anakinra (Biovitrum, Stockholm, Sweden), mice were administered 1 mg drug in 200 μl sterile saline by subcutaneous injection twice daily beginning one day before SAA exposure. For antigen sensitization studies, mice received either saline or SAA (as above) once on day 0, followed by 30 minutes of nebulized 1% ovalbumin (OVA), Fraction V (Sigma-Aldrich, St. Louis, MO) in saline on day 0, 1, and 2. Mice were then challenged with 30 minutes of nebulized 1% OVA on days 14, 15, and 16, and analyzed on day 18. Alum/OVA treated mice were sensitized on day 0 with 100 μg of OVA in Imject Alum (Thermo Scientific, Rockford, IL) and challenged with 30 minutes of nebulized 1% OVA on days 14, 15, and 16, and analyzed on day 18.
BAL collection and lung processing
Lungs were lavaged with 1 ml DPBS (Sigma-Aldrich) from which cells were counted by hemocytometer and differential analysis was performed by cytospin and H&E stain. After lavage, lungs were flash frozen in liquid nitrogen for RNA analysis.
Macrophages
For the isolation of primary macrophages, C57BL/6, TLR2−/−, TLR4−/−, and MyD88−/− mice were administered 1 ml of 4% thioglycollate by intraperitoneal injection. 96 hours later, mice were euthanized and peritoneal lavage was performed to collect peritoneal exudate cells. Cells were plated in RPMI-1640 supplemented with 10% fetal bovine serum, penicillin and streptomycin, L-glutamine, and 2-ME and challenged for 16 hours with apo-SAA followed where indicated by 30 minutes of 5 mM ATP or 8 hours of 500 μg/ml Imject Alum (Thermo Scientific). C57BL/6, NLRP3−/−, ASC−/−, and Caspase1−/− transformed macrophage cell lines (49) were maintained in vitro in RPMI-1640 with 10% FBS, penicillin and streptomycin, L-glutamine, and 2-ME. Following stimulation, cell-free supernatants were flash frozen for later analysis.
Cytokine analysis
Cytokines from BAL and cell supernatants were analyzed by ELISA for IL-1β and TNFα (BD Biosciences, San Jose, CA), as well as IL-23 (R&D Systems, Minneapolis, MN). PGE2 was assessed by EIA (Cayman Chemical, Ann Arbor, MI). Customized Bio-Plex (Bio-Rad, Hercules, CA) and Milliplex assays (Millipore, Billerica, MA) were used to measure IL-5, IL-13, and IL-17, as well as IL-1α, IL-1β, TNFα, IL-6, GM-CSF, G-CSF, KC, MIP-1α, MIP-1β, MCP-1, IL-12p40, and IL-12p70.
Quantitative RT-PCR
Total RNA was extracted from frozen whole lungs or transformed macrophages using the PrepEase RNA Isolation kit (USB Corp., Cleveland, OH) and reversed transcribed to cDNA using the iScript kit from Bio-Rad. Primers were designed for mouse Saa1, Saa2, Saa3, and Il1b and RT-PCR was performed using SYBR Green Supermix (Bio-Rad) and normalized to Gapdh or Actb using the ΔΔCT method, as previously described (47).
Splenocyte restimulation
Splenocytes from experimental mice and C57BL/6 control mice were isolated using Lymphocyte Separation Media (MP Biomedicals, Solon, OH) as previously described (48). 4×106 cells/ml were cultured in RPMI-1640 supplemented with 10% FBS, pen/strep, L-glutamine, and 2-ME, and were activated with 100 μg/ml of OVA in 48-well plates. Following 96 hours of stimulation, supernatants were collected for analysis by Milliplex.
Bone marrow-derived dendritic cells (BMDC)
Bone marrow was flushed from the femurs and tibae and cultured on 24-well plates at 1 × 106 cells/well (1 ml/well) in RPMI-1640 containing 10% serum and 10% conditioned media from X63-GMCSF myeloma cells transfected with murine GM-CSF cDNA (kindly provided by Dr. Brent Berwin, Dartmouth College). Media was replaced on days 2 and 4 and the adherent and lightly-adherent BMDCs, predominantly CD11b+CD11c+ by FACS, were collected on day 6. BMDCs were treated with SAA for 16 hours. For flow cytometry, BMDCs were detached using versene and gentle scraping, washed in FACS buffer (DPBS with 5% FBS and 0.1% sodium azide) and 1×106 cells were incubated with Fc block (2.5 μg/ml anti-CD16/CD32) (BD Pharmingen) for 30 minutes at 4°C, washed in FACS buffer and then stained for 30 minutes at 4°C in 100μl of antibody solution at the optimal concentration. Cells were stained with: anti-CD80-PE (BD Pharmingen), anti-CD86-Alexa 647 (Caltag, Carlsbad, CA), anti-MHC II-PerCP/Cy5.5 (BD Pharmingen), and biotinylated anti-OX40L (BD Pharmingen). Biotinylated antibodies were detected using streptavidin-PE (BD Pharmingen). Following staining, all cells were washed and fixed in DPBS with 5% FBS and 1% paraformaldehyde. Cells were analyzed on a Becton Dickinson LSR II FACS flow cytometer equipped to distinguish as many as 7 fluorophores 1–3 days following staining. Dead cells were excluded from analysis by FSC and SSC gating. Data were analyzed using FlowJo (Tree Star Inc., Ashland, OR).
SAA contaminant analysis
BMDCs were treated with SAA for 16 hours in the presence or absence of Polymxyin B (Sigma-Aldrich) at 25 μg/ml and 1 μg/ml. Proteinase K (Sigma-Aldrich) at 25 μg/ml and 1 μg/ml (or absent) was incubated with apo-SAA at 37°C for 1 hour, heated to 100°C for 5 minutes to deactivate the enzyme, and allowed to cool to room temperature before addition to cells. Cells were treated for 16 hours and cell-free supernatants were flash frozen prior to further analysis.
CD4+ T cell culture with BMDC-conditioned media
Cell-free conditioned media from unstimulated or 24-hour SAA-exposed BMDCs were incubated with 1×106 splenic CD4+ T cells from naïve mice that were stimulated in the presence of 5 μg/ml immobilized anti-CD3 and 1 μg/ml soluble anti-CD28 for 96 hours. Alternatively, CD4+ T cells were polyclonally stimulated in media alone or in the presence of 1μg/ml apo-SAA.
Splenocyte cultures
Splenocytes from C57BL/6, TLR2−/−, NLRP3−/−, ASC−/−, and Caspase-1−/−mice were cultured at 4 × 106 cells/ml in RPMI-1640 containing 10% FBS, pen/strep, L-glutamine, and 2-ME on plates coated with 5 μg/ml anti-CD3, and treated with 1 μg/ml soluble anti-CD28, 10 ng/ml Anakinra, and 1 μg/ml SAA. Cell-free supernatants were analyzed 96 hours after stimulation.
Statistics
Data were analyzed by two-tailed unpaired t-test or one-way ANOVA and Bonferroni post-hoc test using GraphPad Prism 4 for Windows (GraphPad Software, Inc.). A p value smaller than 0.05 was considered statistically significant.
Results
SAA3 expression in mouse lung
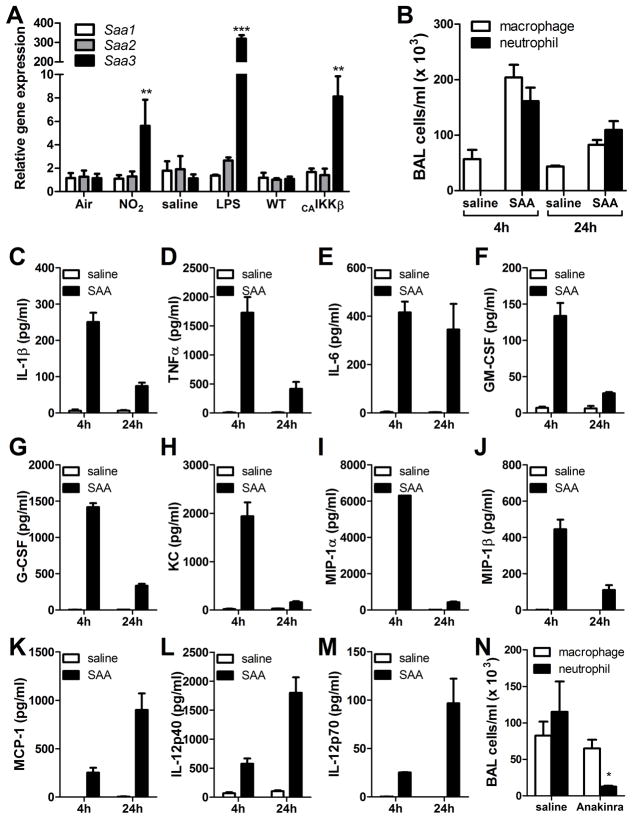
Our studies have employed multiple mechanisms of allergic sensitization, including NO2 exposure, oropharyngeal administration of LPS, and airway epithelial-specific NF-κB activation, each of which can promote mixed TH2/TH17 responses (40, 47, 48). Following exposure to these stimuli, lungs of C57BL/6 mice exhibited a preferential mRNA induction of Saa3 over Saa1 or Saa2 (Fig. 1A). Mice exposed to 15 ppm of NO2 for 1 hour and analyzed 24 hours later showed a 6-fold induction of Saa3 in the lung, very similar to the response in transgenic mice that inducibly express a constitutively-active form of IKKβ in the airway epithelium following 48 hours of doxycycline administration (Fig. 1A). Oropharyngeal administration of 100ng LPS, a low dose used in models of inhalational allergic sensitization (40, 50), induced high mRNA levels of Saa3 24 hours post challenge (Fig. 1A). These results demonstrate expression of Saa3 in the lung under conditions that facilitate mixed TH2/TH17 polarization.
FIGURE 1. SAA is expressed in the lungs during mixed TH2/TH17 allergic sensitization regimens and induces pulmonary inflammation upon inhalational exposure.
Quantitative PCR of whole lung for SAA isoforms in mice exposed to NO2, LPS, or in which NF-κB has been activated in the airway epithelium (A). C57BL/6 mice were administered 10 μg of SAA by oropharyngeal aspiration and analyzed 4 and 24 hours later. Bronchoalveolar lavage (BAL) total cell counts were performed by hemocytometer and differential analysis was by cytospin (B). BAL fluid was analyzed by Milliplex assay for IL-1β (C), TNFα (D), IL-6 (E), GM-CSF (F), G-CSF (G), KC (H), MIP-1α (I), MIP-1β (J), MCP-1 (K), IL-12p40 (L), and IL-12p70 (M). Data are representative of three independent experiments. C57BL/6 mice were administered saline or 1 mg Anakinra (n= 3 per group) by subcutaneous injection twice daily beginning one day prior to oropharyngeal aspiration of 10 μg SAA. At 24 hours, BAL total cell counts were performed by hemocytometer and differential analysis was by cytospin (N). * = p<0.05, ** = p<0.005, and *** = p<0.001 compared to control exposures (A) or saline controls (B–N).
SAA elicits robust IL-1-dependent pulmonary inflammation
Recombinant human apo-SAA (with functional similarity to mouse SAA3) is available commercially from Peprotech, and is reported to contain less than 1 EU/μg endotoxin. Our own Limulus amebocyte lysate assay confirmed that this was indeed true (data not shown). To determine the effects of SAA in the lung, mice were administered 10 μg of apo-SAA or saline by oropharyngeal aspiration and analyzed at 4 and 24 hours. In contrast to saline, SAA induced a robust airway neutrophilia (Fig. 1B) and the production of inflammatory cytokines, as measured from bronchoalveolar lavage (BAL) fluid (Fig. 1C–M). Since IL-1β was present at elevated concentrations in the BAL fluid (Fig. 1C), we administered IL-1 receptor antagonist (Anakinra) or saline to mice prior to apo-SAA aspiration. In the Anakinra-treated mice, airway neutrophilia was significantly reduced compared to apo-SAA-exposed mice treated with saline vehicle (Fig. 1N). These results implicate an important function of IL-1 in SAA-promoted inflammation.
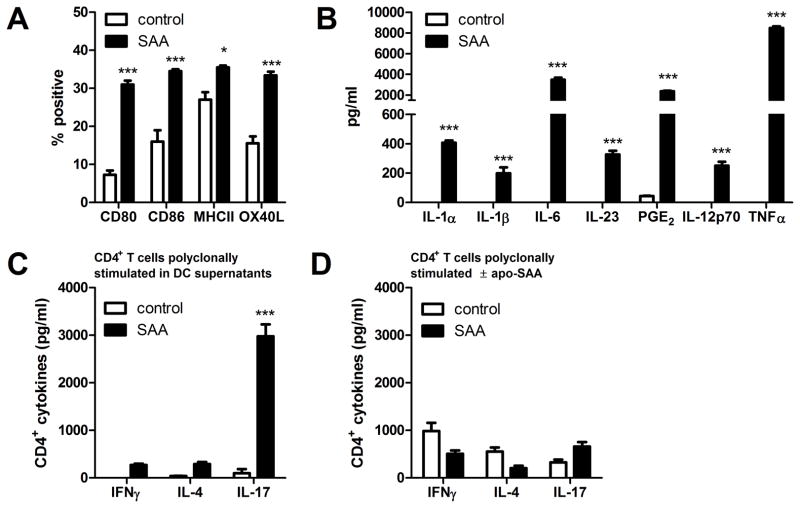
SAA activates dendritic cells that promote IL-17A production from CD4+ T cells
To determine the effects of SAA on antigen presenting cells, which are critical for initiation of CD4+ T cell responses, bone marrow-derived dendritic cells (BMDCs) from C57BL/6 mice were challenged with apo-SAA and analyzed 16 hours later. We observed increases in surface markers of DC maturation, including CD80, CD86, MHCII, and OX40L (Fig. 2A), upon exposure of BMDCs to apo-SAA. In addition, BMDCs secreted IL-1α, IL-1β, IL-6, and IL-23 (Fig. 2B), cytokines that participate in TH17 polarization and maintenance (10, 23, 51). Furthermore, treatment with SAA caused BMDCs to secrete a significant amount of prostaglandin E2 (PGE2) (Fig. 2B), which has recently been shown to induce dendritic cells to preferentially secrete IL-23, and thus contribute to the development of a TH17 response (52, 53). DCs exposed to apo-SAA also secreted small amounts of the Th1-polarizing cytokine, IL-12p70, and substantial amounts of pro-inflammatory TNFα (Fig. 2B). When the cell-free supernatants from these SAA-treated BMDCs were provided to polyclonally-stimulated naïve CD4+ T cells, a significant production of IL-17 was induced, compared to very small amounts of IFN-γ and IL-4 (Fig. 2C). Importantly, treatment of the polyclonally-stimulated naïve CD4+ T cells with SAA resulted in no significant production of any of these cytokines (Fig. 2D), indicating that the effect of SAA is directly on dendritic cells, which in turn drive the polarization of the CD4+ T cells through the secretion of soluble mediators, including IL-1β.
FIGURE 2. SAA elicits inflammatory mediator production and dendritic cell maturationin vitro.
Bone marrow-derived dendritic cells were treated with 1 μg/ml SAA for 16 hours and analyzed for surface markers of maturation (A) and secretion of TH17-polarizing mediators (B). The conditioned media from control or SAA-exposed BMDCs (C) or fresh media with or without SAA (D) was transferred to CD4+ T cells that were polyclonally stimulated with anti-CD3 and anti-CD28. After 96 hours, IFNγ, IL-4, and IL-17A were measured in supernatants. Data are representative of three independent experiments. * = p<0.05, ** = p<0.005, and *** = p<0.001 compared to control exposures.
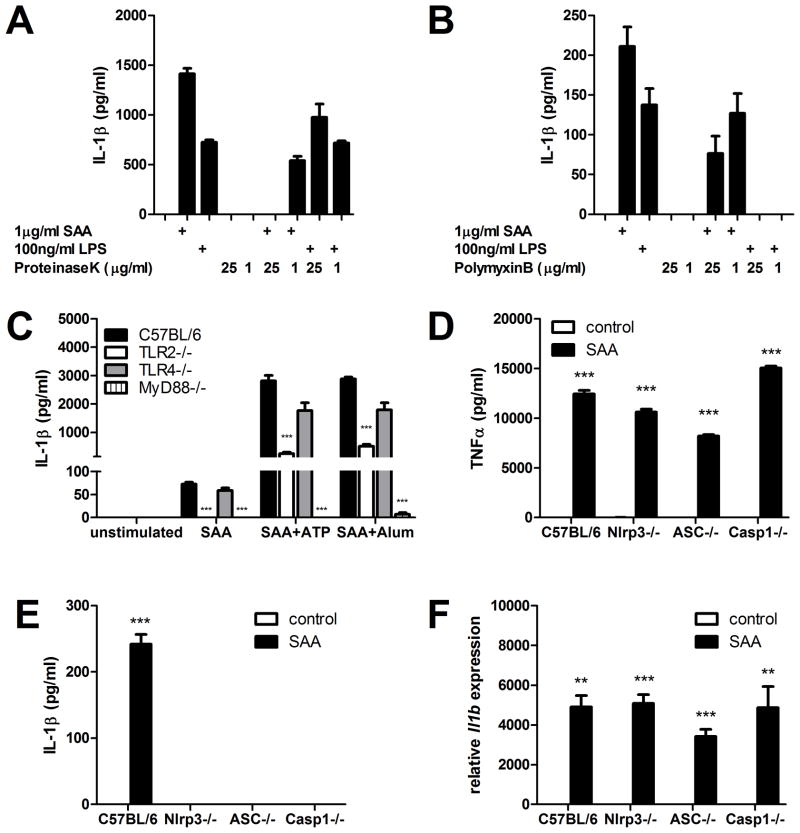
SAA-induced IL-1β secretion requires TLR2 and the NLRP3 inflammasome
Since IL-1β has been implicated as a TH17-polarizing and -priming factor, we determined cell receptors required for SAA-induced secretion of this cytokine. To ensure that the effects of SAA required the recombinant SAA protein and were not due to endotoxin contamination, we cultured bone marrow-derived dendritic cells (BMDCs) for 16 hours with apo-SAA or LPS that had been treated with Proteinase K or left untreated. All samples were then boiled to inactivate the Proteinase K. Under these conditions, we observed a dose-dependent reduction in the response to apo-SAA, but no effect on the LPS-induced IL-1β response (Fig. 3A). In contrast, exposure of BMDCs to LPS in the presence of Polymyxin B completely blocked IL-1β production, whereas the presence of Polymyxin B during apo-SAA treatment still allowed for substantial amounts of IL-1β to be produced (Fig. 3B). Having demonstrated the requirement for SAA protein and the minimal contribution of contaminating endotoxin in the effects of apo-SAA, we exposed peritoneal exudate macrophages from TLR2−/−, TLR4−/−, and MyD88−/− mice to SAA for 16 hours. In these cells, IL-1β secretion is primarily dependent upon TLR2 and MyD88, both in the absence or presence of ATP or aluminum crystals (Fig. 3C), potent inducers of IL-1β secretion. TLR4−/− PECs showed no significant reduction in their ability to secrete IL-1β following SAA treatment, further ruling out the contribution of endotoxin contamination of apo-SAA to its biological effects in these experimental systems. The response to SAA was also examined in transformed macrophage cell lines from wild type, NLRP3−/−, ASC−/−, and Caspase1−/− mice. Whereas apo-SAA-induced levels of TNFα, an inflammasome-independent cytokine, were highly induced in all cells (Fig. 3D), complete abrogation of IL-1β secretion was observed in response to apo-SAA in NLRP3-, ASC-, and Caspase-1-deficient macrophages (Fig. 3E). Indicative of the roles these molecules play in processing the pro-IL-1β protein, levels of Il1b message expression were robustly induced in the wild type, NLRP3-, ASC-, and Caspase-1-deficient macrophages (Fig. 3F).
FIGURE 3. SAA-induced IL-1β production requires TLR2, MyD88, and the NLRP3 inflammasome.
Cell-free supernatants from bone marrow-derived dendritic cells treated with SAA or LPS in the presence or absence of Proteinase K (A) or Polymyxin B (B) were analyzed by ELISA after 24 hours. Peritoneal exudate macrophages from wild type (C57BL/6), TLR2−/−, TLR4−/−, and MyD88−/− mice were unstimulated or were primed overnight with 1 μg/ml SAA alone or followed by 30 minutes of 5 mM ATP or 8 hours of 500 μg/ml Alum, and supernatants were analyzed for IL-1β secretion (C). Transformed macrophages from wild type (C57BL/6), Nlrp3−/−, ASC−/−, and Caspase-1−/− mice were treated for 24 hours with 1 μg/ml SAA and supernatants were analyzed for TNFα (D) and IL-1β (E) secretion. Il1b expression was measured from wild type (C57BL/6), Nlrp3−/−, ASC−/−, and Caspase-1−/− transformed macrophages that had been untreated or exposed for 4 hours to 1 μg/ml SAA (F). * = p<0.05, ** = p<0.005, and *** = p<0.001 compared to wild type (C) or control exposures (D–F).
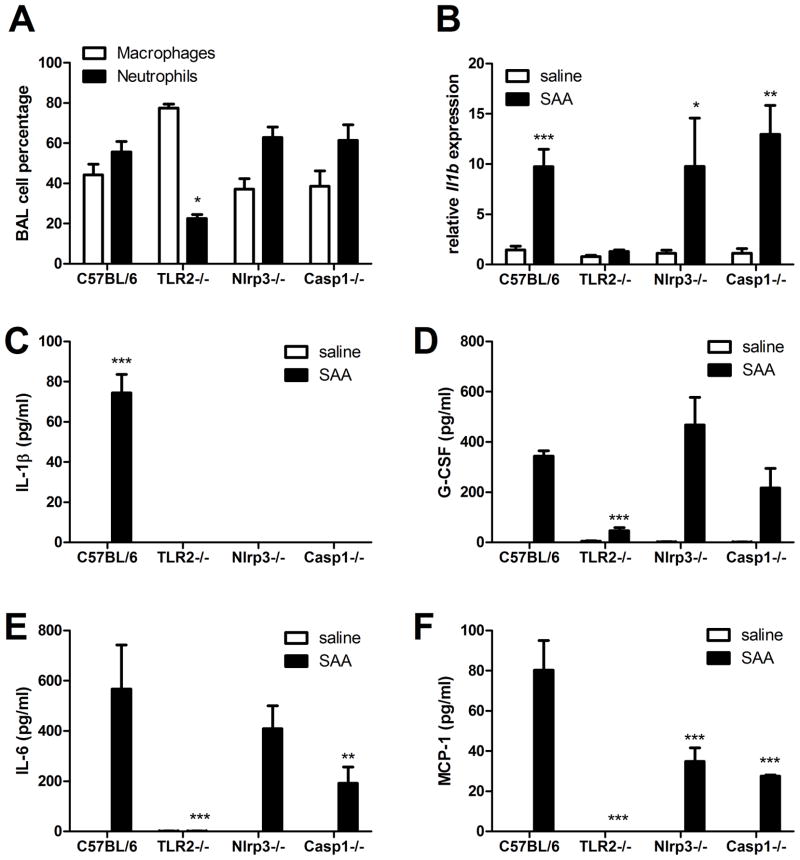
SAA-induced pulmonary inflammation requires TLR2 and involves the NLRP3 inflammasome
Having demonstrated the important functions of TLR2, NLRP3, and Caspase-1 for SAA-induced IL-1β secretion in vitro, we next performed additional pulmonary SAA exposures to determine the contribution of these molecules to inflammation in vivo. Oropharyngeal administration of 10 μg apo-SAA elicited a robust influx of neutrophils into the lung after 24 hours in C57BL/6 mice, a response that was diminished in TLR2−/−, but not NLRP3−/− or Caspase-1−/− mice (Fig. 4A). Measurement of Il1b gene expression in the lung following SAA aspiration revealed increased mRNA abundance in wild type, NLRP3−/− and Caspase1−/− mice, but not in TLR2−/− mice (Fig 4B). Analysis of the bronchoalveolar lavage (BAL) fluid from these mice demonstrated that TLR2 signaling was also required for IL-1β, G-CSF, IL-6, and MCP-1 production, whereas NLRP3 and Caspase-1 were necessary only for the production of IL-1β (Fig. 4C–F). The NLRP3−/− and Caspase1−/− mice also displayed reduced levels of IL-6 and MCP-1, two cytokines that can be induced by IL-1β (54, 55), and are perhaps diminished as a consequence of lost IL-1β signaling.
FIGURE 4. SAA-induced pulmonary inflammation requires TLR2 and involves the NLRP3 inflammasome.
Wild type (C57BL/6), TLR2−/−, Nlrp3−/−, and Caspase-1−/− mice were administered 10μg of SAA by oropharyngeal aspiration and analyzed 24 hours later. Bronchoalveolar lavage (BAL) total cell counts were performed by hemocytometer and differential analysis was by cytospin (A). Il1b expression was measured from whole lung by quantitative RT-PCR (B). BAL fluid was analyzed by Milliplex assay for IL-1β (C), G-CSF (D), IL-6 (E), and MCP-1 (F). Data are representative of three independent experiments. * = p<0.05, ** = p<0.005, and *** = p<0.001 compared to wild type (A) or compared to saline (B–F).
SAA-promoted allergic sensitization favors a TH17 response that requires IL-1Rα signaling
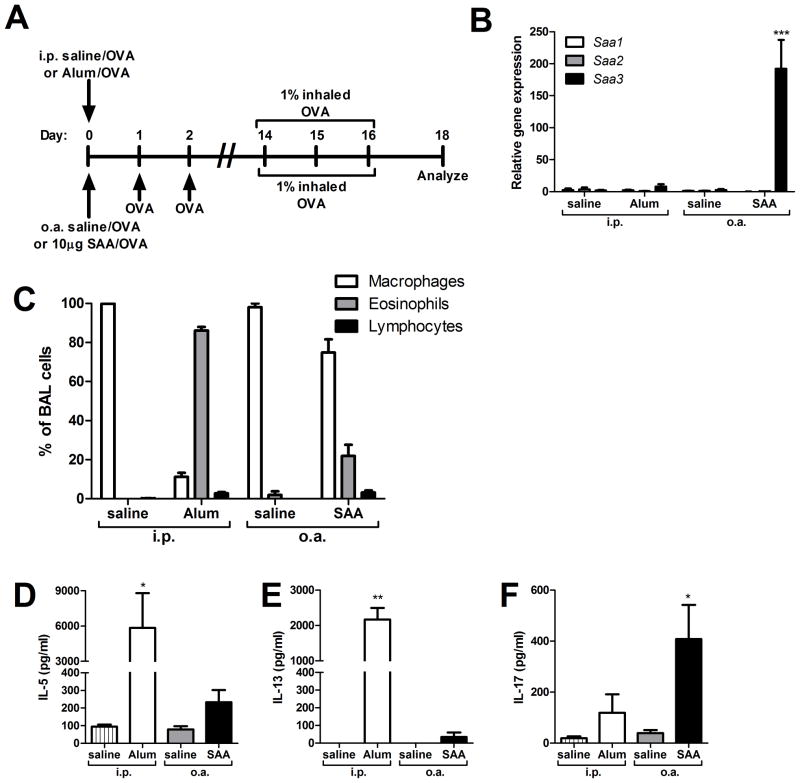
Having demonstrated evidence for dendritic cell maturation and TH17 polarization in response to apo-SAA in vitro, we sought to compare a conventional TH2 allergic sensitization protocol, a well-characterized Alum/OVA model (56, 57), with an experimental model of apo-SAA-promoted antigen sensitization (Fig. 5A). A very distinct profile of SAA gene expression occurred in the lung 24 hours following antigen sensitization in the two models (Fig. 5B). Intraperitoneal (i.p.) injection of the adjuvant Alum elicited little SAA expression in the lung (Fig. 5B, 3.4-fold induction of Saa1, 6.9-fold induction of Saa2, and 3.0-fold induction of Saa3), but induced strong expression of Saa1 (781-fold induction) and Saa2 (199-fold induction) in the liver, with only 4.2-fold induction of Saa3 (data not shown). This is consistent with the reported roles of liver SAA1 and SAA2 in the systemic inflammatory response. In contrast, pulmonary administration of apo-SAA by oropharyngeal aspiration (o.a.) selectively induced mRNA expression of Saa3 in the lungs (Fig. 5B), with no systemic (liver) production of any other isotypes (data not shown).
FIGURE 5. SAA inhalation promotes TH17 allergic sensitization.
Mice underwent antigen sensitization via oropharyngeal aspiration (o.a.) with either saline and OVA (saline/OVA) or SAA and OVA (SAA/OVA), or via intraperitoneal (i.p.) injection with either saline and OVA (saline/OVA) or Alum and OVA (Alum/OVA), according to the schema (A). Saa1, Saa2, and Saa3 gene expression in whole lung was measured on day 1, 24 hours after i.p. injection with saline or Alum, or 24 hours after o.a. administration of saline or SAA (B). On day 18, total cell counts from bronchoalveolar lavage (BAL) fluid were performed by hemocytometer and differential analysis was by cytospin (C). On day 18, splenocytes from i.p. saline, i.p. Alum, o.a. saline, and o.a. SAA mice were restimulated in vitro with OVA for 96 hours and IL-5 (D), IL-13 (E), and IL-17A (F) levels in culture media were measured. Data are representative of three independent experiments. * = p<0.05, ** = p<0.005, and *** = p<0.001 compared to saline controls (B, D–F).
The disparate induction of Saa expression in liver and lung led us to speculate that differences in route of sensitization and adjuvant used modulate local effects that may contribute to distinct responses in the Alum- and SAA-promoted allergic sensitization models. Therefore, we examined the BAL cell profiles from challenged mice subjected to the two models of antigen sensitization. Mice sensitized i.p. with Alum/OVA robustly recruited eosinophils into the lung compared with unsensitized saline/OVA controls, whereas the SAA/OVA sensitized mice showed eosinophilia on the order of 10–20%, which is more representative of that typically present in the BAL fluid in an asthmatic patient (Fig 5C). Splenocytes from sensitized and control mice were cultured and restimulated with OVA for 96 hours. The Alum/OVA mice, as expected, responded by producing copious amounts of the TH2 cytokines IL-5 and IL-13, whereas SAA/OVA mice produced modest but elevated levels of these cytokines compared to the saline control mice (Fig 5D–E). However, SAA/OVA sensitized mice, unlike Alum/OVA mice, displayed significant production of IL-17 in response to antigen (Fig. 5F), recapitulating our in vitro findings from Fig. 2C that the SAA-induced inflammatory response can polarize T cells to secrete primarily IL-17A.
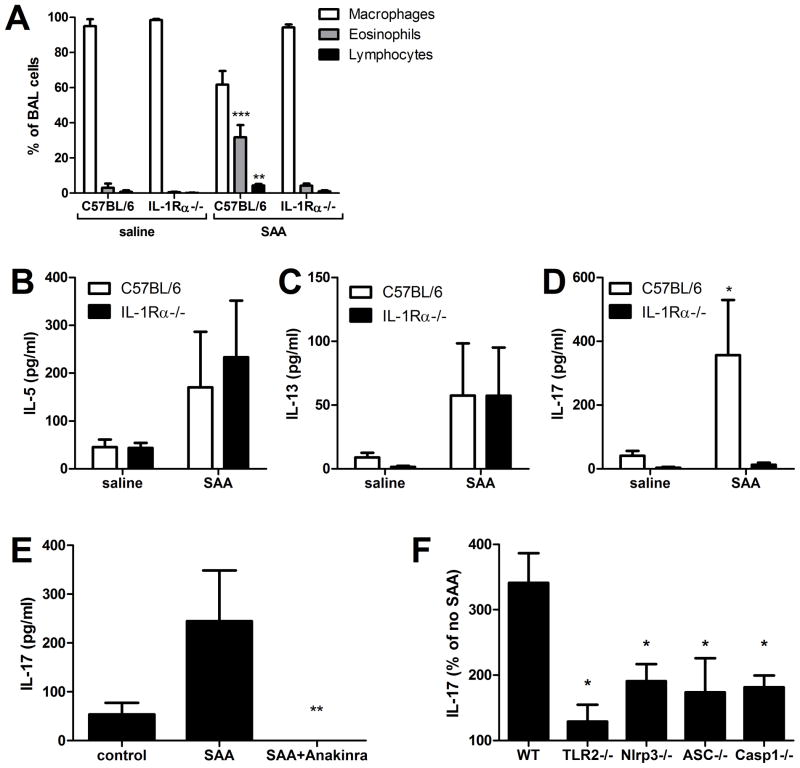
As we have demonstrated, the capacity of antigen-restimulated splenocytes to produce IL-17 may rely on the microenvironmental cytokine milieu that is generated by dendritic cells and other antigen presenting cells in response to SAA. Since SAA promotes IL-1-dependent pulmonary inflammation, we repeated our SAA/OVA sensitization model (Fig. 5A) using IL-1 receptor alpha (IL-1Rα) knockout mice to determine whether IL-1signaling played a critical role in the CD4+ T cell priming and polarization process. Following antigen challenge, the cellular BAL profile revealed that IL-1Rα−/− mice exposed to SAA/OVA recruited fewer eosinophils and lymphocytes to the lung than did wild type mice (Fig. 6A). Furthermore, when splenocytes from these sensitized and challenged mice were restimulated in vitro with OVA, wild type and knockouts showed a similar induction of IL-5 and IL-13, but the IL-17A production was absent in the IL-1Rα−/− mice (Fig. 6B–D). To show that an IL-1Rα ligand was required for SAA to induce IL-17A production, and that the IL-17A deficiency seen in IL-1Rα−/− mice was not due to a developmental defect, splenocytes from wild type mice were polyclonally stimulated in the presence of SAA, with or without Anakinra. The addition of Anakinra, even at a relatively low dose of 10 ng/ml, completely abrogated IL-17A production (Fig. 6E). Finally, to investigate the requirement for TLR2 and NLRP3 inflammasome components for SAA-promoted IL-17A production, splenocytes from C57BL/6, TLR2−/−, NLRP3−/−, ASC−/−, and Caspase-1−/− mice were plated and polyclonally stimulated in the presence or absence of apo-SAA. The TLR2−/− splenocytes showed a nearly complete inability of SAA to augment IL-17A production after 96 hours of stimulation (Fig 6F). IL-17 production was also impaired in splenocytes that lacked components of the NLRP3 inflammasome complex, although the abrogation was more moderate (Fig 6F). Clearly, IL-1 signaling plays a critical role in the induction of SAA-promoted allergic airway disease, specifically in mediating the production of IL-17A.
FIGURE 6. SAA-promoted allergic sensitization and TH17 polarization require IL-1R.
C57BL/6 and IL-1Rα−/− mice were antigen-sensitized with saline and OVA (saline) or SAA and OVA (SAA) by o.a., according to the timeline in 5A. On day 18, total and differential cell counts from BAL fluid were performed (A). Splenocytes were restimulated in vitro with OVA for 96 hours and IL-5 (B), IL-13 (C), and IL-17 (D) levels in culture media were measured. Splenocytes from C57BL/6 mice in the presence or absence of SAA and 10 ng/ml Anakinra (E) and splenocytes from C57BL/6, TLR2−/−, NLRP3−/−, ASC−/−, and Casp1−/− (F) mice were polyclonally stimulated for 96 hours with anti-CD3 and anti-CD28 in the presence or absence of 1 μg/ml SAA and IL-17A was measured by ELISA (F). Data are representative of two independent experiments * = p<0.05, ** = p<0.005, and *** = p<0.001 compared to saline controls (A-D), compared to SAA (E) or compared to wild type (F).
Discussion
Our results reported herein implicate a causal role and a molecular mechanism for SAA in the pathogenesis of allergic asthma. Several of our previous studies have focused on inhalational antigen sensitization via exogenous insults, predominantly the pollutant NO2. Inhalation of NO2 activates airway epithelial NF-κB and promotes a mixed TH2/TH17 response (48, 58), which can be recapitulated through the activation of NF-κB in airway epithelium and inhalation of antigen (47). Within the lung, SAA3 is induced as a consequence of both NO2 inhalation and airway epithelial NF-κB activation. SAA isotypes are differentially expressed in distinct tissues (13). SAA1 and 2 in mice are expressed predominantly in the liver, are most commonly found bound to HDL in the circulation, and are acutely upregulated in systemic disease states (13, 59). In contrast, SAA3 in mice has been shown to be expressed in a wide variety of cells and tissues, including leukocytes and epithelium, and has never been identified bound to HDL (60–62). In humans, SAA1 and 2 are expressed in the liver and in the lung and have been associated locally with the TLR2-dependent development of sarcoidosis (63). The rapid and robust induction of SAA in response to a panel of inhalational stimuli (Fig. 1A) indicates a possible role for SAA as a mediator in both allergic sensitization and during antigen challenge (exacerbation). Based on our results, we speculate that local production of SAA, rather than the particular isoform expressed, is capable of influencing local innate and adaptive immune responses. Therefore, whereas SAA3 is the predominant form of SAA expressed in mouse lungs, SAA1 or SAA2 in human lungs may exert effects similar to those we report herein.
The inflammatory cytokine milieu surrounding naïve dendritic cells is key to their maturation and to the polarization of the CD4+ T cell response, as we have previously demonstrated using a model of inducible airway epithelial NF-κB activation (47). In addition, the work of Ivanov et al. has implicated SAA as an important mediator for TH17 polarization in the gut in response to colonization with segmented filamentous bacteria (20). We have shown a profound effect of apo-SAA on bone marrow-derived dendritic cells that includes antigen-presenting cell maturation and the secretion of the TH17-polarizing mediators IL-1α, IL-1β, IL-6, IL-23, and PGE2. From the studies presented herein using IL-1 receptor antagonism with Anakinra, it is clear that IL-1α and IL-1β are potential mediators induced by SAA that are capable of eliciting predominantly IL-17A production from naïve, polyclonally stimulated CD4+ T cells.
IL-1β requires cleavage via Caspase-1 for proper secretion, which is facilitated as a consequence of inflammasome assembly and activation. The NLRP3 inflammasome has emerged as a critical cytosolic sensor for a number of endogenous mediators, including amyloid proteins (2, 22), that are capable of promoting IL-1β secretion. Our studies demonstrate that regulation of IL-1β production in response to SAA occurs as two distinct levels. At the transcriptional level, SAA-induced IL-1β requires TLR2, a finding first reported in human THP-1 cells by Cheng et al (14). At the post-translational level, use of transgenic mice that are deficient in proteins of the NLRP3 complex demonstrate a clear role for the NLRP3 inflammasome in SAA-induced IL-1β secretion. TLR2−/− mice exhibit severe impairment in the inflammatory response to oropharyngeal administration of apo-SAA, including a diminution in neutrophil recruitment and decreased secretion of inflammatory cytokines in the BAL. The remaining neutrophilia in these mice is likely due to the capacity of apo-SAA to stimulate neutrophil chemotaxis via FPRL1/FPR2 (17, 18), which remains intact in all of the mice we studied. Taken together, our in vitro and in vivo results indicate that proper production and secretion of IL-1β in response to SAA is regulated both at the transcriptional level by TLR2, and at the level of secretion by the NLRP3 inflammasome. Furthermore, they suggest that IL-1β not only participates in the inflammatory cascade, but also amplifies the response through the augmented production of select inflammatory cytokines. Nevertheless, our in vivo data using Anakinra, an antagonist of the IL-1 receptor that blocks the effects of both IL-1α and IL-1β, demonstrate a causal role for IL-1 in SAA-promoted pulmonary neutrophilia, whereas the in vivo data from the NLRP3- and Caspase-1-deficient mice reveal very modest reductions in inflammatory cytokines (aside from IL-1β) and pulmonary neutrophilia following SAA aspiration. Taken together, these findings suggest that there may be an additional role for IL-1α release in response to SAA that requires further investigation.
Models of in vitro and in vivo TH17 polarization require the presence of IL-1, IL-23, IL-6 (23, 64–67) and repression of TH1- and TH2-polarizing cytokines to establish a unique environment that challenge with apo-SAA appears to replicate. The TH2-polarizing Alum/OVA model has long been criticized for the magnitude of the response, whereas inhalational models of allergic sensitization (using different adjuvants such as LPS or cigarette smoke) tend to induce a modest eosinophilia that is more representative of human asthma (68). The more moderate response seen in our SAA/OVA sensitization model could represent a different aspect of the asthma syndrome, one that is less TH2 in nature and more TH17. It has recently been shown that IL-17 production promoted by IL-1β involves conversion of Foxp3+ T regulatory cells to RORγt-expressing TH17 cells (10, 11). In addition, in the human disease and in mouse models of severe allergic asthma, CD4+ T cell production of IL-17 can originate from a population of CRTH2+ effector cells/memory cells that are capable of secreting TH2 cytokines and co-expressing the transcription factors GATA3 and RORγt, a population that can be induced upon stimulation with pro-inflammatory cytokines, including IL-1β (69). Our studies have not identified the precursor CD4+ T cell population that develops the capacity to produce IL-17A in response to SAA, nor have we yet thoroughly investigated the spectrum of TH17-related cytokines produced by these IL-17A-producing CD4+ T cells. Regardless of the mechanism of CD4+ T cell conversion, the role that SAA may play in the process as an endogenous mediator has far-reaching implications for the pathogenesis of IL-17-producing CD4+ T cells in severe allergic asthma.
The conclusions of the studies described herein are three-fold. First, it is clear that the acute phase SAA proteins are more than simply biomarkers of disease severity. Instead, they function as biological mediators through the stimulation of TLR2 and the NLRP3 inflammasome to regulate pulmonary cytokine production and neutrophilia. Second, while the properties of SAA that enable activation of these pathways remain to be determined, the capacity to both induce IL-1β gene expression and allow for IL-1β secretion make SAA distinct from other endogenous amyloid peptides and proteins that function solely in the later step of IL-1β release (2, 22). Third, SAA is sufficient to function as an adjuvant to promote allergy to an innocuous inhaled antigen, in a manner that is dependent upon IL-1 receptor signaling to stimulate the capacity of CD4+ T cells to produce IL-17A. Whether SAA is necessary for Th17 development in response to inhalational antigen sensitization and at what threshold concentration endogenous SAA manifests TLR2/NLRP3 stimulation remain to be determined. These results are first to link pulmonary SAA, IL-1β, and IL-17A in a manner that explains their interrelationships in allergic asthma and their mechanisms of action. Based upon our findings, it is evident that novel models of SAA/IL-1-mediated allergic airway disease may provide new insight into the endogenous mechanisms behind the inappropriate or maladaptive immune responses at play in the complex phenotypes of allergic asthma.
Acknowledgments
This work was supported by R01 HL089177 and http://projectreporter.nih.gov/project_info_details.cfm?aid=7839235&icde=5567292NCRRCOBREP20RR15557.
References
- 1.Glaccum MB, Stocking KL, Charrier K, Smith JL, Willis CR, Maliszewski C, Livingston DJ, Peschon JJ, Morrissey PJ. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 2.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nunez G, Yodoi J, Kahn SE, Lavelle EC, O’Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daheshia M, Yao JQ. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35:2306–2312. doi: 10.3899/jrheum.080346. [DOI] [PubMed] [Google Scholar]
- 4.Clutterbuck AL, Mobasheri A, Shakibaei M, Allaway D, Harris P. Interleukin-1beta-induced extracellular matrix degradation and glycosaminoglycan release is inhibited by curcumin in an explant model of cartilage inflammation. Ann N Y Acad Sci. 2009;1171:428–435. doi: 10.1111/j.1749-6632.2009.04687.x. [DOI] [PubMed] [Google Scholar]
- 5.Gris D, Ye Z, Iocca HA, Wen H, Craven RR, Gris P, Huang M, Schneider M, Miller SD, Ting JP. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J Immunol. 185:974–981. doi: 10.4049/jimmunol.0904145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin WS, Liu L, Li Y, Mrak RE, Barger SW. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J Neuroinflammation. 2006;3:5. doi: 10.1186/1742-2094-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rooney M, Symons JA, Duff GW. Interleukin 1 beta in synovial fluid is related to local disease activity in rheumatoid arthritis. Rheumatol Int. 1990;10:217–219. doi: 10.1007/BF02274836. [DOI] [PubMed] [Google Scholar]
- 8.Radema SA, van Deventer SJ, Cerami A. Interleukin 1 beta is expressed predominantly by enterocytes in experimental colitis. Gastroenterology. 1991;100:1180–1186. [PubMed] [Google Scholar]
- 9.So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Kim J, Boussiotis VA. IL-1beta-mediated signals preferentially drive conversion of regulatory T cells but not conventional T cells into IL-17-producing cells. J Immunol. 185:4148–4153. doi: 10.4049/jimmunol.1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Eynon EE, Flavell RA, Kang I. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 115:530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheng N, He R, Tian J, Ye PP, Ye RD. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J Immunol. 2008;181:22–26. doi: 10.4049/jimmunol.181.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He RL, Zhou J, Hanson CZ, Chen J, Cheng N, Ye RD. Serum amyloid A induces G-CSF expression and neutrophilia via Toll-like receptor 2. Blood. 2009;113:429–437. doi: 10.1182/blood-2008-03-139923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorkman L, Karlsson J, Karlsson A, Rabiet MJ, Boulay F, Fu H, Bylund J, Dahlgren C. Serum amyloid A mediates human neutrophil production of reactive oxygen species through a receptor independent of formyl peptide receptor like-1. J Leukoc Biol. 2008;83:245–253. doi: 10.1189/jlb.0607-408. [DOI] [PubMed] [Google Scholar]
- 17.Liang TS, Wang JM, Murphy PM, Gao JL. Serum amyloid A is a chemotactic agonist at FPR2, a low-affinity N-formylpeptide receptor on mouse neutrophils. Biochem Biophys Res Commun. 2000;270:331–335. doi: 10.1006/bbrc.2000.2416. [DOI] [PubMed] [Google Scholar]
- 18.Su SB, Gong W, Gao JL, Shen W, Murphy PM, Oppenheim JJ, Wang JM. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med. 1999;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vallon R, Freuler F, Desta-Tsedu N, Robeva A, Dawson J, Wenner P, Engelhardt P, Boes L, Schnyder J, Tschopp C, Urfer R, Baumann G. Serum amyloid A (apoSAA) expression is up-regulated in rheumatoid arthritis and induces transcription of matrix metalloproteinases. J Immunol. 2001;166:2801–2807. doi: 10.4049/jimmunol.166.4.2801. [DOI] [PubMed] [Google Scholar]
- 20.Ivanov, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liepnieks JJ, Kluve-Beckerman B, Benson MD. Characterization of amyloid A protein in human secondary amyloidosis: the predominant deposition of serum amyloid A1. Biochim Biophys Acta. 1995;1270:81–86. doi: 10.1016/0925-4439(94)00076-3. [DOI] [PubMed] [Google Scholar]
- 22.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 24.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, Kolls JK. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol. 2008;181:4089–4097. doi: 10.4049/jimmunol.181.6.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buyukozturk S, Gelincik AA, Genc S, Kocak H, Oneriyidogan Y, Erden S, Dal M, Colakoglu B. Acute phase reactants in allergic airway disease. Tohoku J Exp Med. 2004;204:209–213. doi: 10.1620/tjem.204.209. [DOI] [PubMed] [Google Scholar]
- 26.Ozseker F, Buyukozturk S, Depboylu B, Yilmazbayhan D, Karayigit E, Gelincik A, Genc S, Colakoglu B, Dal M, Issever H. Serum amyloid A (SAA) in induced sputum of asthmatics: a new look to an old marker. Int Immunopharmacol. 2006;6:1569–1576. doi: 10.1016/j.intimp.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Wu TL, Chang PY, Tsao KC, Sun CF, Wu LL, Wu JT. A panel of multiple markers associated with chronic systemic inflammation and the risk of atherogenesis is detectable in asthma and chronic obstructive pulmonary disease. J Clin Lab Anal. 2007;21:367–371. doi: 10.1002/jcla.20197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tillie-Leblond I, Pugin J, Marquette CH, Lamblin C, Saulnier F, Brichet A, Wallaert B, Tonnel AB, Gosset P. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med. 1999;159:487–494. doi: 10.1164/ajrccm.159.2.9805115. [DOI] [PubMed] [Google Scholar]
- 29.Al-Ramli W, Prefontaine D, Chouiali F, Martin JG, Olivenstein R, Lemiere C, Hamid Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97:726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 31.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125:1028–1036. e1013. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laan M, Linden IL-17 as a potential target for modulating airway neutrophilia. Curr Pharm Des. 2002;8:1855–1861. doi: 10.2174/1381612023393864. [DOI] [PubMed] [Google Scholar]
- 34.Laan M, Palmberg L, Larsson K, Linden A. Free, soluble interleukin-17 protein during severe inflammation in human airways. Eur Respir J. 2002;19:534–537. doi: 10.1183/09031936.02.00280902. [DOI] [PubMed] [Google Scholar]
- 35.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 36.Wong CK, Ho CY, Ko FW, Chan CH, Ho AS, Hui DS, Lam CW. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001;125:177–183. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Xu CB, Cardell LO. Long-term exposure to IL-1beta enhances Toll-IL-1 receptor-mediated inflammatory signaling in murine airway hyperresponsiveness. Eur Cytokine Netw. 2009;20:148–156. doi: 10.1684/ecn.2009.0156. [DOI] [PubMed] [Google Scholar]
- 38.Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm. J Immunol. 2009;183:6236–6243. doi: 10.4049/jimmunol.0900614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eisenbarth SC. Use and limitations of alum-based models of allergy. Clin Exp Allergy. 2008;38:1572–1575. doi: 10.1111/j.1365-2222.2008.03069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He R, Shepard LW, Chen J, Pan ZK, Ye RD. Serum amyloid A is an endogenous ligand that differentially induces IL-12 and IL-23. J Immunol. 2006;177:4072–4079. doi: 10.4049/jimmunol.177.6.4072. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 43.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 44.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 45.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 47.Ather JL, Hodgkins SR, Janssen-Heininger YM, Poynter ME. Airway Epithelial NF-{kappa}B Activation Promotes Allergic Sensitization to an Innocuous Inhaled Antigen. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2010-0106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hodgkins SR, Ather JL, Paveglio SA, Allard JL, LeClair LA, Suratt BT, Boyson JE, Poynter ME. NO2 inhalation induces maturation of pulmonary CD11c+ cells that promote antigenspecific CD4+ T cell polarization. Respir Res. 11:102. doi: 10.1186/1465-9921-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheibanie AF, Khayrullina T, Safadi FF, Ganea D. Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis. Arthritis Rheum. 2007;56:2608–2619. doi: 10.1002/art.22794. [DOI] [PubMed] [Google Scholar]
- 53.Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–735. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 3:cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 55.Struyf S, Van Collie E, Paemen L, Put W, Lenaerts JP, Proost P, Opdenakker G, Van Damme J. Synergistic induction of MCP-1 and -2 by IL-1beta and interferons in fibroblasts and epithelial cells. J Leukoc Biol. 1998;63:364–372. doi: 10.1002/jlb.63.3.364. [DOI] [PubMed] [Google Scholar]
- 56.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor-kappaB in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol. 2002;160:1325–1334. doi: 10.1016/s0002-9440(10)62559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bates JH, Rincon M, Irvin CG. Animal models of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;297:L401–410. doi: 10.1152/ajplung.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bevelander M, Mayette J, Whittaker LA, Paveglio SA, Jones CC, Robbins J, Hemenway D, Akira S, Uematsu S, Poynter ME. Nitrogen dioxide promotes allergic sensitization to inhaled antigen. J Immunol. 2007;179:3680–3688. doi: 10.4049/jimmunol.179.6.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meek RL, Benditt EP. Amyloid A gene family expression in different mouse tissues. J Exp Med. 1986;164:2006–2017. doi: 10.1084/jem.164.6.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meek RL, Eriksen N, Benditt EP. Murine serum amyloid A3 is a high density apolipoprotein and is secreted by macrophages. Proc Natl Acad Sci U S A. 1992;89:7949–7952. doi: 10.1073/pnas.89.17.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chiba T, Han CY, Vaisar T, Shimokado K, Kargi A, Chen MH, Wang S, McDonald TO, O’Brien KD, Heinecke JW, Chait A. Serum amyloid A3 does not contribute to circulating SAA levels. J Lipid Res. 2009;50:1353–1362. doi: 10.1194/jlr.M900089-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Molenaar AJ, Harris DP, Rajan GH, Pearson ML, Callaghan MR, Sommer L, Farr VC, Oden KE, Miles MC, Petrova RS, Good LL, Singh K, McLaren RD, Prosser CG, Kim KS, Wieliczko RJ, Dines MH, Johannessen KM, Grigor MR, Davis SR, Stelwagen K. The acute-phase protein serum amyloid A3 is expressed in the bovine mammary gland and plays a role in host defence. Biomarkers. 2009;14:26–37. doi: 10.1080/13547500902730714. [DOI] [PubMed] [Google Scholar]
- 63.Chen ES, Song Z, Willett MH, Heine S, Yung RC, Liu MC, Groshong SD, Zhang Y, Tuder RM, Moller DR. Serum amyloid A regulates granulomatous inflammation in sarcoidosis through Toll-like receptor-2. Am J Respir Crit Care Med. 181:360–373. doi: 10.1164/rccm.200905-0696OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chizzolini C, Chicheportiche R, Alvarez M, de Rham C, Roux-Lombard P, Ferrari-Lacraz S, Dayer JM. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Aliahmadi E, Gramlich R, Grutzkau A, Hitzler M, Kruger M, Baumgrass R, Schreiner M, Wittig B, Wanner R, Peiser M. TLR2-activated human langerhans cells promote Th17 polarization via IL-1beta, TGF-beta and IL-23. Eur J Immunol. 2009;39:1221–1230. doi: 10.1002/eji.200838742. [DOI] [PubMed] [Google Scholar]
- 67.Kimura A, Kishimoto T. IL-6: Regulator of Treg/Th17 balance. Eur J Immunol. 40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 68.Finkelman FD, Wills-Karp M. Usefulness and optimization of mouse models of allergic airway disease. J Allergy Clin Immunol. 2008;121:603–606. doi: 10.1016/j.jaci.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, Bernstein JA, Huston DP, Liu YJ. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 207:2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]