Abstract
BACKGROUND
Contemporary therapy for medulloblastoma results in adverse neurocognitive effects on young children, particularly those under the age of three. Stratification of patients by risk group may allow toxic treatment to be avoided.
METHODS
76 patients diagnosed with medulloblastoma and enrolled on CCG-9921 underwent central review of pathology, and histologic subtype was designated as desmoplastic or non-desmoplastic. Non-parametric event-free survival (EFS) and survival (OS) curves were computed using the product limit (Kaplan-Meier) estimates and the log-rank test was used to compare survival according to histologic subtype.
RESULTS
Patients with desmoplastic medulloblastoma experienced a favorable EFS of 77 ± 9% and OS of 85 ± 8% compared to EFS of 17 ± 5% and OS of 29 ± 6% for patients with tumors in the non-desmoplastic group (p < 0.0001 for both EFS and OS comparisons). Patients without disease progression did not receive radiation therapy.
CONCLUSIONS
Children less than three with desmoplastic histology of medulloblastoma represent a lower-risk group for whom reduction of therapy, including elimination of radiation therapy, is an appropriate strategy.
Keywords: Medulloblastoma, Desmoplastic, Survival, Brain Neoplasms
INTRODUCTION
Survival of patients with medulloblastoma has improved greatly with combined modality treatment consisting of surgery, craniospinal radiation and chemotherapy.1 However, this multimodality therapy for young children, particularly the group of patients under three years of age, results in devastating long-term neurocognitive outcome. While direct effect of tumor, hydrocephalus at diagnosis, and neurosurgery may all pose a risk to the developing nervous system;2 long-term neurocognitive decline appears to be related most significantly to timing and dose of radiation therapy, although intensive intraventricular chemotherapy has also been implicated.3–5 Strategies to reduce, delay or even eliminate radiation therapy have been the focus of clinical trials for very young children with brain tumors over the past several decades.6–9 Risk stratification of patients would ideally allow for the prospective identification of low risk groups in which radiation may be safely eliminated.
Several retrospective studies have evaluated medulloblastoma histologically to determine prognostic markers. Eberhart et al retrospectively evaluated 330 patients treated on POG protocols and found an association between anaplasia and worse outcome, but they reported no association between desmoplasia (fibrosis) or nodularity (desmoplastic medulloblastoma) with outcome.10 Von Hoff further identified the molecular marker of c-myc amplification along with large cell histology as associated with particularly poor outcome.11 Gajjar et al reported no association between histology and outcome but identified ERBB2 as a molecular marker with prognostic significance.12 One limitation of these retrospective studies is that they include patients of a variety of ages who were not treated uniformly.
Two prospective trials of children over the age of three treated uniformly with upfront radiation therapy have either not examined or not demonstrated a prognostic significance of desmoplastic medulloblastoma subtype. In the St. Jude study, SJBM96, of children treated with risk-adapted craniospinal radiation therapy followed by high-dose chemotherapy with stem cell rescue, Gajjar et al reported a worse outcome for large cell/anaplastic medulloblastoma, but did not find desmoplastic medulloblastoma to have a more favorable outcome than classic medulloblastoma.13 Anaplasia was similarly associated with worse overall survival in the most recently completed A 9961 international cooperative trial for children with non-disseminated standard risk medulloblastoma who were treated with reduced-dose craniospinal radiation therapy followed by one of two maintenance chemotherapy regimens. On the A 9961 trial Packer et al did not report desmoplastic medulloblastoma separately from classic medulloblastoma.14 Children under the age of three were ineligible for both of the above studies.
Desmoplastic histology has been shown to be associated with improved survival in two individually published cohorts of children under the age of three5, 8–9 and one meta-analysis of children under the age of five treated for medulloblastoma.15. These data suggest young patients with desmoplastic medulloblastoma may represent a target group for therapy de-escalation.
Children less than three years of age diagnosed with medulloblastoma and treated on CCG-9921 protocol had a 5-year EFS of 32 ±5% and OS of 43 ± 5%. 83% of 5-year event-free survivors never received radiation therapy. The prognostic significance of histologic subtype has not been reported for the cohort of children with medulloblastoma treated on CCG-9921.6
MATERIALS AND METHODS
The Children’s Cancer Group (CCG) conducted a trial between April 1993 and June 1997 for infants with malignant brain tumors who were randomized to one of two chemotherapy regimens, the results of which have been previously reported. Treatment on CCG-9921 only included radiation therapy for children with metastatic or residual tumor at the time of completion of chemotherapy.6 Written informed consent was obtained from appropriate guardians for all participants at the time of study entry.
Pathology
The study neuropathologist (DCM) centrally reviewed all available samples to classify them histopathologically. At the time of central review, a variety of immunohistochemical stains, plus reticulin stains if found necessary, were used to aid in tumor classification. The further designation of medulloblastoma tumors specifically as desmoplastic, classic or anaplastic was made according to WHO guidelines. Specifically, tumors histopathologically designated as desmoplastic medulloblastoma consisted of nodules of cells with advanced neurocytomatous differentiation without desmoplasia or reticulin staining surrounded by a background of desmoplasia or reticulin positivity. Tumors were considered desmoplastic medulloblastoma whether they had focal or extensive nodularity, which included tumors designated as nodular/desmoplastic medulloblastoma as well as the newly described entity of medulloblastoma with extensive nodularity.16
Statistical Methods
The primary endpoints for statistical analysis were event-free survival (EFS) and overall-survival (OS). Event-free survival was defined as the minimum time from study entry to disease progression, disease relapse, the occurrence of a second malignant neoplasm, or death from any cause. Overall survival was defined as time to death from any cause. Non-parametric estimates of the EFS and OS were obtained using the product limit estimate, with standard errors computed using the Greenwood formula. Comparisons of EFS and OS between desmoplastic and non-desmoplastic groups were based on the logrank test. Multivariate Cox regression was used to test differences in EFS between desmoplastic and non-desmoplastic groups while controlling for other prognostic factors, such as age, gender, race, extent of resection, residual tumor, M-stage and treatment assigned.
RESULTS
A total of 92 eligible patients diagnosed with medulloblastoma were enrolled on CCG-9921. This analysis excluded six patients without central pathology review and ten patients who did not have medulloblastoma by the central review. Thus, the analysis was based on 76 patients with medulloblastoma by central review, of whom 40 (53%) were male, 53 (70%) were white and 14 (18%) were younger than 12 months; the median age was 21 months. Of the 76 patients, 49 (64.5%) had localized disease (stage M0) and 27 (35%) had metastatic (M+) disease. Metastatic sites included four patients (5.3%) with positive CSF cytology only (stage M1), two patients (2.6%) with intracranial leptomeningeal spread only (stage M2), 11 patients (14.5%) with spinal leptomeningeal disease (stage M3a), nine patients (11.8%) with metastatic disease in both brain and spine (stage M3b); and one patient with extraneural disease (stage M4). The distribution of patient characteristics between the desmoplastic and non-desmoplastic groups is well balanced except with regard to M stage. The details of patient characteristics are presented in Table 1. Metastatic disease was present in 18% of patients with desmoplastic medulloblastoma compared to 43% of patients in the non-desmoplastic group (Fisher’s exact test p=0.06).
Table 1.
Patient Characteristics
| Total | Desmoplastic | Non-desmoplastic | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Frequency | Percent | Frequency | Percent | Frequency | Percent | p-value* | |
| Age | 0 to 11 months | 14 | 18.4% | 4 | 18.2% | 10 | 18.5% | 1 |
| 12 to 36 months | 62 | 81.6% | 18 | 81.8% | 44 | 81.5% | ||
| Gender | Male | 40 | 52.6% | 11 | 50.0% | 29 | 53.7% | 0.77 |
| Female | 36 | 47.4% | 11 | 50.0% | 25 | 46.3% | ||
| Race | White | 53 | 69.7% | 16 | 72.7% | 37 | 68.5% | 0.72 |
| Non-white | 23 | 31.3% | 6 | 27.3% | 17 | 31.5% | ||
| M-Stage | M0 | 49 | 64.5% | 18 | 81.8% | 31 | 57.4% | 0.06 |
| M1 - M4 | 27 | 35.5% | 4 | 18.2% | 23 | 42.6% | ||
| Extent resection | <90% resection | 22 | 28.9% | 6 | 27.3% | 16 | 29.6% | 0.84 |
| >90% resection | 54 | 71.1% | 16 | 72.7% | 38 | 70.4% | ||
| Residual | <1.5 cm. | 49 | 64.5% | 16 | 72.7% | 33 | 61.1% | 0.34 |
| ≥1.5 cm. | 27 | 35.5% | 6 | 27.3% | 21 | 38.9% | ||
| Regimen assigned | A | 44 | 57.9% | 15 | 68.2% | 29 | 53.7% | 0.25 |
| B | 32 | 42.1% | 7 | 31.8% | 25 | 46.3% | ||
Comparing patient characteristics between desmoplastic and non-desmoplastic patients; p-values based on Chi-square or Fisher's exact test.
For the 22 patients with desmoplastic medulloblastoma, five-year event-free survival (EFS) and overall survival (OS) was 77 ± 9% and 85 ± 8% respectively, compared to EFS and OS of 17 ± 5% and 29 ± 6% respectively for the remaining 54 patients with tumors in the non-desmoplastic group (log rank p < 0.0001 for both EFS and OS, Figures 1 and 2). The comparison of outcome between desmoplastic and non-desmoplastic groups remained significant (Cox p<0.0001) after adjusting for other prognostic factors, including M-stage.
Figure 1.
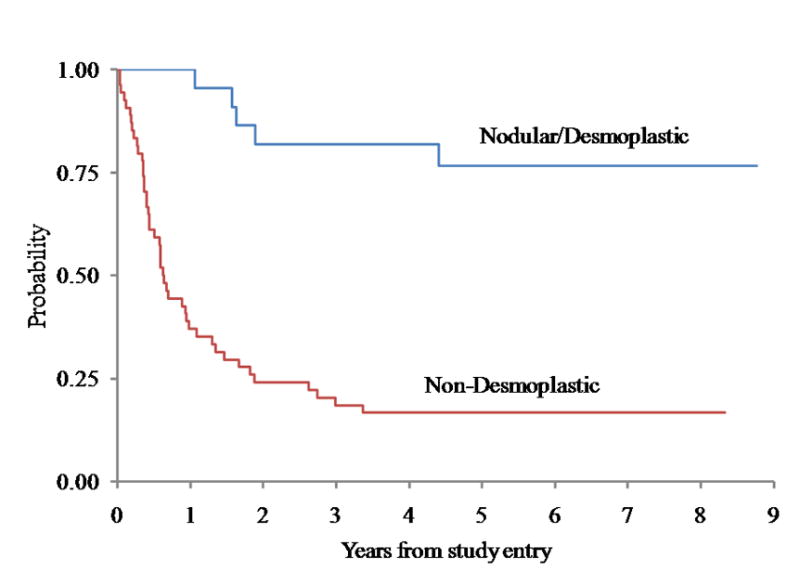
Event-Free Survival for Medulloblastoma comparing Desmoplastic Groups (p < 0.0001)
Figure 2.
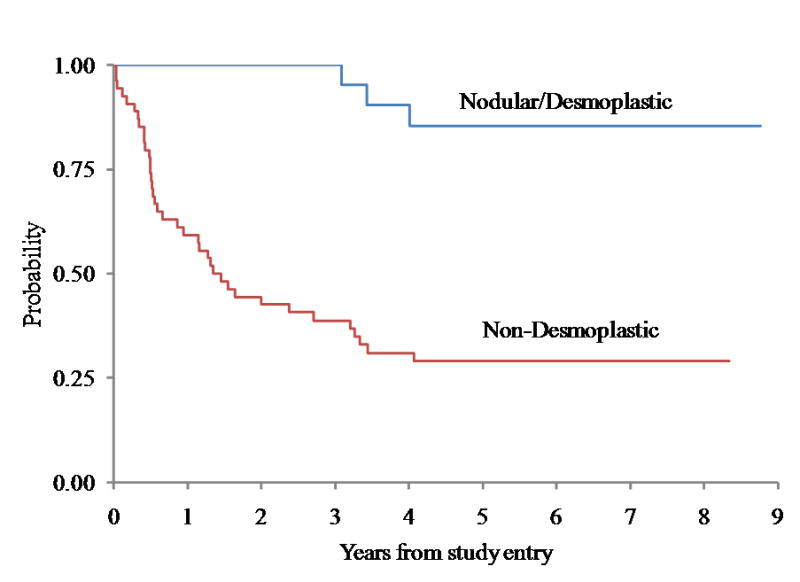
Overall Survival for Medulloblastoma comparing Desmoplastic Groups (p < 0.0001)
Localized Disease and Metastatic Disease
It is of interest to present the comparison between the desmoplastic and non-desmoplastic groups with localized and metastatic disease separately. Of the 49 patients with localized M0 disease, 18 (37%) were classified as nodular/desmoplastic and 31 (63%) were non-desmoplastic. Non-desmoplastic histology included four tumors (8%) designated as anaplastic, and the remaining had classic histology. Event free survival and overall survival were significantly higher in the desmoplastic group. Five year event free and overall survival was 72 ± 11% and 83 ± 9% respectively in the desmoplastic group compared to 16 ± 6.6% and 32 ± 8% in the non-desmoplastic group (p values were < 0.0001 for EFS and 0.0004 for OS).
Of the 27 patients with metastatic disease, only four patients had metastatic desmoplastic medulloblastoma; three were stage M3 (spine) and one was stage M4. All four patients with metastatic desmoplastic medulloblastoma were alive at last contact with no event at 2.2, 5.1 and 8.0 (2 patients) years. The five-year EFS and OS for the 23 patients with metastatic disease in the non-desmoplastic group was 17 ± 8% and 25 ± 9% respectively.
Radiation Therapy
According to CCG-9921 protocol, metastatic patients were to receive radiation therapy while patients with localized disease only received radiation therapy in the event of either less than complete response by the end of induction therapy, disease recurrence or progression. Of the 18 patients with localized desmoplastic medulloblastoma, only four patients received radiation therapy, all of these for disease progression. One of the patients with disease progression was alive at last follow-up. Of the four patients with metastatic desmoplastic medulloblastoma, only two received radiation therapy.
DISCUSSION
This is the first report from a single prospective trial in the United States documenting the effect of histologic subtype on survival in young children with medulloblastoma. The CCG-9921 trial attempted to eliminate the use of radiation therapy in young patients. Children with localized disease and complete response after surgery and chemotherapy did not receive radiation therapy unless they suffered tumor recurrence. In the cohort of patients reported here, desmoplastic medulloblastoma predicted a favorable outcome, with five-year survival of 85 ± 8% in patients with desmoplastic/nodular tumors. More significantly, five-year event-free survival in patients with localized (M0) disease was 72 ± 11% without the use of radiation therapy. Although a small subgroup, it is notable that the four patients on CCG-9921 with metastatic desmoplastic medulloblastoma were all long term survivors, two of whom never received any radiation therapy.
Young children less than three years of age have been identified as a high-risk group since multimodality therapy was first used for patients with medulloblastoma.17 Younger patients have consistently had poorer survival rates 5–9, 18–19 in comparison to older patients treated on contemporary regimens with upfront radiation therapy followed by chemotherapy.13–14 Furthermore, younger patients who do survive are also at increased risk for more severe long-term neurocognitive deficits.3–4, 19 Because these deficits are more closely associated with craniospinal radiation therapy than any other potentially modifiable treatment factors, contemporary clinical trials for young patients have attempted to reduce, delay or even eliminate radiation therapy. More recently, cooperative group studies in young children in the US have attempted to further intensify chemotherapy with the use of high-dose chemotherapy requiring stem cell support with or without the addition of methotrexate.
This report demonstrates survival for the subgroup of young patients with desmoplastic medulloblastoma treated with chemotherapy alone which is comparable to published survival rates for older children treated with combination radiation and chemotherapy.13–14 Although desmoplastic histology was not predictive of better outcome in the published cohorts of older patients treated with upfront radiation therapy, it is possible that older patients with desmoplastic medulloblastoma could retain a similar survival with further reduction, or even elimination, of radiation therapy. The predictive value of desmoplastic medulloblastoma subtype will be important to examine in the subgroup of patients between the ages of 3 to 7 for whom reduction in radiation therapy dose is being attempted in the ongoing Children’s Oncology Group trial. There are two published cohorts and one recently published meta-analysis of patients in the youngest (less than three years) age group in whom desmoplastic histology was examined and also found to favorably predict survival (summarized in Table 2). The treatment regimens used in each of these cohorts as well as patient factors at diagnosis varied significantly. In the German cohort of 43 patients reported by Rutkowski et al.,5 intraventricular and systemic methotrexate was used, although intraventricular therapy was only given to the subset of patients with normal CSF flow. Five-year event-free and overall survival for those with desmoplastic histology on this regimen was 85 ± 8% and 95 ± 5% respectively. However, they demonstrated that intraventricular chemotherapy as well as radiation therapy was associated with a significantly worse neurocognitive outcome, with the lowest cognitive outcome in children who received both radiation and intraventricular methotrexate. The German cohort did not include any patients with anaplastic histology, and metastatic disease predicted poor outcome.
Table 2.
Summary of Studies Reporting Survival Comparing Desmoplastic Groups
| Trial [reference] | Number of patients | Observation time (years) | Event-free survival | Overall survival | Initial treatment | ||||
|---|---|---|---|---|---|---|---|---|---|
| DMB | Non-DMB | DMB | Non-DMB | CSI | HDC ASCR | HDMTX | |||
| 1. HIT-SKK"92 [5] | 43 | 5 | 85% | 34% | 95% | 41% | NO | NO | YES |
| 2. HIT-SKK"87 [9] | 29 | 10 | 89% | 30% | 89% | 40% | YES | NO | YES |
| 3. UKCCSG/SIOP CNS9204 [8] | 31 | 5 | 35%* | 33%* | 53% | 33% | NO | NO | YES |
| 4. meta-analysis [15] | 270 | 8 | 55% | 27% | 77% | 42% | YES^ | YES^ | YES^ |
| 5. CCG-9921, this analysis | 76 | 5 | 77% | 17% | 85% | 29% | NO | NO | NO |
DMB Desmoplastic Medulloblastoma, including desmoplastic nodular medulloblastoma and medulloblastoma with extensive nodularity; CSI Craniospinal irradiation; HDC ASCR High-dose chemotherapy with autologous stem cell rescue; HDMTX High-dose methotrexate.
All differences in survival are statistically significant except for reference #3 event-free survival.
Patient cohorts in meta-analysis treated on six different regimens, including reverences 1–3 above in addition to Italian and Head-Start regimens which used HDC ASCR.
Grundy et al.8 recently reported the outcome for 31 young children with medulloblastoma treated on the United Kingdom Children’s Cancer Study Group (UKCCSG) CNS 9204 trial. The UK group treated children with a prolonged course of alternating chemotherapy in the absence of progression. The UK treatment regimen is approximately twice the length of either the German or CCG regimens, and also included high-dose methotrexate, although did not include intraventricular administration of chemotherapy. A diagnosis of desmoplastic medulloblastoma in this cohort was not predictive for a favorable five-year event-free survival (35.3% vs. 33.3% for classic medulloblastoma), but did predict a better overall survival (52.9% vs. 33.3% for classic medulloblastoma), and metastatic disease was not independently prognostic. The few long-term survivors who did not receive radiation therapy, only three patients in this cohort, all had desmoplastic medulloblastoma.
The recently published combined international analysis of five cohorts of children less than five with medulloblastoma also reported desmoplastic histology as a strong independent factor predictive of survival.15 Most interestingly, this report demonstrated no difference in 8-year event-free-survival between patients with localized or metastatic desmoplastic medulloblastoma (54% and 56% respectively). Significantly different treatment strategies for young children with brain tumors have been employed by each national group, including prolonging total chemotherapy treatment course by altering agents with varying toxicities,7–8 further intensifying therapy with high-dose chemotherapy followed by stem cell support,20–21 or adding intensive intraventricular therapy5 with varying success at eliminating versus delaying radiation therapy.22 Although not designed to compare treatment strategies, there was a statistically significant difference in survival between treatment regimens, possibly suggesting that intensity of therapy is important for patients with desmoplastic as well as classic medulloblastoma.15
In addition to differences in treatment regimens, patient factors which varied between cohorts included the proportion of tumors designated as desmoplastic, presence of anaplastic tumors, as well as the proportion of patients with metastatic disease. Unique to the cohort presented here, the treatment regimens given on the CCG-9921 protocol did not include methotrexate. Also, the majority of survivors in this cohort did not receive radiation therapy.6 As the developing nervous system has been shown to be sensitive to both radiation therapy and methotrexate,3, 5 survival without either of these treatments is encouraging in terms of potential to spare neurocognitive function, although specific neurocognitive follow-up data is lacking.
Biologic subtypes of medulloblastoma are now being identified which are associated with histological subtypes.11, 23–24 As we learn more about the biology of medulloblastoma, it is likely that specific genetic markers may either add to or replace conventional histology to better stratify patients by risk groups. At this time, the presence of desmoplastic histology in young patients with localized medulloblastoma is the best identifier of a lower-risk subgroup in whom attempts should be made to reduce long-term toxicity of treatment. We report here a successful strategy to treat desmoplastic medulloblastoma with an adequately intense regimen of chemotherapy alone; and specifically without radiation therapy, methotrexate, or high-dose chemotherapy requiring stem cell support. It may be appropriate to expand this strategy to include older patients with desmoplastic medulloblastoma.
Acknowledgments
Funding Sources: This work was funded in part by the COG/NIH Grant for Human Specimen Banking U24 CA114766 and Dr. Leary is funded by the Seattle Children’s Center for Clinical and Translational Research Mentored Scholar Program.
Footnotes
Financial Disclosures: The authors have no significant financial interests to disclose.
References
- 1.Gottardo NG, Gajjar A. Current Therapy for Medulloblastoma. Curr Treat Options Neurol. 2006;8(4):319–34. doi: 10.1007/s11940-006-0022-x. [DOI] [PubMed] [Google Scholar]
- 2.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 3.Fouladi M, Gilger E, Kocak M, Wallace D, Buchanan G, Reeves C, et al. Intellectual and functional outcome of children 3 years old or younger who have CNS malignancies. J Clin Oncol. 2005;23(28):7152–60. doi: 10.1200/JCO.2005.01.214. [DOI] [PubMed] [Google Scholar]
- 4.Palmer SL, Reddick WE, Gajjar A. Understanding the cognitive impact on children who are treated for medulloblastoma. J Pediatr Psychol. 2007;32(9):1040–9. doi: 10.1093/jpepsy/jsl056. [DOI] [PubMed] [Google Scholar]
- 5.Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352(10):978–86. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 6.Geyer JR, Sposto R, Jennings M, Boyett JM, Axtell RA, Breiger D, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children's Cancer Group. J Clin Oncol. 2005;23(30):7621–31. doi: 10.1200/JCO.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 7.Grill J, Sainte-Rose C, Jouvet A, Gentet JC, Lejars O, Frappaz D, et al. Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6(8):573–80. doi: 10.1016/S1470-2045(05)70252-7. [DOI] [PubMed] [Google Scholar]
- 8.Grundy RG, Wilne SH, Robinson KJ, Ironside JW, Cox T, Chong WK, et al. Primary postoperative chemotherapy without radiotherapy for treatment of brain tumours other than ependymoma in children under 3 years: results of the first UKCCSG/SIOP CNS 9204 trial. Eur J Cancer. 2010;46(1):120–33. doi: 10.1016/j.ejca.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Rutkowski S, Gerber NU, von Hoff K, Gnekow A, Bode U, Graf N, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro Oncol. 2009;11(2):201–10. doi: 10.1215/15228517-2008-084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eberhart CG, Kepner JL, Goldthwaite PT, Kun LE, Duffner PK, Friedman HS, et al. Histopathologic grading of medulloblastomas: a Pediatric Oncology Group study. Cancer. 2002;94(2):552–60. doi: 10.1002/cncr.10189. [DOI] [PubMed] [Google Scholar]
- 11.von Hoff K, Hartmann W, von Bueren AO, Gerber NU, Grotzer MA, Pietsch T, et al. Large cell/anaplastic medulloblastoma: Outcome according to myc status, histopathological, and clinical risk factors. Pediatr Blood Cancer. 2009 doi: 10.1002/pbc.22339. [DOI] [PubMed] [Google Scholar]
- 12.Gajjar A, Hernan R, Kocak M, Fuller C, Lee Y, McKinnon PJ, et al. Clinical, histopathologic, and molecular markers of prognosis: toward a new disease risk stratification system for medulloblastoma. J Clin Oncol. 2004;22(6):984–93. doi: 10.1200/JCO.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–20. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 14.Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–8. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 15.Rutkowski S, von Hoff K, Emser A, Zwiener I, Pietsch T, Figarella-Branger D, et al. Survival and Prognostic Factors of Early Childhood Medulloblastoma: An International Meta-Analysis. J Clin Oncol. 2010 doi: 10.1200/JCO.2010.30.2299. [DOI] [PubMed] [Google Scholar]
- 16.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans AE, Jenkin RD, Sposto R, Ortega JA, Wilson CB, Wara W, et al. The treatment of medulloblastoma. Results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J Neurosurg. 1990;72(4):572–82. doi: 10.3171/jns.1990.72.4.0572. [DOI] [PubMed] [Google Scholar]
- 18.Thorarinsdottir HK, Rood B, Kamani N, Lafond D, Perez-Albuerne E, Loechelt B, et al. Outcome for children <4 years of age with malignant central nervous system tumors treated with high-dose chemotherapy and autologous stem cell rescue. Pediatr Blood Cancer. 2007;48(3):278–84. doi: 10.1002/pbc.20781. [DOI] [PubMed] [Google Scholar]
- 19.Gajjar A, Mulhern RK, Heideman RL, Sanford RA, Douglass EC, Kovnar EH, et al. Medulloblastoma in very young children: outcome of definitive craniospinal irradiation following incomplete response to chemotherapy. J Clin Oncol. 1994;12(6):1212–6. doi: 10.1200/JCO.1994.12.6.1212. [DOI] [PubMed] [Google Scholar]
- 20.Dallorso S, Dini G, Ladenstein R, Cama A, Milanaccio C, Barra S, et al. Evolving role of myeloablative chemotherapy in the treatment of childhood brain tumours. Bone Marrow Transplant. 2005;35 (Suppl 1):S31–4. doi: 10.1038/sj.bmt.1704841. [DOI] [PubMed] [Google Scholar]
- 21.Dhall G, Grodman H, Ji L, Sands S, Gardner S, Dunkel IJ, et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer. 2008;50(6):1169–75. doi: 10.1002/pbc.21525. [DOI] [PubMed] [Google Scholar]
- 22.Rutkowski S, Cohen B, Finlay J, Luksch R, Ridola V, Valteau-Couanet D, et al. Medulloblastoma in young children. Pediatr Blood Cancer. 2010;54(4):635–7. doi: 10.1002/pbc.22372. [DOI] [PubMed] [Google Scholar]
- 23.Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24(12):1924–31. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 24.Eberhart CG, Kratz J, Wang Y, Summers K, Stearns D, Cohen K, et al. Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and anaplasia. J Neuropathol Exp Neurol. 2004;63(5):441–9. doi: 10.1093/jnen/63.5.441. [DOI] [PubMed] [Google Scholar]


