Abstract
Purpose
Given that carotid vasa vasorum neovascularization is associated with increased risk for stroke and cardiac events, the present in vivo study was designed to investigate molecular imaging of carotid artery vasa vasorum neovascularization via target-specific contrast-enhanced ultrasound (CEU) micro-imaging.
Procedures
Molecular imaging was performed in male transgenic rats with carotid artery disease and non-transgenic controls using dual endothelin1/VEGFsp receptor (DEspR)-targeted microbubbles (MBD) and the Vevo770 micro-imaging system and CEU imaging software.
Results
DEspR-targeted CEU-positive imaging exhibited significantly higher contrast intensity signal (CIS)-levels and pre-/post-destruction CIS-differences in seven of 13 transgenic rats, in contrast to significantly lower CIS-levels and differences in control isotype-targeted microbubble (MBC)-CEU imaging (n =8) and in MBD CEU-imaging of five non-transgenic control rats (P<0.0001). Ex vivo immunofluorescence analysis demonstrated binding of MBD to DEspR-positive endothelial cells; and association of DEspR-targeted increased contrast intensity signals with DEspR expression in vasa vasorum neovessel and intimal lesions. In vitro analysis demonstrated dose-dependent binding of MBD to DEspR-positive human endothelial cells with increasing %cells bound and number of MBD per cell, in contrast to MBC or non-labeled microbubbles (P<0.0001).
Conclusion
In vivo DEspR-targeted molecular imaging detected increased DEspR-expression in carotid artery lesions and in expanded vasa vasorum neovessels in transgenic rats with carotid artery disease. Future studies are needed to determine predictive value for stroke or heart disease in this transgenic atherosclerosis rat model and translational applications.
Keywords: Molecular imaging, DEspR, Dual endothelin1/VEGFsp receptor, Vasa vasorum neovascularization, Carotid artery atherosclerosis model, Pathological angiogenesis
Introduction
The dual endothelin-1 (ET1)/vascular endothelial growth factor-signal peptide (VEGFsp) receptor or dual endothelin1/VEGFsp receptor (DEspR; formerly dear gene as deposited in GenBank) [1] plays a key role in developmental angiogenesis deduced from the embryonic lethal phenotype exhibited by despr−/− knockout mice due to absent embryonic and extraembryonic angiogenesis, aborted dorsal aorta vasculogenesis, and abnormal cardiac development [2]. While exhibiting similar abnormal vasculogenesis and angiogenesis phenotypes with VEGF+/− haploinsufficient mice, despr−/− null mice exhibit distinct neural tube phenotypes [2–4]. Consistent with its role in developmental angiogenesis, DEspR inhibition results in decreased tumor angiogenesis and tumor growth in adult rat mammary tumors and mouse melanomas [2], thus implicating DEspR in pathological angiogenesis and a priori, identifying a putative endothelial target for target-specific imaging technologies of pathological angiogenesis not just in tumors but also in cardiovascular diseases.
Development of target-specific contrast enhanced ultrasonography (CEU) imaging, from hereon referred to as molecular imaging, of vascular disease neovascularization is important since carotid artery vasa vasorum neovascularization is associated with increased risk for stroke [5, 6]. However, successful molecular imaging of vasa vasorum neovessels has not been reported, although detection by non-targeted CEU imaging has [7]. On the other hand, successful molecular imaging in different disease models detecting different targets [8, 9] has shown the potential of molecular imaging in different disease contexts, such as αvβ3 in tumor and hind limb ischemia angiogenesis [10, 11], VEGFR2 in tumor angiogenesis [12], ICAM-1 in transplant rejection [13], L-selectin in malignant lymphnodes [14], and ICAM-1 and VCAM-1 in atherosclerosis [15], P-selectin in myocardial ischemia [16, 17], GIIb/IIIa and fibrinogen in thrombosis [18, 19]. Molecular imaging of vascular disease neovascularization requires more study since studies targeting VEGFR2-, ICAM-1, and VCAM-1 did not detect vasa vasorum neo-vessels in a hyperlipidemic rabbit model of injury-induced vascular neovascularization [9, 20]. As an emerging field, in vivo validation of molecular imaging for different molecular targets and different disease models is imperative.
Here, we provide in vivo proof-of-concept for molecular imaging of DEspR in carotid artery lesions and expanded vasa vasorum neovessels in transgenic-hyperlipidemic, hypertensive carotid artery disease rat model.
Materials and Methods
Animals Used for Study
In order to facilitate molecular imaging studies of pathological angiogenesis in vascular lesions or in expanded vasa vasorum neovessels, we selected to study a carotid artery disease rat model with hypertension-atherosclerosis as risk factors, the Tg25[hCETP] Dahl-S rat model, Tg25, transgenic for human cholesteryl ester transfer protein which develops accelerated stroke [21] or later-onset coronary heart disease [22]. We studied 4-month-old transgenic male rats projected to be around early–midpoint along the disease course of stroke [21] or coronary atherosclerosis phenotype [22], for DEspR-targeted molecular imaging (n=13). DEspR-targeted microbubbles (MBD)-infused non-transgenic, non-atherosclerotic littermates were studied as negative biological controls (n=5). Isotype-specific control isotype-microbubbles (MBC)-infused transgenic rats (n=8), with the following subgroups: four transgenic rats which exhibited MBD-specific CEU positive imaging, and four de novo transgenic rats, were studied concurrently as negative imaging controls.
Target-Specific CEU Molecular Imaging
We used the Vevo770 high resolution ultrasound system with contrast mode software, and streptavidin-coated “target ready” MicroMarker microbubbles (VisualSonics Inc, Canada) previously validated for molecular imaging of VEGFR2 on tumor angiogenesis in mice [12]. To target the microbubble to rat DEspR-positive endothelial cells, we linked target ready-MicroMarker microbubbles to biotinylated anti-DEspR antibody (MBD) via streptavidin-biotin coupling. For control, we linked target ready-MicroMarker microbubbles to biotinylated, isotype-antibody (MBC). Each bolus comprised of 3–4×108 microbubbles in 200 μL saline, infused into the rat tail vein over 8 s.
CEU imaging of rat carotid arteries comprised a sequence of steps aimed at optimizing MB-target binding, eliminating confounders, and ascertaining reproducible CEU imaging. We first obtained baseline images of the carotid artery and immobilized the scanhead to maintain the optimal B-mode view of the common, external, and internal carotid arteries in one 2D image (Figs. 1 and 2). One minute after MB bolus infusion, the MB blood pool was documented by B-mode imaging for all rats to ascertain MB infusion and to demonstrate absence of contrast intensity in surrounding tissue (Figs. 1 and 2). We waited 4–5 min to allow MBD adherence to DEspR-positive endothelial targets [12], and to allow clearance of unbound circulating microbubbles [23]. Clearance of most circulating MBs facilitates detection of increased contrast intensity signals due to adherent MBs validated for detection using the Vevo770 imaging system [23]. Adherent MBs were defined by the loss of contrast-intensity upon acoustic destruction performed using pre-set Contrast Enhanced software (VisualSonics, Inc, Canada) as described [12].
Fig. 1.
Representative CEU-images with contrast intensity signals (CIS) depicted in false-color green. a MBD DEspR-targeted molecular imaging in transgenic rat-R1 demonstrating CEU-positive imaging and the characteristic drop in CIS-peak after acoustic disruption (red line). b Subsequent isotype-microbubble (MBC) imaging in transgenic rat-R1 showing low peak CIS-levels and ‘flat-line’ pattern of CIS pre- and post-destruction indicating CIU-negative imaging. c MBD DEspR-targeted molecular imaging in non-transgenic rat-R2 demonstrating CEU-negative imaging similar to MBC CEU-negative imaging. d Graph of CIS-differences (empty triangle) among different study groups as notated distinguishing CEU-positive imaging in Tg MBD CEU+group from the other CEU-negative groups. e. Graph of CIS difference between all transgenic rats (Tg+) and non-transgenic rats (nonTg). Yellow hatched bar apparent threshold between MBD-infused CEU+ and MBD-infused CEU transgenic rats. Blood pool, CEU image 1 min after bolus injection of MBs, demonstrating equivalent MB infusion among different rats and minimal contrast–intensity signals from movement artifacts. 1 Pre, pre-acoustic destruction CEU images obtained 4 min after bolus infusion, in order to allow MB-adherence to target, if any, and to document minimal, if any, circulating MBs in the lumen. Image corresponds to 1 on CIS-plot. 2 Post, CEU image after acoustic destruction corresponding to 2 on scatter plot. CIS-plot, scatter plot of contrast-intensity signals (CIS) in representative regions of interest (encircled in aqua). 1 CIS detected pre-acoustic destruction, 2 CIS detected post-acoustic destruction. Red line and following gap mark period of acoustic destruction in CIS scatter plots. MBD DEspR-targeted microbubble, MBC control isotype-targeted microbubble, Tg transgenic rat; nonTg, nontransgenic control rat, CEU+ CEU positive imaging, CEU− CEU negative imaging, Δ contrast intensity pre-/post-destruction CIS-difference; aqua dashed-circle, region of interest; ***P<0.0001.
Fig. 2.
Representative MBD-specific CEU-positive images depicting complex pattern of acoustic destruction of adherent MBD-microbubbles in a transgenic rat, R3. a Representative CEU image documenting blood pool of circulating MBDs filling carotid artery lumen 1 min after bolus infusion. CCA common carotid artery, ECA external carotid artery, ICA internal carotid artery, * CCA bifurcation. b–d Scatter plots of contrast–intensity signals marked with same-colored blocks to refer to corresponding regions of interest (ROI) in panel e. b Blue, c yellow, d red ROIs. e Representative CEU image that corresponds to 1 on scatter plots b, c, d documenting adherent DEspR-targeted microbubbles (MBD) just prior to pre-acoustic destruction (red line). Adherent MBDs (false color green) are seen in the three ROIs encircled blue, yellow, and red. f Representative CEU image corresponding to 2 on scatter plots b–d showing a post-acoustic destruction dip in signal intensity compared to levels in 1 in the different ROIs, respectively. g Representative CEU image corresponding to 3 on scatter plots b–d showing a post-acoustic destruction secondary peak in contrast intensity signals in the different ROIs. h Representative CEU image corresponding to 4 on scatter plots documenting the decline in contrast-intensity signals approaching baseline levels observed in isotype control or MBD-infused CEU negative images (Fig. 1b,c) and demonstrating low background CIS levels.
We monitored four regions of interest (ROI) on the carotid artery: the common carotid artery, bifurcation, external and internal carotid arteries. Quantitation of contrast intensity signals (CIS) resulting from backscatter of adherent targeted-microbubbles was done using contrast-enhanced analysis program validated for the Vevo770 imaging platform (VisualSonics Inc, Canada) detecting pre- and post-acoustic disruption contrast intensity signals. The contra-lateral carotid artery was checked immediately, and the same CEU-imaging protocol followed. After a 20-min interval to allow complete clearance of any residual MBs, a pre-set destruction sequence was performed for subsequent CEU imaging with isotype-specific MBCs following identical procedures. For quantitative comparative analyses, the difference in contrast intensity signals between pre- and post-acoustic destruction, CIS difference, as well as their respective pre-destruction CIS peak levels were studied for each carotid artery per rat (see online Supplementary Methods).
Histology and Immunofluorescence Staining of Rat Carotid Arteries
After CEU imaging, carotid arteries were collected en bloc preserving the surrounding tissue around the common (CCA), external (ECA), and internal (ICA) carotid arteries including the carotid artery bifurcation. The ECA was cut longer than the ICA to be able to distinguish the two. Longitudinal serial sections were obtained per carotid artery (50–100 sections) and staining every tenth slide with Masson’s trichrome allowed proper orientation and site-specific analyses corresponding to ROIs in CEU imaging. The flanking serial sections to MT-stained slides of interest were then immunostained. Double immunofluorescence staining was done on deparaffinized sections via sequential antigen retrieval, treatment to reduce background, blocking, incubation with primary antibody at 4°C overnight, secondary antibody incubation overnight at 4°C with AlexaFluor 568 goat anti-mouse IgG and AlexaFluor 488 goat anti-rabbit IgG, washing, and mounting using Prolong Gold with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, CA, USA). Negative controls were run using rabbit-isotype antibody for anti-rat DEspR antibody. A Zeiss Axioskop2plus microscope was used for fluorescence imaging and differential interference contrast (DIC) photomicroscopy to provide morphological information overlay to immunostained sections. Low 2.5× magnification was used for proper orientation and site-specific identification along the carotid artery (see online Supplementary Methods).
In vitro Analysis of MBD and DEspR-Positive Endothelial Cell Interactions
Human-specific DEspR-targeted MBDs were made following identical procedures for rat-specific DEspR molecular imaging with the exception of the use of a pre-validated anti-humanDEspR monoclonal antibody (data not shown). Fixed numbers of human umbilical vein endothelial cells (HUVECs) were seeded onto IBIDI perfusion 6-lane micro-slide VI (ibidiGmbH, Germany). After 24 h, MBD-type microbubbles were infused at the following MB-cell ratios: 8×, 80×, and 800×. Negative controls comprised of 800× MBCs and 800× non-targeted microbubbles, MBOs. These were all infused at 20 dynes/cm2 shear stress one-way flow on the same six-lane micro-flow chamber slide. After 45 min of incubation, DAPI nuclear staining was performed and excess MBs were washed with HUVECs media at same shear stress. Phase contrast and epifluorescence microscopy was performed in six random high power fields. Cells and microbubbles were documented by photomicroscopy and counted as to per cent cells with bound MB, and number of MBs per cell. We compared MBD, MBC, and non-targeted microbubbles MBO.
Statistical Analysis
Values are expressed as mean±SEM. Data was analyzed with Prism 5 statistics software (GraphPad Software Inc, CA, USA). Where applicable, nonparametric analysis of variance (ANOVA) and Dunn’s multiple comparison tests or ANOVA and Tukey’s multiple pairwise comparison tests were used. For two group comparison, nonparametric Kruskal–Wallis test was performed using Prism5 (GraphPad Software Inc, CA, USA).
Results
DEspR-Targeted Molecular Imaging of Carotid Artery
Given the recognized need for detecting vascular disease-associated angiogenesis in carotid artery disease [5, 6], we tested whether DEspR can serve as an endothelial target for CEU imaging of pathological angiogenesis in carotid artery disease lesions or vasa vasorum neovascularization. We used the Tg25 rat model of carotid artery disease comparing 4-month-old male Tg25 rats projected to be at midpoint of atherosclerotic disease course [21, 22], with age-matched non-transgenic male littermates. Compared to coronary artery disease, investigation of carotid artery disease provides a tactical experimental system with less movement artifacts.
Using the Vevo770 ultrasound contrast-enhanced imaging system and MBD compared with MBC, we detected MBD-specific CEU-positive imaging in different ROI along the common CCA, carotid artery bifurcation, proximal internal, and/or external carotid arteries in seven of 13 transgenic rats (Figs. 1 and 2; Table 1). MBD-specific CEU-positive imaging was defined as stably increased contrast intensity signals detected after circulating microbubbles have cleared, and which decreased upon acoustic destruction (Fig. 1a). The peak pre-destruction contrast intensity signals and the differences in pre-/post-destruction contrast intensity signals (CIS-differences) were significantly higher in MBD-specific CEU-positive images (Fig. 1a, Table 1) compared with CEU imaging observed in isotype MBC-infused rats (Fig. 1b) and in MBD-infused non-transgenic control rats (n=5; Fig. 1c), with the latter two empirically defining CEU-negative imaging. Notably, of the seven transgenic rats exhibiting MBD-specific CEU-positive imaging, four exhibited CEU-positive imaging in both carotid arteries, while three exhibited CEU-negative imaging on the contralateral carotid artery, suggesting selectivity of MBD-specific CEU-positive imaging and concordant with specificity (Table 1). Moreover, six transgenic rats exhibited CEU-negative imaging with low peak contrast intensity signals, “flat-line” pre-/post-destruction CIS-plot pattern, and minimal CIS-differences (Fig. 1d,e, Table 1) similar to CEU-negative imaging observed in MBC-control rats (Fig. 1b) and in MBD-infused non-transgenic controls (Fig. 1c). Altogether, these observations provide compelling evidence that MBD-based CEU-positive images are specific and due likely to putative adherent MBDs in said carotid arteries. Statistical analysis by ANOVA and post hoc multiple comparison testing establish that the CIS-differences of MBD-specific CEU-positive imaging are significantly higher, P<0.0001, compared to each CEU negative imaging study group, respectively (Table 1, Fig. 1d). Interestingly, since CEU-positive imaging is detected only in transgenic rats, and with 54% of transgenic rats exhibiting MBD-specific CEU-positive imaging at 4 months of age equivalent to an early–midpoint of the typical model disease course in males [21, 22], average CIS differences are significantly different (P<0.0001) between transgenic rats and their non-transgenic controls (Fig. 1e). With seven of 13 transgenic rats exhibiting CEU-positive imaging, and six of 13 exhibiting CEU-negative imaging upon MBD infusion, a sub-grouping of transgenic rats based on MBD CEU-imaging CIS-differences at the 4-month midpoint of the disease course is apparent (Fig. 1e). Predictive value of this sub-grouping needs further study.
Table 1.
DEspR-targeted molecular imaging in transgenic rat model of carotid artery disease
| Rat groups: 4-month-old male | Tg25+ |
Non-transgenic CEU (−) | |
|---|---|---|---|
| CEU (+) | CEU (−) | ||
| MBD contrast-enhanced image | |||
| Number of rats: both carotid arteries | 4 | 6 | 5 |
| Number of rats: one carotid artery | 3a | 3a | − |
| Contrast intensity signal Δ | |||
| MBD (n =18 rats) | 89.96±11.0b | 2.2±0.9 | 2.0±0.8 |
| MBC (n =8 rats) | 1.9±0.7 | ND | ND |
| Histopathology: | |||
| Intimal lesions, plaque | + | ± | − |
| Vasa vasorum expansion | + | ± | − |
| Immunostaining | |||
| DEspR | +: in vasa vasorum, initimal lesions | ± | − |
Values are group means±SEM, Δ delta or difference, + present, − absent, ± low to no expression
CAD carotid artery disease, MBD DEspR-targeted microbubble, MBC isotype-targeted microbubble, ND not done
Same three rats with one carotid artery CEU+ and the other CEU(−)
ANOVA and Tukey’s multiple pairwise comparison P<0.0001. Tukey’s test for multiple pairwise comparison of CIS levels in Tg25+ MBD CEU(+) with each of the other 3 groups.
Interestingly, the CIS plots of three transgenic rats with the highest MBD-specific CIS differences exhibited the expected post-acoustic destruction drop in signal intensity but had secondary peaks of contrast intensity signals followed subsequently by decline to low/baseline levels (Fig. 2a–h). This post-acoustic destruction/disruption pattern would be consistent with a putative sequence of microbubble events: micro-bubble fragmentation accounting for the drop, residual microbubble acoustic stimulation accounting for the secondary peak, followed by acoustically driven diffusion accounting for the subsequent steady decline to baseline levels.
Histological Analysis Detects MBD-microbubbles on DEspR-positive Endothelial Cells
Unexpectedly, Masson’s trichrome-stained histological analysis detected a few microbubbles still attached to endothelial cells or within intimal lesions (Fig. 3a) obtained from R1: MBD rat with CEU-positive imaging shown in Fig. 1a. Corresponding DEspR-immunostaining on the adjacent serial section confirmed adherence of MBD-microbubbles to DEspR-positive endothelial cells (Fig. 3b,c). Immunostaining with isotype antibody confirms specificity of DEspR-positive immunostaining (Fig. 3d). Altogether, these observations corroborate MBD-binding and specificity of MBD-binding to DEspR-positive endothelium. Survival of percutaneous endoscopic gastrostomy (PEG)-coated Target-ready MicroMarker microbubbles (VisualSonics, Inc., Canada) through PBS-buffered 4% paraformaldehyde fixation, paraffin embedding and deparaffinization parallels our observation that PEG-based biomaterials survive fixation, paraffin embedding, deparaffinization, and Masson’s trichrome staining [24].
Fig. 3.
Representative histological and fluorescence immunostaining analysis of carotid arteries with DEspR-positive molecular imaging corresponding to rat-R1 in Fig. 1 (a–d), and rat-R3 in Fig. 2 (e–h). a Masson’s trichrome-stained section of carotid artery endothelium. b, c Differential interference contrast (DIC) image overlaid with fluorescence immunostaining for DEspR expression (green immunostain) and DAPI nuclear stain (blue stain). d Control isotype-ab immunostaining and DAPI nuclear stain overlaid with DIC image of endothelium. e Carotid artery Masson’s trichrome-stained section showing increased adventitial vasa vasorum neovessels. Boxed area is shown in higher magnification in f documenting rbc-filled vasa vasorum. g Fluorescence immunostaining detects DEspR-positive expression (green immunostain) in vasa vasorum and surrounding cells. h Double immunostaining with α-SMA (red-orange immunostain) and DEspR detects αSMA co-expression in DEspR-positive neovessels (yellow-orange immunostain). Arrow adherent DEspR-targeted microbubble MBD; white arrowheads point to vasa vasorum neovessel in panels g and h; m media; bar=10 microns panels a–d, f; 20-μm panels e, g, h.
Histological analysis of R3:MBD rat shown in Fig. 2a–h also detected increased endothelial DEspR-positive expression and luminal endothelial pathology (data not shown), as well as marked carotid vasa vasoral expansion by neovascularization (Fig. 3e,f) with DEspR-positive expression in vasa vasorum neovessel (Fig. 3g). Double-immunofluorescence immunostaining with DEspR and α-smooth muscle actin (αSMA) detected some co-localization of DEspR+αSMA-positive immunostaining in carotid artery vasa vasorum (Fig. 3h).
Increased DEspR-Expression is Associated with DEspR-Positive Molecular Imaging
To determine whether increased level and/or area of DEspR-expression is associated with MBD-specific CEU-positive imaging defined by higher CIS differences (Fig. 1d) and higher pre-destruction CIS-peak levels (Fig. 4a), we performed double immunofluorescence-staining with anti-DEspR and anti-αSMA antibodies, the latter serving as a positive control for immunostaining of vascular smooth muscle cells in the media. We analyzed serial sections from representative rats (n=3/group) with MBD-specific bilateral CEU-positive imaging, MBD-infused bilateral CEU-negative imaging, and with one-sided CEU-positive/CEU-negative imaging. Analysis of immunofluorescence and DIC microscopy showed that MBD-specific CEU-positive imaging is associated with DEspR+ expression in carotid intimal lesions, vasa vasorum neovascularization, and DEspR+expression in vasa vasorum neovessels (Fig. 3b,c, 4b,c, Table 1). In contrast, rat carotid arteries exhibiting MBD–CEU-negative molecular imaging were associated with minimal, if any, DEspR+endothelial expression (Fig. 4d, Table 1). We also note the low levels of αSMA expression in carotid media smooth muscle cells (SMCs) compared with the expanded vasa vasorum (Fig. 4a), due most likely to the synthetic state of SMCs in these hypertensive rats since αSMA expression is deinduced in synthetic or proliferating SMCs [25]. These observations link MBD-specific CEU-positive imaging in this rat model with increased DEspR expression intensity and area in both intimal lesions and vasa vasorum neovessel density.
Fig. 4.

Representative fluorescence immunostaining analysis of carotid arteries from rats exhibiting MBD-specific CEU positive imaging (a, b, c) and CEU-negative imaging (a, d, e). a Scatter dot plot of pre-destruction CIS peak levels highlighting apparent threshold (yellow hatched bar) between MBD-specific CEU-positive (CEU+) and CEU-negative (CEU−) imaging. b DEspR-positive immunostaining of carotid artery endothelium and expanded vasa vasorum (green immunostain); αSMA-positive immunostaining in smooth muscle cells (SMCs) in the media (red-orange immunostain). Some vasa vasorum neovessels are double-immunostained for DEspR and αSMA (yellow immunostain). c Corresponding DIC-image shows structural layers of carotid artery and vasa vasorum. d Representative minimal to no DEspR-expression in rat carotid artery exhibiting CEU-negative imaging (shown here, nonTg rat-R2, Fig. 1c). Similar images obtained for CEU-negative transgenic rat carotid arteries. αSMA-immunostaining (red immunostain) detects expression in SMCs in the media. Low levels of αSMA-immunostaining in the medial indicates synthetic SMC phenotype in both carotid arteries (a, c), consistent with hypertensive remodeling. d Corresponding DIC-image shows structural layers of carotid artery and adventitia with no vasa vasorum expansion. Bar=20 μm (a, b), 10 μm (c, d). m media, adv adventitia, white small arrow endothelium, white large arrow vasa vasorum, blue fluorescence DAPI nuclear stain.
In vitro Analysis of Dose–Response MBD-Adherence to DEspR-Positive Endothelial Cells
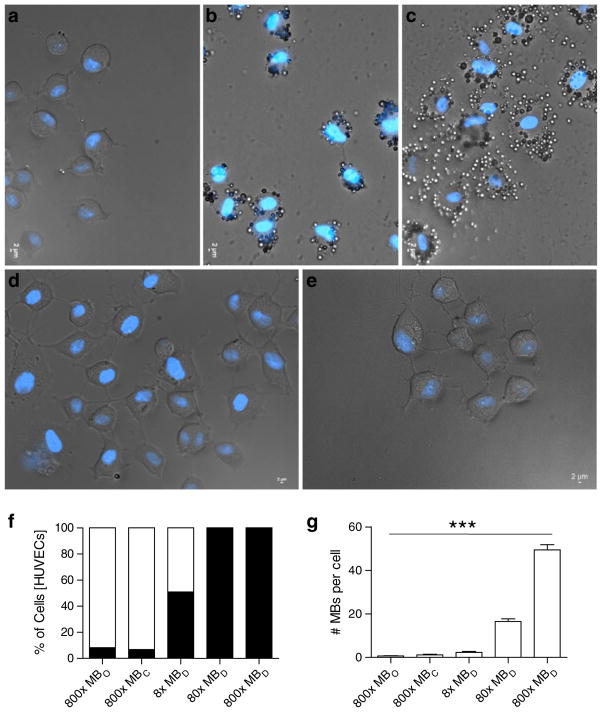
In order to further dissect MBD interactions with DEspR-positive cells, we tested the dose–response of MBD adherence in vitro. In order to avail of standardized primary cultures of endothelial cells and to gain translational insight into molecular imaging in humans, we used HUVECs which express DEspR in proliferating and pro-angiogenesis culture conditions as detected by a human-specific anti-DEspR monoclonal antibody (data not shown). Using increasing number of MBDs from 8×, 80×, and 800× MBD to cell ratio, we observed that HUVECs are increasingly bound by MBDs being 100% bound at 80× MBD:cell ratio (Fig. 5a–c), in contrast to 800× MBCs (Fig. 5d) and non-targeted MBOs (Fig. 5e) which bound 6.8% and 8.2% of HUVECs, respectively (Fig. 5f). Moreover, analysis of number of MBs bound per cell after a 45-min incubation and wash at flow rates with aortic-like shear stress of >20 dyne/cm2 revealed significant differences in number of MBs bound per cell increasing from 8×, 80×, to 800× as follows: 2.3, 17, and 49 MBs/cell, with only 0.6 and 1.1 MB/cell for non-targeted MBs and isotype MBCs (ANOVA P<0.0001). These observations reflect the relative stability and specificity of the MB–cell interaction. Importantly, cell toxicity was not observed upon contact of MB with cells even at high-dose 800× MBDs.
Fig. 5.
Phase contrast-fluorescence microscopy analysis of anti-humanDEspR-targeted microbubbles (MBD) binding to human endothelial cells, HUVECs, in vitro. Increasing DEspR-targeted microbubbles (MBD) to cell ratio a 8×, b 80×, and c 800×. d Isotype control (MBC) at 800×; e non-targeted control MBO at 800×. f % of HUVECs with bound MBs (filled square) and no MB binding (empty square). g Number of MBs (mean±SEM) per bound cell with increasing MB to cell ratio: MBD compared with isotype control MBC and control non-targeted MBO. ***ANOVA P< 0.0001. Blue fluorescence, DAPI staining of dsDNA in cell nuclei.
Discussion
Proof of Concept: DEspR-Targeted Molecular Imaging of Carotid Artery Disease
Although VEGFR2-targeted molecular imaging of tumor angiogenesis has been reported [12], previous VEGFR2-targeted molecular imaging of vasa vasorum neovascularization was not successful, along with other vascular adhesion molecule targets, leading authors to suggest that vasa vasoral flow might be a technical hurdle for target-specific CEU-molecular imaging [9]. With this perspective, the demonstrated molecular imaging of DEspR-positive endothelial cells in carotid artery disease (Figs. 1, 2, 3, and 4) provides critical proof-of-concept that in vivo molecular imaging of carotid artery disease endothelium and expanded vasa vasorum is feasible. Given optimal ultrasound imaging parameters, the likely factors for differential success in target-specific CEU-molecular imaging could be differences in molecular thresholds defined by the level and/or area of expression of the target, and/or in technical thresholds defined by density and size of, as well as flow in target vessel(s). A priori, these thresholds must be surpassed concurrently for detectable targeted CEU-positive imaging or molecular imaging. More specifically, the level of DEspR expression, the degree of luminal endothelial pathology, and the density of vasa vasorum neovascularization, along with the larger size of the rat carotid artery disease model used here, comprise logical factors contributing to successful DEspR-targeted CEU-positive imaging of carotid artery vasa vasorum in the Tg25 rat model of carotid artery disease, in contrast to the negative molecular imaging results targeting VEGFR2 reported for vasa vasorum neovascularization in a carotid artery injury-induced mouse model [9]. Furthermore, differences between CEU-positive transgenic rats from CEU-negative transgenic rats reveal a putative threshold for CIS-differences (Fig. 1e) and pre-disruption CIS-peak levels (Fig. 4a). This observed threshold for CEU-positive imaging suggests the hypothesis that DEspR-targeted CEU-positive imaging could be a non-invasive biomarker for pathological angiogenesis, and could have predictive value for disease progression. These hypotheses need to be studied further.
Insights from In vivo Target-Specific Molecular Imaging
Surpassing the molecular and technical threshold for successful detection of target-specific molecular imaging is concordant with the principle that reflectivity is directly proportional to the concentration of the microbubbles themselves [26]. More specifically, greater DEspR expression and greater density of DEspR-positive endothelial cells, be it at the lumen or in vasa vasorum, will translate to greater concentration of bound microbubbles. This in turn would be expected to translate to greater reflectivity and detection levels since microbubble–cell binding does not dampen microbubble reflectivity in contrast to leukocyte engulfment of microbubble [27]. After clearance of most circulating microbubbles and prior to acoustic disruption, stable binding of target-specific microbubbles exhibits a relatively stable contrast–intensity level that is significantly greater than negative or background contrast–intensity (Fig. 1d, ANOVA P<0.0001). Since high-frequency imaging can induce microbubble fragmentation or gas diffusion per se, a slight decline could also be observed prior to acoustic disruption. However, upon acoustic disruption, a drop in contrast–intensity due to fragmentation is observed to confirm microbubble binding (Fig. 1). Acoustic fragmentation may not be complete due to microbubble interaction in high-density ROIs which could dampen microbubble resonance [28], or from inability of microbubbles within microvessels to reach tenfold diameter fluctuation that underlies acoustic fragmentation [29]. Furthermore, incomplete fragmentation with gas release and relatively low flow, as would be expected in vasa vasorum compared to carotid artery lumen, could account for the secondary peak observed in rat-R3 followed by slow decline back to baseline levels (Fig. 2). The secondary peak is likely not due to refill because at this experimental time point there is minimal, if any, circulating microbubbles (Figs. 1 and 2). The fact that rat-R3 reached higher contrast-intensity levels than rat-R1 suggests greater microbubble concentration, which can also dampen acoustic destruction due to inter-microbubble interactions [28]. Notably, while acoustic fragmentation corroborates microbubble binding, the pattern of acoustic fragmentation or diffusion could also provide further insight into microbubble concentration, as well as binding site vessel caliber and flow. This provides an alternative molecular imaging paradigm to that reported for mouse aortic root atherosclerosis [30]. While CEU imaging in the current set-up is successful, future testing of non-linear imaging of adherent microbubbles could provide greater sensitivity and/or improved quantitation as observed for intravascular ultrasound for vasa vasorum flow imaging [31].
Translational Implications
The detection of dose-dependent increase in %cells targeted by MBDs and dose-dependent increase in number of MBs per cell (Fig. 5), gives insight into the stable interaction, kinetics, specificity and non-toxicity of DEspR-targeted MB–cell interactions. More importantly, given that in vitro studies were performed using human endothelial cells and human-specific anti-DEspR monoclonal antibody for targeting, that MB–cell coupling withstood a high shear stress wash after 45 min and did not elicit cell toxicity on contact, these in vitro observations of MBD–cell interactions provide translational insight into the potential of future DEspR-targeted molecular imaging of pathological angiogenesis.
Conclusions
Altogether, comparative analysis of molecular imaging contrast-intensity levels, histological confirmation of microbubble-to-endothelium binding, immunostaining confirmation that DEspR-positive molecular imaging is associated with DEspR-positive endothelial cell expression, and concordant patterns of bound microbubble behavior after acoustic destruction, demonstrate that target-specific molecular imaging of carotid endothelium and vasa vasorum neovascularization in carotid artery disease rat model is feasible. The identification of DEspR as a successful target for in vivo molecular imaging of vasa vasorum neovascularization and carotid artery disease lesions will facilitate the longitudinal study of vasa vasorum neovascularization and endothelial changes in carotid artery disease progression in animal models. Along with the in vitro observations of MBD–HUVECs stable binding, the data altogether contribute to fulfilling the promise of molecular imaging’s potential in the earlier detection of pathophysiological changes in cardiovascular disease for better estimation of risk for disease progression and complications [32].
Supplementary Material
Acknowledgments
We acknowledge the Ultrasound Micro-Imaging Core established by the Department of Medicine. This study was supported by NIH RO1 AG32649 to Dr. Herrera.
Footnotes
Disclosures. Pending patent application by Boston University for DEspR.
Electronic supplementary material The online version of this article (doi:10.1007/s11307-010-0444-4) contains supplementary material, which is available to authorized users.
References
- 1.Ruiz-Opazo N, Hirayama K, Akimoto K, Herrera VLM. Molecular characterization of a dual endothelin-1/angiotensin II receptor. Mol Med. 1998;4:96–108. [PMC free article] [PubMed] [Google Scholar]
- 2.Herrera VLM, Ponce LRB, Bagamasbad PD, VanPelt BD, Didishvili T, Ruiz-Opazo N. Embryonic lethality in Dear gene-deficient mice: new player in angiogenesis. Physiol Genomics. 2005;23:257–268. doi: 10.1152/physiolgenomics.00144.2005. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P, Ferreira V, Breir G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 5.Dunmore BJ, McCarthy MJ, Naylor AR, Brindle NP. Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J Vasc Surg. 2007;45:155–159. doi: 10.1016/j.jvs.2006.08.072. [DOI] [PubMed] [Google Scholar]
- 6.Giannoni MF, Vicenzini E, Citone M, et al. Contrast carotid ultrasound for the detection of unstable plaques with neoangiogenesis: a pilot study. Eur J Vasc Endovasc Surg. 2009 doi: 10.10.16/j.ejvs.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Vincenzini E, Giannoni MF, Benedetti-Valentini F, Lenzi GL. Imaging of carotid plaque angiogenesis. Cerbrovasc Dis. 2009;27(Suppl 2):48–54. doi: 10.1159/000203126. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann BA, Lindner JR. Molecular imaging with targeted contrast ultrasound. Curr Opin Biotech. 2007;18:11–16. doi: 10.1016/j.copbio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Lindner JR. Molecular imaging of cardiovascular disease with contrast-enhanced ultrasonography. Nat Rev Cardiol. 2009;6:475–481. doi: 10.1038/nrcardio.2009.77. [DOI] [PubMed] [Google Scholar]
- 10.Ellegala DB, Leong-Poi H, Carpenter JE, et al. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation. 2003;108:336–341. doi: 10.1161/01.CIR.0000080326.15367.0C. [DOI] [PubMed] [Google Scholar]
- 11.Leong-Poi H, Christiansen J, Heppner P, et al. Assessment of endogenous and therapeutic arteriogenesis by contrast ultrasound molecular imaging of integrin expression. Circulation. 2005;111:3248–3254. doi: 10.1161/CIRCULATIONAHA.104.481515. [DOI] [PubMed] [Google Scholar]
- 12.Willmann JH, Paulmurugan R, Chen K, et al. US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology. 2008;246:508–518. doi: 10.1148/radiol.2462070536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weller GE, Lu E, Csikari MM, et al. Ultrasound imaging of acute cardiac transplant rejection with microbubbles targeted to intercellular adhesion molecule-1. Circulation. 2003;108:218–224. doi: 10.1161/01.CIR.0000080287.74762.60. [DOI] [PubMed] [Google Scholar]
- 14.Hauff P, Reinhardt M, Briel A, Debus N, Schirner M. Molecular targeting of lymph nodes with L-selectin ligand-specific US contrast agent: a feasibility study in mice and dogs. Radiology. 2004;231:667–673. doi: 10.1148/radiol.2313030425. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann BA, Sanders JM, Davis C, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–284. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 16.Christiansen JP, Leong-Poi H, Klibanov AL, Kaul S, Lindner JR. Noninvasive imaging of myocardial reperfusion injury using leukocyte-targeted contrast echocardiography. Circulation. 2002;105:1764–1767. doi: 10.1161/01.cir.0000015466.89771.e2. [DOI] [PubMed] [Google Scholar]
- 17.Villanueva FS, Wagner WR. Ultrasound molecular imaging of cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2008;5:S26–S32. doi: 10.1038/ncpcardio1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumann PA, Christiansen JP, Quigley RM, et al. Targeted-microbubble binding selectively to GPIIb/IIIa receptors of platelet thrombi. Invest Radiol. 2002;37:587–593. doi: 10.1097/00004424-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton A, Huang SL, Warninck D, et al. Left ventricular thrombus enhancement after intravenous injection of echogenic immunoliposomes: studies in a new experimental model. Circulation. 2002;105:2772–2778. doi: 10.1161/01.cir.0000017500.61563.80. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Carr CL, Belcik TA, et al. Contrast-enhanced ultrasound characterization of inflammation and vasa vasoral proliferation caused by mural hemorrhage and platelet deposition. Circulation. 2008;118:S644. (Abstract 1074) [Google Scholar]
- 21.Decano JL, Viereck JC, McKee AC, Hamilton JA, Ruiz-Opazo N, Herrera VLM. Early-life sodium exposure unmasks susceptibility to stroke in hyperlipidemic, hypertensive heterozygous Tg25 rats transgenic for human cholesteryl ester transfer protein. Circulation. 2009;119:1501–9. doi: 10.1161/CIRCULATIONAHA.108.833327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrera VLM, Tsikoudakis A, Didishvili T, et al. Analysis of gender-specific atherosclerosis susceptibility in transgenic[hCETP]25DS rat model. Atherosclerosis. 2004;177:9–18. doi: 10.1016/j.atherosclerosis.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Loveless ME, Li X, Huamani J, et al. A method for assessing the microvasculature in a murine tumor model using contrast-enhanced ultrasonography. J Ultrasound Med. 2008;27:1699–1709. doi: 10.7863/jum.2008.27.12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera VL, Viereck JC, Lopez-Guerra G, et al. 11.7 Tesla magnetic resonance microimaging of laryngeal tissue architecture. Laryngoscope. 2009;119:2187–94. doi: 10.1002/lary.20643. [DOI] [PubMed] [Google Scholar]
- 25.Blindt R, Vogt F, Lamby D, et al. Characterization of differential gene expression in quiescent and invasive human arterial smooth muscle cells. J Vasc Res. 2002;39:340–352. doi: 10.1159/000065546. [DOI] [PubMed] [Google Scholar]
- 26.Calliada F, Campani R, Bottinelli O, Bozzini A, Sommaruga MG. Ultrasound contrast agents: basic principles. Eur J Radiol. 1998;27 (Suppl 2):S157–160. doi: 10.1016/s0720-048x(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 27.Lankford M, Behm CZ, Yeh J, Klibanov AL, Robinson P, Lindner JR. Effect of microbubble ligation to cells on ultrasound signal enhancement: implications for targeted imaging. Invest Radiol. 2006;41:721–728. doi: 10.1097/01.rli.0000236825.72344.a9. [DOI] [PubMed] [Google Scholar]
- 28.Yasui K, Lee J, Tuziuti T, Towata A, Kozuka T, Iida Y. Influence of the bubble–bubble interaction on destruction of encapsulated microbubbles under ultrasound. J Acoust Soc Am. 2009;126:973–982. doi: 10.1121/1.3179677. [DOI] [PubMed] [Google Scholar]
- 29.Chomas JE, Dayton P, Allen J, Morgan K, Ferrara KW. Mechanisms of contrast agent destruction. IEEE Trans Ultrason Ferroelectr Freq Control. 2001;48:232–248. doi: 10.1109/58.896136. [DOI] [PubMed] [Google Scholar]
- 30.Kaufmann BA, Carr CL, Belcik T, et al. Molecular imaging of the initial inflammatory response in atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:54–59. doi: 10.1161/ATVBAHA.109.196386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goertz DE, Frijlink ME, Tempel D, et al. Subharmonic contrast intravascular ultrasound for vasa vasorum imaging. Ultrasound Med Biol. 2007;33:1859–1872. doi: 10.1016/j.ultrasmedbio.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann BA. Ultrasound molecular imaging of atherosclerosis. Cardiovasc Res. 2009;83:617–625. doi: 10.1093/cvr/cvp179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.