Abstract
Activation of the Ras small GTP-binding protein is necessary for normal T cell development and function. However, which of a number of Ras GTPase activating proteins (RasGAPs) inactivate Ras in T cells is unknown. We used a T cell-specific RASA1-deficient mouse model to investigate the role of the p120 RasGAP protein (RASA1) in T cells. Death of CD4+CD8+ double-positive (DP) thymocytes was increased in RASA1-deficient mice. Despite this, on an MHC class II-restricted TCR transgenic background, evidence was obtained for increased positive selection of thymocytes associated with augmented activation of the Ras-MAPK pathway. In the periphery, RASA1 was found to be dispensable as a regulator of Ras-MAPK activation and T cell functional responses induced by full agonist peptides. However, numbers of naïve T cells were substantially reduced in RASA1-deficient mice. Loss of naïve T cells in the absence of RASA1 could be attributed in part to impaired responsiveness to the IL-7 pro-survival cytokine. These findings reveal an important role for RASA1 as a regulator of DP survival and positive selection in the thymus as well as naïve T cell survival in the periphery.
Introduction
Ras is a small G protein tethered to the inner leaflet of the cell membrane that cycles between inactive GDP-bound and active GTP-bound states (1). In its GTP-bound state, Ras triggers activation of downstream signaling pathways such as the MAPK pathway that regulates cell growth and differentiation (2). A plethora of studies have illustrated the importance of Ras and MAPK for T cell development and function. Thus, in the thymus, Ras-MAPK signal transduction is necessary for pre-TCR-induced transition of CD4−CD8− double-negative (DN)2 thymocytes into CD4+CD8+ double-positive (DP) thymocytes (3, 4). Furthermore, the Ras-MAPK pathway is essential for TCR-mediated positive selection of DP cells resulting in their maturation into CD4+ or CD8+ single positive (SP) T cells (4–7). In peripheral T cells, TCR-induced activation of the Ras-MAPK pathway is necessary for T cell activation and differentiation (8, 9).
The mechanism by which the TCR activates Ras has been well studied and involves mobilization of the guanine nucleotide exchange factors (GEF), mammalian son of sevenless and Ras guanine nucleotide releasing protein 1, to cell membranes (10–12). These GEF activate Ras by ejecting GDP from the Ras guanine nucleotide-binding pocket, thereby permitting Ras to bind GTP. Inactivation of Ras involves Ras-mediated hydrolysis of GTP to GDP. However, Ras has only weak GTP hydrolase activity and, therefore, Ras GTPase-activating proteins (RasGAPs) are required for efficient inactivation of Ras (13, 14). Through physical interaction, RasGAPs increase the ability of Ras to hydrolyse GTP by several orders of magnitude.
At least 10 different RasGAPs have now been identified in mammals (13). However, with the exception of neurofibromin 1 (NF1), which of these RasGAPs inactivate Ras in T cells has been little studied. Thymi and spleens from T cell deficient mice transplanted with bone marrow (BM) from non-conditional NF1-deficient mice contained increased numbers of thymocytes and T cells respectively compared to T cell-deficient mice transplanted with wild type BM, although ratios of thymocyte and T cell subsets were unchanged (15). Furthermore, quiescent T cells in mice that had received NF1-deficient BM showed increased constitutive levels of active MAPK, albeit that MAPK activity was not greater or more prolonged in these cells following TCR engagement compared to wild type T cells. These findings point to a role for NF1 as a constitutive rather than negative feedback regulator of Ras activation in the T cell lineage and, in addition, reveal its function as a regulator of T cell homeostasis. However, which RasGAP(s) regulate Ras activation once Ras-GTP levels have risen at key pre-TCR or TCR driven T cell developmental checkpoints or during the course of T cell activation is unknown.
Another prototypical RasGAP that is well expressed in T cells is p120 RasGAP (RASA1). Biochemical analyses have implicated RASA1 as regulator of Ras activation in T cells beforehand (16). However, non-conditional RASA1-deficient mice succumb at a relatively early point in embryonic development (17). Therefore, it has not been possible to perform BM adoptive transfer experiments to address definitively the importance of RASA1 in T cells. To examine this, we generated T cell-specific RASA1-deficient mice. Studies of these mice have revealed an important role for RASA1 as regulator of thymocyte survival and positive selection and in survival of naïve T cells in the periphery.
Materials and Methods
Mice
The generation of rasa1fl/fl mice with and without plck-cre and ub-ert2cre transgenes has been described (18). HY TCR transgenic (Tg) and AND TCR Tg rasa1fl/fl and rasa1fl/fl plck-cre mice were generated by cross-breeding with HY TCR Tg or AND TCR Tg mice (Taconic and JAX respectively). All mice are on a C57BL/6 (H-2b) genetic background. C57BL/6 and B10.BR (H-2k) mice were purchased from JAX. All mice were 2–3 mo of age at the time of experiments. All experiments were performed in compliance with University of Michigan guidelines and were approved by the University Committee on the Use and Care of Animals.
Tamoxifen administration
Tamoxifen (TM; Sigma) was administered i.p. (two injections of 80 mg/kg body weight on two consecutive days) to rasa1fl/fl ub-ert2cre mice and rasa1fl/fl control mice at 6–8 wk of age. Flow cytometric analysis of splenocytes from TM-injected mice was performed 2–3 mo after TM injection.
Flow cytometry
Subpopulations of thymocytes and splenocytes were enumerated by cell counting and flow cytometry using fluorochrome-conjugated CD4, CD8, CD25, CD69, CD44, CD62L, TCRβ, TCRγδ, HY TCR (T3.70), TCR Vβ3, TCR Vα11, HSA, NK1.1, CD45.1 and CD45.2 specific mAb (Becton Dickinson). For analysis of DN thymocytes, macrophages, DC and epithelial cells were gated out of populations on the basis of light scatter properties whereas NK cells and DN γδ T cells and NKT cells were gated out on the basis of specific antibody staining. For AND and HY TCR Tg thymi, analyses were performed upon Vβ3+ and HY TCR+ populations respectively. In AND TCR Tg mice, A–D stages of positive selection were defined as indicated in Fig. 3A. In female HY TCR Tg mice, populations A–D were similarly defined except that populations C and D were derived from a CD8 SP gate. In non-TCR Tg mice, populations A–D were defined on the basis of TCR versus CD69 expression as described (19, 20). CD8+ intermediate single positive cells in all types of mice were eliminated from CD8 SP gates on the basis of high levels of HSA expression. Cell viability was determined by staining with fluorochrome-coupled annexin V and 7-amino-actinomycin D (7AAD; Becton Dickinson). ERK activation was determined by intracellular staining using a fluorochrome-conjugated phospho-ERK specific mAb (Cell Signaling) as described previously with minor modifications (21). Cell staining was analyzed on a FACSCanto (Becton Dickinson).
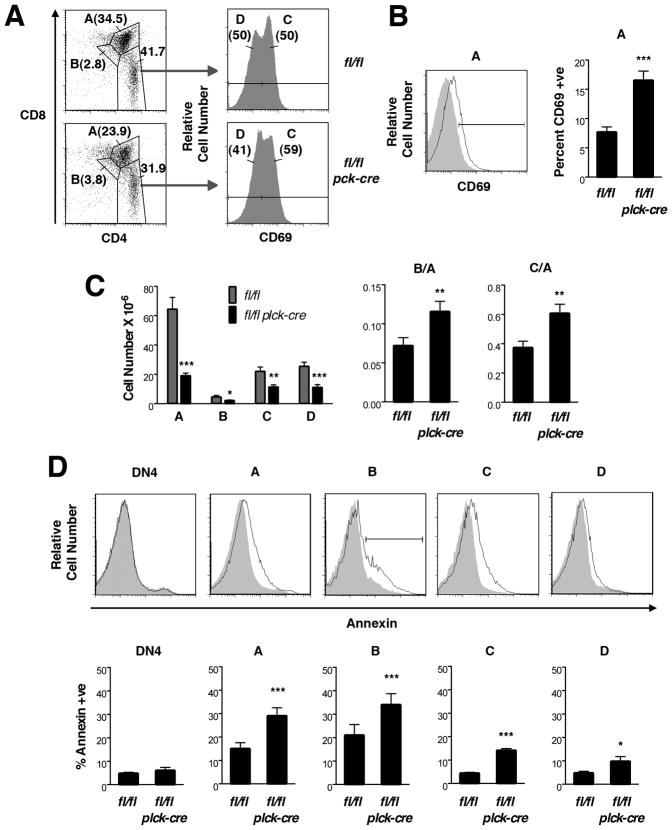
FIGURE 3. Positive selection in T cell-specific RASA1-deficient AND TCR Tg mice.
All experiments were performed with thymi of littermate rasa1fl/fl and rasa1fl/fl plck-cre AND TCR Tg mice. (A) Representative flow cytometry plots of whole thymi showing gating method used to define successive stages of positive selection, A (CD4+CD8+), B (CD4dullCD8dull), C (CD4+CD8loCD69+) and D (CD4+CD8-CD69-). (B) At left is a representative flow cytometry plot showing expression of CD69 upon population A cells from rasa1fl/fl (filled histogram) and rasa1fl/fl plck-cre (open histogram) AND TCR Tg mice. The bar indicates CD69+ cells. At right is shown the mean percent + 1 SEM of CD69+ cells of population A (rasa1fl/fl, n=11; rasa1fl/fl plck-cre, n=10). (C) At left is shown the mean number + 1 SEM of populations A through D in thymi. Bar graphs at right show mean B/A and C/A ratios + 1 SEM (rasa1fl/fl, n=11; rasa1fl/fl plck-cre, n=10). (D) At top are representative histograms showing annexin V staining upon the indicated thymocyte populations from rasa1fl/fl (filled histograms) and rasa1fl/fl plck-cre (open histograms) AND TCR Tg mice. The bar indicates annexin+ cells. 7AAD+ cells were eliminated from analyses. At bottom is shown the mean percent + 1 SEM of annexin+ cells of each population (rasa1fl/fl, n=4; rasa1fl/fl plck-cre, n=4). Statistical significance was determined using the Student’s two-sample t-test.
Cell isolation
CD4+, CD8+, CD4+CD44- and CD8+CD44- T cells were isolated from lymph nodes (LN) and spleen by negative selection using immunobeads (Miltenyi). Cell populations were greater than 90% pure as determined by flow cytometry. Expression of RASA1 in unfractionated and fractionated cell populations was determined by Western blotting using the B4F8 mAb (Santa Cruz). Blots were stripped and reprobed with either β-actin or GAPDH antibodies (Santa Cruz) to verify equivalent protein loading.
Bone marrow transfer experiments
BM from rasa1fl/fl plck-cre (CD45.2) mice and age and sex matched C57BL/6 wild type (CD45.1/CD45.2) mice was depleted of lineage positive cells by complement-mediated lysis. Cells were then co-injected (5 × 106 cells each) into tail veins of lethally-irradiated (950 rad) C57BL/6 wild type recipients (CD45.1). After 6 wk, recipients were euthanized and spleens were analyzed by flow cytometry.
MAPK activation in peripheral T cells
For assessment of ERK activation in peripheral T cells, 1.5 × 106 CD4+CD44- T cells from AND TCR Tg mice were mixed with 1.5 × 106 irradiated splenic adherent cells from B10.BR mice that had been pre-pulsed with pigeon cytochrome c (PCC) peptide 88–104 (10 μg/ml) for 1 h. Cells were co-pelleted and transferred to 37° C for different times. ERK activation was determined by flow cytometry (see above) and Western blotting using a phospho-specific ERK antibody (Cell Signaling). Western blots were stripped and reprobed with an ERK antibody (Santa Cruz) to ascertain equivalent protein loading.
T cell cytokine synthesis and proliferation
For cytokine synthesis and proliferation assays, 2 × 105 CD4+CD44- AND T cells were stimulated with 1 × 106 irradiated B10.BR splenic adherent cells and different concentrations of PCC peptide or CD3 and CD28 mAb (1 μg/ml; eBioscience) in complete medium (RPMI 1640 containing FBS and antibiotics) in wells of 96 well U-bottomed plates. Concentrations of IL-2 in culture supernatants were determined by ELISA after 48 h culture (R&D Systems). T cell proliferation was assessed by dilution of CFSE fluorescence after 48, 72 or 96 h of culture.
Thymocyte apoptosis
Thymocytes were seeded into wells of 96 well flat-bottomed plates at 2 × 105 thymocytes/well in complete medium or RPMI 1640. Wells were pre-coated or not with CD3 and CD28 mAb by overnight incubation in PBS. Dexamethasone or staurosporine (Sigma) were added to wells at the indicated concentrations. After 20 h culture, cells were harvested and viability was assessed by flow cytometry.
Activation induced cell death of peripheral T cells
CD4+CD44- AND T cells were stimulated with irradiated B10.BR splenic adherent cells and 10 μg/ml PCC plus 100 ng/ml of recombinant IL-2 (R&D systems) as indicated in cytokine synthesis and proliferation assays. After 48 h, cells were harvested, washed and recultured in RPMI 1640 medium alone or complete medium supplemented or not with IL-2 (10 ng/ml). After a further 48 h, cell viability was determined by flow cytometry.
Homeostatic proliferation
Five million CFSE-labeled CD8+CD44- T cells isolated from LN and spleen of rasa1fl/fl and rasa1fl/fl plck-cre mice (CD45.2) were injected into the tail veins of sublethally irradiated (500 rad) C57BL/6 mice (CD45.1). After 5 d, LN and spleen were harvested and the extent of proliferation of donor T cells was determined by dilution of CFSE fluorescence.
Peripheral T cell survival assays
Purified whole T cell populations from spleens of rasa1fl/fl and rasa1fl/fl plck-cre mice were cultured in wells of 24 well plastic plates (1 × 106 cells/ml) in complete medium in the presence or absence of 5 ng/ml of recombinant IL-7 (R&D systems). After 3 d culture, cell viability was determined by flow cytometry.
Results
T cell development in T cell-specific RASA1-deficient mice
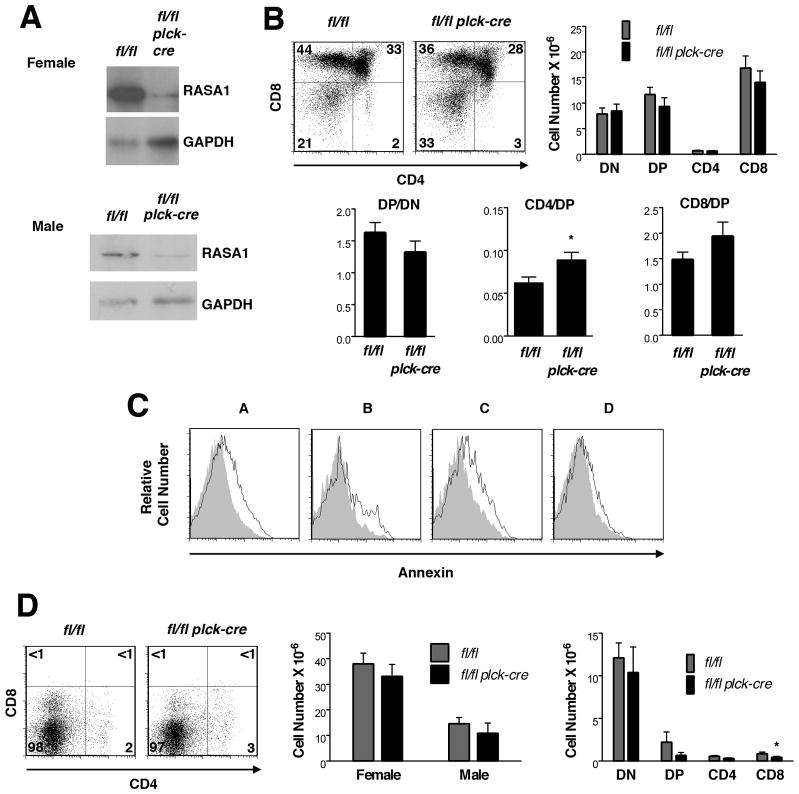
To delete RASA1 in the T cell lineage, rasa1fl/fl mice were crossed with plck-cre mice to generate rasa1fl/fl plck-cre mice (18). In these mice, Cre is expressed from the DN3 (CD44loCD25+) stage of thymocyte development onward (22). We first examined T cell development in these mice (Supplemental Fig. 1). In comparison with littermate control rasa1fl/fl mice, total numbers of DN and SP cells in thymi were largely unaltered. However, a modest reduction in DP number was observed. Although this was not statistically significant, the reduction in DP number was marked enough to cause a significant decrease in the DP to DN ratio in these mice (Supplemental Fig. 1C). Further analysis showed that an increased fraction of DP cells in RASA1-deficient mice stained positively for annexin V, indicating an increased susceptibility to apoptosis (data not shown). From the DP stage onward, we defined different successive stages of development based on expression levels of the αβ TCR and CD69 (19, 20). Each of population A (pre-positive selection; CD4+CD8+TCRintCD69-), population B (post-positive selection; CD4dullCD8dullTCRintCD69+) and population C (post-positive selection; CD4+CD8lo or CD4loCD8+ TCRhiCD69+) thymocytes showed increased annexin and 7AAD staining in RASA1-deficient mice (Supplemental Fig. 1D). By contrast, increased apoptosis was not observed in DN3, DN4 or population D cells (mature; CD4+CD8− or CD8+CD4- TCRhiCD69-). Therefore, the reduced DP/DN ratio in RASA1-deficient thymi is most likely explained by increased susceptibility of population A cells (which constitute the vast majority of DP cells) to apoptotic death rather than indicating impaired DN to DP transition.
T cell development in T cell-specific RASA1-deficient TCR transgenic mice
Since loss of RASA1 did not appear to affect DN to DP transition or positive selection, indicated by increased SP to DP ratios, we next examined T cell development in TCR Tg mice. It was reasoned that a role for RASA1 in these processes might be revealed in TCR Tg mice that would not be readily apparent in a polyclonal setting. To study the selection of MHC class II-restricted T cells, we generated rasa1fl/fl and rasa1fl/fl plck-cre AND TCR Tg mice. The AND TCR has specificity for PCC peptide 88–104 in the context of the MHC class II molecule, I-Ek (23). In H-2b mice, AND TCR Tg DP cells are positively-selected on the MHC class II molecule, I-Ab, and mature into CD4 SP T cells. Use of this model, therefore, affords an opportunity to examine positive selection upon MHC class II at a clonal level.
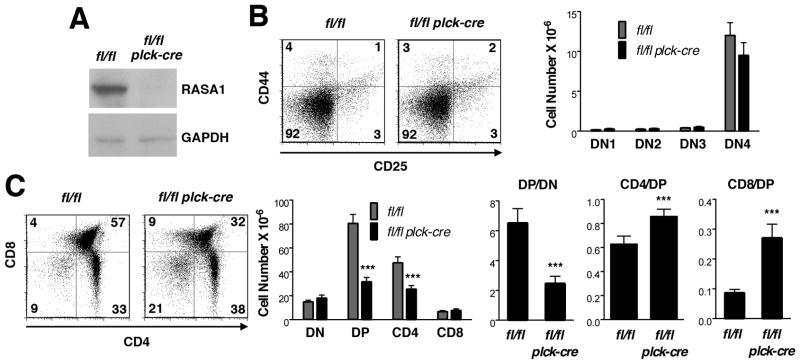
Numbers of DN cells were not affected by loss of RASA1 in AND TCR Tg mice. By contrast, substantial reductions in the number of DP and CD4 SP cells were observed (Fig. 1B and C). Annexin V staining of thymocyte subpopulations revealed that, similar to non-TCR Tg mice, DP cells but not DN cells showed increased apoptosis (data not shown and Fig. 3). Therefore, as before, the increased apoptosis of DP cells likely accounts for the decreased DP to DN ratio in the absence of RASA1 rather than indicating impaired DN to DP transition (Fig. 1C).
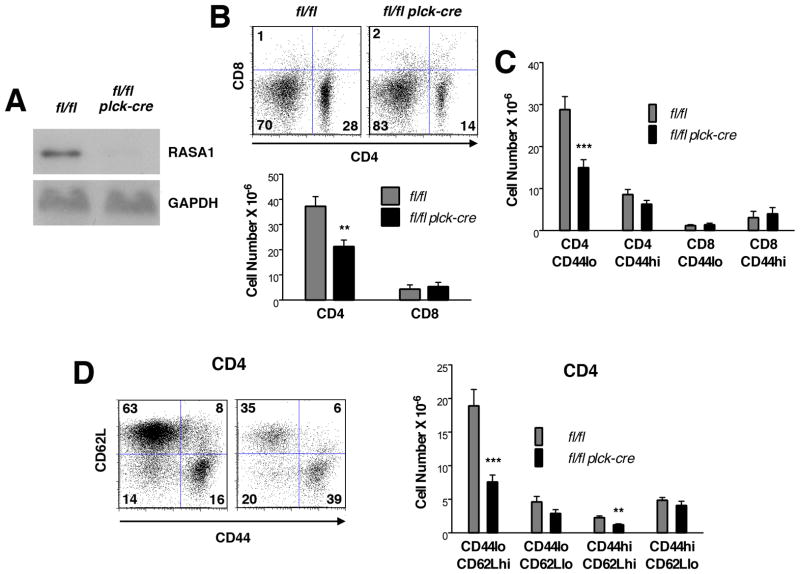
FIGURE 1. T cell development in T cell-specific RASA1-deficient AND TCR Tg mice.
All experiments were performed with thymi of littermate rasa1fl/fl and rasa1fl/fl plck-cre AND TCR Tg mice. (A) Representative Western blot showing RASA1 expression in whole thymi. (B) At left are shown representative two-color flow cytometry plots of CD44 and CD25 expression upon CD4−CD8− DN thymocytes. Numbers indicate the percent of cells in each quadrant. Bar chart at right shows mean number + 1 SEM of DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44loCD25+) and DN4 (CD44−CD25−) cells in thymi (n=5 mice of each genotype). (C) Representative plots of CD4 and CD8 expression (left) and bar chart (middle) showing mean number + 1 SEM of DN, DP, CD4 and CD8 SP cells in thymi (rasa1fl/fl, n=18; rasa1fl/fl plck-cre, n=20). Bar graphs at right depict indicated mean ratios + 1 SEM of thymocyte subsets within each genotype. Statistical significance was determined using the Student’s two-sample t-test.
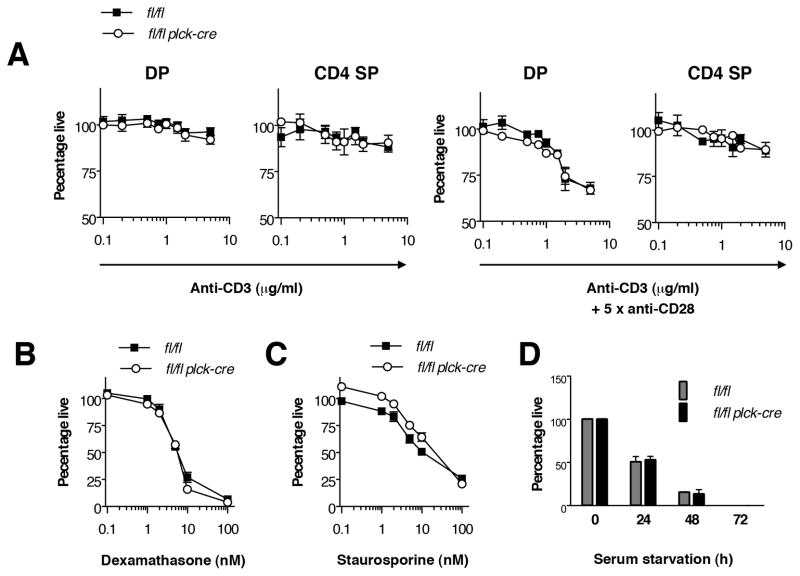
In contrast to the DP to DN ratio, the CD4 SP to DP ratio was increased in the absence of RASA1 (Fig. 1C). This could be taken as evidence for augmented positive selection in this model. However, before exploring this further we wanted to ascertain that the decreased numbers of DP and CD4 SP cells was not a result of increased negative selection of AND TCR Tg cells. To address this, we compared the apoptotic response of wild type and RASA1-deficient thymocytes to increasing concentrations of CD3 mAb (directed to the TCR complex) plus mAb against the CD28 costimulatory receptor or to CD3 mAb alone (Fig. 2A). In wild type thymocytes, CD3 plus CD28 mAb but not CD3 mAb alone induced significant apoptosis in DP cells, as expected (24, 25). In contrast, neither CD3 plus CD28 mAb nor CD3 mAb alone induced significant apoptosis in wild type CD4 SP cells. The apoptotic responses of RASA1-deficient thymocytes to CD3 plus CD28 mAb and CD3 mAb alone were essentially identical to those of wild type thymocytes. In DP cells, CD3 plus CD28 mAb triggered the same extent of apoptosis over a range of mAb concentrations. Furthermore, sensitivity of DP cells to CD3 mAb alone or CD4 SP cells to CD3 plus CD28 mAb or CD3 mAb alone was not acquired with loss of RASA1 expression. Therefore, it is unlikely that the reduced number of DP and CD4 SP cells in RASA1-deficient AND TCR Tg thymi can be explained by increased negative selection. Interestingly, RASA1-deficient thymocytes also showed similar sensitivity to other established apoptosis-inducing agents or conditions in vitro including dexamethasone, staurosporine and serum deprivation (Fig. 2B–D).
FIGURE 2. Apoptosis sensitivity of RASA1-deficient thymocytes.
Experiments were performed with thymocytes of littermate rasa1fl/fl and rasa1fl/fl plck-cre mice. (A) Thymocytes were seeded into wells that had been precoated or not with different concentrations of CD3 mAb or CD3 plus CD28 mAb. After 20 h, the percentage of live annexin− 7AAD− DP or CD4 SP cells in cultures was determined by flow cytometry. Results were normalized to the percentage of live DP or CD4 SP cells harvested from wells that were not coated with mAb. Graphs show the mean percentage of live cells +/−1 SEM under the different stimulation conditions (n=3 mice of each genotype). (B and C) Experiments were conducted as in A except that dexamethasone (B) or staurosporine (C) were added to wells in different concentrations. Graphs show the mean percentage of live whole thymocytes +/− 1 SEM for each condition (n=3 mice each genotype). (D) Thymocytes were cultured in serum free or complete medium for the indicated times. Cell viability was determined as above. Results with serum free medium were normalized to the percentage of viable cells cultured in complete medium for the same time. Bar graphs show the mean percentage of live whole thymocytes +/− 1 SEM at each time in serum free medium (n=3 mice each genotype).
Another alternative interpretation for the increased CD4 SP to DP ratio in RASA1-deficient AND TCR Tg mice is that this ratio is artificially increased as a consequence of the increased death of DP cells. To address this, therefore, we again identified successive stages of T cell development from the DP stage onward. However, owing to the relatively modest upregulation of CD69 on population B cells in AND TCR Tg mice, populations A and B were initially distinguished on the basis of CD4 and CD8 expression rather than TCR versus CD69 expression (Fig. 3A). Population C and D cells were identified within a CD4 SP gate and distinguished based upon high or low expression of CD69 respectively (Fig. 3A). Examination of the expression levels of the TCR and HSA confirmed that populations A–D so defined in AND TCR Tg mice were analogous to the same populations in non-TCR Tg mice (data not shown).
Analysis of populations A–D in AND TCR Tg mice strongly supported the notion of increased positive selection. First, in population A cells, expression of CD69 was consistently increased in the absence of RASA1 (Fig. 3B). Upregulation of CD69 expression is characteristic of positive selection in the thymus (24, 25). Second, ratios of the post-positive selection populations B and C to the pre-positive selection population A were both increased (Fig. 3C). These increases could not be accounted for by increased susceptibility of population A to apoptosis since populations A, B and C showed comparable increases in apoptosis in RASA1-deficient mice (Fig. 3D).
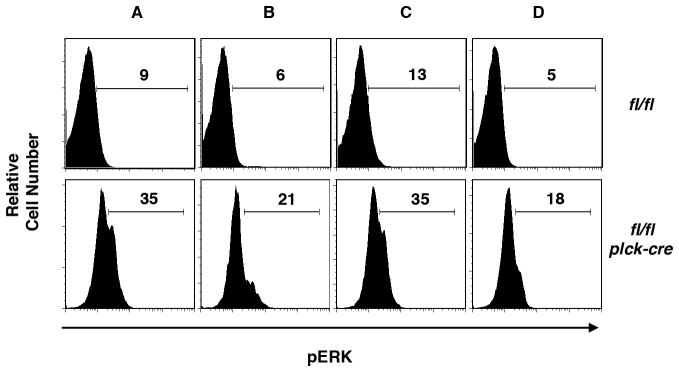
Increased positive selection in RASA1-deficient AND TCR Tg mice is consistent with the known function of RASA1 as a negative regulator of Ras-MAPK signal transduction. To confirm that RASA1 regulates this pathway during the course of positive selection, we examined activation of ERK MAPK in thymocyte subsets in freshly explanted thymi by phospho-flow cytometry (Fig. 4). In control AND TCR Tg thymi, distinct ERK+ve cells were difficult to identify by phospho-flow cytometry in any of populations A–D. By contrast, in RASA1-deficient AND TCR Tg thymi, ERK+ve cells were readily identified. Staining was highest in populations A and C and lowest in population D. Furthermore, background levels of ERK staining were consistently higher in all four thymocyte populations in RASA1-deficient mice (although not in peripheral T cells – see later). Increased activation of ERK, therefore, likely accounts for the increased positive selection in the absence of RASA1 (7).
FIGURE 4. ERK activation during positive selection in T cell-specific RASA1-deficient AND TCR Tg mice.
Representative flow cytometric plots showing expression of phospho-ERK in the indicated thymocyte populations from littermate rasa1fl/fl and rasa1fl/fl plck-cre AND TCR Tg mice (see Fig. 3). Numbers represent the percent phospho-ERK+ cells within each population. The same results were obtained in a repeat experiment.
To examine the selection of MHC class I-restricted T cells, we used HY TCR Tg mice. The HY TCR recognizes a male-specific HY peptide in the context of the MHC class I molecule, H-2Db (26). In female H-2b HY TCR Tg mice, HY TCR Tg DP cells are positively-selected upon recognition of H-2Db and mature into CD8+ SP T cells.
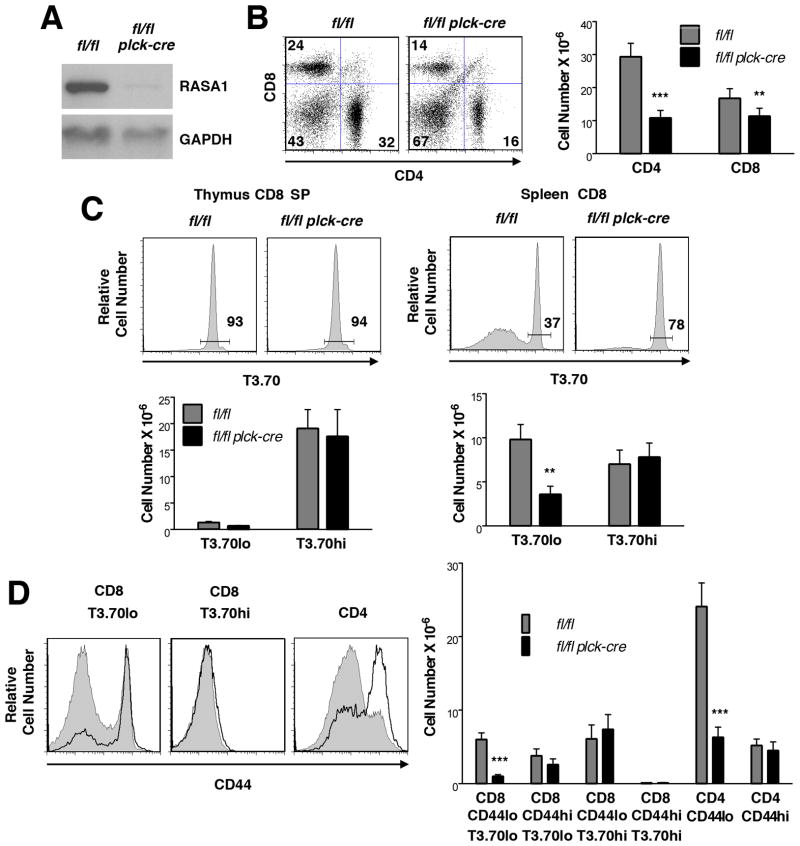
Numbers of DN, DP and SP cells in T cell-specific RASA1-deficient female HY TCR Tg mice were comparable to those observed in control female HY TCR Tg mice (Fig. 5B). A modest, not statistically significant, reduction in DP number was noted that could be accounted for by increased apoptosis of population A cells, similar to non-TCR Tg and AND TCR Tg mice (Fig. 5C). However, the CD8 SP to DP ratio was not significantly changed. This finding indicates that in this particular MHC class I-restricted TCR Tg model, RASA1 does not play a role in positive selection.
FIGURE 5. T cell development in T cell-specific RASA1-deficient HY TCR Tg mice.
All experiments were performed with thymi of littermate rasa1fl/fl and rasa1fl/fl plck-cre HY TCR Tg mice. (A) Representative Western blots showing RASA1 expression in whole thymi of female and male HY TCR Tg mice. (B) Representative plots of CD4 and CD8 expression (top left) and bar chart (top right) showing mean number + 1 SEM of HY TCR+ (T3.70hi) DN, DP, CD4 SP and CD8 SP cells in thymi of female mice (rasa1fl/fl, n=12; rasa1fl/fl plck-cre, n=16). Bar graphs below show mean ratios + 1 SEM of thymocyte subsets within genotypes. (C) Representative histograms of annexin V staining upon the indicated HY TCR+ thymocyte subpopulations in thymi of female rasa1fl/fl (filled histograms) and rasa1fl/fl plck-cre (open histograms) HY TCR Tg mice (see Fig. 3 and Methods). The same results were obtained in three repeat experiments. (D) Representative plots of CD4 versus CD8 expression upon HY TCR+ thymocytes in male thymi are shown at left. Bar charts depict mean number + 1 SEM of total HY TCR+ cells (middle) and indicated HY TCR+ thymocyte subsets (right) in male thymi (rasa1fl/fl, n=6; rasa1fl/fl plck-cre, n=5). Comparisons with total numbers of HY TCR+ cells in female mice are shown (rasa1fl/fl, n=12; rasa1fl/fl plck-cre, n=16) (middle panel). Statistical significance was determined using the Student’s two-sample t-test.
In male HY TCR Tg male mice, expression of the HY peptide upon thymic stromal cells results in negative selection of HY TCR-expressing DP cells (26). Therefore, we examined thymi of male RASA1-deficientHY TCR Tg mice to determine if there was any influence of RASA1 loss upon negative selection in this model (Fig. 5D). However, thymi from RASA1-deficient and control male HY TCR Tg mice showed the same reductions in total cellularity associated with loss of DP and SP cells compared to female HY TCR Tg mice (also compare Fig. 5B). We conclude, therefore, that expression of RASA1 is not necessary for thymic negative selection in HY TCR Tg male mice. Moreover, there are no signs of autoimmune disease development in T cell-specific RASA1-deficient mice, consistent with the notion that negative selection is intact in these animals.
Peripheral T cells in T cell-specific RASA1-deficient mice
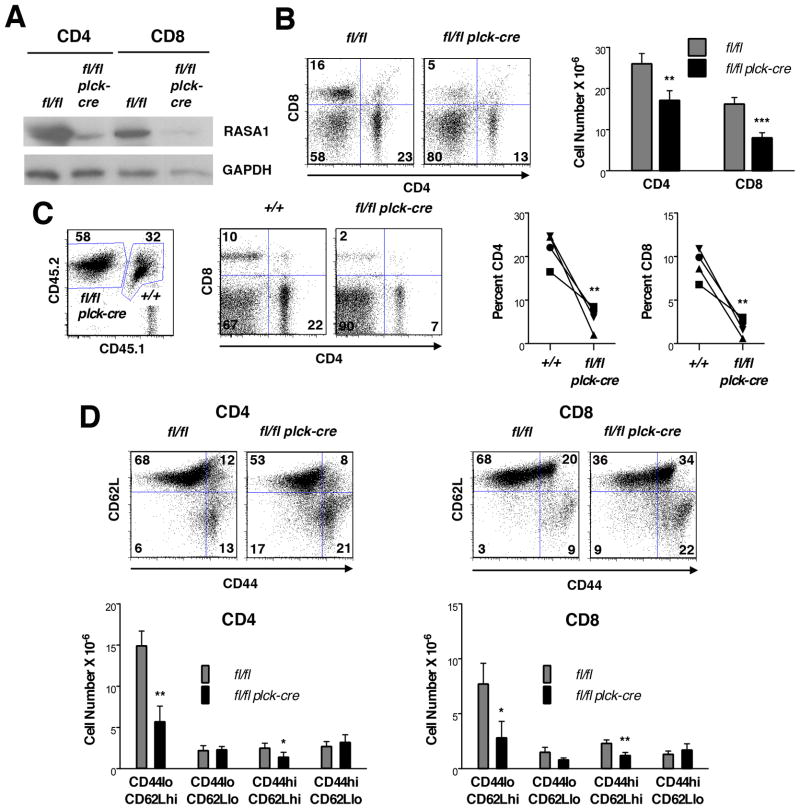
Despite that normal numbers of CD4 and CD8 SP T cells were found in thymi of T cell-specific RASA1-deficient non-TCR Tg mice, substantially reduced numbers of CD4 and CD8 T cells were found in peripheral lymphoid organs of these animals (Fig. 6B) (Figs. 6–9 show results from spleen; the same observations were made in LN throughout). This loss of peripheral T cells was not caused by the plck-cre transgene itself since rasa1+/+ plck-cre mice contained the same number of peripheral T cells as littermate rasa1+/+ mice (Supplemental Fig. 2). Furthermore, TM treatment of adult rasa1fl/fl ub-ert2cre mice (to induce global deletion of RASA1) resulted in a loss of peripheral CD4 and CD8 T cells (Fig. 7). Therefore, expression of RASA1 in the T cell lineage is necessary to maintain normal numbers of T cells in the periphery. The importance of RASA1 in peripheral T cell homeostasis was further illustrated in competitive BM transfer experiments (Fig. 6C). In these experiments, equal numbers of lineage negative BM cells from wild type and rasa1fl/fl plck-cre mice were injected into lethally-irradiated wild type recipient mice. After 6 wk, spleens were harvested and the percentage of CD4 and CD8 T cells among splenocytes of donor wild type and rasa1fl/fl plck-cre BM origin was determined with the use of congenic CD45 markers. As shown, the percentage of CD4 and CD8 T cells among rasa1fl/fl plck-cre splenocytes was substantially less than the percentage of CD4 and CD8 T cells among wild type splenocytes.
FIGURE 6. Peripheral T cells in T cell-specific RASA1-deficient mice.
(A) Representative Western blot showing expression of RASA1 in CD4 and CD8 T cells isolated from spleens of littermate rasa1fl/fl and rasa1fl/fl plck-cre mice. (B) Representative flow cytometry plots of CD4 and CD8 expression (left) and mean number + 1 SEM of CD4 and CD8 T cells (right) in spleens of littermate rasa1fl/fl and rasa1fl/fl plck-cre mice (n=16 mice each genotype). (C) BM from rasa1fl/fl plck-cre (CD45.2) and wild type C57BL/6 (+/+) (CD45.1/CD45.2) mice was adoptively transferred to lethally-irradiated wild type C57BL/6 (CD45.1) recipients. Flow cytometric plots show CD45, CD4 and CD8 expression in a single representative recipient spleen 6 wk after BM transfer. Numbers in CD45 plots indicate percentage of splenocytes of donor wild type or rasa1fl/fl plck-cre origin among total splenocytes. Numbers in CD4/CD8 plots indicate percentage of cells in quadrants among splenocytes of the indicated genotype. Line graphs summarize results from 4 independent recipient mice. Percent CD4 and CD8 T cells among splenocytes of the indicated genotypes are shown. Alike symbols are used to represent data obtained from the same mouse within and between graphs. (D) At top are shown representative flow cytometry plots of CD44 and CD62L expression upon CD4 and CD8 T cells in spleens of littermate rasa1fl/fl and rasa1fl/fl plck-cre mice. Corresponding bar graphs below show mean cell number +1 SEM of the indicated splenic subpopulations (rasa1fl/fl, n=6; rasa1fl/fl plck-cre, n=7). Statistical significance was determined using unpaired (B and D) and paired (C) Students’ t-tests.
FIGURE 9. Peripheral T cells in T cell-specific RASA1-deficient female HY TCR Tg mice.
All analyses were performed upon splenocytes or splenic T cells or thymocytes of littermate rasa1fl/fl and rasa1fl/fl plck-cre female HY TCR Tg mice. (A) Representative Western blot showing expression of RASA1 in purified CD8 splenic T cells from female HY TCR Tg mice. (B) Representative flow cytometric plots of CD4 and CD8 expression (left) and mean number + 1 SEM of CD4 and CD8 T cells (right) in spleens of female HY TCR Tg mice (rasa1fl/fl, n=8; rasa1fl/fl plck-cre, n=9). (C) Representative histograms at top show expression of the T3.70 marker upon thymic CD8 SP T cells and splenic CD8 T cells of female HY TCR mice. Percentages of CD8 T cells that express the HY TCR (T3.70hi cells) are indicated. Bar graphs below shown mean number + 1 SEM of the indicated thymic CD8 SP and splenic CD8 T cell populations (rasa1fl/fl, n=8; rasa1fl/fl plck-cre, n=9). (D) Histograms at left show representative expression of CD44 upon the indicated splenic T cell populations of female HY TCR Tg mice. Filled and bold histograms represent rasa1fl/fl and rasa1fl/fl plck-cre mice respectively. Bar graph at right shows mean number +1 SEM of the indicated populations in spleens (rasa1fl/fl, n=8; rasa1fl/fl plck-cre, n=9). Statistical significance was determined using the Student’s two-sample t-test.
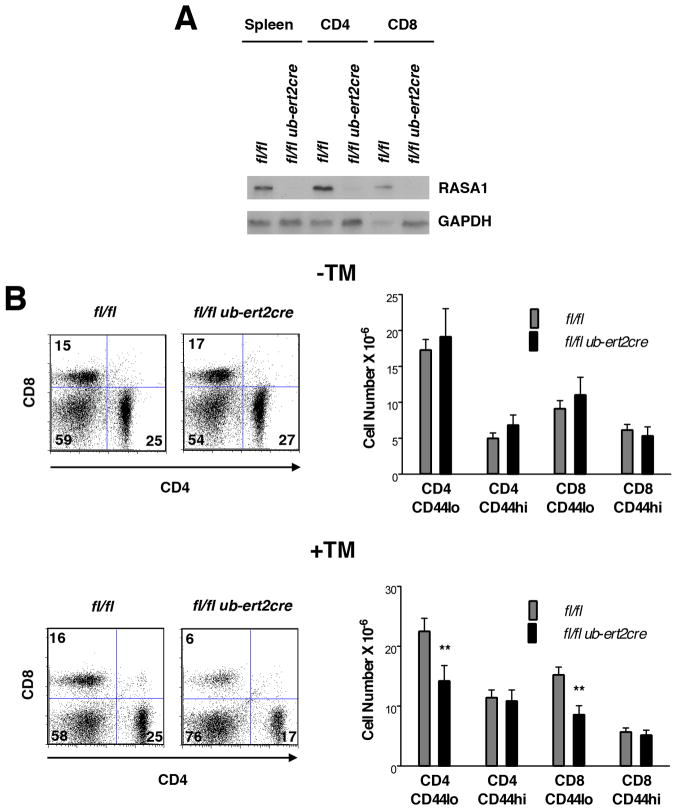
FIGURE 7. Effect of induced loss of RASA1 upon peripheral T cell numbers.
All experiments were performed with littermate rasa1fl/fl and rasa1fl/fl ub-ert2cre mice. (A) Representative Western blot showing expression of RASA1 in spleens and purified splenic CD4+ and CD8+ T cells from mice injected with TM at 6 wk of age. WB were performed 8 wk after TM administration. (B) Representative two color flow cytometry plots (left) showing CD4 and CD8 staining upon splenocytes of mice either not injected with TM (−TM) or injected with TM (+TM) at 6–8 wk of age. Analyses were performed at 3–4 mo of age. Bar graphs at right show mean number + 1 SEM of the indicated splenic T cell populations in mice without TM treatment (rasa1fl/fl, n=8; rasa1fl/fl ub-ert2cre, n=6) and with TM treatment (rasa1fl/fl, n=15; rasa1fl/fl ub-ert2cre, n=16). Statistical significance was determined using the Student’s two-sample t-test.
We examined numbers of naïve (CD44lo), central memory (CD44hiCD62Lhi) and effector memory (CD44hiCD62Llo) CD4 and CD8 T cells in T cell-specific RASA1-deficient non-TCR Tg mice (Fig. 6D). These studies revealed that the diminution of CD4 and CD8 T cell number was primarily accounted for by loss of naïve T cells. A specific loss of naïve T cells was also observed in rasa1fl/fl ub-ert2cre mice treated with TM (Fig. 7).
Analysis of peripheral T cells in RASA1-deficient AND TCR Tg mice revealed a similar diminution in the total number of CD4 T cells that was explained by a reduction in the number of naïve CD4 T cells specifically (Fig. 8B–D). In addition, reduced numbers of CD8 T cells were found in the periphery of RASA1-deficient female HY TCR Tg mice compared to controls (Fig. 9B). In control female HY TCR Tg mice, the majority of peripheral CD8 T cells do not in fact express the clonotypic HY TCR, defined by high-level expression of the T3.70 marker (Fig. 9C, right). This is not a result of inefficient allelic exclusion by the HY TCR during T cell development since the vast majority of thymic CD8 SP T cells express high levels of T3.70 (Fig. 9C, left). Rather, as shown previously, HY TCR-expressing T cells are peculiarly unable to undergo homeostatic expansion in the periphery of female mice (27, 28). This is in contrast to HY TCR negative CD8 T cells and CD4 T cells, which are able to expand in the periphery and which thus outgrow HY TCR T cells despite that far fewer of these cells are generated in the thymus. Notably, numbers of peripheral HY TCR CD8 T cells are the same in female RASA1-deficient and control HY TCR Tg mice. Rather, there are much fewer HY TCR negative CD8 T cells in the periphery of the RASA1-deficient mice, which accounts entirely for the reduction in total CD8 T cell number (Fig. 9C, right). Among HY TCR negative CD8 T cells in RASA1-deficient mice, CD44lo T cells are reduced in number specifically (Fig. 9D). Likewise, the reduced number of CD4 T cells in the periphery of RASA1-deficient HY TCR Tg mice can be explained by a specific reduction in the number of CD44lo T cells (Fig. 9D).
FIGURE 8. Peripheral T cells in T cell-specific RASA1-deficient AND TCR Tg mice.
All analyses were performed upon splenocytes or splenic T cells of littermate rasa1fl/fl and rasa1fl/fl plck-cre AND TCR Tg mice. (A) Representative Western blot showing expression of RASA1 in purified CD4 splenic T cells from AND TCR Tg mice. (B) At top are shown representative flow cytometry plots of CD4 and CD8 expression in AND TCR Tg spleens. Bar graph below depicts mean cell number + 1 SEM of CD4 and CD8 splenic T cells (n=13 mice each genotype). (C) Bar graph shows mean number + 1 SEM of indicated CD4 and CD8 subpopulations in AND TCR Tg spleens (n=13 mice each genotype). (D) At left are shown representative histograms of CD44 and CD62L staining upon CD4+ T cells in AND TCR Tg spleens. Bar graph at right shows mean number + 1 SEM of the indicated CD4+ populations (n=8 each genotype). Statistical significance was determined using the Student’s two-sample t-test.
Functional responses of peripheral RASA1-deficient T cells
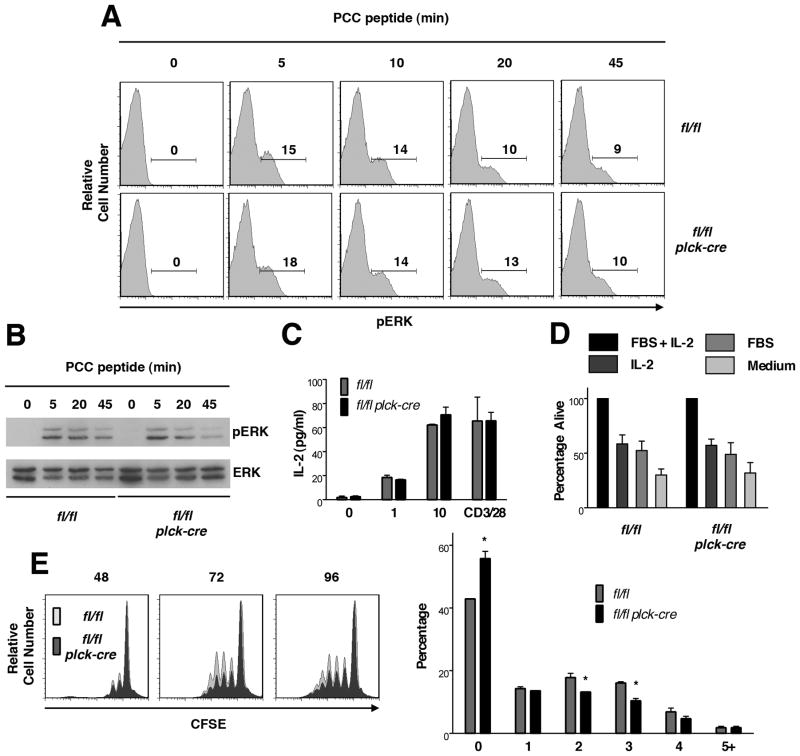
To determine if RASA1 regulates TCR-induced activation of the Ras-MAPK pathway in peripheral T cells, naïve CD44− CD4 peripheral T cells were purified from spleens and LN of AND TCR Tg mice and stimulated with PCC peptide-pulsed I-Ek APC for different times in vitro. Activation of ERK MAPK was then determined by phospho-flow cytometry and Western blotting (Fig. 10A and B). As shown, PCC-pulsed APC induced digital ERK activation responses in control and RASA1-deficient T cells that were similar in nature both in terms of magnitude and duration. In accordance with these findings, amounts of the IL-2 cytokine synthesized by naïve AND TCR Tg T cells in response to PCC-APC stimulation were the same between RASA1-deficient and control mice (Fig. 10C). Some reduction in the extent of PCC-APC induced proliferation of naïve AND RASA1-deficient T cells was observed (Fig. 10E). However, this difference in proliferation was only modest.
FIGURE 10. Functional responses of RASA1-deficient T cells.
All experiments were performed with purified CD4+CD44- peripheral T cells from littermate rasa1fl/fl and rasa1fl/fl plck-cre AND TCR Tg mice. (A and B) T cells were stimulated with PCC peptide-pulsed APC for the indicated times. Expression of phospho-ERK in T cells was determined by flow cytometric staining (A) and Western blotting (B). In flow cytometry histograms, numbers indicate percent of phospho-ERK+ cells. Data are representative of three and twelve repeat experiments respectively. (C) T cells were stimulated with APC and the indicated concentrations of PCC peptide or with CD3 and CD28 antibodies for 24 h. Concentrations of IL-2 in culture supernatants were determined by ELISA. Shown are mean IL-2 concentration + 1 SEM of triplicate determinations. Data are representative of ten repeat experiments. (D) T cells were stimulated with APC plus 10 μg/ml of PCC peptide in the presence of IL-2 for 48h. Cells were washed and then replated in medium alone or medium containing FBS and/or IL-2. After 48 h, the percentage of viable T cells was determined and values were normalized to the value obtained for T cells cultured with FBS plus IL-2. Bar graphs show mean percent viable T cells + 1 SEM (n=5 mice each genotype). (E) CFSE-labeled T cells were stimulated with APC and 10 ug/ml of PCC peptide for the indicated times in hours. At left are shown representative histograms of CFSE fluorescence determined by flow cytometry. Bar graph at right shows mean percentage +1 SEM of T cells at the indicated cell division number after 72 h (n=3 mice each genotype). Statistical significance was determined using the Student’s two-sample t-test.
We also examined activation induced cell death of RASA1-deficient naïve T cells. For this purpose, naïve AND T cells were stimulated with PCC-APC and IL-2. After 2 d, cells were then replated in medium lacking IL-2, FBS or both and the extent of T cell death was determined after an additional 2 d culture (Fig. 10D). No difference in the extent of T cell death was observed between RASA1-deficient and control T cells. Likewise, no difference in T cell death was observed in similar assays in which death of T cells was induced by restimulation with CD3 and CD28 antibodies (data not shown).
In addition to AND TCR Tg T cells, we also examined ERK activation responses, cytokine synthesis, proliferation and death of RASA1-deficient female HY TCR Tg T cells stimulated with HY peptide and H-2Db APC and of non-TCR Tg T cells stimulated with CD3 and CD28 antibodies. In each case, responses were similar to those observed with control T cells (data not shown). We conclude that RASA1 is dispensable as regulator of each of these responses of peripheral T cells.
Homeostatic proliferation and survival of naïve RASA1-deficient T cells
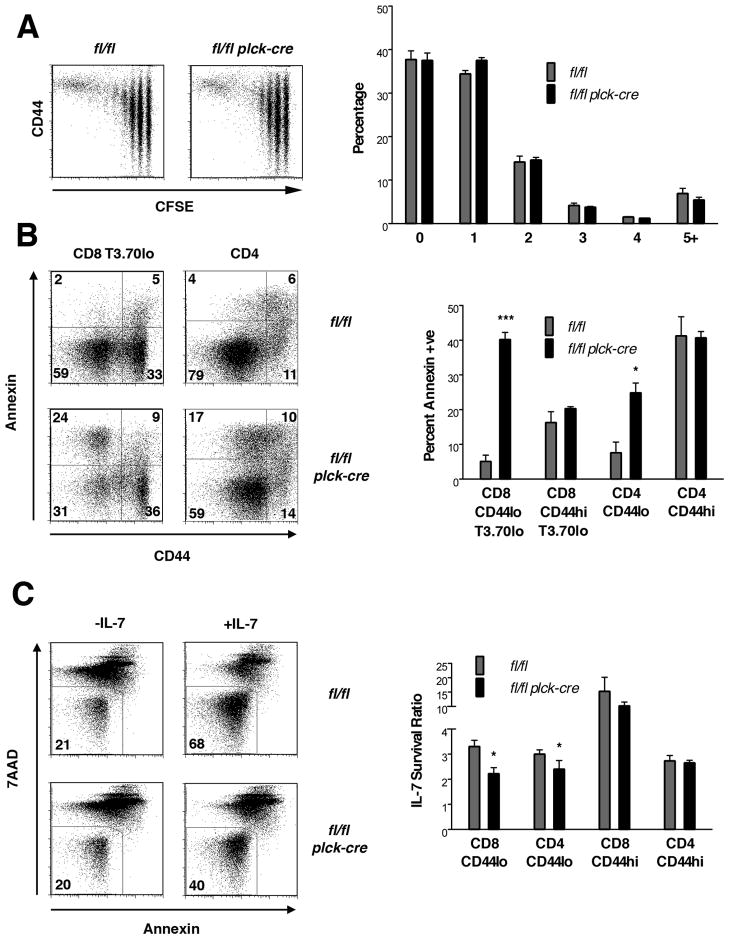
Based upon findings in HY TCR Tg mice, we considered the possibility that a primary defect in the ability of naïve T cells to undergo homeostatic expansion accounts for the reduced number of these cells in the periphery of RASA1-deficient mice (29, 30). To examine this directly, CFSE-labeled naïve CD8 T cells from non-TCR Tg RASA1-deficient or control wild type mice were injected into wild type recipients that had been rendered immunodeficient by sublethal irradiation. After 5 d, dilution of donor T cell CFSE fluorescence was assessed to determine the extent of homeostatic proliferation (Fig. 11A). As shown, RASA1-deficient naïve CD8 T cells were able to undergo homeostatic proliferation to the same extent as wild type naïve CD8 T cells in this model. Furthermore, RASA1-deficient T cells proliferated to the same extent as wild type T cells in an alternative model of homeostatic proliferation in which T cells were adoptively transferred to RAG-deficient immunodeficient recipients (data not show). Thus, a primary defect in homeostatic proliferation potential is unlikely to account for the paucity of naïve T cells in RASA1-deficient mice.
FIGURE 11. Homeostatic proliferation and survival of RASA1-deficient T cells.
(A) Purified CFSE-labeled CD8+CD44- peripheral T cells from littermate rasa1fl/fl and rasa1fl/fl plck-cre mice (CD45.2) were adoptively transferred to sublethally irradiated wild type recipients (CD45.1). Spleens were harvested from recipient mice after 5 d. Plots show representative CFSE fluorescence versus CD44 expression upon CD45.2 gated splenocytes. Bar graph shows mean percentage + 1 SEM of donor T cells at the indicated cell division number (n=3 mice each genotype). (B) At left are representative flow cytometry plots showing expression of CD44 versus annexin V staining upon the indicated T cell populations in spleens of littermate rasa1fl/fl and rasa1fl/fl plck-cre female HY TCR Tg mice. 7AAD+ cells were eliminated from analysis. Bar graph at right shows mean percent + 1 SEM of annexin+ cells among the indicated splenic T cell populations (n=3 mice each genotype). (C) Purified T cells from littermate rasa1fl/fl and rasa1fl/fl plck-cre mice were cultured in complete medium alone with or without IL-7 for 72 h. The percent viable cells (annexin− and 7AAD−) among different T cell populations was then determined by flow cytometry. Plots show representative annexin and 7AAD staining upon CD8+CD44- cells. For each subpopulation a ratio of the percent viable T cells in the presence of IL-7 versus the absence of IL-7 was calculated (IL-7 survival ratio). The bar graph at right shows the mean IL-7 survival ratio + 1 SEM of the indicated T cell populations (n=3 mice each genotype). Statistical significance was determined using the Student’s two-sample test.
An alternative explanation for the reduced numbers of naïve T cells in RASA1-deficient mice is impaired survival in the periphery (29, 30). To address this, we asked if naïve T cells in RASA1-deficient mice showed increased levels of annexin V staining upon isolation from mice. The most clear cut results were obtained in the HY TCR Tg model (Fig. 11B). As shown, CD44lo HY TCR negative CD8 T cells from RASA1-deficient HY TCR Tg mice showed much higher amounts of annexin V staining compared to the same cell population in wild type HY TCR Tg mice. In contrast, memory CD44hi HY TCR negative CD8 T cells from RASA1-deficient HY TCR Tg mice showed the same amount of annexin V staining as their counterparts in wild type mice. This pattern of annexin V staining was also observed amongst CD4 T cells in HY TCR Tg mice. Thus, CD4 CD44lo T cells showed increased annexin V staining in RASA1-deficient animals whereas memory CD4 T cells did not (Fig. 11B). These findings strongly support the conclusion that the diminished number of CD44lo T cells in RASA1-deficient mice is a consequence of decreased survival of these T cells in vivo.
Survival of naïve T cells in the periphery is known to be mediated by the cytokine IL-7 following TCR recognition of self MHC peptide complexes (29, 30). Since loss of RASA1 did not appear to impact upon TCR induced responses of naïve peripheral T cells, we asked instead if RASA1-deficient naïve T cells showed impaired responsiveness to IL-7. For this purpose, whole T cells from RASA1-deficient and control non-TCR Tg mice were cultured in the presence or absence of IL-7. After 3 d, cell viability was then assessed (Fig. 11C). As shown, IL-7 promoted the survival of naïve CD4 and CD8 T cells from both types of mice but was clearly less efficient at promoting survival of CD44lo RASA1-deficient T cells. In contrast, IL-7 promoted the survival of memory phenotype CD4 and CD8 T cells in control and RASA1-deficient mice to the same extent. We conclude, therefore, that impaired IL-7 mediated survival of naïve T cells is the principal reason for the reduced numbers of naïve T cells in RASA1-deficient mice.
Discussion
Knowledge of which RasGAPs regulate Ras activation in T cells is incomplete. To this end, in this study, we asked if RASA1 functioned as regulator of Ras in T cells. Initial analysis of thymocyte differentiation in RASA1-deficient non-TCR Tg mice indicated that RASA1 might not play a role in positive selection. However, when thymocyte differentiation was examined in RASA1-deficient AND TCR Tg mice, a negative regulatory role for RASA1 in the positive selection of CD4 SP T cells was revealed. Thus, despite that total numbers of DP and mature CD4 SP cells were reduced in RASA1-deficient mice in this model, ratios of the number of post-positive selection to pre-positive selection thymocytes were significantly increased. Furthermore, levels of CD69 expression were consistently increased upon pre-selection thymocytes in RASA1-deficient mice. Why a role for RASA1 in positive selection of CD4 T cells is apparent in AND TCR Tg mice but not for polyclonal CD4 T cells in non-TCR Tg mice is unclear at present. However, a relatively high affinity of the AND TCR for self peptide-MHC is likely a contributing factor. Only under such conditions might RASA1 contribute to regulation of the Ras-MAPK response during the course of positive selection.
Importantly, increased positive selection in the absence of RASA1 was associated with increased ERK signal transduction in pre- and post-positive selection thymocytes. MAPK are known critical regulators of thymocyte positive selection (7). Expression of MAPK is essential in order for positive selection to occur (4). Furthermore, Tg mice that express a constitutively-active mutant form of ERK2 known as sevenmaker in the T cell lineage show increased positive selection of CD4 SP cells, although not, interestingly, CD8 SP T cells (31). Therefore, increased MAPK activation is likely the driving force for augmented positive selection of CD4 SP T cells in the absence of RASA1.
With regards other major checkpoints in thymocyte differentiation, loss of RASA1 did not appear to influence DN to DP transition in any of the examined models, despite that RASA1 is well expressed in DN cells. DN to DP transition is also known to be critically dependent upon activation of the Ras-MAPK pathway (3, 4). Therefore, which RasGAP regulate Ras activation during DN to DP transition remains to be defined. In addition, no evidence was obtained that loss of RASA1 increases negative selection or that RASA1 is required for negative selection of thymocytes. Lack of a role for RASA1 in negative selection is consistent with previous evidence that Ras-MAPK signaling pathway is not required for this event (6, 31–33).
In peripheral T cells, no evidence was obtained that RASA1 functions as a negative regulator of Ras activation in response to stimulation with agonist peptide-MHC complexes. Consequently, upon agonist peptide-MHC stimulation, RASA1-deficient T cells produced normal levels of cytokines, showed only a modest decrease in cellular proliferation and showed appropriate levels of activation induced cell death. However, numbers of naïve CD4 and CD8 T cells were substantially reduced (by 50–90%) in the periphery of RASA1-deficient mice. Such a deficit could not be accounted for by impaired homeostatic proliferation but ostensibly by an increased susceptibility of naïve T cells to apoptotic cell death. Studies performed in vitro revealed that IL-7-mediated survival of naïve T cells is impaired in the absence of RASA1. Survival of naïve T cells in vivo is known to depend upon IL-7 (29, 30). Therefore, this finding is likely to account for the diminished number of naïve T cells in RASA1-deficient mice. Since in non-TCR Tg mice gross alterations in thymic development were not apparent, it is likely that the diminution of naive T cell number results from loss of RASA1 expression in that cell type. However, currently, we cannot formally exclude the possibility that the reduced number of naïve T cells in the periphery of RASA1-deficient mice is consequent to an altered T cell developmental program.
At the molecular level, how RASA1 promotes IL-7-mediated survival of naïve T cells is unknown. IL-7 receptor signal transduction is known to involve activation of JAK protein tyrosine kinases leading to recruitment, phosphorylation and activation of the STAT5 transcription factor (34). Activation of STAT5 proceeds normally in naïve RASA1-deficient T cells (data not shown). Furthermore, in naïve T cells from wild type mice, we have observed only minimal ERK activation in response to IL-7 stimulation and this is not increased in RASA1-deficient naive T cells. However, sevenmaker Tg mice also show a reduced number of peripheral naïve T cells, particularly naïve CD8 T cells (31). This finding argues strongly in favor of a role for increased MAPK activation as a cause of the diminished number of naïve T cells in RASA1-deficient mice. It is possible that in RASA1-deficient naïve T cells, persistent low level IL-7-induced ERK activation impairs survival. If so, one potential relevant downstream target of ERK might be the pro-apoptotic Bim protein, a member of the Bcl-2 family of apoptosis-regulating molecules (35). Bim is phosphorylated by MAPK resulting in altered apoptosis-inducing potential (36, 37). This is relevant since Bim promotes peripheral T cell death upon withdrawal of IL-7 (38).
In contrast to naïve T cells, numbers of memory T cells in RASA1-deficient mice were only modestly reduced, if at all. This finding was consistent an inability to detect increased amounts of apoptosis within memory populations as well as normal IL-7-induced survival of memory cells in vitro. Indeed, in those instances where small reductions in the number of memory population were detected, a reduced precursor pool of naïve cells most likely explains this finding. Why IL-7-induced survival of naïve T cells but not memory T cells should be affected by RASA1-deficiency is unclear. One possibility is that RasGAPs other than RASA1 are involved in the regulation of IL-7 signaling in memory cells. On the other hand, increased ERK activation does not appear to affect memory cell survival, as implied by findings in the sevenmaker mouse (31).
Similar to naïve T cells, survival of DP cells in the thymus was also found to be impaired in RASA1-deficient mice. In this instance, it is unlikely that the increased susceptibility to death of all DP subsets can be explained by altered IL-7 signal transduction since the IL-7 receptor is expressed upon only a minority of DP cells, i.e. DP cells undergoing positive selection (39). Therefore, in DP cells, RASA1 must control survival through means other than regulation of IL-7 signal transduction. Loss of RASA1 in thymocytes did not affect apoptosis induced by dexamethasone, staurosporine or serum deprivation. Therefore, the mechanism by which RASA1 controls the survival of DP thymocytes in vivo is uncertain. However, whatever this mechanism, it is again likely that it relates to dysregulated ERK activation. Thus, in the sevenmaker mutant mouse a similar specific reduction in total number of DP cells has also been observed (31).
In conclusion, with the use of T cell-specific RASA1-deficient mice, we demonstrate here a role for RASA1 as a negative regulator of Ras activation during positive selection of CD4 SP T cells in the thymus. In addition, we demonstrate a role for RASA1 as a positive regulator of naïve T cell survival in the periphery. These findings contribute significantly to our understanding of RasGAP-mediated control of T cell development and function.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grants AI050699 to PDK and AI073677 to CC.
Abbreviations: BM, bone marrow; DN, double negative; DP, double positive; GEF, guanine nucleotide exchange factor; LN, lymph node; NF1, neurofibromin 1; PCC, pigeon cytochrome c peptide; RasGAP, Ras GTPase-activating protein; SP, single positive; Tg, transgenic; TM, tamoxifen.
Disclosures
The authors have no conflicting financial interests.
References
- 1.Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 2.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 3.Crompton T, Gilmour KC, Owen MJ. The MAP kinase pathway controls differentiation from double-negative to double-positive thymocyte. Cell. 1996;86:243–251. doi: 10.1016/s0092-8674(00)80096-3. [DOI] [PubMed] [Google Scholar]
- 4.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature. 1995;373:620–623. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- 6.Swan KA, Alberola-Ila J, Gross JA, Appleby MW, Forbush KA, Thomas JF, Perlmutter RM. Involvement of p21ras distinguishes positive and negative selection in thymocytes. Embo J. 1995;14:276–285. doi: 10.1002/j.1460-2075.1995.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberola-Ila J, Hernandez-Hoyos G. The Ras/MAPK cascade and the control of positive selection. Immunol Rev. 2003;191:79–96. doi: 10.1034/j.1600-065x.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 8.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 9.Rincon M, Flavell RA, Davis RJ. Signal transduction by MAP kinases in T lymphocytes. Oncogene. 2001;20:2490–2497. doi: 10.1038/sj.onc.1204382. [DOI] [PubMed] [Google Scholar]
- 10.Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, Barry M, Bleakley RC, Ostergaard HL, Stone JC. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95:3199–3203. [PubMed] [Google Scholar]
- 11.Priatel JJ, Teh SJ, Dower NA, Stone JC, Teh HS. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity. 2002;17:617–627. doi: 10.1016/s1074-7613(02)00451-x. [DOI] [PubMed] [Google Scholar]
- 12.Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol Cell Biol. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta. 2003;1603:47–82. doi: 10.1016/s0304-419x(02)00082-3. [DOI] [PubMed] [Google Scholar]
- 14.Donovan S, Shannon KM, Bollag G. GTPase activating proteins: critical regulators of intracellular signaling. Biochim Biophys Acta. 2002;1602:23–45. doi: 10.1016/s0304-419x(01)00041-5. [DOI] [PubMed] [Google Scholar]
- 15.Ingram DA, Zhang L, McCarthy J, Wenning MJ, Fisher L, Yang FC, Clapp DW, Kapur R. Lymphoproliferative defects in mice lacking the expression of neurofibromin: functional and biochemical consequences of Nf1 deficiency in T-cell development and function. Blood. 2002;100:3656–3662. doi: 10.1182/blood-2002-03-0734. [DOI] [PubMed] [Google Scholar]
- 16.Downward J, Graves JD, Warne PH, Rayter S, Cantrell DA. Stimulation of p21ras upon T-cell activation. Nature. 1990;346:719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- 17.Henkemeyer M, Rossi DJ, Holmyard DP, Puri MC, Mbamalu G, Harpal K, Shih TS, Jacks T, Pawson T. Vascular system defects and neuronal apoptosis in mice lacking ras GTPase-activating protein. Nature. 1995;377:695–701. doi: 10.1038/377695a0. [DOI] [PubMed] [Google Scholar]
- 18.Lapinski PE, Bauler TJ, Brown EJ, Hughes ED, Saunders TL, King PD. Generation of mice with a conditional allele of the p120 Ras GTPase-activating protein. Genesis. 2007;45:762–767. doi: 10.1002/dvg.20354. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Hoyos G, Anderson MK, Wang C, Rothenberg EV, Alberola-Ila J. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity. 2003;19:83–94. doi: 10.1016/s1074-7613(03)00176-6. [DOI] [PubMed] [Google Scholar]
- 20.Lauritsen JP, Kurella S, Lee SY, Lefebvre JM, Rhodes M, Alberola-Ila J, Wiest DL. Egr2 is required for Bcl-2 induction during positive selection. J Immunol. 2008;181:7778–7785. doi: 10.4049/jimmunol.181.11.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 2005;3:e356. doi: 10.1371/journal.pbio.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hennet T, Hagen FK, Tabak LA, Marth JD. T-cell-specific deletion of a polypeptide N-acetylgalactosaminyl-transferase gene by site-directed recombination. Proc Natl Acad Sci U S A. 1995;92:12070–12074. doi: 10.1073/pnas.92.26.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 24.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol. 2002;2:309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 25.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 26.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 27.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 28.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 29.Boyman O, Letourneau S, Krieg C, Sprent J. Homeostatic proliferation and survival of naive and memory T cells. Eur J Immunol. 2009;39:2088–2094. doi: 10.1002/eji.200939444. [DOI] [PubMed] [Google Scholar]
- 30.Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17:183–191. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Sharp LL, Schwarz DA, Bott CM, Marshall CJ, Hedrick SM. The influence of the MAPK pathway on T cell lineage commitment. Immunity. 1997;7:609–618. doi: 10.1016/s1074-7613(00)80382-9. [DOI] [PubMed] [Google Scholar]
- 32.Alberola-Ila J, Hogquist KA, Swan KA, Bevan MJ, Perlmutter RM. Positive and negative selection invoke distinct signaling pathways. J Exp Med. 1996;184:9–18. doi: 10.1084/jem.184.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bommhardt U, Scheuring Y, Bickel C, Zamoyska R, Hunig T. MEK activity regulates negative selection of immature CD4+CD8+ thymocytes. J Immunol. 2000;164:2326–2337. doi: 10.4049/jimmunol.164.5.2326. [DOI] [PubMed] [Google Scholar]
- 34.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor L, Strasser A, O’Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai B, Chang SH, Becker EB, Bonni A, Xia Z. p38 MAP kinase mediates apoptosis through phosphorylation of BimEL at Ser-65. J Biol Chem. 2006;281:25215–25222. doi: 10.1074/jbc.M512627200. [DOI] [PubMed] [Google Scholar]
- 37.Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, Degenhardt K, White E, Cook SJ. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. EMBO J. 2007;26:2856–2867. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li WQ, Guszczynski T, Hixon JA, Durum SK. Interleukin-7 regulates Bim proapoptotic activity in peripheral T-cell survival. Mol Cell Biol. 2010;30:590–600. doi: 10.1128/MCB.01006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–3904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.