Abstract
The dynamic interactions between the amygdala and the medial prefrontal cortex (mPFC) are usefully conceptualized as a circuit that both allows us to react automatically to biologically relevant predictive stimuli as well as regulate these reactions when the situation calls for it. In this review, we will begin by discussing the role of this amygdala-mPFC circuitry in the conditioning and extinction of aversive learning in animals. We will then relate these data to emotional regulation paradigms in humans. Finally, we will consider how these processes are compromised in normal and pathological anxiety. We conclude that the capacity for efficient crosstalk between the amygdala and the mPFC, which is represented as the strength of the amygdala-mPFC circuitry, is crucial to beneficial outcomes in terms of reported anxiety.
Keywords: amygdala, medial prefrontal cortex, connectivity, anxiety, anxiety disorders, emotion regulation
1. Introduction
Accurate evaluation of and response to potentially life threatening or sustaining events are hallmarks of biologically relevant learning in animals and humans. In response to cues of threat, rodents exhibit a distinctive “freezing” or somatomotor arrest behavior. This behavior is critical in a natural environment in which movement may attract a predator to its location. Humans show a similar freezing response to a potentially threatening situation [1]. Rather than having to avoid predators, humans might more ordinarily show such a response when having to speak in front of a large audience. In such threatening instances, performance would be facilitated if able to override the initial freezing behavior.
In psychological terms, instinctive reactions to threat and subsequent regulatory responses are often referred to as bottom-up and top-down processes, respectively. The interplay between these two processes is exemplified by the following example: upon encountering a snake at a zoo, an initial reaction is driven by its appearance (i.e., bottom-up saliency), but the response is then implicitly controlled by the determination that the snake presents no immediate danger because it is behind a sheet of Plexiglas (i.e., top-down control). Of course, the context is critical since the same snake encountered in a field would evoke an initial freezing response followed by a very different type of top-down control in the form of running (or screaming in some cases). Thus, interactions between bottom-up and top-down processes will determine the adaptiveness of behavior is in a given situation.
This conceptualization may be directly applicable to clinical research, as the interaction between these bottom-up and top-down processes is hypothesized to be impaired in psychiatric illnesses – and here we will focus on the anxiety disorders. For example, in specific phobias, perhaps a failure to employ top-down control mechanisms allows initial bottom-up responses to intrude on normal cognitive functioning. Alternatively, it may be the case that the initial bottom-up reactions are so potent and exaggerated that even a normal functioning top-down regulatory system cannot keep these responses in check. Individual differences in the function and structure of this circuitry can also explain differences in normal levels of anxiety.
Numerous studies have highlighted the critical role of the amygdala and the mPFC in behavioral phenomena that involve competition between bottom-up and top-down processes, including fear conditioning and extinction [2–4]. Critically, it is believed that the mPFC regulates and controls amygdala output and the accompanying behavioral phenomena [2–4]. The reciprocal relationship between the amygdala and the mPFC strongly suggests the need to investigate these brain regions as one circuit, rather than studying them separately. That is, while numerous studies have assessed the separate contributions the amygdala and mPFC make to reactivity and regulation, respectively [5–8], more recent studies suggest that the structural and functional connectivity between these two regions is a better predictor of these outcomes than the activity of either region alone [9–11]. The idea here is that the stronger the coupling between the amygdala and the mPFC, the better the behavioral outcome in terms of reported anxiety.
2. The structural and functional connectivity of the human amygdala and prefrontal cortex
2.1. Structural neuroanatomy of amygdala-mPFC circuitry
The amygdala is an almond-shaped brain structure that resides in the medial temporal lobe of the brain [12, 13]. Its structure is comprised of many subnuclei, including the basolateral nucleus (BLA) and the central nucleus (Ce), which have distinct anatomical connections with other brain regions that serve different functions. Comprehensive descriptions of the anatomical connections of the amygdala exist elsewhere [14, 15]. Here, we focus on the connectivity between the amygdala and the prefrontal cortex, especially the mPFC. The mPFC can be roughly divided into two subregions, relative to the genu of the corpus callosum – dorsal mPFC (dmPFC) and the ventral mPFC (vmPFC). Broadly defined, the dmPFC includes the supragenual anterior cingulate and the medial frontal gyrus, whereas the vmPFC includes the subgenual anterior cingulate, ventromedial prefrontal and medial orbitofrontal cortex (Figure 1).
Figure 1.
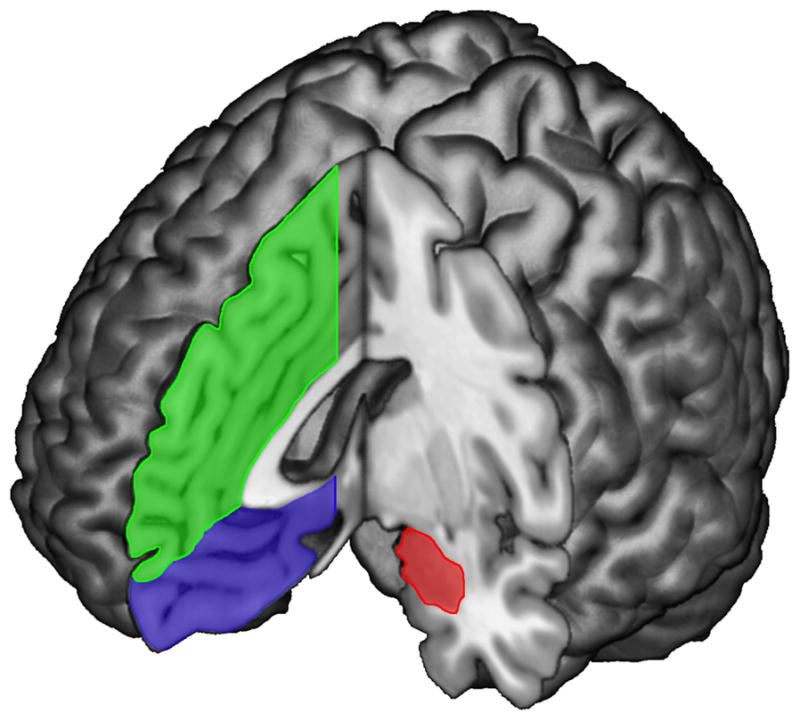
Structural magnetic resonance image of the human brain highlighting the major components of the amygdala-prefrontal circuitry: amygdala (red), ventromedial prefrontal cortex (blue), and dorsomedial prefrontal cortex (green).
Most of the known facts about the anatomical connections of the amygdala-mPFC circuitry are derived from animal studies, especially non-human primates. This is because the invasive nature of the methods that are used to investigate brain connections – such as lesion and tracing studies – are difficult to employ in humans. Data from non-human primate brains show that the majority of the afferent fibers to the amygdala originate in the orbitofrontal cortex and the mPFC, and these projections are denser and heavier from the caudal compared to the rostral aspects of these prefrontal areas [16–21]. In turn, the amygdala sends efferent projections to these orbitofrontal and mPFC regions [17–19, 22, 23], and interestingly these projections are heavier than the reciprocal cortical afferents [19, 24]. Most of the amygdala-prefrontal connections are concentrated in the BLA, as opposed to Ce. Based on animal studies of fear conditioning and extinction, mPFC input to the BLA as well as the intercalated cells (that is adjacent to the BLA) is responsible for inhibiting amygdala output by regulating BLA inputs to the Ce (see section 3. for details).
In humans, the structural connections of the amygdala have been investigated more recently using non-invasive neuroimaging methods such as diffusion tensor imaging (DTI). This imaging method takes advantage of the fact that the movement of water molecules in the brain differs in different types of brain tissue. To elaborate, in an unrestricted tissue environment, such as in the ventricles of the brain, water molecules show isotropic diffusion in all directions equally. Importantly, however, the movement of water molecules is greatly restricted in myelinated axons – that is, water molecules in white matter tend to move in a single direction along the myelinated axons. Thus DTI is a method optimized to assess white matter fiber tracts in the brain. There are two different types of information about which DTI can inform us – 1) the orientation of white matter fiber tracts, and 2) the strength or integrity of white matter fiber tracts. The former can be accomplished by using fiber tracking or tractography to measure the direction of water diffusion [25]. The latter can be calculated by measuring the degree of anisotropic diffusion [26]. Specifically, normalized measures such as fractional anisotropy can be computed for each brain voxel and used to index the structural integrity of the measured white matter fiber tracts [26]. A number of studies have utilized these methods to identify an amygdala-prefrontal pathway in the human with a specific focus on connectivity with the dorsal and ventral aspects of the mPFC [27–29].
2.2. Functional neuroanatomy of the amygdala-mPFC circuitry
The functional counterpart of structural connectivity – functional brain connectivity – can also be used to investigate amygdala-prefrontal interactions. This method is optimal for understanding the relationship between spatially remote brain regions by assessing brain activity across time. Analyses of functional brain connectivity can be defined in two ways: 1) functional connectivity and 2) effective connectivity [30]. Functional connectivity is simply a measure of the temporal correlation of brain activity in two or more regions, whereas effective connectivity seeks to reveal the directional effect of one neuronal system exerted over another [30]. By definition, functional connectivity is purely correlational in nature, and provides no information regarding the directionality of how one brain region affects another. In contrast, effective connectivity attempts to explain the causal relationship between the interactions of different brain regions, relying on more advanced statistical modeling methods such as structural equation modeling [31], psychophysiological interactions [32], and dynamic causal modeling [33]. Based on the extensive anatomical connections between the amygdala and mPFC shown in human and non-human primates, a number of investigations have used these functional and effective connectivity measures to assess the strength of amygdala-mPFC coupling and its relationship with behavioral outcomes [9, 34, 35].
2.3. Amygdala-mPFC circuitry at rest
The majority of the aforementioned functional connectivity studies were task-based, meaning that brain activity was measured in response to particular stimulus presentations and/or task instructions.. For example, amygdala activity measured as subjects viewed surprised facial expressions was used to identify mPFC activity that predicted subjects’ interpretations of these faces as either positively or negatively valenced [36]. Higher mPFC activity predicted lesser amygdala activity and more positive ratings of these expressions. Recently, a growing body of functional neuroimaging studies has emerged investigating brain activity and connectivity at rest, in the absence of presented stimuli or task instructions. The “resting state” can be investigated using fMRI by measuring spontaneous, slow (<0.1 Hz) fluctuations in the brain that occur over time [37]. Identifying the brain’s functional networks at rest can help investigators better understand how brain regions are coupled prior to a task. In fact, investigators have mapped highly detailed resting state functional networks associated with specific brain regions such as the anterior cingulate cortex [38], the striatum [39], and the amygdala [40]. Resting state functional connectivity analyses have also been used to identify distinct neural networks based on specific neurophysiological phenomena, such as repetition priming [41]. Additionally, recent studies have linked the strength of resting state functional connectivity with individual differences in behavioral outcomes, such as behavioral performance on cognitive tasks [42, 43], autistic traits [44] and reported anxiety [11]. Using this method, researchers can test whether the degree to which the amygdala is coupled with mPFC regions at rest influences how well a person regulates their emotional responses when challenged with stimulus presentations during a particular task.
In summary, there are a number of non-invasive methods to investigate the strength of amygdala-mPFC connectivity in vivo. Structural connectivity can be assessed by utilizing DTI, and functional brain connectivity can be evaluated through analyses of functional and effective connectivity. Furthermore, understanding how brain regions are connected with one another at rest may provide new insights that elucidate how the amygdala-mPFC circuitry is engaged during a task.
3. Amygdala-prefrontal circuitry and fear conditioning and extinction
Studies of the non-human animal amygdala have shown that sensory information received by the BLA is then passed to the Ce [45]. Though outputs exist at the level of the BLA, a majority of outputs originate from the Ce [46]. The Ce projects directly to the hypothalamus and brain stem nuclei that drive autonomic and somatomotor responding [47]. The Ce also projects to all major neuromodulatory systems including dopaminergic, cholinergic, serotonergic and noradrenergic systems [46]. Thus, while direct projections can primarily affect physiological and motor responses, these neuromodulatory projections can serve to globally, non-specifically and instantaneously effect neuronal excitability across the brain. Such changes could serve to induce a state of heightened vigilance rendering the organism a more efficient consumer of information in biologically relevant learning situations [46]. One such situation involves the acquisition and expression of learned responses through classical conditioning [48, 49]. For example, in a typical aversive conditioning paradigm, subjects learn that a previously neutral stimulus (e.g., tone) predicts the occurrence of an unconditioned stimulus (US; e.g., electric shock), thereby acquiring the value of a conditioned stimulus (CS) which now elicits a conditioned response (CR; e.g., freezing) to the CS that was previously reserved for the US [50, 51]. The generation of these CS-US associations and their behavioral expressions are known to be amygdala-dependent since manipulations of this structure block or retard such learning [52, 53].
A reversal of this classical conditioning procedure is known as extinction – suppressing previously learned CS-US associations [54, 55]. The inhibition of CS-US associations can be achieved by top-down regulatory input from the mPFC to the BLA [56]. This process is supported by the existence of amygdala-mPFC connectivity that allows direct, reciprocal communication [14, 19, 56]. For example, electric stimulation of the mPFC resulted in the inhibition conditioned responses, emulating the effects of extinction in the rat [56]. In humans, greater cortical thickness of the vmPFC was associated with better behavioral performance during extinction recall [57, 58]. Similar findings have also been demonstrated in humans using fMRI, highlighted by increased vmPFC activity during successful extinction of learned US-CS associations [59–61]. Interestingly, a study using functional connectivity methods [59] showed that the amygdala and the vmPFC were functionally coupled during the entire course of the experiment, which included a combination of fear extinction and emotion regulation tasks. Thus, these structural and functional findings highlight the importance of amygdala-mPFC interactions for the regulation and inhibition necessary for extinction learning and/or memory.
4. Amygdala-prefrontal circuitry and emotion regulation
The ability to regulate our emotions is essential in our everyday lives, and successful emotion regulation begets beneficial outcomes in many social situations. Emotion regulation is a classic example of how top-down and bottom-up processes compete and interact to produce optimal (or counterproductive) behavioral outcomes. For example, one’s instinctive reaction to a frightening scene in a horror movie may include an urge to scream and/or run out of the room. Normally, this bottom-up reaction is controlled by a top-down intervention (e.g., reminding oneself that this is only a movie). Taking the scenario described above into account, it wouldn’t be too difficult to imagine that individuals may employ different strategies to achieve such emotion regulation.
To date, studies investigating the neural basis of emotion regulation have primarily examined two distinctive means of emotion regulation – by simply suppressing what one is feeling (i.e., suppression), or by cognitively reevaluating the stimulus that is evoking the emotion (i.e., reappraisal) [3]. Not surprisingly, emotion regulation and the extinction of fear conditioning are suggested to have overlapping underlying neural mechanisms, since the essence of both processes involves reevaluating biologically relevant stimuli [4, 62]. Like extinction, it is useful to assume that during emotional regulation the prefrontal cortex exerts control over the amygdala in response to an emotional challenge [3, 63]. Based on this framework, numerous functional neuroimaging studies have demonstrated increased prefrontal activity and concomitant decreased amygdala activity during successful emotion regulation [59, 64–71]. Unlike extinction, many emotion regulation studies point to the ventral and dorsal lateral prefrontal cortex (vlPFC and dlPFC, respectively), in addition to the ventral medial prefrontal cortex, as critical for regulating amygdala activity [59, 66, 67, 69, 71]. In general, studies have shown largely overlapping prefrontal-amygdala activity to suppression and reappraisal strategies, which was characterized by decreased activity of the amygdala and increased activity of the prefrontal cortex – usually including both medial and lateral PFC [3, 67]. Furthermore, the frequency of using reappraisal to regulate emotion in everyday life has been shown to be related to decreased amygdala activity, increased prefrontal and parietal activity [72], and greater vmPFC volume [73]. In addition, during an affect labeling task (i.e., putting emotions into words), which can be regarded as a specific form of emotion regulation [66, 74], diminished amygdala activity was again associated with greater activity of the mPFC [64, 65], vlPFC [66] and also with increased cortical thickness of the vmPFC [74]. These findings provide functional and structural evidence for shared neural mechanisms during different types of emotion regulation strategies.
These neuroimaging findings lead us to an interesting question – does emotion regulation share similar underlying neural mechanisms with more classic forms of cognitive control? Or are there unique brain circuitries recruited by these distinct emotion regulation processes? According to a cognitive control model of emotion regulation [3], the neural representation of emotion regulation can be summarized as interactions between prefrontal (both dlPFC and mPFC) and ACC systems and their influence on subcortical systems, including the amygdala. The two major types of emotion regulation – suppression and reappraisal – have yielded similar results in terms of brain activations, and how the prefrontal and anterior cingulate cortices interact is strikingly similar to other top-down control mechanisms that do not involve emotional processing, such as cognitive control [75–77]. However, another study has shown that emotion regulation through mood-incongruent autobiographical recall recruits the ventral mPFC and vlPFC, but not dlPFC, implying that activations of neural circuitry depend on the type of emotion regulation being used [78]. Taken together, we can tentatively conclude that while emotion regulation does to some extent share similar neural circuitry with cognitive control, it also recruits unique brain regions, such as the vlPFC. Other forms of top-down control processes, such as regulation of appetitive behaviors, attitudes or prejudice, have also shown to use an overlapping amygdala-prefrontal circuitry [79].
Recent findings raise the possibility that a more efficient crosstalk between the amygdala and the prefrontal cortex begets a better ability to regulate one’s emotions. Supporting this idea, the strength of amygdala-mPFC coupling was quantified by computing the functional connectivity between these two areas and comparing this connectivity with how effectively participants regulated their emotions [34]. It was found that the functional coupling between the amygdala-mPFC was strengthened during reappraisal, and that the degree of this functional coupling was positively correlated with the self-reported effectiveness of emotion regulation [34]. A selective increase in the functional coupling of the amygdala with the vmPFC and dlPFC during emotion regulation has also been reported [69], highlighting the importance of efficient communication between the amygdala and the prefrontal cortex in successful top-down control of emotion. Further, functional coupling of the amygdala and vmPFC at rest, predicts beneficial outcomes in terms of reported anxiety [11]. Future studies linking the effective success of emotion regulation strategies and the structural and functional connectivity of the amygdala-mPFC circuitry might provide a better understanding of the neural correlates of these emotion regulatory processes.
5. Amygdala-prefrontal circuitry and the interpretation of emotionally ambiguous facial expressions
In humans, patients with selective amygdala lesions have displayed deficits in processing the facial expressions of fear [80], leading to numerous functional neuroimaging studies using presentations of fearful faces to probe amygdala activity [10, 81–86]. These studies have shown that the amygdala is particularly responsive to fearful faces compared to other expressions [87], including angry, happy, and neutral [10, 81, 82, 84–86], except for a few reports [88]. The affinity of the human amygdala for fearful faces compared to these other expressions, provides insights into amygdala function. For example, since the amygdala is more responsive to fearful faces compared to angry faces, which embody a direct threat, it has been suggested that one function of the amygdala is to augment cortical function through the major neuromodulatory centers to assist in the resolution of predictive uncertainty [86, 89]. That is, the inherent ambiguity of fearful faces in that they predict the increased probability of threat without providing information about its nature or location – leads to selective activation of the amygdala [86, 89].
Given that the amygdala plays a major role in the resolution of predictive uncertainty associated with fearful faces, surprised facial expressions provide a particularly important comparison expression. Indeed, there is evidence that surprise may be the second-most compromised expression in patients with selective amygdala damage, following fear [90]. Fearful and surprised faces have common facial features (e.g., eye-widening), and both expressions indicate the detection of a significant, but unknown, eliciting event [36]. Surprised faces are particularly interesting because, unlike fear, they do not predict the valence of the unknown eliciting event. Indeed, research has shown that surprised faces can be interpreted as either positive or negative in nature [35, 36, 91]. Previous research has shown that when individuals make valence judgments of surprised faces, ratings reflect individual differences in one’s positivity/negativity bias [35, 91] and these differences are mirrored by a distinct pattern of brain activity, which critically involves the vmPFC as well as the amygdala [35]. Specifically, decreased amygdala activity accompanied by increased vmPFC activity was observed in people who interpreted surprised faces as positive, with the reverse brain pattern seen in those who interpreted surprised faces as negative [35]. The role of the vmPFC in resolving the emotional ambiguity (i.e., the valence of a given surprised face) could be understood as a top-down regulatory input to the amygdala, much akin to the neural mechanism of fear extinction or emotion regulation [4]. Indeed, greater vmPFC activity predicts both a) more positive ratings of surprise and b) more positive interpretations of an extinguished tone (i.e., tone now predicts no shock) [92]. In a subsequent study [36], positive and negative sentences (e.g., “He just lost $500” or “He just found $500”) were used to provide contextual information for the presented surprised faces, in order to see how brain activity was influenced by information that provided clear resolution to the source ambiguity problem associated with surprised faces. Again, data from this study showed that greater amygdala response to negative versus positive faces was accompanied by diminished vmPFC activity, and interestingly greater vlPFC activity [36]. Thus, similar medial prefrontal-amygdala regions were activated when a context was provided (i.e., valence of the surprised faces were determined by the experimental condition), compared to when the subjects had to judge the valence of the surprised faces themselves – but additional lateral prefrontal regions were recruited in the contextually mediated condition [35].
In summary, data from these experiments collectively suggest that using emotionally ambiguous stimuli such as surprised faces instigates a competition between top-down and bottom-up processes. This engages the amygdala-mPFC circuitry and the balance of activity within this circuit reflects the resolution of the inherent ambiguity of the perceived surprised faces.
6. Amygdala-prefrontal circuitry and anxiety within the normal range
Anxiety is characterized by chronic, nonspecific apprehension and arousal related to the potential occurrence of future threat [93, 94]. Neurobiological theories of anxiety have highlighted the central role of the amygdala in the generation and experience of the fear that can give rise to anxiety [48, 49], and fear extinction investigations in animals support such theories [48, 49]. Similar to the inhibition of previously conditioned fear responses during fear extinction, reduced anxiety is associated with the top-down regulation of amygdala activity by the mPFC [9, 65, 95]. Findings from anatomical investigations of amygdala connectivity [14, 19] and fear extinction studies in animals [56] emphasize the top-down and bottom-up interactions between the amygdala and mPFC regions in anxiety. To put it another way, efficient crosstalk between the amygdala and the mPFC produces a better outcome in terms of controlling anxiety.
Consistent with this framework, a number of functional neuroimaging studies in humans have shown elevated amygdala activity in highly anxious but otherwise healthy individuals [96–100]. For example, increased amygdala activity to unattended fearful faces was associated with higher levels of self-reported anxiety [96], although this effect may be more prominent in women than men [97]. Using backward masking, an experimental paradigm that has been shown to reliably evoke human amygdala activity and mitigate subjective awareness of fearful face stimuli (e.g., [83, 85]), it has been reported that increased amygdala activity was linked to elevated anxiety levels [98]. It is worth noting that in these studies, the relationship between increased amygdala activity and anxiety was evident when the subjects were not attending to or were unaware of the stimuli, not when they were attending to or aware of them. This raises the possibility that attention or awareness may be an important factor that interacts with amygdala activation and subsequent reported anxiety. Anxiety was not only associated with elevated amygdala activity to threat-related stimuli (e.g., fearful faces, emotionally negative pictures), but was also associated with increased activity to non threat-related stimuli (neutral faces; [99]), suggesting that amygdala activity may reflect greater anxiety levels even in the absence of clear threat.
Other evidence from the human neuroimaging literature shows that altered mPFC activity is associated with anxiety [5–8, 95]. Although changes in mPFC activity has been consistently reported in anxiety research, the spatial location of that activity (i.e., whether it is dorsal or ventral) varies across studies. Depending on the experimental task, different studies have reported divergent results (for review, see [2]) – for example, anxiety reduced activity of the vmPFC in one study [7], and dmPFC in another [5]. More recently, a number of studies have shown that higher levels of anxiety are associated with both decreased vmPFC activity and increased dmPFC activity [6, 8], suggesting differential roles for these mPFC subregions in anxiety.
Based on the findings highlighting the importance of both the amygdala and mPFC regions in anxiety, a number of studies have investigated the amygdala-mPFC circuitry in conjunction with anxiety using functional and structural connectivity measures [9–11]. For example, individuals with anxious temperaments had weaker functional coupling between the amygdala and the vmPFC during a task that involved matching fearful and angry faces [9]. Using DTI, it was demonstrated that the structural integrity of an amygdala-vmPFC pathway was compromised in the participants who exhibited high trait anxiety [10]. Furthermore, studies employing resting state functional connectivity methods have shown that the strength of the coupling between the amygdala and mPFC at rest predicted self-reported levels of anxiety ([11, 101]), where a positive correlation between the amygdala and vmPFC predicted beneficial outcomes in terms of reported anxiety [11]). In this study the dmPFC showed an opposite relationship to that observed between the amygdala and vmPFC – dmPFC activity at rest that was negatively correlated with amygdala activity predicted lower levels of anxiety. This latter finding is complemented by a recent task-based fMRI study in which amygdala-dmPFC functional connectivity strength was positively correlated with neuroticism (an anxiety-related personality trait characterized by a bias to interpret normal situations as harmful and threatening; [102]) during viewing emotionally negative faces [103]. Taken together, these data suggest that the strength of amygdala-mPFC functional connectivity during rest may represent efficient crosstalk between the two brain regions, which may be responsible for abolishing the generation of anxious states [11]. This idea is consistent with findings from task-based functional connectivity [9]. Findings from these studies all fit well with the idea that efficient crosstalk between the amygdala and the mPFC, perhaps particularly the vmPFC, is critically involved in lowering anxiety levels.
7. Amygdala-prefrontal circuitry and pathological anxiety
Taking individual differences in normal fluctuations in anxiety as our starting point, disrupted bottom-up and top-down emotional and cognitive processes are thought to be a crucial component of symptomology in pathological anxiety. This model suggests an imbalance between the amygdala and the prefrontal cortex, which is typically characterized by hyperactivity of the amygdala and hypoactivity of the prefrontal cortex [104, 105].
7.1. Social Anxiety Disorder
A prevalent subtype of the anxiety disorders [106], social anxiety disorder (SAD) is characterized by intense anxiety during social situations in which the person is exposed to unfamiliar people [107]. To this end, emotional facial expressions provide a particularly useful paradigm for studying SAD, which is thought to involve exaggerated emotional reactivity to social stimuli and the inability to regulate these responses [108, 109]. Individuals with SAD reliably showed elevated amygdala reactivity when viewing “harsh” faces (facial expressions displaying anger, contempt, or a combination of both) [110–112], and even neutral faces [113], compared to healthy individuals in fMRI studies. Amygdala reactivity was positively correlated with symptom severity and/or trait anxiety in SAD patients, further demonstrating the neurobiological significance of the amygdala in SAD [111, 113, 114]. These individuals also had exaggerated amygdala reactivity to pictures of emotionally negative scenes (i.e., unpleasant and/or aversive) suggesting abnormal neural activity during general emotional, not just social, processing in SAD [114]. Direct examination of neural activity during emotion regulation demonstrated that SAD patients fail to recruit the mPFC [110], implying that the connectivity of the amygdala-mPFC circuitry is disrupted in SAD. A resting state fMRI study showed that SAD patients had markedly reduced functional connectivity between the left amygdala and the medial orbitofrontal cortex [115], corroborating the previous findings assessing a normal range of anxiety [11]. In addition, state anxiety levels in SAD subjects was inversely correlated with the functional connectivity strength between the amygdala and the medial orbitofrontal cortex, further validating the central role of the amygdala-mPFC circuitry in SAD [115]. In addition to these functional abnormalities, SAD patients exhibited compromised structural integrity of the uncinate fasciculus [116], a major white matter fiber tract that is known to connect the amygdala and the orbitofrontal cortex [117]. Each of these studies provides examples of SAD patients’ failure to recruit the proper cognitive regulatory circuits in the brain, and that the functional abnormalities in these circuits may be attributable, in part, to white matter microstructural problems caused by the pathophysiology of SAD.
7.2. Posttraumatic Stress Disorder
Posttraumatic stress disorder (PTSD) is a stress-induced anxiety disorder characterized by re-experiencing the traumatic event, avoidance of stimuli associated with the trauma, and more generalized symptoms of hyperarousal [107]. PTSD patients show a diminished ability to extinguish this conditioned fear, which may be evidence for prefrontal cortex dysfunction and reduced amygdala inhibition [118, 119]. Shin and colleagues [120] have demonstrated that PTSD is marked by heightened amygdala activation and reduced anterior cingulate and prefrontal cortex activity when viewing fearful faces. Consistent with this finding, PTSD patients exhibited diminished activity in the mPFC to unattended fearful faces [121]. Both studies reported that PTSD symptom severity was associated with decreased mPFC activity, demonstrating the neurobiological importance of this brain region in the pathophysiology of PTSD [120, 121]. Likewise, compared to healthy individuals, PTSD patients failed to recruit vmPFC activity when viewing pictures that were threatening, but unrelated to trauma [122]. In patients with PTSD, the default mode network – brain regions that include the mPFC and the posterior cingulate cortex that are believed to be more “active” during rest [123] – has been affected by the pathophysiology of the disorder as well. Specifically, resting state functional connectivity of the posterior cingulate cortex with the perigenual anterior cingulate and the right amygdala is associated with current PTSD symptoms, and that correlation with the right amygdala predicts future PTSD symptoms [124]. Furthermore, supporting these functional studies, there is DTI evidence that the white matter structural integrity of the cingulum bundle is compromised in PTSD patients compared to healthy individuals [125, 126]. Therefore, it is clear that not only the functionality of the amgydala and the mPFC are impaired in PTSD, but also their connectivity is disrupted as well.
Future research exploring the similarities and differences between non-anxious, normal anxious, and pathologically anxious individuals is needed. Based on numerous findings highlighting the relationship between the amygdala-mPFC circuitry and anxiety, developing treatments – whether they involve medication or psychotherapy – for anxiety disorders that target these brain regions will prove to be useful.
8. Conclusions
From normal emotion to pathological anxiety, an organism’s reaction to biologically relevant stimuli and the regulation of these responses can be usefully conceived as a constant struggle between bottom-up and top-down brain processes. A wealth of animal and human neuroimaging studies has shown that the amygdala and the prefrontal cortex, particularly the medial regions, are central to these processes. Investigating the connectivity between the amygdala and the prefrontal cortex has provided a deeper understanding of the role of the amygdala-mPFC circuitry in anxiety. Efficient crosstalk between the amygdala and the prefrontal cortex – represented as stronger structural and functional connectivity – predicts beneficial behavioral outcomes in terms of emotion regulation and anxiety.
Research Highlights.
Amygdala is extensively interconnected with the medial prefrontal cortex
Amygdala-prefrontal circuitry is critical in top-down and bottom-up processes
Stronger amygdala-prefrontal connectivity predicts lower levels of anxiety
Stronger amygdala-prefrontal connectivity predicts effective emotion regulation
Acknowledgments
Supported by the National Institute of Mental Health grants to MJK (F31 MH090672) and PJW (R01 MH080716).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roelofs K, Hagenaars MA, Stins J. Facing freeze: social threat induces bodily freeze in humans. Psychol Sci. 2010;21:1575–81. doi: 10.1177/0956797610384746. [DOI] [PubMed] [Google Scholar]
- 2.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–16. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 6.Simmons A, Matthews SC, Feinstein JS, Hitchcock C, Paulus MP, Stein MB. Anxiety vulnerability is associated with altered anterior cingulate response to an affective appraisal task. Neuroreport. 2008;19:1033–7. doi: 10.1097/WNR.0b013e328305b722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc Natl Acad Sci U S A. 2001;98:688–93. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage. 2009;44:975–81. doi: 10.1016/j.neuroimage.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 10.Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29:11614–8. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. doi: 10.1093/cercor/bhq237. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggleton JP. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. 1992. [Google Scholar]
- 13.Whalen PJ, Phelps EA. The human amygdala. New York, NY: The Guilford Press; 2009. [Google Scholar]
- 14.Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York, NY: Wiley-Liss, Inc; 1992. [Google Scholar]
- 15.Freese JL, Amaral DG. Neuroanatomy of the primate amygdala. In: Whalen PJ, Phelps EA, editors. The human amygdala. New York, NY: The Guilford Press; 2009. [Google Scholar]
- 16.Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–68. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- 17.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. The Journal of Comparative Neurology. 1995;363:615–41. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 18.Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–79. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 19.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leichnetz GR, Astruc J. The efferent projections of the medial prefrontal cortex in the squirrel monkey (Saimiri sciureus) Brain Res. 1976;109:455–72. doi: 10.1016/0006-8993(76)90027-5. [DOI] [PubMed] [Google Scholar]
- 21.Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol. 2002;451:301–23. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- 22.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–96. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 23.Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;300:549–71. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- 24.LeDoux JE. The Emotional Brain. New York: Simon and Shuster; 1996. [Google Scholar]
- 25.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–9. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 27.Bracht T, Tuscher O, Schnell S, Kreher B, Rusch N, Glauche V, et al. Extraction of prefronto-amygdalar pathways by combining probability maps. Psychiatry Res. 2009;174:217–22. doi: 10.1016/j.pscychresns.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, et al. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25:8854–66. doi: 10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–83. doi: 10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- 31.McIntosh AR, Gonzalez-Lim F. Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp. 1994;2:2–22. [Google Scholar]
- 32.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 33.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 34.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–22. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- 36.Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, et al. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16:1730–45. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- 37.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 38.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–88. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–47. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 40.Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–26. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wig GS, Buckner RL, Schacter DL. Repetition priming influences distinct brain systems: evidence from task-evoked data and resting-state correlations. J Neurophysiol. 2009;101:2632–48. doi: 10.1152/jn.91213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–37. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Stevens WD, Buckner RL, Schacter DL. Correlated Low-Frequency BOLD Fluctuations in the Resting Human Brain Are Modulated by Recent Experience in Category-Preferential Visual Regions. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, et al. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiatry. 2009;166:891–9. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis M, Shi C. The amygdala. Curr Biol. 2000;10:R131. doi: 10.1016/s0960-9822(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 46.Kapp BS, Supple WF, Whalen PJ. Stimulation of the amygdaloid central nucleus produces EEG arousal. Behavioral Neuroscience. 1994;108:81–93. doi: 10.1037//0735-7044.108.1.81. [DOI] [PubMed] [Google Scholar]
- 47.Holstege G, Bandler R, Saper CB. The emotional motor system. Prog Brain Res. 1996;107:3–6. doi: 10.1016/s0079-6123(08)61855-5. [DOI] [PubMed] [Google Scholar]
- 48.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 49.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 50.Kim JJ, Fanselow MS, DeCola JP, Landeira-Fernandez J. Selective impairment of long-term but not short-term conditional fear by the N-methyl-D-aspartate antagonist APV. Behav Neurosci. 1992;106:591–6. doi: 10.1037//0735-7044.106.4.591. [DOI] [PubMed] [Google Scholar]
- 51.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–39. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 52.Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–32. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 53.Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–7. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 54.Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–7. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rescorla RA. Retraining of extinguished Pavlovian stimuli. J Exp Psychol Anim Behav Process. 2001;27:115–24. [PubMed] [Google Scholar]
- 56.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 57.Hartley CA, Fischl B, Phelps EA. Brain Structure Correlates of Individual Differences in the Acquisition and Inhibition of Conditioned Fear. Cereb Cortex. doi: 10.1093/cercor/bhq253. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102:10706–11. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–38. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 61.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 62.Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–46. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 64.Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–8. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- 65.Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 66.Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–8. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 67.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 68.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 69.Erk S, Mikschl A, Stier S, Ciaramidaro A, Gapp V, Weber B, et al. Acute and sustained effects of cognitive emotion regulation in major depression. J Neurosci. 2010;30:15726–34. doi: 10.1523/JNEUROSCI.1856-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biol Psychiatry. 2009;65:367–73. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Welborn BL, Papademetris X, Reis DL, Rajeevan N, Bloise SM, Gray JR. Variation in orbitofrontal cortex volume: relation to sex, emotion regulation and affect. Soc Cogn Affect Neurosci. 2009;4:328–39. doi: 10.1093/scan/nsp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Foland-Ross LC, Altshuler LL, Bookheimer SY, Lieberman MD, Townsend J, Penfold C, et al. Amygdala reactivity in healthy adults is correlated with prefrontal cortical thickness. J Neurosci. 2010;30:16673–8. doi: 10.1523/JNEUROSCI.4578-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–81. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 76.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 78.Cooney RE, Joormann J, Atlas LY, Eugene F, Gotlib IH. Remembering the good times: neural correlates of affect regulation. Neuroreport. 2007;18:1771–4. doi: 10.1097/WNR.0b013e3282f16db4. [DOI] [PubMed] [Google Scholar]
- 79.Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn Sci. 2011;15:132–9. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. Journal of Neuroscience. 1995;15:5879–91. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Breiter H, Etcoff N, Whalen PJ, Kennedy W, Rauch SL, Buckner R, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–87. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 82.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 83.Kim MJ, Loucks RA, Neta M, Davis FC, Oler JA, Mazzulla EC, et al. Behind the mask: the influence of mask-type on amygdala response to fearful faces. Soc Cogn Affect Neurosci. 2010;5:363–8. doi: 10.1093/scan/nsq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–5. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 85.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- 87.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–32. [PMC free article] [PubMed] [Google Scholar]
- 88.Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–8. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 89.Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci. 1998;7:177–88. [Google Scholar]
- 90.Adolphs R, Tranel D, Damasio AR, Damasio H. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Letters to Nature. 1994;372:669–72. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 91.Neta M, Norris CJ, Whalen PJ. Corrugator muscle responses are associated with individual differences in positivity-negativity bias. Emotion. 2009;9:640–8. doi: 10.1037/a0016819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oler JA, Quirk GJ, Whalen PJ. CinguloAmygdala interactions in surprise and extinction: Interpreting associative ambiguity. In: Vogt BA, editor. Cingulate Neurobiology and Disease. USA: Oxford University Press; 2009. [Google Scholar]
- 93.Eysenck M. Anxiety: The cognitive perspective. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- 94.Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105:325–50. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- 95.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–34. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24:10364–8. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dickie EW, Armony JL. Amygdala responses to unattended fearful faces: Interaction between sex and trait anxiety. Psychiatry Res. 2008;162:51–7. doi: 10.1016/j.pscychresns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 98.Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–55. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 99.Somerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biol Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 100.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- 101.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Costa PT, Jr, McCrae RR. Stability and change in personality assessment: the revised NEO Personality Inventory in the year 2000. J Pers Assess. 1997;68:86–94. doi: 10.1207/s15327752jpa6801_7. [DOI] [PubMed] [Google Scholar]
- 103.Cremers HR, Demenescu LR, Aleman A, Renken R, van Tol MJ, van der Wee NJ, et al. Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. Neuroimage. 2010;49:963–70. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 104.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 105.Shin LM, Handwerger K. Is posttraumatic stress disorder a stress-induced fear circuitry disorder? J Trauma Stress. 2009 doi: 10.1002/jts.20442. [DOI] [PubMed] [Google Scholar]
- 106.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 107.APA. Diagnostic and statistical manual of mental disorders. 4. Washington DC: American Psychiatric Press; 1994. [Google Scholar]
- 108.Clark DM, McManus F. Information processing in social phobia. Biol Psychiatry. 2002;51:92–100. doi: 10.1016/s0006-3223(01)01296-3. [DOI] [PubMed] [Google Scholar]
- 109.Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behav Res Ther. 1997;35:741–56. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 110.Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch Gen Psychiatry. 2009;66:170–80. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59:1027–34. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 112.Yoon KL, Fitzgerald DA, Angstadt M, McCarron RA, Phan KL. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-Tesla functional MRI study. Psychiatry Res. 2007;154:93–8. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 113.Cooney RE, Atlas LY, Joormann J, Eugene F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiatry Res. 2006;148:55–9. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 114.Shah SG, Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci. 2009;34:296–302. [PMC free article] [PubMed] [Google Scholar]
- 115.Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. doi: 10.1016/j.neuroimage.2011.02.064. in press. [DOI] [PubMed] [Google Scholar]
- 116.Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, et al. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry. 2009;66:691–4. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien) 1992;115:143–8. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- 118.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kolassa IT, Wienbruch C, Neuner F, Schauer M, Ruf M, Odenwald M, et al. Altered oscillatory brain dynamics after repeated traumatic stress. BMC Psychiatry. 2007;7:56. doi: 10.1186/1471-244X-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 121.Kim MJ, Chey J, Chung A, Bae S, Khang H, Ham B, et al. Diminished rostral anterior cingulate activity in response to threat-related events in posttraumatic stress disorder. J Psychiatr Res. 2008;42:268–77. doi: 10.1016/j.jpsychires.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 122.Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63:184–92. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- 123.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 124.Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Theberge J, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121:33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- 125.Kim MJ, Lyoo IK, Kim SJ, Sim M, Kim N, Choi N, et al. Disrupted white matter tract integrity of anterior cingulate in trauma survivors. Neuroreport. 2005;16:1049–53. doi: 10.1097/00001756-200507130-00004. [DOI] [PubMed] [Google Scholar]
- 126.Kim SJ, Jeong DU, Sim ME, Bae SC, Chung A, Kim MJ, et al. Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology. 2006;54:120–5. doi: 10.1159/000098262. [DOI] [PubMed] [Google Scholar]


