Abstract
Childhood dystonia is a disorder that involves inappropriate muscle activation during attempts at voluntary movement. Few studies have investigated the muscle activity associated with dystonia in children, and none have done so in the hands. In this study, we measured surface electromyographic activity in four intrinsic hand muscles while participants attempted to perform an isometric tracking task using one of the muscles. Children with dystonia had greater tracking error with the task-related muscle and greater overflow to non-task muscles. Both tracking error and overflow correlated with the Barry-Albright Dystonia scale of the respective upper limb. Overflow also decreased when participants received visual feedback of non-task muscle activity. We conclude that two of the motor deficits in childhood dystonia—motor overflow and difficulties in actively controlling muscles—can be seen in the surface electromyographic activity of individual muscles during an isometric task. As expected from results in adults, overflow is an important feature of childhood dystonia. However, overflow may be at least partially dependent on an individual’s level of awareness of their muscle activity. Most importantly, poor single-muscle tracking shows that children with dystonia have deficits of individual muscle control in addition to overflow or co-contraction. These results provide the first quantitative measures of the muscle activity associated with hand dystonia in children, and they suggest possible directions for control of dystonic symptoms.
Keywords: Dystonia, childhood, co-contraction, overflow, surface electromyography
INTRODUCTION
Childhood dystonia is defined as “a movement disorder in which involuntary sustained or intermittent muscle contractions cause twisting and repetitive movements, abnormal postures, or both”.1 Dystonia can result from a number of causes, and its symptoms can be present in various parts of the body, including the hands and arms. In addition to postures and impediments to task-oriented movements, dystonia can also involve extra muscle activity in muscles that are not directly related to the task at hand, a phenomenon called overflow.2,3 There are a number of rating scales for gauging the level of dystonia, such as the Barry-Albright Dystonia (BAD) scale4 and Burke-Fahn-Marsden scale5, but little research has investigated the relation between physiological measures of muscle control and the severity of childhood dystonia, and none of this research has focused on dystonia in the hands.
Two studies have suggested that quantitative physiological measures may hold promise for more clearly defining dystonia in children. Lebiedowska et al.6 showed that strength, walking speed, and resistance to passive movement could differentiate participants with dystonia from those with spasticity. Gordon et al.7 showed that a measure of overflow correlated with participants’ ratings on the BAD scale and aspects of the participants’ performance in a reaching task.
Overflow has also been investigated in a number of other populations. Overflow is common in children and the elderly, but absent in young adults except under conditions of high exertion or fatigue. Overflow can also persist in older children with ADHD and be present in adults with focal hand dystonia, Parkinson’s Disease, and other neurological disorders.8–13
In addition to characterizing the existence of overflow, studies have shown that individuals can reduce overflow when provided knowledge of it through visual or auditory feedback.14–16 It is currently not known whether biofeedback approaches can help children with dystonia to reduce overflow, but promising results have previously been reported for children with dystonia and related disorders.17–20
In the present study, we measure surface electromyography (EMG) of muscles on both hands while individuals perform an isometric tracking task with a single muscle. This approach allows us to monitor (a) the ability of participants to modulate their muscle activity on command, as well as (b) the overflow occurring in other muscles on the same and opposite hand. Additionally, by providing visual feedback of the activity of all non-task muscles, we can determine whether children with dystonia can reduce overflow through biofeedback.
METHODS
Participants
Participants consisted of 16 children with dystonia (7 female; ages 9–18 years, mean 13.5 years, standard deviation 2.6 years), and 36 healthy children (14 female; ages 6–17, mean 10.5 years, standard deviation 3.1 years, 4 left-handed). Individuals in the dystonic group were recruited from the Stanford Medical Center and diagnosis was established by a pediatric neurologist based on history and clinical examination according to standard definitions.1 All participants with dystonia were rated on the Barry-Albright dystonia scale.4 To check for adequate intelligence for the task, we tested all participants in the dystonic group with the TONI-3 scale21 to ensure that they had a cognitive level equivalent to, or greater than, 6 years of age. Table 1 provides individual information on all participants in the dystonic group. Participants in the control group were recruited using advertisements in the local community and university, as well as through the families of the participants in the dystonic group. Control participants were excluded if they had any known neurological conditions or motor disorders, and if they had participated in any other experiments involving active control of EMG.
TABLE 1.
Characteristics of participants with dystonia
| Participant | Age | Gender | Diagnosis | Left Arm BAD score | Right Arm BAD score | Handed | Medication |
|---|---|---|---|---|---|---|---|
| D1 | 9 | F | Attention deficit disorder and torticollis, right writer’s cramp | 0 | 1 | Right | Carbidopa-levodopa, Carbidopa |
| D2 | 11 | F | Spastic diplegic cerebral palsy, premature birth | 3 | 2 | Right | Not available |
| D3 | 11 | M | DYT1-positive primary generalized dystonia, DBS implanted | 0 | 0 | Left | None |
| D4 | 11 | F | Right suprasellar dermoid tumor resection | 3 | 0 | Right | Trihexyphenidyl |
| D5 | 11 | M | Left basal ganglia cavernous malformation resection | 0 | 3 | Left | None |
| D6 | 13 | M | Triplegic cerebral palsy and possible dystonia in left upper extremity | 1 | 0 | Right | Not available |
| D7 | 13 | F | Right secondary hemidystonia due to stroke in basal ganglia | 0 | 3 | Left | Trihexyphenidyl |
| D8 | 13 | M | DYT1-positive primary generalized dystonia | 0 | 0 | Right | Trihexyphenidyl |
| D9 | 13 | F | Dyskinetic cerebral palsy | 3 | 3 | Left | Trihexyphenidyl |
| D10 | 14 | F | Primary generalized dystonia | 1 | 1 | Left | Botulinum toxin, Carbidopa-levodopa |
| D11 | 14 | M | Right hemiplegic cerebral palsy | 0 | 3 | Left | Botulinum toxin |
| D12 | 16 | M | Tetraplegic cerebral palsy | 2 | 3 | Left | Carbidopa-levodopa, Baclofen |
| D13 | 16 | M | Generalized secondary dystonia due to glutaric aciduria type 1, DBS implanted | 3 | 4 | Left | Trihexyphenidyl, Baclofen, Riboflavin, Levocarnitine, Oxybutynin, Metronidazole |
| D14 | 16 | M | Left hemiplegic cerebral palsy | 4 | 0 | Right | Not available |
| D15 | 17 | M | Right hemiplegic cerebral palsy | 0 | 3 | Right | None |
| D16 | 18 | F | Dyskinetic cerebral palsy | 3 | 4 | Right | Albuterol, Montelukast, Esomeprazole, Atenolol, Paroxetine |
BAD, Barry-Albright Dystonia scale; DBS, Deep brain stimulator
Stanford University’s Institutional Review Board approved the study protocol, and the study was registered with clinicaltrials.gov (NCT00285870). All participants or their parents gave informed written consent for participation and authorization for use of protected health information.
Apparatus
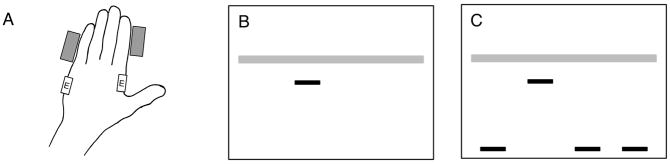
Participants sat on a chair and placed both hands flat on the surface of a table with palms down in a comfortable position. The table was adjusted to a comfortable height for each participant. The second and fifth fingers of both hands were constrained to prevent abduction, using plastic blocks that adhered to the surface of the table (see Fig. 1A). A small number of participants could not maintain their hands flat on the table, so a bandage was wrapped around their fingers to prevent abduction (From Table 1, both hands bandaged: D12, D13, D16; right hand bandaged: D11). Surface EMG electrodes (DE-2.3, Delsys Inc., USA) with a band-pass filter of 20–450 Hz and an amplification of 1,000 times were placed over the bellies of the first dorsal interosseous (FDI) and abductor digiti minimi (ADM) muscles on both hands. The EMG electrode signals were sampled at 1 kHz with an analog to digital interface (Power 1401, CED Technologies Inc., UK) and custom data acquisition software.
FIG. 1.
Experimental setup. (A) Hand placement on table. Rectangular blocks prevented abduction of the second and fifth fingers, and EMG electrodes (labeled E) were placed on the ADM and FDI muscles. Left hand is shown, and right hand was arranged in the same way. (B and C) Tracking task: The horizontal gray bar acted as a target, moving vertically with a randomly distorted sinusoidal motion. Participants tracked the target with one of the dark horizontal bars (cursor), activating their muscle to move the cursor upward, and relaxing their muscle to move it downward. The order of bars on the screen corresponded to the order of muscles with hands lying on table (left to right): LH ADM, LH FDI, RH FDI, and RH ADM. (B) Display for block 1 contained only the target and cursor (LH FDI shown in this case). (C) Display for block 2 also included all non-task muscles.
We obtained isometric muscle activation signals for the experimental task by filtering the EMG signal from each electrode through three steps. Each signal was processed with a high-pass Butterworth filter (4th-order, 1 Hz cutoff), then a Bayesian filter, and finally a low-pass Butterworth filter (2nd-order, 5 Hz cutoff). The Bayesian filter produces a smooth output that estimates the drive underlying the EMG signal, while also allowing fast low-latency changes in the filtered signal.22
Prior to the start of the experiment, we measured the isometric maximal voluntary contraction (MVC) for each muscle. The EMG signal from each electrode was displayed as visual feedback for the participant. The participant performed three attempts of 5 s of maximum contraction for each muscle with encouragement and feedback. MVC was quantified by the data acquisition software as the maximum mean EMG activation measured over a 200 ms period. Following this measurement, all muscle activation levels for the experiment were expressed as normalized EMG, defined as the ratio of the EMG value of each muscle to its MVC.
Procedure
Each participant attended a single experimental session of approximately 1-hour duration. The session started with seating at the table, placement of the EMG electrodes, and measurement of MVC values. The participant then completed a series of trials during which they tracked a target on a computer screen by activating one of their measured muscles to move a cursor.
During each trial, participants watched a computer monitor displaying a target and cursor, as shown in Fig. 1. The target moved vertically in a randomly distorted smooth sinusoidal motion with an average period of 7–10 s. Participants tracked the target with the cursor by isometrically activating one of their ADM or FDI muscles. Gain was adjusted so that when the tracking muscle was at rest, the cursor remained at the bottom of the screen, and the cursor reached the top of the screen at 20% of MVC. The mean target position during the trial was equivalent to approximately 10% of MVC. In some trials, participants were also provided with a visual display of the activity of the muscles that were not being used to control the cursor, as shown in Fig. 1C. The height of each of these additional cursors was normalized to the individual muscle MVC values so that the top of the screen always represented 20% MVC and the bottom always represented rest.
Prior to the start of testing, participants were allowed to practice moving the cursor on the screen for 60 s using each of the four muscles. During this time, the experimenter monitored the participant’s performance and made adjustments or suggestions to ensure that the participant was able to control the cursor adequately. All four cursors were shown during practice.
Following the practice trial, each participant performed 8 test trials, arranged in 2 blocks of 4 trials. In each block, participants performed the tracking task once with each muscle. All trials had a duration of 60 s. Participants were given a 30 s rest between trials, and a 60 s rest between blocks. Participants were also able to indicate if they were tired and desired a longer rest.
During the first block of trials, the monitor showed only the target and the cursor for the active muscle, and there was no indication of the activity of non-task muscles, as shown in Fig. 1A. Participants were instructed to track the target while keeping their hands as relaxed as possible. During the second block, the monitor showed the target, cursor, and the activity of the non-task muscles, as shown in Fig. 1B. Participants were instructed to track the target as the first priority while also trying to keep the activation of non-task muscles as low as possible.
Analysis
For each trial, we measured two aspects of performance: tracking error and overflow. We defined tracking error as the mean absolute difference between the target position and the cursor position, expressed in units of normalized EMG. Tracking error allowed us to measure how well individuals could modulate muscle activity on demand, as well as determine whether any reduction of overflow in block 2 occurred at the expense of tracking performance. Overflow for each non-task muscle was defined as the mean of the normalized activation of that muscle over the entire trial. For each trial, we used the mean of all samples in the trial to obtain a single measure of tracking error for the task muscle and a measure of overflow for each of the three non-task muscles.
For tracking error and mean overflow across all muscles, we used a linear mixed-effects model to test for fixed effects of group (dystonia vs. control) and block (1 vs. 2) while considering participant as a random factor (i.e., Fixed effects: group + block + group×block). Analysis was performed using the lme function from the nlme package23,24 of the R statistical computing environment25 [i.e., R statement: lme(error ~ group * block, random = ~ 1 | participant)]. For the dystonic group, we also calculated the correlation of tracking error and overflow in all trials of block 1 with the BAD scale of participants’ arms using the Pearson correlation coefficient. In order to correct the positive skew and heterogeneity of variance seen in the data, we logarithmically transformed the tracking error and overflow values prior to performing all tests.
RESULTS
Raw signals and distribution of measures
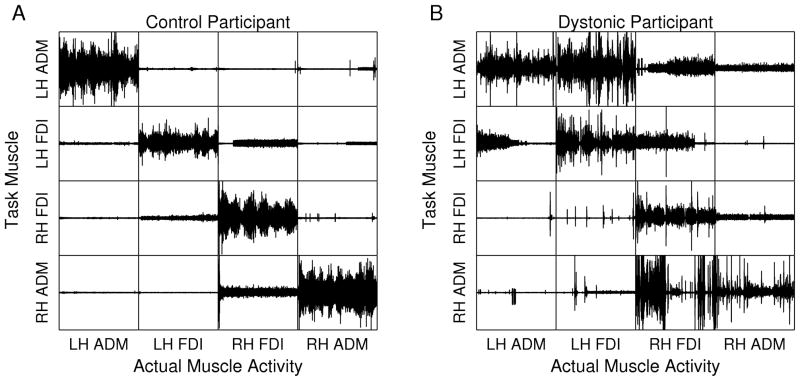
To provide an indication of the nature and relation of the signals analyzed, Fig. 2 shows the raw EMG from block 1, measured from a typical participant in each group. For most participants in the control group, almost all muscle activity occurred in the task muscle of each trial, and there was little activity in the non-task muscles. For many participants in the dystonic group, however, there was considerable activity in the non-task muscles, consistent with overflow. Overflow was also typically greatest for the muscle ipsilateral to the task muscle, followed by the contralateral homologous muscle, and finally the contralateral non-homologous muscle.
FIG. 2.
Representative EMG recordings from all trials in the first block (no feedback). For each participant, EMG recordings of all four muscles during four 60 s trials are organized in a matrix of trials (rows) and muscles (columns). The tracking muscles for each trial are located on the diagonal of the matrix, and non-task muscles are located off of the diagonal. A: Typical participant from the control group shows little muscle activity in non-task muscles. B: Typical participant from the dystonic group shows substantial muscle activity in non-task muscles. In particular, this subject showed significant overflow within each hand and overflow from the left hand to right hand.
We also checked for differences in the MVC values between groups. Both groups had similar values in the left hand, but the dystonic group had a lower mean MVC in the right hand (1.17 mV vs. 1.63 mV, p = 0.001). These differences are likely due to the characteristics of the individuals in the groups: the dystonic group had a much higher proportion of left-handedness than the control group, as well as more dystonia in the right arm than the left (see BAD scores in Table 1).
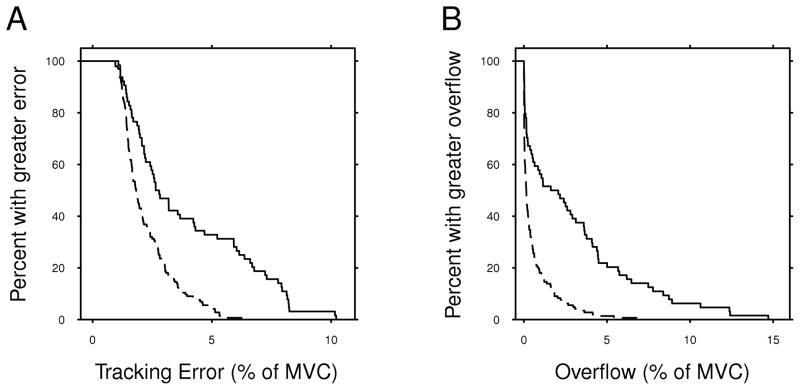
To provide a sense of the distribution of task results for both groups, Fig. 3 shows Kaplan-Meier plots of tracking error and mean overflow from all muscles in block 1. These plots show that both groups had a skewed distribution of results, with most participants having low values of tracking error and overflow, while a smaller number of participants had larger values. It is also evident that the degree of this skew differs between groups. As expected, a much higher proportion of participants in the control group are closer to the lower limit of each measure, and the dystonic group has a higher upper bound for tracking error and overflow than the control group. Nevertheless, many of the dystonic participants have values of tracking error and overflow that are well within the range of the control participants.
FIG. 3.
Kaplan-Meier plots showing mean tracking error (A) and overflow (B) in block 1 for individuals in the control (dashed line) and dystonic (solid line) groups. These plots illustrate the distribution of the measured values for each group, especially the difference between groups in the tails of the data.
Group and condition comparisons
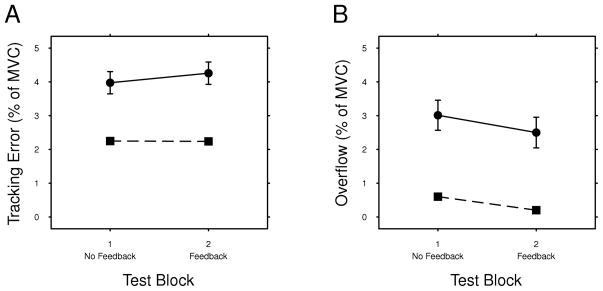
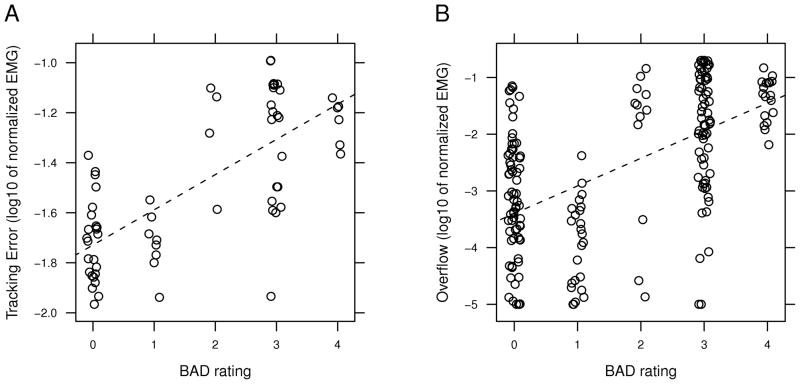
Figure 4A shows the mean tracking error across feedback and no-feedback blocks for each group. Testing for the effects of group and block showed no significant interaction between the factors (F(1,362) = 0.940, p = 0.333). Group was a significant factor (F(1,50) = 11.4, p = 0.001), indicating that the dystonic group had significantly more tracking error than the control group. Block was not a significant factor (F(1,362) = 0.0126, p = 0.911), even though there was a trend for the dystonic group to have greater tracking error in block 2 (post-hoc regression of dystonic group: F(1,111) = 0.900, p = 0.345). Tracking error also correlated positively with the BAD score of individual arms in the dystonic group (r =0.725, p < 0.001, Fig. 5A).
FIG. 4.
Mean tracking error (A) and overflow (B) in both blocks for the Control (squares with dashed line) and Dystonic (circles with solid line) groups. Error bars indicate standard errors of the mean for all participants.
FIG. 5.
Relation of tracking error (A) and overflow (B) to the BAD rating of the respective arm for participants in the dystonic group during block 1. Points are plotted with a log scale and jittered about their location on the x-axis to illustrate their density. The dotted line is the best-fitting regression line.
Figure 4B shows the mean overflow across all conditions for each group. Testing for the effects of group and block showed no significant interaction between the factors (F(1,362) = 0.020, p = 0.966). Group was a significant factor (F(1,50) = 12.7, p < 0.008), indicating that the dystonic group had significantly more overflow than the control group. Block was also a significant factor (F(1,362) = 17.7, p < 0.001), indicated that participants had less overflow in block 2 (with feedback) than block 1 (without feedback). Overflow also correlated positively with the BAD score of individual arms in the dystonic group (r =0.560, p < 0.001, Fig. 5B).
DISCUSSION
Using surface EMG, we measured the activity of four hand muscles while children with and without dystonia performed an isometric tracking task with each muscle. The dystonic group had greater overflow and tracking error than the control group. Tracking error and overflow also correlated with the severity of dystonia, as measured with the BAD scale. Additionally, individuals in both groups were able to reduce their levels of motor overflow when provided with visual feedback, and they did so without significantly increasing their error in the tracking task.
While the dystonic group on average had greater difficulty modulating muscle activity and maintaining non-task muscles at rest, the range of measured values for overflow and tracking error overlapped with that of typically developing children. There are several possible reasons for this. First, several of the children with dystonic symptoms had one or two arms with low or zero ratings of dystonia, and these individual arms generally had lower levels of error and overflow (Fig. 5). Second, as we have seen in other studies of co-contraction in antagonist muscles16,26, the muscle activity of children with dystonia can often be comparable to what is seen in children without dystonia. Other research has made similar observations: individuals with dystonia can often perform as well those without dystonia, but certain constraints—using a particular finger or hand, muscle activation at a particular pace, or movement in a specified direction—can bring out the impairments that can limit their abilities.27 Finally, it is also possible that our measures do not measure only the aspects of muscle activity that define dystonia. In this experiment, we were attempting to quantify the muscle activity underlying the observed difficulties that children with dystonia have in actively controlling muscles and keeping muscles at rest.2,3 At the same time, it is likely that a measure that clearly delineates individuals with dystonia from those without will be a more complex combination of these, and possibly other, measures.
Our measures of task error and overflow correlated with the BAD scores of the individual limbs. This shows that the abilities to modulate individual muscle activity and maintain groups of muscles at rest may be related to the physical manifestation of dystonia. It is also important to note that our measure of tracking error had a greater correlation with the BAD score than our measure of overflow. This may be somewhat surprising, as other researchers have focused on overflow as a measure of dystonia.7 The difference in this case may be due to the consistency of the effect. As shown in Fig. 5, individuals more consistently had difficulty modulating their muscle activation in an arm with a higher BAD rating, resulting in higher tracking error. Overflow, however, was a more variable occurrence—while muscles from arms with a high BAD rating often had greater levels of overflow, there were other trials in which the muscles were not highly active. Similarly, many muscles from arms with a BAD rating of 0 had large amounts of overflow. The variation of dystonia with time, position, and other factors has been noted previously.1 These results suggest that measures of dystonia and dystonia-related symptoms should consider both task-related movements and non-task related muscle activity.
Another important result from this study is that individuals in both groups had lower overflow when provided with visual feedback of overflow. This shows that children with dystonia are able to reduce their overflow to a certain extent, as long as their attention can be drawn specifically to the muscle activity. This result is similar to observations of two recent studies from our laboratory16,17, and it suggests that sensory feedback may play a role in dystonia.
We have presented an approach for measuring the muscle activity from multiple hand muscles in children with dystonia. While our measures correlate with clinical scales of dystonia, there remain important questions about how to use and interpret these measurements. For example, we cannot determine whether the deficits in overflow and tracking that we measure are a pathognomonic feature of dystonia, or whether they are additional deficits associated with the causes of dystonia. Additionally, this study does not have sufficient discriminatory power to distinguish between possible differences in the form of dystonic symptoms associated with different etiologies. These are important questions, but they cannot be answered until definitive and objective characterization of dystonia is achieved, so that dystonia per se can be distinguished from associated impairments. We hope that our results will contribute toward the ability to quantify and distinguish between dystonia and associated motor deficits.
Acknowledgments
We thank Rosemary Bloom for participant recruitment and assistance with data collection. We also thank Allison Przekop for performing neurological examinations. This research was performed at Stanford University and the University of Southern California.
Funding: This study was funded by Grant NS052236 from the National Institute of Neurological Disorders and Stroke (NINDS). Additional funding was provided by the Crowley-Carter Foundation and the Don and Linda Carter Foundation.
Footnotes
Potential conflict of interest: Nothing to report.
AUTHOR ROLES
Young: 1B, 1C, 2A, 2B, 2C, 3A, 3B. van Doornik: 1B, 1C, 2C, 3B. Sanger: 1A, 1B, 2A, 2C, 3B. (Based on categories suggested in Author Guidelines, 1. Research project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript: A. Writing of the first draft, B. Review and Critique;)
FULL FINANCIAL DISCLOSURE FOR THE PREVIOUS 12 MONTHS
Young: Employment, University of Southern California; Employment, Stanford University
van Doornik: None
Sanger:
Grants/Research:
- R01 NS052236 (PI: Sanger) NIH/NINDS
- R01 NS064046 (PI: Sanger) NIH/NINDS
- R01 NS069214 (PI: Sanger) NIH/NINDS
- U13 NS043180 (PI: Sanger) NIH/NINDS/NICHD
- Investigator-initiated clinical research (PI: Sanger) Don and Linda Carter Foundation, Crowley Carter Foundation
- Investigator-initiated clinical research (PI: Sanger) James S. McDonnell Foundation
Salary: University of Southern California, Children’s Hospital Los Angeles
References
- 1.Sanger TD, Delgado MR, Gaebler-Spira D, et al. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111:e89–97. doi: 10.1542/peds.111.1.e89. [DOI] [PubMed] [Google Scholar]
- 2.Sanger TD, Chen D, Fehlings DL, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010;25:1538–1549. doi: 10.1002/mds.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanger TD. Pathophysiology of pediatric movement disorders. J Child Neurol. 2003;18 (Suppl 1):S9–24. doi: 10.1177/0883073803018001S0401. [DOI] [PubMed] [Google Scholar]
- 4.Barry MJ, VanSwearingen JM, Albright AL. Reliability and responsiveness of the Barry-Albright Dystonia Scale. Dev Med Child Neurol. 1999;41:404–411. doi: 10.1017/s0012162299000870. [DOI] [PubMed] [Google Scholar]
- 5.Burke RE, Fahn S, Marsden CD, et al. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35:73–77. doi: 10.1212/wnl.35.1.73. [DOI] [PubMed] [Google Scholar]
- 6.Lebiedowska MK, Gaebler-Spira D, Burns RS, Fisk JR. Biomechanic characteristics of patients with spastic and dystonic hypertonia in cerebral palsy. Arch Phys Med Rehabil. 2004;85:875–880. doi: 10.1016/j.apmr.2003.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Gordon LM, Keller JL, Stashinko EE, et al. Can spasticity and dystonia be independently measured in cerebral palsy? Pediatr Neurol. 2006;35:375–381. doi: 10.1016/j.pediatrneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Cole WR, Mostofsky SH, Larson JC, et al. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71:1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgiou-Karistianis N, Hoy KE, Bradshaw JL, et al. Motor overflow in Huntington’s disease. J Neurol Neurosurg Psychiatry. 2004;75:904–906. doi: 10.1136/jnnp.2003.016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cincotta M, Giovannelli F, Borgheresi A, et al. Surface electromyography shows increased mirroring in Parkinson’s disease patients without overt mirror movements. Mov Disord. 2006;21:1461–1465. doi: 10.1002/mds.20972. [DOI] [PubMed] [Google Scholar]
- 11.Hoy KE, Fitzgerald PB, Bradshaw JL, et al. Motor overflow in schizophrenia. Psychiatry Res. 2004;125:129–137. doi: 10.1016/j.psychres.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Shindo M, Yanagisawa N. Enhanced associated movements in the contralateral limbs elicited by brisk voluntary contraction in choreic disorders. Clin Neurophysiol. 2001;112:1612–1617. doi: 10.1016/s1388-2457(01)00627-7. [DOI] [PubMed] [Google Scholar]
- 13.Sitburana O, Wu LJC, Sheffield JK, et al. Motor overflow and mirror dystonia. Parkinsonism Relat Disord. 2009;15:758–761. doi: 10.1016/j.parkreldis.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus JA, Todor JI. The role of attention in the regulation of associated movement in children. Dev Med Child Neurol. 1991;33:32–39. doi: 10.1111/j.1469-8749.1991.tb14783.x. [DOI] [PubMed] [Google Scholar]
- 15.Lazarus JC. Associated movement in hemiplegia: the effects of force exerted, limb usage and inhibitory training. Arch Phys Med Rehabil. 1992;73:1044–1049. [PubMed] [Google Scholar]
- 16.Young SJ, van Doornik J, Sanger TD. Visual feedback reduces co-contraction in children with dystonia. J Child Neurol. 2010 doi: 10.1177/0883073810371828. [DOI] [PubMed] [Google Scholar]
- 17.Bloom R, Przekop A, Sanger TD. Prolonged electromyogram biofeedback improves upper extremity function in children with cerebral palsy. J Child Neurol. 2010 doi: 10.1177/0883073810369704. [DOI] [PubMed] [Google Scholar]
- 18.Cataldo MF, Bird BL, Cunningham CE. Experimental analysis of EMG feedback in treating cerebral palsy. J Behav Med. 1978;1:311–322. doi: 10.1007/BF00846682. [DOI] [PubMed] [Google Scholar]
- 19.Finley WW, Niman C, Standley J, Ender P. Frontal EMG-biofeedback training of athetoid cerebral palsy patients: a report of six cases. Biofeedback Self Regul. 1976;1:169–182. doi: 10.1007/BF00998584. [DOI] [PubMed] [Google Scholar]
- 20.Neilson PD, McCaughey J. Self-regulation of spasm and spasticity in cerebral palsy. J Neurol Neurosurg Psychiatry. 1982;45:320–330. doi: 10.1136/jnnp.45.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown L, Sherbenou RJ, Johnsen SK. Test of Nonverbal Intelligence, Third Edition Examiner’s Manual. Austin, TX: Pro-Ed; 1997. [Google Scholar]
- 22.Sanger TD. Bayesian filtering of myoelectric signals. J Neurophysiol. 2007;97:1839–1845. doi: 10.1152/jn.00936.2006. [DOI] [PubMed] [Google Scholar]
- 23.Pinheiro J, Bates D, DebRoy S, Sarkar D R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. 2009. R package version 3. 1–96. [Google Scholar]
- 24.Pinheiro J, Bates D. Mixed-Effects Models in S and S-PLUS. New York: Springer-Verlag; 2000. [Google Scholar]
- 25.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2009. URL http://www.R-project.org. [Google Scholar]
- 26.Malfait N, Sanger TD. Does dystonia always include co-contraction? A study of unconstrained reaching in children with primary and secondary dystonia. Exp Brain Res. 2007;176:206–216. doi: 10.1007/s00221-006-0606-4. [DOI] [PubMed] [Google Scholar]
- 27.Ghez C, Gordon J, Hening W. Trajectory control in dystonia. Adv Neurol. 1988;50:141–155. [PubMed] [Google Scholar]