Abstract
Previously, a learning-free measure was used to demonstrate that chronic food restriction (FR) increases the reward magnitude of a wide range of abused drugs. Moreover, a variety of striatal neuroadaptations were detected in FR subjects, some of which are known to be involved in synaptic plasticity but have been ruled out as modulators of acute drug reward magnitude. Little is known about effects of FR on drug-conditioned place preference (CPP) and brain regional mechanisms that may enhance CPP in FR subjects. The purpose of the present study was to compare the expression and persistence of a conditioned place preference (CPP) induced by a relatively low dose of cocaine (7.0 mg/kg, i.p.) in ad libitum fed (AL) and FR rats and take several brain regional biochemical measures following the first CPP conditioning session to probe candidate mechanisms that may underlie the more robust CPP observed in FR subjects. Behaviorally, AL subjects displayed a CPP upon initial testing which extinguished rapidly over the course of subsequent test sessions while CPP in FR subjects persisted. Despite previous reports of elevated BDNF protein in forebrain regions of FR rats, the FR protocol used in the present study did not alter BDNF levels in dorsal hippocampus, nucleus accumbens or medial prefrontal cortex. On the other hand, FR rats, whether injected with cocaine or vehicle, displayed elevated p-ERK1/2 and p-Ser845-GluA1 in dorsal hippocampus. FR rats also displayed elevated p-ERK1/2 in medial prefrontal cortex and elevated p-ERK1 in nucleus accumbens, with further increases produced by cocaine. The one effect observed exclusively in cocaine-treated FR rats was increased p-Ser845-GluA1 in nucleus accumbens. These findings suggest a number of avenues for continuing investigation with potential translational significance.
Keywords: food restriction, cocaine, conditioned place preference, synaptic plasticity, addiction
1. Introduction
The shared neural substrates of ingestive behavior and drug abuse have been established in both human and animal subjects (Kelley & Berridge, 2002; Cardinal & Everitt, 2004; Di Chiara, 2005; Volkow et al., 2008). One source of evidence for, and a consequence of, this relationship is the enhanced rewarding effect of a wide range of abused drugs in subjects that are chronically food-restricted (FR) (Cabeza de Vaca and Carr, 1998). This enhancement has been demonstrated using a learning-free measure of drug reward which capitalizes on threshold-lowering effects in an electrical brain stimulation paradigm. The enhancing effect of FR is robust, regardless of whether drugs are administered systemically (Cabeza de Vaca et al., 2004), intracerebroventricularly (Carr et al., 2000), or directly into the nucleus accumbens (Carr et al., 2009b). It is likely that the increase in drug reward magnitude contributes to the well-documented increased self-administration of drugs by FR subjects (Carroll et al., 1979). While numerous pre- and postsynaptic neuroadaptations have been identified in dorsal and ventral striatum of FR subjects (Pothos et al., 1995; Cadoni et al., 2003; Carr et al., 2003; Haberny et al., 2004; Haberny and Carr, 2005; Zhen et al., 2006; Thanos et al., 2008) the mechanistic basis of increased drug reward magnitude remains to be fully elucidated.
The augmentation of drug reward by FR may have clinical significance in so far as a history of severe dieting is a risk factor for binge eating disorder (Stice et al., 2008), and a high comorbidity of disordered eating and substance abuse is well established (Krahn et al., 1992; Wiederman and Pryor, 1996), most recently among high school students (Pisetsky et al., 2008; Seo and Jiang, 2009). The prevalence of substance abuse in individuals with an eating disorder is 5-6-fold higher than in the general population with the greatest prevalence in individuals with anorexia/bulimia (Root et al., 2010). Moreover, within the general population episodic dieting is prevalent and some drugs of abuse are deliberately sought out as diet aids to facilitate or maintain weight loss (Klesges et al., 1997; Cochrane et al., 1998).
While increased drug reward magnitude in an acute behavioral assay suggests that FR may increase vulnerability to initial use, escalation to abuse and addiction are modeled in other behavioral measures. One such measure is the persistent incentive-motivating effect of environmental cues and contexts that have acquired incentive salience as a result of repeated pairing with drug effects (O'Brien et al., 1962; Childress et al., 1988). In animal subjects, this may be assayed in the conditioned place preference paradigm (CPP), in which one side of a two-chambered apparatus is repeatedly paired with drug administration, the other is repeatedly paired with vehicle and time spent in the one side versus the other during a subsequent drug-free state is taken as a measure of conditioned incentive effects (Carr et al., 1989). Persistence is assessed by tracking the extinction of place preference over successive drug-free test sessions (Mueller and Stewart, 2000). Little is known about effects of FR on drug-induced place preference, its persistence, and brain regional mechanisms that may enhance CPP in FR subjects. The numerous neuroadaptations identified in FR subjects, with known involvement in synaptic plasticity, suggest that a CPP would be more enduring in FR than AL subjects.
One liability of the CPP paradigm is its low sensitivity to dose effects (Aguilar et al., 2009). Nevertheless, two studies have shown that the threshold doses of cocaine and d-amphetamine are lower in FR than AL rats (Bell et al., 1997; Stuber et al., 2002). In those studies, the single post-conditioning CPP test was the terminal measure taken. In the present study, a relatively low but suprathreshold dose of cocaine was used to demonstrate a more robust cocaine-CPP in FR relative to ad libitum fed (AL) rats as indicated by greater resistance to extinction. To probe for brain regional biochemical responses to CPP conditioning that may mediate the more robust effect in FR subjects, a follow-up experiment was conducted in which brain regions were obtained immediately following the first cocaine-place pairing or vehicle place pairing to identify responses that distinguish FR from AL subjects. The brain regions sampled, and biochemical measures taken, were not exhaustive but were based on convergence of their prior implication in synaptic plasticity, effects of FR, and psychostimulant-CPP. Consequently, BDNF protein levels, which are altered in numerous brain regions by FR (Lee et al., 2000; Duan et al., 2001; Lebrun et al., 2006) and modulate cocaine-seeking behavior (McGinty et al., 2010) were measured. ERK1/2 was examined based on findings of greater D-1 receptor-dependent activation in striatum of FR relative to AL rats (Haberny et al., 2004), and involvement in the acquisition and expression of psychostimulant-CPP (Gerdjikov et al., 2004; Miller and Marshall, 2005b). Finally, phosphorylation of the AMPA receptor GluA1 subunit on Ser845 (the PKA phosphorylation site) was measured based on involvement of GluA1 phosphorylation in AMPA receptor trafficking and synaptic strengthening (Barry and Ziff, 2002; Oh et al., 2006), and prior findings that D-1 receptor stimulation and sucrose ingestion produced greater GluA1 phosphorylation and synaptic insertion of AMPA receptors in FR relative to AL rats (Carr et al., 2010; Peng et al., 2011). All biochemical measures were taken in nucleus accumbens (NAc), dorsal hippocampus (dHPC), and medial prefrontal cortex (mPFC) based on their established involvement in CPP and the local involvement of one or more of the aforementioned biochemical responses (see Discussion).
2. Results
2.1 CPP
Prior to conditioning, subjects displayed no preference for one side of the CPP apparatus over the other (F(side) 1,17=0.0; F(side × diet) 1,17 =0.9; Figure 1). In the first CPP test, conducted 48 hrs following the completion of conditioning, a preference was expressed for the cocaine-paired side relative to the saline-paired side, regardless of diet (F(side) 1,17 =31.7, p<.0001; F(diet) 1,17 =0.63; F(side × diet) 1,17 =0.2). However, across the six days of CPP testing, the FR group displayed a sustained preference for the cocaine-paired side (F(side) 1,9=11.9, p<.01) while the AL group did not (F(side) 1,8=3.1, p>.10). In the final test session, aimed at reinstating the extinguished CPP in AL rats with a priming dose of cocaine, a CPP was not expressed (t(8)=0.05, p>.10). The FR group, on the other hand, displayed a strong CPP (t(9)=2.61, <.025) which likely represents a continuation of the CPP which had not yet extinguished, with a possible enhancing effect of the cocaine prime.
Figure 1.
Effect of chronic food restriction on CPP reinforced by cocaine (7.0 mg/kg, i.p). Mean (± S.E.M.) time spent (sec) in the cocaine- and saline-paired sides of the CPP apparatus during the preconditioning session, six subsequent post-conditioning test sessions, and a seventh test session conducted immediately after administration of a “priming” injection of cocaine (7.0 mg/kg, i.p.). The first CPP test was conducted 48 h after the final conditioning session. The seventh test, preceded by a cocaine prime, was conducted 48 h after the AL group had satisfied extinction criterion. #Time spent on the cocaine-paired side relative to the saline-paired side was greater, across feeding conditions, in the first CPP test (p<.0001). *Time spent on the cocaine-paired side relative to the saline-paired side was greater across all drug-free test sessions in the FR (p <.01) but not in the AL group. @In the seventh test, which was preceded by a cocaine prime, time spent on the cocaine-paired side relative to the saline-paired side was greater in FR (p<.025) but not in AL rats. nAL=9, nFR=10.
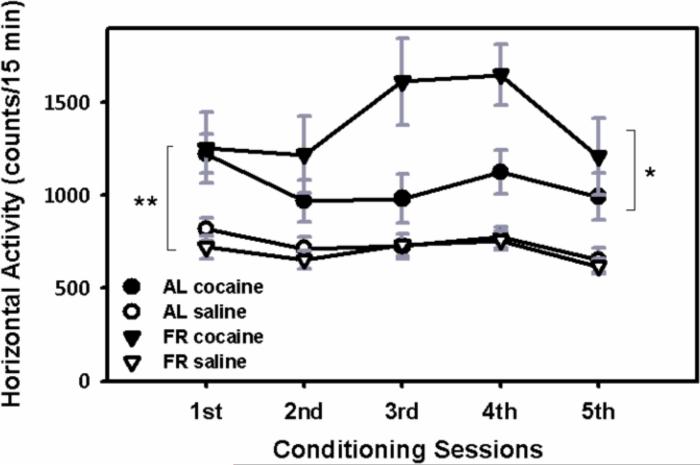
Locomotor activity induced by cocaine during the five conditioning sessions was greater in FR than AL rats (F(drug) 1,17=18.5, p<.001; F(drug × diet) 1,17 =5.4, p<.05; Figure 2). While the interaction of drug × diet × day was not significant (F=1.26), inspection of the data in Figure 2 indicate that the enhanced response of FR rats did not begin to emerge until after the first conditioning session.
Figure 2.
Effect of chronic food restriction on cocaine-induced locomotor activation during CPP conditioning sessions. Mean (± S.E.M.) beam interruptions reflecting horizontal activity (forward locomotion) in response to cocaine administration (7.0 mg/kg, i.p.) during each of the five 15-min conditioning sessions. **Cocaine increased locomotor activity, relative to saline (p<.001), *with a greater effect in FR than AL rats (p<.05). nAL=9, nFR=10.
2.2 BDNF
Levels of BDNF protein were determined by ELISA in diet groups that were not treated with cocaine. No differences were observed between AL and FR rats in dHPC (t(8)=0.18), NAc (t(10)=.09), or mPFC (t(10)=0.49) (Figure 3).
Figure 3.
Effect of chronic food restriction on brain regional BDNF protein levels. Levels of BDNF protein (mean ± S.E.M.), as determined by ELISA, expressed as percentage of control (ad libitum fed group), in medial prefrontal cortex (mPFC, n=6 per group), nucleus accumbens (NAc, n=6 per group), and dorsal hippocampus (dHPC, n=5 per group).
2.3 p-ERK1/2
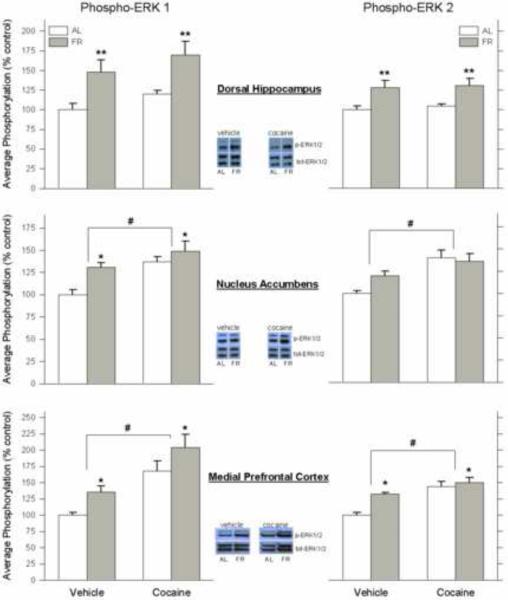
Because bands corresponding to ERK1 and ERK2 were well separated in immunoblots and the two ERK isoforms may be differentially involved in regulating cocaine effects (Ferguson et al., 2006), phosphorylation of ERK1 and ERK2 were analyzed separately. In dHPC, FR increased p-ERK1 across injection conditions (F1,20 =15.6, p<.001), with no effect of cocaine (F1,20 =0.3) or interaction between factors (F1,20 =0.0; Figure 4). FR similarly increased p-ERK2 across injection conditions (F1,20 =15.4, p<.001) with no effect of cocaine (F1,20 =3.0, p>.05) and no interaction (F1,20 =0.02). In NAc, both FR (F1,20 =8.6, p<.01) and cocaine (F1,20 =14.6, p<.001) increased p-ERK1. In contrast, p-ERK2 was increased by cocaine (F1,20 =18.5, p<.001) but not FR (F1,20 =1.5). In mPFC, both FR (F1,20 =7.8, p<.01) and cocaine (F1,20 =26.6, p<.001) increased p-ERK1. In addition, both FR (F1,20 =10.4, p<.01) and cocaine (F1,20 =27.9, p<.001) increased p-ERK2. There were no significant changes in total ERK relative to α-tubulin (data not shown).
Figure 4.
Effect of chronic food restriction on brain regional activation of ERK1/2 MAP kinase during a CPP conditioning session preceded by injection of cocaine or vehicle. Effects of chronic food restriction on ERK1 and ERK2 MAPK phosphorylation in dorsal hippocampus (top, left and right, respectively), nucleus accumbens (middle), and medial prefrontal cortex (bottom). Ad libitum fed (AL) and food-restricted (FR) rats were injected with cocaine (7.0 mg/kg, i. p.) or vehicle, immediately placed in one side compartment of a CPP apparatus and sacrificed following the 15-min conditioning session. Lysates were immunoblotted with anti-phospho-p44/42 MAPK or anti-p44/42 MAPK antibodies. Following densitometry, intensities of bands corresponding to phospho-ERK1 and 2 for each subject were divided by the intensities of the corresponding total ERK bands to correct for small differences in protein loading. Results (mean ± S.E.M.) are expressed in comparison to the normalized control, which was defined as the AL group injected with vehicle. Representative blots (from top to bottom) correspond to phospho-p44 ERK1, phospho-p42 ERK2, total p44 ERK1 and total p42 ERK2. FR greater than AL across drug treatments, **p<.001, *p<.01. #Cocaine greater than saline across diet treatments, p<.001. n=6 per group.
2.4 p-Ser845-GluA1
In dHPC, FR increased levels of p-Ser845-GluA1 across injection conditions (F1,20 =5.59, p<.05) with no effect of cocaine (F1,20 =0.65) and no interaction between factors (F1,20 =0; Figure 5). In NAc, a significant interaction between diet and injection (F1,20 =4.76, p<.05) followed by Tukey HSD pairwise comparisons indicated that p-Ser845-GluA1 was increased in the cocaine-treated FR group relative to all other groups (p at least <.05). In mPFC there were no effects of diet (F1,20 =0.21), cocaine (F1,20 =.0) or interaction between factors (F1,20 =.11) There were no significant changes in total GluA1 relative to α-tubulin (data not shown).
Figure 5.
Effect of chronic food restriction on brain regional phosphorylation of AMPA receptor GluA1 subunit on Ser845 during a CPP conditioning session preceded by administration of cocaine or vehicle. Effects of chronic food restriction on GluA1 phosphorylation on Ser845 in dorsal hippocampus (top), nucleus accumbens (middle), and medial prefrontal cortex (bottom). Ad libitum fed (AL) and food-restricted (FR) rats were injected with cocaine (7.0 mg/kg, i. p.) or vehicle, immediately placed in one side compartment of a CPP apparatus and sacrificed following the 15-min conditioning session. Lysates were immunoblotted with anti-phospho-Ser845 GluA1 or anti-GluA1 antibodies. Following densitometry, intensities of bands corresponding to phospho-GluA1 for each subject were divided by the intensities of the corresponding total GluA1 bands to correct for small differences in protein loading. Results (mean ± S.E.M.) are expressed in comparison to the normalized control, which was defined as the AL group injected with vehicle. Representative blots (from top to bottom) correspond to phospho-Ser845 GluA1 and total GluA1. *FR greater than AL across drug treatments, p<.05. **FR treated with cocaine greater than all other groups, p at least <.05.
3. Discussion
CPP has low sensitivity to dose-response effects (Aguilar et al., 2009), although two previous studies have shown that the threshold doses of cocaine and d-amphetamine in the CPP paradigm are lower in FR than AL rats (Bell et al., 1997; Stuber et al., 2002). While the relatively low dose of cocaine administered in the present study did produce a CPP in both diet groups, there is evidence of a more robust CPP in FR relative to AL rats. Specifically, the CPP persisted across the series of six CPP test sessions in FR rats but did not similarly persist in AL rats. In addition, the extinguished CPP response in AL rats was not reinstated by a priming dose of cocaine. Reinstatement of an extinguished CPP by a priming dose of drug has been demonstrated in numerous studies (for review, see Aguilar et al., 2009). However, it has also been shown that propensity to reinstatement depends on the conditioning dose used. Although magnitude of the initial CPP often does not distinguish suprathreshold doses, conditioning with a low dose precludes future reinstatement (Orsini et al., 2008). Thus, the rapid extinction and absence of reinstatement observed in AL subjects in the present study support the low efficacy of this dose of cocaine in this diet group.
The locomotor activity data collected during the cocaine conditioning sessions are interesting in that they indicate no difference between diet groups upon first exposure to cocaine, followed by a clear separation thereafter, and an overall significant difference between groups. This suggests that neuroplastic changes develop as a consequence of interaction between cocaine and FR and may underlie emergent behavioral differences between groups. As an initial probe for brain regional biochemical responses to CPP conditioning that may contribute to development of a more robust CPP in FR subjects, several brain regions were sampled following the first CPP conditioning session. The brain regions chosen and biochemical measures taken were based on prior convergent findings indicating involvement in CPP, synaptic plasticity, and effects of FR.
BDNF modulates an array of behavioral functions in the mature CNS including mood (Eisch et al, 2003; Nestler and Carlezon, 2006), drug addiction (Horger et al, 1999; Pierce and Bari, 2001; Lu et al., 2004; Graham et al, 2007), and learning (Rattiner et al., 2004; Rademacher et al., 2006). NAc receives convergent BDNF inputs of prefrontal cortical and ventral tegmental origin, which regulate several cocaine-induced behavioral effects including locomotor activation and self-administration (for review see: McGinty et al., 2010). Importantly, FR has been reported to increase BDNF protein levels in neocortical, hippocampal and dorsal striatal regions (Lee et al. 2000; Duan et al., 2001). Furthermore, BDNF-regulated neurons in dHPC play a role in the acquisition of amphetamine-CPP (Shen et al., 2006), and extinction of a classically conditioned response involves dHPC neurons releasing BDNF into the mPFC (Peters et al., 2010). Nevertheless, the present study revealed no effect of FR on BDNF protein levels in dHPC, NAc, or mPFC. The discrepancy between this finding and those of previous investigations (Lee et al. 2000; Duan et al., 2001), which also used ELISA to measure BDNF protein in drug-naïve rat brain regions, is most likely due to significant differences in the FR regimens employed. In the prior studies, subjects were fed every other day for 3 months, while in the present study subjects were fed a limited amount of food every day and assays were conducted after 3–4 weeks of FR. While this regimen is sufficient to increase the reward magnitude of abused drugs and a cocaine-CPP, it appears insufficient to upregulate BDNF, casting doubt on involvement of forebrain BDNF in the augmentation of drug reward and CPP persistence by FR. A caveat, which applies to all measures taken in the present study, is that the interaction between cocaine conditioning and FR may be associated with functionally important changes that emerge during later stages of conditioning and/or subsequent drug-free expression of the CPP. These are questions for future investigation.
Drugs of abuse, including cocaine, activate ERK1/2 MAP kinase in NAc and deep layers of prefrontal cortex in a D-1 DA receptor-dependent manner (Valjent et al., 2004). Striatal ERK is also activated by glutamate (Schwarzschild et al., 1999). The ERK cascade activates RSK2, an S6 kinase which activates transcription factors, including CREB, that bind to the promoter region of the c-fos gene, and mediate synaptic plasticity underlying diverse learning and memory-related processes (Thomas and Huganir, 2004; Sweatt, 2001), including CPP (e.g., Valjent et al., 2000; Gerdjikov et al., 2004). Within the NAc core, which is consistently and strongly activated during expression of a psychostimulant-CPP (Miller and Marshall, 2005b; Rademacher et al., 2006), ERK phosphorylation is necessary for CPP acquisition (Gerdjikov et al., 2004) and expression (Miller and Marshall, 2005b). Moreover, the threshold dose for cocaine CPP correlates with the magnitude of cocaine-induced NAc ERK phosphorylation (Narasimhaiah et al., 2009). It is therefore of interest that FR was previously shown to increase NAc ERK phosphorylation in response to D-1 DA receptor stimulation (Haberny et al., 2004). Further, although the acute rewarding effect of NAc D-1 DA receptor stimulation was greater in FR than AL rats (Carr et al., 2009b), blockade of downstream ERK phosphorylation with a systemically or intra-NAc administered MEK inhibitor did not diminish the rewarding or locomotor activating effects of a D-1 DA receptor agonist or d-amphetamine (Carr et al., 2009a). MEK inhibition did, however, block downstream CREB phosphorylation and immediate early gene expression (Haberny and Carr, 2005; Carr et al., 2009a). Together, these findings led to the expectation that CPP would be increased by FR and that upregulation of ERK signaling, particularly in NAc, would be involved. Results indicate that phosphorylation of ERK1 was increased by both FR and cocaine, leading to a greater net effect in the cocaine-treated FR group relative to others. However, ERK1 is generally less strongly activated by drugs of abuse than ERK2 (Girault et al., 2007) and does not appear to be the ERK isoform within NAc that plays a positive role in drug addiction (Mazzucchelli et al., 2002; Ferguson et al., 2006). ERK2 was activated by cocaine in NAc but, contrary to expectation, did not display greater activation in FR than AL rats. In the mPFC, both ERK isoforms were activated by cocaine and by FR. In dHPC, on the other hand, FR increased activation of ERK1 and ERK2 across injection treatments, without apparent effect of cocaine. Whether this increase was induced by placement of subjects in the CPP compartment or is reflective of the basal state of FR rats cannot be discerned from the present study. In either case, it may correspond to a “priming” of a molecular mechanism of synaptic plasticity which contributes to enhanced contextual learning. Interestingly, FR similarly increased dHPC levels of p-Ser845-GluA1, which may be further indication of “priming” for synaptic plasticity (see discussion of p-Ser-GluA1 below).
This study identified a single localized biochemical response that was unique to FR subjects injected with cocaine, namely, increased phosphorylation of the AMPA receptor GluA1 subunit on Ser845 in NAc. This finding is of interest for several reasons. It was previously shown that FR increases NAc GluA1 phosphorylation on Ser845 in response to D-1 DA agonist treatment, as well as in response to brief sucrose intake (Carr et al., 2010). Phosphorylation of GluA1 on Ser845 increases channel open probability (Banke et al., 2000), thereby increasing neuronal excitability, and increases GluA1 surface expression at extrasynaptic sites (for review, see Wolf, 2010). In the presence of concomitant NMDA receptor stimulation, GluA1 is then translocated to the synaptic membrane (Oh et al, 2006) and mediates synaptic strengthening (Barry and Ziff, 2002). Synaptic insertion of GluA1 in NAc has been shown to correlate with ERK phosphorylation (Boudreau et al., 2007), which is necessary for synaptic delivery of GluA1-containing AMPA receptors downstream of NMDA receptor stimulation (Zhu et al., 2002). Importantly, the upregulated D-1 DA receptor stimulation-induced ERK phosphorylation in NAc of FR rats is blocked by the NMDA antagonist, MK-801 (Haberny and Carr, 2005). Consequently, the increased cocaine-induced phosphorylation of GluA1 on Ser845 observed in the present study may lead to increased synaptic delivery of GluA1-containing AMPA receptors which could play a role in the enhanced acquisition, expression, and/or resistance to extinction of the CPP in FR rats. Indeed, DNQX–a nonspecific AMPA receptor antagonist- microinjected into NAc prior to each cocaine conditioning session blocked the acquisition of CPP (Kaddis et al., 1995)
Acquisition and expression of psychostimulant-CPP appear to require activation of neurons in the amygdala basolateral complex that innervate NAc core and prelimbic mPFC (Miller and Marshall, 2005a), with critical involvement of cdk5 activity in basolateral amygdala (Li et al., 2010), along with convergent glutamatergic inputs to NAc originating in BDNF-regulated neurons of dHPC (Rademacher et al., 2006; Shen et al., 2006). Exposure of rats to a cocaine-paired environment is accompanied by increased phosphorylation of ERK1/2 and GluA1 (Ser845) in the dHPC (Topea et al., 2008), and reversible lesions of dHPC block the acquisition and expression of cocaine CPP (Meyers et al., 2006). Thus, the elevated levels of p-ERK1/2 and p-Ser845-GluA1 in dHPC of FR rats may contribute to development of a more robust CPP in FR relative to AL rats in this study. The elevation of p-ERK1/2 by FR in mPFC is of interest given a suggestion in the literature that mPFC is involved in the enhanced rewarding effect of cocaine and food in FR but not AL subjects. In AL rats lesions of prelimbic mPFC had no effect on acquisition or expression of cocaine CPP (Zavala et al., 2003) or progressive ratio responding for food (Moscarello et al., 2010). In FR rats, these lesions blocked acquisition of cocaine CPP (Tzschentke and Schmidt, 1999) and decreased progressive ratio responding (Moscarello et al., 2010). Thus, in addition to FR-induced elevation of p-ERK1/2 and p-Ser845-GluA1 in dHP, and cocaine-induced p-Ser845-GluA1 in NAc, the increased activation of ERK1/2 in mPFC of FR rats warrants follow-up investigation to identify mechanistic underpinnings of the enhanced CPP of FR rats to low dose cocaine.
The increased rewarding effect of cocaine in FR subjects (Bell et al., 1997; Carr et al., 2000) may be sufficient to explain the greater persistence of CPP. However, it is also possible that FR enhances associative learning in general, diminishes extinction learning and/or increases expression of conditioned incentive motivation. In future studies, confinement of FR to conditioning, expression and extinction phases may yield a more complete functional understanding of the effects of FR. Further, corresponding brain regional biochemical analyses followed by guided, localized pharmacological manipulations and/or use of molecular genetic tools may provide insight into mechanisms with potential translational significance.
4. Experimental Procedures
4.1 Subjects and Food Restriction
All subjects were mature male Sprague-Dawley rats (Taconic Farms, Germantown, NY) weighing 350–400 g at the start of the experiment. Rats were housed, under a 12 h light:dark photoperiod with lights on at 0700 h, in a central animal facility in individual plastic cages with bedding and free access to water and standard lab pellets (Laboratory Rodent Diet #5001, Lab Diet) except when restricted feeding conditions applied (see below). Experimental procedures were approved by the Institutional Animal Care and Use Committee at the New York University School of Medicine and were consistent with the Principles of Laboratory Animal Care (NIH Publication no. 85-23).
Following acclimation to the central animal facility, half of the rats were placed on a food restriction regimen in which daily food allotment was limited to 10 g, delivered to the home cage at 1700h, while the remaining subjects continued to have ad libitum access to food. This FR regimen continued until body weights decreased by 20% (approximately two weeks). Upon attaining the target body weight, animals' daily feeding was titrated to clamp body weight at the new value. Restricted feeding and body weight were maintained until the end of experimentation which, in Experiment 1, extended for an additional ~three weeks and, in Experiment 2, an additional ~ one week.
4.2 CPP Apparatus
An unbiased three-compartment apparatus was used. Each Lucite test chamber (61 × 30.5 × 30.5 cm) consisted of two side compartments (25.4 × 30.5 × 30.5 cm) separated by a small white center area with a smooth ceramic floor (10.2 × 30.5 × 30.5 cm). One side compartment had black walls with white horizontal stripes and a white grid floor composed of parallel metal rods (0.2 cm diameter mounted 1.0 cm apart) while the other side had white walls and a black wire mesh floor (1.3 × 1.3 cm squares). Removable partitions, matching the compartment walls, were used to close off each compartment. Automated data collection, including time spent in each compartment, and horizontal motor activity, was accomplished through 24 infrared photo-beam detectors along the length of the test chamber. Information about beam status, scanned at a rate of 100 times per second, was stored and later transformed into a complete record of each animal's activity (VersaMax System, Accuscan, Columbus, OH). Time spent in each compartment and horizontal activity (beam interruptions) during each session were the two main dependent measures. Pilot testing in several groups of rats revealed no unconditioned preference for one side of the apparatus versus the other.
4.3 Habituation, CPP Pre-Exposure, Conditioning and Testing
Before experimental testing began, all rats were habituated, on at least 6 occasions, to transport (along an interior corridor from animal facility to laboratory) and handling. On the first experimental day, each rat was pre-exposed to the CPP apparatus by being placed in the center compartment with partitions removed and allowed to move freely for 20 min. Time spent in each compartment was recorded and used as baseline preference data to confirm absence of an unconditioned bias for a particular compartment.
Each rat received a total of 10 conditioning sessions over ten consecutive days. Conditioning sessions were of 15 minute duration. During conditioning sessions, the removable partitions were in place and animals were confined to one of the side compartments. On alternate days, rats were injected with cocaine HCL (intraperitoneally, 7.0 mg/kg) immediately prior to placement in the cocaine-paired compartment. On the intervening days, rats received saline-vehicle injections (1.0 ml/kg, i.p.) before confinement to the opposite side compartment. Counterbalancing procedures were used to pair side compartments to cocaine or vehicle across subjects within each feeding group, with matching between feeding groups. The dose of cocaine administered was expected, based on preliminary tests and published studies (e.g., Bell et al., 1997; Zakharova et al., 2008), to be at or just below threshold in AL subjects and suprathreshold in FR subjects.
On test days, no injection was administered before placing each rat in the center compartment with partitions in place for 15 s. Partitions were subsequently removed and rats were allowed to move freely for 20 min. The first test for expression of place preference was conducted 48 hrs following the final conditioning session. Additional CPP tests were conducted on consecutive days until the CPP response was extinguished in at least one group. Extinction was defined as three consecutive CPP test sessions in which time spent on the cocaine-paired side did not differ from time spent on the saline-paired side, as determined by t-test. Forty eight hours later, rats received a priming injection of cocaine (7.0 mg/kg) immediately before being placed in the center compartment for a final CPP test.
4.4 Drugs
Cocaine HCL (NIDA; Research Triangle Institute) was dissolved in sterile 0.9% saline and injected systemically at a dose of 7.0 mg/kg.
4.5 Brain Regional Biochemical Correlates of CPP Conditioning
Groups of AL and FR subjects were prepared as described above through the first CPP conditioning session. Following completion of the first 15-min conditioning session, subjects were briefly exposed to CO2, decapitated by guillotine, and brains were extracted and rapidly frozen in powdered dry ice. 500-μm sections were cut using an IEC Minotome cryostat, and NAc, dHPC, and mPFC were dissected by micropunch, under an Olympus dissecting microscope. Tissue samples were then homogenized in 10 volumes of lysis buffer (320 mM Sucrose, 1 mM NaHCO3, 1 mM MgCl2, 0.5mM CaCl2, 1% SDS) with manufacturer recommended concentrations of Mammalian Protease Inhibitor Cocktail and Phosphatase Inhibitor Cocktails 1 and 2 (Sigma-Aldrich, St. Louis, MO), followed by centrifugation and protein determination using a BCA reagent kit as described by the manufacturer (Pierce, Rockford, IL). Aliquots of supernatants were stored at −80°C until use.
4.6 BDNF Immunoassay
Brain-derived neurotrophic factor protein levels were determined using the commercially available BDNF Emax® ImmunoAssay Systems (Promega, Madison, WI). The ELISAs were performed according to the manufacturer's protocol. On the day of immunoassay, sample aliquots were thawed and 10–20 μg protein was added to wells of a 96-well immunoplate precoated with mouse BDNF specific monoclonal antibody. The plate was incubated at room temperature for 2 h with shaking. The mouse anti-BDNF monoclonal antibody was used as the capture Ab and the anti-human BDNF pAb was used as reporter Ab. After washing, the amount of specifically bound pAb was detected using a species-specific anti-IgY antibody conjugated to horseradish peroxidase as a tertiary reactant. The unbound conjugate was removed by washing followed by incubation with a chromogenic substrate. Absorbance of samples was measured at 450 nm and read using OPTImax microplate reader (Molecular Devices Corp., Sunnyvale, CA). All samples were assayed in duplicate. The readings were normalized to the amount of protein applied.
4.7 Western Blotting
Aliquots of supernatants were mixed with 2X Laemmli SDS-PAGE sample buffer, heated for 5 min at 95°C, cooled on ice and centrifuged. Protein (10–30 μg per lane) was separated by electrophoresis on precast 4–12% polyacrylamide gels (Lonza, Walkersville, MD). Precision Plus protein standard molecular weight markers (Bio-Rad, Hercules, CA) were also loaded to assure complete electrophoretic transfer and to estimate the size of bands of interest. The gels were transferred to Protran® nitrocellulose membrane (Whatman, Mobile, AL) for 2 h, with a constant voltage of 100 V. Membranes were blocked for 1 h at room temperature with blocking buffer, 5% non-fat dry milk in 50 mM Tris–HCl, pH 7.5 containing 150 mM NaCl and 0.05% Tween 20 (TBS-T), then probed overnight at 4 °C using primary antibodies for target proteins or 2 h at room temperature using primary antibody for the protein loading control, α-tubulin. Antibodies used included mouse monoclonal anti-GluA1 (1: 2,000; MAB2263, Millipore, Temecula, CA), rabbit polyclonal anti-phospho-Ser845-GluA1 (1: 1,000; AB5849, Millipore, Temecula, CA), mouse monoclonal anti-phospho-(Thr202/Tyr204)-p44/42 ERK1/2 (1:1000; Cell Signaling, Beverly, MA), rabbit polyclonal anti-p42/44 ERK1/2 (1:2000; Cell Signaling, Beverly, MA), and mouse monoclonal anti-α-tubulin (1: 5,000; T6199, Sigma-Aldrich, St. Louis, MO).
After probing with primary antibodies and washing with TBS-T buffer (3 × 5 min), membranes were incubated with horseradish peroxidase conjugated anti-mouse/rabbit IgG (1:8000; Cell Signaling, Beverly, MA). Proteins were visualized using a chemiluminescence ECL kit (Pierce). Densitometric analysis of the bands was performed using the NIH Image J software. Values for target phospho-proteins were normalized to corresponding total protein and/or tubulin and expressed as percentage of the normalized control, which was the AL group injected with saline vehicle.
4.8 Data Analysis
CPP: (i) Acquisition of CPP, as expressed in the first post-conditioning drug-free test, was assessed by 2-way mixed ANOVA. (ii) By design, at least one diet group had met extinction criteria (see above) before the end of the ensuing series of test sessions. Persistence of CPP over drug-free test sessions was therefore assessed in individual diet groups by 2-way mixed ANOVA. (iii) CPP expression in the final test session, which was preceded by cocaine priming injection, was assessed by t-test.
Cocaine-Induced Locomotor Activity: Horizontal activity during cocaine conditioning trials was analyzed by 3-way mixed ANOVA.
ELISA: Measurement of BDNF protein was limited to saline-treated diet groups and differences between groups were evaluated by t-test.
Western blot: Results for p-ERK1, p-ERK2 and p-Ser845-GluA1 were assessed by 2-way ANOVA, followed by Tukey HSD tests where appropriate.
Acknowledgements
This research was supported by DA03956 and T32 DA007254 from NIDA/NIH.
ABBREVIATIONS
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- DA
dopamine
- BDNF
brain-derived neurotrophic factor
- ERK
extracellular signal-related kinase
- NAc
nucleus accumbens
- mPFC
medial prefrontal cortex
- dHPC
dorsal hippocampus
- FR
food-restriction, food-restricted
- AL
ad libitum fed
- CPP
conditioned place preference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar MA, Rodriguez-Arias M, Minarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev. 2009;59:253–277. doi: 10.1016/j.brainresrev.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MR, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacol. 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Krahne L, Carr KD. A progressive ratio schedule of self-stimulation testing reveals profound augmentation of d-amphetamine reward by food restriction but no effect of a “sensitizing” regimen of d-amphetamine. Psychopharmacol. 2004;175:106–113. doi: 10.1007/s00213-003-1768-4. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Solinas M, Valentini V, Di Chiara G. Selective psychostimulant sensitization by food restriction: differential changes in accumbens shell and core dopamine. Eur J Neurosci. 2003;18:2326–2334. doi: 10.1046/j.1460-9568.2003.02941.x. [DOI] [PubMed] [Google Scholar]
- Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. In: Liebman JM, Cooper SJ, editors. The Neuropharmacological Basis of Reward. Clarendon Press; Oxford: 1989. pp. 264–319. [Google Scholar]
- Carr KD, Cabeza de Vaca S, Sun Y, Chau LS, Pan Y. Effects of the MEK inhibitor, SL-327, on rewarding, motor- and cellular-activating effects of d-amphetamine and SKF-82958, and their augmentation by food restriction. Psychopharmacol. 2009a;201:495–506. doi: 10.1007/s00213-008-1313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Cabeza de Vaca S, Sun Y, DeCarlo LR, Chau LS. Reward-potentiating effects of D-1 dopamine receptor agonist and AMPA GluR1 antagonist in nucleus accumbens shell are increased by food restriction; possible relevance to enhancement of adaptive and maladaptive reward-directed behavior. Psychopharmacol. 2009b;202:731–743. doi: 10.1007/s00213-008-1355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Chau LS, Cabeza de Vaca S, Gustafson K, Stouffer M, Tukey D, Restituito S, Ziff E. AMPA receptor subunit GluR1 downstream of D-1 dopamine receptor stimulation in nucleus accumbens shell mediates increased drug reward magnitude in food-restricted rats. Neurosci. 2010;165:1074–1086. doi: 10.1016/j.neuroscience.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Kim G-Y, Cabeza de Vaca S. Chronic food restriction augments the central rewarding effect of cocaine and the δ-1 opioid agonist, DPDPE, but not the δ-2 agonist, deltorphin-II. Psychopharmacol. 2000;152:200–207. doi: 10.1007/s002130000523. [DOI] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neurosci. 2003;119:1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205:319–321. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- Childress A, Ehrman R, McLellan AT, O'Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 1988;81:74–80. [PubMed] [Google Scholar]
- Cochrane C, Malcolm R, Brewerton T. The role of weight control as a motivation for cocaine abuse. Addict Behav. 1998;23:201–7. doi: 10.1016/s0306-4603(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Dopamine in disturbances of food and drug motivated behavior: A case of homology? Physiol Behav. 2005;86:9–10. doi: 10.1016/j.physbeh.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Duan W, Lee J, Guo Z, Mattson MP. Dietary restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury. J Mol Neurosci. 2001;16:1–12. doi: 10.1385/JMN:16:1:1. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiat. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacol. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- Gerdjikov TV, Ross GM, Beninger RJ. Place preference induced by nucleus accumbens amphetamine is impaired by antagonists of ERK or p38 MAP kinases in rats. Behav Neurosci. 2004;118:740–750. doi: 10.1037/0735-7044.118.4.740. [DOI] [PubMed] [Google Scholar]
- Girault J-A, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nature Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Haberny S, Berman Y, Meller E, Carr KD. Chronic food restriction increases D-1 dopamine receptor agonist-induced ERK1/2 MAP Kinase and CREB phosphorylation in caudate-putamen and nucleus accumbens. Neurosci. 2004;125:289–298. doi: 10.1016/j.neuroscience.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. Food restriction increases NMDA receptor-mediated CaMK II and NMDA receptor/ERK 1/2-mediated CREB phosphorylation in nucleus accumbens upon D-1 dopamine receptor stimulation in rats. Neurosci. 2005;132:1035–1043. doi: 10.1016/j.neuroscience.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddis FG, Uretsky NJ, Wallace LJ. DNQX in the nucleus accumbens inhibits cocaine-induced conditioned place preference. Brain Res. 1995;697:76–82. doi: 10.1016/0006-8993(95)00786-p. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesges RC, Elliott VE, Robinson LA. Chronic dieting and the belief that smoking controls body weight in a biracial, population-based adolescent sample. Tobbac Control. 1997;6:89–94. doi: 10.1136/tc.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn D, Kurth C, Demitrack M, Drewnowski A. The relationship of dieting severity and bulimic behaviors to alcohol and other drug use in young women. J Substance Abuse. 1992;4:341–353. doi: 10.1016/0899-3289(92)90041-u. [DOI] [PubMed] [Google Scholar]
- Lebrun B, Bariohay B, Moyse E, Jean A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: a minireview. Auton Neurosci. 2006;126–127:30–38. doi: 10.1016/j.autneu.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Long JM, Ingram DK, Mattson MP. Dietary restriction increases survival of newly-generated neural cells and induces BDNF expression in the dentate gyrus of rats. J Mol Neurosci. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- Li FQ, Xue YX, Wang JS, Fang Q, Li YQ, Zhu WL, He YY, Liu JF, Xue LF, Shaham Y, Lu L. Basolateral amygdala cdk5 activity mediates consolidation and reconsolidation of memories for cocaine cues. J Neurosci. 2010;30:10351–10359. doi: 10.1523/JNEUROSCI.2112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pagès G, Valverde O, Marowsky A, Porrazzo A, Orban PC, Maldonado R, Ehrengruber MU, Cestari V, Lipp HP, Chapman PF, Pouysségur J, Brambilla R. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Speer CM, Neisewander JL. Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci. 2006;120:401–412. doi: 10.1037/0735-7044.120.2.401. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered Fos expression in neural pathways underlying cueelicited drug seeking in the rat. Eur J Neurosci. 2005a;5:1385–1393. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005b;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Moscarello JM, Ben-Shahar O, Ettenberg A. External incentives and internal states guide goal-directed behavior via the differential recruitment of the nucleus accumbens and the medial prefrontal cortex. Neurosci. 2010;170:468–477. doi: 10.1016/j.neuroscience.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Narasimhaiah R, Kamens HM, Picciotto MR. Effects of galanin on cocaine-mediated conditioned place preference and ERK signaling in mice. Psychopharmacol. 2009;204:95–102. doi: 10.1007/s00213-008-1438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiat. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drugdependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Orsini C, Bonito-Oliva A, Conversi D, Cabib S. Genetic liability increases propensity to prime-induced reinstatement of conditioned place preference in mice exposed to low cocaine. Psychopharmacol. 2008;198:287–296. doi: 10.1007/s00213-008-1137-4. [DOI] [PubMed] [Google Scholar]
- Peng X-X, Carr KD, Ziff EB. Effects of food restriction and sucrose intake on synaptic delivery of AMPA receptors in nucleus accumbens. Synapse. 2011 doi: 10.1002/syn.20931. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-intralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bari AA. The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev Neurosci. 2001;12:95–110. doi: 10.1515/revneuro.2001.12.2.95. [DOI] [PubMed] [Google Scholar]
- Pisetsky EM, Chao YM, Dierker LC, May AM, Striegel-Moore RH. Disordered eating and substance use in high-school students: results from the Youth Risk Behavior Surveillance System. Int J Eat Disord. 2008;41:464–470. doi: 10.1002/eat.20520. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–6650. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher DJ, Kovacs B, Shen F, Napier TC, Meredith GE. The neural substrates of amphetamine conditioned place preference: implications for the formation of conditioned stimulus-reward associations. Eur J Neurosci. 2006;24:2089–2097. doi: 10.1111/j.1460-9568.2006.05066.x. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root TL, Pinheiro AP, Thornton L, Strober M, Fernandez-Aranda F, Brandt H, Crawford S, Fichter MM, Halmi KA, Johnson C, Kaplan AS, Klump KL, La Via M, Mitchell J, Woodside DB, Rotondo A, Berrettini WH, Kaye WH, Bulik CM. Substance use disorders in women with anorexia nervosa. Int J Eat Disord. 2010;43:14–21. doi: 10.1002/eat.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzschild MA, Cole RL, Meyers MA, Hyman SE. Contrasting calcium dependencies of SAPK and ERK activations by glutamate in cultured striatal neurons. J Neurochem. 1999;72:2248–2255. doi: 10.1046/j.1471-4159.1999.0722248.x. [DOI] [PubMed] [Google Scholar]
- Seo D-C, Jiang N. Associations between smoking and severe dieting among adolescents. J Youth Adolesc. 2009;38:1364–1373. doi: 10.1007/s10964-009-9421-0. [DOI] [PubMed] [Google Scholar]
- Shen F, Meredith GE, Napier CT. Amphetamine-induced place preference and conditioned motor sensitization requires activation of tyrosine kinase receptors in the hippocampus. J Neurosci. 2006;26:11041–11051. doi: 10.1523/JNEUROSCI.2898-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Davis K, Miller NP, Marti NC. Fasting increases risk for onset of binge eating and bulimic pathology: A 5-year prospective study. J Abnorm Psychol. 2008;117:941–946. doi: 10.1037/a0013644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Evans SB, Higgins MS, Pu Y, Figlewicz DP. Food restriction modulates amphetamine-conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse. 2002;46:83–90. doi: 10.1002/syn.10120. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Piyis YK, Wang G-J, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo μPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62:50–61. doi: 10.1002/syn.20468. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signaling and synaptic plasticity. Nature Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tropea TF, Kosofsky BE, Rajadhyaksha AM. Enhanced CREB and DARPP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. J Neurochem. 2008;106:1780–1790. doi: 10.1111/j.1471-4159.2008.05518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci. 1999;11:4099–4109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault J-A, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Phil Trans Royal Soc Brit. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederman MW, Pryor T. Substance abuse and impulsive behaviors among adolescents with eating disorders. Addictive Behavior. 1996;21:269–272. doi: 10.1016/0306-4603(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Wolf ME. Regulation of AMPA receptor trafficking in the nucleus accumbens by dopamine and cocaine. Neurotox Res. 2010;184:393–409. doi: 10.1007/s12640-010-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Weber SM, Rice HJ, Alleweireldt AT, Neisewander JL. Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction, and reinstatement of cocaine-conditioned place preference. Brain Res. 2003;990:157–164. doi: 10.1016/s0006-8993(03)03452-8. [DOI] [PubMed] [Google Scholar]
- Zhen J, Reith MEA, Carr KD. Chronic food restriction and dopamine transporter function in rat striatum. Brain Res. 2006;1082:98–101. doi: 10.1016/j.brainres.2006.01.094. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aeist L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]