Abstract
Background and Purpose
Subcortical ischemic vascular disease (SIVD) is a major form of vascular cognitive impairment (VCI) due to small vessel disease. Matrix metalloproteinases (MMPs) are neutral proteases that disrupt the blood-brain barrier (BBB) and degrade myelin basic protein under conditions of neuroinflammation. Brain tissues and CSF of patients with VCI have increased levels of MMPs. We hypothesized that patients with SIVD have increased MMPs in the CSF, which are associated with increased CSF albumin.
Methods
We studied 60 patients with suspected VCI. Twenty-five were classified as SIVD, while other groups included mixed Alzheimer’s disease AD and VCI (MX), multiple strokes (MI), and leukoaraiosis (LA) when white matter lesions were present and the diagnosis of VCI was uncertain. MMP-2 and MMP-9 in CSF and plasma were measured by gel zymography and indexed to CSF and plasma albumin. MMP-3 activity was measured by fluorescent assay.
Results
We found reduced MMP-2 index (p<0.001) in the CSF for the full group of patients (SIVD, MI, MX and LA) compared to controls, whose CSF was obtained during spinal anesthesia. MMP-3 activity was increased in VCI compared to controls (p<0.01). In SIVD, MMP-2 index showed a negative correlation with Qalb, which was absent with the MMP-9 index. Combining MMP-2 index and MMP-3 activity separated the SIVD patients from the controls with high specificity (p<0.0005).
Conclusions
Our results support the hypothesis that MMPs are associated with increased CSF albumin and suggest that they may contribute to the pathophysiology of SIVD.
Introduction
Subcortical ischemic vascular disease (SIVD), which is the small vessel form of vascular cognitive impairment (VCI), is a major cause of dementia in the elderly 1. The characteristic features of SIVD are focal neurological findings, gait imbalance, neuropsychological dysfunction, and large white matter hyperintensities (WMHs) on MRI 2. Arteriolosclerosis of the small vessels in the deep white matter with demyelination and lacunar strokes are the pathological hallmarks 3. As opposed to the large vessel form of VCI, the onset is usually insidious and challenging to separate from other forms of neurodegeneration, such as Alzheimer’s disease 4. Many investigators are searching for biomarkers that can aid in the early diagnosis of SIVD since it is considered to be the optimal form of VCI for treatment trials 5. There is growing evidence from other investigators and us that there is disruption of the blood-brain barrier (BBB) in the white matter 6, 7. We have proposed that the BBB damage is due to the induction of matrix metalloproteinases (MMPs) by hypoxic/ischemic injury 8. Normally, MMPs are present in the brain in latent forms that are activated to remodel the extracellular matrix 9. However, when they are induced and activated under conditions of hypoxia, they can disrupt the basal lamina and tight junctions of the cerebral blood vessels,10 and degrade myelin basic protein 11.
Autopsy studies have demonstrated expression of gelatinase A (MMP-2), gelatinase B (MMP-9) and stromelysin-1 (MMP-3) in brains of patients with white matter lesions from stroke, multiple sclerosis and vascular dementia 12–15. In an earlier study of MMPs in cerebrospinal fluid (CSF) in VCI patients, we observed an increase in MMP-9 16. However, the MMPs were only measured in the CSF and some of the MMP-9 in the CSF may have come from the blood, particularly if the BBB was disrupted. Using albumin, which is produced in the liver, as an index marker for BBB permeability, it is possible to separate endogenous from exogenous MMP production. By indexing the MMPs in the brain and blood compartments to albumin in both, in a manner similar to the use of the IgG index in multiple sclerosis, it is possible to determine intrathecal synthesis of the enzymes 17. Therefore, we determined a MMP index for MMP-2 and MMP-9 by measuring the levels in blood and CSF and forming the MMP index. We hypothesized that MMPs were produced in the CSF compartment in patients with SIVD, but not in the patients with other forms of VCI. Furthermore, we used a newly developed fluorescent immunocapture assay to measure the levels of active MMP-3.
Subjects and Methods
Subjects
Sixty patients with suspected VCI were entered into the study. These patients were part of a larger study of multiple parameters to select the optimal biomarkers for SIVD. This report describes the results of the CSF biomarker studies in a subgroup of 45 patients that had a lumbar puncture as part of the evaluation. Patients were referred to one of the study neurologists (JA, EE, GR) for evaluation and were seen in the Neurology Clinics at the University of New Mexico Hospital and the Albuquerque Veterans Administration Hospital. After obtaining informed consent, they were enrolled in the study. All aspects of the study were conducted in compliance with the regulations of the University of New Mexico Human Research Review Committee (HRRC) and the Albuquerque Veterans Hospital Research Committee. Patients had neurological and neuropsychological testing, lumbar puncture for collection of CSF, and MRI. Imaging studies always preceded lumbar puncture. Study procedures were completed within two contiguous days.
CSF was analyzed for routine studies (cells, protein, glucose, culture) and blood was collected for calculation of a demyelinating profile and albumin index (Qalb=Albumin in CSF/Albumin in plasma). CSF was collected from 20 controls that had spinal anesthesia for non-neurological conditions.
Diagnoses
Patients entered into the study were referred because of WMHs on MRI, cognitive complaints, and suspected VCI. One patient had dementia, but the others were suspected to have VCI-no dementia 4. We separated patients clinically into several diagnostic subgroups of VCI based on the results of the neurological, neuropsychological, and anatomical MRI findings without knowledge of CSF results (Supplemental Table 1). Clinical categories included: 1) subcortical ischemic vascular disease (SIVD) when small vessel disease was suspected in the presence of focal findings and significant WMHs on MRI; 2) lacunar or large vessel infarcts (MI) when they were evident on the MRI and accompanied by hemiparesis; 3) mixed vascular and AD (MX) for those with symmetric periventricular WMHs and dominant memory impairment; and 4) a group of with leukoaraiosis (LA) whose diagnosis was uncertain1. Although patients had diagnoses made by two neurologists with expertise in VCI (JA and GR), they remain provisional without autopsy verification. Only two patients have died and undergone autopsy, which confirmed the diagnosis of MI in one and MX in another.
All patients had medical evaluations to exclude other causes of white matter disease and cognitive impairment. In all patients with CSF studies, the demyelinating profile revealed no oligoclonal bands, reducing the likelihood of including patients with multiple sclerosis. Volume measurements of the WMHs were made by manually tracing the white matter lesions on FLAIR MRI and summing the volumes from each slice.
Gelatin-Substrate Zymography of CSF and Plasma
CSF and plasma were collected at the same time. The levels of MMP-2 and MMP-9 were measured in the CSF and plasma by gelatin-substrate zymography as described previously 18. CSF samples (10 μL) were mixed with an equal volume of loading buffer (62.5 mM Tris -HCl pH 6.8, 10% glycerol, 2% SDS and 0.00125% bromophenol blue). Plasma samples were diluted 1:30 with distilled water and 10 μL of the diluted sample was mixed with 10 μL of loading buffer and applied to the zymography gels. After electrophoresis, gels were washed in 2.5% Triton X-100 to remove the SDS and then incubated for 96 h at 37°C in a developing buffer containing 50 mM Tris pH 7.6, 5 mM CaCl2, 0.2 mM NaCl, and 0.02% Brij-35. Gels were stained with 0.125% Coomassie Brilliant Blue R-250 (Sigma, Saint Louis, MO) for 30 min in 10% acetic acid and 50% methanol. Gels were destained with a solution containing 10% acetic acid until clear bands of gelatinolysis appeared on a dark blue background. Dried gels were scanned and analyzed using AlphaEase software (Alpha Innotech, San Leandro, CA). Media from HT1080 fibrosarcoma cells served as positive controls for human MMP-2 and MMP-9.
Immunocapture Assay and Fluorometric Measurement of MMP-3 Enzymatic Activity
The activity of MMP-3 (stromelysin-1) in CSF and plasma was measured fluorometrically using a 5-FAM/QXL™ 520 fluorescence resonance energy transfer (FRET) peptide (60580; AnaSpec, San Jose, CA). In the intact FRET peptide (5-FAM-Arg-Pro-Lys-Pro-Val-Glu-Nva-Trp-Arg-Lys(QXL 520)-NH2), the fluorescence of 5-FAM (5-carboxyfluorescein) is quenched by QXL™ 520. Upon cleavage into two separate fragments by MMP-3, the fluorescence of 5-FAM is recovered, and can be monitored at excitation/emission wavelengths of 490/520 nm. This peptide has been documented to be cleaved by only MMP-3 and MMP-12, but not by other MMPs 19.
Before conducting the activity assay, the MMP-3 present in the sample was immunocaptured utilizing a rabbit anti-MMP-3 antibody (sc-6839-R, Santa Cruz Biotechnology) to ensure the specificity of the assay for MMP-3. Samples of CSF (50 μL) and plasma (20 μL) were mixed in 1 mL of TCNB buffer (50 mM Tris, 10 mM CaCl2, 150 mM NaCl, 0.05% Brij-35) with 2 μg of the immunocapture antibody at 4 °C for 2 h in a rocker. Twenty microliters of Protein A/G beads (sc-2003, Santa Cruz Biotechnology) were then added to the tubes and samples were incubated overnight at 4°C with gentle rocking. Samples were centrifuged at 1000 ×g for 5 min at 4°C and supernatant was discarded. Beads were washed twice with 500 μL of TCNB buffer. After the last wash, 100 μL of assay buffer (50 mM Tris -HCl pH 7.6, 200 mM NaCl, 5 mM CaCl2, 20 μM ZnSO 4 and 0.05% Brij-35) was added and mixed thoroughly with the beads. Samples were transferred to a black 96-well plate and 100 μL of as say buffer containing 2 μM of the FRET peptide was added. Fluorescence was measured after 8 h of incubation at 37°C and expressed as Relative Fluorescence Units (RFU) after subtraction of the readings for the substrate control (without sample) from the RFU values of the samples.
Statistical Methods
Statistical analyses for between-group differences for neuropsychological tests and clinical history were done using SPSS (SPSS for Windows, 16.0.1). One-way ANOVAs and Chi-Square analyses were conducted as appropriate based on continuous or categorical data. For the BBB and Qalb data, we determined statistical significance with nonparametric t-tests or one-way analysis of variance (Kruskal-Wallis with Dunn’s corrections for multiple comparisons), and with Spearman rank nonparametric correlations or linear correlations, using Prism 5 for Mac (GraphPad, La Jolla, CA). Statistical significance was set at the p<0.05. The data was represented as mean ± standard error of the mean (SEM).
Results
The numbers of patients in each subgroup is shown in Supplemental Table 1. Nineteen patients were classified as SIVD with smaller numbers in the other groups. Prevalence of hypertension was significantly increased in all groups except LA; hyperreflexia and stroke were highest in the MI group; and SIVD patients had the largest volume of WMHs (Table 1). Executive function was lowest in the SIVD group, and memory function worse in the MX group, but neither was statistically significant (Table 2). Clinical information on the 20 control subjects is shown in Supplemental Table 2.
Table 1.
Clinical Information on the patients in the groups identified by Diagnosis (see Text)
| SIVD | MI | MX | LA | Statistics | |
|---|---|---|---|---|---|
| Male Sex (%) | 9 (48%) | 6 (67%) | 6 (100%) | 2 (25%) | χ2(3) = 8.75, p = 0.03 |
| Hypertension | 10 (53%) | 5 (56%) | 4 (67%) | 0 (0%) | χ2(3) = 8.53, p = 0.04 |
| Diabetes | 5 (26%) | 1 (11%) | 2 (33%) | 1 (13%) | χ2(3) = 1.72, n.s. |
| Hyperreflexia | 16 (84%) | 8 (89%) | 3 (50%) | 5 (63%) | χ2(3) = 4.57, n.s. |
| Imbalance | 18 (95%) | 7 (78%) | 4 (67%) | 3 (38%) | χ2(3) = 10.52, p = 0.02 |
| Stroke History | 9 (47%) | 9 (100%) | 1 (17%) | 1 (13%) | χ2(3) = 16.16, p < 0.01 |
| Lesion Size | 0.12 (0.13) | 0.05 (0.08) | 0.06 (0.04) | 0.02 (0.03) | F(3,37) =2.63, p = 0.07 |
Table 2.
Neuropsychological test results for patients in the groups identified by diagnosis
| SIVD | MI | MX | LA | Statistics | |
|---|---|---|---|---|---|
| MMSE | 27.28 (2.76) | 28.12 (2.03) | 24.40 (4.45) | 27.38 (2.50) | F(3,35) = 1.91, n.s. |
| Executive function † | 41.29 (7.49) | 43.00 (9.84) | 41.33 (6.02) | 44.88 (8.32) | F(3,35) = 0.41, n.s. |
| Memory† | 41.82 (13.62) | 38.75 (11.67) | 34.33 (8.17) | 46.12 (8.22) | F(3,35) = 1.31, n.s. |
| Language† | 43.88 (9.64) | 42.12 (4.67) | 37.17 (8.33) | 49.38 (10.17) | F(3,35) = 2.30, n.s. |
| Attention† | 41.82 (7.34) | 42.88 (5.41) | 40.00 (6.96) | 42.62 (4.34) | F(3,43) = 0.27, n.s. |
Mean (SD);
Standardized T-Scores
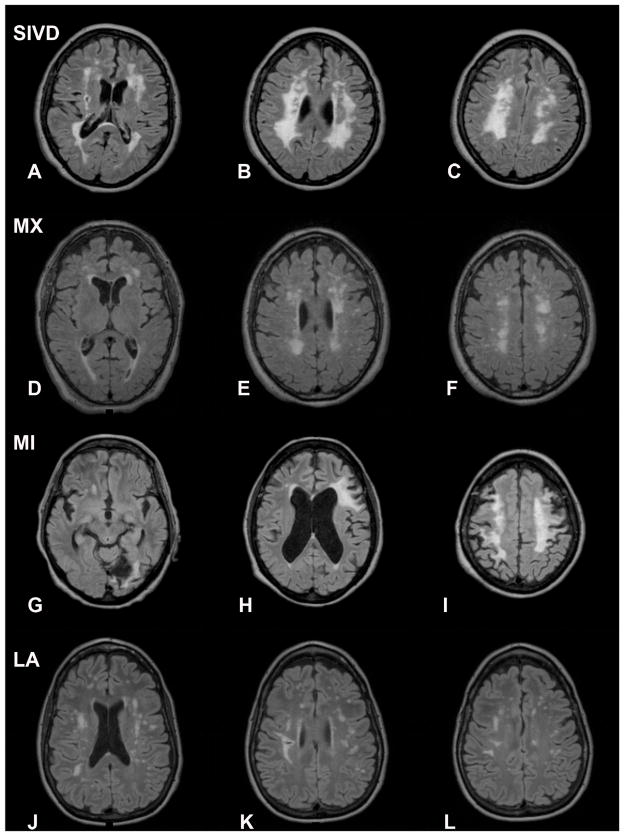
A representative MRI of a patient with SIVD shows extensive white matter changes with lacunar strokes (FIGURE 1A–C). Symmetric periventricular lesions are seen in a MX patient (FIGURE 1D–F). MRI in a MI patient shows a large stroke with white matter changes (FIGURE 1G–I). A patient in the LA group shows scattered WMHs, but an uncertain diagnosis (FIGURE 1J–L). However, the MRI was not diagnostic, particularly in the MX or LA groups.
Figure 1.
FLAIR MRI scans from representative patients in the different subgroups. A–C) Patients in the subcortical ischemic vascular disease (SIVD) group show extensive white matter hyperintensities (WMHs) in a relatively symmetric distribution. D–F) Mixed VCI and AD (MX) patients have WMHs that are also symmetric. G–I) Multiple infarct (MI) patients have asymmetric lesions consistent with strokes. J–L) Leukoaraiosis (LA) patients have different patterns of WMHs that are difficult to characterize.
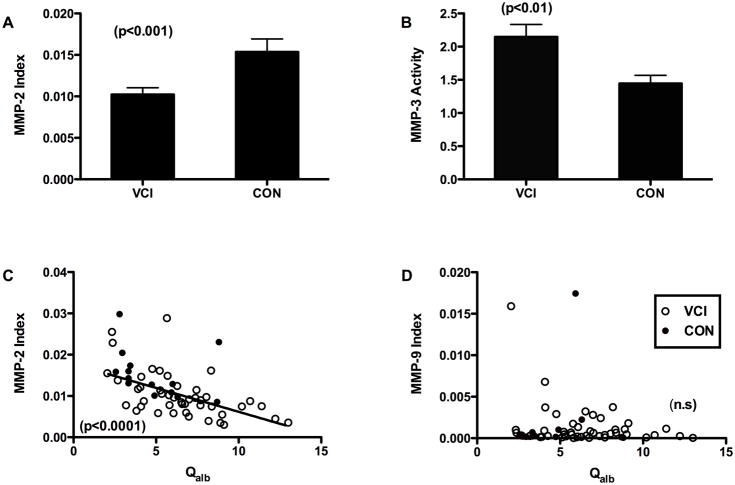
To answer the first question of the relationship of the MMP indexes to Qalb, we compared all patients in the VCI group, including those with LA that had both measurements completed. The MMP-2 index was significantly lower when the full group was compared to the controls (FIGURE 2A). Similarly MMP-3 activity was higher in VCI than in controls (FIGURE 2B). There was a negative correlation between the MMP-2 index and the albumin index (FIGURE 2C), but not in the MMP-9 index (FIGURE 2D).
Figure 2.
Cerebrospinal fluid values for the MMP-2 index and MMP-3 activity. A) MMP-2 index for VCI and controls. The MMP-2 index for the VCI group was significantly lower than controls. B) MMP-3 activity is increased compared to controls (p<0.01). Significance levels are shown in parentheses. C) MMP-2 index values for all VCI patients (open circles) and controls (black dots) are plotted against albumin index (Qalb). There was a significant negative correlation (p<0.0001). D) MMP-9 index plotted similarly failed to show a correlation.
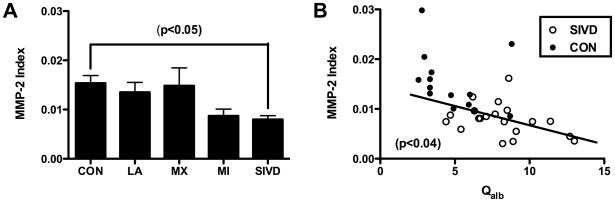
Analyzing the MMP-2 index for the different diagnostic groups, we found a significant difference between the controls and the SIVD patients (p<0.05) (FIGURE 3A). Comparing the SIVD group with Qalb improved the separation between patients and controls, and showed a negative correlation between the MMP-2 index and Qalb for the SIVD patients (p<0.04) (FIGURE 3B).
Figure 3.
A) MMP-2 index values plotted for each of the subgroups. Control values were mainly above 0.01, while the SIVD group was significantly lower (Kruskal-Wallis with Dunn’s correction; p<0.05) B) MMP-2 index showed a linear correlation with Qalb for SIVD patients (p<0.04).
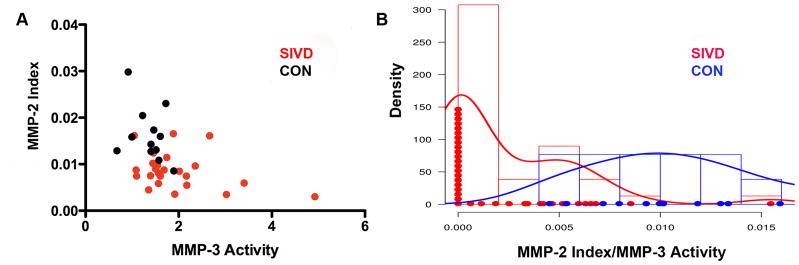
Since we hypothesized that MMPs would be associated with WMHs in the SIVD group, we plotted MMP-2 index against MMP-3 activity for the SIVD and control groups. A scatter plot showed the clustering in different quadrants of the SIVD and control values (FIGURE 4A). Controls had high MMP-2 index and low MMP-3 activity placing them in one quadrant of the graph. On the other hand, SIVD patients had low MMP-2 index and high MMP-3 activity and were mainly clustered in another quadrant. In order to determine the statistical significance of the clusters, we formed the ratio of MMP-2 index to MMP-3 activity for the SIVD and controls. The histogram of MMP-2 index/MMP-3 activity for controls and SIVD patients shows that these two groups could be separated with the mean of controls located at 0.00234 and mean of SIVD located at 0.01232 (p<0.0005) (FIGURE 4B).
Figure 4.
Relationship of MMP-2 index to MMP-3 activity for the SIVD and control groups. A) Scatter plot of MMP-2 index and MMP-3 activity shows that the controls clustered in the high MMP-2 index and low MMP-3 activity quadrant (black dots). The SIVD group clustered in the low MMP-2 index and high MMP-3 activity (red dots). B) A density plot of the ratio of MMP-2 index to MMP-3 activity shows that the two groups could be separated based on their means with a p-value of 0.0005.
Discussion
We found a reduction in the MMP-2 index and an increase in MMP-3 activity in CSF of VCI patients. Albumin index, which is a marker of blood-brain barrier (BBB) damage showed a significant correlation with the MMP-2 index, suggesting an association between the reduction in MMP-2 and opening of the BBB. We identified an active form of MMP-3 in CSF, using a sensitive immunocapture method for the first time in CSF. The ratio of MMP-2 index and MMP-3 activity separated the SIVD group from the controls. Our results support the hypothesis that MMPs are associated with white matter damage, particularly in SIVD.
Pathological studies in patients with SIVD often show loss of myelin and small vessel infarcts that are associated with fibrosis of blood vessels secondary to arteriolosclerosis. Tissue hypoxia is suggested to occur secondary to damaged blood vessels with hypoxic hypoperfusion 20–22. In SIVD, demyelination is often seen around vessels with evidence of leakage across a disrupted BBB 23–25. Hypoxic conditions initiate an inflammatory reaction with the expression of MMPs, which could both contribute to the opening of the BBB and breakdown of myelin.
In our earlier study measuring MMPs in CSF of VCI patients, we found MMP-9 was elevated 16. However, in this study, MMP-9 index failed to show a significant increase over the controls. In the earlier study, which was done with CSF measurements alone, the contribution to the CSF of MMPs in the blood was unknown. Since proteins measured in the CSF could be transferred from the blood into the CSF through a damaged BBB, which is known to be present in VCI, the use of an index is more accurate 17. Another explanation for the differences between the two studies could be the more advanced disease in the earlier series with many of the patients in the vascular dementia category while only one in the current series had that designation.
We found that the MMP-2 index, which was calculated similarly to IgG index in multiple sclerosis, was lower in the patients with VCI as a whole compared to controls and was lowest in the SIVD subgroup. A reduction in MMP-2 was not observed in the CSF in the earlier study. This could have been because the amount of MMP-2 in the CSF is much larger than MMP-9, and small changes could have been missed.
The measurement of MMP-2 in the CSF and plasma was done by zymography, which detects the 72-kDa latent form of the enzyme. There are two possible explanations that could explain the decrease in the latent form of the enzyme. On the one hand, activation of MMP-2 could deplete the latent form. Another possibility is that the astrocytes may be damaged or dying, resulting in a reduction in the production of the latent form. Resolution of this issue is not possible at this time.
Neither MMP-2 index nor MMP-3 activity clearly separates patients and controls, suggesting that these are not biomarkers themselves. However, the relationship between MMP-2 index and the albumin index shows an association between the levels of MMPs and the disruption of the BBB. We propose that elevated MMPs could contribute both to BBB opening and demyelination. The main biomarker for SIVD appears to be the MMP-2 index, which was consistently lower in SIVD compared to the other groups and controls. Astrocytes are a major source of MMP-2, and white matter is gliotic in SIVD. The MMP-3 activity was more variable. Macrophages and pericytes express MMP-3 around blood vessels in regions of demyelination in VCI brains, but the source of MMP-3 is uncertain 15.
The MRI scans aided along with clinical features in identifying the SIVD patients. SIVD produces a pattern of white matter damage in some patients that suggest gradual growth of existing lesions 26. It is unlikely that such a pattern is the result of a series of silent strokes that occur in both hemispheres in a relatively symmetric fashion with sparing of the U-association fibers. We propose that the MMPs participate in an on-going inflammatory reaction with gliosis and macrophage recruitment, responding to hypoxia and blood vessel fibrosis. Although the data support a link between MMPs and BBB disruption, the overlap of the data from the patients and controls precludes its use alone as a biomarker. We propose that hypoxia to deep white matter is a mechanism that could explain progressive growth of relatively symmetric lesions in elderly patients many of whom have compromised circulation secondary to diseases that cause narrowing of the blood vessels, such as hypertension and diabetes.
Our data is only for one time period and cannot be used to determine causality. We cannot exclude the possibility in some patients that sensitized, fibrotic blood vessels form small thrombotic lesions, leading to the formation of a series of small silent strokes in multiple locations. In that situation, the neuroinflammatory response involving MMP production may be secondary to the multiple strokes. Longitudinal studies in larger populations will be necessary to resolve this important question.
Supplementary Material
Acknowledgments
Patient studies were done in the General Clinical Research Center at the University of New Mexico.
Sources of Funding
This work was supported by a grant from the NIH to GAR (R01 NS052305) and by a grant from Bayer Pharmaceutical Corporation.
Footnotes
Disclosures
ECJ: Collaborator on grant R01 NS052305; JT: None; ST: Collaborator on grant R01 NS052305; MG: None; JA: None; EE: None; JP: None; JW; None; GR: Principal investigator on grant R01 NS052305 as well as on a research grant from Bayer Pharmaceutical Corporation.
References
- 1.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 2.Caplan LR. Binswanger’s disease--revisited. Neurology. 1995;45:626–633. doi: 10.1212/wnl.45.4.626. [DOI] [PubMed] [Google Scholar]
- 3.Olszewski J. Subcortical arteriosclerotic encephalopathy. Review of the literature on the so-called Binswanger’s disease and presentation of two cases. World Neurol. 1962;3:359–375. [PubMed] [Google Scholar]
- 4.Bowler JV. Modern concept of vascular cognitive impairment. Br Med Bull. 2007;83:291–305. doi: 10.1093/bmb/ldm021. [DOI] [PubMed] [Google Scholar]
- 5.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 6.Hanyu H, Asano T, Tanaka Y, Iwamoto T, Takasaki M, Abe K. Increased blood-brain barrier permeability in white matter lesions of Binswanger’s disease evaluated by contrast-enhanced MRI. Dement Geriatr Cogn Disord. 2002;14:1–6. doi: 10.1159/000058326. [DOI] [PubMed] [Google Scholar]
- 7.Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Munoz Maniega S, Farrall A, Sudlow C, Dennis M, Dhillon B. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194–202. doi: 10.1002/ana.21549. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009;8:205–216. doi: 10.1016/S1474-4422(09)70016-X. [DOI] [PubMed] [Google Scholar]
- 9.Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 11.Chandler S, Coates R, Gearing A, Lury J, Wells G, Bone E. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995;201:223–226. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- 12.Maeda A, Sobel RA. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996;55:300–309. doi: 10.1097/00005072-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Clark AW, Krekoski CA, Bou SS, Chapman KR, Edwards DR. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett. 1997;238:53–56. doi: 10.1016/s0304-3940(97)00859-8. [DOI] [PubMed] [Google Scholar]
- 14.Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–415. [PubMed] [Google Scholar]
- 15.Rosenberg GA, Sullivan N, Esiri MM. White matter damage is associated with matrix metalloproteinases in vascular dementia. Stroke. 2001;32:1162–1168. doi: 10.1161/01.str.32.5.1162. [DOI] [PubMed] [Google Scholar]
- 16.Adair JC, Charlie J, Dencoff JE, Kaye JA, Quinn JF, Camicioli RM, Stetler-Stevenson WG, Rosenberg GA. Measurement of gelatinase B (MMP-9) in the cerebrospinal fluid of patients with vascular dementia and Alzheimer disease. Stroke. 2004;35:e159–e162. doi: 10.1161/01.STR.0000127420.10990.76. [DOI] [PubMed] [Google Scholar]
- 17.Liuzzi GM, Trojano M, Fanelli M, Avolio C, Fasano A, Livrea P, Riccio P. Intrathecal synthesis of matrix metalloproteinase-9 in patients with multiple sclerosis: implication for pathogenesis. Mult Scler. 2002;8:222–228. doi: 10.1191/1352458502ms800oa. [DOI] [PubMed] [Google Scholar]
- 18.Mandler RN, Dencoff JD, Midani F, Ford CC, Ahmed W, Rosenberg GA. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in cerebrospinal fluid differ in multiple sclerosis and Devic’s neuromyelitis optica. Brain. 2001;124:493–498. doi: 10.1093/brain/124.3.493. [DOI] [PubMed] [Google Scholar]
- 19.Nagase H, Fields CG, Fields GB. Design and characterization of a fluorogenic substrate selectively hydrolyzed by stromelysin 1 (matrix metalloproteinase-3) J Biol Chem. 1994;269:20952–20957. [PubMed] [Google Scholar]
- 20.Bennett DA, Wilson RS, Gilley DW, Fox JH. Clinical diagnosis of Binswanger’s disease. J Neurol Neurosurg Psychiatry. 1990;53:961–965. doi: 10.1136/jnnp.53.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher CM. Binswanger’s encephalopathy: a review. J Neurol. 1989;236:65–79. doi: 10.1007/BF00314400. [DOI] [PubMed] [Google Scholar]
- 22.Zhang WW, Olsson Y. The angiopathy of subcortical arteriosclerotic encephalopathy (Binswanger’s disease): immunohistochemical studies using markers for components of extracellular matrix, smooth muscle actin and endothelial cells. Acta Neuropathol. 1997;93:219–224. doi: 10.1007/s004010050607. [DOI] [PubMed] [Google Scholar]
- 23.Feigin I, Popoff N. Neuropathological Changes Late in Cerebral Edema: The Relationship to Trauma, Hypertensive Disease and Binswanger’s Encephalopathy. J Neuropathol Exp Neurol. 1963;22:500–511. doi: 10.1097/00005072-196307000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Akiguchi I, Tomimoto H, Suenaga T, Wakita H, Budka H. Blood-brain barrier dysfunction in Binswanger’s disease; an immunohistochemical study. Acta Neuropathol. 1998;95:78–84. doi: 10.1007/s004010050768. [DOI] [PubMed] [Google Scholar]
- 25.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt R, Scheltens P, Erkinjuntti T, Pantoni L, Markus HS, Wallin A, Barkhof F, Fazekas F. White matter lesion progression: a surrogate endpoint for trials in cerebral small-vessel disease. Neurology. 2004;63:139–144. doi: 10.1212/01.wnl.0000132635.75819.e5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.