Abstract
Although high mobility group box 1 (HMGB1) in tumor cells is involved in many aspects of tumor progression, its role in tumor immune suppression remains elusive. Host cell-derived interleukin-10 (IL-10) suppressed a naturally acquired CD8 T cell-dependent antitumor response. The suppressive activity of tumor-associated Foxp3+CD4+CD25+ regulatory T cells (Treg) was IL-10-dependent. Neutralizing HMGB1 impaired tumor cell-promoted IL-10 production by Treg. Short hairpin RNA (shRNA)-mediated knockdown of HMGB1 (HMGB1 KD) in tumor cells did not affect tumor cell growth but uncovered naturally acquired long-lasting tumor-specific IFN-γ- or TNF-α-producing CD8 T cell responses and attenuated their ability to induce Treg leading to naturally acquired CD8 T cell- or IFN-γ-dependent tumor rejection. The data suggest that tumor cell-derived HMGB1 may suppress naturally acquired CD8 T cell-dependent antitumor immunity via enhancing Treg to produce IL-10 which is necessary for Treg-mediated immune suppression.
Keywords: HMGB1, Treg, Tumor immune suppression
Introduction
During progression, tumor-associated Treg inhibit naturally acquired antitumor immune responses (1). Tumor cell-derived factors (e.g., TGF-β, indoleamine 2, 3-dioxygenase, stem cell factor, CCL22 and yet-to-be-identified factors) contribute directly to Treg or CD25-CD4+ T cells, and/or indirectly to dendritic cells (DC), plasmacytoid DC, myeloid-derived suppressor cells or B cells for Treg expansion, conversion, activation and/or recruitment (2-9). How tumor cell-derived factors suppress naturally acquired antitumor immunity via Treg is poorly understood from a mechanistic point of view.
IL-10 inhibits antitumor activity in both mice and humans (10-12). CD4+ T regulatory type 1 cells (Tr1) do not express high levels of CD25 or Foxp3 but produce significant IL-10 to mediate immune suppression in vitro and in vivo (13). Treg also produce IL-10 and IL-10-producing Treg have been shown to be highly suppressive (14). In both mouse and human tumor studies, although Treg-derived IL-10 mediates immune suppression in vitro (15-16), whether it happens in vivo remains unclear (17). Importantly, the signals derived by tumor cells acting on Treg to promote IL-10 production are largely unknown (14).
HMGB1, a small protein of 215 amino acid residues with extensive various post-translation modifications, is a highly conserved protein in the nucleus, cytoplasm or extracellular environment (e.g., released from cells via necrosis and autophagy, secreted from inflammatory or cancer cells) with multiple distinguished functions (e.g., binding/bending DNA to facilitate transcription factor assembly on site-specific DNA targets, promoting autophage, inducing cell death, acting as a signaling molecule to alert innate immunity) (18-28). Advanced glycation end products (RAGE), toll-like receptor 2 (TLR2), TLR4, TLR9, and CD24 have been suggested to be the receptors of HMGB1 (26-28).
HMGB1 is highly expressed in tumor cells and elevated levels of HMGB1 in tumor cells are usually associated with a greater tumor angiogenesis, growth, invasion and metastasis (23-28). HMGB1 released by tumor cells is involved with either anti- or pro-tumor effects under certain circumstances and/or models (23-31). As an endogenous adjuvant, HMGB1, released from dying tumor cells post chemo-, viro- or radiation-therapy, promotes DC maturation and tumor antigen presentation via acting on TLR2 or TLR4 or activates innate immunity, thereby resulting in antitumor activity (29-31). As a tumor-promoting factor, tumor cell-derived HMGB1 enhances tumor angiogenesis, growth, invasion and metastasis (23-29).
Although HMGB1 produced by tumor cells exhibits the inhibitory effect on DC in both mouse and human studies (32), it is largely unknown whether and how tumor cell-derived HMGB1 mediates tumor immune suppression. In this study, we examined the impacts of tumor cell-derived HMGB1 on Treg and naturally acquired antitumor immunity.
Materials and methods
Mice and tumor cell lines
BALB/c, BALB/c-IL-10-/- (C.129P2(B6)-IL-10tm1Cgn/J), BALB/c-C.129S7(B6)-Ifngtm1Ts/J, BALB/c-Foxp3-eGFP mice (C.Cg-Foxp3tm2Tch/J) and C57BL/6 (B6) mice (female, 6-8 weeks) were purchased from JAX and Taconic, and housed and bred in specific pathogen-free conditions in the University of Pittsburgh animal facility. All animal procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals. Murine breast tumor 4T1.2-Neu (33), lung cancer 3LL (ATCC) and colon carcinoma CT26 (ATCC) were maintained in DMEM (IRVINE Scientific) supplemented with 10% fetal bovine serum (FBS) (Hyclone), 2mM glutamine (Invitrogen) and 1x antibiotic antimycotic solution (Sigma).
IL-10 production by Treg
BALB/c or BALB/c-Foxp3-eGFP mice were subcutaneously (s.c.) inoculated with 4T1.2-Neu (1×105) in 20μl endotoxin-free 1×PBS (Sigma) at the 4th mammary fat pad (33). B6 mice were s.c. inoculated with 3LL (1×105) at the left flank. After 3-4 weeks, Treg were purified from splenocytes of tumor-bearing mice using mouse CD4+CD25+ regulatory T cell isolation kit according to the vendor's instruction (Miltenyi Biotec) (34). In some experiments, Treg (eGFP+) were sorted from splenocytes or single-cell suspensions of tumors using a BD FACSAria High Speed Cell Sorter (BD Biosciences). (Purity of Foxp3 Treg was confirmed by flow cytometry and consistently resulted in greater than 95%.) To obtain tumor cell culture supernatants, tumor cells (1×104/150μl) were cultured 24 hours and culture media were centrifuged at room temperature, 2,000 rpm for 5 minutes. Treg (2×105) were cultured alone or with tumor cells (1×104) in 200μl RPMI 1640 10% FBS or tumor cell culture supernatants, at 37°C, 5% CO2 for 2 days. In some experiments, functional anti-HMGB1 antibody (Ab) (anti-HMGB1166–181 kindly provided by Dr. Michael T. Lotze, University of Pittsburgh; ab18256 purchased from Abcam) or rabbit IgG (eBioscience) (10-20μg/ml) were added. The concentration of IL-10 in the culture supernatants was determined by ELISA (BD Biosciences, eBioscience).
HMGB1 KD in tumor cells
HMGB1 small interfering RNA (siRNA) (GCUGAAAAGAGCAAGAAAATT) was demonstrated to be effective in specific depletion of HMGB1 in mouse tumor cells (29). Oligonucleotides including HMGB1 siRNA sequence (sense: 5’GATCCGCTGAAAAGAGCAAGAAAATTTCAAGAGAATTTTCTTGCTCTTTTCAGCTTTTTGGAAG’3; antisense: 5’AGCTCTTCCAAAAAGCTGAAAAGAGCAAGAAAATTCTCTTGAAATTTTCTTGCTCTTTTCAGCG’3) were synthesized (IDTDNA). The annealed oligonucleotides were cloned into retrovial vector pRetrosuper (pRS, a generous gift from Dr. Joan Massagué at Memorial Sloan-Kettering Cancer Center) (resultant pRS-HMGB1 shRNA). Inserted shRNA was confirmed by DNA sequencing. DNA was purified using EndoFree plasmid kits (QIAGEN). 4T1.2-Neu or 3LL wild type (WT) were transfected with pRS-HMGB1 shRNA or pRS (vector control) using Lipofectamine™ 2000 (Invitrogen), and selected with puromycin (Invivogen). Selected 4T1.2-Neu shRNA HMGB1 (4T1.2-Neu HMGB1 KD), 4T1.2-Neu vector control (4T1.2-Neu KD control), 3LL shRNA HMGB1 (3LL HMGB1 KD) or 3LL vector control (3LL KD control) were used in experiments. The equivalent amounts of proteins from cell lysate (20μg) or tumor cell culture supernatants (50μg) were loaded to confirm HMGB1 KD in tumor cell lysates or culture supernatants using Western blotting (WB) with rabbit anti-HMGB1 Ab (ab18256) (primary Ab), goat anti-rabbit poly-HRP (Cell Signaling Technology, Pierce) (secondary Ab) and ECL™ WB Detection Reagents (GE Healthcare) or SuperSignal West Femto Chemiluminescent Substrate (Pierce).
Tumor-specific CD8 T cell responses
BALB/c mice (3/group) were s.c. inoculated with 4T1.2-Neu WT, HMGB1 KD or KD control (1×105). Day 21 or 60 post tumor inoculation, CD8 T cells were isolated from splenocytes of those mice (naïve mice as non-tumor-bearing control) using anti-mouse CD8 microbeads according to the vender's instruction (Miltenyi Biotec). Purified CD8 T cells (4×105) were restimulated with mitomycin C (Sigma)–treated 4T1.2-Neu or CT26 (tumor-specific control) (4×104) (35) in the presence or absence of irradiated (4000 Rad) naïve syngenic CD8-splenocytes (served as antigen-presenting cells: APC) (2×106) in 200 μl RPMI 1640 10% FBS at 37°C, 5% CO2 for 3 days. The concentration of IFN-γ or TNF-α in the culture supernatants was determined by ELSA (BD Biosciences).
Treg-mediated immune suppressions
A) Treg-mediated suppression of IFN-γ production by T cells in vitro (34)
Tumor-primed CD4+CD25- (CD4) T cells were obtained from 4T1.2-Neu-bearing BALB/c or 3LL-bearing B6 mice. Splenic DC or CD8 T cells were purified from splenocytes of naïve BALB/c or B6 mice using anti-mouse CD11c or CD8 microbeads (Miltenyi Biotec). Purified DC were loaded with 4T1.2-Neu or 3LL lysates (34-35). Tumor-primed CD4 T cells (2×105), tumor Ag-loaded DC (2×105) and naïve CD8 T cells (2×105) were cultured in the presence or absence of WT-Treg or IL-10-/--Treg (2×105) purified from 4T1.2-Neu-bearing BALB/c, BALB/c-Foxp3-eGFP or BALB/c-IL-10-/- mice, 4T1.2-Neu or 3LL WT-, HMGB1 KD- or KD control-inoculating BALB/c or B6 mice in 200μl RPMI 1640 10%FBS at 37°C, 5% CO2 for 2 days. The concentration of IFN-γ in the culture supernatants was determined by ELISA.
B) Treg-mediated suppression of DC maturation in vitro (34)
Naïve splenic DC and tumor-primed CD4 T cells were prepared as described above. DC (2×105) and tumor-primed CD4 T cells (2×105) were cultured alone or with Treg (2×105) purified from 4T1.2-Neu WT-, HMGB1 KD- or KD control-inoculating BALB/c mice in the presence of LPS (1μg/ml, Sigma) in 200μl RPMI 1640 10%FBS at 37°C, 5% CO2 for 18 hours. The concentration of IL-12(p40) in the culture supernatant was measured by ELISA (BD Biosciences). Those cells were harvested and treated with 5 mM EDTA. Fc receptor binding of Ab was minimized by incubation with rat anti-mouse CD16/CD32 Ab (BD Biosciences) prior to staining with anti-mouse CD11c-APC (HL3) and CD80-PE (16-10A1) or CD86-PE (GL1) (isotype control of each Ab was used in control staining) (BD Biosciences or eBioscience), and analyzed by flow cytometry on a BD LSRII with CellQuest software (BD Biosciences). Propidium iodide (BD Biosciences) was used to check cell viability. Forward scatter and side scatter was used to exclude cell debris. The flow cytometric data was analyzed using Flowjo software (Tree star).
C) Treg-mediated suppression of tumor-specific CD8 T cell activation in vivo (34): Tumor-primed CD4 T cells (1×107) and WT-Treg or IL-10-/--Treg (1×107), purified from 4T1.2-Neu WT-, HMGB1 KD- or KD control-inoculating BALB/c mice or 4T1.2-Neu WT-bearing BALB/c-IL-10-/- mice, were adoptively cotransferred intravenously (i.v.) into naïve BALB/c mice (3/group) on day -1. Those mice were inoculated s.c. with 4T1.2-Neu (1×105) on day 0. On day 5, CD4-CD11c- tumor-draining lymph node (TDLN) cells (4×105) were restimulated with mitomycin C–treated 4T1.2-Neu or CT26 (tumor-specific control) (8×103) in the presence of purified naïve syngenic splenic DC (8×104) in 200 μl RPMI 1640 10% FBS at 37°C, 5% CO2 for 5 days. The concentration of IFN-γ in the culture supernatants was determined by ELISA.
Tumor challenge
BALB/c-WT, -IL-10-/- or -IFN-γ-/- mice (2-5/group) were s.c. inoculated with 1×105 4T1.2-Neu WT, HMGB1 KD or KD control in 20μl endotoxin-free 1×PBS at the 4th mammary fat pad on day 0. B6-WT mice (3-7/group) were s.c. inoculated with 1×105 3LL WT, HMGB1 KD or KD control at the left flank on day 0. In some experiments, to deplete CD8 T cells, anti-mouse CD8 Ab (53-6.7) (200μg/injection) were intraperitoneally (i.p.) injected into WT mice on days -1, 1, 3, 6 and 9. (CD8 T cell depletion was confirmed by flow cytometry and resulted in greater than 95% reduction of CD8 T cells.) Tumors were measured using a digital slide calipers (Fisher Scientific) in the two perpendicular diameters every 2 days. Mice were dead naturally or sacrificed when tumor reached 10mm in mean diameter (33-35).
Statistics
Data were statistically analyzed using Student's t test (Graph Pad Prism version 5). Data from animal survival experiments were statistically analyzed using Log Rank test (Graph Pad Prism version 5). P < 0.05 is considered to be statistically significant.
Results
Host cell-derived IL-10 inhibits naturally acquired CD8 T cell-dependent antitumor immunity
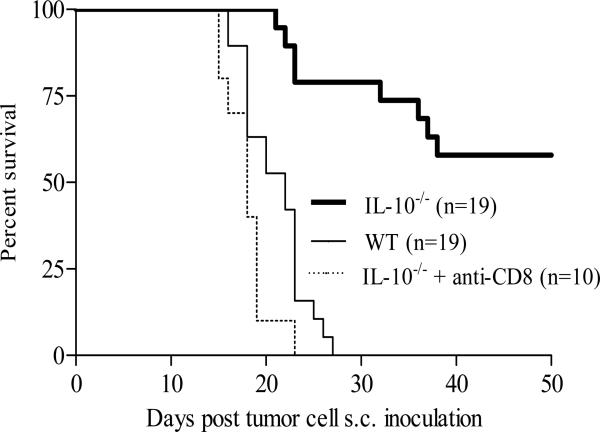
BABL/c mice s.c. inoculated with 4T1.2-Neu (1×105) at mammary fat pad bore a primary solid tumor at the site of injection and metastatic tumors at various distant organs, and were naturally dead or sacrificed within 3-4 weeks (Figure 1) (33-35). Although 4T1.2-Neu initially grew well in BALB/c-IL-10-/- mice (data not shown), the primary tumors were eventually rejected in around 60% mice (Figure 1). Metastatic tumors were not found in those (primary) tumor-rejection mice (data not shown). Moreover, depletion of endogenous CD8 T cells abrogated the tumor rejection (Figure 1). 4T1.2-Neu cultured in vitro did not produce detectable soluble IL-10 (Figure 3A). The data suggest that, in this breast tumor model, host cell-derived IL-10 inhibits naturally acquired CD8 T cell-dependent antitumor immunity.
FIGURE 1.
Host cell-derived IL-10 inhibits naturally acquired CD8 T cell-dependent antitumor immunity. BALB/c-WT or -IL-10-/- mice (female, 4-5 weeks) (2-5/group) were inoculated with 4T1.2-Neu. (Because 13% of homozygotes BALB/c-IL-10-/- mice at 9 weeks had adenocarsinomas and there was a 65% incidence of colorectal carcinoma at 10-31 weeks, BALB/c-IL-10-/- mice bearing an inoculated 4T1.2-Neu with spontaneous adenocarsinomas or colorectal carcinoma were removed out from these experiments.) 19 BALB/c-IL-10-/- mice presented here were adenocarsinomas or colorectal carcinoma free, at least, in the experimental time period. Data are combined from four independent experiments. n: the numbers of animals used. IL-10-/- vs. WT or IL-10-/- + anti-CD8: p<0.0001. Animal survival is presented using Kaplan-Meier survival curves.
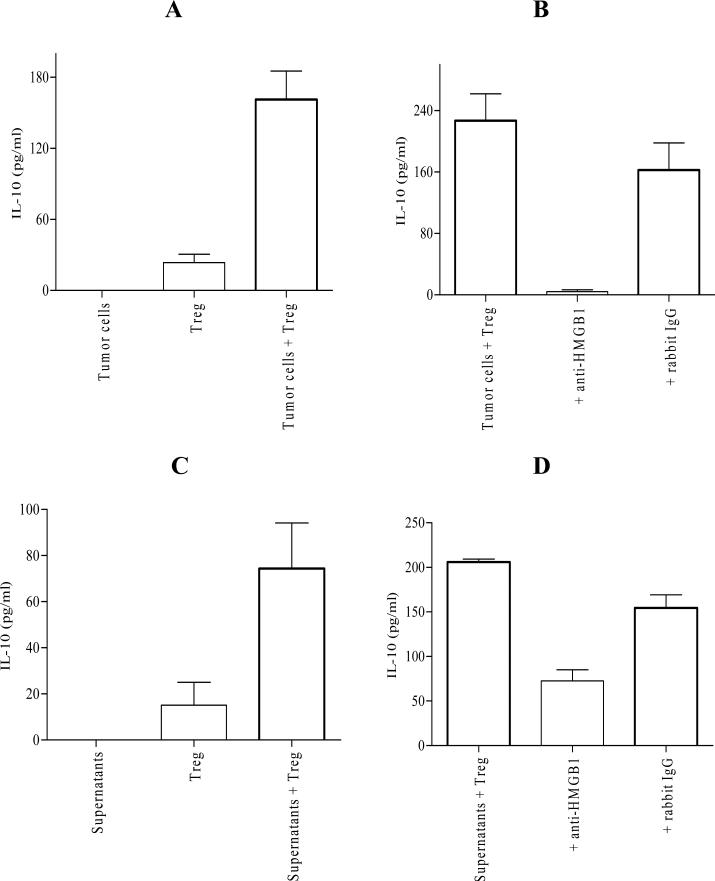
FIGURE 3.
Tumor cell-derived HMGB1 is involved in promoting IL-10 production by Treg in vitro. A, Treg from 4T1.2-Neu-bearing mice were cultured alone or cocultured with tumor cells (4T1.2-Neu). Treg vs. tumor cells + Treg: p<0.0005. B, Treg from 4T1.2-Neu-bearing mice were cocultured with 4T1.2-Neu in the presence or absence of anti-HMGB1 Ab or rabbit IgG. Tumor cells + Treg vs. tumor cells + Treg + anti-HMGB1: p<0.0005, tumor cells + Treg vs. tumor cells + Treg + rabbit IgG: no significant (NS). C, Treg from 4T1.2-Neu-bearing mice were cultured in 4T1.2-Neu culture supernatants. Treg vs. supernatants + Treg: p<0.05. D, Treg from 4T1.2-Neu-bearing mice were cultured in 4T1.2-Neu culture supernatants in the presence or absence of anti-HMGB1 Ab or rabbit IgG. Supernatants + Treg vs. supernatants + Treg + anti-HMGB1: p<0.05, supernatants + Treg vs. supernatants + Treg + rabbit IgG: NS. Data (A-D) are represented as mean ± SEM. Results are combined from four (A) or five (B-C) independent experiments and representative of three (D) independent experiments with at least one to two mice per group in each experiment.
Treg-derived IL-10 is necessary for mediating immune suppression in vitro and in vivo
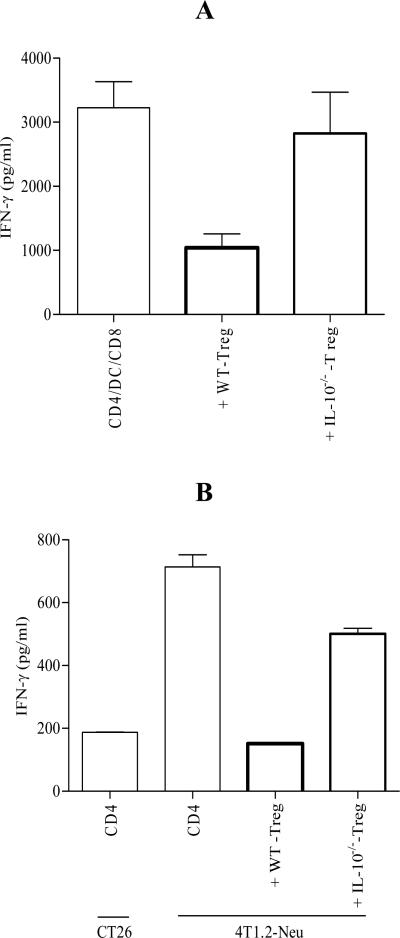
Treg from spleens of tumor-bearing mice (spleen-derived Treg) exhibited potent function in suppressing IFN-γ production by T cells stimulated by DC in vitro (Figure 2A) (34). Since tumor-infiltrating Treg are postulated to suppress tumor-infiltrating CD8 T cells, the phenotype and function of Treg from tumors (tumor-derived Treg) were examined. Although tumor-derived Treg expressed less CD25, more membrane-bound CTLA-4 and comparable GITR compared to spleen-derived Treg (Figure S1A), their suppressive activity was comparable (Figure S1B). Spleen is the most feasible source of Treg, spleen-derived Treg thus were used in the most of experiments in this study. To determine the role for Treg-derived IL-10 in mediating immune suppression, IL-10 signaling was blocked using functional anti-IL-10R Ab (rat IgG1 as isotype control). Blocking IL-10 signaling in the cell culture diminished Treg-mediated immune suppression in vitro (Figure S2). To confirm this observation, IL-10-/--Treg were obtained from 4T1.2-Neu-bearing BALB/c-IL-10-/- mice. As shown in Figure 2A, IL-10-/--Treg lost their suppressive function in vitro. To examine whether Treg-derived IL-10 is necessary for mediating immune suppression of tumor-specific CD8 T cell activation in vivo, tumor-primed CD4 T cells and WT-Treg or IL-10-/--Treg (from tumor-bearing BALB/c-WT or -IL-10-/- mice) were coadoptively transferred into BABL/c mice before tumor inoculation (35). After 5 days, CD11c-CD4- single cells of TDLN were restimulated by naïve syngenic splenic DC in the presence of mitomycin C-treated 4T1.2-Neu or CT26 (tumor-specific control). As previously reported (35), adoptively-transferred tumor-primed CD4 T cells induced tumor-specific CD8 T cell activation (Figure 2B). Although IL-10-/--Treg exhibited a certain degree of suppressive function in vivo, WT-Treg were much more potent to suppress adoptively-transferred CD4 T cell-induced tumor-specific CD8 T cell activation in vivo compared to IL-10-/--Treg (Figure 2B). The data indicate that Treg-derived IL-10 is necessary for mediating immune suppression in vitro and in vivo.
FIGURE 2.
Treg-derived IL-10 is necessary for mediating immune suppression in vitro and in vivo. A, Treg obtained from 4T1.2-Neu-bearing BALB/c-WT (WT-Treg) or -IL-10-/- (IL-10-/--Treg) mice were cocultured with 4T1.2-Neu-primed CD4 T cells (CD4), 4T1.2-Neu-loaded naïve syngenic splenic DC (DC) and naïve syngenic CD8 T cells (CD8). Data are represented as mean ± standard error of the mean (SEM) and combined from three independent experiments with at least one to two mice per group in each experiment. WT-Treg vs. IL-10-/--Treg: p<0.05. B, BALB/c-WT mice (3/group) were adoptively cotransferred with CD4 and WT-Treg or IL-10-/--Treg on day -1, and inoculated with 4T1.2-Neu on day 0. BALB/c-WT mice adoptively transferred with CD4 alone served as positive control. On day 5, CD4-CD11c- TDLN cells were restimulated by mitomycin C–treated 4T1.2-Neu or CT26 (tumor-specific control) in the presence of purified naïve syngenic splenic DC. Data are represented as mean ± SEM and combined from three independent experiments. WT-Treg vs. IL-10-/--Treg: p<0.005.
Tumor cell-derived HMGB1 is involved in promoting IL-10 production by Treg in vitro
Treg-derived IL-10 appears a pro-tumor factor in 4T1.2-Neu growth (Figures 1-2). A lung cancer 3LL, which did not produce detectable soluble IL-10 in cell culture (Figure S3), grows rapidly in IL-10 transgenic mice and T cell-derived IL-10 promotes its growth by suppressing both T cell and APC function (36-37), suggesting that host cell-derived IL-10 is a pro-tumor factor in 3LL progression. Both 4T1.2-Neu and 3LL did not secrete detectable IL-10 in cell culture (Figures 3A, S3). Treg isolated from 4T1.2-Neu- or 3LL-bearing mice secreted detectable (4T1.2-Neu) or undetectable (3LL) IL-10 in cell culture without stimulation and/or activation (Figures 3A, S3). Tumor cells or tumor cell culture supernatants promoted Treg from tumor-bearing mice to produce IL-10 in vitro (Figures 3A, 3C, S3, data not shown). It has been reasoned that the increase of IL-10 production by Treg due to either tumor cell-stimulated Treg proliferation (number) or tumor cell-enhanced Treg capacity to produce IL-10 (function). Tumor cells did not stimulate Treg proliferation in vitro (data not shown). It seems that tumor cell-derived soluble factors promote tumor-associated Treg to produce IL-10. Unexpectedly, neutralization of HMGB1 signaling using functional anti-HMGB1 Ab (anti-HMGB1166–181 or ab18256) (rabbit IgG as isotype control) dampened tumor cell- or tumor cell culture supernatant-promoted IL-10 production by Treg (Figures 3B, 3D, S3). The data suggest that tumor cell-derived HMGB1 is involved in promoting IL-10 production by Treg in vitro.
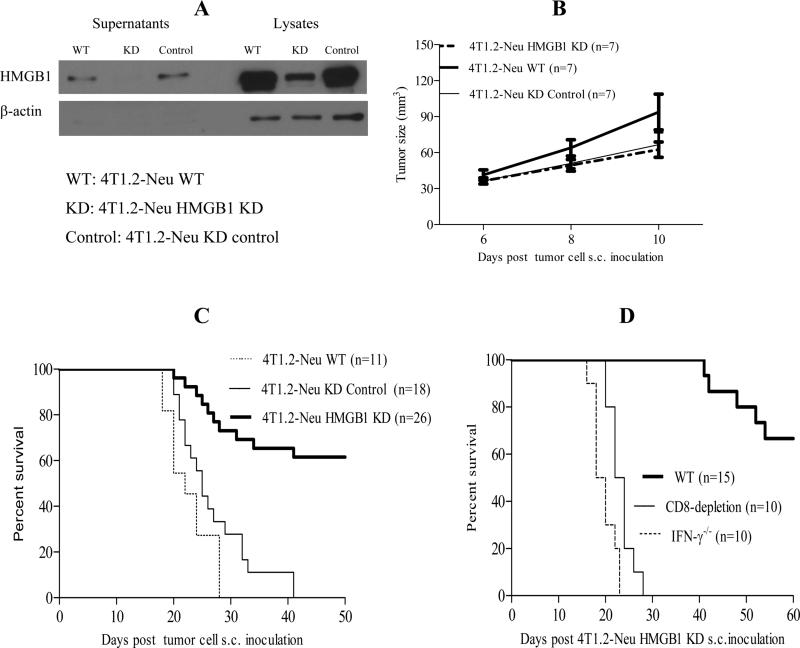
HMGB1 KD does not affect tumor cell growth but uncovers naturally acquired CD8 T cell-dependent antitumor immunity
Tumor cell-derived HMGB1 promoted Treg to produce IL-10 which was important to inhibit naturally acquired CD8 T cell-dependent antitumor immunity (Figures 1-3). Tumor cell-derived HMGB1, in both 4T1.2-Neu and 3LL models, may play a critical role in tumor progression as a pro-tumor factor. Efficient siRNA-mediated HMGB1 KD in tumor cells did not affect the tumor cell growth in those models in vitro (Figures 4A, S4A-C). The growth of 4T1.2-Neu or 3LL HMGB1 KD in syngenic WT mice was comparable to 4T1.2-Neu or 3LL KD control or WT (Figures 4B, S4D). Although they initially grew well (Figures 4B, S4D), substantial 4T1.2-Neu or 3LL HMGB1 KD were eventually rejected (Figures 4C-D, S4E), suggesting that HMGB1 KD uncovers a naturally acquired antitumor activity leading to the effective tumor rejection. Importantly, endogenous CD8 T cell depletion or IFN-γ deficiency abrogated the observed tumor rejection (Figures 4D, S4E), implying that endogenous immune effectors mediate HMGB1 KD-associated tumor rejection in syngenic immune-component mice. The data demonstrate that HMGB1 KD does not impair tumor cell growth but uncovers naturally acquired CD8 T cell-dependent antitumor immunity.
FIGURE 4.
HMGB1 KD does not affect tumor growth but uncovers naturally acquired CD8 T cell- or IFN-γ-dependent antitumor immunity. A, HMGB1 KD in 4T1.2-Neu lysates or culture supernatants was confirmed by WB using rabbit anti-HMGB1 Ab, goat anti-rabbit poly-HRP (Cell Signaling Technology) and ECL™ WB Detection Reagents (GE Healthcare). β-actin was served as an internal control. One representative of three independent experiments is shown. B, BALB/c-WT mice (3-4/group) were inoculated with 4T1.2-Neu WT, HMGB1 KD or KD control on day 0. Tumor size was measured. Data are combined from two independent experiments. HMGB1 KD vs. WT or KD control: NS. C, BALB/c-WT mice (2-5/group) were inoculated with 4T1.2-Neu WT, HMGB1 KD or KD control on day 0. Data are combined from six independent experiments. HMGB1 KD vs. WT or KD control: p<0.0001, WT vs. KD control: NS. D, BALB/c-WT or -IFN-γ-/- mice (3-5/group) were inoculated 4T1.2-Neu HMGB1 KD on day 0. Anti-mouse CD8 Ab were injected into some WT mice on days -1, 1, 3, 6 and 9. Data are combined from three independent experiments. WT vs. CD8 depletion or IFN-γ-/-: p<0.0001. Animal survival is presented using Kaplan-Meier survival curves.
HMGB1 KD uncovers a naturally acquired long-lasting tumor-specific IFN-γ- or TNF-α-producing CD8 T cell response
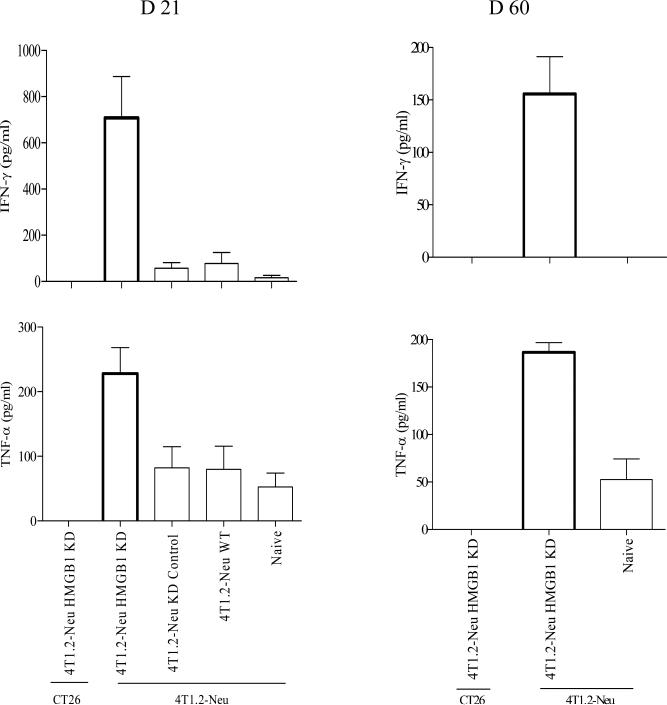
To examine tumor-specific CD8 T cell responses, BALB/c mice were inoculated with 4T1.2-Neu WT, HMGB1 KD or KD control. Day 21 or 60 (only 4T1.2-Neu HMGB1 KD-rejection mice survived at this time point.) post tumor inoculation, CD8 T cells isolated from splenocytes of tumor-bearing mice (naïve mice as non-tumor-bearing control) were restimulated with irradiated 4T1.2-Neu- or CT26 (tumor-specific control)-pulsed naïve syngenic CD8- splenocytes. As shown in Figure 5, HMGB1 KD uncovered a naturally acquired long-lasting tumor-specific IFN-γ- or TNF-α-producing CD8 T cell response.
FIGURE 5.
HMGB1 KD uncovers a naturally acquired long-lasting tumor-specific IFN-γ- or TNF-α-producing CD8 T cell response. BALB/c mice (3/group) were inoculated with 4T1.2-Neu WT, HMGB1 KD or KD control on day 0. Day 21 (A) or 60 (B, only 4T1.2-Neu HMGB1 KD-rejection mice survived at this time point.), CD8 T cells were isolated from splenocytes of those mice (naïve mice as non-tumor-bearing control). Purified CD8 T cells were restimulated with mitomycin C–treated 4T1.2-Neu or CT26 (tumor-specific control) in the presence or absence of irradiated naïve syngenic CD8- splenocytes (served as APC). Data are represented as mean ± SEM and combined from three independent experiments. D21: HMGB1 KD vs. WT, KD control or naïve: p<0.05, WT vs. KD control: NS. D60: HMGB1 KD vs. naïve: p<0.0005.
HMGB1 KD attenuates the ability of tumor cells to in vivo induce Treg
To determine the impacts of HMGB1 KD in tumor cells on tumor-associated Treg frequencies and numbers in tumor-bearing mice, BALB/c-Foxp3-eGFP mice were s.c. inoculated with 4T1.2-Neu WT, HMGB1 KD or KD control. Two weeks post tumor inoculation, the frequencies and absolute numbers of Treg in spleen and TDLN were examined. HMGB1 KD reduced the ability of tumor cells to increase absolute numbers (not frequencies) of Treg in both TDLN and spleen (Figure 6A, data not shown). Three weeks after tumor inoculation, the ability of those splenic Treg in suppressing splenic DC maturation was measured. Treg from 4T1.2-Neu HMGB1 KD-inoculating mice showed a reduced activity of suppressing the expression of CD80 or CD86 on DC and the production of IL-12 by DC compared to Treg from 4T1.2-Neu WT- or KD control-inoculating mice (Figures 6B, S5A). To confirm the suppressive function of Treg in vitro, the ability of those splenic Treg in suppressing T cell activation was examined. Treg from 4T1.2-Neu WT- or KD control-inoculating mice exhibited potent suppressive activity compared to Treg from 4T1.2-Neu HMGB1 KD-inoculating mice (Figure 6C). By using the 3LL HMGB1 KD model, a similar result was observed (Figure S5B). To further determine the suppressive function of Treg in vivo, the ability of those splenic Treg in suppressing CD8 T cell activation in vivo was examined. Although Treg from 4T1.2-Neu HMGB1 KD-inoculating mice exhibited suppressive activity, their suppressive activity was low compared to Treg from 4T1.2-Neu WT or KD control-inoculating mice (Figure 6D). The data suggest that HMGB1 KD attenuates the ability of tumor cells to in vivo induce Treg.
FIGURE 6.
HMGB1 KD attenuates the ability of tumor cells to in vivo induce Treg. A, BALB/c-Foxp3-eGFP mice (3/group) were inoculated with 4T1.2-Neu WT, HMGB1 KD or KD control. After 2 weeks, Treg (eGFP+) in TDLN and spleen were measured by flow cytometry. Absolute numbers of Treg per spleen and TDLN are presented. Data are represented as mean ± SEM and combined from three independent experiments. HMGB1 KD vs. WT or KD control: p<0.05, WT vs. KD control: NS. B, Treg purified from 4T1.2-Neu WT, HMGB1 KD or KD control-inoculating mice were cocultured with CD4 and DC. HMGB1 KD vs. WT or KD control: p<0.05, WT vs. KD control: NS. The in vitro (C) and in vivo (D) suppressive activity of Treg from 4T1.2-Neu WT, HMGB1 KD or KD control-inoculating mice were measured as described in Fig.2A-B. Data (B-D) are represented as mean ± SEM. Results are combined from three (B-C) or two (D) independent experiments with at least one to two mice per group in each experiment. HMGB1 KD vs. WT or KD control: p<0.05, WT vs. KD control: NS.
Discussion
Overproduction of HMGB1, which occurs in various tumor cells, is associated with the hallmarks of cancer (e.g., tumor angiogenesis, growth, inflammation, invasion and metastasis) (27, 38). HMGB1 plays multiple roles in either anti- or pro-tumor effects (23-31). Recombinant human HMGB1 induces a distinct form of cell death in cancer cells that may provide therapeutic benefits for cancer patients (39). HMGB1 released from dying tumor cells after chemo-, viro- or radiation-therapy, as a ‘danger’ molecule, stimulates DC maturation and tumor antigen presentation via TLR2/4 or activates innate immunity, leading to an antitumor immune response (29-31). However, in the physiological condition (tumor progression), the role of HMGB1 in tumor immune suppression is largely unknown even though it has been reported that HMGB1 produced by tumor cells exhibits the inhibitory effect on DC in both mice and humans (32).
The observations in this work suggest that tumor cell-derived HMGB1 may suppress a naturally acquired immune effector cell (CD8)- or cytokine (IFN-γ)-dependent antitumor response, probably by enhancing tumor-associated Treg to produce IL-10, which is necessary for immune suppression in vitro and in vivo. Treg-derived IL-10 may dampen DC, CD4 or CD8 T cell function to diminish the priming of tumor-specific CD8 T cells. DC have been suggested to be the most relevant targets of Treg in vivo and decommissioning of DC probably is a realistic mechanism underlying Treg-mediated immune suppression (40). The mechanisms underlying Treg-derived IL-10-mediated suppression of the priming of antitumor CD8 T cells via a DC effect will be precisely dissected such as using the tetramer analysis of antitumor CD8 T cell frequencies in the future work.
IL-10-producing Treg have been shown to be highly suppressive (14). The in vitro data indicate that tumor cell-derived HMGB1 may act, as an extracellular signal, on tumor-associated Treg to promote IL-10 production for an enhanced suppressive functionality. Whether HMGB1-KD tumor immunity in vivo involves interference with IL-10 producing-Treg needs to be investigated in the future studies. In a burn injury model, massive HMGB1 released from burn injury has been suggested to activate Treg via RAGE to produce (detectable) IL-10 in vitro (41). It is possible that tumor cell-derived HMGB1 may interact with RAGE (or other putative HMGB1 receptors) on Treg to activate p38 MAP kinase, extracellular signal-regulated kinase1/2 and Jun N-terminal kinase, leading to the activation of transcriptional factors (activator protein 1 and NFκB) for IL-10 production in Treg (19, 28). The exact intrinsic molecular pathway triggered by tumor cell-derived HMGB1 and whether anti-HMGB1 treatment alters the downstream signaling pathway need to be elucidated in the future studies.
HMGB1 KD in tumor cells resulted in a CD8 T cell- or IFN-γ-dependent tumor rejection, clearly suggesting that HMGB1 KD-mediated antitumor activity in vivo is due to naturally acquired antitumor immunity but not modulation on tumor cells for death suggested in the in vitro prostate tumor cell study (42). HMGB1 KD may render tumor cell susceptibility to cytotoxic T lymphocyte-mediated killing via altering tumor phenotype. Since Treg depletion provoked antitumor immunity and tumor elevated and activated Treg which suppressed antitumor immunity (34-35), attenuation of the ability of tumor cells to in vivo expand and activate Treg by HMGB1 KD may allow for CD8 T cells to operate in an unopposed manner leading to enhanced CD8 T cell responses. How HMGB1 KD reduce tumor cell capacity to in vivo induce Treg is still mysterious. It is possible that HMGB1 KD may modulate tumor cells to produce immune stimulatory molecules and/or to reduce secrete immune suppressive factors.
The conflicting data shown in the literature and here on HMGB1 in tumor immune responses may be explained by the different sources of HMGB1 under different tumor cell conditions. HMGB1 released from dying tumor cells post chemo-, viro- or radiation-therapy may complex with soluble moieties in tumors (e.g., nucleic acids, microbial products, and cytokines) to exert its inflammatory properties (29-31). HMGB1 released (secreted) from tumor cells in the physiological condition (tumor progression) may not be able to do so or may be specifically modified posttranslationally (e.g., oxidation) to exhibit its ability to promote tumor invasion, metastasis or immune tolerance (23-28, 43). Indeed, the state of oxidation of HMGB1 is critical in determining immune response vs. non-responsiveness (43).
RAGE-HMGB1 blockade by administration of soluble RAGE, anti-RAGE and/or anti-HMGB1 Ab inhibits tumor growth and metastases (44). Since HMGB1 KD in tumor cells uncovered naturally acquired CD8 T cell-dependent antitumor immunity, we are actively investigating whether anti-HMGB1 treatment [e.g., intratumorally blocking HMGB1 signaling using: i) anti-HMGB1 mAb (neutralizing HMGB1 signaling), ii) siRNA HMGB1 (HMGB1 KD) or iii) glycyrrhizin (a specific inhibitor of extracellular HMGB1) (inhibiting HMGB1)] can be used therapeutically to rescue an antitumor CD8 T cell response to established tumors.
In sum, the data suggest a new function for tumor cell-derived HMGB1 in suppressing naturally acquired CD8 T cell-dependent antitumor immunity probably via promoting tumor-associated IL-10-producing Treg.
Supplementary Material
Acknowledgments
We are indebted to M. T. Lotze (University of Pittsburgh) for providing anti-HMGB1 Ab, J. Massagué (Memorial Sloan-Kettering Cancer Center) for providing the retroviral vector pRetrosuper, and W. J. Storkus (University of Pittsburgh) for his valuable discussions.
This work was supported by NIH grant R01CA108813, R01CA108813-04S2 (to Z.Y.), R01AI076060, CA106662, and P01CA73743 (to L.D.F.).
Abbreviation used in this paper
- HMGB1
high mobility group box 1
- Treg
Foxp3+CD4+CD25+ regulatory T cells
- IL-10
interleukin-10
- shRNA
short hairpin RNA
- KD
knockdown
- DC
dendritic cells
- Tr1
CD4+ T regulatory type 1 cells
- RAGE
advanced glycation end products
- TLR
toll-like receptor
- TDLN
tumor-draining lymph nodes
- APC
antigen-presenting cells
Footnotes
Z.L. and Z.Y. designed research; Z.L. performed research; Z.L., L.D.F. and Z.Y. analyzed data; and Z.Y. wrote the paper.
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
- 1.Zou W. Regulatory T cells, tumor immunity and immunotherapy. Nat. Rev. Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 2.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 3.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-β–secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, Zhang Q, Lonning S, Teicher BA, Lee C. Tumor Evasion of the Immune System by Converting CD4+CD25– T Cells into CD4+CD25+ T Regulatory Cells: Role of Tumor-Derived TGF-β. J. Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 5.Sharma MD, Baban B, Chandler P, Hou D-Y, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2, 3-dioxygenase. J. Clin. Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan P-Y, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen S-H. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curiel TJ. Regulatory T cells and treatment of cancer. Curr. Opin. Immunol. 2008;20:241–246. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T regulatory cells. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-4316. Published OnlineFirst March 28, 2011; doi:10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against Cancer: a counterpoint. J. Leukoc. Biol. 2005;78:1043–1051. doi: 10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- 11.O'garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunol. Rev. 2008;223:114–131. doi: 10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 12.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol. Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 14.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 15.Larmonier N, Marron M, Zeng Y, Cantrell J, Romanoski A, Sepassi M, Thompson S, Chen X, Andreansky S, Katsanis E. Tumor-derived CD4(+)CD25(+) regulatory T cell suppression of dendritic cell function involves TGF-beta and IL-10. Cancer Immunol. Immunother. 2007;56:48–59. doi: 10.1007/s00262-006-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, Whiteside TL. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin. Cancer Res. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 17.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Müller S, Iannacone M, Traversari C, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–830. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J. Leukoc. Biol. 2005;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- 20.Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends in Immunology. 2005;26:381–387. doi: 10.1016/j.it.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Bianchi ME, Manfredi AA. High mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol. Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 22.Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ, III, Lotze MT. Endogenous HMGB1 regulates autophagy. J. Cell Biol. 2010;190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, Lotze MT. Masquerader: high mobility group box-1 and cancer. Clin. Cancer Res. 2007;13:2836–2848. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 24.Campana L, Bosurgi L, Rovere-Querini P. HMGB1: a two-headed signal regulating tumor progression and immunity. Curr. Opin. Immunol. 2008;20:518–523. doi: 10.1016/j.coi.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Srikrishna G, Freeze HH. Endogenous Damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11:615–628. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 27.Tang D, Kang R, Zeh HJ, III, Lotze MT. High-mobility group box 1 and cancer. Biochim. Biophys. Acta. 2010;1799:131–140. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rojas A, Figueroa H, Morales E. Fueling inflammation at tumor microenvironment: the role of multiligand/rage axis. Carcinogenesis. 2010;31:334–341. doi: 10.1093/carcin/bgp322. [DOI] [PubMed] [Google Scholar]
- 29.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 30.Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerriero JL, Ditsworth D, Catanzaro JM, Sabino G, Furie MB, Kew RR, Crawford HC, Zong W-X. DNA Alkylating Therapy Induces Tumor Regression through an HMGB1-Mediated Activation of Innate Immunity. J. Immunol. 2011;186:3517–3526. doi: 10.4049/jimmunol.1003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kusume A, Sasahira T, Luo Y, Isobe M, Nakagawa N, Tatsumoto N, Fujii K, Ohmori H, Kuniyasu H. Suppression of Dendritic Cells by HMGB1 Is Associated with Lymph Node Metastasis of Human Colon Cancer. Pathobiology. 2009;76:155–162. doi: 10.1159/000218331. [DOI] [PubMed] [Google Scholar]
- 33.Kim JH, Majumder N, Lin H, Chen J, Falo LD, Jr., You Z. Enhanced immunity by NeuEDhsp70 DNA vaccine is needed to combat an aggressive spontaneous metastatic breast cancer. Mol. Ther. 2005;11:941–949. doi: 10.1016/j.ymthe.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Kim JH, Falo LD, Jr., You Z. Tumor Regulatory T Cells Potently Abrogate Antitumor Immunity. J. Immunol. 2009;182:6160–6167. doi: 10.4049/jimmunol.0802664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Noh HS, Chen J, Kim JH, Falo LD, Jr., You Z. Potent tumor-specific protection ignited by adoptively transferred CD4+ T cells. J. Immunol. 2008;181:4363–4370. doi: 10.4049/jimmunol.181.6.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagenbaugh A, Sharma S, Dubinett S, Wei SH-Y, Aranda R, Cheroutre H, Fowell D, Binder S, Tsao B, Locksley R, et al. Altered immune responses in IL-10 transgenic mice. J. Exp. Med. 1997;185:2101. doi: 10.1084/jem.185.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma S, Stolina M, Lin Y, Gardner B, Miller PW, Kronenberg M, Dubinett SM. T Cell-Derived IL-10 Promotes Lung Cancer Growth by Suppressing Both T Cell and APC Function. J. Immunol. 1999;163:5020–5028. [PubMed] [Google Scholar]
- 38.Hanahan D, Weinberg RA. Hallmarkers of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Gdynia G, Keith M, Kopitz J, Bergmann M, Fassl A, Weber ANR, George J, Kees T, Zentgraf H-W, Wiestler OD, et al. Danger Signaling Protein HMGB1 Induces a Distinct Form of Cell Death Accompanied by Formation of Giant Mitochondria. Cancer Res. 2010;70:8558–8568. doi: 10.1158/0008-5472.CAN-10-0204. [DOI] [PubMed] [Google Scholar]
- 40.Shevach EM. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Huang L-F, Yao Y-M, Zhang L-T, Dong N, Yu Y, Sheng Z-Y. The effect of HMGB1 protein on activity of regulatory T cells after thermal injury in rats. Shock. 2009;31:322–329. doi: 10.1097/SHK.0b013e3181834070. [DOI] [PubMed] [Google Scholar]
- 42.Gnanasekar M, Thirugnanam S, Ramaswamy K. Short hairpin RNA (shRNA) constructs targeting high mobility group box-1 (HMGB1) expression leads to inhibition of prostate cancer cell survival and apoptosis. International Journal of Oncology. 2009;34:425–431. [PubMed] [Google Scholar]
- 43.Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, et al. Blockade of RAGE-amphoterin signaling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.