Abstract
Study Design
A retrospective population based study cross referencing a genealogic database of over 2 million Utah residents with 10 years of clinical diagnosis data from a large tertiary hospital.
Objective
The objective of this study is to determine the presence or absence of an inherited predisposition to the development of cervical spondylotic myelopathy (CSM).
Summary of Background Data
A genetic predisposition for the development of cervical spondylosis has been discussed in the literature with low quality evidence. Families with a high incidence of disease or early onset disease in monozygotic twins have both been reported. However, these suggestions of an inherited predisposition for disease have never been rigorously studied. The purpose of this study is to determine a genetic predisposition among patients diagnosed with cervical spondylotic myelopathy.
Methods
The Utah Population Database (UPDB) combines health and genealogic data on over 2 million Utah residents. ICD-9 codes were used to identify 486 patients in the database with a diagnosis of cervical spondylosis with myelopathy (ICD9 code 721.1). The hypothesis of excessive familial clustering was tested using the Genealogical Index of Familiality (GIF) and Relative risks (RR) in relatives were estimated by comparing rates of disease in relatives with rates estimated in the relatives of 5 matched controls for each case. This methodology has been previously reported and validated for other disease conditions but not for cervical spondylotic myelopathy.
Results
The GIF analysis for patients with CSM showed significant excess relatedness for disease (p<0.001). Relative risks were significantly elevated in both first- (RR=5.21, CI=2.1-13.2, p<0.001) and third-degree relatives (RR=1.95, CI=1.04-3.7, p<0.05).
Conclusions
Excess relatedness of cases and significantly elevated relative risks to both close and distant relatives supports an inherited predisposition to cervical spondylosis with myelopathy.
Level of Evidence
III
Keywords: Cervical spondylosis, Myelopathy, Inheritance, Genetics, Familial predisposition
Introduction
Cervical spondylosis with myelopathy (CSM) is the most commonly acquired cause of spinal cord dysfunction in patients aged greater than 55 years1. Although the symptoms of CSM are most commonly attributed to stenosis from degenerative changes in the spine, individuals with significant spondylotic cord compression may have no symptoms. Furthermore, previous studies have suggested a multifactorial etiology including biochemical changes in the components of the intervertebral disc, bony osteophyte formation, and variable tolerances of spinal cord ischemia2. These other factors, rooted in patient genetics, may contribute to symptomatic rather than asymptomatic disease. A genetic predisposition to the development of degenerative diseases of the cervical spine has been widely speculated 3, 4, 5, 6, 7 but no study to date has shown familial clustering of cervical spondylotic myelopathy on a population based, multigenerational level.
The Utah Population Database (UPDB) is a computerized genealogy of the Utah founding pioneers and their descendents. This database was linked to the University of Utah Health Sciences Center (UUHSC) data warehouse, a resource that contains diagnosis and procedural data on over 1 million patients treated at the University of Utah hospital system. The resultant research tool is a unique resource and has been previously used to evaluate familial clustering in other disease processes8,9,10.
The purpose of this study is to define the familial clustering of cervical spondylotic myelopathy in a large population-based, multigenerational study. We tested the hypothesis of an inherited predisposition using two methods: relative risk in relatives and the Genealogical Index of Familiality.
Materials and Methods
UPDB Data
The Utah Population Data Base is a computerized genealogy of the Utah pioneers and their modern day descendants11 and represents approximately 2.3 million individuals linked into pedigrees from 3 to 11 generations. This genealogy data has been linked to over 1 million patients seen at the University of Utah Hospital and Clinics since 1993. Specific International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes are available for each patient. Selection of the appropriate code (721.1) allowed identification of individuals who had been diagnosed with CSM. To allow investigation of familial patterns, only individuals with 3 or more generations of genealogy data were included.
The review identified 486 patients who had at least 3 generations of genealogy data and at least one diagnosis of CSM (ICD-9 721.1). Two different statistical analyses, the relative risk of disease and Genealogic Index of Familiality, were performed on genetic relationships represented between all CSM patients. For both of these analyses of familial clustering, we compared the observed results among cases to expected results for the Utah population, requiring the identification and analysis of appropriately matched controls. While there is an attempt to link all patients treated at the UUHSC to the Utah genealogical data, and most of them do link, not all patient data could be made accessible for this analysis due to the confidential nature of the information. Instead, a set of patients representing approximately 20% of all UUHSC hospital patients were randomly selected for use as controls. To allow appropriate matching for characteristics that may influence the quality and quantity of genealogical data or record-linking success, multiple cohorts for such characteristics (gender, 5 year birth year range and birth place (Utah or not)) were created. The control set of patients represent 20% of each defined cohort and have at least three generations of genealogy data.
Genealogical Index of Familiality (GIF)
The GIF analysis identifies the degree of relatedness among all cases. It is based on a null hypothesis of no excess familial clustering (or relatedness) among all cases with the diagnosis of interest. The average relatedness of the set of CSM cases was calculated by measuring the pairwise genetic distance between all pairs of cases. The pairwise genetic distance is estimated using the Malecot coefficient of kinship, or the probability that the two individuals share the same allele inherited from a common ancestor at a given locus12. The significance of the hypothesis test is calculated empirically. The same measure of average pairwise relatedness is calculated for all possible pairs among a set of randomly selected, matched controls and this process is repeated 1000 times. The significance is measured as the number of times the control relatedness exceeded the case relatedness. The overall GIF statistic tests for excess relatedness between pairs of cases. The distance GIF test statistic is calculated similarly, but ignores relationships closer than third degree. The distance GIF test statistic thus avoids the confounding variables of environmental, infectious and other exposures that are often common in close relatives and tests for evidence of a genetic contribution to observed familial clustering.
Relative Risks (RR) in Relatives
Estimation of relative risks for a diagnosis among the relatives of cases provides more traditional evidence of a genetic contribution to disease. Excess risk in first-degree relatives may indicate evidence of a genetic contribution, but may also be indicative of a shared physical environment. Excess risks in second- and third-degree relatives, therefore, more strongly support a genetic contribution to disease. Relative risks in first-degree relatives were estimated by counting the number of cases among all first-degree relatives of cases (without duplication), and among all first-degree relatives of 5 randomly selected sets of matched hospital controls. The same methodology was applied to determine second- and third-degree relative risks. For each degree of relative, the significance of the alternative hypothesis RR≥ 1.0 is calculated as a Fisher's exact test, 95% confidence intervals are defined as described in Agresti13.
No patient identifiers were used in this study and all analysis of genetic relationships between affected individuals was non-identifiable. This study was approved by both the University of Utah institutional review board and the oversight body for the Utah Population Database.
Results
GIF Test for Excess Relatedness
To test the null hypothesis of no significant excess relatedness among the CSM cases, the GIF statistic was calculated for the 486 CSM patients and 1,000 sets of matched controls. Each control group has 486 patients, one randomly selected matched control for each case. Table 1 includes the number of cases, the average relatedness of cases (Case Overall GIF), the average relatedness of 1000 matched control analyses (mean Control GIF), the p value for the Overall GIF test of all relationships, and the p-value for the Distant GIF test, which only considers relationships beyond second-degree.
TABLE 1. Genealogicial Index of Familiality (GIF) test of excess relatedness for 486 individuals diagnosed with Cervical Spondylosis with Myelopathy.
| Diagnosis | N | Case Overall GIF | Mean Control GIF | Overall GIF empirical p value | Distance GIF empirical p value |
|---|---|---|---|---|---|
| Cervical Spondylosis with Myelopathy | 486 | 3.74 | 2.75 | <0.001 | 0.291 |
The overall GIF test revealed a significant excess of relationships between cases when compared to controls (p<0.001); this supports the hypothesis of excess familial clustering. The Distance GIF test (p=0.291) did not show a statistically significant excess of distant relationships among CSM patients.
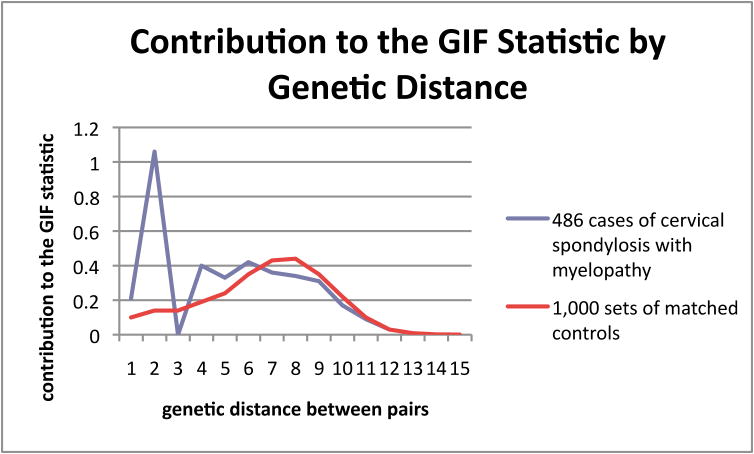
The GIF statistic represents a sum of average pairwise distances by genetic relationship. In order to determine which genetic relationships cases differ from controls, we have displayed the distribution of the GIF statistic for different pairs of genetic relationships observed for cases (and for controls) in Figure 1. The X-axis represents the genetic distance (or relationship) between the pairs of individuals (1= parent/offspring, 2=siblings/grandparent/grandchild, 3=avunculars, 4=first cousins, etc…) for cases and controls. The Y-axis reveals the value of the GIF statistic that was contributed for each genetic distance. Figure 1 shows that most of the excess relatedness of CSM in cases compared to controls was observed for close relationships, which explains the finding of significant excess familial clustering (p<0.001). Although an excess relatedness of cases extends to genetic distance = 6 (second cousins), the Distant GIF test did not detect a significant excess of relationships beyond second degree (genetic distance = 3).
Figure 1. Contribution to the Genealogical Index of Familiality Statistic by Genetic Distance.
Comparison of Genealogical Index of Familiality (GIF) contribution to genetic distance reveals and increased risk of cervical spondlylotic disease in the relatives of affected individuals
Relative Risks
The estimated Relative Risks for CSM in first-, second- and third-degree relatives of patients with CSM are shown in Table 2. The table shows the number of affected relatives of cases and of controls observed, the total number of relatives of cases and controls considered, the estimated relative risk (RR), the significance of the Fisher's Exact Test for the 2×2 table of cases versus controls, and the 95% confidence interval for the RR estimate. The relative risk of developing CSM was significantly elevated in both first- and third-degree relatives of affected individuals, with no cases found among second-degree relatives. The lack of cases among second-degree relatives is not surprising given the narrow time range (13 years) of our hospital diagnosis data. Second-degree relatives are usually of a different generation and thus unlikely to be diagnosed with the same disease as their younger or older family member in a short time period. The relative risk results are in agreement with the GIF analysis and strongly support a genetic contribution to the development of CSM.
TABLE 2. Relative risks (RR) in relatives of individuals Cervical Spondylosis with Myelopathy.
| Relative | # affected Case/controls | # relatives Case/controls | RR | P value | Confidence Interval |
|---|---|---|---|---|---|
| First | 9/9 | 3827 / 20029 | 5.21 | 0.0009 | 2.07 – 13.1 |
| Second | 0/21 | 11371/60869 | 0 | 0.07 | --- |
| Third | 13/36 | 27759 / 150105 | 1.95 | 0.04 | 1.04 - 3.7 |
RR- relative risk; CI – confidence interval
Discussion
Previous reports have suggested a familial predisposition to cervical spondylotic myelopathy but have failed to prove this relationship with a large population based study. The results of this study support a heritable predisposition to CSM, proving an excess relatedness of cases and significantly elevated relative risks for both close and distant relatives in the Utah genealogical database.
The possibility of a genetic contribution to the development of CSM was first published in 1969 by Bull et al3. The authors evaluated several hundred cervical spine radiographs in an attempt to understand cervical spondylosis, noting a higher prevalence of CSM among both monozygous and dizygous twins. They concluded that approximately 75% of monozygotic twins and 25% of dizygotic twins had radiographs that were the “same”. Another study by Sambrook et al6 evaluated the cervical and lumbar spine MRI's of 172 monozygotic and 154 dizygotic twins. Adjusting for age, weight, smoking, occupational manual labor and exercise, the authors concluded that heritability accounted for 73% of all MRI findings and 79% of findings among patients with severe disease. Although these, and other studies like them, suggest a genetic contribution, twin studies suffer from several inherent weaknesses. Twins nearly universally share similar environmental risk factors such as diet and activity level that cannot be adjusted for. Further, twin studies usually have a very small number of patients with the disease of interest, limiting statistical power.
With building interest in the genetic contribution to cervical spondylotic myelopathy, several authors have published case reports of families with curiously high incidences of disease. Yoo et al.7 published a report in 1998 of similar cases of multilevel cervical spondylosis with myelopathy requiring surgical intervention in a woman and both of her two sons. The authors implied that this may represent an extreme case of a genetic influence seen in all cases of CSM or it may denote the presence of a separate entity called familial cervical spondylosis. In 2007, Mukerjii et. Al14 published a case report of identical twins with cervical myelopathy at a young age requiring curative surgery. They offered this example as evidence of a genetic contribution to the development of cervical spondylotic myelopathy. While these case reports have highlighted the potential genetic contributions to CSM, the present study extends this conclusion more generally using objective measures in a large population.
To our knowledge, the literature has been absent of a population-based study to determine the genetic influence on cervical spondylosis with myelopathy. This study provides strong evidence to support a genetic basis for disease as they avoid the recall and ascertainment bias usually present in clinical studies. The methodology utilized in this study has previously been used to demonstrate a familial predisposition to breast cancer15, asthma10, and rotator cuff disease8, among other phenotypes. Identification and study of the extended high-risk pedigrees represented in the UPDB has led to the identification of individual genes associated with a disease process (ex: BRCA1 in breast and ovarian cancer16). Our study solidifies early suggestions of a genetic predisposition for CSM, should promote further research into the possible causes of the observed heritable predisposition for disease, and has identified a resource of Utah high risk pedigrees that can be used to test and understand candidate genes.
The primary limitation of our study is the use of ICD-9 data to identify cases of interest. These codes are dependent upon individual clinicians and accuracy may vary depending upon physician experience and specialization. The use of diagnostic codes prohibited analysis of disease severity or response to treatment. Any affected individual without an appropriate ICD-9 code, who was diagnosed before 1993, was diagnosed at another facility, or was not represented in the Utah genealogy was not included in this study. Such censoring applies across the data source uniformly to relatives of both cases and controls and thus should not affect the overall results, except to lower our power to identify genetic influence. The population of Utah has been shown to be genetically similar to the U.S. and to the Northern European population from which of the Utah founders came17. Therefore the results of this study should be generalizable to the U.S and Northern European populations.
In conclusion, cervical spondylosis with myelopathy likely has a multifactorial etiology including contributions from age dependent degeneration, mechanical stress, biochemical factors and genetics. This study supports a genetic basis to symptomatic CSM. This study has also allowed identification of high-risk CSM pedigrees in the UPDB that can be studied to identify genes responsible for this predisposition. Identification of the specific genetic products responsible for disease may help in the development of potential biologic interventions to prevent and/or treat cervical spondylotic myelopathy.
Acknowledgments
This work was partially supported by National Library of Medicine grant LM009331 (to Lisa Cannon Albright). Partial support for all datasets within the Utah Population Database (UPDB) was provided by the University of Utah Huntsman Cancer Institute.
References
- 1.Fehlings M, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine. 1998;23:2730–7. doi: 10.1097/00007632-199812150-00012. [DOI] [PubMed] [Google Scholar]
- 2.Baptiste D, Fehlings MG. Pathophysiology of cervical myelopathy. The Spine Journal. 2006;6:190–7. doi: 10.1016/j.spinee.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Bull J, el Gammal T, Popham M. A possible genetic factor in cervical spondylosis. Br j Radiol. 1969;42:9–16. doi: 10.1259/0007-1285-42-493-9. [DOI] [PubMed] [Google Scholar]
- 4.Lestini W, Wiesel SW. The pathogenesis of cervical spondylosis. Clin Orthop Relat Res. 1989;239:69–93. [PubMed] [Google Scholar]
- 5.Palmer P, Stadalnick R, Arnon S. The genetic factor in cervical spondylosis. Skeletal Radiology. 1984;11:178–82. doi: 10.1007/BF00349491. [DOI] [PubMed] [Google Scholar]
- 6.Sambrook P, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366–72. doi: 10.1002/1529-0131(199902)42:2<366::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Yoo K, Origiano TC. Familial Cervical Spondylosis. J Neurosurgery. 1998;89:139–41. doi: 10.3171/jns.1998.89.1.0139. [DOI] [PubMed] [Google Scholar]
- 8.Tashjian RZ, Farnham James, Albright Frederick, et al. Evidence for an Inherited Predispostion Contributing to Risk for Rotator Disease. JBJS Am. 2009;91:1136–42. doi: 10.2106/JBJS.H.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albright F, Orlando P, Pavia AT, et al. Evidence for a heritable predisposition to death due to influenza. J Infect Dis. 2008;197:18–24. doi: 10.1086/524064. [DOI] [PubMed] [Google Scholar]
- 10.Teerlink C, Hegewald MJ, Cannon-Albright LA. A genealogical assessment of heritable predisposition to asthma mortality. Am J Respir Crit Care Med. 2007;176:865–70. doi: 10.1164/rccm.200703-448OC. [DOI] [PubMed] [Google Scholar]
- 11.Skolnick M. The Utah genealogical database: a resource for genetic epidemiology. In: Cairns J, Lyon JL, Skolnick M, editors. Banbury Report No 4. New York: 1980. pp. 285–97. [Google Scholar]
- 12.Malecot G. Les Mathematiques de L'Heredite ed. Paris: Masson; 1948. [Google Scholar]
- 13.Agresti A, Min Y. Simple improved confidence intervals for comparing matched proportions. Statistics in Medicine. 2005;24:729–40. doi: 10.1002/sim.1781. [DOI] [PubMed] [Google Scholar]
- 14.Mukerji N, Sinar E. Identical twins with cervical myelopathy: a case for hereditary cervical spondylosis? J Neurosurg Spine. 2007;6:344–349. doi: 10.3171/spi.2007.6.4.10. [DOI] [PubMed] [Google Scholar]
- 15.Allen-Brady K, Camp NJ, Ward JH, et al. Lobular breast cancer: Excess familiality observed in the Utah Population Database. Int J Cancer. 2005;117:655–61. doi: 10.1002/ijc.21236. [DOI] [PubMed] [Google Scholar]
- 16.Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 17.McLellon T, Jorde LB, Skolnick MH. Genetic distances between the Utah Mormons and related populations. Am j Hum Genet. 1984;36:836–57. [PMC free article] [PubMed] [Google Scholar]