Abstract
Renal amyloidosis is a detrimental disease caused by the deposition of amyloid fibrils. A child with renal amyloidosis may present with proteinuria or nephrotic syndrome. Chronic renal failure may follow. Amyloid fibrils may deposit in other organs as well. The diagnosis is through the typical appearance on histopathology. Although chronic infections and chronic inflammatory diseases used to be the causes of secondary amyloidosis in children, the most frequent cause is now autoinflammatory diseases. Among this group of diseases, the most frequent one throughout the world is familial Mediterranean fever (FMF). FMF is typically characterized by attacks of clinical inflammation in the form of fever and serositis and high acute-phase reactants. Persisting inflammation in inadequately treated disease is associated with the development of secondary amyloidosis. The main treatment is colchicine. A number of other monogenic autoinflammatory diseases have also been identified. Among them cryopyrin-associated periodic syndrome (CAPS) is outstanding with its clinical features and the predilection to develop secondary amyloidosis in untreated cases. The treatment of secondary amyloidosis mainly depends on the treatment of the disease. However, a number of new treatments for amyloid per se are in the pipeline.
Keywords: Renal amyloidosis, Children, Autoinflammatory diseases
Introduction
The amyloidoses constitute a group of diseases characterized by extracellular tissue deposition of fibrils composed of low-molecular-weight subunits of a variety of proteins [1–3]. All amyloid fibrils appear faintly red on Congo red staining on microscopic examination and show a typical apple-green birefringence under polarized light. The site of deposition relies on the type of amyloidosis, which can be acquired or hereditary, localized or systemic. At least 25 different precursors of amyloid fibrils are now known; the main protein types leading to amyloidosis are shown in Table 1 [1–3].
Table 1.
Important amyloid fibrils for nephrologists
| Amyloid protein | Precursor protein | Clinical syndrome |
|---|---|---|
| AA | Serum amyloid A protein | Secondary, reactive, associated with chronic inflammatory diseases |
| AL | Monoclonal Ig light chains | Primary, associated with monoclonal plasma cell dyscrasias |
| AH | Monoclonal Ig light chains | Primary, associated with monoclonal plasma cell dyscrasias |
| Aβ2M | β2-microglobulin | Associated with dialysis |
| AFib | Fibrinogen α-chain | Familial autosomal dominant systemic amyloidosis |
| ACys | Cystatin C | Hereditary cerebral hemorrhage with cerebral and systemic amyloidosis |
| ALys | Lysozyme | Familial autosomal dominant systemic amyloidosis |
| AApoAI | Apolipoprotein AI | Familial |
| AApoAII | Apolipoprotein AII | Familial |
| ATTR | Transthyretin | Familial |
| AGel | Gelsolin | Familial |
In pediatrics, the most common form of amyloidosis is reactive AA amyloidosis due to chronic inflammatory diseases. AA amyloidosis secondary to chronic infectious diseases was common in the early 20th century. However, due to the advances in the management of chronic infections such as tuberculosis, this is no longer the case. Juvenile idiopathic arthritis (JIA) was also a cause of secondary amyloidosis, however, with better recognition and treatment, as well as the introduction of biologics, secondary amyloidosis rarely occurs in JIA in this decade. The main cause of childhood amyloidosis is familial Mediterranean fever (FMF) and the other autoinflammatory diseases. AL amyloidosis, which is generally seen in the elderly, is the most common type of amyloidosis in the developed countries today. β2-microglobulin amyloidosis (Aβ2M amyloidosis) is seen in patients with renal failure. AFib and ACys amyloidoses are hereditary, autosomal dominant, and late-onset diseases having rarely been reported in children [1–3].
This review discusses the pathogenesis, common causes such as autoinflammatory diseases with special emphasis on FMF, clinical manifestations, diagnosis, and treatment of amyloidosis.
Pathogenesis
The production of precursor proteins, deposition of amyloid fibrils, and tissue/organ damage are the three major steps in the pathogenesis of amyloidosis. The nature of the precursor protein yields the main component of amyloid fibrils and the propensity for forming amyloid is related to the characteristics of this precursor protein. The amyloidogenic potential of precursor proteins is mainly affected by three factors: overproduction, decreased clearance, and their stability [1–7].
Overproduction
The production of precursor proteins are increased in several conditions such as chronic inflammatory diseases (AA amyloidosis) and immunoglobulin light chain amyloidosis (AL amyloidosis) [2, 4]. The precursor of amyloid A protein is serum amyloid A (SAA), an acute phase protein produced in response to inflammation [8, 9]. Serum amyloid A is produced by inflammatory signals, interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6. Polymerization of SAA into amyloid fibrils requires removal of the C-terminal of the AA protein [10]. The persistent augmentation of an inflammatory pathway through the innate immune system might be crucial in the deposition of the amyloid protein leading to the clinical picture of renal amyloidosis [8].
Decreased clearance
In healthy individuals, β2-microglobulin is synthesized at a constant rate, but retention of it occurs in renal failure. In the case of amyloidosis associated with β2-microglobulin, the clearance of β2-microglobulin is decreased and plasma levels can be elevated as much as 60-fold [5].
Unstable proteins
Genetically transmitted mutations make unstable proteins prone to fibril conversion. Transthyretin [TTR], apolipoprotein A [apoA]-I, apoA-II, fibrinogen, gelsolin, cystatin C and lysozyme are examples of mutated proteins associated with hereditary amyloidosis [3, 7].
Clinical manifestations due to amyloidosis
Amyloidosis is a multisystemic disease. Therefore, the clinical manifestations due to amyloidosis vary widely and depend on the involved organ(s) and the amount of amyloid fibrils deposited. Symptoms and findings due to amyloid deposition may be accompanied by the clinical manifestations of the underlying disease. Amyloid fibrils are most commonly deposited in the kidneys, but may also attack the heart, peripheral nerves, thyroid, gastrointestinal system, and bone marrow. The organs involved are affected by the type of amyloid fibrils. Clinically, it is difficult to distinguish AA and AL amyloidosis from each other because of overlapping clinical presentations. Although more than one organ is generally affected, localized forms can occur as well. Involvement of heart and kidneys are the most important predictors affecting survival. On the other hand, gastrointestinal amyloidosis may also have devastating complications [1, 3, 9]. In secondary amyloidosis, kidney is the most affected organ, the others are rarely involved.
Kidney
Asymptomatic proteinuria is the most common initial presentation of kidney disease. Thus, urinalysis should be routinely examined in patients with a disease associated with the risk of secondary amyloidosis. The most common and earliest sign of amyloidosis in FMF is proteinuria, gradually progressing to nephrotic syndrome and/or renal dysfunction. In the series reported by the Turkish FMF study group, the presenting clinical features of the patients with amyloidosis secondary to FMF were as follows: 32% proteinuria, 40% nephrotic syndrome, and 28% chronic renal failure [11]. Hematuria and hypertension almost never occurs [8]. Edema can exist in nephrotic patients [12].
The amount of proteinuria and renal function vary in each case, which is related to the amount and/or site of amyloid deposition. Uda et al. [13] demonstrated that patients having glomerular amyloid deposition are more common and have a poorer prognosis than patients having vascular and tubular amyloid deposition in rheumatoid arthritis-related AA amyloidosis. Nishi et al. [14] showed that 10–30% of patients with renal amyloidosis might have only mild proteinuria and normal renal function. Some patients present with renal failure with little or no proteinuria due to primary deposition of amyloid fibrils to blood vessels or tubules. Signs of tubular dysfunction such as type 1 (distal) renal tubular acidosis, nephrogenic diabetes insipidus related polyuria, [15] and acquired Fanconi syndrome [16] have been rarely reported as well.
Heart
Systolic or diastolic dysfunction, and arrhythmia are the main presenting features of heart involvement. Cardiac involvement is more rare in AA amyloidosis compared to AL amyloidosis [17–19] and may progress rapidly.
Gastrointestinal tract
The gastrointestinal system is one of the common affected sites in systemic amyloidosis. Since the rectum is commonly affected and easily reachable, rectal biopsy is the initial diagnostic tool used in most of the cases. Gastrointestinal presentations are frequently nonspecific and may include dyspepsia, abdominal pain, nausea, diarrhea, gastrointestinal bleeding, malabsorption, constipation, and obstruction [20, 21]. Acute gastrointestinal system symptoms may resemble FMF attacks.
Liver and spleen
The deposition of amyloid fibrils in liver and spleen leads to hepatomegaly and splenomegaly [22–24]. Serum liver enzymes can elevate, which makes prescription of drugs difficult. Splenomegaly can cause hypersplenism and rarely splenic rupture [25, 26].
Hematopoietic system
Amyloid fibrils can accumulate in the bone marrow [27]. Amyloidosis can cause bleeding diathesis due to factor X deficiency, liver disease, or infiltration of blood vessels [28]. Thrombotic events due to underlying disease, nephrotic syndrome, and amyloidosis can also be seen [29].
Nervous system
Mixed sensory and motor peripheral neuropathy and autonomic neuropathy may occur in amyloidosis albeit rare in the secondary forms. Neuropathy is a prominent feature in some of the hereditary amyloidoses (called familial amyloidotic polyneuropathy) [30]. Central nervous system involvement is unusual in patients with AA amyloidosis.
Miscellaneous
Infiltration of amyloid fibrils may cause enlargement of muscles and arthropathy. The clinical manifestations of Aβ2M amyloidosis include carpal tunnel syndrome, bone cysts, spondyloarthropathy, pathologic fractures, and swollen painful joints [5]. Deposition of amyloid fibrils in endocrine glands can affect organ function causing adrenal insufficiency, hypothyroidism, and hypogonadism [31]. Symptoms related to the respiratory system and skin have also been reported.
Diagnosis
Suspicion is essential in subjects having an underlying disease with a potential to cause amyloidosis. Amyloidosis should be suspected typically in a patient who presents with proteinuria and who has one of the autoinflammatory syndromes. However, organs other than the kidney may be affected as well. In fact, in patients who are candidates for this complication, secondary amyloidosis should also be considered in the differential diagnosis of cardiomyopathy, peripheral neuropathy, hepatomegaly, or in the presence of symptoms related to the gastrointestinal tract.
The diagnosis of amyloidosis is based on the demonstration of amyloid fibrils in the biopsy of the involved tissue (Fig. 1). In addition to renal biopsy, rectal or abdominal fat biopsies may also reveal amyloid deposition. Although renal, rectal, or abdominal biopsies are commonly used, tissues obtained by any diagnostic intervention or surgical procedure may yield the diagnosis: for example a liver biopsy done for hepatomegaly, upper gastrointestinal endoscopy done for vomiting, intestinal biopsy done for malabsorption, pleural biopsy done for pleural effusion, lung biopsy done for restrictive lung disease, bone marrow biopsy done for anemia, excision of a local mass or surgery for carpal tunnel syndrome can reveal amyloidosis [2, 3, 9].
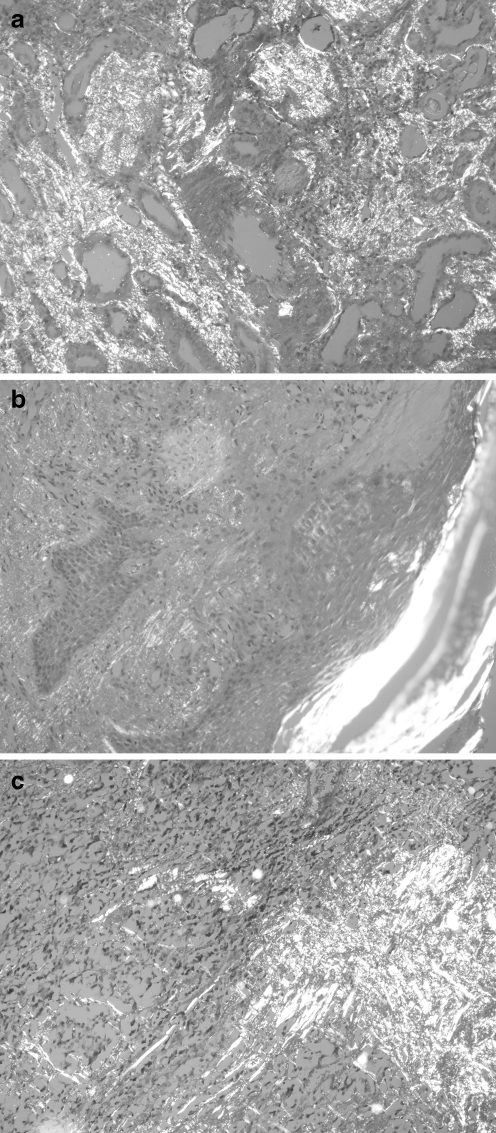
Fig. 1.
Typical apple-green birefringence under polarized light, Congo red staining: a kidney (×200), b skin (×200), and c thyroid gland (×400)
The deposited amyloid fibrils are extracellular, eosinophilic, and metachromatic on light microscopy. Congo red staining is necessary for diagnosis. Amyloid fibrils appear faintly red on Congo red staining and show the characteristic apple-green birefringence under polarized light. Specific investigation is needed for detection of the types of amyloid fibrils. Immunofluorescence or immunohistochemical staining of the tissue using antibodies that are directed against known amyloidogenic proteins are easy, common, and inexpensive methods of typing amyloid fibrils. Immunoelectron microscopy, Western blotting, mass spectrometry, and amino acid sequencing are methods that can be used in the typing of amyloid deposits [32, 33].
Actually, infiltrative renal diseases including amyloidosis must be considered in the differential diagnosis of all patients having chronic kidney disease and normal or large sized kidneys. AA amyloidosis can also be diagnosed using serum amyloid P component scintigraphy [34].
Reporting of renal biopsies The histopathologic changes comprise a spectrum in renal amyloidosis. Clear relationships between the extent of amyloid deposition and the severity of clinical manifestations have not been demonstrated. The studies investigating clinico-pathologic correlations face some difficulties due to the absence of standardization of the relevant biopsy reports. This issue was discussed at several meetings [32, 33] and Sen and Sarsik proposed a histopathologic classification, scoring, and grading system aiming to standardize the biopsy reports of renal amyloidosis [33]. They divided glomerular amyloid deposition into six classes, similar to the classification of systemic lupus erythematosus. Amyloid depositions (glomerular, vascular, and interstitial) and other histopathologic lesions such as interstitial fibrosis, atrophy, inflammation, and glomerular sclerosis were scored. The sum of these scores was termed the renal amyloid prognostic score and was divided into three grades: early, late, or advanced. Ninety percent of the investigated cases had AA amyloidosis mostly related to FMF. Because of the inadequacy of the patients’ records and outcomes, different therapy regimes, and etiologies, clinical validation of this study has not been completed, but this histopathologic classification, scoring, and grading system is promising for further research.
Underlying causes of secondary amyloidosis
Juvenile idiopathic arthritis
Juvenile idiopathic arthritis is the most common rheumatic disease of childhood. The diagnostic criteria require a child of less than 16 years of age with arthritis for at least 6 weeks’ duration with exclusion of other identifiable causes of arthritis. Juvenile idiopathic arthritis has been classified into seven subtypes [35]. Secondary amyloidosis used to be one of the most serious and fatal complications of JIA. Before the 1990s, the incidence of secondary amyloidosis was as high as 2.1%, with lower rates in North America. The form of JIA is important; amyloidosis has been observed mainly in systemic and polyarticular forms [36]. Secondary amyloidosis due to JIA has been decreasing dramatically in recent years, which is due to earlier recognition and better management of the disease and the introduction of new biologic agents. In this decade, amyloidosis is a rare entity in JIA.
Autoinflammatory syndromes
Autoinflammatory syndromes are defined as a group of diseases characterized by unprovoked inflammatory episodes and the lack of autoantibodies [37]. Most of the well-defined diseases in this group are monogenic diseases. The spectrum of autoinflammatory diseases has now expanded from the monogenic diseases such as FMF and TNF-receptor-associated periodic fever syndrome (TRAPS) to the more common polygenic diseases such as Behçet’s disease, Crohn’s disease, and others [38]. The subject of autoinflammatory diseases has become an exciting and growing but debatable one; it will be a difficult challenge to precisely classify inflammatory diseases in the spectrum of autoinflammation to autoimmunity.
Familial Mediterranean fever
Familial Mediterranean fever (FMF) is an autoinflammatory disease characterized by recurrent attacks of fever and serositis. FMF is of interest to nephrologists for a number of reasons:
FMF is the only kidney disease that can be prevented by a cheap drug, where secondary amyloidosis is prevented by colchicine.
FMF is nature’s experiment on inflammation and understanding its pathogenesis teaches us about the pathway of inflammation in other diseases as well.
It is the most frequent autoinflammatory disease around the world, reaching a frequency of 1/500–1/1,000 in certain ethnic groups such as the Jewish, Turkish, Armenian, and Arabic populations [39–41].
And last but not least, FMF is associated with an increased rate of vasculitic diseases that may affect the kidney as well [8].
FMF is a monogenic autoinflammatory disease associated with mutations in a gene called MEFV (from MEditerranean FeVer), identified more than 10 years ago. The mutations in the MEFV gene are associated with excess IL-1 production [42]. This results in clinical attacks of inflammation in the form of fever and serositis in the form of peritoneal, pleural, or synovial inflammation along with increased acute-phase reactants. FMF patients may have laboratory parameters of inflammation in between the attacks as well with increased basal levels of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and SAA [43]. This inflammatory state is what probably results in the variety of problems related to clinical inflammation observed in patients with FMF [8].
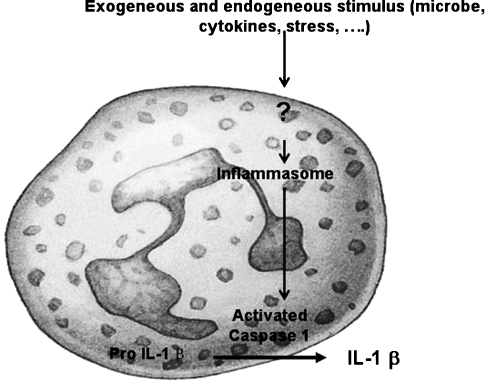
Pathophysiology The MEFV gene encodes a protein called pyrin (from the Greek word for fever) [44]. This protein has also been called marenostrin (from the Latin words mare nostrum, the Mediterranean Sea) by the French Consortium who simultaneously coded the gene, however, the former name has been used more often [45]. FMF is basically inherited in an autosomal recessive fashion. As stated above, the carrier rate is very high in the Eastern Mediterranean. In the past years we have studied the possible causes of this high carrier rate, hypothesizing that the carrier rate offered some advantage to the settlers in the Eastern Mediterranean [46]. However, having failed to blame a certain pathogen, we and others have suggested that the carriers had the simple advantage of mounting a better innate immune response to common insults [47, 48].Indeed mutations in pyrin result in increased inflammation through the innate immune system [42]. Pyrin is localized in the cells of innate immunity; mainly the neutrophils and the monocytes, and is an important element of the inflammasome. Once a ‘danger’ signal is received, the inflammasome assembles. This assembly activates caspase leading to IL-1β production, resulting in high basal levels of acute-phase reactants including the SAA protein [38]. It is believed that persistently elevated SAA reflecting ongoing inflammation plays the key role in the development of amyloidosis (Fig. 2). As the studies on mutations for FMF progressed, we have become aware that there were patients with clear phenotypes of the disease but who lacked mutations on both alleles [38]. Recent studies have addressed this problem, however, we lack definite answers to how patients with one mutation only express the phenotype. Another interesting aspect of this hereditary disease is the impact of environment on the course and possibly the occurrence of kidney complications [49, 50].
Fig. 2.
The role of inflammasome in IL-1β production
Clinical and laboratory features of FMF FMF is typically characterized by recurrent attacks of fever and serositis along with high acute-phase reactants. Onset of the disease occurs before the age of 30 in most of the patients [51]. In a multicenter study from Turkey, representing the largest series of FMF, the mean ages of onset and diagnosis were 9.6 ± 8.6 years and 16.4 ± 11.6 years, respectively [11].FMF attacks are typically accompanied by high fever of 38.5–40.0°C. Recurrent attacks classically last 1/2–3 days on average and resolve spontaneously. The frequency of the cardinal signs and symptoms of FMF are: fever (%92.5), peritonitis (% 93.7), arthritis (47.4%), pleurisy ( 31.2%), amyloidosis (12.9%), and non-amyloid glomerular disease (0.8%) [11]. Other signs and symptoms such as headache, aseptic meningitis, pericarditis, splenomegaly, acute scrotum, and febrile myalgia scattered purpura have also been reported [52, 53]. Symptoms may not always be associated with attacks. For example, erysipelas-like erythema and exertional myalgia of the calves and feet are symptoms that are not always related to the attacks [8].Acute FMF attacks are associated with increases in ESR, CRP, SAA, fibrinogen, and white blood cell count, which return to lower levels after resolving of the symptoms. Levels may remain above normal especially in untreated patients and even carriers [54]. Every patient with FMF or with periodic fever disease should have a work-up of complete blood count, ESR, CRP, SAA if possible, and urinalysis. If the child has not been treated properly and if secondary amyloidosis develops, urinalysis will reveal proteinuria [52]. If proteinuria is not diagnosed, it will progress to full-blown nephrotic syndrome [8].The differential diagnosis of the patient with FMF includes all the other autoinflammatory diseases. These diseases share the common phenotype of recurring inflammatory attacks but all have certain specific features. These will also all be mentioned later in this review.
Factors affecting the development of amyloidosis in FMF The frequency of amyloidosis in FMF patients is not always associated with the frequency of attacks. However, subclinical inflammation reflected with persistently high SAA levels is considered to play a crucial role in the deposition of amyloid fibrils in these patients. The use of colchicine prevents the development of amyloidosis [55]. Many studies have focused on the role of gender, genetic, and environmental factors in the development of amyloidosis in patients with FMF:
Male gender and the presence of secondary amyloidosis in the family are significantly associated with the development of amyloidosis [56].
Although still controversial, many studies have indicated that mutations in Exon 10, in the region between 680 and 694, and especially M694V homozygosity, are associated with amyloidosis [11, 57, 58]. However, patients with different mutations may also develop amyloidosis [59].
A number of polymorphisms related to the modifier genes have been proposed as factors increasing the development of amyloidosis: SAA1.1α/α genotype is associated with a seven-times-higher risk of amyloidosis as compared to the other SAA genotypes and this was confirmed in the Armenian, Jewish, and Turkish populations [60, 61].
Environment: As stated above, environment is believed to affect the course of the disease. In their multicenter multiethnic study, Toitou et al. [49] suggested that country of recruitment was the key risk factor for renal amyloidosis in FMF and they suggested that the patient’s country should be considered along with MEFV genotype as an indication for prophylactic colchicine.
Other glomerular lesions in FMF Renal involvement other than amyloidosis can be seen in FMF patients. In a nationwide study enrolling 2,838 FMF patients from Turkey, 22 had nonamyloid glomerular diseases [11]. Various types of glomerulonephritis have been reported in this study [11]. Microscopic hematuria alone occurs in fewer than 5% of FMF patients [51, 57]. Kidney biopsy is probably not indicated if hematuria does not persist.
Vasculitis involving the kidney in FMF Certain rheumatic diseases have an increased prevalence among patients with FMF [53]. We initially noted in a small series that certain vasculitides were increased among these patients. In the aforementioned multicenter study from Turkey, polyarteritis nodosa (PAN) (0.9%) and Henoch–Schönlein purpura (HSP) (2.7%) indeed had a higher frequency, as expected in the normal population [11]. Interestingly, in patients with PAN and FMF, there are certain specific features such as the overlap of microangiopathic polyangiitis (MPA) and classic PAN, the younger age at onset, and better prognosis [62].
Treatment in FMF Colchicine treatment has changed the course of the disease by both reducing attack frequency and severity and preventing amyloidosis. Goldinger first described its effectiveness in 1972 and since then colchicine became the drug of choice for FMF [63]. Colchicine, an alkaloid, binds to β-tubulin hindering its polarization with consequent defective transfer and mitosis, inhibition of neutrophil chemotaxis, and reduced expression of adhesion molecules [51]. Colchicine is mainly metabolized by the liver. Colchicine is started at lower doses in children (≤ 0.5 mg/day for children <5 years of age, 1.0 mg/day for children 5–10 years of age and 1.5 mg/day for children >10 years of age) compared to adults and the dose should be monitored according to the clinical symptoms and SAA levels [64]. Despite the narrow therapeutic index of the drug, the recommended dose is safe, and side-effects are minimal. The most frequent side-effect is diarrhea. Modification of diet, split doses, and dose reduction is recommended for side-effects. Once symptoms resolve, dosage should be increased in a stepwise fashion [64]. Resistance to colchicine is very rare [52]. If the diagnosis of amyloidosis is established, colchicine dose should be increased to 2 mg/day [64]. Colchicine treatment is the mainstay against the prevention of amyloidosis [55]. Although colchicine treatment is not expected to dissolve amyloid fibrils, it has been shown that colchicine therapy may result in some stabilization or resolution of the proteinuric stage [65, 66]. This may be due to the prevention of further deposition in the renal tissue or the possible antifibrotic effect of colchicine [67]. One of the most important prognostic factors in amyloid nephropathy is interstitial fibrosis [68]. In experimental settings, colchicine impairs collagen synthesis and enhances collagenase activity [69].Renal failure is associated with an increased risk of colchicine toxicity [64, 66, 70, 71]. For patients with severe renal failure (GFR <10 ml/min), the dosage should be reduced by 50% [64, 72].
Cryopyrin-associated periodic syndromes
Cryopyrin-associated periodic syndromes (CAPS) are a group of rare autoinflammatory diseases including familial cold urticaria (FCAS), Muckle-Wells syndrome (MWS), and chronic infantile neurologic cutaneous articular syndrome (CINCA), also known as neonatal onset multisystem inflammatory disease (NOMID). They represent a continuum of severity with FCAS being the mildest and NOMID/CINCA being the most severe disease. CAPS are all caused by mutations in CIAS1 encoding cryopyrin, which is a component of the IL-1β inflammasome [38, 73, 74]. These are all transmitted in an autosomal-dominant fashion.
Clinical and laboratory findings of CAPS Patients with FCAS present with a history of cold-induced episodes of inflammation that manifest with fever, urticaria-like rash, joint pain, conjunctivitis, and headaches. The peak of the attack occurs at 6–8 h and lasts up to 24 h. Amyloidosis is a rare complication of FCAS (2–4%) [75].In MWS, the typical attack includes fever, rash, arthralgia, arthritis, myalgia, headaches, conjunctivitis, episcleritis, and uveitis lasting up to 3 days. Progressive sensorineural hearing loss develops in the second and fourth decades. Amyloidosis develops in 25% of the cases [73, 76].The onset of CINCA-NOMID is at or within several weeks of birth. It is characterized by urticaria-like rash, fever, chronic aseptic meningitis, eye findings including conjunctivitis, uveitis, and papillitis of the optic nerve. By about 2 years, 50% of patients develop a severe arthropathy. Patients have typical morphological changes of short stature, frontal bossing, macrocephaly, saddle nose, short, thick extremities with clubbing of fingers, and wrinkled skin. If untreated, 20% die by age 20 years, and others develop amyloidosis [74, 76].
Treatment in CAPS In FCAS and MWS, corticosteroids may be used but are needed frequently and may cause significant side-effects. Anti-IL-1 agents are very effective in alleviating symptoms and decreasing inflammation in all CAPS patients [76–78].
Hyperimmunoglobulinemia D and periodic fever syndrome
Hyperimmunoglobulinemia D syndrome (HIDS) is an autosomal recessively inherited disease characterized by fever, arthralgia, abdominal pain, diarrhea, maculopapular rash, and lymphadenopathy lasting 3–7 days. The disease is due to mutations in the mevalonate kinase gene [79]. Mutations in this pathway are associated with elevated IL1. A number of treatments have been tried including biologics. Secondary amyloidosis has been reported in 3% of the patients, which is rarer than that reported for the other monogenic autoinflammatory syndromes [43, 80].
Tumor necrosis factor receptor-associated periodic syndrome
Tumor necrosis factor receptor-associated periodic syndrome (TRAPS) is an autosomal-dominant disorder characterized by fever, abdominal pain, pleurisy, myalgia, arthritis, rash, and periorbital edema often lasting more than 1 week [81]. When compared to FMF, attacks take longer and eye and skin symptoms are more prominent [82]. TRAPS has been reported in patients of many ethnicities [83]. It is the second most common periodic fever disorder with mutations in TNFRSF1A [81]. The 55-kDa TNF receptor is widely expressed on cell membranes and mediates proinflammatory action [84]. Patients with TRAPS have an increased state of inflammation due to a shedding defect in the TNF-alpha receptor or possibly due to a misfolding disorder in the receptor [38].
Clinical attacks may include peritoneal inflammation or pleurisy or both. Cutaneous symptoms consist of macular areas or erythema, which is migratory in nature. Ocular inflammation with per-orbital edema or conjunctivitis is common. Arthralgia is more prominent than arthritis [82, 85].
Acute-phase reactants are increased during attacks and may remain elevated in between clinical attacks. The specific diagnosis is defined by mutations in TNFRSF1A [81, 82]. Treatment depends on the severity of the disease. For patients with infrequent attacks and normal SAA, prednisone during attacks may be effective [82]. For patients with more severe disease, etanercept or adalimumab as anti-TNF agents were found to be effective [43, 82]. IL-1 receptor antagonist has also shown to be effective in non-responsive patients [86]. Secondary amyloidosis is a rather frequent complication especially in those who have persistent inflammation despite treatment and affects 10% of patients. The risk of amyloidosis appears to be greater among patients with cysteine mutations [83].
Other autoinflammatory syndromes that may be associated with secondary amyloidosis
The spectrum of autoinflammatory diseases has expanded to include diseases with mutations in the innate immune system pathway to polygenic/complex genetic trait diseases. For example, in some patients with Crohn’s disease, a mutation in NOD2, a molecule resembling cryopyrin, has been identified [38]. On the other hand, Behçet’s disease is now classified as a polygenic autoinflammatory disease due to its specific features [53, 87, 88]. Both of these diseases and more are known to be associated with secondary amyloidosis in severe cases. The mechanism may be speculated to be due to uncontrolled inflammation similar to that in monogenic autoinflammatory diseases.
Rare causes of amyloidosis in childhood
Sickle cell anemia, chronic granulomatous disease-associated aspergillosis, and Hodgkin's disease are other diseases that have been very rarely associated with AA type of amyloidosis in children in the medical literature [89–91].
AL-type amyloidosis
AL-type amyloidosis is the most common type of amyloidosis seen in adults. It is caused by deposition of protein derived from immunoglobulin light-chain fragments. AL amyloidosis is a plasma cell dyscrasia, which should be suspected with the presence of a paraprotein in the serum or urine. Protein immunoelectrophoresis and immunofixation are necessary for the identification of monoclonal proteins. Since AL amyloidosis is a disease of the elderly, it will not be discussed further here [1, 4, 92].
Treatment of amyloidosis
In general, amyloidosis has a poor prognosis if untreated and undiagnosed. The prognosis is greatly influenced by the type of amyloid protein involved and the extent of organ involvement and damage. Therefore, the diagnosis of amyloidosis and typing are crucial for the patient. In practice, specific treatment of the underlying disorder, aiming to suppress the inflammatory activity is the major strategy. Treatment of amyloidosis will be discussed in three sections: Targets and mechanisms of treatment, specific treatment strategies for secondary amyloidosis, and renal replacement therapy.
Targets of treatment Reducing the production of amyloidogenic precursor protein (AA and AL amyloidosis) and enhancing the clearance of amyloidogenic precursor protein (Aβ2M amyloidosis) as well as trying to break down the amyloid deposits are the aims of the therapy.Colchicine is the prototype drug that decreases production of amyloidogenic precursor protein. As stated above, colchicine treatment is the mainstay against the prevention of amyloidosis in FMF. Colchicine suppresses the inflammatory activity, decreases the severity and frequency of attacks, and prevents amyloidosis in patients with FMF. Clinical remission may occur at the stage of proteinuria in a small number of FMF patients after colchicine treatment, as discussed in the previous sections [55, 65, 66].In the other autoinflammatory syndromes, specific therapy for the suppression of inflammation is often achieved with anti-IL1 treatment. Biologic treatment, such as anti-TNF, anti-IL-1 therapy, may have a beneficial effect on amyloidosis per se. However, many patients continue to progress to end-stage renal failure, albeit with a longer renal survival, even with these agents.In FMF patients developing secondary amyloidosis, most centers would now also start using a biologic agent along with colchicine in an attempt to effectively suppress inflammation. There are reports suggesting the effectiveness of anti-TNF and anti IL-1 antagonists on regression of secondary amyloidosis [93–95]. Anti-IL-1 therapy has also been tried in secondary amyloidosis in a patient with some clinical response [96]. However, we await long-term studies to understand whether these biologics prevent renal insufficiency or simply prolong the process.
Specific treatment strategies for secondary amyloidosis New treatment options directed to affect the amyloid structure (e.g., diflunisal for hereditary amyloidosis) or to prevent fibrillogenesis (e.g., eprodisate for AA amyloidosis) or to weaken their structural stability (e.g., iododoxorubicin) are being investigated, but current experience with these drugs is limited [97–100].Eprodisate is a member of a new class of compounds designed to interfere with interactions between amyloidogenic proteins and glycosaminoglycans and thereby inhibit polymerization of amyloid fibrils and deposition of the fibrils in tissues. A multicenter, randomized, placebo-controlled study investigated the ability of eprodisate to prevent the progression of AA amyloidosis-related nephropathy [99]. Eprodisate therapy slowed the progression of renal disease compared to placebo. However, the drug had no significant effect on progression to end-stage renal disease or risk of death [99].
Renal replacement therapy Once end-stage renal disease develops, patients can be treated with either dialysis or renal transplantation [101–103]. Hemodialysis and peritoneal dialysis (continuous ambulatory peritoneal dialysis or automated peritoneal dialysis) appear to be equally effective, but some clinical manifestations complicate the selection of dialysis type for an individual patient. Unsuccessful vascular access due to vascular amyloid infiltration, thrombosis of vascular access, and hypotension make hemodialysis impossible in some patients. Peritoneal dialysis is contraindicated in a patient with malnutrition and hypoalbuminemia [104]. Colchicine should be continued in dialysis patients who have FMF [64]. Renal transplantation is one of the options, but the decision should be based on other involved organs, especially the heart. Renal transplantation, which can regress to amyloidosis, is the most effective treatment in Aβ2M amyloidosis [105]. Combined liver-kidney transplantation can prevent the recurrence of amyloidosis in fibrinogen A alpha chain amyloidosis [106]. Delayed gastric emptying, drug interactions (e.g., cyclosporine-colchicine), and neuropathy (patients using tacrolimus) can cause problems in renal transplant recipients [107, 108]. Treatment of colchicine-resistant FMF patients with interferon could be challenging after renal transplantation [109, 110]. The data regarding biological treatments after renal transplantation is limited [111].
Other Symptomatic treatment is required for affected organs. Angiotensin-converting enzyme inhibitors and cholesterol-lowering statins may be added to the therapeutic regimen.
Conclusions
The causes of secondary amyloidosis in children have been changing over the years. In this decade, the autoinflammatory diseases are the most common cause of reactive amyloidosis in children. The nephrologist should be aware that renal amyloidosis is potentially preventable in these autoinflammatory diseases. Understanding the pathophysiology of this group of diseases has not only improved our understanding of inflammation and the innate immune system but has also enabled us to better treat these diseases and their complications as well.
Questions (answers appear following the reference list)
- The main cause of childhood amyloidosis is
- Juvenile idiopathic arthritis
- Beta2 microglobulin amyloidosis
- AFib amyloidosis
- Familial Mediterranean fever
- Familial cold autoinflammatory disease
- Which one is wrong about the autoinflammatory diseases causing secondary amyloidosis in children?
- The mutations in the MEFV gene causing FMF are associated with excess Il-6 production
- Colchicine is the mainstay treatment for the prevention of amyloidosis in FMF
- CAPS is associated with mutations in cryopyrin
- Hematuria may be seen in FMF
- Secondary amyloidosis may occur in TRAPS
- Which of the following is NOT associated with secondary amyloidosis in FMF?
- Male gender
- Family history
- SAA1.α/α genotype
- Environment
- E148Q homozygosity
- Which one is WRONG about the treatment of amyloidosis?
- Colchicine is not effective in other causes of secondary amyloidosis
- Eprodisate weakens the structural stability of amyloid fibrils
- Hemodialysis and peritoneal dialysis appear to be equally effective
- Colchicine should be continued in dialysis patients having FMF
- Renal transplantation is the most effective treatment in Aβ2M amyloidosis
- What is the MOST common initial manifestation of renal disease secondary to amyloidosis?
- Hypertension
- Asymptomatic hematuria
- Asymptomatic proteinuria
- Renal tubular acidosis
- Chronic renal failure
Footnotes
Answers
1. d
2. a
3. e
4. b
5. c
References
- 1.Perfetto F, Moggi-Pignone A, Livi R, Tempestini A, Bergesio F, Matucci-Cerinic M. Systemic amyloidosis: a challenge for the rheumatologist. Nat Rev Rheumatol. 2010;6:417–429. doi: 10.1038/nrrheum.2010.84. [DOI] [PubMed] [Google Scholar]
- 2.Röcken C, Shakespeare A. Pathology, diagnosis and pathogenesis of AA amyloidosis. Virchows Arch. 2002;440:111–122. doi: 10.1007/s00428-001-0582-9. [DOI] [PubMed] [Google Scholar]
- 3.Picken MM. New insights into systemic amyloidosis: the importance of diagnosis of specific type. Curr Opin Nephrol Hypertens. 2007;16:196–203. doi: 10.1097/MNH.0b013e3280bdc0db. [DOI] [PubMed] [Google Scholar]
- 4.Rysavá R. AL amyloidosis with renal involvement. Kidney Blood Press Res. 2007;30:359–364. doi: 10.1159/000107980. [DOI] [PubMed] [Google Scholar]
- 5.Drüeke TB, Massy ZA. Beta2-microglobulin. Semin Dial. 2009;22:378–380. doi: 10.1111/j.1525-139X.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- 6.Lachmann HJ, Booth DR, Booth SE, Bybee A, Gilbertson JA, Gillmore JD, Pepys MB, Hawkins PN. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med. 2002;6(346):1786–1791. doi: 10.1056/NEJMoa013354. [DOI] [PubMed] [Google Scholar]
- 7.Westermark P, Bergström J, Solomon A, Murphy C, Sletten K (2003)Transthyretin-derived senile systemic amyloidosis: clinicopathologic and structural considerations. Amyloid 10 Suppl 1:48-54 [PubMed]
- 8.Ozen S. Renal amyloidosis in familial Mediterranean fever. Kidney Int. 2004;65:1118–1127. doi: 10.1111/j.1523-1755.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 9.Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- 10.Ben Chetritt R. FMF and renal amyloidosis. Phenotype- genotype correlation, treatment and prognosis. J Nephrol. 2003;16:431–434. [PubMed] [Google Scholar]
- 11.Tunca M, Akar S, Onen F, Ozdogan H, Kasapcopur O, Yalcınkaya F, Tutar E, Ozen S, Topaloglu R, Yılmaz E, Arici N, Bakkaloglu A, Besbas N, Akpolat T, Dinc A, Erken E; Turkish FMF study group (2005) Familial Mediterranean fever in Turkey. Results of a nationwide multicenter study. Medicine 84:1-11
- 12.Tuglular S, Yalcinkaya F, Paydas S, Oner A, Utas C, Bozfakioglu S, Ataman R, Akpolat T, Ok E, Sen S, Düsünsel R, Evrenkaya R, Akoglu E. A retrospective analysis for aetiology and clinical findings of 287 secondary amyloidosis cases in Turkey. Nephrol Dial Transplant. 2002;17:2003–2005. doi: 10.1093/ndt/17.11.2003. [DOI] [PubMed] [Google Scholar]
- 13.Uda H, Yokota A, Kobayashi K, Miyake T, Fushimi H, Maeda A, Saiki O. Two distinct clinical courses of renal involvement in rheumatoid patients with AA amyloidosis. J Rheumatol. 2006;33:1482–1487. [PubMed] [Google Scholar]
- 14.Nishi S, Alchi B, Imai N, Gejyo F. New advances in renal amyloidosis. Clin Exp Nephrol. 2008;12:93–101. doi: 10.1007/s10157-007-0008-3. [DOI] [PubMed] [Google Scholar]
- 15.Neugarten J, Gallo GR, Buxbaum J, Katz LA, Rubenstein J, Baldwin DS. Amyloidosis in subcutaneous heroin abusers ("skin poppers' amyloidosis") Am J Med. 1986;81:635–640. doi: 10.1016/0002-9343(86)90550-4. [DOI] [PubMed] [Google Scholar]
- 16.Orfila C, Lepert JC, Modesto A, Bernadet P, Suc JM. Fanconi's syndrome, kappa light-chain myeloma, non-amyloid fibrils and cytoplasmic crystals in renal tubular epithelium. Am J Nephrol. 1991;11:345–349. doi: 10.1159/000168336. [DOI] [PubMed] [Google Scholar]
- 17.Dubrey SW, Cha K, Simms RW, Skinner M, Falk RH. Electrocardiography and Doppler echocardiography in secondary (AA) amyloidosis. Am J Cardiol. 1996;77:313–315. doi: 10.1016/s0002-9149(97)89403-9. [DOI] [PubMed] [Google Scholar]
- 18.Dubrey SW, Cha K, Anderson J, Chamarthi B, Reisinger J, Skinner M, Falk RH. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. 1998;91:141–157. doi: 10.1093/qjmed/91.2.141. [DOI] [PubMed] [Google Scholar]
- 19.Benson MD. The hereditary amyloidoses. Best Pract Res Clin Rheumatol. 2003;17:909–927. doi: 10.1016/j.berh.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Sattianayagam P, Hawkins P, Gillmore J. Amyloid and the GI tract. Expert Rev Gastroenterol Hepatol. 2009;3:615–630. doi: 10.1586/egh.09.59. [DOI] [PubMed] [Google Scholar]
- 21.Ebert EC, Nagar M. Gastrointestinal manifestations of amyloidosis. Am J Gastroenterol. 2008;103:776–787. doi: 10.1111/j.1572-0241.2007.01669.x. [DOI] [PubMed] [Google Scholar]
- 22.Chopra S, Rubinow A, Koff RS, Cohen AS. Hepatic amyloidosis. A histopathologic analysis of primary (AL) and secondary (AA) forms. Am J Pathol. 1984;115:186–193. [PMC free article] [PubMed] [Google Scholar]
- 23.Looi LM, Sumithran E. Morphologic differences in the pattern of liver infiltration between systemic AL and AA amyloidosis. Hum Pathol. 1988;19:732–735. doi: 10.1016/s0046-8177(88)80181-3. [DOI] [PubMed] [Google Scholar]
- 24.Renzulli P, Schoepfer A, Mueller E, Candinas D. Atraumatic splenic rupture in amyloidosis. Amyloid. 2009;16:47–53. doi: 10.1080/13506120802676922. [DOI] [PubMed] [Google Scholar]
- 25.Elvy A, Harbach L, Bhangu A (2010) Atraumatic splenic rupture: a 6-year case series. Eur J Emerg Med. doi:10.1097/MEJ.0b013e32833ddeb5 [DOI] [PubMed]
- 26.Renzulli P, Schoepfer A, Mueller E, Candinas D. Atraumatic splenic rupture in amyloidosis. Amyloid. 2009;16:47–53. doi: 10.1080/13506120802676922. [DOI] [PubMed] [Google Scholar]
- 27.Sungur C, Sungur A, Ruacan S, Arik N, Yasavul U, Turgan C, Caglar S. Diagnostic value of bone marrow biopsy in patients with renal disease secondary to familial Mediterranean fever. Kidney Int. 1993;44:834–836. doi: 10.1038/ki.1993.318. [DOI] [PubMed] [Google Scholar]
- 28.Sucker C, Hetzel GR, Grabensee B, Stockschlaeder M, Scharf RE. Amyloidosis and bleeding: pathophysiology, diagnosis, and therapy. Am J Kidney Dis. 2006;47:947–955. doi: 10.1053/j.ajkd.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 29.Zangari M, Elice F, Fink L, Tricot G. Hemostatic dysfunction in paraproteinemias and amyloidosis. Semin Thromb Hemost. 2007;33:339–349. doi: 10.1055/s-2007-976169. [DOI] [PubMed] [Google Scholar]
- 30.Benson MD, Kincaid JC. The molecular biology and clinical features of amyloid neuropathy. Muscle Nerve. 2007;36:411–423. doi: 10.1002/mus.20821. [DOI] [PubMed] [Google Scholar]
- 31.Keven K, Oztas E, Aksoy H, Duman N, Erbay B, Ertürk S. Polyglandular endocrine failure in a patient with amyloidosis secondary to familial Mediterranean fever. Am J Kidney Dis. 2001;38:E39. doi: 10.1053/ajkd.2001.29295. [DOI] [PubMed] [Google Scholar]
- 32.Herrera GA, Turbat-Herrera EA. Renal diseases with organized deposits: an algorithmic approach to classification and clinicopathologic diagnosis. Arch Pathol Lab Med. 2010;134:512–531. doi: 10.5858/134.4.512. [DOI] [PubMed] [Google Scholar]
- 33.Sen S, Sarsik B. A proposed histopathologic classification, scoring, and grading system for renal amyloidosis: standardization of renal amyloid biopsy report. Arch Pathol Lab Med. 2010;134:532–544. doi: 10.5858/134.4.532. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins PN. Serum amyloid P component scintigraphy for diagnosing and monitoring amyloidosis. Curr Opin Nephrol Hypertens. 2002;11:649–655. doi: 10.1097/00041552-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Petty RE, Soutwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J, Prieur A, Suarez Almazor ME, Woo P. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 36.Besbas N, Saatci U, Bakkaloğlu A, Ozen S. Amyloidosis of juvenile chronic arthritis in Turkish children. Scand J Rheumatol. 1992;21:257–259. doi: 10.3109/03009749209099235. [DOI] [PubMed] [Google Scholar]
- 37.Stojanov S, Kastner DL. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr Opin Rheumatol. 2005;17:586–599. doi: 10.1097/bor.0000174210.78449.6b. [DOI] [PubMed] [Google Scholar]
- 38.Masters SL, Simon A, Ksentijevic I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu Rev Immunol. 2009;27:621–628. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozen S, Karaaslan Y, Ozdemir O, Saatci U, Bakkaloglu A, Koroglu E, Tezcan S. Prevalence of juvenile chronic arthritis and familial Mediterranean fever in Turkey. J Rheumatol. 1998;25:2445–2449. [PubMed] [Google Scholar]
- 40.Sohar E, Gafni J, Pras M, Heller H. Familial Mediterranean fever. A survey of 470 cases and review of literature. Am J Med. 1967;43:227–253. doi: 10.1016/0002-9343(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 41.Majeed HA, El-Shanti H, Al-Khateeb MS, Rabia ZA. Genotype/phenotype correlations in Arab patients with FMF. Semin Arthritis Rheum. 2002;31:371–376. doi: 10.1053/sarh.2002.32551. [DOI] [PubMed] [Google Scholar]
- 42.Chae JJ, Wood G, Richard K, Jaffe H, Colburn NT, Masters SL, Gumucio DL, Shoham NG, Kastner DL. The familial Mediterranean fever protein, pyrin is cleaved by caspase-1 and activates NF-kappa B through its N-terminal fragment. Blood. 2008;11:1794–1803. doi: 10.1182/blood-2008-01-134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samuels J, Ozen S. Familial Mediterranean fever and the other auto inflammatory syndromes: evaluation of the patient with recurrent fever. Curr Opin Rheumatol. 2006;18:108–117. doi: 10.1097/01.bor.0000198006.65697.5b. [DOI] [PubMed] [Google Scholar]
- 44.International FMF Consortium Ancient missense mutations in a new number of the Roret gene family are likely to cause FMF. Cell. 1997;90:797–807. doi: 10.1016/s0092-8674(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 45.French FMF consortium A candidate gene for familial Mediterranean fever. Nat Genet. 1997;17:25–31. doi: 10.1038/ng0997-25. [DOI] [PubMed] [Google Scholar]
- 46.Ozen S, Balci B, Ozkara S, Ozcan A, Yilmaz E, Besbas N, Ozguc M, Kastner DL, Bakkaloglu A. Is there a heterozygote advantage for familial Mediterranean fever carriers against tuberculous infections? Clin Exp Rheumatol. 2002;20:S57–S58. [PubMed] [Google Scholar]
- 47.Ozen S. Mutations/polymorphisms in a monogenic auto inflammatory disease is a susceptibility marker for certain rheumatic diseases. Lessons from the bedside for the benchside. Curr Opin Rheumatol. 2009;27:S29–S31. [PubMed] [Google Scholar]
- 48.Booth DR, Lachman JJ, Gillmor JD, Booth SE, Hawkins PN. Prevalence and significance of the familial Mediterranean gene mutation encoding pyrin Q148. QJM. 2002;95:332–333. doi: 10.1093/qjmed/94.10.527. [DOI] [PubMed] [Google Scholar]
- 49.Touitou I, Sarkisian T, Medlej-Hashim M, Tunca M, Livneh A, Cattan D, Yalcınkaya F, Ozen S, Majeed H, Ozdogan H, Kastner D, Booth D, Ben-Chetrit E, Pugnere D, Michelon C, Seguret F, Gershoni-Baruch R. Country as the primary risk factor for renal amyloidosis in familial Mediterranean fever. Arthritis Rheum. 2007;56:1706–1712. doi: 10.1002/art.22507. [DOI] [PubMed] [Google Scholar]
- 50.Ozen S, Aktay N, Lainka E, Duzova A, Bakkaloglu A, Kallinich T. Disease severity in children and adolescents with familial Mediterranean fever: a comparative study to explore environmental effects on a monogenic disease. Ann Rheum Dis. 2009;68:246–248. doi: 10.1136/ard.2008.092031. [DOI] [PubMed] [Google Scholar]
- 51.Ben Chetrit E, Levy M. Familial Mediterranean fever. Lancet. 1998;351:659–664. doi: 10.1016/S0140-6736(97)09408-7. [DOI] [PubMed] [Google Scholar]
- 52.Lidar M, Livneh A. Familial Mediterranean fever: clinical, molecular and management advances. Neth J Med. 2007;65:318–324. [PubMed] [Google Scholar]
- 53.Ozen S. The "other" vasculitis syndromes and kidney involvement. Pediatr Nephrol. 2010;25:1633–1639. doi: 10.1007/s00467-009-1327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lachmann HJ, Sengul B, Yavuzsen TU, Booth DR, Booth SE, Bybee A, Gallimore JR, Soytürk M, Akar S, Tunca M, Hawkins PN. Clinical and subclinical inflammation in patients with familial Mediterranean fever and in heterozygous carriers of MEFV mutations. Rheumatology. 2006;45:746–750. doi: 10.1093/rheumatology/kei279. [DOI] [PubMed] [Google Scholar]
- 55.Zemer D, Pras M, Sohar E, Modan M, Cabili S, Gafni J. Colchicine in the prevention and treatment of amyloidosis of familial Mediterranean fever. N Engl J Med. 1986;314:1001–1005. doi: 10.1056/NEJM198604173141601. [DOI] [PubMed] [Google Scholar]
- 56.Saatci U, Ozen S, Ozdemir S, Bakkaloglu A, Besbas N, Topaloglu R, Arslan S. FMF in children: report of a large series and discussions of the risk and prognostic factors of amyloidosis. Eur J Pediatr. 1997;156:619–623. doi: 10.1007/s004310050677. [DOI] [PubMed] [Google Scholar]
- 57.Sohar E, Gafni J, Pras M, Heller H. Familial Mediterranean fever. A survey of 470 cases and review of literature. Am J Med. 1967;43:227–253. doi: 10.1016/0002-9343(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 58.Shohat M, Magal N, Shohat T, Chen X, Dagan T, Mimouni A, Danon Y, Laron R, Ogur G, Schlezinger M, Halpern GJ, Schwabe A, Kastner D, Rotter JI, Fischel-Ghodslan N. Phenotype-genotype correlation in FMF: evidence for an association between M694V and amyloidosis. Eur J Hum Genet. 1999;7:287–292. doi: 10.1038/sj.ejhg.5200303. [DOI] [PubMed] [Google Scholar]
- 59.Cakar N, Yalcınkaya F, Ozkaya N, Tekin M, Akar N, Koçak H, Mısırlıoğlu M, Akar E, Tümer N. FMF associated amyloidosis in childhood. Clinical features, course and outcome. Clin Exp Rheumatol. 2001;19:S63–S67. [PubMed] [Google Scholar]
- 60.Cazeneuve C, Ajrapetyan H, Papin S, Rpudot-Thoraval F, Genevieve D, Mindjoyan E, Papazian M, Sarkisian A, Babloyan A, Boissier B, Duquesnay P, Kouyoumdjian JC, Girodon-Boulandet E, Grateau G, Sarkisian T, Amselem S. Identification of MEFV independent modifying genetic factors for FMF. Am J Hum Genet. 2000;67:1136–1143. doi: 10.1016/s0002-9297(07)62944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gershoni-Baruch R, Brik R, Zacks N, Shinawi M, Lidar M, Livneh A. The contribution of genotypes at the MEFV and SAA1 loci to amyloidosis and disease severity in patients with FMF. Arthritis Rheum. 2003;48:1149–1155. doi: 10.1002/art.10944. [DOI] [PubMed] [Google Scholar]
- 62.Ozen S, Ben-Chetrit E, Bakkaloglu A, Gur H, Tınaztepe K, Calguneri M, Turgan C, Turkman A, Akpolat I, Danaci M, Besbas N, Akpolat T. Polyarteritis nodosa in patients with FMF: a concomitant disease or a feature of FMF? Semin Arthritis Rheum. 2001;30:281–287. doi: 10.1053/sarh.2001.19958. [DOI] [PubMed] [Google Scholar]
- 63.Goldinger SE. Colchicine for familial Mediterranean fever. N Engl J Med. 1972;287:1302. doi: 10.1056/NEJM197212212872514. [DOI] [PubMed] [Google Scholar]
- 64.Kallinich T, Haffner D, Niehues T, Huss K, Lainka E, Neudorf U, Schaefer C, Stajanov S, Timmann C, Keitzer R, Ozdogan H, Ozen S. Colchicine use in children and adolescents with familial Mediterranean fever: literature review and consensus statement. Pediatrics. 2007;119:e474–e483. doi: 10.1542/peds.2006-1434. [DOI] [PubMed] [Google Scholar]
- 65.Oner A, Erdogan O, Demircin G, Bulbul M, Memis L. Efficacy of colchicine therapy in amyloid nephropathy of familial Mediterranean fever. Pediatr Nephrol. 2003;18:521–526. doi: 10.1007/s00467-003-1129-x. [DOI] [PubMed] [Google Scholar]
- 66.Livneh A, Zemer D, Langevitz P, Laor A, Sohar E, Pras M. Colchicine treatment of AA amyloidosis of familial Mediterranean fever : an analysis of factors affecting outcome. Arthritis Rheum. 1994;37:1804–1811. doi: 10.1002/art.1780371215. [DOI] [PubMed] [Google Scholar]
- 67.Schattner A. Colchicine-expanding horizons. Postgrad Med J. 1991;67:223–226. doi: 10.1136/pgmj.67.785.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bohle A, Wehrmann M, Fissele R, Gise HV, Mackensen-Haen S, Müller C. The long-term prognosis of AA and al amyloidosis and the pathogenesis of chronic renal failure in renal amyloidosis. Pathol Res Pract. 1993;89:316–331. doi: 10.1016/s0344-0338(11)80516-9. [DOI] [PubMed] [Google Scholar]
- 69.Bauer EZ, Valle KJ. Colchicine-induced modulation of collagenase in human skin fibroblast cultures. Stimulation enzyme synthesis normal cells. J Invest Dermatol. 1982;79:398–402. doi: 10.1111/1523-1747.ep12530243. [DOI] [PubMed] [Google Scholar]
- 70.Montseny JJ, Meyrier A, Gherardi RK. Colchicine toxicity in patients with chronic renal failure. Nephrol Dial Transplant. 1996;11:2055–2058. doi: 10.1093/oxfordjournals.ndt.a027096. [DOI] [PubMed] [Google Scholar]
- 71.Wallace SL, Singer JZ, Duncan GJ, Wigley FM, Kunel RW. Renal function predicts colchicine toxicity: guidelines for the prophylactic use of colchicine in gout. J Rheumatol. 1991;18:264–269. [PubMed] [Google Scholar]
- 72.Anderson-Haag T, Patel B. Safety of colchicine in dialysis patients. Semin Dial. 2003;16:412–413. doi: 10.1046/j.1525-139x.2003.16091.x. [DOI] [PubMed] [Google Scholar]
- 73.Hoffmann HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin like protein causes familial cold autoinflammatory syndrome and Muckle Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, Teilac-Hamel D, Fischer A, Saint BG. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoffman HM, Wanderer AA, Broide DH. Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. J Allergy Clin Immunol. 2001;108:615–620. doi: 10.1067/mai.2001.118790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Toker O, Hashkes PJ. Critical appraisal of canakinumab in the treatment of adults and children with cryopyrin-associated periodic syndrome (CAPS) Biologics. 2010;25:131–138. doi: 10.2147/btt.s7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum. 2004;50:607–612. doi: 10.1002/art.20033. [DOI] [PubMed] [Google Scholar]
- 78.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, Gitton X, Widmer A, Patel N, Hawkins PN. Use of canakinumab in the cryopyrin associated periodic syndrome. N Engl J Med. 2009;360:2416–2425. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 79.Drenth JP, Cuisset L, Grateau G, Vasseur C, Velde-Visser SD, Jong JG, Beckmann JS, Meer JW, Delpech M. Mutations in the gene encoding mevolanate kinase cause hyper-IgD and periodic fever syndrome. Nat Genet. 1999;22:178–181. doi: 10.1038/9696. [DOI] [PubMed] [Google Scholar]
- 80.Goldbach-Mansky R, Kastner DL. Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses. J Allergy Clin Immunol. 2009;124:1141–1149. doi: 10.1016/j.jaci.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, Mansfield E, Godina M, Karenko L, Petterson T, McCarthy J, Frucht DM, Aringer M, Torosyan Y, Teppo AM, Wilson M, Karaaslan HM, Wan Y, Todd I, Wood G, Schlimgen R, Kumarajeewa TR, Cooper SM, Vella JP, Amos EI, Mulley J, Quane KA, Mollay MG, Ranki A, Powell RJ, Hitman GA, Shea JJ O, Kastner DL. Germline mutations in the extracellular domains of the 55-kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 82.Hull KM, Drewe E, Aksentijevich I, Singh HK, Wong K, McDermott EM, Dean J, Powell RJ, Kastner DL. The TNF receptor–associated periodic syndrome (TRAPS): emerging concepts of an auto inflammatory disorder. Medicine. 2002;81:349–368. doi: 10.1097/00005792-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 83.Aksentijevich I, Galon J, Soares M, Mansfield E, Hull K, Oh HH, Goldbach-Mansky R, Dean J, Athreya B, Regianato AJ, Henrickson M, Pons-Estel B, O’Shea JJ, Kastner DL. The tumor necrosis factor receptor associated periodic syndrome: new mutations in TNFRSF1A, ancestral origins, genotype-phenotype studies, and evidence for further heterogeneity of periodic fevers. Am J Hum Genet. 2001;69:301–314. doi: 10.1086/321976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reddy P, Slack JL, Davis R, Cerretti DP, Kozlosky CJ, Blanton RA, Shows D, Peschon JJ, Black RA. Functional analysis of the domain structure of tumor necrosis factor–alpha converting enzyme. J Biol Chem. 2000;275:14608–14614. doi: 10.1074/jbc.275.19.14608. [DOI] [PubMed] [Google Scholar]
- 85.Toro JR, Aksentijevich I, Hull K, Dean J, Kastner DL. Tumor necrosis factor receptor associated periodic syndrome: a novel syndrome with cutaneous manifestations. Arch Dermatol. 2000;136:1487–1494. doi: 10.1001/archderm.136.12.1487. [DOI] [PubMed] [Google Scholar]
- 86.Gottorno M, Pelagatti MA, Meini A, Obici L, Barcellona R, Federici S, Buoncompagni A, Plebani A, Merlini G, Martini A. Persistent efficacy of anakinra in patients with tumor necrosis factor receptor associated periodic syndrome. Arthritis Rheum. 2008;58:1516–1520. doi: 10.1002/art.23475. [DOI] [PubMed] [Google Scholar]
- 87.Akpolat T, Akkoyunlu M, Akpolat I, Dilek M, Odabas AR, Ozen S. Renal Behçet’s disease: a cumulative analysis. Semin Arthritis Rheum. 2002;31:317–337. doi: 10.1053/sarh.2002.31721. [DOI] [PubMed] [Google Scholar]
- 88.Penza R, Brunetti L, Francioso G, Tricarico A, Lospalluti M. Renal amyloidosis in a child with Behçet's syndrome. Int J Pediatr Nephrol. 1983;4:35–37. [PubMed] [Google Scholar]
- 89.Kaltenis P, Mudeniené V, Maknavicius S, Seinin D. Renal amyloidosis in a child with chronic granulomatous disease and invasive aspergillosis. Pediatr Nephrol. 2008;23:831–834. doi: 10.1007/s00467-007-0702-0. [DOI] [PubMed] [Google Scholar]
- 90.Simşek B, Bayazit AK, Ergin M, Soran M, Dursun H, Kilinc Y. Renal amyloidosis in a child with sickle cell anemia. Pediatr Nephrol. 2006;21:877–879. doi: 10.1007/s00467-006-0069-7. [DOI] [PubMed] [Google Scholar]
- 91.Büyükpamukçu M, Hazar V, Tinaztepe K, Bakkaloğlu A, Akyüz C, Kutluk T. Hodgkin's disease and renal paraneoplastic syndromes in childhood. Turk J Pediatr. 2000;42:109–114. [PubMed] [Google Scholar]
- 92.Kyle RA, Rajkumar SV. Epidemiology of the plasma-cell disorders. Best Pract Res Clin Haematol. 2007;20:637–664. doi: 10.1016/j.beha.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 93.Mor A, Pillinger MH, Kishimoto M, Abeles AM, Livneh A. Familial Mediterranean fever successfully treated with etanercept. J Clin Rheumatol. 2007;13:38–40. doi: 10.1097/01.rhu.0000255772.25658.7c. [DOI] [PubMed] [Google Scholar]
- 94.Gottenberg JE, Merle-Vincent F, Bentaberry F, Allanore Y, Berenbaum F, Fautrel B, Combe B, Durbach A, Sibilia J, Dougados M, Mariette X. Anti-tumor necrosis factor alpha therapy in fifteen patients with AA amyloidoses secondary to inflammatory arthritis. Arthritis Rheum. 2003;48:2019–2024. doi: 10.1002/art.11163. [DOI] [PubMed] [Google Scholar]
- 95.Ozen S, Bilginer Y, Ayaz NA, Calguneri M (2010) Anti IL1 treatment for FMF patients resistant to colchicines: reasoning treatment from laboratory experience. J Rheumatol. doi:10.3899/jrheumatol.100718
- 96.Bilginer Y, Ayaz NA, Ozen S. Anti-IL-1 treatment for secondary amyloidosis in an adolescent with FMF and Behçet's disease. Clin Rheumatol. 2009;29:209–210. doi: 10.1007/s10067-009-1279-8. [DOI] [PubMed] [Google Scholar]
- 97.Dember LM. Modern treatment of amyloidosis: unresolved questions. J Am Soc Nephrol. 2009;20:469–472. doi: 10.1681/ASN.2008070793. [DOI] [PubMed] [Google Scholar]
- 98.Sekijima Y, Kelly JW, Ikeda S. Pathogenesis of and therapeutic strategies to ameliorate the transthyretin amyloidoses. Curr Pharm Des. 2008;14:3219–3230. doi: 10.2174/138161208786404155. [DOI] [PubMed] [Google Scholar]
- 99.Dember LM, Hawkins PN, Hazenberg BP, Gorevic PD, Merlini G, Butrimiene I, Livneh A, Lesnyak O, Puéchal X, Lachmann HJ, Obici L, Balshaw R, Garceau D, Hauck W, Skinner M. Eprodisate for AA Amyloidosis Trial Group. Eprodisate for the treatment of renal disease in AA amyloidosis. N Engl J Med. 2007;356:2349–2360. doi: 10.1056/NEJMoa065644. [DOI] [PubMed] [Google Scholar]
- 100.Rysavá R, Merta M, Spicka I, Tesar V. Current therapeutic possibilities in primary and secondary amyloidosis and our experience with 31 patients. Nephrol Dial Transplant. 2003;18(Suppl 5):v38–v40. doi: 10.1093/ndt/gfg1043. [DOI] [PubMed] [Google Scholar]
- 101.Keven K, Sengul S, Kutlay S, Ekmekci Y, Anadol E, Nergizoglu G, Ates K, Erturk S, Erbay B. Long-term outcome of renal transplantation in patients with familial Mediterranean fever amyloidosis: a single-center experience. Transplant Proc. 2004;36:2632–2634. doi: 10.1016/j.transproceed.2004.09.065. [DOI] [PubMed] [Google Scholar]
- 102.Altiparmak MR, Pamuk ON, Ataman R, Serdengeçti K. Continuous ambulatory peritoneal dialysis in familial Mediterranean fever amyloidosis patients with end-stage renal failure: a single-centre experience from Turkey. Nephron Clin Pract. 2004;98:c119–c123. doi: 10.1159/000081553. [DOI] [PubMed] [Google Scholar]
- 103.Ari JB, Zlotnik M, Oren A, Berlyne GM. Dialysis in renal failure caused by amyloidosis of familial Mediterranean fever. A report of ten cases. Arch Intern Med. 1976;136:449–451. [PubMed] [Google Scholar]
- 104.Sahin S, Sahin GM, Ergin H, Kantarci G. The effect of dialytic modalities on clinical outcomes in ESRD patients with familial Mediterranean fever. Ren Fail. 2007;29:315–319. doi: 10.1080/08860220601166560. [DOI] [PubMed] [Google Scholar]
- 105.Tan SY, Irish A, Winearls CG, Brown EA, Gower PE, Clutterbuck EJ, Madhoo S, Lavender JP, Pepys MB, Hawkins PN. Long-term effect of renal transplantation on dialysis-related amyloid deposits and symptomatology. Kidney Int. 1996;50:282–289. doi: 10.1038/ki.1996.313. [DOI] [PubMed] [Google Scholar]
- 106.Gillmore JD, Lachmann HJ, Rowczenio D, Gilbertson JA, Zeng CH, Liu ZH, Li LS, Wechalekar A, Hawkins PN. Diagnosis, pathogenesis, treatment, and prognosis of hereditary fibrinogen A alpha-chain amyloidosis. J Am Soc Nephrol. 2009;20:444–451. doi: 10.1681/ASN.2008060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saglam F, Celik A, Cavdar C, Sifil A, Atila K, Kaya GC, Bora S, Gulay H, Camsari T. A renal transplant recipient with delayed gastric emptying in amyloidosis due to familial Mediterranean fever improved with erythromycin: a case report. Transplant Proc. 2008;40:308–309. doi: 10.1016/j.transproceed.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 108.Terkeltaub RA. Colchicine update: 2008. Semin Arthritis Rheum. 2009;38:411–419. doi: 10.1016/j.semarthrit.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 109.Tweezer-Zaks N, Rabinovich E, Lidar M, Livneh A. Interferon-alpha as a treatment modality for colchicine-resistant familial Mediterranean fever. J Rheumatol. 2008;35:1362–1365. [PubMed] [Google Scholar]
- 110.Fabrizi F, Messa P, Basile C, Martin P. Hepatic disorders in chronic kidney disease. Nat Rev Nephrol. 2010;6:395–403. doi: 10.1038/nrneph.2010.37. [DOI] [PubMed] [Google Scholar]
- 111.Moser C, Pohl G, Haslinger I, Knapp S, Rowczenio D, Russel T, Lachmann HJ, Lang U, Kovarik J. Successful treatment of familial Mediterranean fever with Anakinra and outcome after renal transplantation. Nephrol Dial Transplant. 2009;24:676–678. doi: 10.1093/ndt/gfn646. [DOI] [PubMed] [Google Scholar]