Abstract
Congenital CNS abnormalities have been targets for prenatal intervention since the founding of fetal surgery 30 years ago, but with historically variable results. Open fetal neurosurgery for myelomenigocele has demonstrated the most promising results of any CNS malformation. Improvements in the understanding of congenital diseases and in fetal surgical techniques have reopened the door to applying fetal surgery to other congenital CNS abnormalities. Advances in gene therapy, bioengineering and neonatal neuroprotection will aid in the future expansion of fetal neurosurgery to other CNS disorders.
Keywords: congenital neurodegenerative disorder, fetal neurosurgery, fetal surgery, gene therapy, hydrocephalus, myelomeningocele, spina bifida, stem cell
CNS malformations are among the most common prenatally diagnosed congenital anomalies. Given their high neurological morbidity and reliable prenatal diagnosis, they were one of the earliest targets for fetal surgeons. However, 30 years later, the application of fetal surgery for CNS abnormalities remains delayed despite progress for other congenital disorders. The story of fetal neurosurgery is one of early promise, hard-learned lessons and new possibilities in the modern era.
Early promise: historical interventions in neurosurgery
The early experience of fetal surgeons with congenital CNS abnormalities laid the groundwork for the future of fetal surgery. Hydrocephalus was the first condition targeted by modern fetal interventionists in the early 1980s [1]. Prior to 1980, insufficient imaging techniques had prevented a reliable prenatal diagnosis of hydrocephalus. Hydrocephalus was often only discovered emergently at delivery due to labor arrest or uterine rupture. In the setting of these complicated deliveries, obstetricians had developed a technique to reduce the size of the fetal head by transabdominal aspiration of the ventricle. Since the goal was maternal survival, the fetus would often not survive the intervention. After prenatal ultrasound became routine, the obstetric experience with these ‘transabdominal encephalocentesis’ inspired the first reported fetal intervention for a prenatally diagnosed congenital CNS disorder: serial cephalocenteses for the treatment of hydrocephalus [2]. From diagnosis at 25 weeks’ gestation to delivery at 34 weeks, obstetricians at Brigham and Women’s Hospital (MA, USA) carried out bimonthly ventricular aspirations under sonographic guidance in a fetus with hydrocephalus. Although the procedure itself was groundbreaking, interval ventricular drainage did little to accommodate the constant production of cerebrospinal fluid. In addition, multiple aspirations increased the risk of hemorrhage and porencephaly in the undeveloped brain.
In response to the report of serial cephalocentesis, fetal interventionists hypothesized that continuous drainage of the ventricles was needed to effectively treat fetal hydrocephalus. Therefore, the ventriculoamniotic (VA) shunt was developed to divert cerebrospinal fluid from the ventricles to the amniotic fluid space in a male fetus with hydrocephalus [3]. In the first reported case, a Silastic® shunt (Dow Corning, MI, USA) was inserted percutaneously and under sonographic guidance into a fetal ventricle at 24 weeks’ gestation [1]. The fetal head subsequently grew normally until 32 weeks. However, the shunt failed 2 weeks later, necessitating delivery at 34 weeks. Despite this, the treating team tentatively concluded that the child had benefited from the intervention, arguing that both the lack of typical disfigurement and relatively mild postnatal neurologic symptoms were a direct result of the VA shunt.
Further studies on VA shunting were not as encouraging. Notably, Frigoletto et al. reported the results of a VA shunt inserted at 24 weeks’ gestation in a fetus with hydrocephalus and a facial cleft [4], and Depp et al. reported shunting a fetus with hydrocephalus and Dandy–Walker malformation [5]. Unfortunately, the first infant developed diabetes insipidus, seizures and sepsis, ultimately dying at 5 weeks of age. The second child developed spastic diplegia, hemiparesis and significant developmental delay. Both of these cases helped underscore the importance of patient selection prior to the undertaking of any fetal intervention.
The Fetal Surgery Registry
In response to continued interest in fetal intervention, the Kroc Foundation Symposium (later renamed the International Fetal Medicine and Surgery Society [IFMSS]) was formed in 1982. With the help of Michael Harrison and colleagues at the University of California, San Francisco (UCSF), fetal surgeons established patient selection guidelines for the fetal treatment of hydrocephalus [6]. In 1986, the Fetal Surgery Registry reported the results of 41 fetal interventions for progressive hydrocephalus [7]. The effectiveness of VA shunting for hydrocephalus was deemed inconclusive. Moreover, they reported technical issues common with postnatal shunting, such as malfunction and dislodgement. According to the group, the repeated procedures associated with addressing these issues unnecessarily increased morbidity for both mother and fetus without providing any established benefit.
In summary, the main problem that had plagued earlier procedures – patient selection – joined new, equally challenging problems: technical failures, the morbidity of repeat procedures, the lack of a control group and even abandonment of the Registry’s guidelines. Ultimately, fetal surgeons placed a de facto moratorium on fetal surgery for hydrocephalus – a moratorium that remains in place today [1]. A decade that had begun with promise for fetal neurosurgery had ended without major successes. However, these initial disappointments highlighted important lessons and paved the way for future applications of fetal surgery.
Lessons learned
In response to the first report on serial cephalocentesis in 1981, there were already concerns in the medical community for ‘unwarranted meddling without adequate information’ [8]. Critics pointed out that by focusing solely on one easily identifiable process such as hydrocephalus, early interventionists neglected the fact that these fetuses often had other more serious abnormalities. At the original meeting of the IFMSS, the issue of patient and disease selection was “the most difficult problem in prenatal management” [6]. The goal would be to exclude those patients with the most severe lesions (for whom there was little benefit to justify the risks), but avoid intervening in patients for whom standard postnatal management was deemed curative. This dilemma would shape the future application of fetal surgery and remains one of the most important questions facing fetal surgeons today.
In addition, these early attempts at fetal intervention highlighted the gap in medical knowledge as to the pathophysiology of congenital diseases. Critics rightfully noted that many attempts at fetal intervention (e.g., in the case of hydrocephalus) were based on assumptions drawn from observations in the newborn period [8]. The “assumption that increased intracranial pressure during fetal life retards cerebral development, and the converse proposition that relieving that pressure will ameliorate that process” were both unproven at the time of the first human reports [2]. For some congenital anomalies, we now know that the fetal environment does not translate directly to the postnatal environment [9]. This understanding has led to stricter criteria for offering fetal intervention. Given the leap to human intervention, the focus needed to be shifted back towards better documentation of the natural history of congenital diseases and a greater emphasis placed on experimental animal models.
In addition to a better understanding of basic fetal processes, improvements in prenatal diagnosis and advances in surgical technique have contributed greatly to the expansion of the number of congenital anomalies treated by fetal surgery. Improved fetal ultrasonography has been the most significant recent advance in prenatal diagnosis. Experienced ultrasonographers now routinely assess not only CNS-related anatomy (i.e., ventricles, posterior fossa and spine), but also other anomalies that may indicate increased risk for a genetic syndrome [10]. Ultrasonography also provides prognostic information that may be used to counsel families (e.g., by determining the level of a spinal defect in a myelomeningocele [MMC]). In addition, ultrasonography is used routinely in the operating room for a variety of purposes, from mapping the placenta prior to uterine incision to monitoring the fetal heart rate. As an adjunct to ultrasound, fetal MRI provides additional information that was not available to early fetal surgeons, especially regarding structural brain abnormalities such as heterotopias.
Advances in surgical instrumentation have also vastly improved the surgical technique of fetal surgery. Initially, open surgery had to be used for any major fetal operation. However, most fetal surgeries today are accomplished either wholly or in combination with a percutaneous or minimally invasive method [11]. Unique surgical instruments such as absorbable staplers and back-biting uterine clamps were developed specifically for use in fetal surgery. In addition, advances in anesthesia and tocolytics have minimized preterm labor and membrane rupture. Despite these improvements, the application of fetal surgery to CNS disorders remained delayed due to the early setbacks associated with the treatment of hydrocephalus [12]. One notable exception to this delay has been fetal surgery to treat MMC [13].
Modern applications of fetal neurosurgery
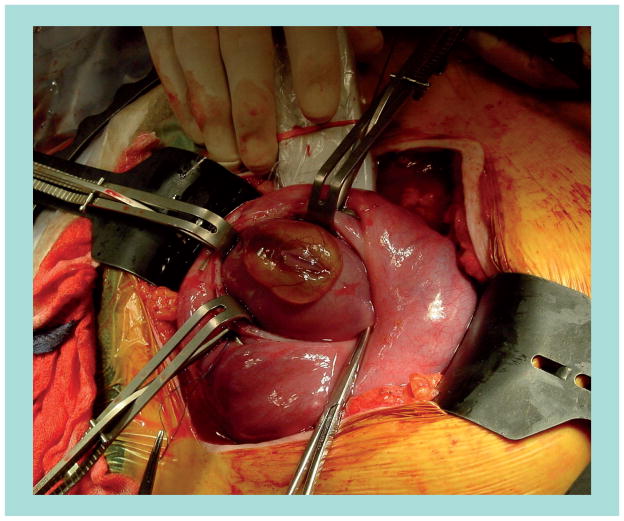
Neural tube defects are the most common CNS abnormalities diagnosed by prenatal ultrasound [14]. Along with hydrocephalus, MMC was one of the first proposed targets for fetal surgery [15,16]. In striking contrast to the abandonment of interventions for hydrocephalus, MMC repair is the most common open fetal surgery performed today (Figure 1) [17]. An estimated 300 open fetal surgeries have been performed for MMC as of February 2011.
Figure 1. Open fetal surgery for myelomeningocele.
Following maternal laparotomy and hysterotomy, the myelomeningocele is exposed using unique back-biting clamps. Intraoperative ultrasonography is used for fetal cardiac monitoring.
Why myelomeningocele?
The primary rationale for the in utero repair of MMC is the ‘two-hit hypothesis’ [18]. That is, despite the primary developmental defect in neurulation, a secondary process of neuronal injury occurs in the intrauterine environment. In support of this theory, researchers have demonstrated the progressive loss of spinal cord function in the prenatal period [19,20]. In addition, amniotic fluid itself may be damaging to the neural tissue [18]. Consequently, much as the rationale for early postnatal repair is a superficial closure to prevent further spinal cord trauma or infection, prenatal closure of the open defect minimizes intrauterine and birth-related damage to the spinal cord.
Animal studies
Various small and large animal models have been described, dating back to rabbits [16] and primates in 1984 [18]. The large animal model for MMC became the most relevant because it reproduced the secondary intrauterine damage postulated by the two-hit hypothesis better. Since the 1990s, the most widely used model for MMC is a surgically created defect in fetal lambs. The low incidence of preterm labor in pregnant sheep, compared with other large animals, makes them an especially good candidate, given that they endure two major operations. In this model, a MMC defect is made in the fetal lamb at gestational age 75 days (corresponding to ~20 weeks in the human). It is then repaired at age 100 days (term = 145 days). The most promising of these studies showed reversal of both lower extremity damage and hindbrain herniation in lambs [21,22]. These results provided the major impetus for the transition to human repair.
The disadvantage of all surgically created animal models of MMC is that they fail to address the ‘first hit’ (i.e., the developmental failure in neurulation). An ideal animal model would thus recreate both the developmental and traumatic causes of MMC, but such a model has yet to be designed. However, despite their limitations, the sheep experiments of the mid-1990s are greatly responsible for advancing the field of fetal surgery for MMC and, by extension, fetal neurosurgery in general.
Human experience
The development of intracranial abnormalities, specifically Chiari malformations and hydrocephalus, has a clear association with MMC. Although the pathophysiology of cranial disturbances has yet to be fully explained, the most promising outcome of human intrauterine MMC repair has been a decreased incidence of postnatal shunt placement [23,24]. However, the benefit of fetal MMC repair on other manifestations of MMC (e.g., extremity neuropathy, bladder and bowel dysfunction or musculoskeletal deformity) has yet to be demonstrated. Given the uncertain benefit of fetal versus traditional postnatal repair, a multi-institutional NIH-sponsored randomized controlled trial (Management of Myelomeningocele Study [MOMS]) was initiated in 2003 to address this question [13]. It aims to enroll 200 patients and is nearing completion, although final outcomes will not be known for several more years.
Minimally invasive repair
Given the morbidity associated with maternal laparotomy and hysterotomy in open fetal MMC repair, a minimally invasive approach would be one of the most promising improvements in fetal MMC repair. Fetoscopic techniques have been attempted to minimize maternal and fetal morbidity. However, these approaches were abandoned due to technical difficulties in performing an adequate back closure [24,25]. Since then, researchers have published reports on newer fetoscopic [25], percutaneous [17] and gasless [26] approaches to human MMC repair. Results of these minimally invasive techniques have been mixed, and their use for MMC repair has not been widely adopted. Given continued improvements in technology and the overall progression towards minimally invasive surgery, it is expected that a less invasive method for MMC repair will eventually gain favor.
At present, newer technologies using biodegradable scaffolds [27], bioadhesives [28] and stem cells [29] are being studies in animals. Preliminary results using these materials in fetal lambs have thus far been no worse than traditional repair when compared separately. Stem cell therapy is particularly enticing if it lives up to its hypothesized potential for neuronal restoration and functional recovery of the spinal cord [30]. Researchers hope that these methods can demonstrate superiority over traditional closure and are able to be translated to human fetal repair in the near future. The results of these animal experiments and their translational potential to humans will shape the future of fetal MMC repair.
Future directions
Besides early studies on hydrocephalus and continued interest in MMC, few other neurological diseases have been studied with fetal intervention in mind. With an increased understanding of congenital diseases and improvements in surgical technique, the time is now ripe for an expansion of the role of fetal neurosurgery. Encephaloceles and vascular CNS anomalies represent just two examples where the role of fetal surgery may be expanded [1,31]. For example, fetal repair of select posterior encephaloceles could, in theory, be approached similarly to fetal MMC repair in order to minimize neurological damage. Another theoretical advantage over postnatal surgery is that some lesions, such as vein of Galen malformations, could be approached by endovascular techniques to prevent steal and brain resorption during the fetal period. In addition, success with MMC repair, a better understanding of pathophysiological processes and advancements in fetal surgery for other congenital diseases have intensified calls for a revisit of the 25-year moratorium on fetal surgery for hydrocephalus [32–34]. Ultimately, successful outcomes with in utero MMC repair will most contribute to the expansion of fetal neurosurgery to other congenital diseases.
Gene therapy
In the meantime, other indirect applications of fetal neurosurgery are emerging. One of the most interesting is gene therapy for congenital neurodegenerative disorders. Gene therapy has already been used clinically to treat adult neurodegenerative diseases such as Huntington’s disease and Parkinson’s disease with some degree of success [35]. In 2009, researchers at the UCSF, in conjunction with the biotechnology company StemCells, Inc. (CA, USA), began the first ever Phase I clinical trial in the congenital myelination disorder Pelizaeus–Merzbacher disease [101]. Pelizaeus–Merzbacher disease is one of the classes of inherited leukodystrophies characterized by a progressive degeneration of myelin. It has variable symptomology but in its severest form leads to progressive neurological dysfunction and premature death. The current trial aims to enroll four children with the severe form and to intracranially transplant human neural stem cells. The aim is to initiate regeneration of myelin around demyelinated nerve fibers. Human neural stem cells have also been applied to the lysosomal storage disease neuronal ceroid lipofuscinosis, also known as Batten disease. Initial results have demonstrated safety, but further studies will be needed to demonstrate efficacy [36].
A unique restriction of gene therapy for CNS disorders is the necessity of using surgically infused viral vectors because, unlike peripheral manifestations of inherited metabolic disorders, the BBB limits enzyme-replacement therapy. Because all clinical trials must address this challenge, a neurosurgeon is essential to the implementation of current gene therapies. As with surgical therapy for any progressive congenital disease, earlier (i.e., prenatal) intervention would theoretically increase its therapeutic potential by promoting healing prior to irreversible organ damage [37]. If results of adult CNS gene therapy trials do show efficacy in ameliorating symptoms, then fetal surgery could play a role in the application of stem cells even prior to birth. In time, one could envision fetal neurosurgeons applying gene therapy prenatally for patients with genetically confirmed adult neurodegenerative diseases such as Huntington’s disease or familial forms of Parkinson’s disease. In addition, evidence of prenatal immunological tolerance may one day obviate the need for long-term immunosupression and is one of the most exciting aspects of in utero stem cell transplantation.
Specialized neurocritical care
Supportive measures in the neonatal period will be vital if we are to expand fetal neurosurgery to other CNS disorders. Because fetal surgery predisposes to fetal prematurity and thus neonatal cerebral injury, the postnatal management of these patients is dependant upon neonatal neuroprotective measures. The world’s first neurointensive care nursery was opened in 2008 to specifically study and treat neonatal cerebral injury [38]. The goal of the neurointensive care nursery is to employ specialized neuroprotective treatment strategies, such as therapeutic hypothermia, continuous EEG monitoring and regular-interval neuroimaging, on at-risk newborns including fetal surgery patients. The hope is that specialized neurocritical care will improve neurodevelopmental outcomes in these newborns, as it has in adults.
Conclusion
Fetal neurosurgery is an exciting addition to the narrative of fetal surgery. A total of 30 years of research and progress on congenital disorders have reopened the door for the application of fetal surgery to CNS disorders. With the advent of new technologies, gene therapy and specialized neurocritical care, the future of fetal neurosurgery is more promising than ever before.
Future perspective
Physicians could hardly envision operating on a human fetus 30 years ago, let alone imagine the variety of complex fetal surgeries performed today. From the first simple needle aspirations to the coordinated intricacy of an ex utero intrapartum treatment (also known as EXIT) procedure of today, fetal surgery has been performed for over two dozen major diseases. Within this greater context, the field of fetal neurosurgery is still in its infancy and its potential remains untapped. It is hoped that within the next decade, promising outcomes from the fetal repair of MMC will inaugurate a renewed interest in fetal surgery for both congenital and adult neurological diseases. As this interest translates into an expansion of fetal neurosurgery as a true specialty, the role of the fetal neurosurgeon will become ever more defined and vital as a specialist in the greater field of fetal surgery.
Executive summary.
Early promise
Despite early promise, there has been a delay in the application of fetal surgery to congenital CNS malformations.
Lessons learned
Improvements in patient selection, our understanding of congenital pathophysiology and improved fetal surgical techniques have reopened the potential for applying fetal neurosurgery to other congenital CNS malformations.
Modern applications
Open fetal neurosurgery for myelomeningocele represents one relative success and has the most potential for changing current standard-of-care treatment.
Modern stem cell and bioengineering technologies are being used in animal experiments to improve upon fetal neurosurgery for myelomeningocele.
Future directions
Fetal neurosurgery can potentially be expanded to other congenital CNS disorders.
Fetal application of neural stem cells may be used to treat debilitating congenital neurological diseases prenatally.
Advancements in neonatal neuroprotective measures will be vital to the expansion of fetal neurosurgery to other CNS disorders.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial and competing interests disclosure
Diana L Farmer is a principal investigator on the Management of Myelomeningocele Study (MOMS), who received NIH funding of this work (grant no. NICHD 3U10 HD041669). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪ ▪ of considerable interest
- 1.Sutton LN, Sun P, Adzick NS. Fetal neurosurgery. Neurosurgery. 2001;48(1):124–142. doi: 10.1097/00006123-200101000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Birnholz JC, Frigoletto FD. Antenatal treatment of hydrocephalus. N Engl J Med. 1981;304 (17):1021–1023. doi: 10.1056/NEJM198104233041706. [DOI] [PubMed] [Google Scholar]
- 3.Clewell WH, Johnson ML, Meier PR, et al. A surgical approach to the treatment of fetal hydrocephalus. N Engl J Med. 1982;306(22):1320–1325. doi: 10.1056/NEJM198206033062202. [DOI] [PubMed] [Google Scholar]
- 4.Frigoletto FD, Birnholz JC, Greene MF. Antenatal treatment of hydrocephalus by ventriculoamniotic shunting. JAMA. 1982;248(19):2496–2497. [PubMed] [Google Scholar]
- 5.Depp R, Sabbagha RE, Brown JT, et al. Fetal surgery for hydrocephalus: successful in utero ventriculoamniotic shunt for Dandy–Walker syndrome. Obstet Gynecol. 1983;61(6):710–714. [PubMed] [Google Scholar]
- 6▪.Harrison MR, Filly RA, Golbus MS, et al. Fetal treatment 1982. N Engl J Med. 1982;307(26):1651–1652. doi: 10.1056/NEJM198212233072623. Landmark consensus statement by fetal surgeons at the Kroc Foundation Symposium providing the first patient selection guidelines for fetal hydrocephalus and other congenital anomalies. [DOI] [PubMed] [Google Scholar]
- 7.Manning FA, Harrison MR, Rodeck C. Catheter shunts for fetal hydronephrosis and hydrocephalus: report of the International Fetal Surgery Registry. N Engl J Med. 1986;315(5):336–340. doi: 10.1056/NEJM198607313150532. [DOI] [PubMed] [Google Scholar]
- 8.Sunder TR. Antenatal treatment of hydrocephalus. N Engl J Med. 1981;305(7):403. [PubMed] [Google Scholar]
- 9.Kunisaki SM, Barnewolt CE, Estroff JA, et al. Large fetal congenital cystic adenomatoid malformations: growth trends and patient survival. J Pediatr Surg. 2007;42(2):404–410. doi: 10.1016/j.jpedsurg.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins LM, Feldstein VA. The use of ultrasound in fetal surgery. Clin Perinatol. 2009;36(2):255–272. doi: 10.1016/j.clp.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Cortes RA, Farmer DL. Recent advances in fetal surgery. Semin Perinatol. 2004;28(3):199–211. doi: 10.1053/j.semperi.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 12▪ ▪.Oi S. Diagnosis, outcome, and management of fetal abnormalities: fetal hydrocephalus. Childs Nerv Syst. 2003;19(7–8):508–516. doi: 10.1007/s00381-003-0790-5. Excellent review of the current status of fetal surgery for hydrocephalus. [DOI] [PubMed] [Google Scholar]
- 13.Sutton LN. Fetal surgery for neural tube defects. Best Pract Res Clin Obstet Gynaecol. 2008;22(1):175–188. doi: 10.1016/j.bpobgyn.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grisoni ER, Gauderer MW, Wolfson RN, Izant RJ., Jr Antenatal ultrasonography: the experience in a high risk perinatal center. J Pediatr Surg. 1986;21(4):358–361. doi: 10.1016/s0022-3468(86)80204-4. [DOI] [PubMed] [Google Scholar]
- 15▪.Bannister CM. Fetal neurosurgery – a new challenge on the horizon. Dev Med Child Neurol. 1984;26(6):827–830. doi: 10.1111/j.1469-8749.1984.tb08179.x. First major publication to delineate fetal neurosurgery as its own field. [DOI] [PubMed] [Google Scholar]
- 16.Brunelli G, Brunelli F. Experimental foetal microsurgery as related to myelomeningocele. Microsurgery. 1984;5(1):24–29. doi: 10.1002/micr.1920050106. [DOI] [PubMed] [Google Scholar]
- 17.Kohl T, Tchatcheva K, Merz W, et al. Percutaneous fetoscopic patch closure of human spina bifida aperta: advances in fetal surgical techniques may obviate the need for early postnatal surgical intervention. Surg Endosc. 2008;23(4):890–895. doi: 10.1007/s00464-008-0153-0. [DOI] [PubMed] [Google Scholar]
- 18.Fichter MA, Dornseifer U, Henke J, et al. Fetal spinal bifida repair – current trends and prospects of intrauterine neurosurgery. Fetal Diagn Ther. 2008;23(4):271–286. doi: 10.1159/000123614. [DOI] [PubMed] [Google Scholar]
- 19.Meuli M, Meuli-Simmen C, Hutchins GM, Seller MJ, Harrison MR, Adzick NS. The spinal cord lesion in human fetuses with myelomeningocele: implications for fetal surgery. J Pediatr Surg. 1997;32(3):448–452. doi: 10.1016/s0022-3468(97)90603-5. [DOI] [PubMed] [Google Scholar]
- 20.Stiefel D, Meuli M. Scanning electron microscopy of fetal murine myelomeningocele reveals growth and development of the spinal cord in early gestation and neural tissue destruction around birth. J Pediatr Surg. 2007;42(9):1561–1565. doi: 10.1016/j.jpedsurg.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 21▪.Meuli M, Meuli-Simmen C, Hutchins GM, et al. In utero surgery rescues neurological function at birth in sheep with spina bifida. Nat Med. 1995;1(4):342–347. doi: 10.1038/nm0495-342. Landmark experimental sheep study of fetal surgery for myelomeningocele that demonstrated the preservation of neurological function after in utero repair. [DOI] [PubMed] [Google Scholar]
- 22.Paek BW, Farmer DL, Wilkinson CC, et al. Hindbrain herniation develops in surgically created myelomeningocele but is absent after repair in fetal lambs. Am J Obstet Gynecol. 2000;183(5):1119–1123. doi: 10.1067/mob.2000.108867. [DOI] [PubMed] [Google Scholar]
- 23▪ ▪.Tulipan N, Sutton LN, Bruner JP, Cohen BM, Johnson M, Adzick NS. The effect of intrauterine myelomeningocele repair on the incidence of shunt-dependent hydrocephalus. Pediatr Neurosurg. 2003;38(1):27–33. doi: 10.1159/000067560. Along with [24], demonstrates the reversal of the Chiari malformation after fetal surgery for myelomeningocele in humans. [DOI] [PubMed] [Google Scholar]
- 24▪ ▪.Farmer DL, von Koch CS, Peacock WJ, et al. In utero repair of myelomeningocele: experimental pathophysiology, initial clinical experience, and outcomes. Arch Surg. 2003;138(8):872–878. doi: 10.1001/archsurg.138.8.872. Along with [23], demonstrates the reversal of the Chiari malformation after fetal surgery for myelomeningocele in humans. [DOI] [PubMed] [Google Scholar]
- 25.Bruner JP, Tulipan NB, Richards WO, Walsh WF, Boehm FH, Vrabcak EK. In utero repair of myelomeningocele: a comparison of endoscopy and hysterotomy. Fetal Diagn Ther. 2000;15(2):82–88. doi: 10.1159/000020981. [DOI] [PubMed] [Google Scholar]
- 26.Pedreira DA, Oliveira RC, Valente PR, Abou-Jamra RC, Araujo A, Saldiva PH. Gasless fetoscopy: a new approach to endoscopic closure of a lumbar skin defect in fetal sheep. Fetal Diagn Ther. 2008;23(4):293–298. doi: 10.1159/000123616. [DOI] [PubMed] [Google Scholar]
- 27.Hosper NA, Eggink AJ, Roelofs LA, et al. Intra-uterine tissue engineering of full-thickness skin defects in a fetal sheep model. Biomaterials. 2010;31(14):3910–3919. doi: 10.1016/j.biomaterials.2010.01.129. [DOI] [PubMed] [Google Scholar]
- 28.Fontecha CG, Peiro JL, Aguirre M, et al. Inert patch with bioadhesive for gentle fetal surgery of myelomeningocele in a sheep model. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):174–179. doi: 10.1016/j.ejogrb.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Fauza DO, Jennings RW, Teng YD, Snyder EY. Neural stem cell delivery to the spinal cord in an ovine model of fetal surgery for spina bifida. Surgery. 2008;144(3):367–373. doi: 10.1016/j.surg.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Lee DH, Kim EY, Park S, et al. Reclosure of surgically induced spinal open neural tube defects by the intraamniotic injection of human embryonic stem cells in chick embryos 24 hours after lesion induction. J Nurosurg. 2006;105(2 Suppl):127–133. doi: 10.3171/ped.2006.105.2.127. [DOI] [PubMed] [Google Scholar]
- 31.Oi S, Babapour B, Klekamp K, Samii M. Prerequisites for fetal neurosurgery: management of central nervous system anomalies toward the 21st Century. Crit Rev Neurosurg. 1999;9(4):252–261. doi: 10.1007/s003290050139. [DOI] [PubMed] [Google Scholar]
- 32.Davis GH. Fetal hydrocephalus. Clin Perinatol. 2003;30 (3):531–539. doi: 10.1016/s0095-5108(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 33.Von Koch CS, Gupta N, Sutton LN, Sun PP. In utero surgery for hydrocephalus. Childs Nerv Syst. 2003;19(7–8):574–586. doi: 10.1007/s00381-003-0775-4. [DOI] [PubMed] [Google Scholar]
- 34.Al-Anazi AR, Rahman A. In utero ventricuol-uterine shunt treatment for fetal hydrocephalus: preliminary study of Al-Anazi ventriculo–uterine shunt. Neurosurg Q. 2010;20(1):1–4. [Google Scholar]
- 35▪.Schwarz SC, Schwarz J. Translation of stem cell therapy for neurological diseases. Transl Res. 2010;156(3):155–160. doi: 10.1016/j.trsl.2010.07.002. Excellent overview and good insight into the potential of stem cells for fighting neurological diseases. [DOI] [PubMed] [Google Scholar]
- 36.Souweidane MM, Fraser JF, Arkin LM, et al. Gene therapy for late infantile neuronal ceroid lipofuscinosis: neurosurgical considerations. J Neurosurg Pediatr. 2010;6(2):115–122. doi: 10.3171/2010.4.PEDS09507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roybal JL, Santore MT, Flake AW. Stem cell and genetic therapies for the fetus. Semin Fetal Neonatal Med. 2010;15(1):46–51. doi: 10.1016/j.siny.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 38▪.Glass HC, Bonifacio SL, Peloquin S, et al. Neurocritical care of neonates. Neurocrit Care. 2010;12(3):421–429. doi: 10.1007/s12028-009-9324-7. Highlights the concept of an intensive care unit focusing specifically on neurocritical care and neuroprotection in newborns. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 101.PMD clinical trial. UCSF Neonatology. http://neonatology.ucsf.edu/nbri/pmd-trial/default.aspx.