Abstract
Many neurochemical systems interact to generate wakefulness and sleep. Wakefulness is promoted by neurons in the pons, midbrain, and posterior hypothalamus that produce acetylcholine, norepinephrine, dopamine, serotonin, histamine, and orexin/hypocretin. Most of these ascending arousal systems diffusely activate the cortex and other forebrain targets. NREM sleep is mainly driven by neurons in the preoptic area that inhibit the ascending arousal systems, while REM sleep is regulated primarily by neurons in the pons, with additional influence arising in the hypothalamus. Mutual inhibition between these wake- and sleep-regulating regions likely helps generate full wakefulness and sleep with rapid transitions between states. This up-to-date review of these systems should allow clinicians and researchers to better understand the effects of drugs, lesions, and neurologic disease on sleep and wakefulness.
Citation:
España RA; Scammell TE. Sleep neurobiology from a clinical perspective. SLEEP 2011;34(7):845-858.
Keywords: Waking, arousal, locus coeruleus, tuberomammillary nucleus, dorsal raphe nucleus, thalamus, ventrolateral preoptic area
INTRODUCTION
Sleep medicine physicians often encounter questions that require an understanding of the neurobiology of sleep: How do certain brain injuries produce coma or hypersomnolence? Why do antidepressants often reduce REM sleep? Why do people with narcolepsy have trouble staying awake? How do amphetamines improve alertness and wakefulness? To help with these and similar questions, this paper provides an overview of the basic circuits that control sleep and wakefulness. This paper has evolved from one we wrote several years ago1 and has been updated to include many of the latest discoveries on the circuits and neurochemistry of sleep, more information on drugs that are used in clinical practice, and some thoughts on medications that are now in clinical trials. We hope this will provide the reader with useful perspectives on sleep disorders, how drugs influence sleep and wakefulness, and how injuries in different brain regions may affect sleep.
Almost 100 years ago, clinicians and pioneer neuroscientists began to identify the general brain regions that regulate sleep and wakefulness. After an epidemic of encephalitis lethargica around 1915–1920, Baron Constantin von Economo found that patients with encephalitis of the posterior hypothalamus and rostral midbrain often had crushing sleepiness, whereas those with injury to the preoptic area usually had severe insomnia.2 He thus hypothesized that the preoptic area and adjacent anterior hypothalamus contained neurons that promoted sleep, whereas neurons in the posterior hypothalamus and rostral midbrain promoted wakefulness. In the 1940s, Moruzzi and Magoun found that stimulation of the rostral reticular formation caused the EEG of an anesthetized animal to switch from slow waves to the low-voltage desynchronized pattern typical of wakefulness, suggesting that this general region is capable of promoting arousal.3 Soon after the discovery of REM sleep in the mid-1950s, Jouvet and others established that this state is driven by circuitry in the pons.4–6 Over the last few decades, the latest generations of researchers and clinicians have built on these ideas and identified many distinct systems, each of which contributes to specific aspects of sleep-wake behavior.
The Reticular Formation
The reticular formation is a heterogeneous region that runs through the core of the brainstem from the medulla up to the midbrain and into the posterior hypothalamus. Soon after Moruzzi and Magoun showed that the rostral reticular formation can activate the cortex, experimental lesions in animals and clinical observations in patients with strokes or tumors confirmed that the rostral reticular formation is necessary for generating wakefulness, as these injuries often produce hypersomnolence or coma.7,8 Thus, many researchers hypothesized that the reticular formation received inputs from a number of sensory systems and promoted wakefulness via excitatory projections to the thalamus, hypothalamus, and basal forebrain. More recently, researchers have reconsidered the idea of a monolithic reticular formation and instead attribute its functions to the activity of specific systems that promote arousal using acetylcholine, glutamate, or monoamine neurotransmitters (e.g., norepinephrine, histamine, serotonin, and dopamine). These neurotransmitters are generally considered to produce arousal through widespread, often excitatory effects on target neurons. In addition, they can act as neuromodulators to enhance other excitatory or inhibitory inputs to these cells. This modulation can thus amplify neuronal signals over much of the brain to recruit the many systems necessary for waking behaviors. Thus, the term reticular formation is helpful anatomically, but more insight can be gained from understanding the specific cells and pathways contained within this general region.
Wake-Promoting Neurochemical Systems
Acetylcholine (ACh)
The basal forebrain (BF) and brainstem contain large groups of cholinergic neurons that promote wakefulness and REM sleep and also participate in learning, memory, and cognition. The BF is a region surrounding the front of the hypothalamus that includes the medial septum, magnocellular preoptic nucleus, diagonal band of Broca, and substantia innominata (Figure 1). Most BF cholinergic neurons are active during wakefulness and REM sleep, and they directly promote fast EEG rhythms via projections to the cortex and hippocampus (Table 1).9–12 The BF also contains a large population of neurons that produce the inhibitory neurotransmitter γ-aminobutyric acid (GABA), and these likely activate the cortex by reducing activity in inhibitory cortical interneurons.13,14
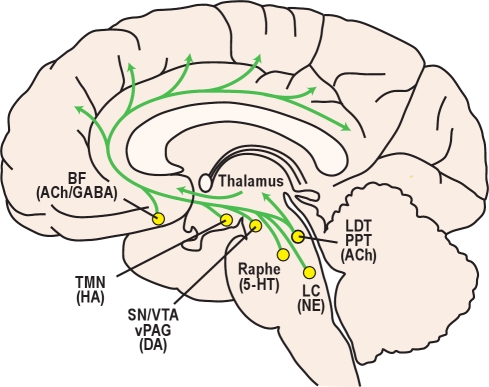
Figure 1.
A variety of neurochemical systems promote arousal via projections to the forebrain. Cortical and subcortical regions are excited by monoaminergic neurotransmitters including norepinephrine (NE) from the locus coeruleus (LC), serotonin (5-HT) from the dorsal and median raphe nuclei, histamine (HA) from the tuberomammillary nucleus (TMN); and dopamine (DA) from the substantia nigra, ventral tegmental area, and ventral periaqueductal gray (SN/VTA/vPAG). Neurons of the basal forebrain (BF) promote cortical activation using acetylcholine (ACh) and γ-aminobutyric acid (GABA). Neurons in the laterodorsal and pedunculopontine tegmental nuclei (LDT/PPT) release ACh to excite neurons in the thalamus, hypothalamus, and brainstem.
Table 1.
Activity profiles of neurotransmitter systems across sleep/wakefulness
| Neurotransmitter | Wakefulness | NREM sleep | REM sleep |
|---|---|---|---|
| Acetylcholine | ↑↑ | — | ↑↑ |
| Monoamines | ↑↑ | ↑ | — |
| Orexin/Hypocretin | ↑↑ | — | — |
| MCH | — | — | ↑↑ |
| VLPO/MNPO | — | ↑↑ | ↑↑ |
Neuronal activity: ↑↑, rapid firing rate; ↑, slower firing rate; —, little or no firing.
A second major group of cholinergic neurons is found in the pons within the laterodorsal and pedunculopontine tegmental nuclei (LDT/PPT). In contrast to the BF, LDT/PPT neurons primarily project to subcortical regions including the thalamus, lateral hypothalamus, and BF.15,16 Like most BF neurons, cholinergic neurons in the LDT/PPT are mainly active during wakefulness and REM sleep, and promote cortical activation by releasing ACh into the thalamus.17,18
Pharmacological studies that manipulate ACh neurotransmission offer further evidence for its importance in the control of sleep and wakefulness. ACh, nicotine, and muscarinic receptor agonists such as pilocarpine produce desynchronized cortical activity and increases wakefulness.19,20 Similar effects occur with physostigmine which blocks enzymatic degradation of ACh. In contrast, agents that reduce ACh signaling, including the muscarinic antagonists scopolamine and atropine, produce immobility and EEG slow waves.21,22
Norepinephrine (NE)
NE is produced by several brainstem nuclei and may help generate arousal during conditions that require high attention or activation of the sympathetic nervous system. The major source of NE to the forebrain is the locus coeruleus (LC), an elongated nucleus just beneath the floor of the fourth ventricle. LC neurons fire most rapidly during wakefulness, are much less active during NREM sleep, and are nearly silent during REM sleep.23,24 Extracellular levels of NE are linearly related to LC neuronal activity, with the highest rates of release observed during wakefulness.25 NE is also made by neurons in the ventral medulla that mediate autonomic responses, and though much less studied, these cells may also promote arousal.26
Pharmacological studies provide some of the strongest evidence that NE regulates wakefulness and sleep. For example, infusion of NE or the noradrenergic agonists isoproterenol and phenylephrine into the lateral ventricle or BF increases behavioral and EEG indices of wakefulness.27 LC neurons are normally inhibited by NE via α2 receptors, and blockade of this negative feedback with yohimbine increases LC activity and also increases wakefulness.28,29 Conversely, bilateral inactivation of the LC with the α2-agonist clonidine, or co-administration of both α1 and β noradrenergic antagonists (prazosin and timolol) increases NREM sleep.30,31
The NE system may be especially important in promoting arousal under conditions that require responding to a behaviorally important stimulus, a cognitive challenge, or stress. In broad terms, an animal may be drowsy and inattentive if LC activity is too low, distractible and anxious if LC activity is too high, but optimally attentive and aroused with intermediate levels of activity. NE tone is clearly linked to cognition as LC neurons in monkeys fire phasically in response to a salient stimulus that signals a reward such as food, but these cells do not respond to a distracting stimulus.32 Integrating these ideas, Aston-Jones and colleagues have proposed that activity in the LC may promote arousal in a way that optimizes attention and task performance.33 In addition, the LC and NE neurons in the ventral medulla are active during stress.23,34 The necessity of this system is clear in mice lacking NE because after exposure to a mild stressor, they fall asleep more rapidly than control mice.35 Similarly, rats with lesions of the LC show considerably less behavioral arousal and cortical activation when confronted with novel stimuli.36 On the other hand, excessive NE tone with anxiety might contribute to insomnia, and the α1 antagonist prazosin can reduce the vivid nightmares and nighttime arousal of PTSD.37
Histamine (HA)
HA plays an essential role in promoting wakefulness, yet little is known about which aspects of arousal it governs.38 The tuberomammillary nucleus (TMN) is a small cluster of cells adjacent to the mammillary body at the base of the posterior hypothalamus. Though few in number, these cells innervate much of the forebrain and brainstem and are the sole source of HA in the brain. Similar to the pattern seen in the LC and other monoaminergic nuclei, TMN firing rates and HA release are highest during wakefulness, lower during NREM sleep and lowest during REM sleep.39,40 Administration of HA or an H1-receptor agonist increases cortical activation and wakefulness while reducing NREM and REM sleep.41,42 In contrast, drugs that reduce HA signaling, including classical antihistamines, such as the H1 receptor antagonists diphenhydramine, pyrilamine, and low dose doxepin increase NREM and REM sleep (Table 2).41–46
Table 2.
Effects of commonly used drugs on sleep and waking
| Drug Type | Examples | Pharmacologic Effect | Neurobiologic Mechanism | Clinical Effects |
|---|---|---|---|---|
| Selective serotonin reuptake inhibitors (SSRIs) | Fluoxetine | Increase extracellular levels of 5-HT | 5-HT inhibits REM sleep-producing cells | Decreased REM sleep |
| Fluvoxamine | ||||
| Citalopram | ||||
| Tricyclic antidepressants | Amitriptyline | Increase extracellular levels of 5-HT and NE | 5-HT and NE inhibit REM sleep-producing cells | Decreased REM sleep |
| Nortriptyline | ||||
| Clomipramine | ||||
| Desipramine | ||||
| Traditional, amphetamine-like stimulants | Amphetamine | Increase extracellular levels of DA and NE | Increased DA and NE signaling | Increased wakefulness |
| Dextroamphetamine | ||||
| Methylphenidate | ||||
| Wake-promoting, non-traditional stimulants | Modafinil | Increase extracellular levels of DA | Increased DA signaling | Increased wakefulness |
| Armodafinil | ||||
| Benzodiazepines | Diazepam | Enhance GABA signaling via GABAA receptors | GABA inhibits the arousal systems | Increased sleep |
| Clonazepam | ||||
| Lorazepam | ||||
| Triazolam | ||||
| Non-benzodiazepine sedative hypnotics | Zolpidem | Enhance GABA signaling via GABAA receptors | GABA inhibits the arousal systems | Increased sleep |
| Zaleplon | ||||
| Zopiclone | ||||
| Classic antihistamines | Diphenhydramine | Block HA H1 receptors | Reduced HA signaling | Increased sleep |
| Triprolidine | ||||
| Typical antipsychotics | Haloperidol | Block DA receptors | Reduced DA signaling | Increased sleep |
| Chlorpromazine | ||||
Over the last several years, a new class of wake-promoting drugs has been developed to target the autoinhibitory histamine H3 receptors.47,48 The clinical rationale for these agents appears strong, as many people with narcolepsy or idiopathic hypersomnia have reduced HA levels,49 and blockade of H3 receptors should increase HA signaling. For example, H3 antagonists (or reverse agonists) such as ciproxifan or tiprolisant promote wakefulness and EEG desynchrony and improve the excessive daytime sleepiness observed with narcolepsy.50–54 This wake-promoting effect is likely mediated by increased HA tone as the response to an H3 antagonist is absent in mice lacking H1 receptors.55
Which aspects of arousal are mediated by the HA system remains unclear. HA improves attention and psychomotor performance,56 and it may promote motivated behaviors such as food seeking.57 In addition, mice lacking HA have less wakefulness at the beginning of their active period,58 suggesting that HA may be especially important for initiating arousal. As sleep inertia upon awakening is common in many patients with idiopathic hypersomnia, it is possible that low HA signaling is a contributing factor.
Serotonin (5-HT)
Understanding how 5-HT promotes arousal is challenging because: there are many sources of 5-HT; 5-HT binds to at least 15 different receptors with varied effects, and 5-HT has been shown to influence many other aspects of behavior including mood, anxiety, aggression, and appetite. 5-HT is produced by neurons in the dorsal raphe nucleus and other raphe nuclei scattered along the midline of the brainstem, and together these neurons innervate many brain regions that can influence sleep/wake behavior, including the preoptic area, basal forebrain, hypothalamus, and thalamus. Early studies suggested that 5-HT might help produce NREM and possibly REM sleep, but more recent work indicates 5-HT generally promotes wakefulness and suppresses REM sleep. The firing rates of dorsal raphe neurons and extracellular 5-HT levels are highest during wakefulness, much lower during NREM sleep, and lowest during REM sleep—a pattern very similar to that of the NE and HA systems.59,60 In support of this wake-promoting role, agonists of the 5-HT1A, 5-HT1B, 5-HT2, or 5-HT3 receptors increase wakefulness.61–65 Of clinical relevance, similar effects occur with selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine and citalopram that increase wakefulness and reduce REM sleep in both people and rodents.66–70 In addition, drugs that block 5-HT2 receptors such as ritanserin or agomelatine are thought to promote NREM sleep and thus are under development as treatments for insomnia.62,64,71–73
Dopamine (DA)
DA has been implicated in the regulation of a variety of behavioral and physiological processes including motor function, motivation, reward, and learning. Additionally, DA exerts potent wake-promoting effects that are of great clinical relevance. For example, sleepiness is common with DA antagonists such as haloperidol or chlorpromazine or in patients with Parkinson's disease who have a loss of DA-producing neurons.74–76 Additionally, D2 agonists like ropinirole can produce sleepiness via activation of autoinhibitory D2 receptors that reduce DA signaling.77,78
However, it is unclear which DA neurons actually promote arousal. DA-producing neurons are most abundant in the substantia nigra and ventral tegmental area, yet cells in these regions fire in relation to movement or reward but, in general, have not been found to alter their rates of firing across sleep and wakefulness.79–82 Nevertheless, extracellular levels of DA are high during periods of wakefulness and lower during NREM sleep, suggesting that some DA neurons must be wake-active.81 One candidate population sits in the ventral periaqueductal gray of the pons, and lesions of these wake-active DA neurons produce moderate reductions in wakefulness.83 The conditions under which these or other DA wake-promoting neurons fire are unknown, but in general, DA may naturally promote arousal when an individual is highly motivated or physically active.
Drugs that increase DA signaling are used frequently to improve excessive daytime sleepiness. Classical stimulants such as methylphenidate and amphetamine increase extracellular levels of DA by disrupting the function of the DA transporter (DAT), thereby increasing extracellular levels of DA (Figure 2).84 These drugs are usually very effective, but because they enhance DA signaling in reward and motor pathways, they have high abuse potential and can elicit tics or other movement disorders. At higher doses, these stimulants can also block the reuptake of NE and 5-HT which can result in tachycardia, arrhythmias, mania, and psychosis.
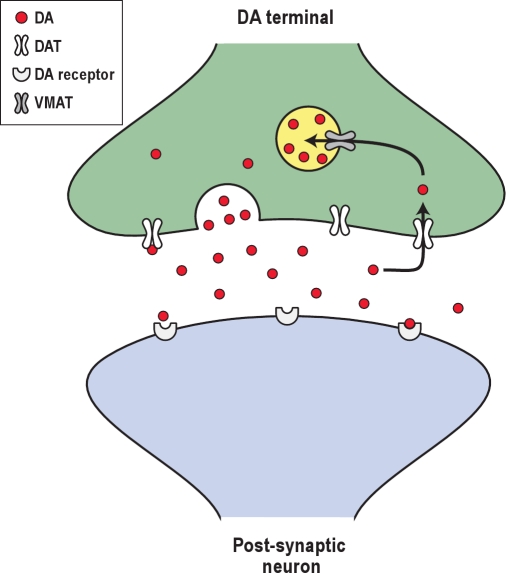
Figure 2.
A prototypical dopamine synapse. Under normal conditions, action potentials in a DA nerve terminal cause DA-filled vesicles to fuse with the presynaptic membrane, and DA is released into the synaptic cleft where it can bind to postsynaptic DA receptors. DA is removed from the synaptic cleft primarily by the DA transporter (DAT). Once DA is back inside the presynaptic terminal, it is repackaged into synaptic vesicles for future release via the vesicular monoamine transporter (VMAT). Amphetamines increase the synaptic concentration of DA through two main mechanisms: Amphetamines interfere with the reuptake of DA through the DAT, and they disrupt vesicular packaging of DA which increases cytosolic levels of DA which can then leak out through the DAT via reverse transport.
Modafinil is frequently prescribed for treating the sleepiness of narcolepsy and some other disorders. Clinically, it promotes wakefulness effectively, usually with fewer side effects than encountered with classical stimulants. Like amphetamines, modafinil disrupts DAT function in humans and rodents,85,86 and this is a necessary part of its wake-promoting mechanism, as mice lacking the DAT show no increase in wakefulness with modafinil,87 and D1 and D2 receptor antagonists can block modafinil-induced wakefulness.88 Still, if modafinil acts via the DAT, it seems surprising that it has less abuse potential than amphetamines. One possible explanation is that amphetamines produce a dramatic efflux of DA into the synapse via reverse transport through the DAT, and this may be very reinforcing. In contrast, modafinil may simply block reuptake of DA through the DAT, leading to more modest rises in DA that are not as reinforcing. A better understanding of these mechanisms could drive the discovery of even better wake-promoting medications.
Orexin/Hypocretin
The excitatory neuropeptides orexin-A and -B (also known as hypocretin-1 and −2) are synthesized by neurons in the lateral and posterior hypothalamus and play essential roles in the regulation of wakefulness and sleep.89,90 The orexin neurons project widely and heavily innervate all the arousal regions described above, with particularly dense innervation of the LC and TMN (Figure 3).89,91 Orexins excite target neurons through the OX1 and OX2 receptors. Like most other wake-promoting neurons, orexin neurons fire mainly during wakefulness, especially during active exploration, and are silent during NREM and REM sleep.92,93 Orexin levels are highest during wakefulness,94 and when injected into the brain, orexins increase arousal and behavioral activity while suppressing NREM and REM sleep.95–97 Consistent with this, selective optogenetic activation of orexin neurons can trigger brief awakenings from sleep.98,99 Additionally, orexin receptor antagonists such as almorexant reduce sleep latency and increase the amounts of REM and NREM sleep.100–102
Figure 3.
Orexin/hypocretin-producing neurons in the lateral hypothalamus innervate all of the ascending arousal systems, as well as the cerebral cortex.
The most compelling evidence that orexins are necessary for the regulation of wakefulness and sleep was the discovery that narcolepsy with cataplexy is associated with a loss of orexin signaling.103–107 Dogs with a mutation of the OX2 receptor gene display many of the classic symptoms of narcolepsy, including cataplexy when presented with palatable foods.104 Further, mice lacking the orexin peptides or the orexin-producing neurons have severe sleepiness and cataplexy.103,108–111 Most importantly, people with narcolepsy with cataplexy have a severe (85% to 95%) loss of the orexin neurons and very low CSF levels of orexin-A.106,107,112 Less severe loss (20% to 60%) of the orexin neurons also occurs in other disorders that can cause sleepiness such as Parkinson disease, multiple system atrophy, and traumatic brain injury.113–117
In just the last 10 years, much has been learned about the ways in which orexins promote arousal. In general, it may be best to think of this as a system for sustaining wakefulness as people and mice with narcolepsy have approximately normal amounts of wakefulness, but have great difficulty maintaining long periods of wakefulness.110 Orexins may also stabilize sleep as people with narcolepsy often have fragmented sleep, and orexins certainly regulate REM sleep as discussed below. In addition, orexins promote arousal responses to homeostatic challenges and drive motivated behaviors such as seeking food. Orexins directly excite neurons of the mesolimbic reward pathways, and orexin antagonists can reduce the motivation to seek drugs of abuse.118–121 The orexin neurons are also activated by humoral indicators of hunger such as low glucose or high levels of ghrelin,122,123 and while normal mice have a clear increase in arousal when deprived of food, mice lacking the orexin neurons show little response.124 Thus, one can view the orexin system as helping sustain wakefulness across much of the day, and increasing arousal in motivating conditions.
Cortical and Thalamic Activity across Sleep and Wakefulness
All the arousal systems we have discussed thus far are located in the BF, hypothalamus, or brainstem and exert diffuse effects on the cortex and many other target regions. These subcortical systems are essential for the generation of sleep/wake states and for the regulation of the transitions between these states. However, patterns of EEG activity and consciousness itself arise from interactions between these subcortical systems, the thalamus, and the cortex.
Thalamic neurons relay information to and from the cortex and have intrinsic electrical characteristics that help generate some of the cortical rhythms seen in NREM sleep.125,126 The thalamus contains two major types of neurons, glutamatergic thalamocortical projection neurons that relay sensory, motor, and limbic information to the cortex, and GABAergic neurons in the reticular nucleus of the thalamus that are innervated by the projection neurons and cortex and in turn inhibit the projection neurons. These reciprocal connections are thought to drive some cortical rhythms, including sleep spindles.127 Thalamic neurons are hyperpolarized during NREM sleep, promoting a pattern of burst firing and reducing their responsiveness to incoming sensory stimuli.128 During wakefulness and REM sleep, ACh depolarizes thalamic neurons to suppress spindles and slow waves and promote the transmission of single spikes that efficiently transmit information to the cortex and drive desynchronized cortical activity.129 During wakefulness, monoamines bolster this effect.119 Extensive damage to the thalamus severely impairs consciousness and the ability to interact with the environment, but the general patterns of wakefulness, NREM, and REM sleep persist, suggesting that the thalamus is not required for the basic generation of sleep states.130–133
The cortex contains a wide variety of neurons, and much less is known about their activity in relation to sleep/wake states. The EEG reflects broad patterns of excitatory and inhibitory post-synaptic potentials, mainly arising from the dendrites of pyramidal neurons. During wakefulness and REM sleep, these potentials are desynchronized, resulting in low-amplitude fast activity, but during NREM sleep these signals are synchronized, resulting in high-amplitude slow activity. Release of ACh and monoamines during wakefulness generally excites cortical neurons and increases their responsiveness to incoming sensory stimuli. Delta waves likely arise from interactions amongst cortical neurons and may also be influenced by the BF and other subcortical sites. Recent work has identified a population of widely projecting GABAergic neurons within the cortex that are uniquely active during NREM sleep, suggesting that these cells may broadly inhibit other cortical neurons, helping generate slow waves during NREM sleep.134 In addition, the intensity of cortical slow waves may reflect prior local activity and changes in synaptic strength, as slow waves during NREM sleep are increased over supplementary motor cortex after learning a motor task but decreased with arm immobilization.135–137
The Arousal Network: Interactions among Wake-Promoting Neurotransmitter Systems
Each of the arousal systems presented above is independently capable of promoting wakefulness, yet these systems work together to generate behavioral arousal. Anatomically, there are many interconnections between the systems. For instance, ACh and 5-HT fibers innervate and excite LC neurons, and nearly all wake-promoting neurons respond to HA, NE, and orexin. In addition, these neurotransmitters often produce similar effects on their targets. For example, all the arousal systems excite thalamic and cortical neurons. These interconnections and parallel effects may explain why injury to any one of the arousal systems often produces little lasting effect on wakefulness. Functionally, this is adaptive, as it helps ensure that wakefulness will still occur after injury to any one of the arousal systems. In fact, there are only a few brain regions in which lesions produce lasting reductions in arousal. One is the rostral reticular formation in the midbrain and posterior hypothalamus in which lesions from strokes or tumors can produce severe hypersomnolence or even coma, probably from damage to many of the ascending monoaminergic and cholinergic pathways.
Wakefulness is a complex and dynamic state, arising from networks of neurons driven by homeostatic, affective, cognitive, and motivational processes. Thus, it is likely that each arousal system helps promote specific aspects of behavioral arousal so that individuals can detect sensory and internal stimuli and generate appropriate motor and affective responses.138 For example, NE, HA, and ACh may be particularly important for enhancing attention and responding to novel, stressful, or salient stimuli. Similarly, through its limbic and striatal projections, DA may promote arousal especially when an individual is motivated or physically active. The orexin peptides help sustain wakefulness and also may help drive goal-oriented behaviors and locomotion. So, while lesions of some arousal systems appear to have little effect on the amounts of wakefulness, deficits in arousal may be best revealed by carefully examining the response to specific circumstances and challenges.
NREM Sleep-Promoting Systems
Preoptic area
In the early 20th century, most researchers thought that sleep was a passive consequence of inactivity in the arousal systems, but many experiments have now shown that specific neurons actively promote sleep. Baron von Economo first observed that insomnia was common in patients with encephalitis injuring the preoptic area (the rostral end of the hypothalamus, just above the optic chiasm) and the adjacent BF.2 This observation suggested that this region might contain neurons that promote sleep, and subsequent research in animals identified sleep-active neurons in the ventrolateral preoptic area (VLPO) and median preoptic area (MNPO).139,140 Many neurons in these nuclei fire most frequently during NREM sleep and to some degree during REM sleep but are virtually silent during wakefulness.141–143 Interestingly, these sleep active VLPO neurons show particularly high rates of firing during deep NREM sleep, and MNPO neurons often begin firing just before NREM sleep. Lesions of the preoptic area and specifically of the VLPO markedly reduce sleep, and the sleep that does occur is light and fragmented.144,145 Collectively, these observations suggest that MNPO neurons may help initiate sleep, whereas VLPO neurons may be necessary for the maintenance of sleep.
Anatomically, the VLPO and MNPO are well positioned to promote sleep. The neurons in these nuclei contain the inhibitory neurotransmitter GABA and the inhibitory neuropeptide galanin,146,147 and they innervate all the arousal-promoting regions, including the LDT/PPT, LC, DR, TMN, and also the orexin neurons (Figure 4). Thus, the VLPO and MNPO are hypothesized to promote sleep by coordinating the inhibition of arousal regions during NREM and REM sleep.146,148,149
Figure 4.
NREM sleep pathways. Ventrolateral preoptic area (VLPO) neurons are active during NREM sleep and reduce activity in the ascending arousal systems using GABA and galanin. A subset of VLPO neurons is also active during REM sleep.
Other brain regions contain neurons active in NREM sleep, but these populations are less well understood. For example, parts of the BF and lateral hypothalamus contain scattered GABAergic neurons that are active during NREM sleep.150–152 Some of these cells may directly innervate the cortex, and it is possible that they modulate cortical networks to promote slow wave activity.
Many of the medications now used to treat insomnia do so by promoting GABA signaling. Benzodiazepines, (e.g., diazepam), barbiturates (e.g., pentobarbital), and the newer non-benzodiazepine agents (e.g., zolpidem) all bind to GABA-A receptors to enhance the effects of GABA.153,154 Gamma hydroxybutyrate (sodium oxybate) promotes very deep sleep, most likely by binding to GABA-B receptors.155 These drugs may promote sleep by boosting signaling by the VLPO and other NREM sleep-active populations, but GABA can inhibit neurons throughout the brain, and the precise pathways through which these drugs work remain unclear.
REM Sleep-Promoting Systems
Soon after the discovery of REM sleep in the mid-1950s,4,5 researchers learned that the pons plays an essential role in the generation of REM sleep.6 After several decades of work, much has been learned, but the specific pathways that generate this state are still debated (Figure 5).
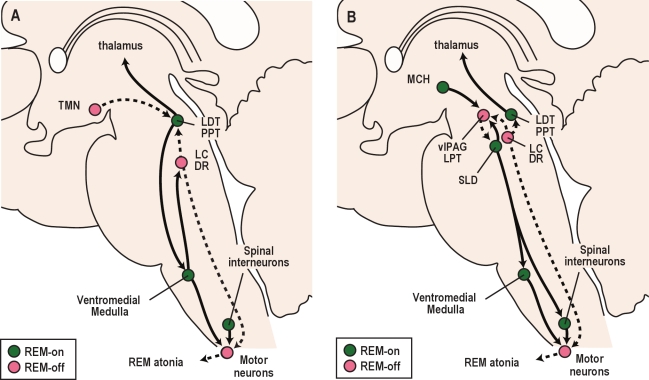
Figure 5.
Pathways that control REM sleep. (A) A classic perspective on REM sleep control involves interactions between the cholinergic and aminergic systems. REM sleep-active cholinergic neurons in the LDT/PPT activate thalamo-cortical signaling and drive atonia by exciting neurons in the ventromedial medulla that inhibit motor neurons. During REM sleep, monoaminergic neurons including the LC, DR, and TMN become silent, which disinhibits the LDT/PPT and lessens the excitation of motor neurons by NE and 5-HT. (B) Recent observations have expanded on the classic view of REM sleep control. In this model, mutual inhibition between REM sleep-on neurons of the sublaterodorsal nucleus (SLD) and REM sleep-off neurons of the ventrolateral periaqueductal gray and lateral pontine tegmentum (vlPAG/LPT) is thought to regulate transitions into and out of REM sleep. During REM sleep, SLD neurons activate GABA/glycine neurons in the ventromedial medulla and spinal cord that inhibit motor neurons. At most times, the vlPAG/LPT inhibits the SLD, but during REM sleep, the vlPAG/LPT may be inhibited by neurons making melanin concentrating hormone (MCH) and other neurotransmitters. Solid lines depict pathways active during REM sleep, while dashed lines are pathways inactive during REM sleep.
Acetylcholine
Many researchers have hypothesized that REM sleep is controlled by cholinergic neurons located in the LDT/PPT. These are the same nuclei that contain wake-promoting cells, but a subpopulation of these cholinergic neurons are active in both wakefulness and REM sleep or are selectively active in REM sleep.9,156–158 When injected into the lateral pontine tegmentum (LPT; a heterogeneous region extending rostrally from the PPT that is lateral to the periaqueductal gray), drugs that enhance ACh signaling such as the cholinergic agonist carbachol or the acetylcholinesterase blocker, neostigmine, elicit intense and long-lasting REM sleep.159–161 Conversely, cholinergic antagonists reduce the duration of REM sleep bouts.162,163 Furthermore, large lesions that include the LDT/PPT produce significant reductions in REM sleep,164,165 suggesting that the LDT/PPT is necessary for REM sleep.
Neurons in the LDT/PPT may help generate the cortical activation and atonia of REM sleep. The LDT/PPT is the main source of ACh to the thalamus, and ACh depolarizes thalamic neurons to promote the transmission of information through the thalamus, driving the cortical activation that is probably required for the complex dreams of REM sleep. The LDT/PPT neurons may also activate atonia-promoting neurons in the ventromedial medulla.158,166 These medullary cells release GABA and another inhibitory neurotransmitter glycine onto spinal and brainstem motor neurons during REM sleep, producing hyperpolarization and inhibition.167 This descending inhibition is clearly important for atonia as drugs that block glycine signaling such as strychnine can markedly increase muscle tone in REM sleep and wakefulness.168,169
Monoamines
Monoamines such as NE and 5-HT increase muscle tone by directly exciting motor neurons.170–173 In the genioglossus muscle, withdrawal of this excitatory tone contributes more to atonia than the inhibitory effects of GABA and glycine.174,175 Whether this applies to most muscles is unknown, but it is clear that atonia during REM sleep is probably due to a combination of inhibition (GABA and glycine) and a loss of excitation (NE and 5-HT).
Monoamines also inhibit REM sleep itself. During wakefulness, and to some degree during NREM sleep, the REM-active cholinergic neurons are inhibited by 5-HT, NE, and HA.176 This interaction between cholinergic and monoaminergic populations forms the foundation of the classic model explaining the alternation of NREM and REM sleep across the night (Figure 5A).177
These monoaminergic effects on motor tone and REM sleep may account for many phenomena commonly seen by sleep clinicians. NE and 5-HT reuptake inhibitors often increase muscle tone during sleep and can unmask REM sleep behavior disorder (RBD) and worsen periodic limb movements of sleep.178 These drugs and other antidepressants also strongly suppress REM sleep, and thus can markedly reduce REM sleep during overnight polysomnograms or during the MSLT.179
GABA
Over the last few years, new observations have expanded on the classic model of REM sleep control (Figure 5B). One region that has received significant attention is the sublaterodorsal nucleus (SLD; also termed the subcoeruleus, or LCα), which is a small cluster of cells ventral to the LC that produce GABA or glutamate.180 Many neurons in the SLD are active during REM sleep,158,181–183 and they project to the ventromedial medulla and ventral horn of the spinal cord, providing pathways through which they may inhibit motor neurons. Activation of the SLD region elicits atonia and REM sleep-like EEG activity,158 while inhibition of the SLD promotes wakefulness and reduces REM sleep. Most importantly, lesions of the SLD region disrupt REM sleep atonia and reduce REM sleep.165,180,182,184,185 Neuronal loss near the SLD has been reported in some patients with RBD,186 suggesting that injury to the SLD may contribute to the inadequate atonia of RBD.
Another new perspective on the classic view of REM sleep is that the SLD neurons may be strongly inhibited by REM sleep-suppressing neurons in the mid-pons.149,182,187 These GABAergic cells are scattered from the ventral part of the periaqueductal gray out into the lateral pontine tegmentum (vlPAG/LPT) and lesions of this region substantially increase REM sleep.188 The vlPAG/LPT inhibits the SLD, and the SLD may in turn inhibit the vlPAG/LPT, giving rise to a mutually inhibitory circuit that may regulate transitions between NREM and REM sleep.149,182
Melanin-concentrating hormone (MCH)
Mixed in with the orexin neurons of the lateral hypothalamus are a large number of REM sleep-active neurons that produce both MCH and GABA.189–191 These cells innervate nearly all the same target regions as the orexin neurons including the DR and LC,192,193 yet in contrast to the excitatory effects of orexins, both MCH and GABA are inhibitory. Electrophysiological recordings demonstrate that MCH neurons fire at a high rate during REM sleep, with much less firing during NREM sleep and complete inactivity during wakefulness.152 The amount of REM sleep is increased by infusions of MCH into the lateral ventricles and decreased by a MCH antagonist.191,194 Consistent with these observations, mice lacking MCH spend less time in NREM and REM sleep.195 Thus, it seems likely that the MCH neurons promote REM sleep by inhibiting the arousal regions. This pattern is strikingly opposite to that of the orexin neurons and much remains to be learned about how the activity of these intertwined systems is organized.
Mechanisms that Regulate the Transitions between Sleep and Wakefulness
The systems that promote wakefulness, NREM, and REM sleep dynamically interact in a variety of ways to ensure rapid and complete transitions between sleep/wake states.148,149 The VLPO and other sleep-promoting preoptic neurons inhibit monoaminergic and cholinergic wake-promoting neurons, and the preoptic neurons themselves are inhibited by NE, 5-HT, and ACh.196,197 During wakefulness, high monoaminergic and cholinergic tone should thoroughly silence the VLPO, thus disinhibiting the arousal regions and helping ensure the production of complete wakefulness. Conversely, during sleep, preoptic neurons become active and inhibit the arousal regions, thus disinhibiting their own firing. This mutual inhibition should produce stable wakefulness and sleep while facilitating rapid transitions between sleep and wakefulness and minimizing time in drowsy, intermediate states. Similar mutually inhibitory circuits may regulate REM sleep as REM sleep-active neurons in the SLD inhibit and are inhibited by neurons in the vlPAG/LPT that are inactive in REM sleep.182
The orexin neuropeptides probably reinforce these mutually inhibitory systems. Orexins may stabilize wakefulness by enhancing activity in the arousal systems, ensuring full alertness and long periods of wakefulness despite rising homeostatic pressure across the day.94 During wakefulness and perhaps to a lesser degree in NREM sleep, orexins may excite a variety of neurons that inhibit REM sleep, including monoaminergic neurons, the vlPAG/LPT, and GABAergic inputs to the SLD.96,198–201 However, loss of the orexin neurons in narcolepsy with cataplexy results in persistent sleepiness, frequent transitions between states, and odd states such as cataplexy and hypnagogic hallucinations in which it seems that elements of REM sleep mix into wakefulness. Collectively, these symptoms may be best thought of as behavioral state instability, a phenomena that is likely caused by loss of the stabilizing effects of orexins on the mutually inhibitory circuits that regulate wakefulness, NREM, and REM sleep.110,202
Somnogens
Most of the preceding text describes neural pathways that regulate sleep/wake states, but these states can also be influenced by diffusible or circulating factors that act upon many brain regions to promote sleep. In fact, more than 100 years ago, researchers found that the CSF of sleep deprived dogs contained somnogens, substances that promote sleep.203,204 Much evidence now suggests that adenosine, cytokines, prostaglandins, and probably additional substances serve as natural sleep-generating signals.205
Adenosine
During wakefulness, brain metabolic activity is high, and adenosine may promote sleep in response to this metabolic challenge.206–208 When cells have ample energy, nearly all adenosine is phosphorylated to ATP and adenosine levels are low. However, when cells are fatigued, ATP production is lower, adenosine levels rise, and then adenosine acts as an inhibitory neuromodulator. For example, adenosine reduces the activity of most wake-promoting neurons, but disinhibits VLPO neurons. With prolonged wakefulness, adenosine levels rise in the basal forebrain and other regions, and levels then fall during recovery sleep.209 Most likely, the extracellular levels of adenosine are governed by the activity of astrocytes, the support cells of the brain, because manipulations of astrocytes reduce the usual increases in sleep and delta power after sleep deprivation.207,209,210 Furthermore, adenosine receptor agonists increase sleep and NREM delta power, while caffeine and other drugs that block adenosine receptors promote wakefulness.207,211–213 As caffeine promotes arousal after sleep deprivation,214 it seems likely that adenosine is an important mediator of everyday sleepiness.
Cytokines
Cytokines are intercellular signaling peptides released by immune cells, neurons, and astrocytes, and several cytokines, including interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), promote sleep.215 Administration of IL-1β into the preoptic area of rats reduces firing rates of wake-active neurons and promotes NREM sleep.216 Similarly, TNF-α infusions into the preoptic area also promote NREM sleep.217 During infections, bacterial cell wall products such as lipopolysaccharide and muramyl dipeptide may trigger production of these cytokines that then increase NREM sleep and reduce REM sleep.218 In addition, these cytokines may promote spontaneous, physiological sleep as IL-1β and TNF-α mRNA and protein levels are highest around sleep onset, and blockade of IL-1β and TNF-α signaling with antagonists, antibodies, or deletion of their receptors reduces spontaneous NREM sleep.219–221
Prostaglandin D2
Prostaglandin D2 (PGD2) is a lipid derived from fatty acids that potently promotes NREM sleep.211 Unlike adenosine or cytokines which are made in the brain parenchyma, PGD2 is likely synthesized in the basal meninges just below the hypothalamus.222 PGD2 levels in cerebrospinal fluid are highest during the sleep period,223 and PDG2 levels increase with sleep deprivation.224 Infusions of PGD2 just below or within the preoptic area activate neurons in the VLPO and increase NREM and REM sleep, perhaps by increasing local concentrations of adenosine.225–229 Like cytokines, PGD2 may contribute to the sleepiness seen with inflammation as patients with African sleeping sickness display increased CSF levels of PGD2.230
Process C and Process S
The two-process model provides a useful macroscopic perspective on the dynamic control of sleep and wakefulness. It is likely that a homeostatic factor (process S) accumulates during wakefulness and declines during sleep, and this factor interacts with a circadian process (process C) that helps regulate the timing of wakefulness and REM sleep.231–233 After a period of wakefulness, delta power in NREM sleep is thought to be a good indicator of Process S,234,235 and somnogens such as adenosine may be the neurobiologic equivalent of Process S as disruption of adenosine signaling can blunt the usual increase in NREM sleep and the intense EEG delta power seen after sleep deprivation.210
Process C is driven by the suprachiasmatic nucleus (SCN), the master pacemaker that regulates the circadian rhythms of sleep, wakefulness, and most other physiologic rhythms.236 The activity of individual SCN neurons is strikingly rhythmic, especially when coupled with other SCN neurons.237 This rhythmicity arises from positive and negative feedback loops in the transcription and translation of several genes.238 To synchronize its activity with the environmental light-dark cycle, the SCN uses luminance information from photosensitive retinal ganglion cells that contain the photopigment melanopsin.239 SCN neurons then relay these timing signals to the adjacent subparaventricular zone, using neuropeptides such as prokineticin 2 or transforming growth factor-α.240,241 This signal is then passed through the dorsomedial nucleus of the hypothalamus and on to brain regions that regulate sleep and wakefulness such as the LC, VLPO, and lateral hypothalamus.242 The SCN also regulates the daily rhythm of body temperature, and through these cycles in temperature, the SCN can entrain circadian activity in cells throughout the body.243 Circadian rhythms are closely linked to metabolism, and a breakdown in this coordination of central and peripheral rhythms may contribute to the obesity and glucose intolerance that is common in people with shift work sleep disorder or insufficient sleep.244
CONCLUSIONS
Since the days of von Economo and then Moruzzi and Magoun, much has been learned about the neurobiology of sleep and wakefulness. We now know that neurons producing ACh and monoamines such as NE, 5-HT, DA, and HA promote various aspects of wakefulness. In addition, orexins/hypocretins help sustain long periods of wakefulness while suppressing REM sleep. NREM sleep is mainly regulated by neural pathways originating in the VLPO and other preoptic regions, yet it is also influenced by diffusible somnogens such as adenosine. REM sleep is driven by neurons in the pons that make ACh and GABA. These discoveries provide a useful framework to better understand sleep disorders and the effects of medications on sleep.
Nevertheless, despite these advances, many questions of clinical importance remain unanswered. What goes wrong in these circuits to cause parasomnias such as sleepwalking and periodic limb movements of sleep? Under what conditions are specific wake- and sleep-promoting systems especially necessary? How is sleep restorative? What are the functions of NREM and REM sleep? Undoubtedly, future sleep research will provide helpful insights into the underlying causes of sleep disorders and lead to new and more powerful therapeutics to treat them.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Scammell has consulted for Merck and Cephalon. Dr. España has indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. P. Valko, D. Kroeger, and C. Burgess for their thoughtful comments on this manuscript. Writing of this article was partially supported by research grants from the NIH (NS055367, HL095491).
REFERENCES
- 1.España RA, Scammell TE. Sleep neurobiology for the clinician. Sleep. 2004;27:811–20. [PubMed] [Google Scholar]
- 2.von Economo C. Sleep as a problem of localization. J Nerv Ment Dis. 1930;71:249–59. [Google Scholar]
- 3.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–73. [PubMed] [Google Scholar]
- 4.Aserinsky E, Kleitman N. A motility cycle in sleeping infants as manifested by ocular and gross bodily activity. J Appl Physiol. 1955;8:11–8. doi: 10.1152/jappl.1955.8.1.11. [DOI] [PubMed] [Google Scholar]
- 5.Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol. 1957;9:673–90. doi: 10.1016/0013-4694(57)90088-3. [DOI] [PubMed] [Google Scholar]
- 6.Jouvet M, Michel F. New research on the structures responsible for the paradoxical phase of sleep. J Physiol (Paris) 1960;52:130–1. [PubMed] [Google Scholar]
- 7.Lindsley DB, Bowden JW, Magoun HW. Effect upon the EEG of acute injury to the brain stem activating system. Electroencephalogr Clin Neurophysiol. 1949;1:475–86. [PubMed] [Google Scholar]
- 8.Posner J, Saper CB, Schiff N, Plum F. Plum and Posner's diagnosis of stupor and coma. 4th ed. Oxford University Press; 2007. [Google Scholar]
- 9.el Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res. 1989;76:519–29. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- 10.Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–59. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee MG, Manns ID, Alonso A, Jones BE. Sleep-wake related discharge properties of basal forebrain neurons recorded with micropipettes in head-fixed rats. J Neurophysiol. 2004;92:1182–98. doi: 10.1152/jn.01003.2003. [DOI] [PubMed] [Google Scholar]
- 12.Boucetta S, Jones BE. Activity profiles of cholinergic and intermingled GABAergic and putative glutamatergic neurons in the pontomesencephalic tegmentum of urethane-anesthetized rats. J Neurosci. 2009;29:4664–74. doi: 10.1523/JNEUROSCI.5502-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–77. [PubMed] [Google Scholar]
- 14.Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27:654–70. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satoh K, Fibiger HC. Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol. 1986;253:277–302. doi: 10.1002/cne.902530302. [DOI] [PubMed] [Google Scholar]
- 16.Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol. 1987;262:105–24. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- 17.Williams JA, Comisarow J, Day J, Fibiger HC, Reiner PB. State-dependent release of acetylcholine in rat thalamus measured by in vivo microdialysis. J Neurosci. 1994;14:5236–42. doi: 10.1523/JNEUROSCI.14-09-05236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marrosu F, Portas C, Mascia MS, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671:329–32. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto KI, Domino EF. Cholinergic agonist-antagonist interactions on neocortical and limbic EEG activation. Int J Neuropharmacol. 1967;6:357–73. doi: 10.1016/0028-3908(67)90028-7. [DOI] [PubMed] [Google Scholar]
- 20.Davila DG, Hurt RD, Offord KP, Harris CD, Shepard JW., Jr Acute effects of transdermal nicotine on sleep architecture, snoring, and sleep-disordered breathing in nonsmokers. Am J Respir Crit Care Med. 1994;150:469–74. doi: 10.1164/ajrccm.150.2.8049831. [DOI] [PubMed] [Google Scholar]
- 21.Spehlmann R, Norcross K. Cholinergic mechanisms in the production of focal cortical slow waves. Experientia. 1982;38:109–11. doi: 10.1007/BF01944557. [DOI] [PubMed] [Google Scholar]
- 22.Vanderwolf CH. Neocortical and hippocampal activation relation to behavior: effects of atropine, eserine, phenothiazines, and amphetamine. J Comp Physiol Psychol. 1975;88:300–23. doi: 10.1037/h0076211. [DOI] [PubMed] [Google Scholar]
- 23.Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77:3033–7. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–86. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berridge CW, Abercrombie ED. Relationship between locus coeruleus discharge rates and rates of norepinephrine release within neocortex as assessed by in vivo microdialysis. Neuroscience. 1999;93:1263–70. doi: 10.1016/s0306-4522(99)00276-6. [DOI] [PubMed] [Google Scholar]
- 26.España RA, Vlasaty J, McCormack SL, Llewellyn-Smith IJ, Scammell TE. Aminergic inputs to the hypocretin/orexin neurons. Washington, DC: Society for Neuroscience Meeting; 2005. [Google Scholar]
- 27.Berridge CW, Isaac SO, España RA. Additive wake-promoting actions of medial basal forebrain noradrenergic alpha1- and beta-receptor stimulation. Behav Neurosci. 2003;117:350–9. doi: 10.1037/0735-7044.117.2.350. [DOI] [PubMed] [Google Scholar]
- 28.De Sarro GB, Ascioti C, Froio F, Libri V, Nistico G. Evidence that locus coeruleus is the site where clonidine and drugs acting at alpha 1- and alpha 2-adrenoceptors affect sleep and arousal mechanisms. Br J Pharmacol. 1987;90:675–85. doi: 10.1111/j.1476-5381.1987.tb11220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci. 1991;11:3135–45. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–93. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- 31.Berridge CW, España RA. Synergistic sedative effects of noradrenergic alpha(1)- and beta- receptor blockade on forebrain electroencephalographic and behavioral indices. Neuroscience. 2000;99:495–505. doi: 10.1016/s0306-4522(00)00215-3. [DOI] [PubMed] [Google Scholar]
- 32.Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 1994;14:4467–80. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 34.Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci. 2001;14:1143–52. doi: 10.1046/j.0953-816x.2001.01733.x. [DOI] [PubMed] [Google Scholar]
- 35.Hunsley MS, Palmiter RD. Norepinephrine-deficient mice exhibit normal sleep-wake states but have shorter sleep latency after mild stress and low doses of amphetamine. Sleep. 2003;26:521–6. [PubMed] [Google Scholar]
- 36.Gompf HS, Mathai C, Fuller PM, et al. Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci. 2010;30:14543–51. doi: 10.1523/JNEUROSCI.3037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byers MG, Allison KM, Wendel CS, Lee JK. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30:225–9. doi: 10.1097/JCP.0b013e3181dac52f. [DOI] [PubMed] [Google Scholar]
- 38.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 39.Sakai K, el Mansari M, Lin JS, Zhang ZG, Vanni-Mercier G. The posterior hypothalamus in the regulation of wakefulness and paradoxical sleep. In: Mancia M, Marini M, editors. The diencephalon and sleep. New York: Raven Press; 1990. pp. 171–98. [Google Scholar]
- 40.Mochizuki T, Yamatodani A, Okakura K, Horii A, Inagaki N, Wada H. Circadian rhythm of histamine release from the hypothalamus of freely moving rats. Physiol Behav. 1992;51:391–4. doi: 10.1016/0031-9384(92)90157-w. [DOI] [PubMed] [Google Scholar]
- 41.Lin JS, Sakai K, Jouvet M. Evidence for histaminergic arousal mechanisms in the hypothalamus of cat. Neuropharmacology. 1988;27:111–22. doi: 10.1016/0028-3908(88)90159-1. [DOI] [PubMed] [Google Scholar]
- 42.Monti JM, Pellejero T, Jantos H. Effects of H1- and H2-histamine receptor agonists and antagonists on sleep and wakefulness in the rat. J Neural Transm. 1986;66:1–11. doi: 10.1007/BF01262953. [DOI] [PubMed] [Google Scholar]
- 43.Roehrs TA, Tietz EI, Zorick FJ, Roth T. Daytime sleepiness and antihistamines. Sleep. 1984;7:137–41. doi: 10.1093/sleep/7.2.137. [DOI] [PubMed] [Google Scholar]
- 44.Tasaka K, Chung YH, Sawada K, Mio M. Excitatory effect of histamine on the arousal system and its inhibition by H1 blockers. Brain Res Bull. 1989;22:271–5. doi: 10.1016/0361-9230(89)90053-1. [DOI] [PubMed] [Google Scholar]
- 45.Hajak G, Rodenbeck A, Voderholzer U, et al. Doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study. J Clin Psychiatry. 2001;62:453–63. doi: 10.4088/jcp.v62n0609. [DOI] [PubMed] [Google Scholar]
- 46.Krystal AD, Durrence HH, Scharf M, et al. Efficacy and safety of doxepin 1 mg and 3 mg in a 12-week sleep laboratory and outpatient trial of elderly subjects with chronic primary insomnia. Sleep. 2010;33:1553–61. doi: 10.1093/sleep/33.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Celanire S, Wijtmans M, Talaga P, Leurs R, de Esch IJ. Keynote review: histamine H3 receptor antagonists reach out for the clinic. Drug Discov Today. 2005;10:1613–27. doi: 10.1016/S1359-6446(05)03625-1. [DOI] [PubMed] [Google Scholar]
- 48.Lin JS, Sergeeva OA, Haas HL. Histamine H3 receptors and sleep-wake regulation. J Pharmacol Exp Ther. 2011;336:17–23. doi: 10.1124/jpet.110.170134. [DOI] [PubMed] [Google Scholar]
- 49.Kanbayashi T, Kodama T, Kondo H, et al. CSF histamine contents in narcolepsy, idiopathic hypersomnia and obstructive sleep apnea syndrome. Sleep. 2009;32:181–7. doi: 10.1093/sleep/32.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin JS, Sakai K, Vanni-Mercier G, et al. Involvement of histaminergic neurons in arousal mechanisms demonstrated with H3-receptor ligands in the cat. Brain Res. 1990;523:325–30. doi: 10.1016/0006-8993(90)91508-e. [DOI] [PubMed] [Google Scholar]
- 51.Monti JM, Jantos H, Ponzoni A, Monti D. Sleep and waking during acute histamine H3 agonist BP 2.94 or H3 antagonist carboperamide (MR 16155) administration in rats. Neuropsychopharmacology. 1996;15:31–5. doi: 10.1016/0893-133X(95)00151-3. [DOI] [PubMed] [Google Scholar]
- 52.Bonaventure P, Letavic M, Dugovic C, et al. Histamine H3 receptor antagonists: from target identification to drug leads. Biochem Pharmacol. 2007;73:1084–96. doi: 10.1016/j.bcp.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 53.Ligneau X, Perrin D, Landais L, et al. BF2.649 [1-{3-[3-(4-Chlorophenyl)propoxy] propyl}piperidine, hydrochloride], a nonimidazole inverse agonist/antagonist at the human histamine H3 receptor: Preclinical pharmacology. J Pharmacol Exp Ther. 2007;320:365–75. doi: 10.1124/jpet.106.111039. [DOI] [PubMed] [Google Scholar]
- 54.Lin JS, Dauvilliers Y, Arnulf I, et al. An inverse agonist of the histamine H(3) receptor improves wakefulness in narcolepsy: studies in orexin-/- mice and patients. Neurobiol Dis. 2008;30:74–83. doi: 10.1016/j.nbd.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Huang ZL, Mochizuki T, Qu WM, et al. Altered sleep-wake characteristics and lack of arousal response to H3 receptor antagonist in histamine H1 receptor knockout mice. Proc Natl Acad Sci U S A. 2006;103:4687–92. doi: 10.1073/pnas.0600451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van RP, Vermeeren A, Riedel WJ. Cognitive domains affected by histamine H(1)-antagonism in humans: a literature review. Brain Res Rev. 2010;64:263–82. doi: 10.1016/j.brainresrev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 57.Passani MB, Blandina P, Torrealba F. The histamine H3 receptor and eating behavior. J Pharmacol Exp Ther. 2011;336:24–9. doi: 10.1124/jpet.110.171306. [DOI] [PubMed] [Google Scholar]
- 58.Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22:7695–711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–50. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- 60.Portas CM, Bjorvatn B, Fagerland S, et al. On-line detection of extracellular levels of serotonin in dorsal raphe nucleus and frontal cortex over the sleep/wake cycle in the freely moving rat. Neuroscience. 1998;83:807–14. doi: 10.1016/s0306-4522(97)00438-7. [DOI] [PubMed] [Google Scholar]
- 61.Dzoljic MR, Ukponmwan OE, Saxena PR. 5-HT1-like receptor agonists enhance wakefulness. Neuropharmacology. 1992;31:623–33. doi: 10.1016/0028-3908(92)90140-k. [DOI] [PubMed] [Google Scholar]
- 62.Dugovic C, Wauquier A, Leysen JE, Marrannes R, Janssen PA. Functional role of 5-HT2 receptors in the regulation of sleep and wakefulness in the rat. Psychopharmacology (Berl) 1989;97:436–42. doi: 10.1007/BF00439544. [DOI] [PubMed] [Google Scholar]
- 63.Bjorvatn B, Ursin R. Effects of the selective 5-HT1B agonist, CGS 12066B, on sleep/waking stages and EEG power spectrum in rats. J Sleep Res. 1994;3:97–105. doi: 10.1111/j.1365-2869.1994.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 64.Boutrel B, Franc B, Hen R, Hamon M, Adrien J. Key role of 5-HT1B receptors in the regulation of paradoxical sleep as evidenced in 5-HT1B knock-out mice. J Neurosci. 1999;19:3204–12. doi: 10.1523/JNEUROSCI.19-08-03204.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ponzoni A, Monti JM, Jantos H. The effects of selective activation of the 5-HT3 receptor with m-chlorophenylbiguanide on sleep and wakefulness in the rat. Eur J Pharmacol. 1993;249:259–64. doi: 10.1016/0014-2999(93)90520-r. [DOI] [PubMed] [Google Scholar]
- 66.Bakalian MJ, Fernstrom JD. Effects of L-tryptophan and other amino acids on electroencephalographic sleep in the rat. Brain Res. 1990;528:300–7. doi: 10.1016/0006-8993(90)91671-3. [DOI] [PubMed] [Google Scholar]
- 67.Maudhuit C, Jolas T, Lainey E, Hamon M, Adrien J. Effects of acute and chronic treatment with amoxapine and cericlamine on the sleep-wakefulness cycle in the rat. Neuropharmacology. 1994;33:1017–25. doi: 10.1016/0028-3908(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 68.Vasar V, Appelberg B, Rimon R, Selvaratnam J. The effect of fluoxetine on sleep: a longitudinal, double-blind polysomnographic study of healthy volunteers. Int Clin Psychopharmacol. 1994;9:203–6. doi: 10.1097/00004850-199409000-00009. [DOI] [PubMed] [Google Scholar]
- 69.Monaca C, Boutrel B, Hen R, Hamon M, Adrien J. 5-HT 1A/1B receptor-mediated effects of the selective serotonin reuptake inhibitor, citalopram, on sleep: studies in 5-HT 1A and 5-HT 1B knockout mice. Neuropsychopharmacology. 2003;28:850–6. doi: 10.1038/sj.npp.1300109. [DOI] [PubMed] [Google Scholar]
- 70.Vazquez-Palacios G, Hernandez-Gonzalez M, Guevara Perez MA, Bonilla-Jaime H. Nicotine and fluoxetine induce arousing effects on sleep-wake cycle in antidepressive doses: a possible mechanism of antidepressant-like effects of nicotine. Pharmacol Biochem Behav. 2010;94:503–9. doi: 10.1016/j.pbb.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Monti JM. Serotonin 5-HT(2A) receptor antagonists in the treatment of insomnia: present status and future prospects. Drugs Today (Barc) 2010;46:183–93. doi: 10.1358/dot.2010.46.3.1437247. [DOI] [PubMed] [Google Scholar]
- 72.Teegarden BR, Al SH, Xiong Y. 5-HT(2A) inverse-agonists for the treatment of insomnia. Curr Top Med Chem. 2008;8:969–76. doi: 10.2174/156802608784936700. [DOI] [PubMed] [Google Scholar]
- 73.Lemoine P, Guilleminault C, Alvarez E. Improvement in subjective sleep in major depressive disorder with a novel antidepressant, agomelatine: randomized, double-blind comparison with venlafaxine. J Clin Psychiatry. 2007;68:1723–32. doi: 10.4088/jcp.v68n1112. [DOI] [PubMed] [Google Scholar]
- 74.Neylan TC, van Kammen DP, Kelley ME, Peters JL. Sleep in schizophrenic patients on and off haloperidol therapy. Clinically stable vs relapsed patients. Arch Gen Psychiatry. 1992;49:643–9. doi: 10.1001/archpsyc.1992.01820080051008. [DOI] [PubMed] [Google Scholar]
- 75.Ongini E, Bonizzoni E, Ferri N, Milani S, Trampus M. Differential effects of dopamine D-1 and D-2 receptor antagonist antipsychotics on sleep-wake patterns in the rat. J Pharmacol Exp Ther. 1993;266:726–31. [PubMed] [Google Scholar]
- 76.Arnulf I, Leu S, Oudiette D. Abnormal sleep and sleepiness in Parkinson's disease. Curr Opin Neurol. 2008;21:472–7. doi: 10.1097/WCO.0b013e328305044d. [DOI] [PubMed] [Google Scholar]
- 77.Paus S, Brecht HM, Koster J, Seeger G, Klockgether T, Wullner U. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson's disease. Mov Disord. 2003;18:659–67. doi: 10.1002/mds.10417. [DOI] [PubMed] [Google Scholar]
- 78.Arnulf I. Excessive daytime sleepiness in parkinsonism. Sleep Med Rev. 2005;9:185–200. doi: 10.1016/j.smrv.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 80.Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–88. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 81.Trulson ME. Simultaneous recording of substantia nigra neurons and voltammetric release of dopamine in the caudate of behaving cats. Brain Res Bull. 1985;15:221–3. doi: 10.1016/0361-9230(85)90140-6. [DOI] [PubMed] [Google Scholar]
- 82.Trulson ME, Preussler DW. Dopamine-containing ventral tegmental area neurons in freely moving cats: activity during the sleep-waking cycle and effects of stress. Exp Neurol. 1984;83:367–77. doi: 10.1016/S0014-4886(84)90105-5. [DOI] [PubMed] [Google Scholar]
- 83.Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006;26:193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci. 2010;1187:316–40. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- 85.Volkow ND, Fowler JS, Logan J, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–54. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mignot E, Nishino S, Guilleminault C, Dement WC. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep. 1994;17:436–7. doi: 10.1093/sleep/17.5.436. [DOI] [PubMed] [Google Scholar]
- 87.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–94. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28:8462–9. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 90.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peyron C, Tighe DK, van Den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–60. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hagan JJ, Leslie RA, Patel S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bourgin P, Huitron-Resendiz S, Spier AD, et al. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–5. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.España RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 98.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–4. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci. 2009;29:10939–49. doi: 10.1523/JNEUROSCI.1205-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brisbare-Roch C, Dingemanse J, Koberstein R, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–5. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 101.Dugovic C, Shelton JE, Aluisio LE, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330:142–51. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- 102.Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–66. doi: 10.1146/annurev-pharmtox-010510-100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 104.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 105.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 106.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 107.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.España RA, McCormack SL, Mochizuki T, Scammell TE. Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep. 2007;30:1417–25. doi: 10.1093/sleep/30.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hara J, Beuckmann CT, Nambu T, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–54. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 110.Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:6291–300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scammell TE, Willie JT, Guilleminault C, Siegel JM. A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32:111–6. [PMC free article] [PubMed] [Google Scholar]
- 112.Crocker A, Espana RA, Papadopoulou M, et al. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–8. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson's disease. Brain. 2007;130:1586–95. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fronczek R, Overeem S, Lee SY, et al. Hypocretin (orexin) loss in Parkinson's disease. Brain. 2007;130:1577–85. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- 115.Benarroch EE, Schmeichel AM, Sandroni P, Low PA, Parisi JE. Involvement of hypocretin neurons in multiple system atrophy. Acta Neuropathol. 2007;113:75–80. doi: 10.1007/s00401-006-0150-0. [DOI] [PubMed] [Google Scholar]
- 116.Baumann CR, Bassetti CL, Valko PO, et al. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol. 2009;66:555–9. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fronczek R, Baumann CR, Lammers GJ, Bassetti CL, Overeem S. Hypocretin/orexin disturbances in neurological disorders. Sleep Med Rev. 2009;13:9–22. doi: 10.1016/j.smrv.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 118.Borgland SL, Chang SJ, Bowers MS, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–25. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–21. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–48. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.España RA, Melchior JR, Roberts DCS, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 2010:1–12. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moriguchi T, Sakurai T, Nambu T, Yanagisawa M, Goto K. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci Lett. 1999;264:101–4. doi: 10.1016/s0304-3940(99)00177-9. [DOI] [PubMed] [Google Scholar]
- 123.Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–33. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yamanaka A, Beuckmann CT, Willie JT, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–13. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 125.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 126.Huguenard JR, McCormick DA. Thalamic synchrony and dynamic regulation of global forebrain oscillations. Trends Neurosci. 2007;30:350–6. doi: 10.1016/j.tins.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 127.Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–73. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- 128.Livingstone MS, Hubel DH. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981;291:554–61. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- 129.Hu B, Steriade M, Deschenes M. The cellular mechanism of thalamic ponto-geniculo-occipital waves. Neuroscience. 1989;31:25–35. doi: 10.1016/0306-4522(89)90028-6. [DOI] [PubMed] [Google Scholar]
- 130.Vanderwolf CH, Robinson TE. Reticulo-cortical activity and behavior: A critique of the arousal theory and a new synthesis. Behavioral Brain Science. 1981;4:459–514. [Google Scholar]
- 131.Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–26. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kinney HC, Korein J, Panigrahy A, Dikkes P, Goode R. Neuropathological findings in the brain of Karen Ann Quinlan. The role of the thalamus in the persistent vegetative state. N Engl J Med. 1994;330:1469–75. doi: 10.1056/NEJM199405263302101. [DOI] [PubMed] [Google Scholar]
- 133.Fuller P, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519:933–56. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gerashchenko D, Wisor JP, Burns D, et al. Identification of a population of sleep-active cerebral cortex neurons. Proc Natl Acad Sci U S A. 2008;105:10227–32. doi: 10.1073/pnas.0803125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–8. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 136.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 137.Huber R, Ghilardi MF, Massimini M, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–76. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 138.Pfaff D, Banavar JR. A theoretical framework for CNS arousal. Bioessays. 2007;29:803–10. doi: 10.1002/bies.20611. [DOI] [PubMed] [Google Scholar]
- 139.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–9. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 140.Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–46. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–88. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 142.Takahashi K, Lin JS, Sakai K. Characterization and mapping of sleep-waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience. 2009;161:269–92. doi: 10.1016/j.neuroscience.2009.02.075. [DOI] [PubMed] [Google Scholar]
- 143.Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol. 2002;543:665–77. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–5. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- 145.Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–42. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–21. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–94. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 148.Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. J Comp Neurol. 2005;493:92–8. doi: 10.1002/cne.20770. [DOI] [PubMed] [Google Scholar]
- 149.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–42. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Manns ID, Alonso A, Jones BE. Discharge profiles of juxtacellularly labeled and immunohistochemically identified GABAergic basal forebrain neurons recorded in association with the electroencephalogram in anesthetized rats. J Neurosci. 2000;20:9252–63. doi: 10.1523/JNEUROSCI.20-24-09252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hassani OK, Lee MG, Henny P, Jones BE. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J Neurosci. 2009;29:11828–40. doi: 10.1523/JNEUROSCI.1259-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hassani OK, Henny P, Lee MG, Jones BE. GABAergic neurons intermingled with orexin and MCH neurons in the lateral hypothalamus discharge maximally during sleep. Eur J Neurosci. 2010;32:448–57. doi: 10.1111/j.1460-9568.2010.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111:231–9. doi: 10.1016/s0306-4522(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 154.Sanger DJ. The pharmacology and mechanisms of action of new generation, non-benzodiazepine hypnotic agents. CNS Drugs. 2004;18(Suppl 1):9–15. doi: 10.2165/00023210-200418001-00004. [DOI] [PubMed] [Google Scholar]
- 155.Vienne J, Bettler B, Franken P, Tafti M. Differential effects of GABAB receptor subtypes, {gamma}-hydroxybutyric Acid, and Baclofen on EEG activity and sleep regulation. J Neurosci. 2010;30:14194–204. doi: 10.1523/JNEUROSCI.3145-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–59. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kayama Y, Ohta M, Jodo E. Firing of ‘possibly' cholinergic neurons in the rat laterodorsal tegmental nucleus during sleep and wakefulness. Brain Res. 1992;569:210–20. doi: 10.1016/0006-8993(92)90632-j. [DOI] [PubMed] [Google Scholar]
- 158.Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P, Luppi PH. The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study. Eur J Neurosci. 2002;16:1959–73. doi: 10.1046/j.1460-9568.2002.02257.x. [DOI] [PubMed] [Google Scholar]
- 159.Velazquez-Moctezuma J, Gillin JC, Shiromani PJ. Effect of specific M1, M2 muscarinic receptor agonists on REM sleep generation. Brain Res. 1989;503:128–31. doi: 10.1016/0006-8993(89)91712-5. [DOI] [PubMed] [Google Scholar]
- 160.Marks GA, Birabil CG. Comparison of three muscarinic agonits injected into the medial pontine reticular formation of rats to enhance REM sleep. Sleep Res Online. 2001;4:17–24. [Google Scholar]
- 161.Baghdoyan HA. Location and quantification of muscarinic receptor subtypes in rat pons: implications for REM sleep generation. Am J Physiol. 1997;273:R896–R904. doi: 10.1152/ajpregu.1997.273.3.R896. [DOI] [PubMed] [Google Scholar]
- 162.Imeri L, Bianchi S, Angeli P, Mancia M. Selective blockade of different brain stem muscarinic receptor subtypes: effects on the sleep-wake cycle. Brain Res. 1994;636:68–72. doi: 10.1016/0006-8993(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 163.Benington JH, Heller HC. Monoaminergic and cholinergic modulation of REM-sleep timing in rats. Brain Res. 1995;681:141–6. doi: 10.1016/0006-8993(95)00305-a. [DOI] [PubMed] [Google Scholar]
- 164.Webster HH, Jones BE. Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states. Brain Res. 1988;458:285–302. doi: 10.1016/0006-8993(88)90471-4. [DOI] [PubMed] [Google Scholar]
- 165.Shouse MN, Siegel JM. Pontine regulation of REM sleep components in cats: integrity of the pedunculopontine tegmentum (PPT) is important for phasic events but unnecessary for atonia during REM sleep. Brain Res. 1992;571:50–63. doi: 10.1016/0006-8993(92)90508-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Morales FR, Engelhardt JK, Soja PJ, Pereda AE, Chase MH. Motoneuron properties during motor inhibition produced by microinjection of carbachol into the pontine reticular formation of the decerebrate cat. J Neurophysiol. 1987;57:1118–29. doi: 10.1152/jn.1987.57.4.1118. [DOI] [PubMed] [Google Scholar]
- 167.Curtis DR, Hosli L, Johnston GA, Johnston IH. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp Brain Res. 1968;5:235–58. doi: 10.1007/BF00238666. [DOI] [PubMed] [Google Scholar]
- 168.Chase MH, Soja PJ, Morales FR. Evidence that glycine mediates the postsynaptic potentials that inhibit lumbar motoneurons during the atonia of active sleep. J Neurosci. 1989;9:743–51. doi: 10.1523/JNEUROSCI.09-03-00743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yamuy J, Fung SJ, Xi M, Morales FR, Chase MH. Hypoglossal motoneurons are postsynaptically inhibited during carbachol-induced rapid eye movement sleep. Neuroscience. 1999;94:11–5. doi: 10.1016/s0306-4522(99)00355-3. [DOI] [PubMed] [Google Scholar]
- 170.Fedirchuk B, Dai Y. Monoamines increase the excitability of spinal neurones in the neonatal rat by hyperpolarizing the threshold for action potential production. J Physiol. 2004;557:355–61. doi: 10.1113/jphysiol.2004.064022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol. 2001;532:467–81. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Perrier JF, Delgado-Lezama R. Synaptic release of serotonin induced by stimulation of the raphe nucleus promotes plateau potentials in spinal motoneurons of the adult turtle. J Neurosci. 2005;25:7993–9. doi: 10.1523/JNEUROSCI.1957-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lai YY, Kodama T, Siegel JM. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. J Neurosci. 2001;21:7384–91. doi: 10.1523/JNEUROSCI.21-18-07384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morrison JL, Sood S, Liu H, et al. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J Physiol. 2003;552:975–91. doi: 10.1113/jphysiol.2003.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fenik V, Davies RO, Kubin L. Combined antagonism of aminergic excitatory and amino acid inhibitory receptors in the XII nucleus abolishes REM sleep-like depression of hypoglossal motoneuronal activity. Arch Ital Biol. 2004;142:237–49. [PubMed] [Google Scholar]
- 176.Leonard CS, Llinas R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study. Neuroscience. 1994;59:309–30. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 177.McCarley RW, Hobson JA. Neuronal excitability modulation over the sleep cycle: a structural and mathematical model. Science. 1975;189:58–60. doi: 10.1126/science.1135627. [DOI] [PubMed] [Google Scholar]
- 178.Hoque R, Chesson AL., Jr Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010;6:79–83. [PMC free article] [PubMed] [Google Scholar]
- 179.Wilson S, Argyropoulos S. Antidepressants and sleep: a qualitative review of the literature. Drugs. 2005;65:927–47. doi: 10.2165/00003495-200565070-00003. [DOI] [PubMed] [Google Scholar]
- 180.Sakai K, Sastre JP, Salvert D, Touret M, Tohyama M, Jouvet M. Tegmentoreticular projections with special reference to the muscular atonia during paradoxical sleep in the cat: an HRP study. Brain Res. 1979;176:233–54. doi: 10.1016/0006-8993(79)90981-8. [DOI] [PubMed] [Google Scholar]
- 181.Verret L, Leger L, Fort P, Luppi PH. Cholinergic and noncholinergic brainstem neurons expressing Fos after paradoxical (REM) sleep deprivation and recovery. Eur J Neurosci. 2005;21:2488–504. doi: 10.1111/j.1460-9568.2005.04060.x. [DOI] [PubMed] [Google Scholar]
- 182.Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–94. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 183.Xi MC, Morales FR, Chase MH. Interactions between GABAergic and cholinergic processes in the nucleus pontis oralis: neuronal mechanisms controlling active (rapid eye movement) sleep and wakefulness. J Neurosci. 2004;24:10670–8. doi: 10.1523/JNEUROSCI.1987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sastre JP, Jouvet M. Oneiric behavior in cats. Physiol Behav. 1979;22:979–89. doi: 10.1016/0031-9384(79)90344-5. [DOI] [PubMed] [Google Scholar]
- 185.Hendricks JC, Morrison AR, Mann GL. Different behaviors during paradoxical sleep without atonia depend on pontine lesion site. Brain Res. 1982;239:81–105. doi: 10.1016/0006-8993(82)90835-6. [DOI] [PubMed] [Google Scholar]
- 186.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–88. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 187.Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur J Neurosci. 2003;18:1627–39. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- 188.Sastre JP, Buda C, Kitahama K, Jouvet M. Importance of the ventrolateral region of the periaqueductal gray and adjacent tegmentum in the control of paradoxical sleep as studied by muscimol microinjections in the cat. Neuroscience. 1996;74:415–26. doi: 10.1016/0306-4522(96)00190-x. [DOI] [PubMed] [Google Scholar]
- 189.Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–31. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Koyama Y, Takahashi K, Kodama T, Kayama Y. State-dependent activity of neurons in the perifornical hypothalamic area during sleep and waking. Neuroscience. 2003;119:1209–19. doi: 10.1016/s0306-4522(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 191.Verret L, Goutagny R, Fort P, et al. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bittencourt JC, Presse F, Arias C, et al. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–45. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 193.Kilduff TS, de Lecea L. Mapping of the mRNAs for the hypocretin/orexin and melanin- concentrating hormone receptors: Networks of overlapping peptide systems. J Comp Neurol. 2001;435:1–5. doi: 10.1002/cne.1189. [DOI] [PubMed] [Google Scholar]
- 194.Ahnaou A, Drinkenburg WH, Bouwknecht JA, Alcazar J, Steckler T, Dautzenberg FM. Blocking melanin-concentrating hormone MCH1 receptor affects rat sleep-wake architecture. Eur J Pharmacol. 2008;579:177–88. doi: 10.1016/j.ejphar.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 195.Willie JT, Sinton CM, Maratos-Flier E, Yanagisawa M. Abnormal response of melanin-concentrating hormone deficient mice to fasting: hyperactivity and rapid eye movement sleep suppression. Neuroscience. 2008;156:819–29. doi: 10.1016/j.neuroscience.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22:977–90. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gallopin T, Fort P, Eggermann E, et al. Identification of sleep-promoting neurons in vitro. Nature. 2000;404:992–5. doi: 10.1038/35010109. [DOI] [PubMed] [Google Scholar]
- 198.Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–9. doi: 10.1016/s0028-3908(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 199.Eggermann E, Serafin M, Bayer L, et al. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–81. doi: 10.1016/s0306-4522(01)00512-7. [DOI] [PubMed] [Google Scholar]
- 200.Marcus JN, Aschkenasi CJ, Lee CE, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 201.Arrigoni E, Saper CB, Lu J. Chicago, IL: Society for Neuroscience Meeting; 2009. Modulation of the spinal cord projecting neurons of the sublaterodorsal nucleus. [Google Scholar]
- 202.Broughton R, Valley V, Aguirre M, Roberts J, Suwalski W, Dunham W. Excessive daytime sleepiness and the pathophysiology of narcolepsy-cataplexy: a laboratory perspective. Sleep. 1986;9:205–15. doi: 10.1093/sleep/9.1.205. [DOI] [PubMed] [Google Scholar]
- 203.Ishimori K. True cause of sleep: A hypnogenic substance as evidenced in the brain of sleep-deprived animals. Tokyo Igakkai Zasshi. 1909;23:429–57. [Google Scholar]
- 204.Legendre R, Pieron H. Recherches sur le besoin de sommeil consecutif a une veille prolongee. Z Allgem Physiol. 1913;14:235–62. [Google Scholar]
- 205.Obal F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:d520–d550. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- 206.Radulovacki M, Virus RM, Djuricic-Nedelson M, Green RD. Adenosine analogs and sleep in rats. J Pharmacol Exp Ther. 1984;228:268–74. [PubMed] [Google Scholar]
- 207.Benington JH, Kodali SK, Heller HC. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692:79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- 208.Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–96. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 209.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–8. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Halassa MM, Florian C, Fellin T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–9. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Huang ZL, Urade Y, Hayaishi O. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Curr Opin Pharmacol. 2007;7:33–8. doi: 10.1016/j.coph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 212.Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2008;105:19992–7. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Scammell TE, Gerashchenko DY, Mochizuki T, et al. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107:653–63. doi: 10.1016/s0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- 214.Penetar D, McCann U, Thorne D, et al. Caffeine reversal of sleep deprivation effects on alertness and mood. Psychopharmacology (Berl) 1993;112:359–65. doi: 10.1007/BF02244933. [DOI] [PubMed] [Google Scholar]
- 215.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Alam MN, McGinty D, Bashir T, et al. Interleukin-1beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: role in sleep regulation. Eur J Neurosci. 2004;20:207–16. doi: 10.1111/j.1460-9568.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- 217.Kubota T, Li N, Guan Z, Brown RA, Krueger JM. Intrapreoptic microinjection of TNF-alpha enhances non-REM sleep in rats. Brain Res. 2002;932:37–44. doi: 10.1016/s0006-8993(02)02262-x. [DOI] [PubMed] [Google Scholar]
- 218.Krueger JM, Majde JA. Microbial products and cytokines in sleep and fever regulation. Crit Rev Immunol. 1994;14:355–79. doi: 10.1615/critrevimmunol.v14.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 219.Bredow S, Guha-Thakurta N, Taishi P, Obal F, Jr, Krueger JM. Diurnal variations of tumor necrosis factor alpha mRNA and alpha-tubulin mRNA in rat brain. Neuroimmunomodulation. 1997;4:84–90. doi: 10.1159/000097325. [DOI] [PubMed] [Google Scholar]
- 220.Floyd RA, Krueger JM. Diurnal variation of TNF alpha in the rat brain. Neuroreport. 1997;8:915–8. doi: 10.1097/00001756-199703030-00020. [DOI] [PubMed] [Google Scholar]
- 221.Taishi P, Bredow S, Guha-Thakurta N, Obal F, Jr, Krueger JM. Diurnal variations of interleukin-1 beta mRNA and beta-actin mRNA in rat brain. J Neuroimmunol. 1997;75:69–74. doi: 10.1016/s0165-5728(97)00002-7. [DOI] [PubMed] [Google Scholar]
- 222.Mizoguchi A, Eguchi N, Kimura K, et al. Dominant localization of prostaglandin D receptors on arachnoid trabecular cells in mouse basal forebrain and their involvement in the regulation of non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2001;98:11674–9. doi: 10.1073/pnas.201398898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pandey HP, Ram A, Matsumura H, Satoh S, Hayaishi O. Circadian variations of prostaglandins D2, E2, and F2 alpha in the cerebrospinal fluid of anesthetized rats. Biochem Biophys Res Commun. 1995;213:625–9. doi: 10.1006/bbrc.1995.2177. [DOI] [PubMed] [Google Scholar]
- 224.Ram A, Pandey HP, Matsumura H, et al. CSF levels of prostaglandins, especially the level of prostaglandin D2, are correlated with increasing propensity towards sleep in rats. Brain Res. 1997;751:81–9. doi: 10.1016/s0006-8993(96)01401-1. [DOI] [PubMed] [Google Scholar]
- 225.Scammell T, Gerashchenko D, Urade Y, Onoe H, Saper C, Hayaishi O. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2. Proc Natl Acad Sci U S A. 1998;95:7754–9. doi: 10.1073/pnas.95.13.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ueno R, Honda K, Inoue S, Hayaishi O. Prostaglandin D2, a cerebral sleep-inducing substance in rats. Proc Natl Acad Sci U S A. 1983;80:1735–7. doi: 10.1073/pnas.80.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Inoue S, Honda K, Komoda Y, Uchizono K, Ueno R, Hayaishi O. Differential sleep-promoting effects of five sleep substances nocturnally infused in unrestrained rats. Proc Natl Acad Sci U S A. 1984;81:6240–4. doi: 10.1073/pnas.81.19.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Onoe H, Ueno R, Fujita I, Nishino H, Oomura Y, Hayaishi O. Prostaglandin D2, a cerebral sleep-inducing substance in monkeys. Proc Natl Acad Sci U S A. 1988;85:4082–6. doi: 10.1073/pnas.85.11.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kantha SS. Histamine-interleukin-prostaglandin pathway: a hypothesis for a biochemical cycle regulating sleep and wakefulness. Med Hypotheses. 1994;42:335–9. doi: 10.1016/0306-9877(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 230.Pentreath VW, Rees K, Owolabi OA, Philip KA, Doua F. The somnogenic T lymphocyte suppressor prostaglandin D2 is selectively elevated in cerebrospinal fluid of advanced sleeping sickness patients. Trans R Soc Trop Med Hyg. 1990;84:795–9. doi: 10.1016/0035-9203(90)90085-s. [DOI] [PubMed] [Google Scholar]
- 231.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–68. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 232.Achermann P. The two-process model of sleep regulation revisited. Aviat Space Environ Med. 2004;75:A37–A43. [PubMed] [Google Scholar]
- 233.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dijk DJ, Brunner DP, Borbely AA. Time course of EEG power density during long sleep in humans. Am J Physiol. 1990;258:R650–R661. doi: 10.1152/ajpregu.1990.258.3.R650. [DOI] [PubMed] [Google Scholar]
- 235.Franken P, Dijk DJ, Tobler I, Borbely AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 236.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 237.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–77. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ukai H, Ueda HR. Systems biology of mammalian circadian clocks. Annu Rev Physiol. 2010;72:579–603. doi: 10.1146/annurev-physiol-073109-130051. [DOI] [PubMed] [Google Scholar]
- 239.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Cheng MY, Bullock CM, Li C, et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–10. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 241.Kramer A, Yang FC, Snodgrass P, et al. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294:2511–5. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- 242.Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–85. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–54. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]