Abstract
Study Objectives:
To determine the prevalence and polysomnographic correlates of insomnia in subjects with self-reported medical disorders.
Design:
Prospective cross-sectional study.
Participants:
Community-based sample of 3282 men and women aged 18 to 65 years old, with a subset who underwent polysomnography.
Measurements:
Self-reported measures of sleep habits and current health, and polysomnographic sleep variables.
Results:
The prevalence of insomnia was 21.4%. The adjusted odds of insomnia were 2.2 times as high in persons with any medical disorders as in those without medical disorders. Specifically, odds of insomnia were higher in people with heart disease (OR = 1.6 [95% CI: 1.2-23], P = 0.004), hypertension (1.5 [12-18], P < 0.001), diabetes (1.4 [105-20], P = 0.04), stomach ulcers (2.1 [1.6-2.7], P < 0.001), arthritis (1.8 [1.5-2.2], P < 0.001), migraine (1.8 [1.5-2.1], P < 0.001), asthma (1.6 [1.3-2.0], P = 0.04), COPD (1.9 [1.5-2.5], P < 0.001), neurological problems (2.0 [1.5-2.7], P < 0.001), and menstrual problems (1.7 [1.3-2.1], P < 0.001) than in people without these disorders. Prevalence of insomnia increased with increasing number of medical disorders. However, polysomnographic sleep was not significantly different in persons with or without medical disorders for most disorders assessed.
Conclusion:
This large population-based study suggests that insomnia is highly prevalent in diverse chronic medical disorders. However, polysomnographic evidence of disturbed sleep is present in only a subset of comorbid insomnia populations.
Citation:
Budhiraja R; Roth T; Hudgel DW; Budhiraja P; Drake CL. Prevalence and polysomnographic correlates of insomnia comorbid with medical disorders. SLEEP 2011;34(7):859-867.
Keywords: Insomnia, comorbid, medical disorders, prevalence, hypertension, stroke, arthritis
INTRODUCTION
Insomnia is defined as difficulty in initiating or maintaining sleep or nonrestorative sleep associated with daytime consequences such as fatigue, decreased concentration, or daytime distress.1–3 Chronic insomnia is defined as persistence of these symptoms for more than a month. It is a common disorder in the general population with a prevalence of approximately 10% to 15%.4–7 Insomnia is a major public health problem8 associated with diverse consequences such as increased risk for major depression,4 decreased productivity,9 increased traffic accidents,10 and reduced quality of life.11,12
While much of the research on insomnia and its treatment has focused on primary insomnia, insomnia is comorbid with other conditions in the majority of cases.13 Some recent studies have assessed the relationship between insomnia and medical disorders. One study in 9,000 participants from the general population aged 65 years and older found insomnia to be associated with an increasing number of respiratory symptoms, physical disabilities, use of nonprescription medications, depressive symptoms, and generally poor health.14 A 3-year follow-up of a subgroup of this cohort reported an increased incidence of insomnia symptoms among those with medical conditions such as heart disease, stroke, and diabetes.15 Another study of 1506 community dwellers found a higher prevalence of insomnia among those with depression, heart disease, or bodily pain.16 Finally, one study of 772 persons found high prevalence of insomnia in diverse medical disorders.17
Further evidence for this association between insomnia and medical disorders is provided by studies performed in patients with specific medical conditions. One study of 3445 patients with ≥ 1 of 5 physician-identified chronic conditions (hypertension, diabetes, congestive heart failure, myocardial infarction, or depression) found severe insomnia in 16% and mild insomnia in 34% of the patients at baseline.13 A 2-year follow up of a subgroup from this cohort found persistence of insomnia in majority of these patients. A higher prevalence of insomnia is also seen in patients with end stage renal disease18 and cancer.19
The current study investigated the association between self-reported medical disorders, a diagnosis of insomnia, and polysomnographically measured sleep in a large community sample. We hypothesized that respondents with a history of medical disorders would have a higher prevalence of chronic insomnia than those without the condition, and that this risk would vary as a function of the number of medical disorders reported.
METHODS
Participants and Definitions
Individuals participating in this study were assessed in conjunction with a larger population-based study investigating the prevalence and causes of daytime sleepiness in general population. The details of recruitment of these subjects have been provided in an earlier study.20,21 Briefly, participants were drawn from the general population of the tri-county Detroit area. The tri-county area includes 84% of the population of southeastern Michigan and is similar to the United States as a whole, with the exception of a different racial/ethnic distribution. The research design was composed of 2 components: (1) a random digit dial, computer-assisted telephone survey and (2) a laboratory-based evaluation. The telephone interviews were conducted by DataStat Inc., Ann Arbor, Michigan, by trained interviewers. For eligibility, the calling address had to be a residence and the participant an adult between the ages of 18 and 65 years. A random-probability selection procedure was used to determine the sex of the target adult. If 2 or 3 adults within a target sex were present in a household, a random-probability selection procedure (oldest/second, oldest/youngest) was used to determine the target respondent. If ≥ 4 adults with the target sex were present in the household, last-birthday method was used to determine the target respondent. In order to maintain an unbiased sample, only individuals who could not answer the questionnaire due to sensory or mental impairment were excluded from the sample. From 4682 eligible participants, 3283 individuals (70.1%) aged 18-65 completed the telephone survey. The sociodemographic characteristics of this population have been described earlier22,23 and are similar to that of the U.S. population based on the 2000 census, with the exception of higher proportion of African American and lower proportion of Hispanic participants.
Thirty-seven percent of the total interview sample (n = 1198) was randomly selected to participate in a laboratory study. In order to enrich the laboratory sample for individuals with excessive sleepiness, all remaining individuals from the overall interview sample who scored high on a validated self-report measure of sleepiness (Epworth Sleepiness Scale) were also asked to participate in the laboratory study (N = 668). Participation rate was 33% for the laboratory study. However, there were no differences between those electing to participate in the laboratory study and those who declined participation for age, gender, race, income, employment, marital status, or reported total sleep time. The laboratory study included 399 random individuals and 222 individuals selected based on the Daytime Sleepiness Scale (total N = 621). There were no demographic differences between the 2 samples. The institutional review board approved all procedures, and informed consent was obtained from all participants.
Insomnia
All respondents were asked about their sleep. Insomnia was assessed using DSM-IV criteria for insomnia.2 Specifically, respondents had to have presence of chronic sleep complaints with daytime impairment. In order to meet insomnia criteria, individuals must have reported difficulty falling asleep, staying asleep, or non-restorative sleep at least sometimes or often for one month or more. Finally, respondents were asked (a) how many days in the last 3 months had they missed work or school because of sleep problems or had productivity at work or school reduced by half or more because of sleep problems (b) how many days in the last 3 months did they not do any household work because of sleep problems or had productivity in household work reduced by half or more because of sleep problems (c) how many days in the last 3 months did they miss social or leisure activities because of sleep problems? If the answer to any of these questions was > 26 (roughly more than twice per week), or their Epworth Sleepiness score was > 10, they were considered to have daytime impairment. Three-month duration was used to ensure that daytime impairment from sleep problems had significant chronicity.
Medical disorders
The respondents were asked whether they ever had any of the following 14 medical disorders: heart disease, hypertension or high blood pressure, diabetes, thyroid problems, cancer, stomach ulcers, colitis, arthritis, migraines, asthma, emphysema or chronic bronchitis, stroke, epilepsy, or other neurological problems. Women were also asked if they currently have menstrual or gynecological problems. No and don’t know responses were collapsed together for analyses. The menstrual or gynecological problems are hereafter referred to as menstrual problems; stomach ulcers as ulcers; stroke, epilepsy or other neurological conditions as neurological problems; and emphysema or chronic bronchitis as COPD (chronic obstructive pulmonary disease).
Polysomnography
The polysomnograms were conducted in the sleep laboratory. The montage of the diagnostic polysomnography included recordings of electroencephalograms (C3, C4, and OZ referenced to mastoid), electrooculogram, submental and leg electromyograms, and electrocardiogram. Airflow was measured by a nasal thermistor. All studies were scored using previously published criteria.24 Subjects were given a scheduled bedtime consistent with their average 2-week sleep diary bedtime. Time in bed was set at 8.5 h. Sleep efficiency was calculated as the total sleep time divided by the time in bed multiplied by 100. Sleep latency was defined as time from lights off to the first epoch of any stage of sleep.
Analyses
Statistical analyses were conducted using SPSS 10.0 for Windows (SSPS Inc, Chicago, IL).We compared the demographic variables in respondents with or without insomnia; in those with or without medical disorders; and in those undergoing polysomnography using Student’s t-test or χ2 test where appropriate. The respondents were dichotomized. The prevalence of insomnia was compared in those with a specific medical disorder (e.g., hypertension) and those without that particular disorder using χ2 test. This process was repeated for each disorder. The odds ratios for insomnia in the presence of each medical disorder, while controlling for age and gender, were calculated using binary logistic regression with insomnia as the dependent variable and each disorder serially used as an independent variable (Table 2). The entire study population of 3,282 respondents was utilized for these analyses. Thereafter, the mean sleep efficiency in the subset of subjects who underwent PSG (n = 621) was calculated. Analysis of covariance (ANCOVA) was used to compare the proportion of different sleep stages in persons with any medical disorder to those with no medical disorder, controlling for the effects of gender and age (Table 3). The χ2 test was used to compare proportion of respondents with sleep efficiency ≤ 82% in presence or absence of specific disorders (Table 4). This cutoff (82%) was chosen since this represented the 25th percentile of sleep efficiency values in persons without any medical disorders. ANCOVA was then used to test the effect of presence or absence of a particular medical disorder on sleep efficiency and sleep latency, controlling for the effects of gender and age. Sleep efficiency and sleep latency were dependent variables for these analyses. The process was repeated for each disorder (Table 5). Next, we chose only respondents in whom one of the medical disorders (such as hypertension) was present and compared sleep between those with and without insomnia to assess whether presence of insomnia is associated with polysomnographically determined sleep disruption in those with a given medical disorder (Table 6). The process was repeated for each disorder. In a similar manner, we assessed the proportion of different stages in each disorder and compared sleep stages in presence or absence of insomnia within that disorder. Again, the process was repeated for each disorder (Table 7). Finally, we compared sleep only in insomniacs in presence or absence of each disorder to assess whether insomnia comorbid with medical disorders was associated with more polysomnographically determined sleep disruption than insomnia without the comorbid disorder (Table 8). Data are expressed as mean ± standard deviation unless otherwise stated. For all tests, 2-tailed statistical significance was set at P < 0.05.
Table 2.
Prevalence of DSM-IV insomnia in presence or absence of specific medical disorders.
| Medical condition (number with disease/total n, % with disease) | Unadjusted prevalence of insomnia if disease present (%) | Unadjusted prevalence of insomnia if disease absent (%) | Adjusted odds ratio (95% CI) | P |
|---|---|---|---|---|
| Ulcer (307/2919) | 33.0 | 20.1 | 2.1 (1.6-2.7) | < 0.001 |
| Neurological problem (691/2532) | 33.3 | 20.6 | 2.0 (1.5-2.7) | < 0.001 |
| COPD (282/2944) | 32.9 | 20.3 | 1.9 (1.5-2.5) | < 0.001 |
| Migraines (767/2461) | 30.5 | 18.5 | 1.8 (1.5-2.1) | < 0.001 |
| Arthritis (800/2430) | 27.1 | 19.5 | 1.8 (1.5-2.2) | < 0.001 |
| Menstrual (388/1252) | 32.2 | 22.3 | 1.7 (1.3-2.1) | < 0.001 |
| Asthma (409/2824) | 29.9 | 20.1 | 1.6 (1.3-2.0) | < 0.001 |
| Heart disease (202/3027) | 26.8 | 21.0 | 1.6 (1.2-2.3) | 0.004 |
| Hypertension (829/2045) | 24.7 | 20.2 | 1.5 (1.2-1.8) | < 0.001 |
| Diabetes (202/3030) | 25.6 | 21.1 | 1.4 (1.05-2.0) | 0.047 |
| Colitis (93/3125) | 27.4 | 21.2 | 1.4 (0.9-2.3) | 0.13 |
| Cancer (152/3081) | 23.0 | 21.3 | 1.2 (0.8-1.8) | 0.32 |
| Thyroid disorders (251/2979) | 23.8 | 21.2 | 1.1 (0.8-1.6) | 0.41 |
Adjusted odds ratio refers to the odds of developing insomnia in presence of that particular disorder, adjusted for age and gender. P values denote significance for adjusted odds ratios.
Table 3.
Comparison of sleep stages in respondents with any medical disorders compared with respondents with no medical disorders
| Sleep stage | Respondents with any medical disorder (n = 341) (mean ± SE) | Respondents with no medical disorders (n = 242) (mean ± SE) | P |
|---|---|---|---|
| Stage N1 (%) | 10.9 ± 0.4 | 9.9 ± 0.4 | 0.13 |
| Stage N2 (%) | 56.7 ± 0.5 | 58.7 ± 2.0 | 0.34 |
| Stage N3 (%) | 14.6 ± 0.5 | 14.5 ± 0.6 | 0.95 |
| Stage REM (%) | 17.6 ± 0.3 | 19.0 ± 0.4 | 0.02 |
Table 4.
Percentage of respondents with sleep efficiency ≤ 82% in presence or absence of specific disorders
| Medical disorder (number with disorder/number without disorder) | Percentage of respondents with sleep efficiency ≤ 82% |
||
|---|---|---|---|
| Disease present | Disease absent | P | |
| Heart disease (43/575) | 62.8 | 29.9 | 0.001 |
| Hypertension (172/449) | 42.4 | 28.5 | 0.001 |
| Diabetes (47/573) | 51.1 | 30.9 | 0.006 |
| Thyroid disorders (51/569) | 47.1 | 31.1 | 0.028 |
| Cancer (30/591) | 30.0 | 32.5 | 0.84 |
| Ulcer (48/572) | 33.3 | 32.2 | 0.87 |
| Colitis (19/598) | 42.1 | 31.9 | 0.45 |
| Arthritis (171/447) | 41.5 | 28.6 | 0.003 |
| Migraines (149/472) | 30.9 | 32.8 | 0.69 |
| Asthma (100/521) | 31.0 | 32.6 | 0.82 |
| COPD (54/562) | 44.4 | 31.0 | 0.04 |
| Neurological problem (60/561) | 31.2 | 44.6 | 0.06 |
| Menstrual (83/237) | 27.7 | 29.1 | 0.88 |
Table 5.
Mean sleep latency and sleep efficiency in presence or absence of specific disorders (adjusted for age, gender, and apnea hypopnea index)
| Disorder (number with disease/number without disease) | Sleep latency (min) |
Sleep efficiency (%) |
||||
|---|---|---|---|---|---|---|
| Disease Present | Disease absent | P | Disease present | Disease absent | P | |
| Heart disease (41/552) | 12.7 ± 3.7 | 15.9 ± 1.0 | 0.41 | 82.3 ± 1.7 | 84.0 ± 0.46 | 0.34 |
| Hypertension (165/431) | 19.6 ± 1.8 | 14.1 ± 1.1 | 0.01 | 82.4 ± 0.87 | 84.9 ± 0.53 | 0.04 |
| Diabetes (46/549) | 11.2 ± 3.4 | 16.0 ± 1.0 | 0.17 | 83.2 ± 1.6 | 83.9 ± 0.46 | 0.66 |
| Thyroid disorders (50/545) | 12.9 ± 3.3 | 15.9 ± 1.0 | 0.40 | 84.2 ± 1.6 | 83.9 ± 0.47 | 0.87 |
| Cancer (30/566) | 13.5 ± 4.2 | 15.8 ± 1.0 | 0.61 | 86.1 ± 2.0 | 83.8 ± 0.46 | 0.26 |
| Ulcer (46/549) | 14.0 ± 3.4 | 15.8 ± 1.0 | 0.60 | 84.0 ± 1.6 | 83.9 ± 0.46 | 0.94 |
| Colitis (17/575) | 9.1 ± 5.5 | 15.8 ± 1.0 | 0.23 | 85.4 ± 2.6 | 83.9 ± 0.45 | 0.58 |
| Arthritis (167/426) | 12.8 ± 1.8 | 16.9 ± 1.1 | 0.07 | 84.5 ± 0.88 | 83.8 ± 0.53 | 0.50 |
| Migraines (142/454) | 17.9 ± 1.9 | 14.9 ± 1.1 | 0.18 | 83.4 ± 0.92 | 84.1 ± 0.51 | 0.51 |
| Asthma (97/499) | 17.5 ± 2.3 | 15.3 ± 1.0 | 0.38 | 83.8 ± 1.1 | 83.9 ± 0.49 | 0.92 |
| COPD (53/538) | 13.9 ± 3.1 | 15.7 ± 1.0 | 0.60 | 83.4 ± 1.5 | 84.0 ± 0.46 | 0.72 |
| Neurological problem (58/538) | 19.8 ± 3.0 | 15.2 ± 1.0 | 0.14 | 81.3 ± 1.4 | 84.2 ± 0.47 | 0.06 |
| Menstrual (82/228) | 13.0 ± 2.2 | 17.2 ± 1.3 | 0.14 | 85.0 ± 1.1 | 84.8 ± 0.64 | 0.86 |
Data are expressed as mean ± standard error.
Table 6.
Mean sleep latency and sleep efficiency in persons with medical disorders in presence or absence of insomnia (adjusted for age, gender, and apnea hypopnea index)
| Disorder (Number with insomnia/number without insomnia) | Sleep Latency |
Sleep Efficiency |
||||
|---|---|---|---|---|---|---|
| Insomnia | No Insomnia | P | Insomnia | No Insomnia | P | |
| Heart disease (21/18) | 16.8 ± 3.7 | 13.2 ± 4.0 | 0.53 | 76.9 ± 2.7 | 78.1 ± 2.9 | 0.78 |
| Hypertension (54/109) | 21.8 ± 4.4 | 20.1 ± 3.1 | 0.75 | 80.8 ± 1.7 | 79.6 ± 1.2 | 0.58 |
| Diabetes (19/27) | 14.8 ± 2.6 | 12.1 ± 3.1 | 0.52 | 86.1 ± 2.4 | 74.7 ± 2.0 | 0.001 |
| Thyroid disorders (11/39) | 16.1 ± 2.1 | 14.4 ± 4.0 | 0.69 | 83.4 ± 3.1 | 80.7 ± 1.6 | 0.45 |
| Cancer (13/16) | 12.2 ± 5.2 | 18.6 ± 5.8 | 0.43 | 83.0 ± 2.3 | 84.0 ± 2.1 | 0.75 |
| Ulcer (17/29) | 14.7 ± 3.7 | 15.4 ± 4.8 | 0.92 | 80.4 ± 3.0 | 83.5 ± 2.3 | 0.43 |
| Colitis (7/10) | 12.1 ± 2.4 | 9.7 ± 2.0 | 0.47 | 78.8 ± 2.5 | 84.6 ± 2.1 | 0.12 |
| Arthritis (60/107) | 18.3 ± 2.4 | 13.2 ± 1.8 | 0.09 | 81.3 ± 1.5 | 81.9 ± 1.1 | 0.77 |
| Migraines (58/84) | 20.5 ± 2.9 | 15.4 ± 2.4 | 0.18 | 83.4 ± 1.5 | 85.0 ± 1.2 | 0.38 |
| Asthma (42/53) | 17.1 ± 3.1 | 16.6 ± 2.8 | 0.89 | 84.9 ± 1.4 | 85.8 ± 1.2 | 0.65 |
| COPD (23/30) | 16.4 ± 4.3 | 13.8 ± 3.8 | 0.65 | 80.5 ± 2.2 | 83.8 ± 1.9 | 0.28 |
| Neurological problem (21/37) | 27.7 ± 5.4 | 17.1 ± 4.0 | 0.12 | 80.7 ± 2.8 | 78.8 ± 2.1 | 0.61 |
| Menstrual (37/45) | 12.4 ± 2.1 | 13.9 ± 1.9 | 0.61 | 85.8 ± 1.6 | 84.6 ± 1.4 | 0.56 |
Data are expressed as mean ± standard error.
Table 7.
Distribution of different sleep stages (as percentage of total sleep) in persons with medical disorders in absence or presence of insomnia (adjusted for age, gender, and apnea hypopnea index)
| Disorder (N with insomnia/ N without insomnia) | Stage N1 (%) |
P |
Stage N2 (%) |
P |
Stage N3 (%) |
P |
Stage REM (%) |
P |
|---|---|---|---|---|---|---|---|---|
| Heart disease (21/18) | 0.60 | 0.50 | 0.28 | 0.32 | ||||
| Insomnia | 12.3 ± 1.5 | 57.6 ± 3.0 | 16.1 ± 2.5 | 13.9 ± 1.6 | ||||
| No insomnia | 11.1 ± 1.4 | 60.4 ± 2.8 | 12.3 ± 2.3 | 16.1 ± 1.5 | ||||
| All respondents | 11.8 ± 1.0 | 59.0 ± 2.0 | 16.8 ± 1.1 | 15.0 ± 1.1 | ||||
| Hypertension (54/109) | 0.78 | 0.75 | 0.48 | 0.91 | ||||
| Insomnia | 11.9 ± 0.8 | 55.5 ± 1.1 | 15.2 ± 1.1 | 17.5 ± 6.4 | ||||
| No insomnia | 12.3 ± 1.2 | 56.1 ± 1.6 | 13.9 ± 1.5 | 17.6 ± 9.1 | ||||
| All respondents | 12.1 ± 0.7 | 55.9 ± 1.0 | 14.5 ± 1.0 | 17.6 ± 0.5 | ||||
| Diabetes (19/27) | 0.17 | 0.39 | 0.31 | 0.06 | ||||
| Insomnia | 13.8 ± 1.7 | 58.8 ± 2.1 | 13.6 ± 1.9 | 13.8 ± 1.2 | ||||
| No insomnia | 9.8 ± 2.1 | 55.8 ± 2.5 | 16.8 ± 2.3 | 17.5 ± 1.4 | ||||
| All respondents | 11.8 ± 1.3 | 57.3 ± 1.5 | 15.2 ± 1.4 | 15.6 ± 0.9 | ||||
| Thyroid disorders (11/39) | 0.10 | 0.054 | 0.006 | 0.88 | ||||
| Insomnia | 9.5 ± 0.8 | 60.6 ± 1.5 | 13.2 ± 1.3 | 17.1 ± 1.1 | ||||
| No insomnia | 6.4 ± 1.6 | 54.4 ± 2.8 | 21.7 ± 2.6 | 17.4 ± 2.0 | ||||
| All respondents | 9.8 ± 1.0 | 57.5 ± 1.5 | 17.5 ± 1.5 | 17.2 ± 1.1 | ||||
| Cancer (13/16) | 0.26 | 0.39 | 0.29 | 0.44 | ||||
| Insomnia | 11.8 ± 1.3 | 56.2 ± 2.4 | 13.0 ± 2.5 | 19.0 ± 1.1 | ||||
| No insomnia | 9.5 ± 1.4 | 52.9 ± 2.8 | 17.2 ± 2.8 | 20.3 ± 1.3 | ||||
| All respondents | 10.6 ± 0.9 | 54.5 ± 1.8 | 15.1 ± 1.8 | 19.6 ± 0.8 | ||||
| Ulcer (17/29) | 0.50 | 0.53 | 0.75 | 0.05 | ||||
| Insomnia | 10.6 ± 1.1 | 57.9 ± 2.0 | 12.6 ± 1.8 | 18.9 ± 1.3 | ||||
| No insomnia | 11.8 ± 1.4 | 60.0 ± 2.6 | 13.5 ± 2.3 | 14.6 ± 1.6 | ||||
| All respondents | 11.2 ± 0.9 | 58.9 ± 1.6 | 13.0 ± 1.4 | 16.7 ± 1.0 | ||||
| Colitis (7/10) | 0.95 | 0.58 | 0.44 | 0.91 | ||||
| Insomnia | 10.7 ± 2.0 | 55.9 ± 3.4 | 14.2 ± 2.7 | 19.1 ± 3.5 | ||||
| No insomnia | 10.5 ± 2.5 | 59.1 ± 4.2 | 10.6 ± 3.4 | 19.7 ± 4.3 | ||||
| All respondents | 10.6 ± 1.6 | 57.5 ± 2.6 | 12.4 ± 2.0 | 19.4 ± 2.6 | ||||
| Arthritis (60/107) | 0.39 | 0.63 | 0.44 | 0.11 | ||||
| Insomnia | 12.4 ± 0.8 | 56.1 ± 1.0 | 14.7 ± 1.0 | 16.8 ± 6.1 | ||||
| No insomnia | 11.2 ± 1.0 | 56.9 ± 1.4 | 13.3 ± 1.4 | 18.5 ± 0.8 | ||||
| All respondents | 11.6 ± 0.6 | 56.5 ± 0.8 | 14.0 ± 0.9 | 17.7 ± 0.5 | ||||
| Migraines (58/84) | 0.50 | 0.13 | 0.71 | 0.02 | ||||
| Insomnia | 10.6 ± 0.8 | 55.6 ± 1.2 | 14.1 ± 1.2 | 19.5 ± 0.7 | ||||
| No insomnia | 9.8 ± 1.0 | 58.5 ± 1.5 | 14.8 ± 1.4 | 16.9 ± 0.9 | ||||
| All respondents | 10.2 ± 0.7 | 57.1 ± 0.9 | 14.5 ± 9.1 | 18.2 ± 0.6 | ||||
| Asthma (42/53) | 0.16 | 0.62 | 0.42 | 0.70 | ||||
| Insomnia | 8.9 ± 0.7 | 56.1 ± 1.2 | 15.7 ± 1.5 | 19.2 ± 0.9 | ||||
| No insomnia | 10.5 ± 0.8 | 57.0 ± 1.3 | 13.8 ± 1.6 | 18.6 ± 1.9 | ||||
| All respondents | 9.7 ± 0.5 | 56.5 ± 0.8 | 14.7 ± 1.1 | 18.9 ± 0.6 | ||||
| COPD (23/30) | 0.71 | 0.29 | 0.53 | 0.98 | ||||
| Insomnia | 12.5 ± 2.0 | 56.8 ± 1.9 | 13.2 ± 2.0 | 17.3 ± 1.5 | ||||
| No insomnia | 11.4 ± 2.3 | 59.9 ± 2.1 | 11.3 ± 2.3 | 17.4 ± 1.7 | ||||
| All respondents | 12.0 ± 1.5 | 58.4 ± 1.4 | 12.3 ± 1.6 | 17.4 ± 1.1 | ||||
| Neurological problem (21/37) | 0.55 | 0.09 | 0.35 | 0.38 | ||||
| Insomnia | 13.0 ± 1.8 | 55.0 ± 2.0 | 13.5 ± 1.4 | 18.4 ± 1.2 | ||||
| No insomnia | 11.1 ± 2.4 | 60.8 ± 2.7 | 11.4 ± 1.9 | 16.6 ± 1.6 | ||||
| All respondents | 12.1 ± 1.5 | 57.9 ± 1.7 | 12.5 ± 1.2 | 17.5 ± 1.9 | ||||
| Menstrual (37/45) | 0.57 | 0.25 | 0.6 | 0.2 | ||||
| Insomnia | 10.0 ± 0.8 | 58.2 ± 1.3 | 15.2 ± 1.5 | 16.4 ± 1.0 | ||||
| No insomnia | 9.2 ± 0.9 | 55.9 ± 1.5 | 16.5 ± 1.6 | 18.4 ± 1.1 | ||||
| All respondents | 9.6 ± 0.6 | 57.1 ± 1.0 | 15.9 ± 1.1 | 17.4 ± 0.7 |
P values denote significance for comparison between respondents without insomnia and those with insomnia within the disorder. Data are expressed as mean ± standard error.
Table 8.
Mean sleep latency and sleep efficiency within insomniacs in presence or absence of specific disorders (adjusted for age, gender, and apnea hypopnea index)
| Sleep latency (min) |
Sleep efficiency (%) |
|||||
|---|---|---|---|---|---|---|
| Disorder (number with disease/number without disease) | Disease present | Disease absent | P | Disease present | Disease absent | P |
| Heart disease (21/162) | 14.3 ± 4.5 | 15.9 ± 1.6 | 0.74 | 81.5 ± 2.4 | 84.5 ± 0.8 | 0.23 |
| Hypertension (54/129) | 21.5 ± 2.8 | 13.3 ± 1.8 | 0.02 | 82.9 ± 1.5 | 84.7 ± 0.9 | 0.33 |
| Diabetes (19/164) | 10.8 ± 4.6 | 16.3 ± 1.6 | 0.27 | 89.7 ± 2.4 | 83.5 ± 8.0 | 0.02 |
| Thyroid disorders (11/172) | 10.0 ± 6.3 | 16.1 ± 1.5 | 0.35 | 86.0 ± 3.2 | 84.1 ± 0.8 | 0.57 |
| Cancer (13/170) | 16.2 ± 5.6 | 15.7 ± 1.5 | 0.92 | 86.5 ± 2.9 | 84.0 ± 0.8 | 0.41 |
| Ulcer (17/166) | 15.3 ± 4.9 | 15.7 ± 1.5 | 0.93 | 81.4 ± 3.2 | 84.4 ± 0.8 | 0.26 |
| Colitis (7/174) | 9.4 ± 7.6 | 15.8 ± 1.5 | 0.43 | 82.1 ± 3.9 | 84.5 ± 0.8 | 0.56 |
| Arthritis (60/123) | 16.4 ± 2.7 | 15.3 ± 1.8 | 0.74 | 84.2 ± 1.4 | 84.2 ± 0.9 | 0.99 |
| Migraines (58/125) | 21.5 ± 2.6 | 13.0 ± 1.8 | 0.008 | 82.4 ± 1.4 | 85.1 ± 0.9 | 0.12 |
| Asthma (42/151) | 17.3 ± 3.2 | 15.2 ± 1.7 | 0.57 | 83.8 ± 1.6 | 84.2 ± 0.9 | 0.81 |
| COPD (23/157) | 13.8 ± 4.3 | 15.9 ± 1.6 | 0.65 | 82.0 ± 2.2 | 84.3 ± 0.8 | 0.39 |
| Neurological problems (21/162) | 25.6 ± 4.2 | 14.4 ± 1.5 | 0.01 | 83.4 ± 2.3 | 84.2 ± 0.8 | 0.80 |
| Menstrual problems (37/77) | 12.2 ± 3.5 | 19.9 ± 2.4 | 0.08 | 86.2 ± 1.7 | 83.8 ± 1.1 | 0.24 |
Data are expressed as mean ± standard error.
RESULTS
Table 1 shows the demographic characteristics and the prevalence of the various disorders in the entire sample and the persons who underwent PSG. Of the 3282 respondents, 3247 responded to the survey questions needed to make the diagnosis of insomnia. Of these, 21.4% (N = 695) met criteria for insomnia disorder. The insomnia subjects were slightly younger than the non-insomniacs (40.2 ± 11.5 y vs. 42.1 ± 12.9 y, P = 0.001) and were more likely to be female (58.6% vs. 48.7%, P < 0.001). The prevalence of insomnia was 24.7% in women and 18.0% in men (P < 0.001).
Table 1.
Population characteristics and prevalence of diverse disorders in all subjects and in those who underwent polysomnography
| All subjects (N = 3282) N (%) | Polysomnography sample (N = 621) N (%) | |
|---|---|---|
| Sex | ||
| Female | 1668 (50.8) | 320 (51.5) |
| Male | 1614 (49.2) | 301 (48.5) |
| Age (y) | 41.7 ± 12.7 | 41.6 ± 12.8 |
| Heart disease | 211/3276 (6.4) | 43/618 (7.0) |
| Hypertension | 846/3281 (25.8) | 172/621 (27.7) |
| Diabetes | 207/3278 (6.3) | 47/620 (7.6) |
| Thyroid disorders | 256/3275 (7.8) | 51/620 (8.2) |
| Cancer | 153/3279 (4.7) | 30/621 (4.8) |
| Ulcer | 314/3273 (9.6) | 48/620 (7.7) |
| Colitis | 98/3263 (3.0) | 19/617 (3.1) |
| Arthritis | 817/3277 (24.9) | 171/618 (27.7) |
| Migraines | 778/3275 (23.8) | 149/621 (24.0) |
| Asthma | 420/3279 (12.8) | 100/621 (16.1) |
| COPD | 286/3273 (8.7) | 54/616 (8.8) |
| Neurological problems | 229/3270 (7.0) | 60/621 (9.7) |
| Menstrual problems | 393/1273 (23.6) | 83/320 (25.9) |
At least one medical disorder was reported by 54.9% of the respondents. Participants reporting medical disorders were older than those with no medical disorders (43.2 ± 12.4 y vs. 37.1 ± 12.5 y, P < 0.001). The prevalence of insomnia in those with any medical disorder was significantly higher than in those with no medical disorders (26.3% vs. 14.8%, P < 0.001). Logistic regression revealed that participants with any medical disorders (OR = 2.2 [95% CI 1.8-2.7], P < 0.001) and women (OR = 1.6 [95% CI 1.2-2.2], P < 0.001) had higher odds of insomnia, while older respondents had slightly lower odds of insomnia (OR = 0.93 [95% CI 0.88-0.98], P = 0.04).
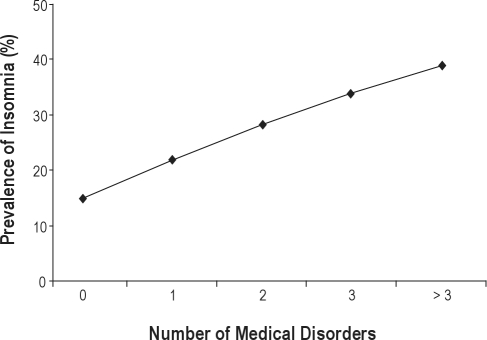
Of all persons, 45.3% had no medical disorders, 29.4% had 1 medical disorder, 14.9% had 2 medical disorders, 6.7% had 3 medical disorders, and 3.7% had > 3 medical disorders. Prevalence of insomnia increased with increasing number of medical disorders (P < 0.001, Figure 1).
Figure 1.
Prevalence of insomnia increased with increasing number of medical disorders: The prevalence in those with no medical disorder (n = 1395) was 14.8%, with 1 medical disorder (n = 904) was 21.9% with 2 disorders (n = 459) was 28.3% with 3 disorders (n = 207) was 33.8% and with > 3 disorders (n = 113) was 38.9%.
Prevalence of Insomnia in Specific Medical Disorders
Table 2 shows the prevalence of insomnia in subjects with each disorder as compared with those without that particular disorder. We calculated the odds of presence of insomnia in each disorder using logistic regression, adjusting for gender and age. People with heart disease, hypertension, diabetes, stomach ulcers, arthritis, migraine, asthma, COPD, neurological, and menstrual problems had significantly higher odds of having insomnia than those without the conditions (Table 2).
Sleep in Persons who Underwent Polysomnography
The mean sleep efficiency in subjects who underwent in-laboratory PSG (n = 621) was 83.9% ± 12.4%. Sleep efficiency correlated inversely with age (r = −0.44, P < 0.001). Women (n = 320) had a higher mean sleep efficiency than men (n = 301) (84.9% vs. 82.8%, P = 0.04). There was a negative correlation between sleep efficiency and the apnea hypopnea index (r = −0.12, P = 0.003). However, the apnea hypopnea index was not significantly different in individuals with or without insomnia (1.0 ± 0.7 vs. 1.9 ± 0.2, P = 0.26). Sleep efficiency expectedly correlated inversely with sleep latency (r = −0.48, P < 0.001) and latency to persistent sleep (r = −0.61, P < 0.001).
Polysomnographic Sleep in Medical Disorders
Respondents with any medical disorder had lower proportion of REM sleep than those without any medical disorders (Table 3). We compared the percentage of respondents with disorders who had sleep efficiency of ≤ 82% compared to the percentage in those who did not have that medical disorder. The percentage was significantly higher in those with heart disease, hypertension, diabetes, thyroid disorders, arthritis, and COPD compared to those without these disorders (Table 4). This cutoff (82%) represented the 25th percentile of sleep efficiency values in persons without any medical disorders. However, when adjusted for age, gender, and AHI, mean sleep efficiency and latency in most disorders were similar to that in absence of those disorders (Table 5). We compared sleep in persons with or without insomnia within each specific medical disorder. Presence of insomnia was not associated with significantly different sleep efficiency or sleep latency in most disorders (Table 6). Distribution of different sleep stages was similar in persons with medical disorders in presence or absence of insomnia when adjusted for age, gender, and apnea hypopnea index (Table 7).
Sleep in Insomniacs with or without Specific Medical Disorders
We compared sleep in subjects with insomnia comorbid with specific medical disorders and in insomniacs without those disorders. Hence, for these analyses, only respondents with insomnia were included. Persons with hypertension, migraines, or neurological problems had significantly higher sleep latency (adjusted for age, gender, and AHI) than those without these disorders (Table 8). The adjusted sleep efficiency was higher in persons with diabetes than those without diabetes (Table 8).
DISCUSSION
The current study evaluated the effect of several disorders on self-report and PSG-determined sleep parameters. The study findings suggest that a history of heart disease, hypertension, diabetes, stomach ulcers, arthritis, migraine, asthma, COPD, neurological problems (including epilepsy or stroke), or menstrual problems is associated with a higher prevalence of insomnia. Prevalence of insomnia increased with increasing number of medical disorders suggesting an additive adverse effect of medical disorders on insomnia.
Virtually all of the disorders studied were associated with higher odds of having insomnia. The odds were especially high in conditions associated with significant somatic discomfort. For example, arthritis and COPD, where pain and dyspnea, respectively, may contribute to sleep disturbances,25 were associated with adjusted odds of insomnia of 1.8 and 1.9, respectively. Other conditions with chronic pain or discomfort such as ulcer and migraines also had a higher prevalence of insomnia. The association between stomach ulcers and insomnia is consistent with prior findings of sleep disturbances in persons with GERD.26 While determining the specific etiologies of the insomnia disorder in these diverse disorders is beyond the scope of the current study, one may speculate that insomnia, in some cases, results not only from the health condition itself, but from the treatment thereof, e.g., β-blockers for hypertension or β-agonists for COPD; or from nonspecific psychological factors associated with chronic disease. Another possibility is that the symptoms of disorders per se, such as nocturnal chest pain and dyspnea in some patients with hypertension and heart disease, may predispose to sleep disturbances. These data are consistent with prior studies reporting a relationship between higher reported sleep disturbances and hypertension or heart complaints.27–29 However, in contrast to previous studies,30–32 we did not find higher prevalence of insomnia in persons with self-reported cancer. However, the small number of persons with cancer in the current study and a poor characterization of the type or stage of cancer limit conclusions from our data.
While medical disorders may result in sleep disturbances, sleep disturbances themselves may contribute to the severity of many of the disorders that give rise to insomnia. Immune function is altered in patients with insomnia. Insomniacs have lower levels of CD3+, CD4+, and CD8+ cells.33 Insomniac men have a lower IFN-γ than non-insomniac men.34 One study showed a reduction in natural killer cell activity, interleukin-2 production, and lymphocyte enumeration in participants with insomnia.35 Such alteration in immune function may be one of the factors mediating this reciprocal relation between insomnia and medical disorders. Furthermore, inflammation is believed to be an important pathogenetic component of disorders such as hypertension and cardiovascular disease.36–39 Inflammation has been demonstrated in patients with insomnia and sleep deprivation, and may be another factor underlying the association between sleep problems and other medical disorders. Finally, it is plausible that the psychosocial stress from poor sleep may affect the health conditions. Emerging data suggest a role for poor sleep in causing or worsening health conditions. Indeed, short sleep duration, often found among patients with insomnia, has been shown to increase the risk of hypertension.40
The prevalence of insomnia in the current study was higher than that reported in some prior population studies utilizing DSM-IV criteria (6% to 10%).5,41,42 However, many of these studies based their diagnoses primarily on the Sleep-EVAL,43 which may underestimate the prevalence of insomnia.44 Our insomnia estimate is close to that reported in a recent study (22.1%) in 10,094 US commercial health plan subscribers,44 as well as a large (n = 12,778) French study that reported a 19.0% prevalence estimate.45 We also found lower prevalence of DSM-IV insomnia in elderly. This is similar to that reported in the aforementioned study by Roth et al.44 This might be because elderly may report less daytime impairment compared to younger people, plausibly because of less demanding daytime social and/or professional responsibilities many elderly people may have.
In addition to relating medical disorders to insomnia diagnoses, the current study also assessed objective sleep parameters in medical disorders in a large sample. We did not find significant polysomnographic differences in sleep efficiency or sleep latency in presence of most disorders or insomnia. The absence of significant impairment in polysomnographic sleep architecture in individuals with COPD is similar to what has been noted in analysis of Sleep Heart Health Study data.46 Similarly, paucity of PSG findings has been demonstrated in patients with rheumatoid arthritis.47 Notably, frequency analysis of EEG in this study by Drewes et al. showed an increase in alpha activity in NREM sleep in most sleep cycles despite minimal PSG changes. Another study in gastroesophageal reflux disease patients has shown that EEG spectral power during sleep is shifted towards higher frequencies despite no difference in PSG sleep architecture when compared to controls.48 We did find that hypertension was associated with a more pronounced polysomnographically defined sleep disruption, a finding similar to that noted by Redline et al. in an analysis of Sleep Heart Health Study data.49 However, while assessment of PSG variables yielded significant differences at P values less than 0.05 for some disorders, the clinical significance of these differences is unclear, especially since no correction was made for multiple comparisons.
This study has several limitations. First, self-reporting may have limited the reliability of the presence of various disorders. While prevalence of disorders such as COPD, asthma, hypertension, and thyroid disorders is close to what has been shown in prior population studies, prevalence of migraines was very high in the current study. This may have been because of reporting of any significant headaches by respondents as migraines. The lower numbers of subjects in less prevalent disorders also limits conclusions regarding sleep in those disorders. Studies with larger samples are required to better elucidate the sleep patterns in specific disorders. Second, while the study demonstrated a higher prevalence of insomnia in several medical disorders, it is not clear whether the insomnia predated the medical disorder, started concurrently, or followed the medical disorder; and whether it worsened with progression of the medical disorder. Prospective longitudinal studies in specific conditions are needed to determine the temporal concordance of medical complaints with sleep problems and to elucidate whether a given condition is a cause of insomnia. Third, the study was designed with the aim of broadly encompassing different systems of the body to include widely prevalent and important conditions.50 Still, patients may have had other medical problems which were not identified.
In conclusion, this study demonstrates a significantly increased prevalence of insomnia in most medical disorders in a community-based sample. The results provide a platform for more focused research aimed at determining the causes and consequences of insomnia in specific disorders. Studies analyzing adverse effects of insomnia on daily functioning and healthcare use over and above those conferred by medical disorders will provide an estimate of the individual and public health burden of these sleep abnormalities. Furthermore, studies need to be designed to assess the effects of various therapies for insomnia on the comorbid disorders. The recognition and therapy of insomnia may have the potential of improving the symptoms and severity of the medical disorders themselves, as has been shown recently for multiple sclerosis,51 osteoarthritis,52 and depression.53
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Drake has received research support from Cephalon and Merck and research and equipment support from Zeo; he has also received honoraria from Cephalon unrelated to this study. Dr. Hudgel has received research support from ResMed and serves on the board of directors for YRT Corporation unrelated to this study. Dr. Roth has received grants from Aventis, Cephalon, GlaxoSmithKline, Neurocrine, Pfizer, Sanofi, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth, and Xenoport. He has served as a consultant for Abbott, Acadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstraZeneca, Aventis, AVER, BMS, BTG, Cephalon, Cypress, Dove, elan, Eli Lilly, Evotec, Forest, GlaxoSmithKline, Hypnion, Impax, Intec, Intra-Ceullular, Jazz, Johnson and Johnson, King, Lundbeck McNeil, MediciNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Prestwick, Proctor and Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport. Additionally, Dr. Roth has served as a speaker for Cephalon, Sanofi, and Takeda. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was funded by NIMH Grants MH59338 (to Dr. Roth) and MH068372 (to Dr. Drake).
REFERENCES
- 1.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3:S7–10. [PMC free article] [PubMed] [Google Scholar]
- 2.Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453–62. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 3.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 4.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 5.Ohayon MM, Smirne S. Prevalence and consequences of insomnia disorders in the general population of Italy. Sleep Med. 2002;3:115–20. doi: 10.1016/s1389-9457(01)00158-7. [DOI] [PubMed] [Google Scholar]
- 6.Ohayon M. Epidemiological study on insomnia in the general population. Sleep. 1996;19:S7–15. doi: 10.1093/sleep/19.suppl_3.s7. [DOI] [PubMed] [Google Scholar]
- 7.Roth T, Roehrs T. Insomnia: epidemiology, characteristics, and consequences. Clin Cornerstone. 2003;5:5–15. doi: 10.1016/s1098-3597(03)90031-7. [DOI] [PubMed] [Google Scholar]
- 8.Littner M, Hirshkowitz M, Kramer M, et al. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26:754–60. doi: 10.1093/sleep/26.6.754. [DOI] [PubMed] [Google Scholar]
- 9.Leger D, Guilleminault C, Bader G, Levy E, Paillard M. Medical and socio-professional impact of insomnia. Sleep. 2002;25:625–9. [PubMed] [Google Scholar]
- 10.Roth T. Prevalence, associated risks, and treatment patterns of insomnia. J Clin Psychiatry. 2005;66:10–3. quiz 42-3. [PubMed] [Google Scholar]
- 11.Leger D, Scheuermaier K, Philip P, Paillard M, Guilleminault C. SF-36: evaluation of quality of life in severe and mild insomniacs compared with good sleepers. Psychosom Med. 2001;63:49–55. doi: 10.1097/00006842-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Zammit GK, Weiner J, Damato N, Sillup GP, McMillan CA. Quality of life in people with insomnia. Sleep. 1999;22:S379–85. [PubMed] [Google Scholar]
- 13.Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998;158:1099–107. doi: 10.1001/archinte.158.10.1099. [DOI] [PubMed] [Google Scholar]
- 14.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 15.Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22:S366–72. [PubMed] [Google Scholar]
- 16.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 18.Gigli GL, Adorati M, Dolso P, et al. Restless legs syndrome in end-stage renal disease. Sleep Med. 2004;5:309–15. doi: 10.1016/j.sleep.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 20.Drake CL, Roehrs TA, Burduvali E, Bonahoom A, Rosekind M, Roth T. Effects of rapid versus slow accumulation of eight hours of sleep loss. Psychophysiology. 2001;38:979–87. doi: 10.1111/1469-8986.3860979. [DOI] [PubMed] [Google Scholar]
- 21.Drake C, Roehrs T, Breslau N, et al. The 10-year risk of verified motor vehicle crashes in relation to physiologic sleepiness. Sleep. 33:745–52. doi: 10.1093/sleep/33.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jefferson CD, Drake CL, Scofield HM, et al. Sleep hygiene practices in a population-based sample of insomniacs. Sleep. 2005;28:611–5. doi: 10.1093/sleep/28.5.611. [DOI] [PubMed] [Google Scholar]
- 23.Singh M, Drake CL, Roth T. The prevalence of multiple sleep-onset REM periods in a population-based sample. Sleep. 2006;29:890–5. doi: 10.1093/sleep/29.7.890. [DOI] [PubMed] [Google Scholar]
- 24.Rechtschaffen A, Kales . A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles, CA: Brain Information Service, University of California, Los Angeles; 1968. [Google Scholar]
- 25.Wilson KG, Eriksson MY, D’Eon JL, Mikail SF, Emery PC. Major depression and insomnia in chronic pain. Clin J Pain. 2002;18:77–83. doi: 10.1097/00002508-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Fass R, Quan SF, O’Connor GT, Ervin A, Iber C. Predictors of heartburn during sleep in a large prospective cohort study. Chest. 2005;127:1658–66. doi: 10.1378/chest.127.5.1658. [DOI] [PubMed] [Google Scholar]
- 27.Appels A, de Vos Y, van Diest R, Hoppner P, Mulder P, de Groen J. Are sleep complaints predictive of future myocardial infarction? Act Nerv Super (Praha) 1987;29:147–51. [PubMed] [Google Scholar]
- 28.Eaker ED, Pinsky J, Castelli WP. Myocardial infarction and coronary death among women: psychosocial predictors from a 20-year follow-up of women in the Framingham Study. Am J Epidemiol. 1992;135:854–64. doi: 10.1093/oxfordjournals.aje.a116381. [DOI] [PubMed] [Google Scholar]
- 29.Newman AB, Spiekerman CF, Enright P, et al. Daytime sleepiness predicts mortality and cardiovascular disease in older adults. The Cardiovascular Health Study Research Group. J Am Geriatr Soc. 2000;48:115–23. doi: 10.1111/j.1532-5415.2000.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 30.Degner LF, Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symptom Manage. 1995;10:423–31. doi: 10.1016/0885-3924(95)00056-5. [DOI] [PubMed] [Google Scholar]
- 31.Kurtz ME, Kurtz JC, Given CW, Given B. Loss of physical functioning among patients with cancer: a longitudinal view. Cancer Pract. 1993;1:275–81. [PubMed] [Google Scholar]
- 32.Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res. 1994;3:183–9. doi: 10.1007/BF00435383. [DOI] [PubMed] [Google Scholar]
- 33.Savard J, Laroche L, Simard S, Ivers H, Morin CM. Chronic insomnia and immune functioning. Psychosom Med. 2003;65:211–21. doi: 10.1097/01.psy.0000033126.22740.f3. [DOI] [PubMed] [Google Scholar]
- 34.Sakami S, Ishikawa T, Kawakami N, et al. Coemergence of insomnia and a shift in the Th1/Th2 balance toward Th2 dominance. Neuroimmunomodulation. 2002;10:337–43. doi: 10.1159/000071474. [DOI] [PubMed] [Google Scholar]
- 35.Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun. 2003;17:365–72. doi: 10.1016/s0889-1591(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 36.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 37.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. Bmj. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lisman KA, Stetson SJ, Koerner MM, Farmer JA, Torre-Amione G. The role of inflammation in the pathogenesis of heart failure. Curr Cardiol Rep. 2002;4:200–5. doi: 10.1007/s11886-002-0051-3. [DOI] [PubMed] [Google Scholar]
- 39.Hasper D, Hummel M, Kleber FX, Reindl I, Volk HD. Systemic inflammation in patients with heart failure. Eur Heart J. 1998;19:761–5. doi: 10.1053/euhj.1997.0858. [DOI] [PubMed] [Google Scholar]
- 40.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 41.Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–8. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- 42.Ohayon MM, Zulley J. Correlates of global sleep dissatisfaction in the German population. Sleep. 2001;24:780–7. [PubMed] [Google Scholar]
- 43.Ohayon MM, Guilleminault C, Zulley J, Palombini L, Raab H. Validation of the sleep-EVAL system against clinical assessments of sleep disorders and polysomnographic data. Sleep. 1999;22:925–30. doi: 10.1093/sleep/22.7.925. [DOI] [PubMed] [Google Scholar]
- 44.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR, ICD-10, and RDC/ICSD-2 criteria: Results from the America Insomnia Survey. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.10.023. In Press. [DOI] [PubMed] [Google Scholar]
- 45.Leger D, Guilleminault C, Dreyfus JP, Delahaye C, Paillard M. Prevalence of insomnia in a survey of 12,778 adults in France. J Sleep Res. 2000;9:35–42. doi: 10.1046/j.1365-2869.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 46.Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167:7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 47.Drewes AM, Svendsen L, Taagholt SJ, Bjerregard K, Nielsen KD, Hansen B. Sleep in rheumatoid arthritis: a comparison with healthy subjects and studies of sleep/wake interactions. Br J Rheumatol. 1998;37:71–81. doi: 10.1093/rheumatology/37.1.71. [DOI] [PubMed] [Google Scholar]
- 48.Budhiraja R, Quan SF, Punjabi NM, Drake CL, Dickman R, Fass R. Power spectral analysis of the sleep electroencephalogram in heartburn patients with or without gastroesophageal reflux disease: a feasibility study. J Clin Gastroenterol. 44:91–6. doi: 10.1097/MCG.0b013e3181a92a57. [DOI] [PubMed] [Google Scholar]
- 49.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–18. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 50.E Kramarow HL, Rooks R, Weeks J, Saydah S. Hyattsville (MD): National Center for Health Statistics; 1999. In: Health and aging chartbook. Health, United States, 1999; pp. 40–1. [Google Scholar]
- 51.Baron KG, Corden M, Jin L, Mohr DC. Impact of psychotherapy on insomnia symptoms in patients with depression and multiple sclerosis. J Behav Med. 2010 Aug 31; doi: 10.1007/s10865-010-9288-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5:355–62. [PMC free article] [PubMed] [Google Scholar]
- 53.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]