Abstract
Study Objectives:
To describe the prevalence, persistence, and characteristics associated with sleep disordered breathing (SDB) symptoms in a population-based cohort followed from 6 months to 6.75 years.
Design:
Avon Longitudinal Study of Parents and Children (ALSPAC).
Setting:
England, 1991-1999.
Participants:
12,447 children in ALSPAC with parental report of apnea, snoring, or mouth-breathing frequency on any one of 7 questionnaires.
Measurements:
Symptom prevalence rates—assessed as “Always” and “Habitually”—are reported at 0.5, 1.5, 2.5, 3.5, 4.75, 5.75, and 6.75 years of age. The proportion of children in whom symptoms develop, persist or abate between observation points is reported. Exploratory multivariate analyses identified SDB risk factors at 1.5, 4.75, and 6.75 years.
Results:
The prevalence of apnea (“Always”) is 1%-2% at all ages assessed. In contrast, snoring “Always” ranges from 3.6% to 7.7%, and snoring “Habitually” ranges from 9.6% to 21.2%, with a notable increase from 1.5- 2.5 years. At 6 years old, 25% are habitual mouth-breathers. The “Always” and “Habitual” incidence of each symptom between time points is 1%-5% and 5%-10%, respectively. In multivariate analyses of combined symptoms, socioeconomic factors have stronger, more persistent effects upon increased SDB risk than gestational age, gender, or race (aside from 1.5 years); adenoidectomy decreases risk by 40%-50%.
Conclusions:
This is the first natural history study of the primary symptoms of SDB across a key 6-year period in the development of SDB symptoms. Snoring rates are higher and spike earlier than previously reported. Symptoms are dynamic, suggesting the need for early and continued vigilance in early childhood.
Citation:
Bonuck KA; Chervin RD; Cole TJ; Emond A; Henderson J; Xu L; Freeman K. Prevalence and persistence of sleep disordered breathing symptoms in young children: a 6-year population-based cohort study. SLEEP 2011;34(7):875-884.
Keywords: Epidemiological, sleep disordered breathing, snoring, apnea, mouth-breathing, children
INTRODUCTION
Obstructive sleep apnea is estimated to affect about 1% to 4% of children. Yet many more may suffer significant medical consequences from less obvious forms of obstructive sleep disordered breathing (SDB) arising from variations in upper airway anatomy, local tissue compliance, and neurophysiological control. SDB disrupts nocturnal respiration and sleep, with adverse consequences for cognition,1–4 behavior,2–4 cardiac function, and growth.3–6 For most children, adenotonsillar hypertrophy is a primary cause of SDB. Tonsillectomy and adenoidectomy is curative in many cases,7,8 though recent data suggest lower success rates than previously believed, particularly in children who are > 7 years and/or obese.9 SDB reportedly peaks between 2-6 years of age, given the adenotonsillar hypertrophy found at this time,10 but may be found in younger and older children.11
Despite an exponential increase in research–a more than 10-fold increase in the number of scientific publications on sleep disordered breathing and children between 1990-1999 and 2000-2010–population-based data on the natural history of SDB in children are scarce. Existing natural history studies have enrolled either preschool12 or school-aged13–15 children, leaving important questions during the earliest years unresolved.16 Only one study follows children longer than 3 years,15 and only one was based upon a large population-based cohort.14
In contrast, longitudinal, population-based cohort studies have yielded data on behaviorally related sleep disorders in young17 and school-aged children,18 children transitioning from pre- to school-age,19 and adolescents20 based upon reported symptoms. One reason for the gap in longitudinal population-based studies of SDB in children may be the infeasibility of employing polysomnography (PSG) for epidemiological purposes, given the expense, time, and possible selection bias of those undergoing PSG. However, as baseline SDB symptoms in comparison to PSG may predict treatment responses just as well,21 epidemiological studies of SDB symptoms may actually have considerable clinical relevance, at least until PSG methods can be made more informative and less cost-prohibitive for large samples.
This paper fills a critical gap in the literature by presenting the first-ever population-based cohort data on 3 key symptoms of SDB–snoring, mouth-breathing, and apnea–assessed at 7 time points from birth through 6.75 years of age. Two research questions were at the core of the study: (1) “What is the prevalence of snoring, mouth-breathing, and apnea in the cohort, over time?” and (2) “In what proportions of children do each of these symptoms develop, resolve, or persist in the shorter term (i.e., approximately 12-month period)”? Secondarily, we conducted exploratory multivariate analyses to identify factors associated with the prevalence of each symptom prior to (1.5 years), during (4.75 years), and at the end (6.75 years) of the previously reported peak prevalence of symptoms.
METHODS
Population
The Avon Longitudinal Study of Parents and Children (ALSPAC), a geographically based cohort study of children, enrolled pregnant women residing in a defined part of the former county of Avon in southwest England with an expected date of delivery between April 1991 and December 1992. A total of 14,541 pregnant women were enrolled. The cohort has been described in detail elsewhere and is broadly representative of the UK population in terms of socioeconomic status (SES), although with a slight under-representation of ethnic minority families, and slight overrepresentation of wealthier families. Data for these analyses represent the 14,049 live births. Analyses were conducted on the ALSPAC sample because it is, to the best of our knowledge, the only existing population-based study to assess SDB symptoms in children from infancy through the early school years.
SDB Symptom Assessment
SDB symptoms were assessed through parent report of snoring, apnea, and mouth-breathing via preexisting questionnaires developed by ALSPAC that were mailed to participants. Snoring and apnea lie along the continuum of symptoms used to define SDB; mouth-breathing is a common clinical finding in younger22 and older23 children with SDB, and often resolves post-tonsillectomy or adenoidectomy,24 along with snoring and apnea. As a secondary data analysis, objective sleep evaluation measures were unavailable. However, items similar to, or the same as those used in ALSPAC have consistently been validated against polysomnographic data obtained from sleep laboratories. Some of these validation studies have employed parent report of all 3 SDB symptoms–snoring, mouth-breathing, and apnea,21,25–28–while a few have included only snoring and apnea.29,30 As data were collected in the 1990s, parent responses are unlikely to have been affected by the increased media and clinician attunement to pediatric sleep disorders since that time.
SDB symptoms were assessed as follows:
Mouth-Breathing: “Does she breathe through her mouth rather than her nose?” Through 3.5 years, mouth breathing was assessed at 4 levels: Always, Much of the Time, Rarely, Never. Beginning at 4.75 years, the scale was expanded to 5 levels.
Apnea: When asleep, does she seem to stop breathing or hold breath for several seconds at a time? Apnea was assessed in this manner at 3 levels (Yes/Often, Yes/Sometimes, No) except at 1.5 years, when the additional category Yes, But Rarely was included.
Snoring: Does she snore for more than a few minutes at a time? At 6 months, snoring was assessed at 3 levels: Most Nights, Quite Often, Rarely. At 1.5 years, responses were expanded to 4 levels: Most Nights, Quite Often, Sometimes, Rarely/Never. Options reverted to 3 levels at 2.5 years (Yes/Often, Yes/Sometimes, No) and 3.5 years (Most Nights, Quite Often, Rarely). From 4.75 years onward, snoring was assessed at 5 levels: Most Nights, Quite Often, Sometimes, Only Rarely, Never.
Covariate Assessment
We selected relevant covariates for examining potential attrition bias in our data on prevalence and persistence (primary aims), and for our exploratory multivariate analyses (secondary aim). Initially, we selected potential risk factors for SDB based upon existing literature and clinical judgment. Bivariate analyses of more than 300 such variables' association with both “Always” and “Habitual” reduced these to:
Maternal and family characteristics
Maternal cigarette smoking: defined as “ever” vs. “never” prior to pregnancy.
Ethnicity of child: defined as white or non-white.
Housing inadequacy: coded “Yes” if crowdedness (< 1 room/person); “Yes” for “homelessness”; “Yes” for the periods from the index child's birth-2 years, and/or from 2-4 years. Otherwise, housing inadequacy was coded “No”.
Paternal social class: assessed as manual vs. professional.
Maternal education: assessed as low or high. “Low” was defined as “O” level education or less (the end of compulsory education, resulting in a school leaving certificate at 16 in the UK), from 5 original groupings.
Child characteristics
Birthweight and gestational age, analyzed as continuous and categorical variables. For the latter, low birthweight was defined as < 2500 grams, and prematurity as < 37 weeks gestation.
Breastfeeding: identifies whether the child was ever breastfed or not.
Other children: number of other children in the house when the index child was 6 months old.
Asthma: On each questionnaire, parents were asked whether their child had wheezed in the prior 12 months and if he or she had, the total number of days. This number was used as the primary indicator for asthma.
Adenoidectomy/Tonsillectomy: On the questionnaires for 4.75, 5.75, and 6.75 years, parents were asked whether their child had ever had their tonsils or adenoids removed. Exact surgery dates for ALSPAC participants were unavailable. In population-based data from another study begun in the 1990s in Northern Ireland, the median ages for these surgeries were 6.7 years and 5.6 years, respectively.31
BMI z-scores: We computed age- and sex-specific BMI z-scores for ages 0.5, 1.5, and 3.5 years or younger,32 when universal measurements are taken by health visitors in U.K. homes, and recorded in personal child health records. If anthropometric data were available for the 10% subsample directly measured by ALSPAC staff at study clinics, those data were used instead of the home visitor measures, though the accuracy of the latter measures has been established.33
Statistical Analyses
SDB symptom prevalence rates were derived from ALSPAC's preexisting response options. As noted above, these response options were not uniform either across symptoms or over time. This is a common limitation in definitions of parent-reported snoring.34 For dichotomous outcomes of symptom presence/absence, we addressed this problem by employing both the “Always” and “Habitual” terminology, consistent with prior work. “Always” was defined as the left-most extreme response option, while “Habitual” was defined as the 2 left-most extreme response options (e.g., “Yes/Often” and “Yes/Sometimes” for apnea). Note that this was not an issue for combined symptom outcomes in multivariate analyses, because we used standardized scores (see below), which mitigate the effects of varying response options. The cohort's prevalence of “Always” and “Habitual” SDB symptoms is shown for each time point.
The persistence of symptoms was based upon children with non-missing data for 2 consecutive time points. Changes in “Always” having the symptom were described as: “Develops”, “Resolves”, or “Persists”. These changes reflect the differences in proportions from one time point to the next who go on to develop the symptom, in whom the symptom is no longer present, and in whom the symptom remains present. Corresponding data for “Habitual” transitions are in Supplemental Appendices.
Exploratory multivariate logistic regression analyses were employed to describe the characteristics associated with each of the 3 symptoms separately (i.e., having the symptom or not) at 1.5, 4.75, and 6.75 years. These 3 time points were selected to simplify data presentation, and because they reflect periods prior to, during, and at the end of the peak prevalence for SDB. (We note that given the complexity of the data, results from any one time point should not be overinterpreted; thus these are considered exploratory analyses). To analyze the effect of the 3 symptoms combined, we derived z-scores by extrapolating the codes across an interval from 0-100 with higher values denoting more symptoms (e.g., Apnea, 0 = No, 50 = Yes/sometimes, 100 = Yes/often). At each time point, the assigned scores of the 3 symptoms were summed, yielding a score from 0-300, which was then standardized to derive the SDB z-scores used as dependent variables in multiple linear regression models.
Both the multivariate logistic and regression analyses incorporate all putative covariates in the model. In Supplemental Tables S1–S2 we present the multivariate logistic regression analyses for the subsample of children with SDB data at all 7 time points.
First- and second-order interactions of wheezing, tonsillectomy (“ever” by 4.75 and 6.75 years), and adenoidectomy (“ever” by 4.75 and 6.75 years) were included in the above multivariate models given that (a) SDB is a prime indication for both surgeries, (b) wheezing history in this cohort is predictive of physician-diagnosed asthma,35 which is a known risk factor for SDB,36,37 and (c) tonsillectomy mediates the effect of wheeze upon SDB.36 Multivariate models were derived with the complete set of maternal, family, and child characteristics. Prior work found an association between low socioeconomic (SES) and increased risk of SDB, that was mediated by a third variable, high BMI.38 Thus, to determine the effects of BMI, we incorporated BMI z-score changes from 0.6-1.5 years and from 1.5-3.5 years upon SDB outcomes at later time points for the subset of children with non-missing data for both BMI z-score changes and the SDB variables at these ages.
RESULTS
Data Completeness and Attrition Bias
Of the base sample of 14,049 children, respondents with ≥ 1 SDB symptom assessed (not shown) were as follows: 0.5 years = 80.8%; 1.5 years = 78.5%; 2.5 years = 72.8%; 3.5 years = 70.8%; 4.75 years = 67%; 5.75 years = 61.2%; and 6.75 years = 59%. Of the base sample, 45.4% provided at least some SDB data for all 7 time points (n = 6,219). The following proportions of the sample provided partial data: ≥ 6/7 time points = 57.6%; ≥ 5/7 time points = 65.8%; ≥ 4/7 = 72.1%; ≥ 3/7 time points = 77.5%; ≥ 2/7 time points = 83.1%; and ≥ 1/7 time points = 88.7%. Of the base sample, 11.4% did not provide any SDB data. Note, overall ALSPAC questionnaire response rates were nearly identical to those for respondents with ≥ 1 SDB symptom.
Table 1 compares the characteristics of respondents with and without snoring data at 1.5, 4.75, and 6.75 years. Children with missing data were more likely to have SDB symptom risk factors, i.e., prior wheezing, low birthweight, preterm, born to primiparous mother, never breastfed, and lower SES. Children with mouth-breathing data (not shown) at 1.5 (n = 9,970), 4.75 (n = 8,525), and 6.75 (n = 7,460) years and with apnea data at 1.5 (n = 9,774), 4.75 (n = 8,102), and 6.75 (n = 6,998) years exhibited nearly identical patterns of attrition bias to those shown in Table 1.
Table 1.
Characteristics of the study population with and without snoring data at 1.5, 4.75, and 6.75 years
| Covariates | 1.5 years |
4.75 years |
6.75 years |
|||
|---|---|---|---|---|---|---|
| With Data N = 10,653 | Missing N = 3,396 | With Data N = 9,007 | Missing N = 5,042 | With Data N = 7,895 | Missing N = 6,154 | |
| Wheezed, ever† | 34.58% | 60.75%* | 49.24% | 83.63%* | 53.39% | 87.89%* |
| LBW (< 2500 g) | 4.99% | 7.93%* | 4.60% | 7.66%* | 4.48% | 7.27%* |
| Preterm (< 37 weeks) | 5.80% | 7.86%* | 5.33% | 8.03%* | 5.32% | 7.56% |
| Housing, inadequate | 9.34% | 8.11%* | 12.53% | 12.25% | 11.80% | 13.52% |
| Girl | 48.47% | 46.67% | 47.97% | 48.81% | 48.45% | 48.05% |
| White‡ | 97.85% | 95.21%* | 98.11% | 95.67%* | 98.14% | 96.14%* |
| Smoked, ever | 48.66% | 58.63%* | 47.77% | 56.83%* | 47.29% | 55.75%* |
| Other child, ≥ 1 | 54.82% | 57.75%* | 54.10% | 57.76%* | 53.70% | 57.61%* |
| Breastfed, never | 23.32% | 34.71%* | 22.32% | 31.94%* | 21.39% | 31.41%* |
| Paternal employment, manual | 35.12% | 54.41%* | 41.37% | 50.97%* | 40.53% | 50.43%* |
| Maternal education, lower | 62.29% | 75.54%* | 60.83% | 73.55%* | 59.47% | 72.98%* |
Significant at P < 0.05. P values are from χ2 test.
Based upon report of ever wheezing, prior 12 months.
Participants reporting a race other than white are, in descending order: Black Caribbean (n = 75), Indian (n = 53), Other Black (n = 44), Chinese (n = 8), Pakistani (n = 22), Black African (n = 11), and Bangladeshi (n = 7).
Prevalence of SDB Across Early Childhood
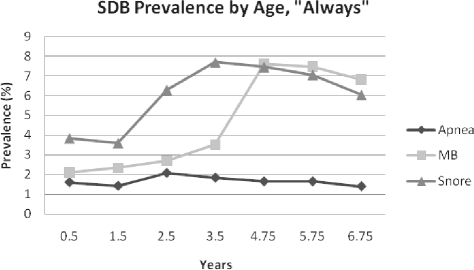
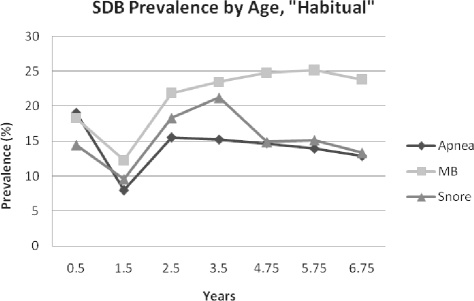
The prevalence of “Always” snoring, mouth-breathing, and apnea is shown in Figure 1. “Always” apnea prevalence ranged from 1% to 2%; “Always” snoring ranged from 3.6% at 1.5 years, to a peak of 7.7% at 3.5 years, and “Always” mouth-breathing ranged from 2.1% at 0.5 years to 7.6% at 4.75 years, with a doubling from 3.5-4.75 years. As shown in Figure 2, “Habitual” apnea peaked at 19% at 0.5 years, fell to a nadir of 8% at 1.5 years (likely due to a one-time change in response categories as stated above), then plateaued at 12.9% at 6.75 years. Habitual snoring had a low of 9.6% at 1.5 years and reached its height at 3.5 years at 21%. Habitual mouth-breathing was at its lowest point at 1.5 years (12.3%), and then peaked later than either snoring or apnea, at 5.75 years (25.2%). Prevalence data for the subsample of children with SDB symptom data for all 7 time points (n = 6,219) are shown in Supplemental Figures S1–S2. Snoring and mouth-breathing prevalence rates were approximately 1% lower in this subsample, while apnea rates appeared to be unchanged.
Figure 1.
SDB Prevalence by Age, “Always”
Figure 2.
SDB Prevalence by Age, “Habitual”
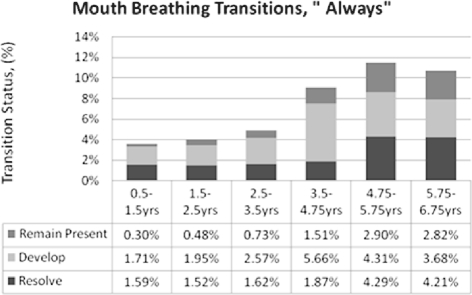
Persistence of SDB Symptoms Across Early Childhood
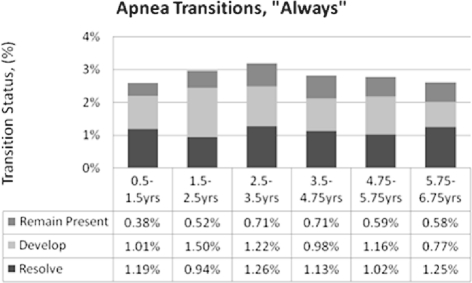
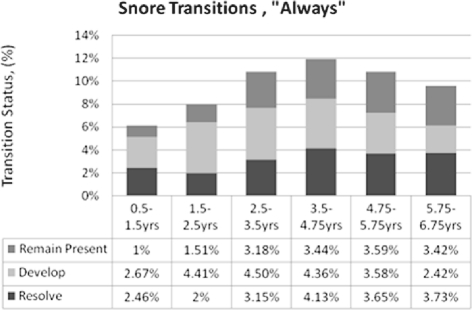
Figures 3–5 depict the proportions of the sample whose symptoms resolved, developed, or remained present between time points per the “Always” measure. Apnea remained stable throughout, at 1% to 2% (Figure 3). Snoring incidence peaked in the middle 4 intervals (Figure 4). Mouth-breathing patterns increased most from 3.5-4.75 years, resolved most from 4.75-5.75 years, and had the most overall shifts in the last 3 intervals (Figure 5). The primary differences for “Habitual” outcomes for all 3 symptoms (Supplemental Figures S3–S5) were relatively greater resolution in the first interval and relatively greater development in the second interval. Overall, each of the 3 “Always” symptoms developed in 1%-5% of the cohort from one time point to the next, while “Habitual” symptoms developed in approximately 5%-10% between time points.
Figure 3.
Apnea “Always” Persistence
Figure 4.
Snoring “Always” Persistence
Figure 5.
Mouth-breathing “Always” Persistence
Maternal, Family, and Child Characteristics Associated with Snoring, Mouth-Breathing, and Apnea
Table 2 shows risk factor associations with our “Always” outcomes in the full sample model (vs. the subset with non-missing BMI data); Table 3 presents corresponding data for “Habitual” outcomes. Significant ORs (95% CIs) are shown in bold. For maternal and family characteristics, 2- to 3-fold increases in race-associated risks of “Always” snoring and mouth-breathing, and “Habitual” snoring, mouth-breathing, and apnea at 1.5 years, were not significant at later outcomes. (NB: persons reporting race other than white were primarily Black Caribbean or Indian.) Manual paternal employment and lower maternal education increased most SDB symptoms risks by 20%-100% in “Always” models, with fewer and more modest significant “Habitual” effects, although inadequate housing became a significant risk factor (≈ 30% to 55%) in “Habitual” models across symptoms and time points.
Table 2.
Maternal, family, and child characteristics* associated with mouth-breathing, snoring, or apnea at 1.5, 4.75, and 6.75 years (always vs. not always), odds ratios (95% CIs)
| Covariates # Always/Total # | 1.5 years |
4.75 years |
6.75 years |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mouth- breathing 133/7672 | Snoring 221/8116 | Apnea 94/7457 | Mouth- breathing 376/6264 | Snoring 429/6561 | Apnea 89/5910 | Mouth- breathing 337/5506 | Snoring 319/5774 | Apnea 66/5120 | |
| Maternal and family characteristics: | |||||||||
| Maternal smoking, ever | 1.07 (0.75, 1.52) | 0.90 (0.68, 1.19) | 0.74 (0.49,1.14) | 0.98 (0.76,1.22) | 1.11 (0.91,1.35) | 1.88 (1.21,2.93) | 1.10 (0.87,1.37) | 1.11 (0.88,1.40) | 1.20 (0.73,1.98) |
| Race (other v. white) | 2.76 (1.09, 6.99) | 2.80 (1.38, 5.70) | 1.85 (0.56,6.06) | † | 1.24 (0.57,2.71) | † | 1.06 (0.38,2.95) | † | † |
| Housing, inadequate (yes v. no) | 0.92 (0.50, 1.68) | 1.49 (0.99, 2.25) | 1.75 (0.97,3.16) | 1.58 (1.17,2.12) | 1.23 (0.92,1.65) | 1.72 (0.99,2.99) | 1.53 (1.10,2.12) | 1.44 (1.03,2.01) | 1.81 (0.93,3.50) |
| Paternal employment, manual v. professional | 1.63 (1.13, 2.37) | 1.19 (0.89, 1.59) | 1.60 (1.03,2.50) | 1.29 (1.03,1.61) | 1.31 (1.06,1.62) | 0.99 (0.63,1.57) | 1.29 (1.01,1.64) | 1.28 (1.00,1.64) | 1.20 (0.71, 2.04) |
| Maternal education, lower v. higher | 2.06 (1.32, 3.21) | 1.82 (1.30, 2.55) | 1.51 (0.92,2.47) | 1.46 (1.15,1.87) | 1.18 (0.94,1.48) | 1.06 (0.65,1.71) | 1.39 (1.08,1.80) | 1.08 (0.84,1.40) | 0.89 (0.52,1.53) |
| Other child: | |||||||||
| 1 v. 0 | 1.33 (0.92, 1.94) | 0.81 (0.61, 1.09) | 0.92 (0.58,144) | 0.86 (0.69,1.08) | 1.06 (0.86,1.30) | 0.74 (0.47,1.16) | 0.59 (0.47,0.75) | 1.07 (0.84,1.37) | 1.33 (0.79,2.25) |
| > 1 v. 0 | 1.20 (0.51, 2.79) | 1.82 (1.10, 3.00) | 1.88 (0.90,3.93) | 0.88 (0.52,1.49) | 1.00 (0.60,1.65) | 1.25 (0.52,2.96) | 0.83 (0.48,1.43) | 1.52 (0.90,2.57) | 0.97 (0.27,3.47) |
| Child characteristics: | |||||||||
| Gestational age, (continuous, in weeks) | 1.02 (0.91, 1.15) | 1.00 (0.92, 1.09) | 0.96 (0.84,1.10) | 0.99 (0.92,1.06) | 1.00 (0.94,1.07) | 1.05 (0.91,1.22) | 0.96 (0.89,1.04) | 1.00 (0.92,1.08) | 1.23 (1.02,1.47) |
| Gender (male v. female) | 1.49 (1.04, 2.13) | 1.46 (1.10, 1.93) | 1.22 (0.81,1.86) | 1.03 (0.83,1.27) | 1.00 (0.82,1.22) | 0.78 (0.51,1.20) | 1.12 (0.89,1.41) | 0.89 (0.71, 1.13) | 0.84 (0.51,1.38) |
| Breastfed (no v. yes) | 1.26 (0.86, 1.86) | 1.64 (1.22, 2.21) | 0.81 (0.49,1.34) | 1.20 (0.93,1.53) | 1.16 (0.92,1.47) | 1.33 (0.81,2.17) | 1.44 (1.11,1.87) | 1.41 (1.08,1.84) | 1.34 (0.76,2.38) |
| Adenoids removed | ‡ | ‡ | ‡ | 0.52 (0.28,0.97) | 0.38 (0.22,0.66) | 0.33 (0.12,0.94) | 0.33 (0.21,0.53) | 0.39 (0.24,0.63) | 0.49 (0.22,1.09) |
| Wheezing days | 1.02 (0.98, 1.06) | 1.06 (1.04, 1.09) | 1.09 (1.06, 1.13) | 1.04 (1.02,1.06) | 1.05 (1.03,1.07) | 1.09 (1.05,1.12) | 1.05 (1.03,1.08) | 1.06 (1.03,1.08) | 1.09 (1.05,1.14) |
Only ORs (95% CIs) for putative risk factors significantly associated with ≥ 1 outcome measure in analyses of full sample data are shown (i.e., not the sample subset with BMI z-score change data at 0.5-1.5 years and 1.5-3.5 years).
Race not included in these models as the reported race for all children with SDB “Always” symptoms at these time points is white.
N/A because history of adenoidectomy was not assessed at 1.5 years. Bold indicates significant ORs at the level of 0.05.
Table 3.
Maternal, family, and child characteristics associated* with mouth-breathing, snoring, or apnea at 1.5, 4.75, and 6.75 years (“Habitual” vs. “not habitual”), odds ratios (95% CIs)
| Covariates Habitual # Case/Total # | 1.5 years |
4.75 years |
6.75 years |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mouth-Breathing 824/7672 | Snoring 660/8116 | Apnea 574/7457 | Mouth-Breathing 1443/6246 | Snoring 883/6561 | Apnea 826/5910 | Mouth-Breathing 1270/5506 | Snoring 719/5774 | Apnea 629/5120 | |
| Maternal and family characteristics: | |||||||||
| Maternal smoking, ever | 1.17 (1.01,1.35) | 0.95 (0.80,1.12) | 1.07 (0.90,1.28) | 1.06 (0.94, 1.19) | 1.06 (0.92,1.23) | 1.20 (1.03,1.39) | 1.13 (0.99,1.28) | 1.02 (0.87,1.20) | 1.25 (1.05,1.48) |
| Race (other v. white) | 2.06 (1.29,3.28) | 1.89 (1.14,3.12) | 1.85 (1.10,3.13) | 1.16 (0.70,1.91) | 1.38 (0.80,2.39) | 1.13 (0.60,2.10) | 0.76 (0.41,1.44) | 0.91 (0.45,1.84) | 0.91 (0.43,1.92) |
| Housing, inadequate (Yes v. No) | 0.86 (0.66,1.13) | 1.42 (1.09,1.84) | 1.56 (1.18,2.06) | 1.27 (1.05,1.54) | 1.33 (1.07,1.66) | 0.99 (0.78,1.26) | 1.36 (1.10,1.67) | 1.30 (1.01,1.66) | 1.05 (0.79,1.39) |
| Paternal employment, manual v. professional | 1.22 (1.04,1.42) | 1.17 (0.98,1.39) | 1.09 (0.90,1.31) | 1.09 (0.96,1.24) | 1.09 (0.93,1.27) | 1.11 (0.94,1.30) | 1.18 (1.03,1.36) | 1.02 (0.85,1.21) | 1.08 (0.90,1.30) |
| Maternal education, lower v. higher | 1.48 (1.25,1.76) | 1.23 (1.02,1.49) | 1.12 (0.92,1.35) | 1.47 (1.29,1.68) | 1.04 (0.89,1.23) | 1.13 (0.96, 1.34) | 1.34 (1.16,1.54) | 1.18 (0.99,1.40) | 1.09 (0.90,1.31) |
| Other child: | |||||||||
| 1 v. 0 | 1.02 (0.87,1.19) | 0.89 (0.75,1.06) | 0.91 (0.76,1.09) | 0.77 (0.68,0.88) | 0.94 (0.81,1.09) | 0.85 (0.73,0.99) | 0.78 (0.68,0.89) | 0.90 (0.76,1.06) | 0.91 (0.76,1.08) |
| > 1 v. 0 | 1.44 (1.03,2.01) | 1.64 (1.17,2.29) | 1.03 (0.69,1.54) | 0.73 (0.52,1.01) | 0.71 (0.48,1.06) | 1.04 (0.71,1.53) | 0.74 (0.52,1.06) | 1.28 (0.86,1.89) | 0.84 (0.52,1.35) |
| Child characteristics: | |||||||||
| Gestational age, (continuous, in weeks) | 1.01 (0.96,1.06) | 0.99 (0.94,1.04) | 1.02 (0.97,1.08) | 0.95 (0.91,0.99) | 0.95 (0.90,0.99) | 1.02 (0.97,1.07) | 1.02 (0.97,1.06) | 1.02 (0.96,1.07) | 1.00 (0.95,1.06) |
| Gender (male v. female) | 1.15 (0.99,1.33) | 1.15 (0.98,1.35) | 0.98 (0.83,1.17) | 0.95 (0.84,1.07) | 1.01 (0.87,1.17) | 1.04 (0.89,1.21) | 1.06 (0.93,1.21) | 0.93 (0.79,1.09) | 1.19 (1.01,1.42) |
| Breastfed (no v. yes) | 1.32 (1.11,1.56) | 1.47 (1.22,1.77) | 0.99 (0.80,1.22) | 1.26 (1.08,1.45) | 1.28 (1.08,1.53) | 1.04 (0.86,1.25) | 1.29 (1.10,1.51) | 1.20 (0.99,1.46) | 109 (0.88,1.34) |
| Adenoids removed | † | † | † | 0.48 (0.32,0.71) | 0.35 (0.23,0.52) | 0.58 (0.36,0.95) | 0.47 (0.34,0.65) | 0.38 (0.27,0.55) | 1.01 (0.63,1.61) |
| Wheezing days | 1.03 (1.02,1.05) | 1.06 (1.05,1.08) | 1.06 (1.04, 1.08) | 1.04 (1.03,1.05) | 1.04 (1.03,1.06) | 1.06 (1.05,1.08) | 1.04 (1.03,1.06) | 1.06 (104,1.08) | 1.05 (1.03,1.07) |
Only ORs (95% CIs) for putative risk factors significantly associated with ≥ 1 outcome measure in analyses of full sample data are shown (i.e., not the sample subset with BMI Z score change data at 0.5-1.5 years and 1.5-3.5 years).
N/A because history of adenoidectomy was not assessed at 1.5 years. Bold indicates significant ORs at the level of 0.05.
Among the significant child characteristics in Tables 2 and 3, wheezing had the smallest (3%-9%)–albeit consistent–effects across symptoms and time points. Other putative SDB risk factors, e.g., gestational age and gender had only limited significance. In contrast, a history of adenoidectomy was associated with 50%-65% reduced “Always” and “Habitual” risks across all symptoms and time points, except apnea at 6.75 years. Not breastfeeding was associated with an increased risk of “Always” snoring at 1.5 (≈ 65%) and 6.75 (≈ 40%) years and “Habitual” snoring at 1.5 (≈ 50%) and 4.75 (≈ 30%) years. Outcomes for the subset of children with SDB data for all 7 time points (Supplemental Tables S1-S2) were quite similar to those in Tables 2 and 3 for maternal education, adenoidectomy, and wheezing days, but differed markedly in the pattern and magnitude of effects for other variables.
We also conducted subsample analyses with BMI z-score change data (not shown). These found small but significant positive associations between the 0.5-1.5 year BMI z-score Δ and “Always” mouth-breathing at 4.75 years, and between the 0.5-1.5 year BMI z-score Δ and “Habitual” mouth-breathing at both 4.75 and 6.75 years. Given smaller sample sizes in these analyses–averaging n = 4,500 at 1.5 years, n = 2,800 at 4.75 years, and n = 2,500 at 6.75 years across SDB symptoms–risk factor effects were not directly comparable to those found in the full sample.
Maternal, Family, and Child Characteristics Associated with Combined SDB Outcomes
Factors associated with significant changes in mean combined SDB z-scores are shown in Table 4. Positive standardized β values (SE) indicate an increased combined SDB z-score, and negative standardized β values (SE) indicate a decreased combined SDB z-score, compared to the reference category. For example, the 1.5-year mean combined SDB z-score of non-white children was 0.415 higher than for white children, but race was not significant thereafter. Adenoidectomy had the greatest effects, lowering combined SDB z-scores by 0.656 at 4.75 years, and by 0.525 at 6.75 years. Tonsillectomy had the second greatest overall effect, increasing combined SDB z-scores by 0.302 at 4.75 years. Maternal smoking effects increased from 1.5, to 4.75, to 6.75 years, as did the lack of breastfeeding, from 1.5 to 6.75 years (not significant at 4.75 years), while inadequate housing effects became attenuated from 1.5 to 6.75 years (not significant at 4.75 years). Maternal smoking, maternal education, and wheezing days were the only significant variables at all 3 time points. In subsample analyses with BMI z-score data, only the 0.5-1.5 year change was significant, at 4.75 years
Table 4.
Maternal, family, and child characteristics associated with combined mouth-breathing, snoring, and apnea standardized scores at 1.5, 4.75, and 6.75 years*
| 1.5 years Z Score Parameter Estimate |
4.75 years Z Score Parameter Estimate |
6.75 years Z Score Parameter Estimate |
||||
|---|---|---|---|---|---|---|
| Full Sample, N = 8,393 | Subsample, N = 4,895 | Full Sample, N = 7,240 | Subsample, N = 3,154 | Full Sample, N = 6,673 | Subsample, N = 2,957 | |
| Maternal and family characteristics: | ||||||
| Maternal smoking, ever (yes v. no) | 0.055 (0.021) | 0.033 (0.028) | 0.072 (0.029) | 0.040 (0.035) | 0.104 (0.024) | 0.071 (0.036) |
| Race (other v. white) | 0.415 (0.081) | 0.308 (0.115) | 0.054 (0.092) | 0.0194 (0.164) | 0.008 (0.099) | 0.144 (0.167) |
| Housing, inadequate (yes v. no) | 0.155 (0.039) | 0.194 (0.053) | 0.049 (0.037) | 0.054 (0.059) | 0.114 (0.040) | 0.130 (0.061) |
| Paternal employment, manual v. professional | 0.065 (0.023) | 0.081 (0.030) | 0.069 (0.025) | 0.096 (0.037) | 0.044 (0.026) | 0.052 (0.038) |
| Maternal education, lower v. higher | 0.078 (0.023) | 0.064 (0.030) | 0.100 (0.025) | 0.092 (0.038) | 0.060 (0.026) | 0.041 (0.039) |
| Other child: 2 > 2, 1-1&2, 0-0 | -0.006 (0.019) | -0.012 (0.025) | -0.087 (0.020) | -0.086 (0.031) | -0.088 (0.022) | -0.070 (0.032) |
| Child characteristics: | ||||||
| Gestational age, (continuous, in weeks) | 0.002 (0.007) | -0.002 (0.009) | -0.011 (0.008) | -0.014 (0.012) | 0.006 (0.008) | -0.009 (0.012) |
| Gender (male v. female) | 0.071 (0.021) | 0.079 (0.027) | 0.032 (0.023) | 0.035 (0.035) | 0.021 (0.024) | 0.078 (0.036) |
| Breastfed (no v. yes) | 0.081 (0.026) | 0.059 (0.034) | 0.053 (0.092) | 0.040 (0.044) | 0.112 (0.031) | 0.072 (0.046) |
| BMI Z Δ, 0.05-1.5 y | † | 0.011 (0.118) | † | 0.038 (0.016) | † | 0.031 (0.016) |
| Tonsils removed (vs. not removed) | † | † | 0.302 (0.123) | 0.208 (0.192) | 0.178 (0.090) | 0.190 (0.133) |
| Adenoids removed (vs. not removed) | † | † | -0.656 (0.086) | -0.641 (0.126) | -0.525 (0.071) | -0.553 (0.099) |
| Wheezing days | 0.039 (0.003) | 0.039 (0.004) | 0.032 (0.003) | 0.030 (0.004) | 0.036 (0.003) | 0.035 (0.005) |
Significant β coefficient estimates and (standard errors) of risk factor effects upon standardized SDB scores. Positive β values signify an increase, and negative β values signify a decrease in mean combined SDB z-score compared to the reference group for categorical variables.
N/A for BMI Z Δ, 0.05-1.5 y, because BMI data were not available for all participants, and; for Tonsils Removed and Adenoids Removed because history of these procedures was not assessed at 1.5 years. Bold indicates significant ORs at the level of 0.05.
DISCUSSION
This is the first study to report the natural history of snoring, mouth-breathing, and apnea in a population-based cohort, with multiple observation points, across a key 6-year period in the development of SDB symptoms. The large spike in snoring (“Always”) prevalence from 1.5-2.5 years was earlier than previously reported. Similarly, this population's rates of “Always” snoring (range = 3.6%-7.7%) and “Habitually” snoring (range = 9.6%-21.2%) were considerably higher than analogous rates of 1.5%-6% and 5%-12%, respectively, from a systematic review incorporating data from 2- to 18-year-olds.34 The presence of apnea (“Always”) remained stable at 1% to 2%, consistent with the literature. Aside from being population-based, this study's other considerable strengths include large sample size, extended follow-up, control for multiple potential confounders, and measurement of 3 different but commonly recognized symptoms of SDB.
SDB is dynamic, as shown by data for children in whom “Always” symptoms developed, resolved, or remained present between the approximate one-year age intervals. Overall, the persistence of symptoms on a population basis is less common than either their resolution or development. Snoring, mouth-breathing, and apnea each developed in roughly 1% to 5% of this population-based sample from one interval to the next per the “Always” measure, and in 5% to 10% per the “Habitual” measure. Absent a longitudinal analyses of combined symptom patterns–beyond the scope of this paper–our findings cannot discern phenotypes of children for whom (and for how long) watchful waiting is the optimal course.
As with any longitudinal data, ascertainment bias is possible. Families with missing data at a particular time point had more of the known risk factors for SDB: mothers who smoked; children who were preterm, low birthweight, and who had a history of wheezing; and families who were of lower SES. This bias was reflected in the lower rates (≈ 1%) of snoring and mouth-breathing in the subsample with SDB symptom data for all 7 time points, and in the symptom-specific analyses of this subsample (Supplemental Tables S1–S2). If this were a random subsample of the n = 12,447 in the study's analytic sample, the ORs in Tables S1 and S2 would be the same as those in Tables 2 and 3, respectively, but with wider 95% CIs. However, this is not the case, with some ORs significant in the subsample not significant in the broader sample, and vice-versa. Thus, it appears that the subsample with SDB data at all 7 time points is a biased sample. For this reason, we present results for the broader sample in the main body of the paper.
A secondary goal of the study was to conduct exploratory analyses of putative risk factors. The complexity of these data–multiple observation points with varying schema for measuring symptoms–should caution against overinterpretation of results from one particular time point, in favor of examining consistent trends. Inadequate housing, paternal manual employment, maternal smoking, and lower maternal education significantly increased combined SDB risk at 1.5 years, and at either or both of the later time points. In contrast, initial race and gender effects did not persist. In a large English sample of 1- to 4-year-olds that measured parent report of multiple SES, environmental and biological risk factors, habitual snoring (7.9% prevalence) was positively associated with socioeconomic deprivation in bivariate analyses, but in analyses adjusted for wheeze and other atopic disorders, respiratory symptoms, BMI, and exposure to pollutants, SES was no longer significant.39 Our SES findings may thus be proxies for other, unobserved clinical and environmental characteristics. Lower SES families are more likely to live in crowded housing, increasing the possibility of bedroom sharing, particularly among younger children, and therefore greater likelihood of symptom recognition.
Not breastfeeding increased the risk of combined SDB symptoms, an effect that intensified from 1.5 to 6.75 years, but, curiously, was not significant at 4.75 years (data complexity cited above may be a factor). In a study of snoring children undergoing polysomnography, having been breastfed for ≥ 2 months reduced SDB severity on every measure assessed. This may occur via breastfeeding reducing infections that lead to adenotonsillar hypertrophy, or anatomically, by promoting an upper airway less vulnerable to narrowing and collapse during sleep.40 Prematurity increased SDB risk among 8- to 11-year olds,41 while very low birthweight increased risk for SDB in young adulthood.42 In contrast, neither birthweight nor gestational age was associated with a significant increase in combined SDB symptoms.
Adenoidectomy had the strongest effect upon reducing combined SDB symptoms; this is particularly notable given tonsillectomy's lesser but opposite effect. We speculate that this is because adenoidectomy alone is performed earlier than either tonsillectomy or adenotonsillectomy (and has fewer risks than those surgeries)43 to relieve chronic nasal obstruction. Such nasal obstruction can lead to remodeling of the upper airway, predisposing to obstructive sleep apnea. In contrast, tonsillectomies and adenotonsillectomies may be performed later, as often for suspected apnea as for recurrent pharyngitis (perhaps with comorbid undiagnosed SDB). Waiting periods longer than 12 months for either surgery were common in England during the study period, thus surgeries were preferentially performed in the more severe and absolute indications. That likely would have included demonstrable SDB, though SDB related morbidity was not as widely recognized at the time.
Our findings have implications for clinical care. They suggest that SDB risk, as identified by chronic nasal obstruction at age 4 years or earlier, may merit treatment well before SDB actually develops later in childhood. Once SDB develops, treatment by tonsillectomy and even adenotonsillectomy may not as reliably eliminate SDB, especially in the context of obesity. Although such conclusions, from a cohort study rather than a randomized trial are speculative, they align with data showing that snoring at baseline is more predictive of hyperactive behavior (an important SDB outcome) 4 years later than are concurrent snoring patterns at that 4-year follow-up.44
The study has several limitations. First, results are based upon parent-report of each symptom, by a single item. Objective data may yield different results; newer cohort studies in schoolchildren employing polysomnography yielded a 1.2% prevalence of moderate SDB45 and a 2.8% prevalence of apnea. However, our parent-reported SDB symptom prevalence data are consistent with results from a systematic review.34 Furthermore our epidemiological study of symptoms aligns with current clinical practice in the US and abroad, which relies primarily upon symptoms–vs. PSG or other objective tests–regarding decisions about adenotonsillectomy.46,47
Second, the ALSPAC question on observed apneas was not phrased to elicit a distinction between obstructive and central apneas. Brief, generally benign central apneas are not uncommon in the youngest children.48,49 While the 19% prevalence of “Habitual” apneas in 6-month-olds could reflect parent observation of central apneas, one would not expect central (vs. obstructive) apneas to occur in a similar pattern as both snoring and mouth-breathing at this time. Given that all three symptoms were “Habitually” present at comparable rates at this point, this suggests that physiological central apneas were not the major component of those reported by parents.
Third, there was inconsistency in the SDB item response categories across symptoms and over time. We sought to address this by presenting dichotomous SDB outcomes in two ways (“Always” and “Habitual”) and by standardizing combined SDB scores.
Fourth, anthropometric data were only available for three time points, the latest being 3.5 years. This may be one reason why BMI z-score changes between 0.5, 1.5, and 3.5 years had only minimal effects upon SDB. Alternatively, controlling for SES may have attenuated the effects of BMI upon SDB risk, as found by others.39
Understanding the trajectory of pediatric SDB has significant clinical implications for deciding whether and in whom watchful waiting might be a valid long-term strategy for children with mild SDB.50 Findings from this large, population-based cohort study suggest the necessity for early and continued screening for SDB symptoms. Secondarily, our findings reaffirm that modifiable characteristics such as those related to SES and infant feeding, as well as adenoidectomy may have a substantial impact upon SDB risk.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Chervin formerly served on the scientific advisory board and received stock options from Pavad Medical and has received honoraria from the AASM, ABSM, and ASMF. Dr. Freeman has consulted for Ortho-McNeil-Janssen, Osiris, and Schlesinger Law. The other authors indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Susan Garetz, MD, for her assistance in interpretation of our findings regarding tonsillectomy and adenotonsillectomy. In addition, the authors thank the following members of the study's advisory group:
Dr. Raanan Arens (Montefiore Medical Center/Albert Einstein College of Medicine), Dr. John Bent (Montefiore Medical Center/Albert Einstein College of Medicine), Dr. Peter Blair (University of Bristol), Dr. Pauline Emmett (University of Bristol), Dr. Peter Fleming (University of Bristol), Dr. Jon Heron (University of Bristol), Dr. Carole Marcus (Children's Hospital of Philadelphia), Dr. Kenneth Ong (Cambridge University), Dr. Sanjay Parikh (Montefiore Medical Center/Albert Einstein College of Medicine), and Dr. Susan Redline (Case Western Reserve University).
This work was performed at the University of Bristol, and the Albert Einstein College of Medicine. This project was supported with a grant from the National Heart Lung and Blood Institute R21HL091241 to Dr. Bonuck.
SDB Prevalence by Age, “Always”, Children with Complete SDB Data only
Prevalence by Age, “Habitual”, Children with Complete SDB Data only
Apnea Transitions for “Habitual”
Mouth-Breathing Transitions for “Habitual”
Snore Transitions for “Habitual”
Table S1.
Maternal, Family, and Child Characteristics Associated* with Mouth-Breathing, Snoring, or Apnea at 1.5, 4.75, and 6.75 years (“Always” vs. “Not Always”), Odds Ratios (95% CIs) of Subsample with SDB Data for 7/7 Timepoints
| Covariates Habitual # Case/Total # | 1.5 years |
4.75 years |
6.75 years |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mouth-Breathing 63/5022 | Snoring 129/5249 | Apnea 64/4894 | Mouth-Breathing 280/4930 | Snoring 324/5122 | Apnea 70/4724 | Mouth-Breathing 273/4743 | Snoring 261/4901 | Apnea 60/4423 | |
| Maternal and Family Characteristics: | |||||||||
| Maternal Smoking, Ever | 1.00 (0.60,1.66) | 0.75 (0.52,1.08) | 0.81 (0.49,1.36) | 1.03 (0.81,1.32) | 1.02 (0.81,1.28) | 2.07 (1.26,3.40) | 1.11 (0.86,1.42) | 1.15 (0.89,1.48) | 1.42 (0.84,2.41) |
| Race (Other v. White) | 3.20 (0.75,13.77) | 3.69 (1.42,9.59) | 2.58 (0.60,11.06) | 0.57 (0.14,2.34) | 1.25 (0.49,3.15) | † | 1.56 (0.55,4.42) | 0.29 (0.04,2.12) | † |
| Housing, Inadequate (Yes v. No) | 0.92 (0.35,2.41) | 1.60 (0.91,2.79) | 1.01 (0.42,2.42) | 1.67 (1.18,2.37) | 1.19 (0.84,1.70) | 1.71 (0.89,3.29) | 1.61 (1.12,2.32) | 1.72 (1.20,2.47) | 1.68 (0.82,3.46) |
| Paternal Employment, Manual v. Professional | 1.19 (0.70,2.02) | 1.18 (0.81,1.72) | 1.55 (0.91,2.65) | 1.41 (1.09,1.83) | 1.25 (0.98,1.60) | 0.85 (0.51,1.44) | 1.30 (0.99,1.70) | 1.30 (0.99,1.71) | 1.32 (0.76,2.29) |
| Maternal Education, Lower v. Higher | 1.82 (1.01,3.29) | 2.00 (1.31,3.04) | 1.77 (0.99,3.17) | 1.48 (1.12,1.96) | 1.27 (0.99,1.64) | 1.07 (0.63,1.83) | 1.41 (1.06,1.86) | 1.06 (0.80,1.41) | 0.87 (0.49,1.55) |
| Other Child: | |||||||||
| 1 v. 0 | 1.50 (0.87,2.57) | 0.77 (0.53,1.13) | 0.81 (0.48,1.38) | 0.73 (0.57,0.95) | 0.95 (0.75,1.20) | 0.57 (0.34,0.95) | 0.57 (0.44,0.74) | 1.06 (0.81,1.38) | 1.25 (0.73,1.27) |
| > 1 v. 0 | 1.05 (0.23,4.80) | 1.72 (0.83,3.53) | 1.53 (0.53,4.39) | 0.70 (0.37,1.34) | 1.03 (0.57,1.86) | 1.42 (0.56,3.64) | 1.05 (0.60,1.85) | 1.45 (0.81,2.59) | 1.05 (0.29,3.87) |
| Child Characteristics: | |||||||||
| Gestational Age, (Continuous, in weeks) | 1.01 (0.85,1.20) | 0.95 (0.85,1.06) | 0.87 (0.74,1.02) | 0.98 (0.90,1.06) | 1.00 (0.93,1.08) | 1.08 (0.91,1.29) | 1.00 (0.92,1.09) | 1.03 (0.95,1.13) | 2.21 (0.99,1.46) |
| Gender (Male v. Female) | 1.76 (1.04,2.99) | 1.55 (1.07,2.24) | 0.73 (0.44,1.21) | 1.03 (0.81,1.32) | 0.85 (0.68,1.07) | 0.79 (0.49,1.28) | 1.17 (0.91,1.51) | 0.86 (0.66,1.11) | 0.86 (0.51,1.46) |
| Breastfed (No v. Yes) | 1.51 (0.85,2.66) | 1.21 (0.80,1.84) | 0.97 (0.53,1.78) | 1.18 (0.88,1.58) | 1.12 (0.85,1.48) | 1.29 (0.73,2.29) | 1.51 (1.13,2.02) | 1.34 (0.99,1.81) | 1.36 (0.75,2.49) |
| Adenoids Removed | ‡ | ‡ | ‡ | 0.44 (0.23,0.87) | 0.28 (0.16,0.49) | 0.26 (0.09,0.74) | 0.35 (0.21,0.60) | 0.36 (0.21,0.66) | 0.51 (0.16,1.57) |
| Wheezing Days | 0.95 (0.87,1.04) | 1.07 (1.03,1.10) | 1.09 (1.05,1.14) | 1.04 (1.02,1.06) | 1.05 (1.03,1.07) | 1.08 (1.04,1.12) | 1.06 (1.03,1.09) | 1.06 (1.03,1.09) | 1.10 (1.06,1.14) |
Only ORs (95% CIs) for putative risk factors significantly associated with ≥1 outcome measure in analyses of full sample data are shown (i.e., not the sample subset with BMI Z score change data at 0.5-1.5 years and 1.5-3.5 years).
Race not included in these models as the reported race for all children with SDB “Always” symptoms at these timepoints is White.
N/A because history of adenoidectomy was not assessed at 1.5 years.
Table S2.
Maternal, Family, and Child Characteristics Associated* with Mouth-Breathing, Snoring, or Apnea at 1.5, 4.75, and 6.75 years (“Habitual” vs. “Not Habitual”), Odds Ratios (95% CIs) for Subsample with SDB Data for 7/7 Timepoints.
| Covariates Habitual # Case/Total # | 1.5 years |
4.75 years |
6.75 years |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mouth-Breathing 479/5022 | Snoring 396/5249 | Apnea 353/4894 | Mouth-Breathing 1083/4930 | Snoring 677/5122 | Apnea 655/4724 | Mouth-Breathing 1046/4743 | Snoring 601/4901 | Apnea 551/4423 | |
| Maternal and Family Characteristics: | |||||||||
| Maternal Smoking, Ever | 1.16 (0.96,1.41) | 0.89 (0.72,1.10) | 1.07 (0.85,1.33) | 1.07 (0.93,1.23) | 1.00 (0.85,1.18) | 1.26 (1.06,1.49) | 1.17 (1.02,1.35) | 1.05 (0.88,1.25) | 1.32 (1.10,1.59) |
| Race (Other v. White) | 1.51 (0.71,3.21) | 2.99 (1.60,5.58) | 2.12 (1.03,4.38) | 1.16 (0.64,2.09) | 1.43 (0.75,2.70) | 1.07 (0.50,2.27) | 0.79 (0.35,1.63) | 0.97 (0.43,2.16) | 1.23 (0.57,2.63) |
| Housing, Inadequate (Yes v. No) | 0.84 (0.57,1.22) | 1.46 (1.03,2.08) | 1.28 (0.86,1.89) | 1.22 (0.97,1.52) | 1.32 (1.01,1.71) | 1.05 (0.79,1.38) | 1.32 (1.05,1.67) | 1.24 (0.94,1.65) | 1.05 (0.77,1.42) |
| Paternal Employment, Manual v. Professional | 1.05 (0.86,1.29) | 1.03 (0.82,1.29) | 1.14 (0.89,1.44) | 1.07 (0.92,1.24) | 1.05 (0.88,1.26) | 1.06 (0.88,1.27) | 1.14 (0.98,1.33) | 1.02 (0.84,1.23) | 1.11 (0.91,1.35) |
| Maternal Education, Lower v. Higher | 1.33 (1.07,1.64) | 1.29 (1.02,1.63) | 1.00 (0.79,1.27) | 1.40 (1.21,1.63) | 1.06 (0.88,1.27) | 1.15 (0.96,1.38) | 1.33 (1.14,1.55) | 1.20 (0.99,1.45) | 1.11 (0.91,1.36) |
| Other Child: | |||||||||
| 1 v. 0 | 1.06 (0.87,1.29) | 0.87 (0.70,1.09) | 0.85 (0.68,1.07) | 0.70 (0.61,0.81) | 0.88 (0.74,1.04) | 0.83 (0.70,0.99) | 0.75 (0.65,0.87) | 0.86 (0.72,1.03) | 0.91 (0.75,1.09) |
| > 1 v. 0 | 1.27 (0.77,2.09) | 1.49 (0.92,2.42) | 0.99 (0.56,1.75) | 0.75 (0.51,1.09) | 0.69 (0.43,1.11) | 1.02 (0.65,1.59) | 0.69 (0.46,1.03) | 1.30 (0.84,1.99) | 0.86 (0.52,1.44) |
| Child Characteristics: | |||||||||
| Gestational Age, (Continuous, in weeks) | 1.02 (0.96,1.09) | 0.95 (0.89,1.02) | 1.02 (0.95,1.10) | 0.95 (0.91,0.99) | 0.96 (0.91,1.01) | 1.04 (0.98,1.10) | 1.01 (0.97,1.06) | 1.03 (0.97,1.09) | 1.01 (0.95,1.07) |
| Gender (Male v. Female) | 1.11 (0.92,1.35) | 1.24 (1.00,1.53) | 0.94 (0.76,1.18) | 0.95 (0.83,1.09) | 0.93 (0.79,1.09) | 1.00 (0.85,1.19) | 1.06 (0.92,1.22) | 0.90 (0.75,1.07) | 1.23 (1.03,1.48) |
| Breastfed (No v. Yes) | 1.34 (1.07,1.69) | 1.26 (0.98,1.62) | 1.10 (0.83,1.45) | 1.23 (1.04,1.46) | 1.21 (0.99,1.49) | 1.09 (0.88,1.34) | 1.29 (1.09,1.54) | 1.18 (0.95,1.47) | 1.03 (0.82,1.29) |
| Adenoids Removed | N/A† | N/A† | N/A† | 0.43 (0.28,0.67) | 0.32 (0.20,0.50) | 0.60 (0.35,1.02) | 0.45 (0.31,0.64) | 0.40 (0.27,0.60) | 1.15 (0.68,1.93) |
| Wheezing Days | 1.03 (1.01,1.05) | 1.06 (1.04,1.08) | 1.06 (1.04,1.09) | 1.03 (1.02,1.04) | 1.05 (1.03,1.06) | 1.06 (1.05,1.08) | 1.05 (1.03,1.07) | 1.06 (1.04,1.08) | 1.06 (1.04,1.08) |
Only ORs (95% CIs) for putative risk factors significantly associated with ≥ 1 outcome measure in analyses of full sample data are shown (i.e., not the sample subset with BMI Z score change data at 0.5-1.5 years and 1.5-3.5 years).
N/A because history of adenoidectomy was not assessed at 1.5 years.
REFERENCES
- 1.Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep. 2006;29:1115–34. doi: 10.1093/sleep/29.9.1115. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell RB, Kelly J. Behavior, neurocognition and quality-of-life in children with sleep-disordered breathing. Int J Pediatr Otorhinolaryngol. 2006;70:395–406. doi: 10.1016/j.ijporl.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Owens JA. Neurocognitive and behavioral impact of sleep disordered breathing in children. Pediatr Pulmonol. 2009;44:417–22. doi: 10.1002/ppul.20981. [DOI] [PubMed] [Google Scholar]
- 4.Schechter MS. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:e69. doi: 10.1542/peds.109.4.e69. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery P, Dunne D. Sleep disorders in children. Clin Evid (Online) 2007. 2007 [PMC free article] [PubMed] [Google Scholar]
- 6.Bonuck K, Parikh S, Bassila M. Growth failure and sleep disordered breathing: a review of the literature. Int J Pediatr Otorhinolaryngol. 2006;70:769–78. doi: 10.1016/j.ijporl.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Rosen G. Identification and evaluation of obstructive sleep apnea prior to adenotonsillectomy in children: is there a problem? Sleep Med. 2003;4:273–4. doi: 10.1016/s1389-9457(03)00115-1. [DOI] [PubMed] [Google Scholar]
- 8.Chan J, Edman JC, Koltai PJ. Obstructive sleep apnea in children. Am Fam Physician. 2004;69:1147–54. [PubMed] [Google Scholar]
- 9.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182:676–83. doi: 10.1164/rccm.200912-1930OC. [DOI] [PubMed] [Google Scholar]
- 10.Halbower AC, Marcus CL. Sleep disorders in children. Curr Opin Pulm Med. 2003;9:471–6. doi: 10.1097/00063198-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–12. doi: 10.1542/peds.109.4.704. [DOI] [PubMed] [Google Scholar]
- 12.Ali NJ, Pitston D, Stradling JR. Natural history of snoring and related behaviour problems between the ages of 4 and 7 years. Arch Dis Child. 1994;71:74–6. doi: 10.1136/adc.71.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anuntaseree W, Kuasirikul S, Suntornlohanakul S. Natural history of snoring and obstructive sleep apnea in Thai school-age children. Pediatr Pulmonol. 2005;39:415–20. doi: 10.1002/ppul.20207. [DOI] [PubMed] [Google Scholar]
- 14.Eitner S, Urschitz MS, Guenther A, et al. Sleep problems and daytime somnolence in a German population-based sample of snoring school-aged children. J Sleep Res. 2007;16:96–101. doi: 10.1111/j.1365-2869.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin JL, Vasquez MM, Silva GE, Quan SF. Incidence and remission of sleep-disordered breathing and related symptoms in 6-to 17-year old children-The Tucson Children's Assessment of Sleep Apnea Study. J Pediatr. 2010;157:57–61. doi: 10.1016/j.jpeds.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb DJ, Young TB. Natural history of sleep-disordered breathing: shedding light on the early years. Commentary on Bixler et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32:715–6. doi: 10.1093/sleep/32.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenni OG, Fuhrer HZ, Iglowstein I, Molinari L, Largo RH. A longitudinal study of bed sharing and sleep problems among Swiss children in the first 10 years of life. Pediatrics. 2005;115:233–40. doi: 10.1542/peds.2004-0815E. [DOI] [PubMed] [Google Scholar]
- 18.Gregory AM, Caspi A, Moffitt TE, Poulton R. Sleep Problems in childhood predict neuropsychological functioning in adolescence. Pediatrics. 2009;123:1171–6. doi: 10.1542/peds.2008-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quach J, Hiscock H, Canterford L, Wake M. Outcomes of child sleep problems over the school-transition period: Australian population longitudinal study. Pediatrics. 2009;123:1287–92. doi: 10.1542/peds.2008-1860. [DOI] [PubMed] [Google Scholar]
- 20.Patten CA, Choi WS, Gillin JC, Pierce JP. Depressive symptoms and cigarette smoking predict development and persistence of sleep problems in US adolescents. Pediatrics. 2000;106:E23. doi: 10.1542/peds.106.2.e23. [DOI] [PubMed] [Google Scholar]
- 21.Chervin RD, Weatherly RA, Garetz SL, et al. Pediatric sleep questionnaire: Prediction of sleep apnea and outcomes. Arch Otolaryngol Head Neck Surg. 2007;133:216–22. doi: 10.1001/archotol.133.3.216. [DOI] [PubMed] [Google Scholar]
- 22.Li HY, Lee LA. Sleep-disordered breathing in children. Chang Gung Med J. 2009;32:247–57. [PubMed] [Google Scholar]
- 23.Sahin U, Ozturk O, Ozturk M, Songur N, Bircan A, Akkaya A. Habitual snoring in primary school children: prevalence and association with sleep-related disorders and school performance. Med Princ Pract. 2009;18:458–65. doi: 10.1159/000235895. [DOI] [PubMed] [Google Scholar]
- 24.Huang Q, Wu H, Chen X. Clinical analysis of 68 patients with obstructive sleep disordered breathing in children. Lin Chuang Er Bi Yan Hou Ke Za Zhi[Abstract- article in Chinese] 2005;19:971–3. [PubMed] [Google Scholar]
- 25.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric Sleep Questionnaire: validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 26.Franco RA, Jr, Rosenfeld RM, Rao M. First place--resident clinical science award 1999. Quality of life for children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;123:9–16. doi: 10.1067/mhn.2000.105254. [DOI] [PubMed] [Google Scholar]
- 27.Li AM, Cheung A, Chan D, et al. Validation of a questionnaire instrument for prediction of obstructive sleep apnea in Hong Kong Chinese children. Pediatr Pulmonol. 2006;41:1153–60. doi: 10.1002/ppul.20505. [DOI] [PubMed] [Google Scholar]
- 28.Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr. 1982;100:31–40. doi: 10.1016/s0022-3476(82)80231-x. [DOI] [PubMed] [Google Scholar]
- 29.Bruni O, Ottaviano S, Guidetti V, et al. The sleep disturbance scale for children (SDSC) construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996;5:251–61. doi: 10.1111/j.1365-2869.1996.00251.x. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira VR, Carvalho LB, Ruotolo F, de Morais JF, Prado LB, Prado GF. Sleep disturbance scale for children: translation, cultural adaptation, and validation. Sleep Med. 2009;10:457–63. doi: 10.1016/j.sleep.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Cardwell CR, Carson DJ, McNaboe EJ, Patterson CC. Tonsillectomy and adenoidectomy are not associated with an altered risk of childhood-onset type 1 diabetes. Diabetes Care. 2007;30:2564–5. doi: 10.2337/dc07-0655. [DOI] [PubMed] [Google Scholar]
- 32.Cole T, Pan H. United Kingdom: Medical Research Council; User's guide to ImsGrowth (Microsoft Excel add-in) 2002-2007. [Google Scholar]
- 33.Howe LD, Tilling K, Lawlor DA. Accuracy of height and weight data from child health records. Arch Dis Child. 2009;94:950–4. doi: 10.1136/adc.2009.162552. [DOI] [PubMed] [Google Scholar]
- 34.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–52. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63:974–80. doi: 10.1136/thx.2007.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaditis AG, Kalampouka E, Hatzinikolaou S, et al. Associations of tonsillar hypertrophy and snoring with history of wheezing in childhood. Pediatr Pulmonol. 2010;45:275–80. doi: 10.1002/ppul.21174. [DOI] [PubMed] [Google Scholar]
- 37.Desager KN, Nelen V, Weyler JJ, De Backer WA. Sleep disturbance and daytime symptoms in wheezing school-aged children. J Sleep Res. 2005;14:77–82. doi: 10.1111/j.1365-2869.2004.00432.x. [DOI] [PubMed] [Google Scholar]
- 38.Chervin RD, Clarke DF, Huffman JL, et al. School performance, race, and other correlates of sleep-disordered breathing in children. Sleep Med. 2003;4:21–7. doi: 10.1016/s1389-9457(02)00243-5. [DOI] [PubMed] [Google Scholar]
- 39.Kuehni CE, Strippoli M-PF, Chauliac ES, Silverman M. Snoring in preschool children: prevalence, severity and risk factors. Eur Respir J. 2008;31:326–33. doi: 10.1183/09031936.00088407. [DOI] [PubMed] [Google Scholar]
- 40.Montgomery-Downs HE, Crabtree VM, Capdevila OS, Gozal D. Infant-feeding methods and childhood sleep-disordered breathing. Pediatrics. 2007;120:1030–5. doi: 10.1542/peds.2007-0722. [DOI] [PubMed] [Google Scholar]
- 41.Paavonen EJ, Strang-Karlsson S, Raikkonen K, et al. Very low birth weight increases risk for sleep-disordered breathing in young adulthood: the Helsinki Study of Very Low Birth Weight Adults. Pediatrics. 2007;120:778–84. doi: 10.1542/peds.2007-0540. [DOI] [PubMed] [Google Scholar]
- 42.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 43.Gallagher TQ, Wilcox L, McGuire E, Derkay CS. Analyzing factors associated with major complications after adenotonsillectomy in 4776 patients: Comparing three tonsillectomy techniques. Otolaryngol Head Neck Surg. 2010;142:886–92. doi: 10.1016/j.otohns.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Chervin RD, Ruzicka DL, Archbold KH, Dillon JE. Snoring predicts hyperactivity four years later. Sleep. 2005;28:885–90. doi: 10.1093/sleep/28.7.885. [DOI] [PubMed] [Google Scholar]
- 45.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32:731–6. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell RB, Pereira KD, Friedman NR. Sleep-disordered breathing in children: Survey of current practice. Laryngoscope. 2006;116:956–8. doi: 10.1097/01.MLG.0000216413.22408.FD. [DOI] [PubMed] [Google Scholar]
- 47.Weatherly RA, Ruzicka DL, Marriott DJ, Chervin RD. Polysomnography in children scheduled for adenotonsillectomy. Otolaryngol Head Neck Surg. 2004;131:727–31. doi: 10.1016/j.otohns.2004.06.699. [DOI] [PubMed] [Google Scholar]
- 48.O'Driscoll DM, Foster AM, Ng ML, et al. Central apnoeas have significant effects on blood pressure and heart rate in children. J Sleep Res. 2009;18:415–21. doi: 10.1111/j.1365-2869.2009.00766.x. [DOI] [PubMed] [Google Scholar]
- 49.Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–53. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 50.Marcus CL. Childhood obstructive sleep apnoea: to treat or not to treat, that is the question. Thorax. 2010;65:4–5. doi: 10.1136/thx.2009.123141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDB Prevalence by Age, “Always”, Children with Complete SDB Data only
Prevalence by Age, “Habitual”, Children with Complete SDB Data only
Apnea Transitions for “Habitual”
Mouth-Breathing Transitions for “Habitual”
Snore Transitions for “Habitual”
Table S1.
Maternal, Family, and Child Characteristics Associated* with Mouth-Breathing, Snoring, or Apnea at 1.5, 4.75, and 6.75 years (“Always” vs. “Not Always”), Odds Ratios (95% CIs) of Subsample with SDB Data for 7/7 Timepoints
| Covariates Habitual # Case/Total # | 1.5 years |
4.75 years |
6.75 years |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mouth-Breathing 63/5022 | Snoring 129/5249 | Apnea 64/4894 | Mouth-Breathing 280/4930 | Snoring 324/5122 | Apnea 70/4724 | Mouth-Breathing 273/4743 | Snoring 261/4901 | Apnea 60/4423 | |
| Maternal and Family Characteristics: | |||||||||
| Maternal Smoking, Ever | 1.00 (0.60,1.66) | 0.75 (0.52,1.08) | 0.81 (0.49,1.36) | 1.03 (0.81,1.32) | 1.02 (0.81,1.28) | 2.07 (1.26,3.40) | 1.11 (0.86,1.42) | 1.15 (0.89,1.48) | 1.42 (0.84,2.41) |
| Race (Other v. White) | 3.20 (0.75,13.77) | 3.69 (1.42,9.59) | 2.58 (0.60,11.06) | 0.57 (0.14,2.34) | 1.25 (0.49,3.15) | † | 1.56 (0.55,4.42) | 0.29 (0.04,2.12) | † |
| Housing, Inadequate (Yes v. No) | 0.92 (0.35,2.41) | 1.60 (0.91,2.79) | 1.01 (0.42,2.42) | 1.67 (1.18,2.37) | 1.19 (0.84,1.70) | 1.71 (0.89,3.29) | 1.61 (1.12,2.32) | 1.72 (1.20,2.47) | 1.68 (0.82,3.46) |
| Paternal Employment, Manual v. Professional | 1.19 (0.70,2.02) | 1.18 (0.81,1.72) | 1.55 (0.91,2.65) | 1.41 (1.09,1.83) | 1.25 (0.98,1.60) | 0.85 (0.51,1.44) | 1.30 (0.99,1.70) | 1.30 (0.99,1.71) | 1.32 (0.76,2.29) |
| Maternal Education, Lower v. Higher | 1.82 (1.01,3.29) | 2.00 (1.31,3.04) | 1.77 (0.99,3.17) | 1.48 (1.12,1.96) | 1.27 (0.99,1.64) | 1.07 (0.63,1.83) | 1.41 (1.06,1.86) | 1.06 (0.80,1.41) | 0.87 (0.49,1.55) |
| Other Child: | |||||||||
| 1 v. 0 | 1.50 (0.87,2.57) | 0.77 (0.53,1.13) | 0.81 (0.48,1.38) | 0.73 (0.57,0.95) | 0.95 (0.75,1.20) | 0.57 (0.34,0.95) | 0.57 (0.44,0.74) | 1.06 (0.81,1.38) | 1.25 (0.73,1.27) |
| > 1 v. 0 | 1.05 (0.23,4.80) | 1.72 (0.83,3.53) | 1.53 (0.53,4.39) | 0.70 (0.37,1.34) | 1.03 (0.57,1.86) | 1.42 (0.56,3.64) | 1.05 (0.60,1.85) | 1.45 (0.81,2.59) | 1.05 (0.29,3.87) |
| Child Characteristics: | |||||||||
| Gestational Age, (Continuous, in weeks) | 1.01 (0.85,1.20) | 0.95 (0.85,1.06) | 0.87 (0.74,1.02) | 0.98 (0.90,1.06) | 1.00 (0.93,1.08) | 1.08 (0.91,1.29) | 1.00 (0.92,1.09) | 1.03 (0.95,1.13) | 2.21 (0.99,1.46) |
| Gender (Male v. Female) | 1.76 (1.04,2.99) | 1.55 (1.07,2.24) | 0.73 (0.44,1.21) | 1.03 (0.81,1.32) | 0.85 (0.68,1.07) | 0.79 (0.49,1.28) | 1.17 (0.91,1.51) | 0.86 (0.66,1.11) | 0.86 (0.51,1.46) |
| Breastfed (No v. Yes) | 1.51 (0.85,2.66) | 1.21 (0.80,1.84) | 0.97 (0.53,1.78) | 1.18 (0.88,1.58) | 1.12 (0.85,1.48) | 1.29 (0.73,2.29) | 1.51 (1.13,2.02) | 1.34 (0.99,1.81) | 1.36 (0.75,2.49) |
| Adenoids Removed | ‡ | ‡ | ‡ | 0.44 (0.23,0.87) | 0.28 (0.16,0.49) | 0.26 (0.09,0.74) | 0.35 (0.21,0.60) | 0.36 (0.21,0.66) | 0.51 (0.16,1.57) |
| Wheezing Days | 0.95 (0.87,1.04) | 1.07 (1.03,1.10) | 1.09 (1.05,1.14) | 1.04 (1.02,1.06) | 1.05 (1.03,1.07) | 1.08 (1.04,1.12) | 1.06 (1.03,1.09) | 1.06 (1.03,1.09) | 1.10 (1.06,1.14) |
Only ORs (95% CIs) for putative risk factors significantly associated with ≥1 outcome measure in analyses of full sample data are shown (i.e., not the sample subset with BMI Z score change data at 0.5-1.5 years and 1.5-3.5 years).
Race not included in these models as the reported race for all children with SDB “Always” symptoms at these timepoints is White.
N/A because history of adenoidectomy was not assessed at 1.5 years.
Table S2.
Maternal, Family, and Child Characteristics Associated* with Mouth-Breathing, Snoring, or Apnea at 1.5, 4.75, and 6.75 years (“Habitual” vs. “Not Habitual”), Odds Ratios (95% CIs) for Subsample with SDB Data for 7/7 Timepoints.
| Covariates Habitual # Case/Total # | 1.5 years |
4.75 years |
6.75 years |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mouth-Breathing 479/5022 | Snoring 396/5249 | Apnea 353/4894 | Mouth-Breathing 1083/4930 | Snoring 677/5122 | Apnea 655/4724 | Mouth-Breathing 1046/4743 | Snoring 601/4901 | Apnea 551/4423 | |
| Maternal and Family Characteristics: | |||||||||
| Maternal Smoking, Ever | 1.16 (0.96,1.41) | 0.89 (0.72,1.10) | 1.07 (0.85,1.33) | 1.07 (0.93,1.23) | 1.00 (0.85,1.18) | 1.26 (1.06,1.49) | 1.17 (1.02,1.35) | 1.05 (0.88,1.25) | 1.32 (1.10,1.59) |
| Race (Other v. White) | 1.51 (0.71,3.21) | 2.99 (1.60,5.58) | 2.12 (1.03,4.38) | 1.16 (0.64,2.09) | 1.43 (0.75,2.70) | 1.07 (0.50,2.27) | 0.79 (0.35,1.63) | 0.97 (0.43,2.16) | 1.23 (0.57,2.63) |
| Housing, Inadequate (Yes v. No) | 0.84 (0.57,1.22) | 1.46 (1.03,2.08) | 1.28 (0.86,1.89) | 1.22 (0.97,1.52) | 1.32 (1.01,1.71) | 1.05 (0.79,1.38) | 1.32 (1.05,1.67) | 1.24 (0.94,1.65) | 1.05 (0.77,1.42) |
| Paternal Employment, Manual v. Professional | 1.05 (0.86,1.29) | 1.03 (0.82,1.29) | 1.14 (0.89,1.44) | 1.07 (0.92,1.24) | 1.05 (0.88,1.26) | 1.06 (0.88,1.27) | 1.14 (0.98,1.33) | 1.02 (0.84,1.23) | 1.11 (0.91,1.35) |
| Maternal Education, Lower v. Higher | 1.33 (1.07,1.64) | 1.29 (1.02,1.63) | 1.00 (0.79,1.27) | 1.40 (1.21,1.63) | 1.06 (0.88,1.27) | 1.15 (0.96,1.38) | 1.33 (1.14,1.55) | 1.20 (0.99,1.45) | 1.11 (0.91,1.36) |
| Other Child: | |||||||||
| 1 v. 0 | 1.06 (0.87,1.29) | 0.87 (0.70,1.09) | 0.85 (0.68,1.07) | 0.70 (0.61,0.81) | 0.88 (0.74,1.04) | 0.83 (0.70,0.99) | 0.75 (0.65,0.87) | 0.86 (0.72,1.03) | 0.91 (0.75,1.09) |
| > 1 v. 0 | 1.27 (0.77,2.09) | 1.49 (0.92,2.42) | 0.99 (0.56,1.75) | 0.75 (0.51,1.09) | 0.69 (0.43,1.11) | 1.02 (0.65,1.59) | 0.69 (0.46,1.03) | 1.30 (0.84,1.99) | 0.86 (0.52,1.44) |
| Child Characteristics: | |||||||||
| Gestational Age, (Continuous, in weeks) | 1.02 (0.96,1.09) | 0.95 (0.89,1.02) | 1.02 (0.95,1.10) | 0.95 (0.91,0.99) | 0.96 (0.91,1.01) | 1.04 (0.98,1.10) | 1.01 (0.97,1.06) | 1.03 (0.97,1.09) | 1.01 (0.95,1.07) |
| Gender (Male v. Female) | 1.11 (0.92,1.35) | 1.24 (1.00,1.53) | 0.94 (0.76,1.18) | 0.95 (0.83,1.09) | 0.93 (0.79,1.09) | 1.00 (0.85,1.19) | 1.06 (0.92,1.22) | 0.90 (0.75,1.07) | 1.23 (1.03,1.48) |
| Breastfed (No v. Yes) | 1.34 (1.07,1.69) | 1.26 (0.98,1.62) | 1.10 (0.83,1.45) | 1.23 (1.04,1.46) | 1.21 (0.99,1.49) | 1.09 (0.88,1.34) | 1.29 (1.09,1.54) | 1.18 (0.95,1.47) | 1.03 (0.82,1.29) |
| Adenoids Removed | N/A† | N/A† | N/A† | 0.43 (0.28,0.67) | 0.32 (0.20,0.50) | 0.60 (0.35,1.02) | 0.45 (0.31,0.64) | 0.40 (0.27,0.60) | 1.15 (0.68,1.93) |
| Wheezing Days | 1.03 (1.01,1.05) | 1.06 (1.04,1.08) | 1.06 (1.04,1.09) | 1.03 (1.02,1.04) | 1.05 (1.03,1.06) | 1.06 (1.05,1.08) | 1.05 (1.03,1.07) | 1.06 (1.04,1.08) | 1.06 (1.04,1.08) |
Only ORs (95% CIs) for putative risk factors significantly associated with ≥ 1 outcome measure in analyses of full sample data are shown (i.e., not the sample subset with BMI Z score change data at 0.5-1.5 years and 1.5-3.5 years).
N/A because history of adenoidectomy was not assessed at 1.5 years.