Abstract
Study Objectives:
To determine, in a clinical sample of obese adolescents, whether shorter sleep duration is associated with metabolic risk and obesity severity.
Design:
Cross-sectional study.
Setting:
Tertiary care weight-management clinic in Cincinnati, OH, USA.
Participants:
133 obese adolescents aged 10-16.9 years.
Interventions:
N/A.
Measurements:
Multifaceted sleep duration data were examined with fasting venipuncture and anthropometric data collected during clinical care. Primary Outcome: presence of metabolic syndrome. Secondary Outcomes: waist circumference, triglycerides, HDL-cholesterol, blood pressure, glucose, insulin resistance (HOMA-IR), and body mass index (BMI). Predictors: Sleep duration by (1) parent-report, (2) self-report, and (3) multi-night actigraphy. Analysis: Relationships between sleep duration and each outcome were examined via regression models, adjusted for potential confounders.
Results:
Regardless of how measured, sleep duration showed no strong association with metabolic syndrome (OR 1.1 to 1.5, P = 0.2 to 0.8), BMI (β −0.03 to −0.01, P = 0.2 to 0.8), or most other outcomes. Lower triglycerides were predicted by shorter sleep duration by self-report (β 12.3, P = 0.01) and actigraphy (β 13.6, P = 0.03), and shorter parent-reported sleep duration was associated with higher HDL-cholesterol (β = −2.7, P = 0.002).
Conclusions:
Contrary to expectations, sleep duration was not associated with metabolic outcomes, and showed limited associations with lipid profiles. Although inadequate sleep may affect other areas of functioning, it appears premature to expect that lengthening sleep will improve BMI or metabolic outcomes in clinical samples of obese adolescents.
Citation:
Sung V; Beebe DW; VanDyke R; Fenchel MC; Crimmins NA; Kirk S; Hiscock H; Amin R; Wake M. Does sleep duration predict metabolic risk in obese adolescents attending tertiary services? A cross-sectional study. SLEEP 2011;34(7):891-898.
Keywords: Sleep, obesity, metabolic syndrome, adolescent, cross sectional studies
INTRODUCTION
Childhood Obesity and the Metabolic Syndrome
The epidemic of childhood obesity presents a major public health problem, with up to 32% of US children and adolescents overweight or obese, and up to 16% obese (Centers for Disease Control [CDC] definitions).1 Complications from obesity involve multiple organ systems, which further compound the health risk to these children.
One cluster of obesity-linked complications, known as the metabolic syndrome, strongly predicts diabetes and early cardiovascular morbidity.2–5 In 2007, the International Diabetes Foundation defined metabolic syndrome in children as the presence of central obesity together with any 2 of the following 4 age-dependent factors: hypertriglyceridemia, low high-density lipoprotein (HDL) cholesterol, high blood pressure, and high fasting blood glucose.6 Metabolic syndrome has been reported in 25% to 50% of obese children and adolescents from clinical samples,7,8 and in 2% to 10% of obese and non-obese adolescents in population samples.9,10 Finding modifiable risk factors for metabolic syndrome could therefore prevent substantial cardiovascular morbidity, particularly in obese adolescents.
Sleep Duration, Childhood Obesity, and Metabolic Syndrome
A trend toward decreasing sleep duration has paralleled the obesity epidemic. A 2005 US national survey showed that 45% of adolescents have insufficient sleep (< 8 h/night) and a further 31% have borderline (8-9 h) sleep.11 Further, many studies now support an inverse linear relationship between short sleep duration and body mass index (BMI) in preadolescent children.12–16 This raises the possibility of a causal relationship, and therefore the potential for sleep related interventions.
However, results for adolescents have been less consistent,13 with some17–21 but not all22–24 reporting similar findings in population-based samples. One case-control study found differences between obese and demographically matched lean adolescents across a number of sleep variables, including duration, but did not report on associations between sleep duration and adiposity within the obese subgroup.25 A major weakness of research in this area is the exclusive reliance on subjective parent- or self-reported sleep measures, with one study of 7-year-old children and 3 studies of adolescents including actigraphy20,25–27 as objective measures of sleep.
Adult studies have shown sleep duration to be associated with the metabolic syndrome and diabetes.28 The very few metabolic studies exploring associations in children suggest relationships between sleep duration and insulin resistance and waist circumference,29–31 but are inconsistent. No study has found associations between sleep duration and hypertension, glucose levels, or lipid abnormalities in the pediatric population. Further, although obstructive sleep apnea (OSA) is common among obese youth,25 there is considerable disagreement as to whether its presence or severity does32–37 or does not38–40 contribute to adolescent metabolic syndrome, over and above the contribution of obesity itself. Thus OSA could obscure or confound the relationship between sleep duration and the metabolic syndrome, especially in obese adolescents.
Aims and Hypotheses
Despite this conflicting literature on associations among child and adolescent sleep duration, BMI, and metabolic risk, sleep is being explored as a possible treatment target to minimize the risk for cardiovascular morbidity. This seems premature until associations are confirmed and quantified and, if found to be important, causal directions established. To address these issues, we studied a large clinical sample of children and adolescents attending a tertiary hospital outpatient weight management program for whom measured sleep duration, anthropometry, and markers of metabolic syndrome were all available.
Specifically, we aimed to determine, in obese 10- to 16.9-year-olds, cross-sectional associations between sleep duration and the metabolic syndrome. We also aimed to determine if associations existed between sleep duration and individual components of the metabolic syndrome (i.e., lipid markers, blood pressure, fasting glucose), as well as insulin resistance, and adiposity. We hypothesized that sleep duration would be inversely associated with metabolic syndrome, its individual components, adiposity, and insulin resistance in a manner that is statistically independent of OSA.
METHODS
Design and Setting
A sample of obese adolescents was consecutively recruited between 2003 and 2008 at intake into the Cincinnati Children's Hospital Medical Center (CCHMC) multidisciplinary pediatric weight-management clinic, as part of a larger study investigating the relationship between sleep and neurobehavioral functioning in adolescents.41 Demographic and sleep data were prospectively collected, and anthropometric and metabolic data were collected as part of each family's comprehensive intake evaluation for the clinic. Both the original study and the merging of research and clinical databases for current analyses were approved and overseen by the Institutional Review Board (IRB) of CCHMC. All parents provided written informed consent, and all participants provided written assent for collection of research-specific data and for review of the participant's medical record for data collected during clinical care.
Sample
Adolescents attending the clinic were eligible if they were 10 to 16.9 years of age and had a body mass index (BMI, kg/m2) ≥ 95th percentile for age and sex, using CDC definitions.42 Exclusion criteria for current analyses included neurological history (e.g., seizures), craniofacial abnormalities, developmental disorders (e.g., Down syndrome), conditions involving daytime hypoxia (e.g., poorly controlled asthma), use of psychiatric or neurological medication, and treatment for OSA in the past 2 years.
Overview of Procedures
At the initial visit to the clinic, demographics and anthropometric data, including weight, height, waist circumference, and blood pressure were routinely collected. Metabolic markers, including triglycerides, HDL-cholesterol, glucose, and insulin, were routinely collected by a 12-h fasting blood test performed within a month of the initial medical visit. All serum analyses were conducted at the same clinically accredited lab at CCHMC, and the procedure for obtaining the fasting laboratory measures was the same in all cases. During the initial clinic visit, an investigator (DWB) briefly introduced himself and the study, then followed up with interested families via telephone to conduct a more detailed discussion and screening interview. Once eligibility and participation were confirmed, a booking was made for an overnight polysomnogram (PSG) and for actigraphy, usually in the week immediately before the PSG. The PSGs were conducted at a mean of 14 weeks after the initial clinic visit. Parent and participant questionnaires were independently completed in the context of an afternoon neurobehavioral assessment (detailed in our prior report),41 which in nearly all cases occurred the afternoon prior to the PSG, and in all cases occurred within 1 month of the PSG.
MEASURES
Sleep Duration Predictor Measures
(1) Subjective sleep parameters: Parent-reported adolescent sleep duration was recorded by the Child Sleep Habits Questionnaire,43 and self-reported sleep duration by the Youth Sleep Habits Survey.44,45 Both reporters were asked how long the participant typically slept, as well as when he or she typically goes to bed and rises for the day, on school nights. Similar questions are widely used in sleep surveys, and have been found to correlate strongly with objective actigraphy data, especially on school nights.44 For each reporter, we computed sleep duration as the mean of the direct report of sleep duration and the arithmetic difference between reported bedtime and rise time.
(2) Objective actigraphy: As previously described,25 adolescent participants were asked to wear motion-sensitive actigraphs (Mini-Motionlogger, Ambulatory Monitoring, Inc., NY) the size of sports wristwatches on their non-dominant wrists during evening and overnight hours for a week. The units were provided in person by study staff or via overnight mail, accompanied by detailed written instructions that were also reviewed via telephone. Subjects were asked to wear the units at home during evening and overnight hours during their normal sleep routine. Raw data on participant movement patterns were cross-checked against a nightly sleep diary to help screen for artifacts (e.g., actigraph removal) prior to being submitted to an automated scoring algorithm that estimated sleep onset and offset based upon movement patterns; the results of this algorithm have been shown to correlate highly with PSG-determined sleep.46 Sleep duration was calculated as the offset minus onset times averaged over the nights the unit was worn.
Laboratory Measures
All serum analyses were conducted at the same clinically accredited lab at CCHMC, and the procedure for obtaining the fasting blood work and laboratory measures was the same in all cases. Plasma glucose was measured using a Hitachi model 704 glucose analyzer. Plasma insulin was measured by RIA using an anti-insulin serum raised in guinea pegs, 125I labeled insulin (Linco, St. Louis, MO), and a double antibody method to separate bound from free tracer. Assays of fasting plasma lipid profiles were carried out in a laboratory that is National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention standardized, with the low-density lipoprotein (LDL) cholesterol concentration calculated using the Friedewald equation.
Anthropometric Measures
Weight was measured by a digital scale, and height measured using a calibrated wall-mounted stadiometer while subjects were in light clothing and without shoes. Waist circumference was measured using a standardized protocol where the circumference measurement is taken just above the uppermost lateral border of the right iliac crest.47 Blood pressure was measured using an appropriate-sized cuff, and the mean blood pressure value was taken from 2 readings.
Definitions and Calculations
The presence of metabolic syndrome was defined according to the 2007 International Diabetes Foundation criteria6,48 as: waist circumference ≥ 90th percentile for age/gender49 and 2 of the following: (a) fasting triglycerides ≥ 150 mg/dL, (b) fasting HDL-cholesterol < 40 mg/dL, (c) systolic or diastolic BP ≥ 90th percentile,50 and (d) fasting glucose ≥ 100 mg/dL. HOMA-IR was calculated by [fasting serum insulin (μU/mL) × fasting plasma glucose (mmol/L)] divided by 22.5.51–53 BMI (kg/m2) was transformed to BMI z-scores using published CDC tables that adjust for age and sex.42
Potential Confounders/Covariate Measures
All potential confounders were identified a priori. Parent-reported demographic variables included age, gender, race (coded as white/non-white, with 96% of non-white participants being African American) and socioeconomic status, all of which are known to be associated with sleep duration, metabolic risk, or both. Socioeconomic status was represented as a SES composite index, computed as the mean z-score within the sample across 4 variables: maternal education, paternal education, annual family income and median local income based upon the 2000 US Census for the subject's postal code of residence, prorating for any missing fields (e.g., paternal education unreported).41 For the metabolic analyses, BMI z-score was also included because of its previously reported independent associations with both sleep duration and metabolic syndrome.
Finally, OSA was entered as a covariate because OSA severity may independently influence metabolic markers, is associated with obesity, may affect sleep duration,25,41 and may introduce variance into the quality of sleep of any duration. OSA, as indexed by the apnea+hypopnea index (AHI), was measured during overnight PSG, conducted during a single night in an inpatient pediatric sleep unit.25,41 The AHI was computed as the sum of obstructive apneas and hypopneas divided by hours slept. Obstructive apnea was defined as > 80% decline in airflow over ≥ 2 breaths, despite continued chest/abdominal wall movement. Obstructive hypopneas were defined as a decrease of 50% to 80% in airflow over ≥ 2 breaths associated with (a) paradoxical respiration and (b) oxyhemoglobin desaturation (≥ 4%) or a subsequent arousal.
Statistical Analysis
For our primary analysis, 3 logistic regression models examined the change in odds of participants displaying metabolic syndrome by sleep duration for each of the 3 sleep duration measures (parent-report, self-report, and actigraphy). Similarly, the secondary outcomes (all continuous variables) were examined using multiple linear regression models. All regression models included the covariates identified above, with AHI log-transformed to approximate normality, and BMI z-score included only in the metabolic outcome analyses only.
Analyses were conducted using SAS 9.2 software (SAS Institute, Cary, NC). Post hoc power analyses were conducted based upon the available sample size and our observed distributions of the predictors, outcomes, and covariates. Assuming a population base rate of metabolic syndrome of 25% in obese adolescents (the observed rate in this sample was 23%), α = 0.05, and 120 subjects, our primary analyses had 80% power to detect an odds ratio of 1.95 for a 1-h increase in sleep duration. Power would be even higher if the base rate of metabolic syndrome in obese adolescents exceeded 25, as suggested by some authors.7,8,54,55
RESULTS
Sample Description
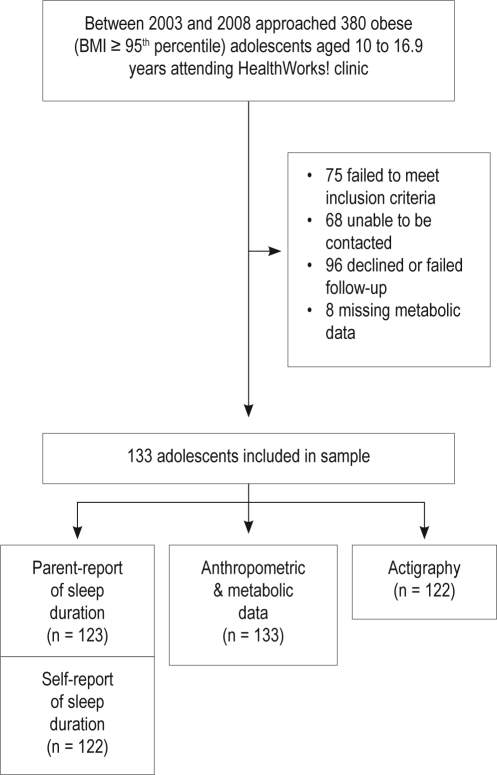
Figure 1 shows the participant flow and reasons for non-participation. Of the 380 families attending clinic, 247 were approached and deemed to be eligible for the study, of whom 133 (54%) participated and provided anthropometric and metabolic data. Of these, 92% of parents and adolescents also reported sleep duration, and 91% had actigraphy data (worn on average 5.8 ± 1.3 nights).
Figure 1.
Participant flow chart
Table 1 summarizes the demographic, sleep, anthropometric, and metabolic features of the sample. Adolescents who participated in the study did not significantly differ from non-participants in age or sex (P = 0.4-0.5). Because we did not have consent to access additional information on non-participants, it is not known whether participants and non-participants differed on other study variables. By design, the sample was obese, with a mean BMI of 37 kg/m2; and participants' mean sleep duration was < 9 h. More than half of the sample (56%) experienced some degree of sleep disordered breathing, and 23% met criteria for the diagnosis of metabolic syndrome. A minority had individually significant markers of metabolic risk, except for low HDL-cholesterol, which occurred in more than half of the sample, and high waist circumference, which was present in almost the entire sample.
Table 1.
Demographic, sleep and metabolic characteristics of the sample (n = 133)
| Characteristics | % or Mean (SD) or Median (range) |
|---|---|
| Child | |
| Male sex (%) | 34 |
| Age (years), mean (SD) | 13.2 (1.8) |
| Race: white (%) | 32 |
| BMI (kg/m2), mean (SD) | 37.0 (7.2) |
| BMI-z score, mean (SD) | 2.5 (0.3) |
| Sleep duration (hours per night), median (range) | |
| Parent-report | 8.5 (6.3 – 11.8) |
| Self-report | 8.3 (5.5 – 13.2) |
| Actigraphy | 7.8 (4.9 – 9.5) |
| Obstructive sleep apnea: AHI ≥ 1 (%) | 56 |
| Metabolic Syndrome present (%) | 23 |
| Markers of metabolic risk (%) | |
| Waist circumference ≥ 90th percentile | 98 |
| Triglycerides ≥ 150 mg/dL | 17 |
| HDL cholesterol < 40 mg/dL | 52 |
| Systolic blood pressure ≥ 90th percentile | 13 |
| Diastolic blood pressure ≥ 90th percentile | 12 |
| Fasting glucose levels ≥ 100 mmol/L | 11 |
Primary Outcome
There were no significant differences in sleep duration among adolescents with a diagnosis of metabolic syndrome and those without (Table 2), although small increases in the odds of having metabolic syndrome were observed for each h increase in sleep duration (parent-report OR = 1.2, 95% CI = 0.7-2.0; self-report OR = 1.1, 95% CI = 0.6-1.8; actigraphy OR = 1.5, 95% CI = 0.8-2.7, P = 0.2-0.8). Further, AHI was not a significant covariate in any of the logistic regression models.
Table 2.
Sleep duration amongst adolescents with and without the diagnosis of metabolic syndrome
| Sleep duration (hours per night), median (range) | With metabolic syndrome (n = 28) | Without metabolic syndrome (n = 105) |
|---|---|---|
| Parent-report | 8.5 (6.5 – 11.5) | 8.5 (6.3 – 11.8) |
| Self-report | 8.5 (6.2 – 9.7) | 8.1 (5.5 – 13.2) |
| Actigraphy | 7.9 (5.6 – 9.5) | 7.8 (4.9 – 9.3) |
Secondary Outcomes
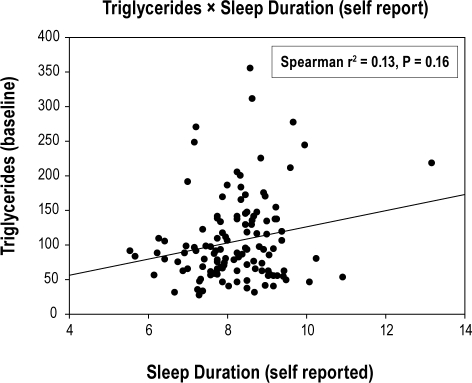
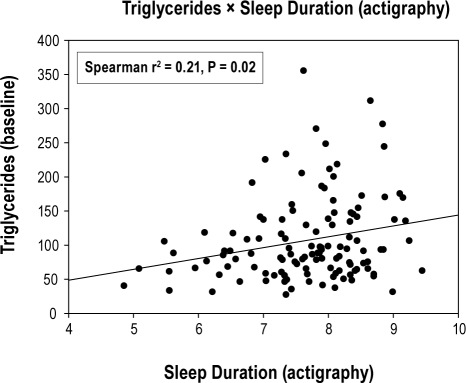
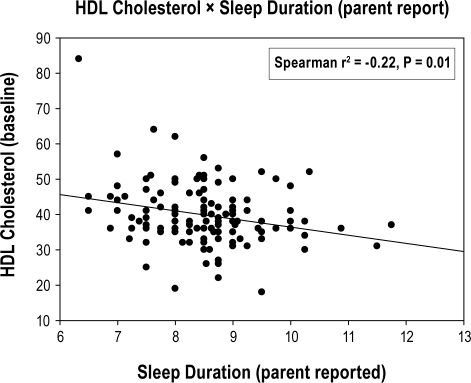
There was a lack of relationship between sleep duration and all secondary outcomes, except for triglycerides and HDL-cholesterol that were in the opposite direction to that hypothesized (Table 3). Longer sleep duration by self-report and actigraphy predicted worse (i.e., higher) triglyceride levels (β = 12.3 [P = 0.01] and 13.6 [P = 0.03], respectively; Figs. 2A and 2B), while longer sleep duration by parent-report predicted worse (i.e., lower) HDL-cholesterol levels (β = −2.7 [P = 0.002]; Figure 2C). These unanticipated relationships between sleep duration and triglycerides/cholesterol displayed heteroscedasticity—i.e., there was greater variability at one end of the sleep duration than at the other. Re-analysis of each secondary outcome using multiple logistic regression and dichotomizing according to cutpoints used to determine metabolic syndrome yielded nearly identical conclusions to the linear analyses.
Table 3.
Regression of (Secondary) BMI and metabolic outcomes on sleep duration
| Sleep duration (hours/night) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome Variable | Parent-report |
Self-report |
Actigraphy |
||||||
| Beta Coeff | 95% CI | P | Beta Coeff | 95% CI | P | Beta Coeff | 95% CI | P | |
| BMI z-scorea | -0.02 | -0.07, 0.02 | 0.4 | -0.01 | -0.05, 0.04 | 0.8 | -0.03 | -0.09, 0.02 | 0.2 |
| Metabolic markersb | |||||||||
| Waist circumference (cm) | -1.6 | -3.6, 0.5 | 0.1 | 0.6 | -1.2, 2.3 | 0.5 | 0.3 | -1.8, 2.4 | 0.8 |
| Triglycerides (mg/dL) | 2.3 | -9.6, 14.1 | 0.7 | 12.3* | 2.5, 22.1 | 0.01 | 13.6* | 1.1, 26.1 | 0.03 |
| HDL-cholesterol (mg/dL) | -2.7* | -4.4, -1.0 | 0.002 | -0.9 | -2.6, 0.7 | 0.3 | -0.8 | -2.8, 1.2 | 0.4 |
| Systolic blood pressure (mmHg) | 0.05 | -0.2, 0.3 | 0.6 | -0.04 | -0.2, 0.1 | 0.7 | 0.05 | -0.2, 0.3 | 0.7 |
| Diastolic blood pressure(mmHg) | 0.01 | -0.2, 0.2 | 1.0 | -0.03 | -0.2, 0.1 | 0.7 | -0.2 | -0.2, 0.02 | 0.1 |
| Fasting glucose (mg/dL) | -0.5 | -2.1, 1.1 | 0.5 | -1.3 | -2.6, 0.1 | 0.07 | -0.8 | -2.6, 0.9 | 0.4 |
| HOMA-IR | 0.2 | -0.8, 1.2 | 0.7 | -0.02 | -0.8, 0.8 | 1.0 | 0.8 | -0.2, 1.8 | 0.1 |
Significant association in opposite direction to hypothesized relationship.
Adjusted for age, gender, race, socio-economic status, and obstructive apnea.
Adjusted for age, gender, race, socio-economic status, BMI z-score, and obstructive apnea.
Figure 2A.
Relationship between self-reported sleep duration (hours/night) and fasting triglyceride levels (mg/dL)
Figure 2B.
Relationship between actigraphy-measured sleep duration (hours/night) and fasting triglyceride levels (mg/dL)
Figure 2C.
Relationship between parent-reported sleep duration (hours/night) and fasting HDL cholesterol levels (mg/dL)
After controlling for covariates, sleep duration did not predict BMI, BMI z-score, or waist circumference, nor did it predict insulin resistance as measured by HOMA-IR and fasting insulin levels (all P > 0.05). AHI was a significant covariate for modeling waist-circumference against self-reported sleep duration (β estimate for log-AHI = 5.68, P = 0.03) and baseline-BMI z-score against actigraphy sleep duration (β estimate for log-AHI = 0.14, P = 0.04). AHI was not a significant covariate in any of the other models for secondary outcomes.
DISCUSSION
Shorter sleep duration did not predict the presence of the metabolic syndrome, adiposity, or insulin resistance in this sample of obese children and adolescents. Moreover, short sleep duration was unexpectedly associated with better triglyceride and HDL-cholesterol levels, although the strength and statistical significance of these effects varied across three different measures of sleep duration.
This study is the first to comprehensively describe the relationship between measured sleep duration and all aspects of the metabolic syndrome, while adjusting for potential confounders, in a group of high-risk obese children and adolescents. While conventional statistical methods are not designed to accept the null hypothesis, this study had a number of strengths that lend confidence that, at least within this subpopulation, any relationship between short sleep duration and the presence of metabolic syndrome or its elements is, at most, quite small. A post hoc power analysis suggested that the sample was of adequate size to detect even a relatively small effect at a liberal significance level of 0.05. For our primary outcome and several secondary outcomes, the nonsignificant trends ran opposite to predictions, suggesting that increasing the sample size would be extremely unlikely to find effects in the direction hypothesized. Further, the failure to find significant effects across multiple measures of sleep duration, including multi-night actigraphy as well as parent- and self-report, strongly argues against measure invalidity as a cause of nonsignificant results. The only other commonly used sleep measure in this age range, PSG, is the gold standard for measuring OSA, but is a poor measure of typical free-living sleep duration because it introduces artificial influences that can fundamentally alter sleep patterns (e.g., presence of monitoring leads and equipment).
Complementing our strong sleep measurement, this study adopted an internationally recognized classification of metabolic syndrome. Individual metabolic markers were also analyzed to ensure that potentially important findings did not emerge for specific elements of the metabolic syndrome, suggesting that alternate metabolic syndrome classifications would not change our conclusions. Further, aside from waist circumference, there was good variability in both the predictors and outcomes, suggesting that associations were not attenuated by a restriction in range. Finally, potential confounders were identified a priori and included in the analyses. In particular, OSA, which was prevalent in this sample, was objectively measured and included as a covariate.
There are some limitations to this study. As this was a cross-sectional study, it remains possible that short sleep increases the risk for the initial development of obesity and/or precursors of metabolic syndrome, which are then maintained and exacerbated by other factors. Such an effect would only be evident in a longitudinal model, perhaps complemented by within-subjects experimental sleep restriction studies that allow for better control of between-subjects variance. Measures for the current study were obtained at different time-points, and there was a general time-lag between the metabolic and sleep measures; insofar as sleep or metabolic functioning might have fluctuated over time this could have attenuated associations. While our recruitment from a weight management clinic allowed us to efficiently enroll significantly obese adolescents, these findings may not generalize to obese, non–treatment-seeking individuals. Detailed information on physical activity, diet, medication, smoking, and alcohol drinking were not systematically collected in the study, and therefore could not be considered as potential confounders. Although we observed considerable variability in sleep duration and the large majority of participants obtained less than the recommended 9 hours of sleep, very few had sleep duration < 6 hours (< 2% per parent-report or self-report, 6% per actigraphy). Thus it remains possible that markedly short sleep duration is associated with metabolic syndrome. Finally, this was not an intervention study that could more definitively speak to whether sleep duration modification alters metabolic risk factors within individuals; however, across obese adolescents, there appears to be minimal relationship between sleep duration and metabolic risk.
Some of our results are similar to, and some differ from, previous studies in the pediatric population. We did not find shorter sleep duration to be associated with insulin resistance (in contrast to Flint's retrospective chart review of 40 obese 3.5- to 18.5-year-olds undergoing overnight PSG29) or waist circumference (in contrast to Verhulst's PSG study of 104 obese 6- to 17-year-olds30 and Hitze's population study of 414 6- to 20-year-olds31). However, in common with all three of these studies, sleep duration was not associated with blood pressure.29–31
To our knowledge, this is the only study that has found an association between longer sleep duration and worse triglycerides and HDL-cholesterol. While it is possible that this is a chance finding, it seems unlikely that we have missed a true association in the opposite direction, particularly given the objective measurement of free-living sleep duration. Several factors could explain the differences in results. No previous study that examined sleep and triglycerides or HDL-cholesterol included OSA or BMI z-score as covariates in the analyses. Flint's and Verhulst's studies reported sleep duration from a single night's PSG which, for reasons noted above, may not accurately reflect free-living sleep duration, while Hintze's study measured sleep duration only by child report in a non-overweight sample. Further research using at least equally reliable measures is needed to ascertain any true associations between sleep duration and lipids in obese children and adolescents. Such research should entertain the possibility of non-linear relationships, such as the U-shaped associations of sleep duration with obesity56 and some metabolic markers57,58 seen in some adult studies.
Like Flint and Verhulst, we did not find an association between short sleep duration and high BMI within the subpopulation of obese adolescents. This contrasts with the widely reported inverse relationship between sleep duration and BMI in preadolescent and adolescent non-obese samples.12–21 While a range of adiposity was represented within our sample, that range was nevertheless restricted by definition, which could have statistically attenuated any sleep-weight relationship. Also, body mass index and related age- and sex-adjusted BMI scores may become less sensitive measures of adiposity in the most obese subpopulations.59 Alternatively, the impact of sleep on body mass may be greatest within the large majority of the population which, despite secular trends towards overweight, continues to fall well below eligibility criteria for the current study.
Given that the relationship between obesity and metabolic risk is well established, the current science suggests that primary interventions to prevent or address obesity are more likely to reduce metabolic risk than are interventions to improve sleep in adolescents who are already obese. This is not to say that sleep is unimportant. There is evidence that OSA, which is common in this population, is associated with poor attention and classroom learning41 and that the effects of inadequate sleep on neurobehavioral functioning have been impressively larger than the relationships between short sleep and either obesity or metabolic syndrome.60 Thus, interventions to improve the sleep of obese youth may yield important health benefits, even if not in the weight or metabolic arenas.
CONCLUSION
Despite evidence in adults that sleep duration is associated with the metabolic syndrome, hypertension, diabetes, cardiovascular morbidity, and obesity, these associations may not apply to clinically obese adolescents. Obese adolescents often have poor sleep quality, and improving this may have a number of health benefits (e.g., improved behavioral functioning and quality of life). However, prolonging sleep duration may not improve metabolic risk in this clinically at-risk group.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was conducted at the Cincinnati Children's Hospital Medical Center, Cincinnati, OH, USA. The study and current analyses/write-up were supported by the American Sleep Medicine Foundation (22-YI-03), National Institutes of Health (K23 HL075369, UL1 RR026314), and Australian NHMRC Career Development Awards 546405 and 607351 and Capacity Building Grant 436914.
REFERENCES
- 1.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003-2006.[see comment] JAMA. 2008;299:2401–5. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 2.Nathan BM, Moran A. Metabolic complications of obesity in childhood and adolescence: more than just diabetes. Curr Opin Endocrinol Diabetes Obes. 2008;15:21–9. doi: 10.1097/MED.0b013e3282f43d19. [DOI] [PubMed] [Google Scholar]
- 3.Fennoy I. Metabolic and respiratory comorbidities of childhood obesity. Pediatr Ann. 2010;39:140–6. doi: 10.3928/00904481-20100223-08. [DOI] [PubMed] [Google Scholar]
- 4.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120:340–45. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 5.Berenson GS, Srinivasan SR, Bao W, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. New Engl J Med. 1998;338:1650–56. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 6.Zimmet P, Alberti G, Kaufman F, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–61. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 7.Sabin MA, Ford AL, Holly JM, Hunt LP, Crowne EC, Shield JP. Characterisation of morbidity in a UK, hospital based obesity clinic. Arch Dis Child. 2006;91:126–30. doi: 10.1136/adc.2005.083485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. New Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 9.Pirkola J, Tammelin T, Bloigu A, et al. Prevalence of metabolic syndrome at age 16 using the International Diabetes Federation paediatric definition. Arch Dis Child. 2008;93:945–51. doi: 10.1136/adc.2007.132951. [DOI] [PubMed] [Google Scholar]
- 10.Cook SP, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med. 2003;157:821–27. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 11.National Sleep Foundation. Sleep in America Poll. [Accessed 19th October 2010]. http://www.sleepfoundation.org/sites/default/files/2006_summary_of_findings.pdf. Accessed.
- 12.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults.[see comment] Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity. 2008;16:265–74. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 14.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence.[see comment] Sleep Med Rev. 2008;12:289–98. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Hart CN, Jelalian E. Shortened sleep duration is associated with pediatric overweight. Behav Sleep Med. 2008;6:251–67. doi: 10.1080/15402000802371379. [DOI] [PubMed] [Google Scholar]
- 17.Chen MY, Wang EK, Jeng YJ. Adequate sleep among adolescents is positively associated with health status and health-related behaviors. BMC Public Health. 2006;6 doi: 10.1186/1471-2458-6-59. doi: 10.1186/1471-2458-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landis AM, Parker KP. A retrospective examination of the relationship between body mass index and polysomnographic measures of sleep in adolescents. J Adolesc Health. 2007;40:89–91. doi: 10.1016/j.jadohealth.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seicean A, Redline S, Seicean S, et al. Association between short sleeping hours and overweight in adolescents: results from a US Suburban High School survey. Sleep Breath. 2007;11:285–93. doi: 10.1007/s11325-007-0108-z. [DOI] [PubMed] [Google Scholar]
- 20.Benefice E, Garnier D, Ndiaye G. Nutritional status, growth and sleep habits among Senegalese adolescent girls. Eur J Clin Nutr. 2004;58:292–301. doi: 10.1038/sj.ejcn.1601781. [DOI] [PubMed] [Google Scholar]
- 21.Bawazeer NM, Al-Daghri NM, Valsamakis G, et al. Sleep duration and quality associated with obesity among Arab children. Obesity. 2009;17:2251–3. doi: 10.1038/oby.2009.169. [DOI] [PubMed] [Google Scholar]
- 22.Eisenmann JC, Ekkekakis P, Holmes M. Sleep duration and overweight among Australian children and adolescents. Acta Paediatr. 2006;95:956–63. doi: 10.1080/08035250600731965. [DOI] [PubMed] [Google Scholar]
- 23.Gibson S, Lambert J, Neate D. Associations between weight status, physical activity, and consumption of biscuits, cakes and confectionery among young people in Britain. Nutrition Bulletin. 2004;29:301–09. [Google Scholar]
- 24.Knutson KL. Sex differences in the association between sleep and body mass index in adolescents. J Pediatr. 2005;147:830–4. doi: 10.1016/j.jpeds.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Beebe DW, Lewin D, Zeller M, et al. Sleep in overweight adolescents: shorter sleep, poorer sleep quality, sleepiness, and sleep-disordered breathing. J Pediatr Psychol. 2007;32:69–79. doi: 10.1093/jpepsy/jsj104. [DOI] [PubMed] [Google Scholar]
- 26.Nixon GM, Thompson JM, Han DY, et al. Short sleep duration in middle childhood: risk factors and consequences. Sleep. 2008;31:71–8. doi: 10.1093/sleep/31.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta NK, Mueller WH, Chan W, Meininger JC. Is obesity associated with poor sleep quality in adolescents? Am J Hum Biol. 2002;14:762–8. doi: 10.1002/ajhb.10093. [DOI] [PubMed] [Google Scholar]
- 28.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9:S23–S28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flint J, Kothare SV, Zihlif M, et al. Association between inadequate sleep and insulin resistance in obese children.[see comment] J Pediatr. 2007;150:364–9. doi: 10.1016/j.jpeds.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 30.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep duration and metabolic dysregulation in overweight children and adolescents. Arch Dis Child. 2008;93:89–90. doi: 10.1136/adc.2007.124768. [DOI] [PubMed] [Google Scholar]
- 31.Hitze B, Bosy-Wesphal A, Bielfeldt F, et al. Determinants and impact of sleep duration in children and adolescents: data of the Kiel Obesity Prevention Study. Eur J Clin Nutr 2008. doi: 10.1038/ejcn.2008.41. doi: 10.1038/ejcn.2008.41. [DOI] [PubMed] [Google Scholar]
- 32.Waters KA, Mast BT, Vella S, et al. Structural equation modeling of sleep apnea, inflammation, and metabolic dysfunction in children. J Sleep Res. 2007;16:388–95. doi: 10.1111/j.1365-2869.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- 33.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing and the metabolic syndrome in overweight and obese children and adolescents. Journal of Pediatrics. 2007;150:608–12. doi: 10.1016/j.jpeds.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 34.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176:401–8. doi: 10.1164/rccm.200703-375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de la Eva RC, Baur LA, Donaghue KC, Waters KA. Metabolic correlates with obstructive sleep apnea in obese subjects.[see comment] J Pediatr. 2002;140:654–9. doi: 10.1067/mpd.2002.123765. [DOI] [PubMed] [Google Scholar]
- 36.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177:1142–9. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sans Capdevila O, Kheirandish-Gozal L, Tauman R, et al. Sleep disordered breathing and metabolic dysfunction in children: a pre- vs. post-adenotonsillectomy study. Sleep. 2006;29:A74. [Google Scholar]
- 38.Sharma SK, Kumpawat S, Goel A, Banga A, Ramakrishnan L, Chaturvedi P. Obesity, and not obstructive sleep apnea, is responsible for metabolic abnormalities in a cohort with sleep-disordered breathing. [see comment] Sleep Med. 2007;8:12–7. doi: 10.1016/j.sleep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 39.Waters KA, Sitha S, O'Brien L M, et al. Follow-up on metabolic markers in children treated for obstructive sleep apnea. Am J Respir Crit Care Med. 2006;174:455–60. doi: 10.1164/rccm.200401-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tauman R, O'Brien LM, Ivanenko A, Gozal D. Obesity rather than severity of sleep-disordered breathing as the major determinant of insulin resistance and altered lipidemia in snoring children. Pediatrics. 2005;116:e66–73. doi: 10.1542/peds.2004-2527. [DOI] [PubMed] [Google Scholar]
- 41.Beebe DW, Ris MD, Kramer ME, Long E, Amin R. The association between sleep-disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep. doi: 10.1093/sleep/33.11.1447. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 43.Owens , S A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–51. [PubMed] [Google Scholar]
- 44.Wolfson AR, Carskadon MA, Acebo C, et al. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26:213–16. doi: 10.1093/sleep/26.2.213. [DOI] [PubMed] [Google Scholar]
- 45.Acebo C, Carskadon MA. Cambridge, UK: Cambridge University Press; 2002. Influence of irregular sleep patterns on waking behavior. [Google Scholar]
- 46.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-week identification: An empirical test of methodological issues. Sleep. 1994;17:201–7. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 47.National Center for Health Statistics. Washington, DC: U.S. Government Printing Office; 1996. NHANES III Anthropometric Video. [Google Scholar]
- 48.Zimmet P, Alberti KG, Kaufman F, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–44. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 50.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and A. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents.[see comment] Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 51.McAuley KA, Mann JI, Chase JG, Lotz TF, Shaw GM. Point: HOMA - satisfactory for the time being. Diabetes Care. 2007;30:2411–13. doi: 10.2337/dc07-1067. [DOI] [PubMed] [Google Scholar]
- 52.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 53.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 54.Viner RM, Segal TY, Lichtarowicz-Krynska E, Hindmarsh P. Prevalence of insulin resistance syndrome in obesity. Arch Dis Child. 2005;90:10–14. doi: 10.1136/adc.2003.036467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. New Engl J Med. 2002;346:802–10. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 56.Marshall NS, Glozier N, Grunstein RR, Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12:289–98. doi: 10.1016/j.smrv.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 57.Choi KM, Lee JS, Park HS, Baik SH, Choi DS, Kim SM. Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. Int J Obes. 2008;32:1091–7. doi: 10.1038/ijo.2008.62. [DOI] [PubMed] [Google Scholar]
- 58.Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635–43. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woo JG. Using body mass index Z-score among severely obese adolescents: A cautionary note. Int J Pediatr Obes. 2009:1–6. doi: 10.3109/17477160902957133. iFirst article. [DOI] [PubMed] [Google Scholar]
- 60.Horne J. Short sleep is a questionable risk factor for obesity and related disorders: statistical versus clinical significance. Biol Psychol. 2008;77:266–76. doi: 10.1016/j.biopsycho.2007.12.003. [DOI] [PubMed] [Google Scholar]